Progress in the Study of Toxic Effects of Microplastics on Organisms in Freshwater Environments and Human Health
Abstract
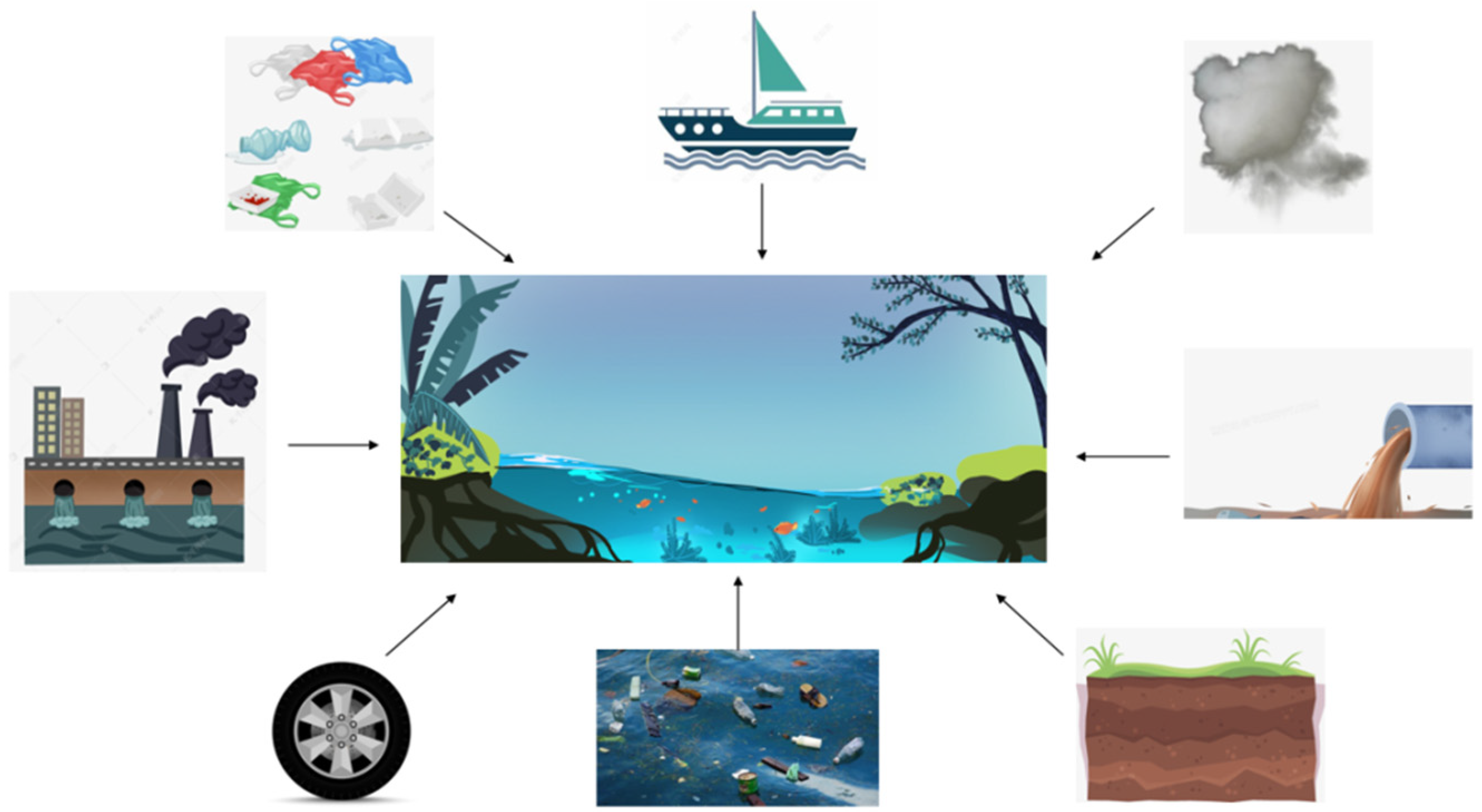
:1. Introduction
2. Toxic Effects of MPs on Algae and Aquatic Animals in Freshwater
2.1. Toxic Effects of MPs on Algae
2.2. Toxic Effects of MPs on Aquatic Animals
3. Combined Toxicity of MPs and Pollutants
4. Impact of MPs on Human Health
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Orose, E.; Wokeh, O.K.; Okey-Wokeh, C.G. Some Behavioural and Physiological Effects of Plastics (Polyethylene) on Fish. Trop. Aquat. Soil Pollut. 2023, 3, 46–57. [Google Scholar] [CrossRef]
- Ingrid, d.S.F.; Luiza, F.M.; Benetis, P.T.; Fiorelini, P.B.; Koppe, G.C. Multilevel Toxicity Evaluations of Polyethylene Microplastics in Zebrafish (Danio rerio). Int. J. Environ. Res. Public Health 2023, 20, 3617. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Emad, Y.; Raghad, H. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar]
- Simon, M.; Hartmann, N.B.; Vollertsen, J. Accelerated Weathering Increases the Release of Toxic Leachates from Microplastic Particles as Demonstrated through Altered Toxicity to the Green Algae Raphidocelis subcapitata. Toxics 2021, 9, 185. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Francois, G.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Q. Development status and prospect of biodegradable plastics industry in China. Chem. Manag. 2024, 27, 100–103. (In Chinese) [Google Scholar]
- Borrelle, S.B.; Jeremy, R.; Lavender, L.K.; Cole, M.C.; Laurent, L.; Alexis, M.; Erin, M.; Jenna, J. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.; Dajana, K.G.; Tomislav, B.; Šime, U.; Matija, C.; Vesna, O.B.; Dionysiou, D.D.; Hrvoje, K. Ecotoxicological Assessment of Microplastics in Freshwater Sources—A Review. Water 2020, 13, 56. [Google Scholar] [CrossRef]
- Kellyn, B. Why small plastic particles may pose a big problem in the oceans. Environ. Sci. Technol. 2008, 42, 8995. [Google Scholar]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef]
- Chang, Y.H.; Ku, C.R.; Lu, H.L. Effects of aquatic ecological indicators of sustainable green energy landscape facilities. Ecol. Eng. 2014, 71, 144–153. [Google Scholar] [CrossRef]
- Kishore, G.; Kashian, D.R. Extracellular polymeric substances in green alga facilitate microplastic deposition. Chemosphere 2022, 286, 131814. [Google Scholar]
- Chae, Y.; Kim, D.; An, Y.-J. Effects of micro-sized polyethylene spheres on the marine microalga Dunaliella salina: Focusing on the algal cell to plastic particle size ratio. Aquat. Toxicol. 2019, 216, 105296. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiang, Y.; He, D.; Li, Y.; Zhao, Y.; Wang, S.; Pan, X. Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Sci. Total Environ. 2019, 678, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, X.; Zhang, L.; Wang, Z.; Yuan, Y.; Li, J.; Li, Y.; Huang, H.; Cao, X.; Fan, Z. Toxicity mechanism of Nylon microplastics on Microcystis aeruginosa through three pathways: Photosynthesis, oxidative stress and energy metabolism. J. Hazard. Mater. 2022, 426, 128094. [Google Scholar] [CrossRef]
- Guschina, I.A.; Hayes, A.J.; Ormerod, S.J. Polystyrene microplastics decrease accumulation of essential fatty acids in common freshwater algae. Environ. Pollut. 2020, 263, 114425. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Tong, L.; Zhang, W. Microplastics in Freshwater Environments: Sources, Fates and Toxicity. Water Air Soil Pollut. 2021, 232, 181. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, X.; Liao, Y.; Zhao, W.; Zhao, P.; Li, M. Adverse physiological and molecular level effects of polystyrene microplastics on freshwater microalgae. Chemosphere 2020, 255, 126914. [Google Scholar] [CrossRef]
- Sjollema, S.B.; Paula, R.-H.; Leslie, H.A.; Kraak, M.H.S.; Vethaak, A.D. Do plastic particles affect microalgal photosynthesis and growth? Aquat. Toxicol. 2016, 170, 259–261. [Google Scholar] [CrossRef]
- Marta, S.; Carmen, G.-F.; Philippe, S.; Arnaud, H.; Marta, E.; Cid, Á.; Ika, P.-P. Polystyrene microbeads modulate the energy metabolism of the marine diatom Chaetoceros neogracile. Environ. Pollut. 2019, 251, 363–371. [Google Scholar]
- Yang, W.; Gao, P.; Li, H.; Huang, J.; Zhang, Y.; Ding, H.; Zhang, W. Mechanism of the inhibition and detoxification effects of the interaction between nanoplastics and microalgae Chlorella pyrenoidosa. Sci. Total Environ. 2021, 783, 146919. [Google Scholar] [CrossRef]
- Cao, Q.; Sun, W.; Yang, T.; Zhu, Z.; Jiang, Y.; Hu, W.; Wei, W.; Zhang, Y.; Yang, H. The toxic effects of polystyrene microplastics on freshwater algae Chlorella pyrenoidosa depends on the different size of polystyrene microplastics. Chemosphere 2022, 308, 136135. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, L.; Lin, Y.; Pan, B.; Li, M. Micrometer scale polystyrene plastics of varying concentrations and particle sizes inhibit growth and upregulate microcystin-related gene expression in Microcystis aeruginosa. J. Hazard. Mater. 2021, 420, 126591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wangjin, X.; Zhang, Y.; Wang, N.; Wang, Y.; Meng, G.; Chen, Y. The toxicity of virgin and UV-aged PVC microplastics on the growth of freshwater algae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 749, 141603. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Zhang, Q.; Fan, Z.; Qi, H.; Wang, Z.; Peng, L. Aged microplastics polyvinyl chloride interact with copper and cause oxidative stress towards microalgae Chlorella vulgaris. Aquat. Toxicol. 2019, 216, 105319. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, Q.; Zhao, H.; Wang, L.; Guo, S.; Li, X. Ecotoxicological effects of graphene oxide on the protozoan Euglena gracilis. Chemosphere 2015, 128, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, P.; Zhang, X.; Zhang, Y.; Xie, S.; Deng, J. Effect of microplastics exposure on the photosynthesis system of freshwater algae. J. Hazard. Mater. 2019, 374, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Shi, X.; Li, H.; Xie, P.; Zhang, H.; Deng, J.; Liang, Y. Effects of lead on tolerance, bioaccumulation, and antioxidative defense system of green algae, Cladophora. Ecotoxicol. Environ. Saf. 2015, 112, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Ali, H.; Gillani, S.; Zinck, P.; Souissi, S. Polylactic acid synthesis, biodegradability, conversion to microplastics and toxicity: A review. Environ. Chem. Lett. 2023, 21, 1761–1786. [Google Scholar] [CrossRef]
- Benson, N.U.; Agboola, O.D.; Fred-Ahmadu, O.H.; De-la-Torre, G.E.; Oluwalana, A.; Williams, A.B. Micro(nano)plastics Prevalence, Food Web Interactions, and Toxicity Assessment in Aquatic Organisms: A Review. Front. Mar. Sci. 2022, 9, 85128. [Google Scholar] [CrossRef]
- Gardon, T.; Reisser, C.; Claude, S.; Virgile, Q.; Gilles, L.M. Microplastics Affect Energy Balance and Gametogenesis in the Pearl Oyster Pinctada margaritifera. Environ. Sci. Technol. 2018, 52, 5277–5286. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.; Zeidi, A.; Sinha, R.; Faggio, C. Individual and Combined Toxic Effects of Nano-ZnO and Polyethylene Microplastics on Mosquito Fish (Gambusia holbrooki). Water 2023, 15, 1660. [Google Scholar] [CrossRef]
- Jabeen, K.; Su, L.; Li, J.; Yang, D.; Tong, C.; Mu, J.; Shi, H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [CrossRef]
- Magni, S.; Gagné, F.; André, C.; Torre, C.D.; Auclair, J.; Hanana, H.; Parenti, C.C.; Bonasoro, F.; Binelli, A. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci. Total Environ. 2018, 631–632, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Greven, A.-C.; Teresa, M.; Filiz, K.; Kristin, M.; Markus, K.; Boris, J.; Dušan, P. Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2016, 35, 3093–3100. [Google Scholar] [CrossRef]
- Ory, N.C.; Gallardo, C.; Lenz, M.; Thiel, M. Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish. Environ. Pollut. 2018, 240, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Sá, L.C.D.; Luís, L.G.; Guilhermino, L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015, 196, 359–362. [Google Scholar]
- Ye, G.; Zhang, X.; Liu, X.; Liao, X.; Zhang, H.; Yan, C.; Lin, Y.; Huang, Q. Polystyrene microplastics induce metabolic disturbances in marine medaka (Oryzias melastigmas) liver. Sci. Total Environ. 2021, 782, 146885. [Google Scholar] [CrossRef]
- Bhuyan, M.S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef]
- Zhao, Y.; Bao, Z.; Wan, Z.; Fu, Z.; Jin, Y. Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ. 2020, 710, 136279. [Google Scholar] [CrossRef] [PubMed]
- Sathisaran, U.; Sheela, P.; Krishna, K.; Mathan, R. Polystyrene microplastics induce apoptosis via ROS-mediated p53 signaling pathway in zebrafish. Chem.-Biol. Interact. 2021, 345, 109550. [Google Scholar]
- Shi, Y.; Chen, C.; Han, Z.; Chen, K.; Wu, X.; Qiu, X. Combined exposure to microplastics and amitriptyline caused intestinal damage, oxidative stress and gut microbiota dysbiosis in zebrafish (Danio rerio). Aquat. Toxicol. 2023, 260, 106589. [Google Scholar] [CrossRef]
- Chubarenko, I.; Bagaev, A.; Zobkov, M.; Esiukova, E. On some physical and dynamical properties of microplastic particles in marine environment. Mar. Pollut. Bull. 2016, 108, 105–112. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As (III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere 2020, 239, 124792. [Google Scholar] [CrossRef]
- Diepens, N.J.; Koelmans, A.A. Accumulation of Plastic Debris and Associated Contaminants in Aquatic Food Webs. Environ. Sci. Technol. 2018, 52, 8510–8520. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Chen, Y.; Zhang, W.; Zhao, J.; He, S.; Yang, C.; Zhang, T.; Tang, C.; Zhang, C.; et al. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 2020, 766, 142572. [Google Scholar] [CrossRef] [PubMed]
- Llorca, M.; Gabriella, S.; Martínez, M.; Barceló, D.; Farré, M. Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ. Pollut. 2018, 235, 680–691. [Google Scholar] [CrossRef]
- Wu, P.; Cai, Z.; Jin, H.; Tang, Y. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 2019, 650, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Ashton, K.; Holmes, L.; Turner, A. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 2010, 60, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Dennis, B.; Bernardo, D.; Filipa, P.; Isabel, C.; João, C.-C. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, S.; Du, X.; Han, Y.; Tang, Y.; Zhou, W.; Shi, W.; Liu, G. Toxic impacts of microplastics and tetrabromobisphenol A on the motility of marine microalgae and potential mechanisms of action. Gondwana Res. 2022, 108, 158–170. [Google Scholar] [CrossRef]
- Feng, L.; Shi, Y.; Li, X.; Sun, X.; Xiao, F.; Sun, J.; Wang, Y.; Liu, X.; Wang, S.; Yuan, X. Behavior of tetracycline and polystyrene nanoparticles in estuaries and their joint toxicity on marine microalgae Skeletonema costatum. Environ. Pollut. 2020, 263, 114453. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zeng, Z.; Zhang, Y.; Zhang, H.; Tang, N.; Guo, Y.; Lu, L.; Li, X.; Zhu, Z.; Gao, X.; et al. Higher toxicity induced by co-exposure of polystyrene microplastics and chloramphenicol to Microcystis aeruginosa: Experimental study and molecular dynamics simulation. Sci. Total Environ. 2023, 866, 161375. [Google Scholar] [CrossRef]
- Fonte, E.; Ferreira, P.; Guilhermino, L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 2016, 180, 173–185. [Google Scholar] [CrossRef]
- Haghi, B.N.; Banaee, M. Effects of micro-plastic particles on paraquat toxicity to common carp (Cyprinus carpio): Biochemical changes. Int. J. Environ. Sci. Technol. 2017, 14, 521–530. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Qu, H.; Zhao, W.; Duan, L.; Zhang, Y.; Zhou, Y.; Yu, G. The influence of nanoplastics on the toxic effects, bioaccumulation, biodegradation and enantioselectivity of ibuprofen in freshwater algae Chlorella pyrenoidosa. Environ. Pollut. 2020, 263, 114593. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.; Zeng, H.; Zhu, L.; Lu, X. Microplastics decrease the toxicity of sulfamethoxazole to marine algae (Skeletonema costatum) at the cellular and molecular levels. Sci. Total Environ. 2022, 824, 153855. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Z.; Liu, F.; Chen, S.; Liu, G. Effects of polystyrene and triphenyl phosphate on growth, photosynthesis and oxidative stress of Chaetoceros meülleri. Sci. Total Environ. 2021, 797, 149180. [Google Scholar] [CrossRef]
- Karami, A.; Romano, N.; Galloway, T.; Hamzah, H. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ. Res. 2016, 151, 58–70. [Google Scholar] [CrossRef]
- Hüffer, T.; Hofmann, T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Takada, H.; Mizukawa, K.; Hirai, H.; Iwasa, S.; Endo, S. International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437–1446. [Google Scholar] [CrossRef]
- Mukherjee, A.; Rochman, C.M.; Parnis, J.M.; Browne, M.A.; Serrato, S.; Reiner, E.J.; Robson, M.; Young, T.; Diamond, M.L.; Teh, S.J. Direct and indirect effects of different types of microplastics on freshwater prey (Corbicula fluminea) and their predator (Acipenser transmontanus). PLoS ONE 2017, 12, e0187664. [Google Scholar]
- Zhu, J.; Li, J. Can Microplastics Accumulate Toxic dye in Water? An adsorption-desorption Study under Different Experimental Conditions. Bull. Environ. Contam. Toxicol. 2024, 112, 37. [Google Scholar] [CrossRef] [PubMed]
- Afmataj, D.; Kordera, O.; Maragkaki, A.; Tzanakakis, V.A.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Adsorption of Reactive Red 120 Dye by Polyamide Nylon 6 Microplastics: Isotherm, Kinetic, and Thermodynamic Analysis. Water 2023, 15, 1137. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Guo, K.; Wu, W.; Yao, M. Effect of chlorination and ultraviolet on the adsorption of pefloxacin on polystyrene and polyvinyl chloride. J. Environ. Sci. 2025, 149, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Xia, S.; Zhao, J. New insights into oxytetracycline (OTC) adsorption behavior on polylactic acid microplastics undergoing microbial adhesion and degradation. Chem. Eng. J. 2021, 416, 129085. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Chen, H.; Zhang, Y. Toxicity effects of microplastics and nanoplastics with cadmium on the alga Microcystis aeruginosa. Environ. Sci. Pollut. Res. Int. 2022, 30, 17360–17373. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, F.; Olivier, O.; Zanella, M.; Daniel, P.; Hiard, S.; Caruso, A. Microplastic interactions with freshwater microalgae: Hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ. Pollut. 2016, 215, 331–339. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Khoironi, A.; Dianratri, I.; Suherman, S.; Muhammad, F.; Vaidyanathan, S. Interactions between polyethylene and polypropylene microplastics and Spirulina sp. microalgae in aquatic systems. Heliyon 2021, 7, e07676. [Google Scholar] [CrossRef]
- Zwollo, P.; Quddos, F.; Bagdassarian, C.; Seeley, M.E.; Hale, R.C.; Abderhalden, L. Polystyrene microplastics reduce abundance of developing B cells in rainbow trout (Oncorhynchus mykiss) primary cultures. Fish Shellfish Immunol. 2021, 114, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Yin, S.; Xiao, K.; Xiong, Q.; Bian, S.; Liang, S.; Hou, H.; Hu, J.; Yang, J. Metabolomics revealing the response of rice (Oryza sativa L.) exposed to polystyrene microplastics. Environ. Pollut. 2020, 266, 115159. [Google Scholar] [CrossRef]
- Song, U.; Kim, J.; Rim, H. Assessing phytotoxicity of microplastics on aquatic plants using fluorescent microplastics. Environ. Sci. Pollut. Res. Int. 2023, 30, 74186–74195. [Google Scholar] [CrossRef] [PubMed]
- Kalčíková, G.; Žgajnar Gotvajn, A.; Kladnik, A.; Jemec, A. Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ. Pollut. 2017, 230, 1108–1115. [Google Scholar] [CrossRef]
- Urbina, M.A.; Correa, F.; Aburto, F.; Ferrio, J.P. Adsorption of polyethylene microbeads and physiological effects on hydroponic maize. Sci. Total Environ. 2020, 741, 140216. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, M.; Chen, X.; Yang, C.; Wu, L. Combined toxicity of microplastics and cadmium on the zebrafish embryos (Danio rerio). Sci. Total Environ. 2020, 743, 140638. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Yuan, Y.; Sepúlveda, M.S.; Bi, B.; Huang, Y.; Kong, L.; Yan, H.; Gao, Y. Acute polyethylene microplastic (PE-MPs) exposure activates the intestinal mucosal immune network pathway in adult zebrafish (Danio rerio). Chemosphere 2023, 311, 137048. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Cheng, H.; Duan, X.; Zhang, H.; Wang, Y.; Gong, Z.; Zhang, H.; Sun, H.; Wang, L. Diet preference of zebrafish (Danio rerio) for bio-based polylactic acid microplastics and induced intestinal damage and microbiota dysbiosis. J. Hazard. Mater. 2022, 429, 128332. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; Saal, F.S.v.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Ormsby, R.T.; Cantley, M.; Kogawa, M.; Solomon, L.B.; Haynes, D.R.; Findlay, D.M.; Atkins, G.J. Evidence that osteocyte perilacunar remodelling contributes to polyethylene wear particle induced osteolysis. Acta Biomater. 2016, 33, 242–251. [Google Scholar] [CrossRef]
- Alonzo, A.N.; Diana, A.; Lenin, C.F.; Bastidas, L.; Soto Villegas, C.; Macay, K.C.; Christensen, J.H. Author Correction: Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galápagos. Sci. Rep. 2022, 12, 3502. [Google Scholar]
- Goswami, P.; Vinithkumar, N.V.; Dharani, G. First evidence of microplastics bioaccumulation by marine organisms in the Port Blair Bay, Andaman Islands. Mar. Pollut. Bull. 2020, 155, 111163. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.-K.; Syrovets, T.; Haas, K.A.; Loos, C.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Simmet, T. Carboxyl-and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials 2016, 85, 78–87. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Q.; An, X.; Yang, X.; Christie, P.; Ke, X.; Wu, L.; Zhu, Y. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Nutrition, the visceral immune system, and the evolutionary origins of pathogenic obesity. Proc. Natl. Acad. Sci. USA 2019, 116, 723–731. [Google Scholar] [CrossRef]
- Wu, S.; Wu, M.; Tian, D.; Qiu, L.; Li, T. Effects of polystyrene microbeads on cytotoxicity and transcriptomic profiles in human Caco-2 cells. Environ. Toxicol. Chem. 2020, 35, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Thubagere, A.; Reinhard, B.M. Nanoparticle-Induced Apoptosis Propagates through Hydrogen-Peroxide-Mediated Bystander Killing: Insights from a Human Intestinal Epithelium In Vitro Model. ACS Nano 2010, 4, 3611–3622. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Yacobi, N.R.; DeMaio, L.; Xie, J.; Hamm-Alvarez, S.F.; Borok, Z.; Jin, K.K.; Crandall, E.D. Polystyrene nanoparticle trafficking across alveolar epithelium. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 139–145. [Google Scholar] [CrossRef]
- Geiser, M.; Barbara, R.R.; Kapp, N.; Samuel, S.; Kreyling, W.; Schulz, H.; Semmler, M.; Hof, V.I.; Heyder, J.; Gehr, P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005, 113, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Farhat, S.C.L.; Silva, C.A.; Orione, M.A.M.; Campos, L.M.A.; Sallum, A.M.E.; Braga, A.L.F. Air pollution in autoimmune rheumatic diseases: A review. Autoimmun. Rev. 2011, 11, 14–21. [Google Scholar] [CrossRef]
- Fernandes, E.C.; Silva, C.A.; Braga, A.L.F.; Sallum, A.M.E.; Campos, L.M.A.; Farhat, S.C.L. Exposure to Air Pollutants and Disease Activity in Juvenile-Onset Systemic Lupus Erythematosus Patients. Arthritis Care Res. 2015, 67, 1609–1614. [Google Scholar] [CrossRef]
- Bernatsky, S.; Smargiassi, A.; Barnabe, C.; Svenson, L.W.; Brand, A.; Martin, R.V.; Hudson, M.; Clarke, A.E. Fine particulate air pollution and systemic autoimmune rheumatic disease in two Canadian provinces. Environ. Res. 2016, 146, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Costa, J.P.d.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]


| Types of MPs | Environmental Conditions | Targets | Specific Hazards | Reference |
|---|---|---|---|---|
| PS | Particle size of 50 μm, combined with Cd2+ | Microcystis aeruginosa | Disrupted cell permeability, induced ROS production, damaged algal cell DNA, and inhibited growth. | [76] |
| Combined with CAP | Microcystis aeruginosa | Exacerbating photosynthetic toxicity, damaging the cell membranes, inducing oxidative stress, and promoting the entry of CAP into cells | [60] | |
| Particle size of 5 μm | Euglena gracilis | Vesicles increased, chloroplasts were deformed, induced POD and SOD activities significantly, and reduced the ‘genetic information processing’ and ‘metabolic’ pathways significantly. | [19] | |
| Particle size of 1 μm or 5 μm | Chlorella pyrenoidosa | Reduced photosynthetic pigment content, induced oxidative stress, disrupted cell membrane integrity, and altered transcript levels of genes related to photosynthesis and energy metabolism | [23] | |
| Particle size less than 70 μm, concentration of 60 mg/L | Chlorella sorokiniana | Affected a range of lipid molecules in living organisms, reducing the tolerance of algal cells to natural stressors (e.g., temperature changes) | [17] | |
| PP | Particle size of 400~1000 μm | Chlamydomas reinhardtii | Did not affect microalgae growth over a period of time of 60 days, but hetero-aggregates constituted of microalgae, microplastics and exopolysaccharides were formed. | [77] |
| PP or PE | Particle size of 0.5~1 μm, concentration of 500 mg/500 mL | Spirulina sp. | Reduced growth rate, damage to the surface of Spirulina sp. cells, losing the carboxyl part of the protein in Spirulina sp. | [78] |
| PVC | Particle size of 50~100 μm, concentration of 10~200 mg/L | Chlamydomonas reinhardtii | Inhibited the growth and photosynthetic effect of algae, the toxicity increased with the increase in concentration and time of action, and the UV-aged PVC had greater oxidative damage to algae. | [25] |
| PVC or PP | PVC particle size of 111~216 μm or PP particle size of 64~236 μm | Chlorella pyrenoidosa and Microcystis flos-aquae | Inhibited the photosynthetic system, affecting the rate of electron transfer, leading to the accumulation of electrons and exacerbating the elevated levels of ROS, promoting the lipid peroxidation of cell membranes; PVC had a greater negative impact on the photosynthetic activity of algae than PP. | [28] |
| PA | Concentration of 1000 mg/L | Microcystis aeruginosa | Obstructed photosynthetic electron transfer, reduced algal bile protein synthesis, damaged algal cell membrane, enhanced the release of extracellular polymers, and induced oxidative stress. | [16] |
| Types of MPs | Environmental Conditions | Targets | Specific Hazards | Reference |
|---|---|---|---|---|
| PS | Particle size of 5~70 μm | Danio rerio | Induced hepatic inflammation and lipid accumulation, generated oxidative stress, induced alterations in hepatic metabolic profiles, disrupted lipid and energy metabolism, PS accumulated in zebrafish. | [44] |
| Particle size of 5 μm or 50 μm; concentration 100 μg/L or 1000 μg/L | Larval Danio rerio | Induced changes in the metabolic profile of Larval Danio rerio, differential metabolites involved in various metabolisms in vivo, produced inflammation and oxidative stress, and interfered with glycolipid and energy metabolism. | [45] | |
| Particle size of 5 μm; concentration of 20~100 μg/L | Danio rerio | Reduced body weight and the status factor, reduced transcript levels of major biochemical markers in the liver and major genes related to glucose and lipid metabolism significantly. | [46] | |
| Particle size of 0.10~0.12 μm, concentration of 10 μg/L or 100 μg/L | Danio rerio | Altered the expression profile of antioxidant genes, affected oxidative and immune defence mechanisms, and inhibited neurotransmission in Danio rerio, leading to alterations in gill histogram laminar structure, capillary dilatation and necrosis | [47] | |
| Particle size of 10 μm; with green fluorescence packaged as 2.5% aqueous suspension | zebrafish embryos | Easy to adhere to the surface of embryonic chorion, increased embryonic and larval mortality, and reduced embryonic and larval heart rate. The lethal toxicity of embryos increased with the concentration of MPs | [84] | |
| Particle size of 5 μm; PS concentration of 10 μg/L with Cd2+ concentration of 20 μg/L or PS concentration of 10 μg/L with Cd2+ concentration of 200 μg/L | Danio rerio | Ps-MPs increased the accumulation of Cd2+ in the liver (46% and 184%), intestine (10% and 25%), and gills (9% and 46%) of zebrafish, and joint exposure caused oxidative damage and inflammation in zebrafish tissues. | [85] | |
| Particle size of 0.83~16.5 μm | Oncorhynchus mykiss | Impaired B cells growing in the developing anterior kidney, reduced the RAG1 gene expression, altered membrane shape of immunoglobulin heavy chain mu and tau | [79] | |
| PVC or PS | PVC concentration of 4.2 mg/L combined with a PCBs concentration of 30 ng/g or PS concentration of 3.2 mg/L combined with a PCBs concentration of 30 ng/g | Corbicula fluminea | More prone to moderate and severe tubular dilatation | [69] |
| PA, PE, PP, or PVC | Particle size of about 70 nm, the concentration of 0.001~10 mg/L | Danio rerio | Intestinal villi rupture, intestinal epithelial cell division | [43] |
| PE | Concentration of 100 μg/L combined with ZnONPs of concentration of 50 μg/L | Gambusia holbrooki | ZnO-NPs assisted in the hepatic accumulation of PE-MPs produced the Oxidative stress response, and promoted the induction of toxic effects. | [33] |
| PE concentration of 100 μg/mL and 1000 μg/mL | Danio rerio | Increased the intestinal microbial diversity index, intestinal innate immunity–complement C3 and C4 content first increased and then declined in a dose-dependent manner and increased the infection probability in the intestinal mucosa. | [86] | |
| PLA | Particle size of 135.35 ± 37.12 μm | Danio rerio | Caused gastrointestinal damage in zebrafish, causing specific changes in gut microbiota diversity, and reducing gut pH value | [87] |
| Types of MPs | Environmental Conditions | Targets | Specific Hazards | Reference |
|---|---|---|---|---|
| PS | Particle size of 8.5~30.7 μm | Oryza sativa L. | Reduced the rice stem biomass, reduced the rice branches length, inhibition effect increased with increasing concentration | [80] |
| Fluorescent microplastics (FMP) and PS | Particle size of 1 μm (1%) | Phragmites australis (Cav.) | Inhibition of height and aboveground biomass | [81] |
| PE | Particle size of 4~12 μm | Lemna minor | Presentation mechanical blocking, affection root growth significantly, reducing the viability of root cells | [82] |
| Particle size of 3 μm | Hydroponic Maize | PE accumulated in the root system and reduced the root transpiration, Nitrogen content and growth. | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, Q.; Ren, Z.; Zheng, Y.; Wang, L.; Lu, H. Progress in the Study of Toxic Effects of Microplastics on Organisms in Freshwater Environments and Human Health. Water 2025, 17, 229. https://doi.org/10.3390/w17020229
Cong Q, Ren Z, Zheng Y, Wang L, Lu H. Progress in the Study of Toxic Effects of Microplastics on Organisms in Freshwater Environments and Human Health. Water. 2025; 17(2):229. https://doi.org/10.3390/w17020229
Chicago/Turabian StyleCong, Qiao, Zixuan Ren, Yang Zheng, Lijun Wang, and Hai Lu. 2025. "Progress in the Study of Toxic Effects of Microplastics on Organisms in Freshwater Environments and Human Health" Water 17, no. 2: 229. https://doi.org/10.3390/w17020229
APA StyleCong, Q., Ren, Z., Zheng, Y., Wang, L., & Lu, H. (2025). Progress in the Study of Toxic Effects of Microplastics on Organisms in Freshwater Environments and Human Health. Water, 17(2), 229. https://doi.org/10.3390/w17020229







