Abstract
In this research, a tungsten oxide was prepared via a green (biogenic) synthesis route where sodium tungstate dihydrate and Punica granatum peel extract were used as a precursor and a reducing/capping agent, respectively. The characterization of the prepared tungsten oxide was performed through various spectroscopic and microscopic techniques. The characterization results revealed the preparation of highly crystalline and nanorod-shaped (length = 123 nm and width = 31.3 nm) tungsten oxide with a probable chemical formula of W5O14. Various functional groups on the W5O14 surface were also reported. The prepared nanorods were further used for the removal of Bismarck Brown R (BBR) dye from water in a batch manner. By varying the dose of nanorods (0.5–3.0 g L−1), BBR solution pH (2−10), contact time (15–120 min), BBR concentration in solution (10–60 mg L−1), and temperature of BBR solution (30, 40, and 50 °C), the optimized condition for maximum adsorption efficiency was measured. The results revealed that 2.0 g L−1 amount of nanorods of tungsten oxide were used to remove ~98% of BBR dye from its 10 mg L−1 at 30 °C and 7.0 pH. The temperature-dependent adsorption data were fitted to different types of non-linear isotherm models (e.g., Langmuir and Freundlich) to assess the adsorption potential and adsorption mechanisms in relation to temperature impacts. The synthesized nano-adsorbent fits the Langmuir as well as the Freundlich isotherm model with a maximum adsorption capacity of 17.84 mg g−1. Pseudo-first-order, pseudo-second-order, and Elovich kinetic models were used for the study of adsorption kinetics. BBR adsorption onto the W5O14 nanorods follows the pseudo-second-order rates. The present adsorption is governed by physico-chemical adsorption with predominant chemical interactions.
1. Introduction
The pollution of colored substances in water is becoming a significant problem [1]. These colored substances are released in freshwater along with wastewater from various industries such as textiles and paint [2,3]. The colored substances mainly contain dyes with complex structures which are very harmful to aquatic life as well as human beings [1,2,3]. Out of various dyes, Bismarck Brown R (BBR) dye is used as a coloring matter in many industries, which is released in freshwater bodies in high quantities [4,5]. BBR is a cationic basic diazo dye, which is carcinogenic and known to have very adverse effects on our ecosystem and human health [4,5]. It causes redness and irritation to the eyes and skin on contact [4]. It causes nausea, vomiting, diarrhea, pain, and redness of the mouth and throat upon ingestion [4]. Considering these effects of BBR, it is very important to find a way to remove it from water. Many types of techniques were used to remove BBR dye from water [6,7], of which adsorption is considered the easiest method [4,5]. The use of nano-adsorbent makes it the most advanced method [8].
Until now, many nanoparticles (NPs) have been used in the adsorption process like tin oxides [9], copper oxides [10], titanium oxides [11], iron oxides [12], zinc oxides [13], and so on [14].
Currently, tungsten oxide NPs (WOx) are being used in wastewater treatment [15]. Tungsten oxide is an n-type semiconductor that has a wide band gap, with large surface area and oxygen defects, i.e., lacking a large number of oxygen species [16]. Various functional groups have also been incorporated into tungsten oxide [17,18], which increases its absorption capacity for pollutants in water [19].
Notably, the properties of NPs such as functionality, stability, surface area, affordability, and sustainability depend on their preparative methods [20,21,22]. Many methods were used for their preparation such as precipitation [23], electrochemical reaction [24], microwave [25], etc. [23]. Similarly, WOx nanorods were also synthesized by different methods, including chemical vapor deposition [26], acid precipitation methods [27], solvothermal synthesis [28], sol–gel methods [29], and hydrothermal process [30].
Currently, the concept of green nanotechnology is gaining importance over conventional methods, including physical and chemical ones [31]. It embraces the principles of green chemistry by fusion with nanotechnology [31,32]. Green chemistry mainly emphasizes the designing of nanomaterials (NMs, 1–100 nm) that are made from renewable and less hazardous substances such as bio-materials [33]. Bio-materials belonging to different groups from microorganisms [34] to plants [35] possess different phytochemicals, which act as stabilizing and reducing agents, thus aiding in the synthesis of a wide variety of NMs [36]. The plant materials are preferred over microbes due to abundance availability, no use of culture media, inexpensiveness, and no requirement of trained staff for handling purposes [37,38]. Plant materials (extract) have the advantages of using natural resources which are biodegradable and biocompatible, less toxic solvents, low cost, shorter time of metal ions reductions, larger-scale NM production, and no requirement for high pressure/energy [37,38,39]. Therefore, focusing on the advantages of biological processes using the plant extract, in this study, tungsten oxide was made with the plant extract of pomegranate peel (Punica granatum).
Punica granatum (P. granatum), a deciduous fruit-bearing shrub, belongs to the Punicaceae family, fruits with great health benefits, and is globally cultivated [40,41,42]. P. granatum peel extract contains various phytochemical constituents, such as tannins, flavonoids, and alkaloids, and high amounts of polyphenol contents, such as ellagitannins (hydrolysable tannins), anthocyanins (condensed tannins), ellagic acid, and gallic acid [43,44,45,46]. Abundant phytochemicals present in the P. granatum extract are responsible for various medicinal properties such as antioxidant [47], antimicrobial [48], anti-carcinogenic [49], and antiparasitic [49] properties.
The phytochemicals found in the P. granatum extract were used during the preparation of NPs and acted as capping and stabilizing agents [40,41]. They were also responsible for the functional groups on the surface of the NPs [50]. Additionally, NPs made using plant extract are affordable, sustainable, and non-toxic [35,36,37].
Various metal oxides, including Fe3O4 [51] and ZnO [52], were already synthesized from the P. granatum peel extract.
Considering all these features of the P. granatum peel extract, in the present study, tungsten oxide was prepared through the green (biogenic) method using a pomegranate peel extract with the idea that the prepared adsorbent would be an affordable, sustainable, and low-toxic material that has many functional groups. The characterization of the prepared tungsten oxide sample along with its adsorption application (for toxic BBR dye) was also carried out. The results obtained in this study were described in detail, which may present better and improved conclusions.
2. Experimental Details
2.1. Materials
The pomegranate was purchased from a local market near Jamia Millia Islamia, New Delhi. The peels of pomegranate were washed and shadow-dried to make an extract. Sodium tungstate dihydrate (Chemical formula = Na2WO4·2H2O; purity = 99%; molecular mass = 329.86 g mol−1) was procured from Loba Chemie Private Limited, Maharashtra, India. Hydrochloric acid (Chemical formula = HCl; purity = 37%; molecular mass = 36.46 g mol−1), sodium hydroxide (Chemical formula = NaOH; purity = 99%; molecular mass = 39.99 g mol−1), and Bismarck Brown R (BBR) (Chemical formula = C21H26Cl2N8 (Figure 1); absorption maximum (λmax) = 484 nm; purity = 99%; molecular mass = 461.395 g mol−1) were purchased from Merck Limited, Maharashtra, India.
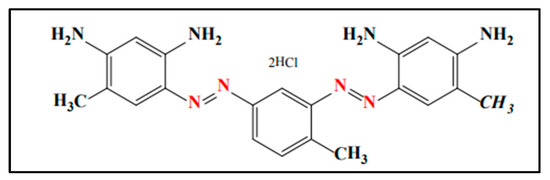
Figure 1.
Structure of BBR dye [53].
2.2. Methods
2.2.1. Preparation of P. granatum Peel Extract
The fine powder of the shadow-dried P. granatum peel was made in the mixer grinder. For extract preparation, 10.0 g of fine P. granatum peel powder was added to 100 mL of double-distilled water and heated at 82 °C for one hour. The prepared extract was filtered using Whatman paper No.1 and stored in a refrigerator for further use.
2.2.2. Green (Biogenic) Synthesis of W5O14 Nanorods
W5O14 nanorods were prepared using green (biogenic) synthesis method using P. granatum peel extract in one step [54]. In a one-step synthesis of W5O14 nanorods, 100 mL of 0.2M solution of Na2WO4·2H2O was made by stirring it for 10.0 min. To this solution of Na2WO4·2H2O, 10 mL of pomegranate peel extract and 5.0 mL of concentrated HCl were added, and a brown precipitate was formed immediately. The solution mixture was heated for 1 h at 80 °C on a magnetic stirrer. After that, the washing of the precipitate was conducted several times with distilled water and ethanol to remove the impurities. The precipitate was separated from the mixture solution after centrifuging at a speed of 3000 rpm for 3.0 min and then dried at 60 °C in a drying oven for about 24 h. The prepared dried brown sample was crushed into a fine powder with the help of a mortar pestle. The sample powder was annealed at a temperature of 800 °C using the furnace for 2 h by maintaining the temperature with a ramp rate of 30 °C per 2.0 min.
2.3. Characterization
The prepared sample was characterized by using several instrumentation techniques. The diffuse reflectance measurement of the powder sample was conducted using a spectrophotometer (U3900, Hitachi, Japan). The UV-Visible spectrophotometer (UV-1900i, Shimadzu, Japan) was used for recording the absorption spectrum during the study of BBR adsorption by W5O14 nanorods. The FTIR spectrum of the sample was recorded on a Vertex 70V (Bruker Optik, Ettlingen, Germany) spectrometer in the range of wavenumber 4000–400 cm−1. The X-ray diffraction patterns of the powdered sample were recorded on XtaLAB Synergy Make Rigaku (Tokyo, Japan) diffractometer. The diffractometer contains a Cu-filter, Cu-Kα radiation (λ = 1.54 Å) source, 40 kV generator voltage, 30 mA current, and a proportional counter detector. The SEM micrographs of the samples were obtained using the scanning electron microscope (JSM-6510LV, JEOL, Tokyo, Japan). A highly sophisticated transmission electron microscopy (TEM) instrument (JEM-2100, JEOL, Japan) was used for getting TEM images which were analyzed.
Point of Zero Charge Determination with Temperature Variation
The zero-point charge (ZPC) is the pH at which solid NM surfaces exhibit zero charge or are electrically neutral in an electrolyte and above or below the charge which the surface acquires. For determining the surface charge of the adsorbent, whether it is positively or negatively charged, H+/OH− plays a very important role. Protonation, i.e., the addition of H+, makes the surface positive, and deprotonation, i.e., the addition of OH− makes the surface negative. The H+/OH− present in the medium either dissociates or associates with amphoteric bio-functional groups. These functional groups come from the plant extract found on the adsorbent surface, causing a build-up of positive and negative charge on the adsorbent. For determining the ZPC, the salt addition method was used, which involved the following steps [55,56].
First, the aqueous solutions of 0.1N HCl, 0.1M NaOH, and 0.1M KNO3 were prepared. The pH of 0.1M KNO3 solutions was set in between 2 and 12 by the addition of the 0.1N HCl (acidic) and 0.1M NaOH (basic) solutions. In a set of 3, batches of 6 Erlenmeyer flasks (for pH 2, 4, 6, 8, 10, and 12) carrying 20 mL of KNO3 solutions with 20 mg of W5O14 nanorods were prepared. The 3 sets of Erlenmeyer flasks containing suspension were agitated for 4 h at three different temperatures of 30, 40, and 50 °C. The pH of the solution at starting was measured as pHi, while after agitation, it was measured as pHf. The plot of ∆pH (pHf-pHi) vs. pHi gives the ZPC of the adsorbent surface [56].
2.4. Adsorption Study
For the study of BBR adsorption by W5O14 nanorods, experiments were conducted in a batch mode. A series of samples were prepared in an Erlenmeyer flask (50 mL), which contained varied amounts of W5O14 (5–30 mg) in 10 mL of the BBR solution (10 ppm as an initial concentration) at different pH values (2–12). The solution was agitated in the temperature-controlled water bath shaker at 150 rpm for agitating time 2.0 h with 15 min intervals. Fixing one variable while varying another, the effect of a dose of W5O14, temperature (30, 40, and 50 °C), pH, and contact time on adsorption was studied. To separate the adsorbent from the BBR solution after adsorption, the solutions after shaking were centrifugated using a centrifugation machine (Remi, R-8C, Remi Electrotechnikk Limited, Mumbai, India) at 3000 rpm. The concentrations of the BBR dye (in filtrate with unknown concentrations) after each adsorption experiments were determined using a UV–vis spectrophotometer.
The capability of a particular adsorbent for the adsorbing dye was calculated in terms of percentage adsorption/removal efficiency (%) using the following relationships (Equations (1) and (2)) [57].
where Co and Ce are the initial and equilibrium concentrations of BBR in mg L−1, Qe is the adsorbed BBR amount on to a unit mass of W5O14 in mg g−1, V is the sample volume in L, and m is the weight of W5O14 nanorods in g L−1.
The adsorption experiment was repeated three times, and its mean values were recorded. The obtained experimental data was further verified by thermodynamics, isotherms, and kinetics.
2.5. Thermodynamics
In order to understand insights into the temperature-dependent mechanism of the adsorption process, the determination of thermodynamic parameters such as Gibbs free energy (∆G), enthalpy (∆H), and entropy (∆S), changes are necessary. ∆G describes the spontaneity of the adsorption process, while ∆H ascribes the endo/exothermic nature of the adsorption process. The randomness extent in the adsorption system can be measured by the ∆S.
The relation between these thermodynamic parameters can be derived from the Equations (3)–(5) [58].
A plot of ΔG versus T (temperature) gave the values of ΔH and ΔS by its intercept and slope, respectively [58].
To determine the ∆G value, the formula given in Equation (4) is applied [58].
where
Hence, through these equations (Equations (3)–(5)), the values of ∆G, ∆H, and ∆S were measured for BBR adsorption on W5O14.
2.6. Isotherms
In the present study, the obtained temperature-dependent BBR adsorption data were also fitted into isotherm models such as Langmuir isotherm and Freundlich isotherm, and the mechanism obtained was expressed.
The Langmuir model is for monolayer adsorption, which describes chemical adsorption on uniform surfaces, while the Freundlich model represents adsorbate adsorption on heterogeneous solid surfaces. In this study, nonlinear isotherm models were used as these non-linear models are considered to be more advanced and accurate compared to the linear models [59]. The Langmuir and Freundlich models can be represented as Equations (6) and (7), respectively [60].
where Ce (mg L−1) is the equilibrium concentration, Qe is the equilibrium capacity, Qmax (mg g−1) is the maximum monolayer adsorption, and b (L mg−1) is the Langmuir constant.
where KF [(mg g−1)(L mg−1)1/n] and n are the Freundlich constants. When the value of n falls between 1 to 10, it confirms that the adsorption exists in a heterogeneous form.
Therefore, through these isotherms equations, the values of maximum adsorption capacity (Qmax), heterogeneity present in the adsorbent (1/n), and adsorbent–adsorbate-binding strength (b) was estimated.
The separation factor (RL) can be estimated from the value of the Langmuir constant (b) obtained from the Langmuir model and equilibrium concentration (Co). This separation factor confirms whether the tested adsorption process is favorable (0 < RL < 1) or unfavorable (RL > 1). Equation (8) was used to estimate the value of RL [61].
2.7. Kinetics
The adsorption data obtained by changing the time were kinetically verified using the kinetic model. For this verification, two major non-linear kinetic models, pseudo-first-order (PFO) and pseudo-second-order (PSO), were applied. PFO kinetic model indicates whether the adsorption rates observed depend only on the adsorbent’s adsorptive site numbers or not, while PSO assumes that the adsorption rate depends on the adsorbent’s adsorptive site numbers as well as the adsorbate concentration.
The mathematical expressions of non-linear PFO and PSO kinetic models are shown through Equations (9) and (10), respectively [60].
where k1 (minute−1) and k2 (g mg−1 min−1) are the PFO and PSO rate constants, and Qe and Qt (mg g−1) are the adsorption capacity at equilibrium and time t (minutes), respectively.
Further, to check whether the BBR adsorption on W5O14 is physical or chemical, an Elovich kinetic model was used. This model also affirms whether the adsorption rate is more or less than the desorption. This model can be represented by Equation (11), where α (mg g−1 min−1) is the rate of adsorption and β (g mg−1) is the rate of desorption [62].
In the adsorption process, it is very important to determine whether the adsorption is multistep (mass transfer, film diffusion, or intraparticle diffusion) or single step, which helps in determining the slow (rate determining) step. To understand this, Weber and Morris gave a model called interparticle diffusion model (IPD), which can be visualized through Equation (12) [60], where Kipd (mg g−1 min−1/2) is the IPD coefficient and C (mg g−1) is the thickness of the boundary layer (liquid film formed).
The boundary layer thickness value is an important parameter that confirms that, if its value is zero, then IPD is only responsible for an adsorption and is the sole rate-determining step; if it is not zero, then some other step (such as boundary layer (liquid film diffusion)), along with IPD, controls the adsorption [60].
The kinetic mechanism and rate of BBR adsorption were determined using the error function of these nonlinear kinetic model plots.
3. Results and Discussion
3.1. Characterization of W5O14 Nanorods
3.1.1. FTIR Analysis
The FTIR spectrum of the synthesized W5O14 nanorods was recorded in the range of 4000–400 cm−1 for identifying the surface functional groups of nanorods.
In Figure 2A (black line), a broadband around 3444 cm−1 due to -OH stretching band confirms the presence of alcohol and carboxylic acid [63]. Small peaks at 2928 and 2859 cm−1 correspond to the C=C stretching band of the alkyne group [61,64]. The peaks around 1641 cm−1 and 1745 cm−1 relate to -OH/or -NH and -C=O groups that come from the plant extract, respectively [63,64,65]. The bands at 1459 cm−1 are due to -O-CH3 deformation. The peak at 949 cm−1 is due to W=O and W-O stretching vibrations; 904 cm−1, 655 cm−1, and 434 cm−1 are assigned to W-O-W bond stretching vibrations; 850 cm−1 corresponds to O-W-O stretching modes [66,67]. Also, vibration bands at 770 cm−1 confirm C-H out-of-plane deformation related to the ring vibrations [63]. Through FTIR analysis of W5O14, it is proved that many functional groups are present on the surface of the prepared nanorods. These functional groups were used as active sites for the adsorption process. Several previous studies showed the mechanism of adsorption of dye adsorption through such functional groups [60,61,64].
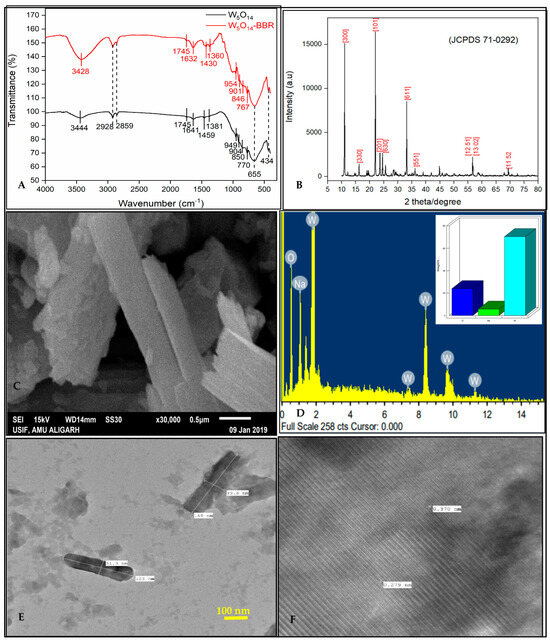
Figure 2.
Results of characterization analysis: (A) FTIR, (B) XRD, (C) SEM, (D) EDX (inset: elemental composition), (E) TEM, and (F) HR-TEM.
The interaction between the functional groups of W5O14 and charged BBR molecule was also demonstrated through comparative studies using the FTIR spectrum of W5O14 before adsorption (Figure 2A black line) and after adsorption (Figure 2A red line). The shifting and appearance of some peaks are noticed in the FTIR spectrum of W5O14 after adsorption (Figure 2A red line). The broad-spectrum peak attributed to the O-H stretching vibrations shifted to a lower wavenumber from 3444 cm−1 to 3428 cm−1, the band for aliphatic C=C stretch at 1641 cm−1 shifted to 1632 cm−1, and the band for -O-CH3 deformation from 1459 cm−1 to 1430 cm−1. The peaks for W=O, W–O, W-O-W, and O-W-O stretching modes, and C-H out-of-plane deformation vibration bands also get shifted. The shifting of these peaks suggested the interaction between the functional groups of W5O14 nanorods and charged BBR molecules. Therefore, it can be said that in the present study, adsorption is governed through the functional groups of W5O14 nanorods.
3.1.2. XRD Analysis
The confirmation of the phase and composition analysis was carried out using the powder XRD technique. The prepared sample was scanned from a 10 to 80° angular range (2θ), as shown in Figure 2B. The XRD diffraction profile revealed the occurrence of diffraction peaks at 2θ of 11.04°, 16.26°, 22.64°, 24.54°, 25.62°, 33.16°, 36.16°, 56.62°, and 69.32°, which correspond to the lattice planes [300], [330], [101], [201], [630], [611], [551], [12 5 1 and 13 0 2], and [11 5 2], respectively, as calculated from the powder X software (https://www.xpowder.com/). W5O14 nanorods have a tetragonal symmetry with cell dimensions a = 23.3300 Å, c = 3.7970 Å, and a space group P −4 21 m, which was determined by using the Match program (Entry number 96-152-7784) [68,69].
Furthermore, the XRD pattern of developed nanorods yields very sharp XRD peaks, demonstrating the rich crystalline nature of the W5O14. Some peaks are found to be more intense compared to other peaks. It shows more growth along a particular crystallographic direction that might be due to nanorod formation.
With the help of characteristic XRD peaks, the crystallite size of any crystal can also be determined by applying Debye–Scherer’s equation (Equation (13)) [70].
where K is a crystalline size factor with a numerical value of 0.9, λ is the wavelength of X-ray radiation (λ= 0.154 nm), β is full width at half maximum (FWHM), and θ is Bragg’s angle.
The crystallite size of the W5O14 nanorods was found to be 61.25 nm.
3.1.3. SEM Analysis
The morphology of the prepared W5O14 nanorods was confirmed by the SEM analysis. Figure 2C shows the SEM micrographs in which W5O14 particles appear as a bundle of elongated cylindrical structures (nanorods) in an aggregated state or clusters. This results in heterogeneity or uneven surfaces, leading to non-uniform adsorption. Note that the aggregation of NPs reduces their surface area, which has a direct effect on the adsorption performance. The elemental composition was confirmed with EDX analysis at the microscopic level. The results from the EDX pattern (Figure 2D) confirm the existence of elements O, Na, and W. The weight % of detected elements were 23.97 (O), 5.65 (Na), and 70.38 (W). The atomic % was found to be 70.44 (O), 11.56 (Na), and 18.00 (W). The Na signal was found due to Na impurity in the prepared sample.
3.1.4. TEM Analysis
TEM and HRTEM analyses were performed to investigate the size and formation of W5O14 nanorods (Figure 2E). The TEM image shows that the NPs have a cylindrical-type structure with a length of ~123 nm and a width of ~31.3 nm. This confirmed that NPs are long rod-shaped. These nanorods have a smooth surface which agrees with the SEM image. Figure 2F shows the enlarged lattice-resolved HRTEM image of the area. The inter-planar distance is found to be approximately 3.7 nm.
3.1.5. ZPC Analysis
The values of ZPC were 5.1, 5.3, and 5.5 at 30, 40, and 50 °C, respectively, as shown in Figure S1a–c. With an increase in temperature, the ZPC increases slightly due to the availability of more H+/OH− ions. This may be due to several factors such as (i) an increase in temperature causing structural changes in the adsorbent, which may lead to changes in the charge density of the adsorbent surface, (ii) an increase in temperature causing the surface functional groups to ionize resulting in higher concentration of charged species on the surface, and (iii) an increase in temperature leading to protonation, and hence more H+ ions. For all these reasons, ZPC may increase with an increase in temperature. This result is in agreement with previous literature [71].
The BBR dye, which is a basic dye, i.e., positively charged in the solution, may show slightly more repulsion with W5O14 at higher temperatures as ZPC increases. This is because the surface becomes more positive with temperature (due to the shift in ZPC towards higher pH), which causes its removal efficiency (%) to slightly decrease with temperature. The effect of pH change on BBR adsorption can be understood from Scheme 1. The effect of pH on BBR adsorption is further shown in detail in the pH effect section.
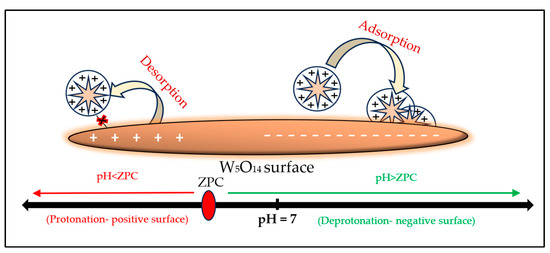
Scheme 1.
Schematic diagram for the adsorption of BBR dye at different pH ranges considering ZPC.
3.2. Adsorption Studies
The present adsorption process was observed with different adsorbent dose, dye concentration, temperature, pH, and contact time.
3.2.1. Effect of W5O14 Dose
The result of the adsorbent dose is shown in the column chart given in Figure 3A. It can be observed that as the adsorption dose increases (0.5 to 3.0 g L−1), the removal efficiency (%) increases from ~15 to ~99%. The reason is that as the adsorbent becomes more concentrated in the solution, the number of active adsorption sites, which is directly proportional to the quantity of adsorbent, increases, due to which the removal efficiency (%) increases for a fixed concentration of dyes.
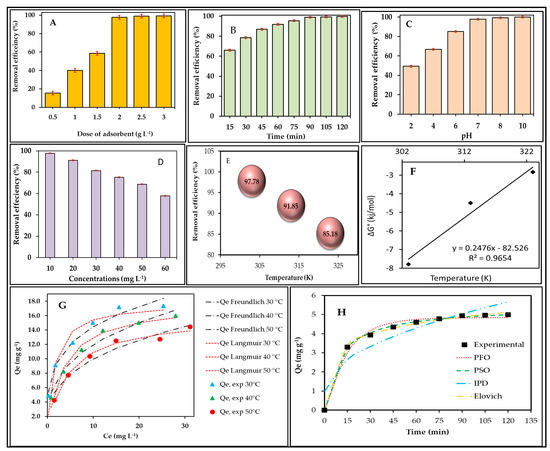
Figure 3.
Effect of (A) adsorption dose, (B) contact time, (C) pH, (D) BBR concentrations, (E) temperature on BBR removal efficiency (%), (F) thermodynamic plot, (G) isotherm plot, and (H) kinetic plot.
When at a certain amount of adsorbents, where the adsorption sites become higher than the concentration of dye (charged molecules) in solution, equilibrium is reached. At this stage, the dye is completely adsorbed on the adsorbent surface; therefore, the removal efficiency (%) becomes almost constant.
This phenomenon can also be seen in this study that after 2 g L−1, there is almost constant in the removal efficiency (%) of BBR, which confirms that, in the present adsorption study, for 10 mg L−1 BBR, 2 g L−1 of W5O14 was the optimized value, at which about 98% of BBR was removed. This result shows good agreement with the previous study [72].
3.2.2. Effect of Contact Time
By keeping the adsorbent dose (2.0 g L−1), concentration (10 mg L−1), pH (7.0), and temperature (30 °C) fixed, the optimized contact time of the BBR adsorption was determined by varying contact time (15–120 min). To note this observation, the removal efficiency (%) was checked at every 15 min interval, the result of which can be seen through Figure 3B. The results confirm that as time increases, removal efficiency (%) increases. However, it can also be seen that in the beginning, at 0–15 min, adsorption efficiency had increased up to more than 60%, and then as time passed, although removal efficiency (%) increased, its rate was very slow, which confirms that the present adsorption was a multi-step adsorption process. It can be explained that, in the beginning, when the adsorbent was used, the surface sites present on the surface of the W5O14 was completely empty and easily absorbed every BBR molecule coming on its surface. In this way, the BBR coming on W5O14 surface does not face any problem, and it becomes easily absorbed on the surface of the W5O14 without any specific adsorption. However, as the contact time increases and the W5O14 surface also gets filled, the incoming BBR molecule is not able to be absorbed easily. Some effort has to be made using BBR to find the remaining adsorptive sites on the W5O14 surface, for which BBR needs specific sites for adsorption, and thus the rate of adsorption decreases when compared to earlier [57].
It can also be seen here that, after 90 min, the removal efficiency (%) almost reached equilibrium, i.e., after this, the BBR content was no longer left in the solution, and thus, it can be said that the BBR was almost completely absorbed at 90 min on W5O14. Therefore, 90 min is the optimized time for the present study.
3.2.3. Effect of pH
Since the pH of wastewater depends on the quality of wastewater, it can vary from acidic to alkaline. Therefore, to provide a practical application of the present adsorbent, the pH effect was also considered here.
It can be seen in the pH-dependent adsorption result (Figure 3C) that, at the alkaline pH environment (above the 7.0 pH), the BBR dye showed better removal efficiency (%) using W5O14. While as the pH environment was moving towards acidic conditions, the ultimate removal efficiency (%) of BBR was also decreasing. The mechanism of this pH effect can be understood through the pH-ZPC of W5O14 (Scheme 1).
In this study, when the pH of the BBR solution was increased or decreased to pH-ZPC (i.e., 5.1 at 30 °C), the removal efficiency (%) of BBR was largely affected. The acidic medium (particularly below the pH-ZPC) is attributed to the protonation on the W5O14 surface (as -COOH2+ and -OH2+); therefore, at low pH levels, the removal efficiency (%) for positively charged BBR gradually decreases (due to repulsion as W5O14-COOH2+---X---BBR+, W5O14-OH2+---X---BBR+). On the other hand, upon increasing the water pH (greater to pH-ZPC), deprotonation occurs (-COO−, OH−); then, the concentration of the negative charged adsorptive sites increases, and due to this, the positively charged BBR is easily attracted towards the negative W5O14 surface (W5O14-COO−------BBR+, W5O14−OH−------BBR+), and this increases the removal efficiency (%) at higher pH levels.
In the present study, 7.0 pH showed better removal efficiency (%) as, at this pH, the W5O14 surface was already negatively charged (-COO−, OH−) due to the positively charged BBR showing better removal efficiency (%) with W5O14 (-COO−------BBR+, OH−------BBR+). Therefore, pH 7.0 was used for the present BBR adsorption investigation of the entire experiment. Scheme 1 can be used to understand the pH effect on BBR adsorption [73].
3.2.4. Effect of Concentration
By fixing the time (90 min), temperature (30 °C), adsorbent dose (2.0 g L−1), and pH (7.0), the effect of BBR concentration (10–60 mg L−1) was also observed in the present adsorption study (Figure 3D). It was found that when the concentration of W5O14 increased from 10 mg L−1 to 60 mg L−1, the removal efficiency (%) gradually decreased, and upon reaching a concentration of 60 mg L−1, it remained only ~57%. Since the W5O14 doses (adsorptive sites) were fixed but the concentration of the incoming BBR increased, the fixed adsorptive sites limited the incoming BBR adsorption at higher concentrations of the dye. Thus, the content of BBR remained in the solution, which reduced the overall removal efficiency (%) at higher concentrations. This explanation can also be understood from the formula of removal efficiency (%) (Equation (2)) in which removal efficiency (%) is inversely proportional to the initial concentration.
It can also be seen here that, at a BBR concentration of 20 mg L−1, there is no significant decrease in removal efficiency (%). At 20 mg L−1 of BBR, the W5O14 showed more than 90% removal efficiency (%), which decreased to ~80% at 30 mg L−1 and ultimately to 57% at 60 mg L−1. Therefore, it can be said that up to 30 mg L−1, W5O14 established itself as an efficient adsorbent. This type of study is also confirmed by many previous studies [72].
3.2.5. Temperature Effect
To understand the effect of temperature on the adsorption of BBR on the surface of W5O14, the current adsorption process was investigated at three temperatures, i.e., 30 °C (303 K), 40 °C (313 K), and 50 °C (323 K). The effect of temperature on BBR removal efficiency (%) is shown in Figure 3E. It can be seen here that as the temperature increased (30 to 50 °C), the removal efficiency (%) for BBR decreased. This may be because, as the temperature increases, the bond (weak physical bond) between the W5O14 surface and BBR gradually breaks [74], or it may also be due to the weakening of residual forces [75]. This result shows good agreement with previous studies [74,75].
3.2.6. Thermodynamic Result
The result of thermodynamic analysis for BBR adsorption onto W5O14 surface is given in Table 1. The value of ΔH yielded a negative sign, which indicates the exothermic adsorption process of BBR on the W5O14 surface. The value of ∆H was found to be around −80 KJ mol−1, which affirms that physico-chemical adsorption (not pure physical or pure chemical adsorption) was responsible for the BBR adsorption on the W5O14 in the present study [58,74]. It is worth noting here that the dye molecule can show both physical (on the top layer) and chemical (inherent layer) adsorption on the surface of the adsorbent [60].

Table 1.
The thermodynamic parameters for the adsorption of BBR onto W5O14.
The values of ΔG were also found to be negative (Figure 3F), indicating the spontaneity of BBR adsorption on the W5O14 at all tested temperatures. The related ∆S value was also measured as negative, which shows the decrease in randomness at the adsorbate–adsorbent interface (i.e., adsorption system). This result shows good agreement with previous studies [75].
3.2.7. Isotherm Results
The resulting parameters of isotherm plots (Figure 3G) are given in Table 2. As can be seen from Langmuir isotherm analysis, with increased temperature, Qmax and b values continually decreased. The decrease in the values of Qmax and b with temperature confirmed that the current BBR adsorption was exothermic, i.e., energy was being released during adsorption, and bond weakening also increased with increased temperatures [74,75]. The values of RL were greater than zero but less than one (0–1), confirming that the BBR adsorption on W5O14 was favorable at every tested temperature [74].

Table 2.
The isothermal parameters for the adsorption of BBR onto W5O14.
The result analysis of the Freundlich isotherm revealed that the values of 1/n fell between 0 and 1, which shows the favorability of the BBR adsorption at all temperatures [61,74]. Moreover, the values of 1/n (less than unity and close to zero) suggest the chemical adsorption of BBR on the heterogeneous surface of W5O14. This result can be confirmed through previous studies given in the literature [60].
The fitting of BBR adsorption data to isotherm models can be confirmed from the fitting and error function (sum of squares regression—SSR and Chi-square (χ2)) of the non-linear graph of Langmuir and Freundlich isotherm plots (Figure 3G) [60]. A smaller SSR and χ2 indicate a better fit of the plot to the data. It is clearly visible that, initially, the Freundlich plot predominantly fits, whereas later, both the Freundlich and Langmuir isotherm plots fit (Langmuir isotherm fits predominantly at 50 °C) to the BBR adsorption data.
As mentioned above in the thermodynamic analysis, the dye molecules can show both physical (on the upper layer) and chemical (on the underlying layer) adsorption on the surface of the adsorbent. Therefore, from the current isotherm plot fitting results, it can be concluded that, as the temperature increases, the physisorption (for instance, H bond or weak Van der Waals forces) weakens, while the chemisorption (electrostatic bonds) remains intact. This is the reason that, at a lower temperature, the Freundlich isotherm plot fits in the BBR adsorption data, while as temperature increases, the Langmuir isotherm plot fitting to BBR adsorption also increases. Therefore, it can be said that the present resulting adsorption was due to the physico-chemical adsorption of BBR on the W5O14 surface. The same result was obtained from current thermodynamic analysis, and it can also be seen in the previous literature [60].
3.2.8. Kinetic Results
The non-linear kinetic plots are shown in Figure 3H, the results of which are given in Table 3. The error functions of the kinetic plots were used to determine which model best fit the experimental data. The kinetic results confirmed that the PSO model proved to be a better fit, as the rate constant and the value of error function (SSR and χ2) for the PSO plot were lower than those for PFO. Moreover, the better agreement between the experimental equilibrium absorption capacity, Qe,exp, and the equilibrium absorption capacity, Qe,cal, calculated from the PSO model also suggested that the PSO model is a predominant model for the BBR absorption study. However, there is not much difference in the error functions (SSR and χ2) and Qe,cal between PFO and PSO, which indicates the simultaneous occurrence of physical and predominant chemical adsorption in the BBR adsorption process on the W5O14 surface [60].

Table 3.
Kinetic parameter for adsorption of BBR onto W5O14.
The predominant chemical adsorption was further confirmed by the Elovich (chemisorption) model, which fits the experimental adsorption data (Qe,exp) well. The values of SSR and χ2 were also found to be very low for the Elovich model. Moreover, the adsorption rate (α) was confirmed to be higher than the desorption rate (β), which also demonstrated the compatibility of the present adsorption process [62]. These findings are also in agreement with the above thermodynamic and isothermal studies.
The plot of the IPD model for this study did not show good fitting with the experimental adsorption data, and the value of C was obtained positive. The positive C value obtained in this study was greater than zero, which confirms that, in the present adsorption, IPD alone was not responsible for determining the rate of BBR adsorption, but the boundary layer (film diffusion) was also responsible for determining the rate [60].
Therefore, the overall kinetic results indicate that, in the present study, the adsorption of BBR on W5O14 occurred through the physico-chemical interaction between the charged W5O14 surface and charged BBR molecules, with the chemical interaction being the predominant factor. Similar results were shown in many previous studies [60,62,75].
3.2.9. Mechanism of BBR Adsorption onto the W5O14 Nanorods
Thermodynamic, isotherm, and kinetic studies revealed that the physicochemical adsorption process was responsible for the adsorption of BBR on the W5O14 surface. This can be justified through the FTIR analysis of W5O14 after adsorption. The FTIR analysis of BBR-loaded W5O14 (Figure 2A, red line) suggested that the physico-chemical interaction was triggered through the electrostatic and non-electrostatic (H bonding and Van der Waals) interaction between the functional groups of W5O14 and the BBR dye. The shifting in -OH peaks confirm that the interaction between W5O14 and BBR might be through the hydrogen bond interaction. Apart from this, other shifted peaks, such as O-CH3, -NH, and W-O, in BBR-loaded W5O14 suggested the electrostatic interaction between functionalized W5O14 and charged BBR dye molecules. Notably, some peaks in the FTIR spectrum of BBR-loaded W5O14 (Figure 2A red line) did not shift, which revealed that there was only some specific interaction between W5O14 and BBR. Additionally, the analysis of the pH effect on BBR adsorption revealed that the BBR adsorption is also governed through the intermolecular (Ven der Waal) forces between cationic BBR and W5O14. Due to these intermolecular forces, W5O14 showed a significant removal efficiency for cationic BBR even at very low pH (acidic condition).
4. Conclusions
In this study, a cationic toxic dye (Bismarck Brown R) was adsorbed on the newly prepared tungsten oxide nanorods. Green synthesis, using an extract of pomegranate peel, was adopted to prepare the tungsten oxide with the chemical formula of W5O14. The reported W5O14 showed a crystalline nature with tetragonal symmetry. The W5O14 particles showed a cylindrical (rod-like) structure of ~123 nm length and ~31.3 nm width. Additionally, the W5O14 showed many functional groups on its surface, which proved to be helpful for the adsorption of the Bismarck Brown R dye. In this study, the Bismarck Brown R removal efficiency (%) was optimized under different variable conditions, and the optimal result was found: 2.0 g L−1 of W5O14 was able to remove about 98% of the Bismarck Brown R dye from its 10 mg L−1 concentration at 30 °C and 7.0 pH. Thermodynamic, isotherm, and kinetic models were used to analyze the mechanism. The results showed that the physico-chemical adsorption (with predominantly chemical adsorption) was responsible for Bismarck Brown R dye adsorption on the surface of W5O14. At 30 °C, the Langmuir adsorption capacity for the present study was found to be 17.65 mg g−1. Further, the current adsorption process was exothermic. The synthesized W5O14 nanorods seem to be a promising candidate in wastewater treatment applications. In the near future, W5O14 should be used for the removal of other toxic pollutants such as heavy metals. Its adsorptive potential should also be explored for natural water.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17020196/s1. Figure S1: Point of zero charge of W5O14 nanorods at (a) 30 °C, (b) 40 °C and (c) 50 °C.
Author Contributions
B.F., R.A., N.M.A. and A.A.A.-G.: Formal analysis; data curation; writing—original draft; composite preparation; adsorption experiments; reagent, material, analysis tool, and data contribution; experiment conception and design; and data analysis and interpretation. S.I.S.: Formal analysis, data curation, writing—original draft, writing—review and editing, data analysis and interpretation. S.O.: Writing—review and editing and English proof reading. R.H.A. and E.A.A.: Formal analysis, data curation, writing—original draft, writing—review and editing, experiment conception and design, data analysis and interpretation, and English proof reading. M.K.K.: Formal analysis, data curation, writing—original draft, writing—review and editing, data analysis and interpretation, and English proof reading. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partial support (to Seungdae Oh) from the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science, ICT and Future Planning) (No. RS-2024-00350751).
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
Acknowledgments
The authors acknowledge Jamia Millia Islamia for providing laboratory facility and the University Grant Commission, New Delhi, India, for financial support in the form of non-NET fellowships to two authors (Bushra Fatima and Sharf Ilahi Siddiqui).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alsukaibi, A.K. Various approaches for the detoxification of toxic dyes in wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Khan, W.U.; Ahmed, S.; Dhoble, Y.; Madhav, S. A critical review of hazardous waste generation from textile industries and associated ecological impacts. J. Indian Chem. Soc. 2023, 100, 100829. [Google Scholar] [CrossRef]
- Viktoryová, N.; Szarka, A.; Hrouzková, S. Recent developments and emerging trends in paint industry wastewater treatment methods. Appl. Sci. 2022, 12, 10678. [Google Scholar] [CrossRef]
- Sivalingam, S.; Sowmiya, A. Industrial biomass waste as an economical, potential adsorbent for removing the Bismarck Brown R dye and zinc metal ions from effluents. Environ. Sci. Adv. 2024, 3, 732–750. [Google Scholar] [CrossRef]
- Solanki, S.; Sinha, S.; Seth, C.S.; Tyagi, S.; Goyal, A.; Singh, R. Enhanced adsorption of Bismark Brown R dye by chitosan conjugated magnetic pectin loaded filter mud: A comprehensive study on modeling and mechanisms. Int. J. Biol. Macromol. 2024, 270, 131987. [Google Scholar] [CrossRef]
- Jawhari, A.H.; Malik, M.A.; Hasan, N.; Fatima, B. MgO-CdWO4: A visible-light-active heterojunction photocatalyst for Bismark brown dye degradation. J. Mol. Struct. 2024, 1305, 137594. [Google Scholar] [CrossRef]
- Medrano-Rodríguez, F.; Picos-Benítez, A.; Brillas, E.; Bandala, E.R.; Pérez, T.; Peralta-Hernández, J.M. Electrochemical advanced oxidation discoloration and removal of three brown diazo dyes used in the tannery industry. J. Electroanal. Chem. 2020, 873, 114360. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Mahdavi, S.; Hassani, A.; Merrikhpour, H. Aqueous phosphorous adsorption onto SnO2 and WO3 nanoparticles in batch mode: Kinetic, isotherm and thermodynamic study. J. Exp. Nanosci. 2020, 15, 242–265. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Sagboye, P.A.; Umenweke, G.; Ajala, O.J.; Omoarukhe, F.O.; Adeyanju, C.A.; Ogunniyi, S.; Adeniyi, A.G. CuO nanoparticles (CuO NPs) for water treatment: A review of recent advances. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100443. [Google Scholar] [CrossRef]
- Jayalath, S.; Wu, H.; Larsen, S.C.; Grassian, V.H. Surface adsorption of Suwannee River humic acid on TiO2 nanoparticles: A study of pH and particle size. Langmuir 2018, 34, 3136–3145. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, K.Q.; Barzinjy, A.A.; Hamad, S.M. Iron oxide nanoparticles: Preparation methods, functions, adsorption and coagulation/flocculation in wastewater treatment. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100661. [Google Scholar] [CrossRef]
- Al-Arjan, W.S. Zinc oxide nanoparticles and their application in adsorption of toxic dye from aqueous solution. Polymers 2022, 14, 3086. [Google Scholar] [CrossRef]
- Mehta, P.; Chelike, D.K.; Rathore, R.K. Adsorption-Based Approaches for Exploring Nanoparticle Effectiveness in Wastewater Treatment. ChemistrySelect 2024, 9, e202400959. [Google Scholar] [CrossRef]
- Hkiri, K.; Mohamed HE, A.; Abodouh, M.M.; Maaza, M. Experimental and theoretical insights into the adsorption mechanism of methylene blue on the (002) WO3 surface. Sci. Rep. 2024, 14, 26991. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Lin, Q.; Chen, T.; Wei, X.; Li, J.; Zhang, Z. Oxygen vacancy regulation on tungsten oxides with specific exposed facets for enhanced visible-light-driven photocatalytic oxidation. Nanoscale 2018, 10, 2908–2915. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xiao, D.; Zhang, D.; Liu, Y.; Sun, M.; Chen, S.; Sun, M. Progress in Functionalized WO3-based Gas Sensors for Selective H2S and NH3: A Review. Ceram. Int. 2024, 50, 40631–40665. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Karimi-Maleh, H.; Chen, F.; Zhao, S. Innovations in WO3 gas sensors: Nanostructure engineering, functionalization, and future perspectives. Heliyon 2024, 10, e27740. [Google Scholar] [CrossRef]
- Hsini, A.; Naciri, Y.; Laabd, M.; Bouziani, A.; Navío, J.; Puga, F.; Boukherroub, R.; Lakhmiri, R.; Albourine, A. Development of a novel PANI@ WO3 hybrid composite and its application as a promising adsorbent for Cr (VI) ions removal. J. Environ. Chem. Eng. 2021, 9, 105885. [Google Scholar] [CrossRef]
- El-Khawaga, A.M.; Zidan, A.; Abd El-Mageed, A.I. Preparation methods of different nanomaterials for various potential applications: A review. J. Mol. Struct. 2023, 1281, 135148. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fuh, J.Y.H.; Dheen, S.T.; Senthil Kumar, A. Synthesis methods of functionalized nanoparticles: A review. Bio-Des. Manuf. 2021, 4, 379–404. [Google Scholar] [CrossRef]
- Gaur, J.; Kumar, S.; Pal, M.; Kaur, H.; Batoo, K.M.; Momoh, J.O. Current trends: Zinc oxide nanoparticles preparation via chemical and green method for the photocatalytic degradation of various organic dyes. Hybrid Adv. 2024, 5, 100128. [Google Scholar] [CrossRef]
- Ahmadi, H.; Khalaj, G.; Soleymani, F.; Moalem, M.; Pourabdollah, M.; Mahmoudan, M. Electrochemical synthesis and characterization of Cu2O nanoparticles: Effect of electrolyte composition. J. Solid State Electrochem. 2024, 28, 2269–2281. [Google Scholar] [CrossRef]
- Reyes-Blas, M.; Maldonado-Luna, N.M.; Rivera-Quiñones, C.M.; Vega-Avila, A.L.; Roman-Velázquez, F.R.; Perales-Perez, O.J. Single step microwave assisted synthesis and antimicrobial activity of silver, copper and silver-copper nanoparticles. J. Mater. Sci. Chem. Eng. 2020, 8, 13–29. [Google Scholar] [CrossRef]
- Lai, W.H.; Su, Y.H.; Teoh, L.G.; Tsai, Y.T.; Hon, M.H. Synthesis of tungsten oxide particles by chemical deposition method. Mater. Trans. 2007, 48, 1575–1577. [Google Scholar] [CrossRef]
- Pham, N.L.; Luu, T.L.A.; Nguyen, H.L.; Nguyen, C.T. Effects of acidity on the formation and adsorption activity of tungsten oxide nanostructures prepared via the acid precipitation method. Mater. Chem. Phys. 2021, 272, 125014. [Google Scholar] [CrossRef]
- Juelsholt, M.; Lindahl Christiansen, T.; Jensen, K.M. Mechanisms for tungsten oxide nanoparticle formation in solvothermal synthesis: From polyoxometalates to crystalline materials. J. Phys. Chem. C 2019, 123, 5110–5119. [Google Scholar] [CrossRef]
- Mirtaheri, B.; Shokouhimehr, M.; Beitollahi, A. Synthesis of mesoporous tungsten oxide by template-assisted sol–gel method and its photocatalytic degradation activity. J. Sol-Gel Sci. Technol. 2017, 82, 148–156. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, G.; Wang, D.; Chen, Z.; Zhao, M.; Xi, G. Crystallographic phase and morphology dependent hydrothermal synthesis of tungsten oxide for robust hydrogen evolution reaction. J. Alloys Compd. 2021, 875, 160054. [Google Scholar] [CrossRef]
- Rathod, S.; Preetam, S.; Pandey, C.; Bera, S.P. Exploring synthesis and applications of green nanoparticles and the role of nanotechnology in wastewater treatment. Biotechnol. Rep. 2024, 41, e00830. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Banjara, R.A.; Kumar, A.; Aneshwari, R.; Satnami, M.L.; Sinha, S.K. A comparative analysis of chemical vs green synthesis of nanoparticles and their various applications. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100988. [Google Scholar] [CrossRef]
- Singh, S.; Tiwari, H.; Verma, A.; Gupta, P.; Chattopadhaya, A.; Singh, A.; Singh, S.; Kumar, B.; Mandal, A.; Kumar, R.; et al. Sustainable synthesis of novel green-based nanoparticles for therapeutic interventions and environmental remediation. ACS Synth. Biol. 2024, 13, 1994–2007. [Google Scholar] [CrossRef] [PubMed]
- Villagrán, Z.; Anaya-Esparza, L.M.; Velázquez-Carriles, C.A.; Silva-Jara, J.M.; Ruvalcaba-Gómez, J.M.; Aurora-Vigo, E.F.; Rodríguez-Lafitte, E.; Rodríguez-Barajas, N.; Balderas-León, I.; Martínez-Esquivias, F. Plant-Based Extracts as Reducing, Capping, and Stabilizing Agents for the Green Synthesis of Inorganic Nanoparticles. Resources 2024, 13, 70. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of green synthesized metal nanoparticles—A review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.; Al Abboud, M.A.; Alawlaqi, M.M.; Ganash Magdah, A.; Helmy, E.A.; Mabrouk, A.S. Recent advances in green synthesis of silver nanoparticles and their applications: About future directions. A review. BioNanoScience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Adetunji, T.L.; Olisah, C.; Acho, M.A.; Oyetunde-Joshua, F.; Amoo, S.O. Global Research Trends and Recent Advances in Medicinal Plant-Synthesized Nanoparticles for Cancer Treatment. Plants 2024, 13, 2836. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Hari Krishna, R.; Vittal, M.; Likhitha, C.; Pooja, N.; Chaudhary, V. Recent advances in pomegranate peel extract mediated nanoparticles for clinical and biomedical applications. Biotechnol. Genet. Eng. Rev. 2024, 40, 3379–3407. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, P.R.; Varshan, E.I.; Shanmugam, R. In Vitro Anti-diabetic Activity of Pomegranate Peel Extract-Mediated Strontium Nanoparticles. Cureus 2023, 15, e51356. [Google Scholar]
- Ge, S.; Duo, L.; Wang, J.; Zhula, G.; Yang, J.; Li, Z.; Tu, Y. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 2021, 271, 113877. [Google Scholar] [CrossRef]
- Khadivi, A.; Rezagholi, M.; Shams, M. Phytochemical properties and bioactive compounds of pomegranate (Punica granatum L.). J. Hortic. Sci. Biotechnol. 2024, 99, 639–652. [Google Scholar] [CrossRef]
- Kiran, S.; Aslam, A.; Parveen, A.; Dilshad, M.; Hussain, S. Phytochemistry of Punica granatum Fruit: Its Nutritional and Biological Potential. Bioactivities 2024, 2, 57–73. [Google Scholar] [CrossRef]
- Sharma, J.; Maity, A. Pomgerenate Phytochemicals: Neutraceutical and Therapeutic values. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 56–76. [Google Scholar]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Moneim, A.E.A. Antioxidant activities of Punica granatum (pomegranate) peel extract on brain of rats. J. Med. Plants Res. 2012, 6, 195–199. [Google Scholar]
- Emam-Djomeh, Z.; Moghaddam, A.; Yasini Ardakani, S.A. Antimicrobial activity of pomegranate (Punica granatum L.) peel extract, physical, mechanical, barrier and antimicrobial properties of pomegranate peel extract-incorporated sodium caseinate film and application in packaging for ground beef. Packag. Technol. Sci. 2015, 28, 869–881. [Google Scholar] [CrossRef]
- Kelleci, K. In Vitro Anticarcinogenic, Antiparasitic and Antimicrobial Effects of P. granatum Extract. Biol. Bull. Rev. 2023, 13 (Suppl. S3), S264–S269. [Google Scholar] [CrossRef]
- Chau, T.P.; Veeraragavan, G.R.; Narayanan, M.; Chinnathambi, A.; Alharbi, S.A.; Subramani, B.; Brindhadevi, K.; Pimpimon, T.; Pikulkaew, S. Green synthesis of Zirconium nanoparticles using Punica granatum (pomegranate) peel extract and their antimicrobial and antioxidant potency. Environ. Res. 2022, 209, 112771. [Google Scholar] [CrossRef] [PubMed]
- Yusefi, M.; Shameli, K.; Ali, R.R.; Pang, S.W.; Teow, S.Y. Evaluating anticancer activity of plant-mediated synthesized iron oxide nanoparticles using Punica granatum fruit peel extract. J. Mol. Struct. 2020, 1204, 127539. [Google Scholar] [CrossRef]
- Shaban, A.S.; Owda, M.E.; Basuoni, M.M.; Mousa, M.A.; Radwan, A.A.; Saleh, A.K. Punica granatum peel extract mediated green synthesis of zinc oxide nanoparticles: Structure and evaluation of their biological applications. Biomass Convers. Biorefinery 2024, 14, 12265–12281. [Google Scholar] [CrossRef]
- Kamil, A.M.; Abdalrazak, F.H.; Halbus, A.F.; Hussein, F.H. Adsorption of Bismarck Brown R Dye onto Multiwall Carbon Nanotubes. J. Environ. Anal. Chem. 2014, 1, 104. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Kasi, G.; Kadhiravan, S.; Arumugam, A.; Al-Ghanim, K.A.; Riaz, M.N.; Govindarajan, M. Synthesis of Tungsten Oxide Nanoflakes and Their Antibacterial and Photocatalytic Properties. Fermentation 2023, 9, 54. [Google Scholar] [CrossRef]
- Muhammad, S.T.H.S.; Hussain, S.T.; Waseem, M.; Naeem, A.; Hussain, J.; Tariq Jan, M. Surface charge properties of zirconium dioxide. Iran. J. Sci. 2012, 36, 481–486. [Google Scholar]
- Fatima, B.; Siddiqui, S.; Ahmed, R.; Chaudhry, S.A. Preparation of functionalized CuO nanoparticles using Brassica rapa leave extract for water purification. Desalin. Water Treat. 2019, 164, 192–205. [Google Scholar] [CrossRef]
- Fatima, B.; Alwan, B.A.; Siddiqui, S.I.; Ahmad, R.; Almesfer, M.; Khanna, M.K.; Mishra, R.; Ravi, R.; Oh, S. Facile synthesis of cu-zn binary oxide coupled cadmium tungstate (Cu-znbo-cp-ct) with enhanced performance of dye adsorption. Water 2021, 13, 3287. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S. Insight into adsorption thermodynamics. In Thermodynamics; Tadashi, M., Ed.; Intech Open: London, UK, 2011. [Google Scholar]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020, 10, 132. [Google Scholar] [CrossRef]
- Ahmad, R.; Alzahrani, E.A.; Dwivedi, P.; Hafeez, S.; Deswal, J.; Fatima, B.; Siddiqui, S.I.; Oh, S. Biodegradable Acid-Based Fe2MnO4 Nanoparticles for Water Remediation. Molecules 2024, 29, 3867. [Google Scholar] [CrossRef]
- Falahian, Z.; Torki, F.; Faghihian, H. Synthesis and application of polypyrrole/Fe3O4 nanosize magnetic adsorbent for efficient separation of Hg2+ from aqueous solution. Glob. Chall. 2018, 2, 1700078. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar]
- Narasimharao, K.; Al-Thabaiti, S.; Rajor, H.K.; Mokhtar, M.; Alsheshri, A.; Alfaifi, S.Y.; Siddiqui, S.I.; Abdulla, N.K. Fe3O4@ date seeds powder: A sustainable nanocomposite material for wastewater treatment. J. Mater. Res. Technol. 2022, 18, 3581–3597. [Google Scholar] [CrossRef]
- Lim, C.S. Microwave-assisted synthesis of CdWO4 by solid-state metathetic reaction. Mater. Chem. Phys. 2012, 131, 714–718. [Google Scholar] [CrossRef]
- Bhuyan, B.; Paul, B.; Dhar, S.S.; Vadivel, S. Facile hydrothermal synthesis of ultrasmall W18O49 nanorods and studies of their photocatalytic activity towards degradation of methylene blue. Mater. Chem. Phys. 2017, 188, 1–7. [Google Scholar] [CrossRef]
- Kumar, V.B.; Mohant, D.B. Formation of nanoscale tungsten oxide structures and colouration characteristics. Bull. Mater. Sci. 2011, 34, 435–442. [Google Scholar] [CrossRef]
- McColm, I.J.; Steadman, R.; Wilson, S.J. Iron-promoted phases in the tungsten-oxygen system. J. Solid State Chem. 1978, 23, 33–42. [Google Scholar] [CrossRef]
- Saqib, M.; Jelenc, J.; Pirker, L.; Škapin, S.D.; De Pietro, L.; Ramsperger, U.; Knápek, A.; Müllerová, I.; Remškar, M. Field emission properties of single crystalline W5O14 and W18O49 nanowires. J. Electron Spectrosc. Relat. Phenom. 2020, 241, 146837. [Google Scholar] [CrossRef]
- Vinila, V.S.; Isac, J. Synthesis and structural studies of superconducting perovskite GdBa2Ca3Cu4O10.5+δ nanosystems. In Design, Fabrication, and Characterization of Multifunctional Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 319–341. [Google Scholar]
- Akratopulu, K.C.; Vordonis, L.; Lycourghiotis, A. Effect of temperature on the point of zero charge and surface dissociation constants of aqueous suspensions of γ-Al2O3. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 3697–3708. [Google Scholar] [CrossRef]
- Ahmed, R.; Siddiqui, S.I.; Al Alwan, B.; Almesfer, M.; Khanna, M.K.; Fatima, B.; Mishra, R.; Ansari, M.A.; Oh, S. Biodegradable acid based nanocomposite-CuO-ZnO-Ni (OH) 2/PA: A novel material for water cleansing. J. Clean. Prod. 2022, 341, 130860. [Google Scholar] [CrossRef]
- Desai, H.; Kannan, A. Sustainable and Circular Adsorption of Anionic Dye from Textile Wastewater Using Economical Adsorbent. Sep. Sci. Technol. 2024, 59, 395–406. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef]
- Gupta, V.; Agarwal, A.; Singh, M.K.; Singh, N.B. Kail sawdust charcoal: A low-cost adsorbent for removal of textile dyes from aqueous solution. SN Appl. Sci. 2019, 1, 1271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).