Evaluating Agro-Based Waste Materials for Cyanotoxin Sorption for Future Incorporation in Nature-Based Solution Units (NBSUs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sorbent Materials
2.2. Cyanotoxin Preparation
2.3. Sorption Studies
2.3.1. Screening of MC-LR and CYN Sorption by Selected Sorbents
2.3.2. Sorption Kinetics
2.3.3. Sorption Isotherm Studies
2.4. Cyanotoxin Analysis
2.5. Characterization of Sorbent Materials
2.6. Data Treatment
3. Results
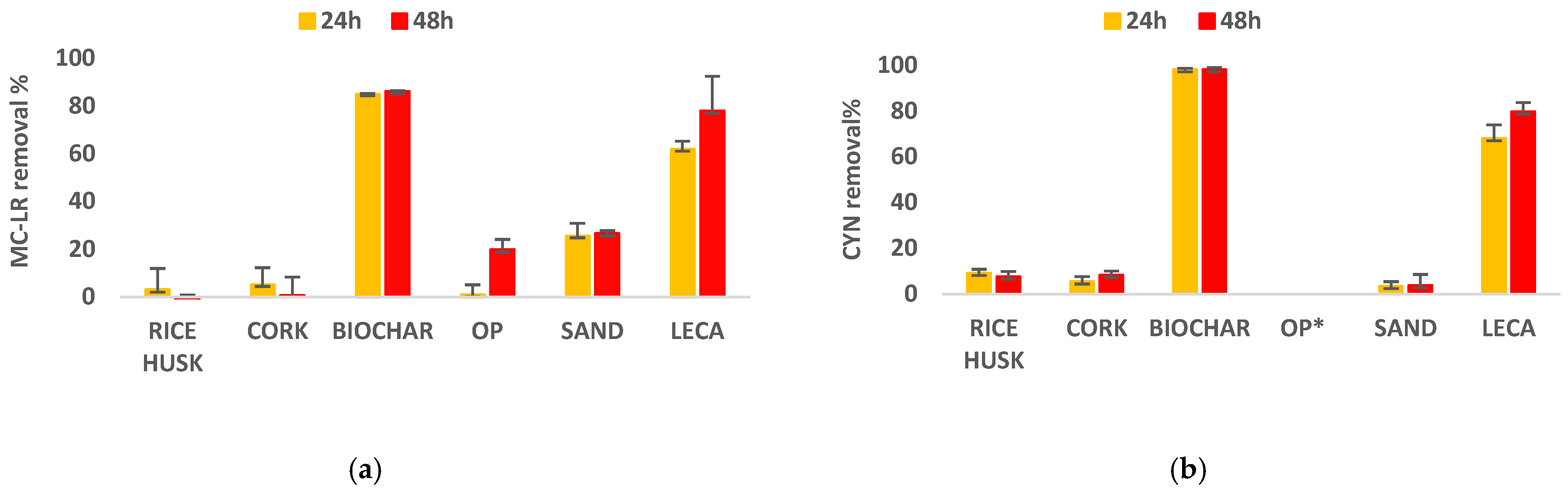
3.1. Pre-Screening of Sorbents
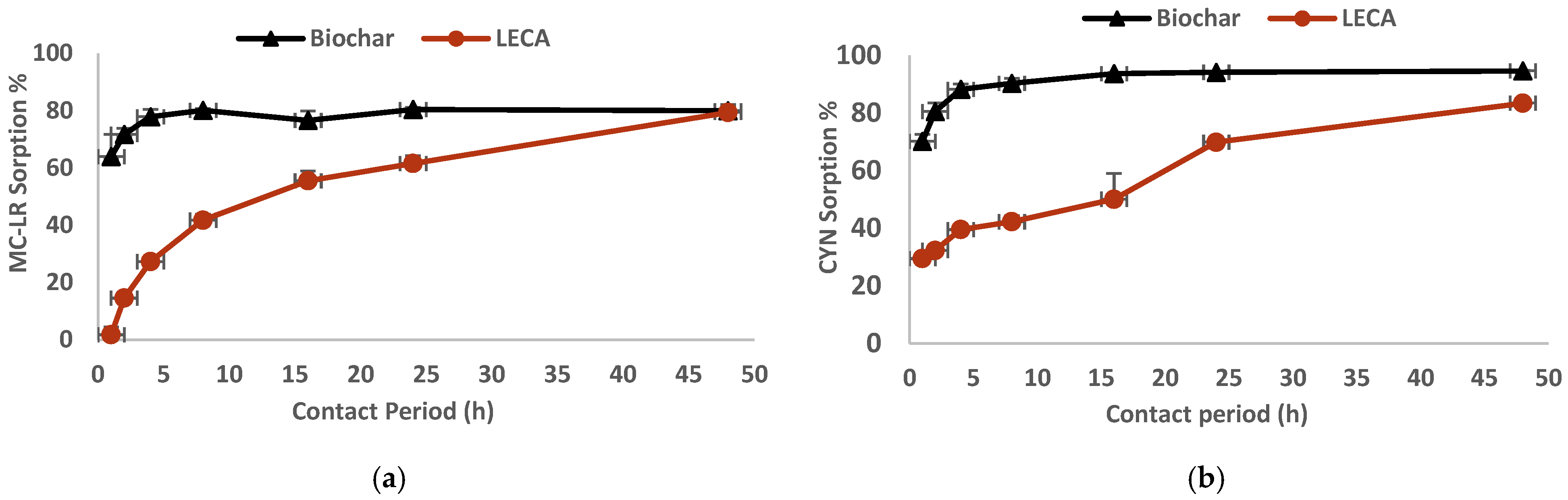
3.2. Kinetic Studies
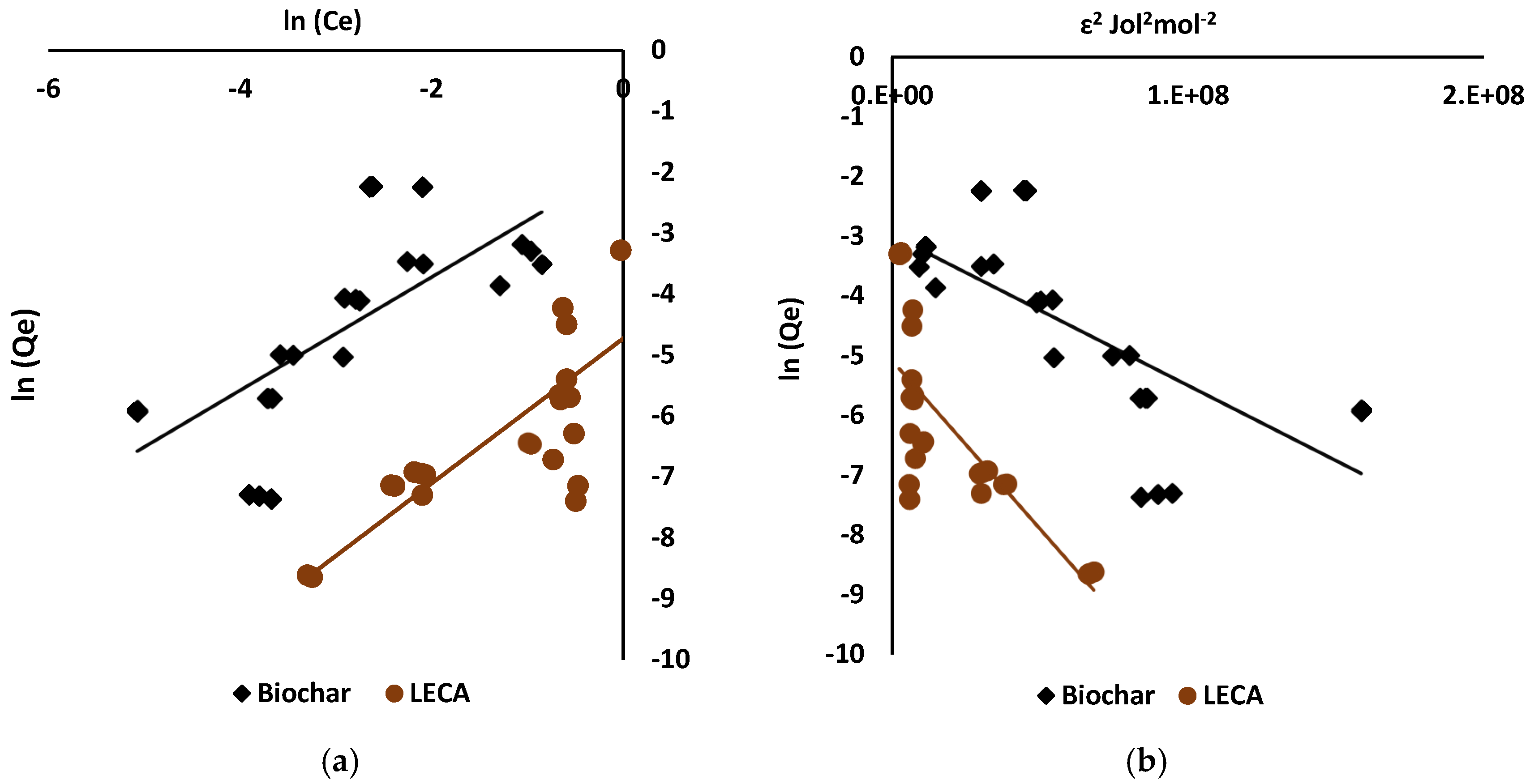
3.3. Sorption Isotherms
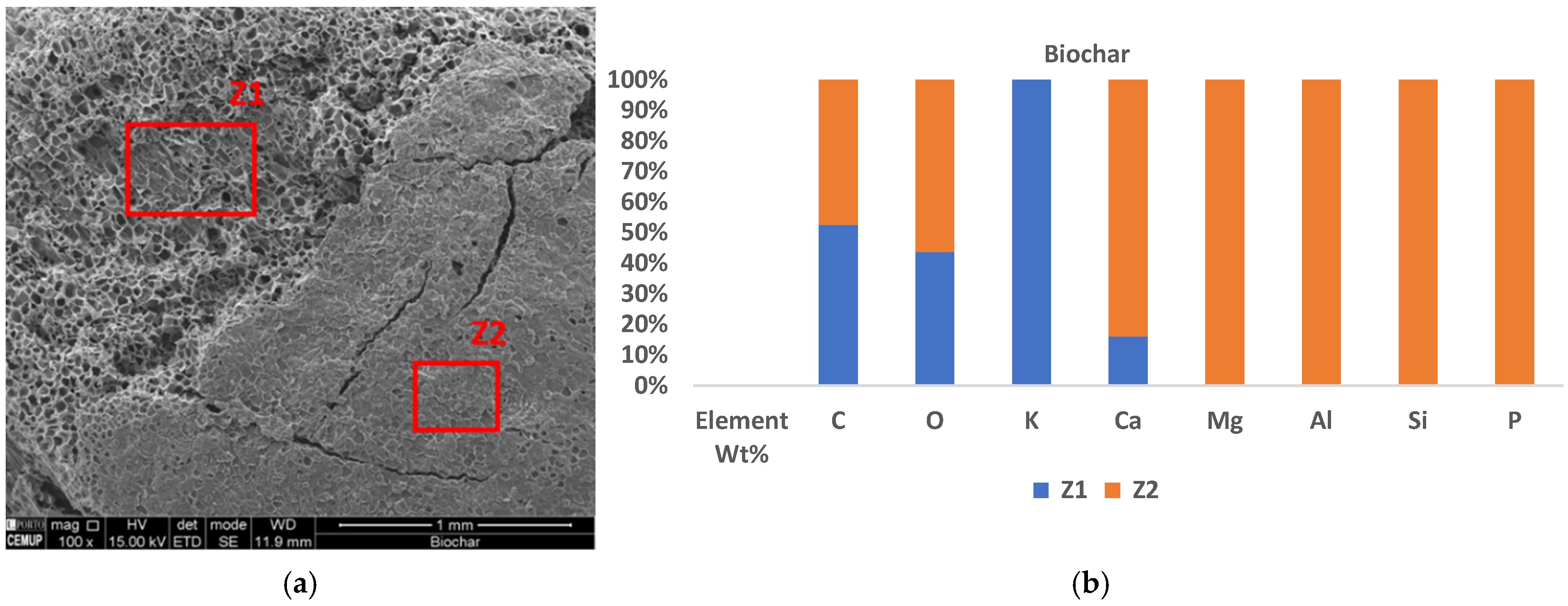
3.4. SEM/EDS of Sorbents
3.4.1. Biochar
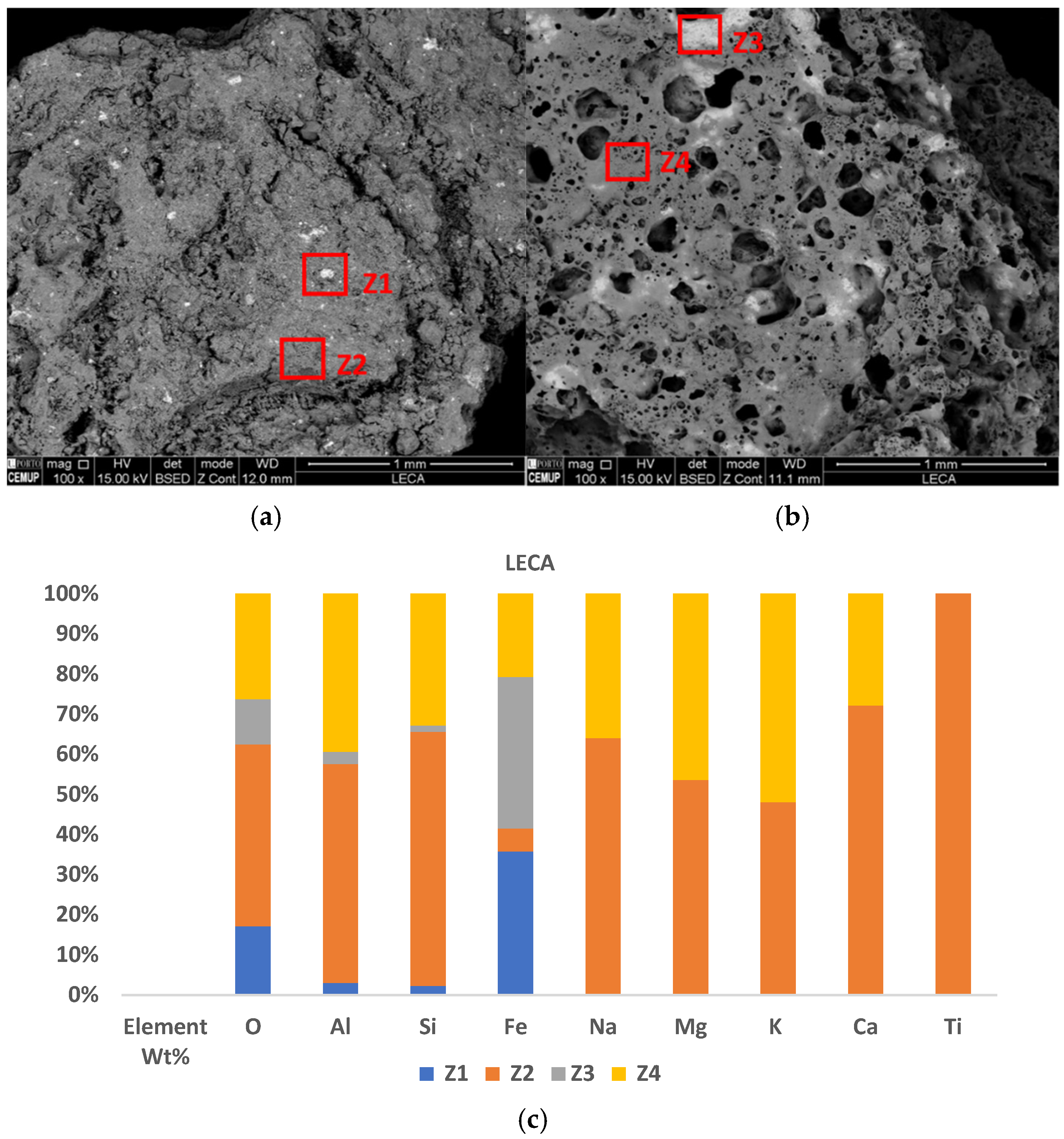
3.4.2. LECA
4. Discussion
4.1. Pre-Screening of Sorbents
4.2. Kinetics
4.3. Sorption Isotherms
4.4. Influence of Surface Element Composition and Morphology of Sorbents on Sorption Potential
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haque, F.; Banayan, S.; Yee, J.; Chiang, Y.W. Extraction and applications of cyanotoxins and other cyanobacterial secondary metabolites. Chemosphere 2017, 183, 164–175. [Google Scholar] [CrossRef]
- Mowe, M.A.; Mitrovic, S.M.; Lim, R.P.; Furey, A.; Yeo, D.C. Tropical cyanobacterial blooms: A review of prevalence, problem taxa, toxins and influencing environmental factors. J. Limnol. 2015, 74, 205–224. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Van Hassel, W.H.R.; Andjelkovic, M.; Wilmotte, A.; Rajkovic, A. Cyanotoxins and Food Contamination in Developing Countries: Review of Their Types, Toxicity, Analysis, Occurrence and Mitigation Strategies. Toxins 2021, 13, 786. [Google Scholar] [CrossRef]
- Cyanobacterial Toxins: Microcystins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; WHO/HEP/ECH/WSH/2020.6; World Health Organization: Geneva, The Netherland, 2020.
- Brient, L.; Lengronne, M.; Bormans, M.; Fastner, J. First occurrence of cylindrospermopsin in freshwater in France. Environ. Toxicol. 2009, 24, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, T.; Khojasteh, D.; Cohen-Shacham, E.; Glamore, W.; Haghani, M.; Bosch, M.v.D.; Rizzi, D.; Greve, P.; Felder, S. The evolution and future of research on Nature-based Solutions to address societal challenges. Commun. Earth Environ. 2024, 5, 132. [Google Scholar] [CrossRef]
- Nature-Based Solutions for Wastewater Treatment; IWA Publishing: London, UK, 2021. [CrossRef]
- Wang, J.; Long, Y.; Yu, G.; Wang, G.; Zhou, Z.; Li, P.; Zhang, Y.; Yang, K.; Wang, S. A Review on Microorganisms in Constructed Wetlands for Typical Pollutant Removal: Species, Function, and Diversity. Front. Microbiol. 2022, 13, 845725. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Abbas, T.; Kajjumba, G.W.; Ejjada, M.; Masrura, S.U.; Marti, E.J.; Khan, E.; Jones-Lepp, T.L. Recent advancements in the removal of cyanotoxins from water using conventional and modified adsorbents—A contemporary review. Water 2020, 12, 2756. [Google Scholar] [CrossRef]
- Couto, R.S.P.; Guarino, A.; Branco, W.S.; Palermo, C.W.C.; Azero, E.F.A. Application of Clay Minerals and Polymeric Resins to Remove Dissolved Microcystin-LR from Water. Int. J. Environ. Res. 2013, 7, 435–442. [Google Scholar]
- Wu, X.; Xiao, B.; Li, R.; Wang, C.; Huang, J.; Wang, Z. Mechanisms and Factors Affecting Sorption of Microcystins onto Natural Sediments. Environ. Sci. Technol. 2011, 45, 2641–2647. [Google Scholar] [CrossRef]
- Gęca, M.; Wiśniewska, M.; Nowicki, P. Biochars and activated carbons as adsorbents of inorganic and organic compounds from multicomponent systems—A review. Adv. Colloid Interface Sci. 2022, 305, 102687. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, N.; Sundaram, B. Adsorption of Pollutants from Wastewater by Biochar: A Review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J. Adsorption of microcystin-LR by rice straw biochars with different pyrolysis temperatures. Environ. Technol. Innov. 2021, 23, 101609. (In English) [Google Scholar] [CrossRef]
- Zeng, S.; Kan, E. Adsorption and regeneration on iron-activated biochar for removal of microcystin-LR. Chemosphere 2021, 273, 129649. (In English) [Google Scholar] [CrossRef]
- Song, H.J.; Gurav, R.; Bhatia, S.K.; Bin Lee, E.; Kim, H.J.; Yang, Y.-H.; Kan, E.; Lee, S.H.; Choi, Y.-K. Treatment of microcystin-LR cyanotoxin contaminated water using Kentucky bluegrass-derived biochar. J. Water Process. Eng. 2021, 41, 102054. (In English) [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Zhai, X.; Wang, Z. Characteristics and mechanisms of microcystin-LR adsorption by giant reed-derived biochars: Role of minerals, pores, and functional groups. J. Clean. Prod. 2018, 176, 463–473. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Yuan, Y.; Wang, R.; Wen, Y.; Man, J. Comparative study for microcystin-LR sorption onto biochars produced from various plant- and animal-wastes at different pyrolysis temperatures: Influencing mechanisms of biochar properties. Bioresour. Technol. 2018, 247, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiu, Y.; Huang, J.; Li, F.; Sheng, G.D. Mechanisms and Factors Influencing Adsorption of Microcystin-LR on Biochars. Water Air Soil Pollut. 2014, 225, 2220. [Google Scholar] [CrossRef]
- Hagemann, N.; Schmidt, H.-P.; Kägi, R.; Böhler, M.; Sigmund, G.; Maccagnan, A.; McArdell, C.S.; Bucheli, T.D. Wood-based activated biochar to eliminate organic micropollutants from biologically treated wastewater. Sci. Total Environ. 2020, 730, 138417. [Google Scholar] [CrossRef] [PubMed]
- Klitzke, S.; Beusch, C.; Fastner, J. Sorption of the cyanobacterial toxins cylindrospermopsin and anatoxin-a to sediments. Water Res. 2011, 45, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; Chatterjee, S.; Vairale, M.G.; Dwivedi, S.K.; Gupta, D.K. Constructed wetland, an eco-technology for wastewater treatment: A review on types of wastewater treated and components of the technology (macrophyte, biolfilm and substrate). J. Environ. Manag. 2021, 283, 111986. [Google Scholar] [CrossRef] [PubMed]
- Mlih, R.; Bydalek, F.; Klumpp, E.; Yaghi, N.; Bol, R.; Wenk, J. Light-expanded clay aggregate (LECA) as a substrate in constructed wetlands—A review. Ecol. Eng. 2020, 148, 105783. [Google Scholar] [CrossRef]
- Deng, S.; Chen, J.; Chang, J. Application of biochar as an innovative substrate in constructed wetlands/biofilters for wastewater treatment: Performance and ecological benefits. J. Clean. Prod. 2021, 293, 126156. [Google Scholar] [CrossRef]
- Valencia-Cárdenas, D.; Tavares, T.S.; Silveira, R.; Brandão, C.C.S.; Soares, R.M.; Ginoris, Y.P. Evaluation of the Removal and Effects of Cylindrospermopsin on Ripened Slow Sand Filters. Toxins 2023, 15, 543. [Google Scholar] [CrossRef]
- Pavagadhi, S.; Tang, A.L.L.; Sathishkumar, M.; Loh, K.P.; Balasubramanian, R. Removal of microcystin-LR and microcystin-RR by graphene oxide: Adsorption and kinetic experiments. Water Res. 2013, 47, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q.; Gao, N.Y.; Deng, Y.; Gu, J.S.; Shen, Y.C.; Wang, S.X. Adsorption of Microcystin-LR from Water with Iron Oxide Nanoparticles. Water Environ. Res. 2012, 84, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Walker, H.W. Adsorption of microcystin-Lr onto iron oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 373, 94–100. [Google Scholar] [CrossRef]
- Park, J.-A.; Kang, J.-K.; Jung, S.-M.; Choi, J.-W.; Lee, S.-H.; Yargeau, V.; Kim, S.-B. Investigating Microcystin-LR adsorption mechanisms on mesoporous carbon, mesoporous silica, and their amino-functionalized form: Surface chemistry, pore structures, and molecular characteristics. Chemosphere 2020, 247, 125811. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Palagama, D.S.W.; Devasurendra, A.M.; Baliu-Rodriguez, D.; Kirchhoff, J.R.; Isailovic, D. Treated rice husk as a recyclable sorbent for the removal of microcystins from water. Sci. Total Environ. 2019, 666, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Prodana, M.; Bastos, A.C.; Amaro, A.; Cardoso, D.; Morgado, R.; Machado, A.L.; Verheijen, F.; Keizer, J.; Loureiro, S. Biomonitoring tools for biochar and biochar-compost amended soil under viticulture: Looking at exposure and effects. Appl. Soil Ecol. 2019, 137, 120–128. [Google Scholar] [CrossRef]
- Ramos, V.; Morais, J.; Castelo-Branco, R.; Pinheiro, Â.; Martins, J.; Regueiras, A.; Pereira, A.L.; Lopes, V.R.; Frazão, B.; Gomes, D.; et al. Cyanobacterial diversity held in microbial biological resource centers as a biotechnological asset: The case study of the newly established LEGE culture collection. J. Appl. Phycol. 2018, 30, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Kotai, J. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae; Norwegian Institute for Water Research: Oslo, Norway, 1972; Volume 11, p. 5. [Google Scholar]
- Welker, M.; Fastner, J.; Erhard, M.; von Döhren, H. Applications of MALDI-TOF MS analysis in cyanotoxin research. Environ. Toxicol. 2002, 17, 367–374. [Google Scholar] [CrossRef]
- Ramanan, S.; Tang, J.; Velayudhan, A. Isolation and preparative purification of microcystin variants. J. Chromatogr. A 2000, 883, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bavithra, G.; Azevedo, J.; Oliveira, F.; Morais, J.; Pinto, E.; Ferreira, I.M.; Vasconcelos, V.; Campos, A.; Almeida, C.M.R. Assessment of Constructed Wetlands’ Potential for the Removal of Cyanobacteria and Microcystins (MC-LR). Water 2019, 12, 10. [Google Scholar] [CrossRef]
- Machado, J.; Campos, A.; Vasconcelos, V.; Freitas, M. Effects of microcystin-LR and cylindrospermopsin on plant-soil systems: A review of their relevance for agricultural plant quality and public health. Environ. Res. 2017, 153, 191–204. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, H.; Peng, Q.; Chen, G.; Liu, J.; Cao, X.; Xiong, S.; Li, G.; Liu, Q. Elimination of Pharmaceutical Compounds from Aqueous Solution through Novel Functionalized Pitch-Based Porous Adsorbents: Kinetic, Isotherm, Thermodynamic Studies and Mechanism Analysis. Molecules 2024, 29, 463. [Google Scholar] [CrossRef]
- Rwiza, M.J.; Oh, S.-Y.; Kim, K.-W.; Kim, S.D. Comparative sorption isotherms and removal studies for Pb(II) by physical and thermochemical modification of low-cost agro-wastes from Tanzania. Chemosphere 2018, 195, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Mittal, A.; Jain, R.; Mathur, M.; Sikarwar, S. Adsorption of Safranin-T from wastewater using waste materials— activated carbon and activated rice husks. J. Colloid Interface Sci. 2006, 303, 80–86. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Sheha, R.; El-Shazly, E. Kinetics and equilibrium modeling of Se(IV) removal from aqueous solutions using metal oxides. Chem. Eng. J. 2010, 160, 63–71. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Lawton, L.A.; Edwards, C.; Codd, A. Extraction and high-performance liquid chromatographic method for the determination of micro-cystins in raw and treated waters. Analyst 1994, 119, 1525–1530. [Google Scholar] [CrossRef]
- Guzmán-Guillén, R.; Prieto, A.I.; González, A.G.; Soria-Díaz, M.E.; Cameán, A.M. Cylindrospermopsin determination in water by LC-MS/MS: Optimization and validation of the method and application to real samples. Environ. Toxicol. Chem. 2012, 31, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Pekar, H.; Westerberg, E.; Bruno, O.; Lääne, A.; Persson, K.M.; Sundström, L.F.; Thim, A.M. Fast, rugged and sensitive ultra high-pressure liquid chromatography tandem mass spectrometry method for analysis of cyanotoxins in raw water and drinking water—First findings of anatoxins, cylindrospermopsins and microcystin variants in Swedish source waters and infiltration ponds. J. Chromatogr. A 2016, 1429, 265–276. [Google Scholar] [PubMed]
- Zervou, S.-K.; Christophoridis, C.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. New SPE-LC-MS/MS method for simultaneous determination of multi-class cyanobacterial and algal toxins. J. Hazard. Mater. 2017, 323, 56–66. [Google Scholar] [CrossRef] [PubMed]
- El Bouaidi, W.; Enaime, G.; Loudiki, M.; Yaacoubi, A.; Douma, M.; Ounas, A.; Lübken, M. Adsorbents Used for Microcystin Removal from Water Sources: Current Knowledge and Future Prospects. Processes 2022, 10, 1235. [Google Scholar] [CrossRef]
- Antonieti, C.C.; Ginoris, Y.P. Removal of Cylindrospermopsin by Adsorption on Granular Activated Carbon, Selection of Carbons and Estimated Fixed-Bed Breakthrough. Water 2022, 14, 1630. [Google Scholar] [CrossRef]
- Xie, R.; Jin, Y.; Chen, Y.; Jiang, W. The importance of surface functional groups in the adsorption of copper onto walnut shell derived activated carbon. Water Sci. Technol. 2017, 76, 3022–3034. [Google Scholar] [CrossRef] [PubMed]
- Thenuwara, S.I.; Kiridena, H.S.; Kirchhoff, J.R.; Isailović, D. Evaluation of the mechanisms of adsorption of microcystins and nodularin-R onto rice husk-based biochar. Environ. Adv. 2022, 9, 100314. [Google Scholar] [CrossRef]
- Kumar, P.; Pérez, J.A.E.; Cledon, M.; Brar, S.K.; Duy, S.V.; Sauvé, S.; Knystautas, É. Removal of microcystin-LR and other water pollutants using sand coated with bio-optimized carbon submicron particles: Graphene oxide and reduced graphene oxide. Chem. Eng. J. 2020, 397, 125398. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Kinetics, Isotherm and Thermodynamic Studies for Efficient Adsorption of Congo Red Dye from Aqueous Solution onto Novel Cyanoguanidine-Modified Chitosan Adsorbent. Polymers 2021, 13, 4446. [Google Scholar] [CrossRef]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Competitive sorption of Cd, Cr, Cu, Ni, Pb and Zn from stormwater runoff by five low-cost sorbents; Effects of co-contaminants, humic acid, salinity and pH. J. Hazard. Mater. 2022, 423, 126938. [Google Scholar] [CrossRef] [PubMed]
- Campinas, M.; Viegas, R.M.; Rosa, M.J. Modelling and understanding the competitive adsorption of microcystins and tannic acid. Water Res. 2013, 47, 5690–5699. [Google Scholar] [CrossRef] [PubMed]
- Orta-Ortiz, M.S.; Geneletti, D. What variables matter when designing nature-based solutions for stormwater management? A review of impacts on ecosystem services. Environ. Impact Assess. Rev. 2022, 95, 106802. [Google Scholar] [CrossRef]
- Varma, M.; Gupta, A.K.; Ghosal, P.S.; Majumder, A. A review on performance of constructed wetlands in tropical and cold climate: Insights of mechanism, role of influencing factors, and system modification in low temperature. Sci. Total Environ. 2021, 755, 142540. [Google Scholar] [CrossRef]
- Frazer-Williams, R.A. A Review of the Influence of Design Parameters on the Performance of Constructed Wetlands. J. Chem. Eng. 1970, 25, 29–42. [Google Scholar] [CrossRef]
- Chu, K.H. Revisiting the Temkin Isotherm: Dimensional Inconsistency and Approximate Forms. Ind. Eng. Chem. Res. 2021, 60, 13140–13147. [Google Scholar] [CrossRef]
- Vigdorowitsch, M.; Pchelintsev, A.; Tsygankova, L.; Tanygina, E. Freundlich Isotherm: An Adsorption Model Complete Framework. Appl. Sci. 2021, 11, 8078. [Google Scholar] [CrossRef]
- Wang, F.; Wu, P.; Shu, L.; Guo, Q.; Huang, D.; Liu, H. Isotherm, kinetics, and adsorption mechanism studies of diethylenetriaminepentaacetic acid—Modified banana/pomegranate peels as efficient adsorbents for removing Cd(II) and Ni(II) from aqueous solution. Environ. Sci. Pollut. Res. 2022, 29, 3051–3061. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Walker, H.W.; Lenhart, J.J. Adsorption of microcystin-LR onto kaolinite, illite and montmorillonite. Chemosphere 2019, 220, 696–705. [Google Scholar] [CrossRef] [PubMed]

| Equation | Isotherm Model | Non-Linearized Form | Linearized Form |
|---|---|---|---|
| (1) | N/A | N/A | |
| (2) | N/A | N/A | qe = (C0 − Ce) V/m |
| (3) | N/A | N/A | ln (qe − qt) = ln qe − K1t |
| (4) | N/A | N/A | t/qt = t/qe + 1/qe2K2 |
| (5) | Langmuir | ||
| (6) | Freundlich | ||
| (7) | D–R | ||
| (8) | Temkin | ||
| (9) | N/A | N/A |
| Sorbent | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||||
|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | qe (cal) (mg/g) | k1 (h−1) | R2 | qe (cal) (mg/g) | k2 (g mg−1 h−1) | R2 | ||
| Biochar | MC-LR | 0.0007 | 0.0001 | 0.0010 | 0.33 | 0.0006 | 6736 | 0.99 |
| CYN | 0.0034 | 0.0007 | 0.0018 | 0.95 | 0.0035 | 497 | 0.99 | |
| LECA | MC-LR | 0.0004 | 0.0004 | 0.0013 | 0.95 | 0.0005 | 210 | 0.98 |
| CYN | 0.0012 | 0.0012 | 0.0022 | 0.92 | 0.0012 | 134 | 0.94 | |
| Isotherm Model | Sorbent | Cyanotoxin | R2 | qmax (mg/g) | b | RL |
|---|---|---|---|---|---|---|
| Langmuir | Biochar | MC-LR | 0.06 | 0.164 | 0.276 | 0.162 |
| CYN | 2 × 10−4 | 0.802 | 0.141 | 0.359 | ||
| LECA | MC-LR | 0.09 | 0.024 | 0.193 | 0.238 | |
| CYN | 1 × 10−5 | 0.469 | 0.009 | 0.897 | ||
| R2 | Kf (L/g) | n | 1/n | |||
| Freundlich | Biochar | MC-LR | 0.83 | 0.050 | 1.056 | 0.95 |
| CYN | 0.53 | 0.155 | 1.076 | 0.93 | ||
| LECA | MC-LR | 0.77 | 0.017 | 0.793 | 1.26 | |
| CYN | 0.67 | 0.009 | 0.840 | 1.19 | ||
| R2 | Qmax (mg/g) | KDR | ||||
| Dubinin–Radushkevich (D–R) | Biochar | MC-LR | 0.87 | 0.092 | 5 × 10−8 | |
| CYN | 0.51 | 0.051 | 3 × 10−8 | |||
| LECA | MC-LR | 0.62 | 0.045 | 7 × 10−8 | ||
| CYN | 0.58 | 0.006 | 6 × 10−8 | |||
| R2 | AT (L/g) | BT | ||||
| Temkin | Biochar | MC-LR | 0.01 | 116.5 | 4.758 | |
| CYN | 0.23 | 129.6 | 4.865 | |||
| LECA | MC-LR | 0.01 | 298.9 | 5.700 | ||
| CYN | 0.34 | 9.897 | 2.292 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bavithra, G.; Azevedo, J.; Campos, A.; Almeida, C.M.R.; Carvalho, P.N. Evaluating Agro-Based Waste Materials for Cyanotoxin Sorption for Future Incorporation in Nature-Based Solution Units (NBSUs). Water 2025, 17, 285. https://doi.org/10.3390/w17020285
Bavithra G, Azevedo J, Campos A, Almeida CMR, Carvalho PN. Evaluating Agro-Based Waste Materials for Cyanotoxin Sorption for Future Incorporation in Nature-Based Solution Units (NBSUs). Water. 2025; 17(2):285. https://doi.org/10.3390/w17020285
Chicago/Turabian StyleBavithra, Guna, Joana Azevedo, Alexandre Campos, C. Marisa R. Almeida, and Pedro N. Carvalho. 2025. "Evaluating Agro-Based Waste Materials for Cyanotoxin Sorption for Future Incorporation in Nature-Based Solution Units (NBSUs)" Water 17, no. 2: 285. https://doi.org/10.3390/w17020285
APA StyleBavithra, G., Azevedo, J., Campos, A., Almeida, C. M. R., & Carvalho, P. N. (2025). Evaluating Agro-Based Waste Materials for Cyanotoxin Sorption for Future Incorporation in Nature-Based Solution Units (NBSUs). Water, 17(2), 285. https://doi.org/10.3390/w17020285









