Sustainable Utilization of Dewatering Sludge for the Development of Reinforcement Grouting Materials in Downhole Applications
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design and Methods
3. Experimental Results Analysis
3.1. Compressive Strength
3.1.1. The Effect of Sludge Content on Compressive Strength
3.1.2. The Effect of Alkali Activator Content on Compressive Strength
3.1.3. The Effect of Liquid-to-Solid Ratio on Compressive Strength
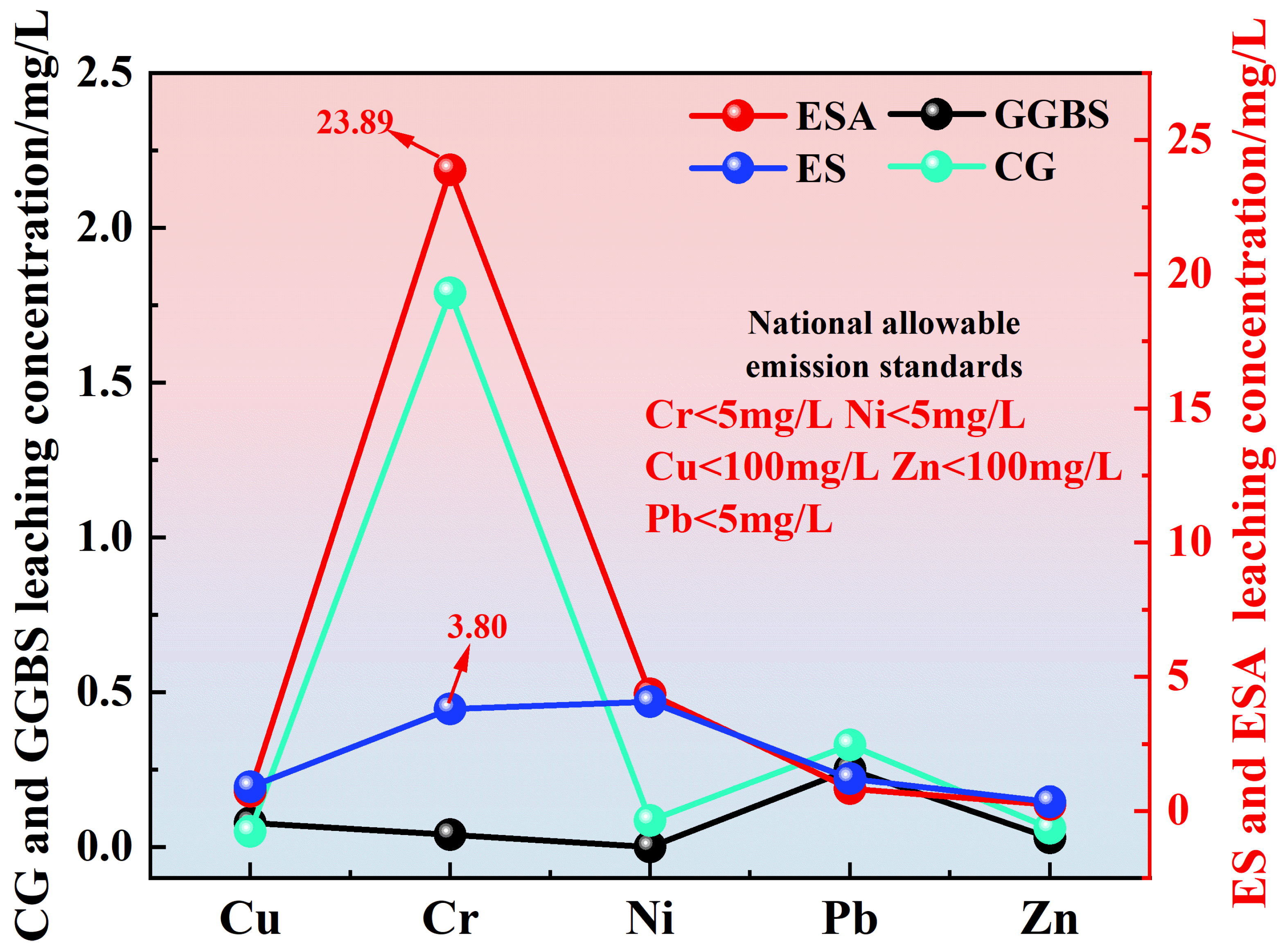
3.2. Concentration and Efficiency of Leaching from Solid Waste Raw Materials and Agglomerates
3.2.1. Leaching Concentration of Raw Materials
3.2.2. Leaching Concentration of Stone Bodies
3.3. Study of the Solidification Behaviour of Cr and Ni by Solid Waste Grouted Agglomerates
3.3.1. Sludge Proportion
3.3.2. Dosage of Alkali Exciters
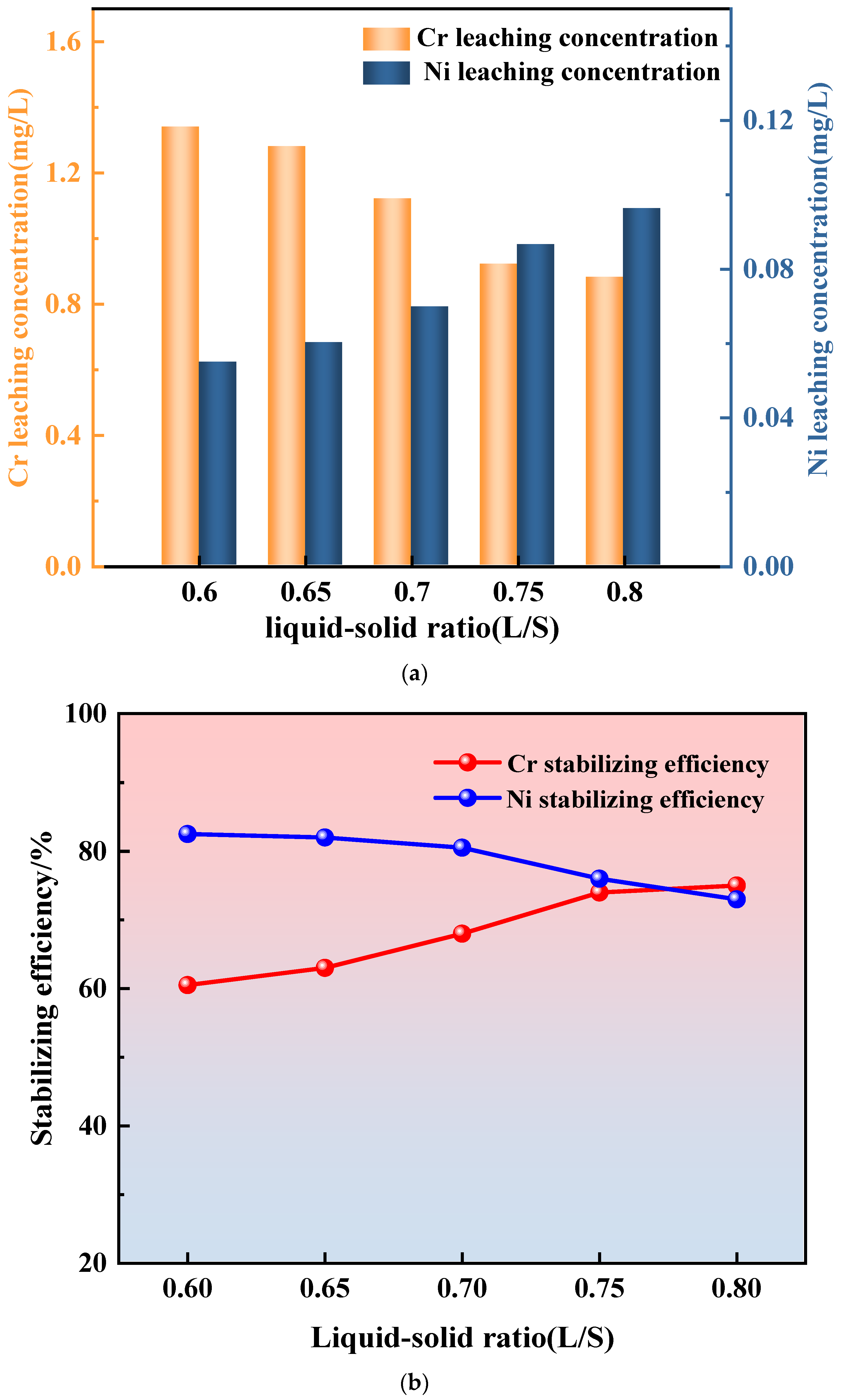
3.3.3. Water–Ash Ratio
3.4. Microscopic Analysis
3.5. Mechanistic Analysis of Solid Waste Grouting Materials for Solidifying Heavy Metals Cr
3.5.1. Mechanism of the Effect of Sludge Incineration on the Leaching of the Heavy Metal Cr
3.5.2. Mechanisms of Reduction and Immobilization of Heavy Metal Cr by Coal-Based Solid Waste Materials
4. Conclusions
- (1)
- With increasing ESA contents, the compressive strength first rises and then declines, peaking at 31.5 MPa with 5% ESA and remaining at 20.8 MPa with 25% ESA, a decrease of only 26.2%. In contrast, the addition of ES leads to a continuous strength reduction of 81.9%, attributed to its high organic content and low pozzolanic activity, which hinder hydration. This comparison highlights the significant contribution of ESA, post incineration, to mechanical strength. While ES offers slightly better fluidity than ESA, its limitations in mechanical strength make it unsuitable for large-scale use as a supplementary cementitious material. The alkali activator significantly affects strength, with an optimal dosage of 8%;
- (2)
- Raw sludge poses potential environmental risks due to Cr (chromium) and Ni (nickel) leaching. The grouting stone effectively immobilized heavy metals, with post-stabilization leaching levels significantly below national environmental standards. The stabilization efficiency of heavy metals in the stone with 25% ESA is in the following order: Cu (86.4%) > Ni (84.3%) > Pb (81.4%) > Zn (72.8%) > Cr (66.1%). ESA-amended samples demonstrated superior heavy metal stabilization compared to that of ES due to the higher compressive strength, which resulted in a denser polymer matrix with reduced porosity;
- (3)
- A decrease in sludge content and an increase in the alkali activator dosage reduced the leaching concentrations of Cr and Ni in the grouting stone. However, a higher liquid-to-solid ratio led to a decrease in Cr leaching but an increase in Ni leaching. The increased water content facilitated the diffusion of Cr(VI), enhancing its reduction by reductive substances (S2− and Fe2+) in GGBS (Ground Granulated Blast-Furnace Slag) and CG, forming Cr(III) with lower mobility and toxicity. This demonstrates that Cr stabilization is not solely reliant on physical encapsulation by hydration gels but also significantly influenced by the chemical reduction of Cr(VI) to a less mobile valence state;
- (4)
- The reductive components in the CGS reduced Cr(VI), subsequently fixing it through the formation of chemically stable chromite phases in the geopolymer matrix. These phases enhance Cr immobilization, minimizing the risk of leaching even under external environmental influences. Thus, using ESA as a cementitious material in CGS offers a safe and resource-efficient strategy for the solidification of heavy metals. This work highlights the potential of ESA as an environmentally sustainable material for the stabilization and solidification of heavy metals in industrial and municipal waste management.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, Y.; Wang, X.K.; Xu, T.; Wang, C.W. A novel sludge Acidification combined Multistage Elutriation (AME) pretreatment strategy for sludge dewaterability improvement, inorganic components separation and heavy metals removal. Resour. Conserv. Recycl. 2022, 185, 106498. [Google Scholar] [CrossRef]
- Lin, W.; Liu, X.; Ding, A.; Ngo, H.H.; Zhang, R.; Nan, J.; Ma, J.; Li, G. Advanced oxidation processes (AOPs)-based sludge conditioning for enhanced sludge dewatering and micropollutants removal: A critical review. J. Water Process Eng. 2021, 45, 102468. [Google Scholar] [CrossRef]
- Ramadan, H.; El Sayed, A.A. Optimization of alum recovery from water treatment sludge-case study: Samannoud water treatment plant, Egypt. Water Environ. J. 2022, 34, 464–473. [Google Scholar] [CrossRef]
- Lo, K.V.; Tan, H.J.; Tunile, I. Microwave enhanced advanced oxidation treatment of municipal wastewater sludge. Chem. Eng. Process. 2018, 128, 143–148. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Y.; Hu, J.H.; Wang, H. A novel method for effective solidifying chromium and preparing crude stainless steel from multi-metallic electroplating sludge. J. Hazard. Mater. 2023, 465, 133068. [Google Scholar]
- Li, D.; Shan, R.; Jiang, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y. A review on the migration and transformation of heavy metals in the process of sludge pyrolysis. Resour. Conserv. Recycl. 2022, 185, 106452. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, D.; Feng, P.; Hao, B.; Guo, Y.; Wang, S. Municipal sewage sludge incineration and its air pollution control. J. Clean. Prod. 2021, 295, 126456. [Google Scholar] [CrossRef]
- Lin, W.; Chen, R.L.; Gong, C.X.; Desmond, P. Sustained oxidation of Tea-Fe(III)/H2O2 simultaneously achieves sludge reduction and carbamazepine removal: The crucial role of EPS regulation. J. Hazard. Mater. 2024, 470, 134182. [Google Scholar] [CrossRef] [PubMed]
- Gailius, A.; Vacenovska, B.; Drochytka, R. Hazardous Wastes Recycling by Solidification/Stabilization Method. Mater. Sci. 2010, 16, 165–169. [Google Scholar]
- Pandey, B.; Kinrade, S.D.; Catalan, L.J.J. Effects of carbonation on the leachability and compressive strength of cement-solidified and geopolymer-solidified synthetic metal wastes. J. Environ. Manag. 2012, 101, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, F.; Stark, J. Activation of blast furnace slag by a new method. Cem. Concr. Res. 2009, 39, 644–650. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.S.; Zhan, B.J. Compressive strength and microstructural properties of dry-mixed geopolymer pastes synthesized from GGBS and sewage sludge ash. Constr. Build. Mater. 2018, 182, 597–607. [Google Scholar] [CrossRef]
- Liang, S.H.; Wang, J.; Wang, Y.X. Experimental study on solidification of concentrated solution sludge by alkali-activated GGBS and MSWl fly ash cementitious materials. China Civil Eng. J. 2023, 56, 75–89. [Google Scholar]
- Wang, A.H.; Zhang, Y.F.; Ni, J.J. Performance and microscopic characteristics of industrial waste residues and bentonite-solidified sludge. Chin. J. Environ. Eng. 2024, 18, 857–865. [Google Scholar]
- Roy, A.; Stegemann, J.A. Nickel speciation in cement-stabilized/solidified metal treatment filtercakes. J. Hazard. Mater. 2017, 321, 353–361. [Google Scholar] [CrossRef]
- Tian, S.C.; Jiang, J.G.; Zhang, C. Influence of flue gas SO2 on the toxicity of heavy metals in municipal solid waste incinerator fly ash after accelerated carbonation stabilization. J. Hazard. Mater. 2010, 192, 1609–1615. [Google Scholar]
- Garrabrants, A.C.; Sanchez, F.; Kosson, D.S. Changes in constituent equilibrium leaching and pore water characteristics of a Portland cement mortar as a result of carbonation. Waste Manag. 2004, 24, 19–36. [Google Scholar] [CrossRef]
- Lin, M.; Ning, X.A.; Liang, X.; Wei, P.; Wang, Y.; Liu, J. Study of the heavy metals residual in the incineration slag of textile dyeing sludge. J. Taiwan Inst. Chem. Eng. 2014, 45, 1814–1820. [Google Scholar] [CrossRef]
- Mao, L.; Gao, B.; Deng, N.; Zhai, J.; Zhao, Y.; Li, Q.; Cui, H. The role of temperature on Cr(VI) formation and reduction during heating of chromium-containing sludge in the presence of CaO. Chemosphere 2015, 138, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, C.; Zhao, M.; Yang, K.; Shen, R.; Zheng, Y. Immobilization potential of Cr(VI) in sodium hydroxide activated slag pastes. J. Hazard. Mater. 2017, 321, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Verbinnen, B.; Billen, P.; Van Coninckxloo, M.; Vandecasteele, C. Heating temperature dependence of Cr (III) oxidation in the presence of alkali and alkaline earth salts and subsequent Cr (VI) leaching behavior. Environ. Sci. Technol. 2013, 47, 5858–5863. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, H.; Mao, L.; Hashmi, M.Z.; Xu, F.; Tang, X. Stabilization/solidification of chromium-bearing electroplating sludge with alkali-activated slag binders. Chemosphere 2020, 240, 124885. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Liu, M.Y.; Luo, F.W.; Lu, H.J. Environmental Characteristics, Mechanical and Hydraulic Behavior of Solidified Cr(VI) Contaminated Soil with Industrial Waste Residue. Water Air Soil Pollut. 2023, 234, 117. [Google Scholar] [CrossRef]
- Yi, C.; Ma, H.Q.; Chen, H.Y.; Wang, J.; Shi, J.; Li, Z.; Yu, M. Preparation and characterization of coal gangue geopolymers. Constr. Build. Mater. 2018, 187, 318–326. [Google Scholar]
- Liao, C.; Tang, Y.; Liu, C.; Shih, K.; Li, F. Double-Barrier mechanism for chromium immobilization: A quantitative study of crystallization and leachability. J. Hazard. Mater. 2016, 311, 246–253. [Google Scholar] [CrossRef]
- Su, P.D.; Zhang, J.K.; Li, Y.D. Chemical fixation of toxic metals in stainless steel pickling residue by Na2S∙xH2O, FeSO4∙6H2O and phosphoric acid for beneficial uses. J. Environ. Sci. 2020, 90, 364–374. [Google Scholar] [CrossRef]
- Wang, X.; Cui, S.P.; Wang, L.; Liu, J. Solidification and Stability of Ettringite Doped Cr lon with Different Valence. Bull. Chin. Ceram. Soc. 2015, 34, 3308–3314. [Google Scholar]
- Guo, Q. Study on Preparation and Adsorption Properties on the Heavy Metal lons of Chitosan/Geopolymer Permeable; Lanzhou Jiaotong University: Lanzhou, China, 2023. [Google Scholar]
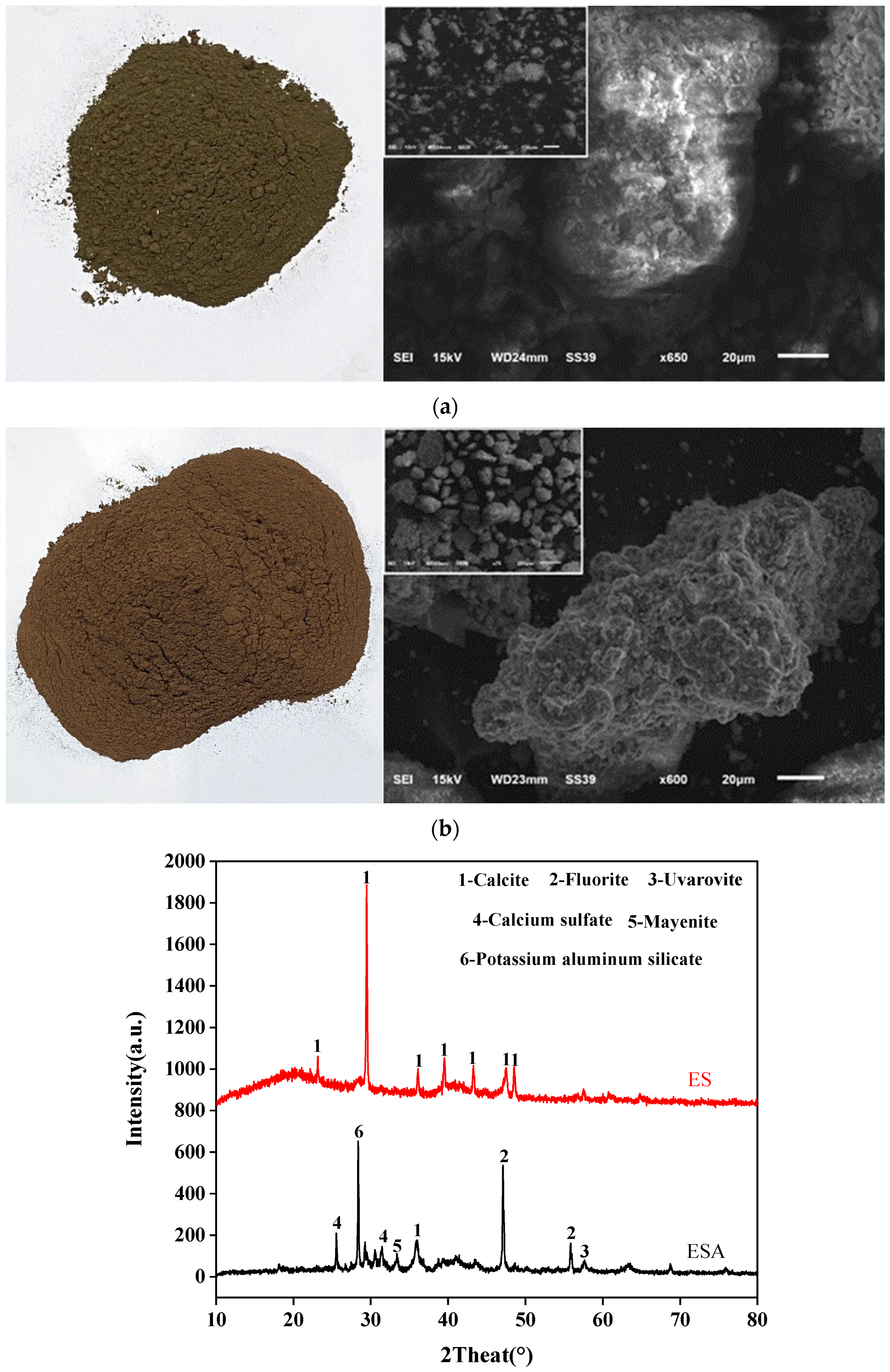
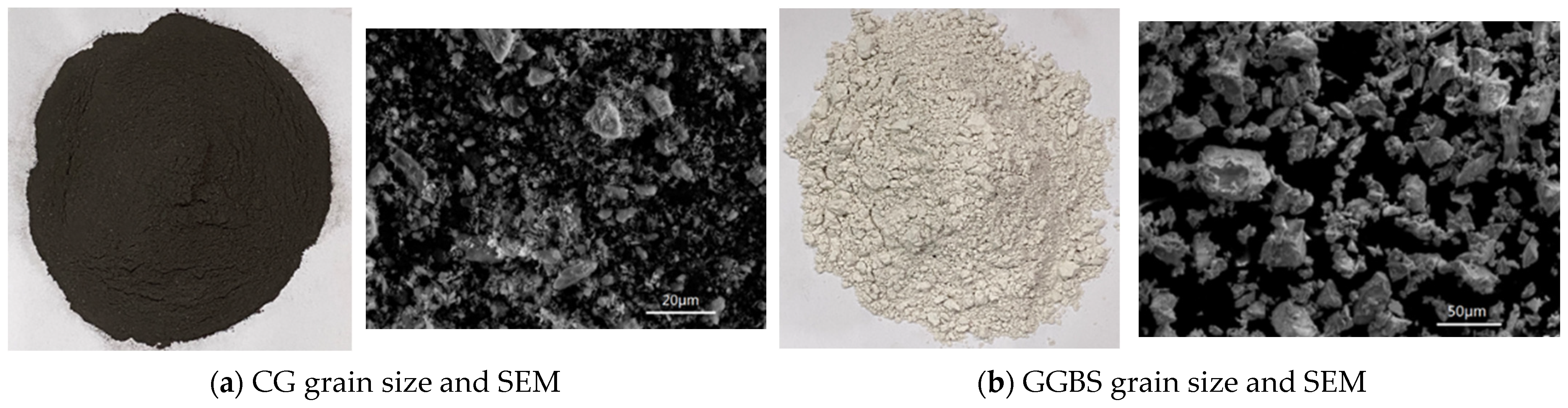








| Raw Material | Al2O3 | SiO2 | CaO | Fe2O3 | MgO | SO3 | Na2O |
|---|---|---|---|---|---|---|---|
| CG | 56.20 | 32.34 | 3.62 | 1.78 | 0.56 | 1.1 | 0.34 |
| GGBS | 30.28 | 13.97 | 41.26 | 0.44 | 9.80 | 2.79 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Du, Y.; Li, S. Sustainable Utilization of Dewatering Sludge for the Development of Reinforcement Grouting Materials in Downhole Applications. Water 2025, 17, 192. https://doi.org/10.3390/w17020192
Zhu X, Du Y, Li S. Sustainable Utilization of Dewatering Sludge for the Development of Reinforcement Grouting Materials in Downhole Applications. Water. 2025; 17(2):192. https://doi.org/10.3390/w17020192
Chicago/Turabian StyleZhu, Xianxiang, Yanhui Du, and Song Li. 2025. "Sustainable Utilization of Dewatering Sludge for the Development of Reinforcement Grouting Materials in Downhole Applications" Water 17, no. 2: 192. https://doi.org/10.3390/w17020192
APA StyleZhu, X., Du, Y., & Li, S. (2025). Sustainable Utilization of Dewatering Sludge for the Development of Reinforcement Grouting Materials in Downhole Applications. Water, 17(2), 192. https://doi.org/10.3390/w17020192







