Degradation of X-Ray Contrast Media in Anaerobic Membrane Bioreactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Standards
2.2. Membrane Bioreactors Setup
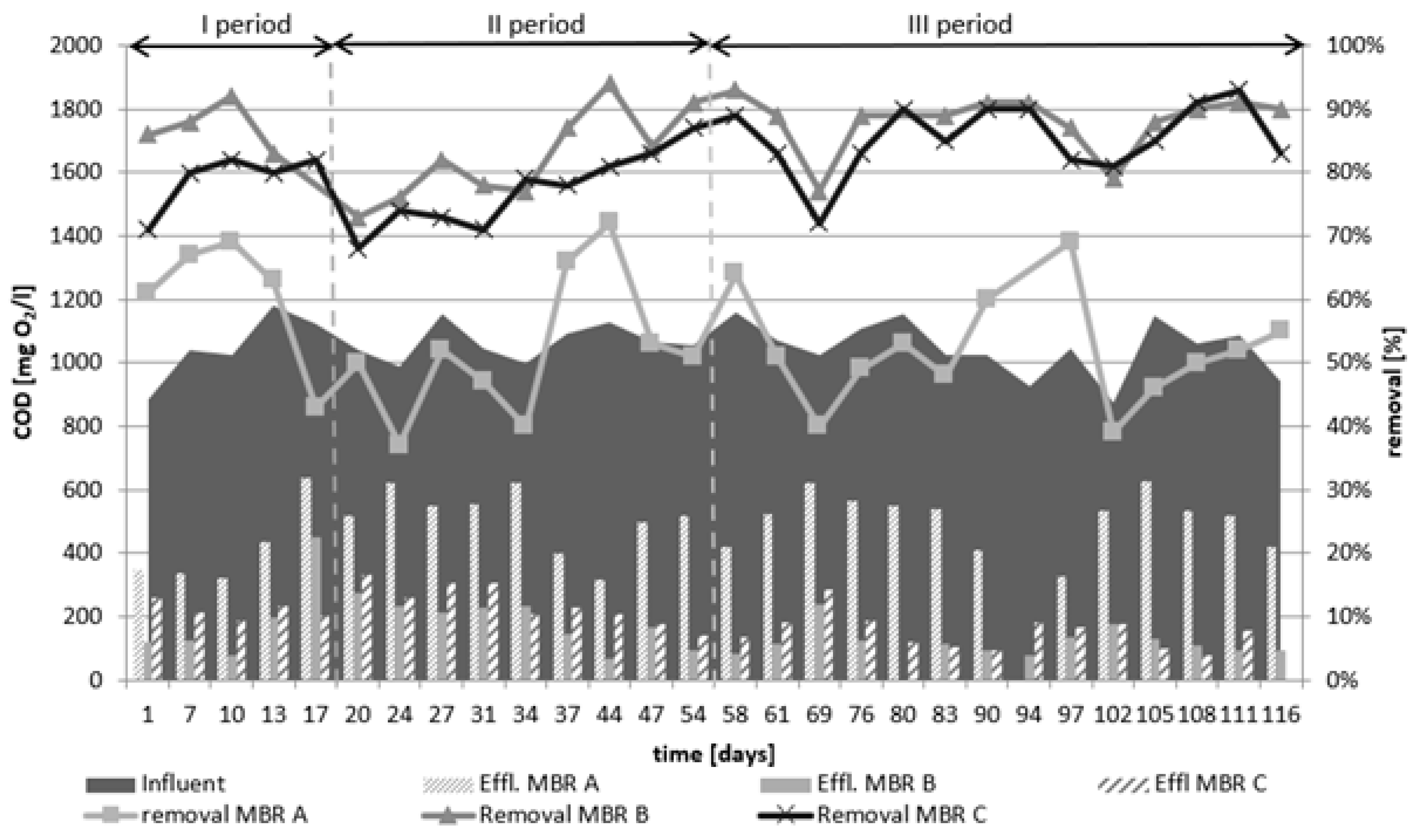
- The first period was from 1 to 17 days, which corresponded to the last 17 days of the adaptation period with regulation of the sludge age;
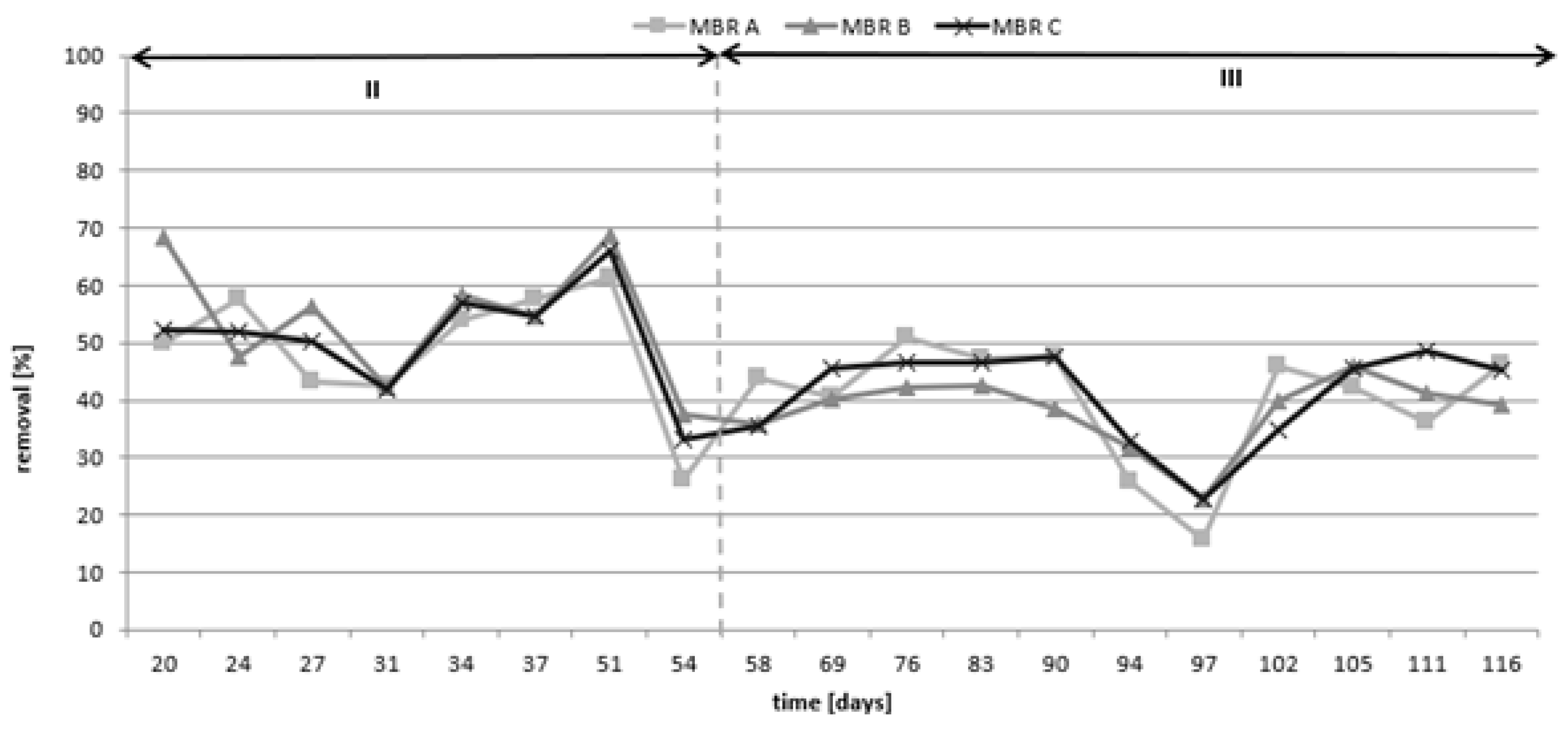
- The second period was from 30 to 54 days, and the synthetic wastewater included ICM and regulation of the sludge age;
- The third period was from 58 to 116 days maintaining a constant sludge age.
2.3. Conventional Measurements
2.4. Detection of ICM by HPLC
- 1–3.5 min: 7% methanol, 93% phosphate buffer;
- 3.5–10 min: 20% methanol, 80% phosphate buffer;
- 10.5–14 min: 7% methanol, 93% phosphate buffer.
3. Results
3.1. COD Elimination
3.2. ICM Elimination
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sengar, A.; Vijayanandan, A. Comprehensive review on iodinated X-ray contrast media: Complete fate, occurrence, and formation of disinfection byproducts. Sci. Total Environ. 2021, 769, 144846. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.D.; D’Aco, V.J.; Shanahan, P.; Chapra, S.C.; Buzby, M.E.; Cunningham, V.L.; Duplessie, B.M.; Hayes, E.P.; Mastrocco, F.J.; Parke, N.J.; et al. Screening analysis of human pharmaceutical compounds in U.S. surface waters. Environ. Sci. Technol. 2004, 38, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107 (Suppl. S6), 907–938. [Google Scholar] [CrossRef]
- Giger, W.; Alder, A.C.; Golet, E.M.; Kohler, H.P.E.; McArdell, C.S.; Molnar, E.; Siegrist, H.; Suter, M.J.F. Occurrence and fate of antibiotics as trace contaminants in wastewaters, sewage sludges, and surface waters. Chimia 2003, 57, 485–491. [Google Scholar] [CrossRef]
- Joss, A.; Żabczyński, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H. Biological degradation of pharmaceuticals in municipal wastewater treatment: Proposing a classification scheme. Water Res. 2006, 40, 1686–1696. [Google Scholar] [CrossRef]
- McArdell, C.S.; Molnar, E.; Suter, M.J.F.; Giger, W. Occurrence and fate of macrolide antibiotics in wastewater treatment plants and in the Glatt Valley Watershed, Switzerland. Environ. Sci. Technol. 2003, 37, 5479–5486. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Miao, X.-S.; Koenig, B.G.; Struger, J. Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes, Canada. Environ. Toxicol. Chem. 2003, 22, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Barceló, D. Fate and occurrence of X-ray contrast media in the environment. Anal. Bioanal. Chem. 2007, 387, 1235–1246. [Google Scholar] [CrossRef]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S.R. Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ. Sci. Technol. 2003, 37, 1061–1608. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.; Ternes, T.A.; Lindart, A.; Haberer, K.; Wilken, R.-D. A sensitive method for the determination of iodine-containing diagnostic agents in aqueous matrices using LC electrospray tandem-MS detection. Fresenius J. Anal. Chem. 2000, 366, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources, and fate of pharmaceuticals and personal care products in groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental endocrine-disrupting chemical exposure: Role in non-communicable diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Barros, R. Pharmaceuticals in water: Risks to aquatic life and remediation strategies. Hydrobiology 2023, 2, 395–409. [Google Scholar] [CrossRef]
- Węgrzyn, A.; Żabczyński, S. Monitoring of bacteria biodiverisity in anaerobic membrane bioreactors (AnMBRs) dealing with wastewater containing X-ray contrast media compounds. Environ. Prot. Eng. 2014, 1, 151–164. [Google Scholar]
- Yan, H.; Zhang, T.; Yang, Y.; Li, J.; Liu, Y.; Qu, D.; Feng, L.; Zhang, L. Occurrence of iodinated contrast media (ICM) in water environments and their control strategies with a particular focus on iodinated by-products formation: A comprehensive review. J. Environ. Manag. 2024, 351, 119931. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Hirsch, R. Occurrence and behavior of X-ray contrast media in sewage facilities and the aquatic environment. Environ. Sci. Technol. 2000, 34, 2741–2748. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Kovalova, L.; Siegrist, H.; Singer, H.; Wittmer, A.; McArdell, C.S. Hospital wastewater treatment by membrane bioreactor: Performance and efficiency for organic micropollutant elimination. Environ. Sci. Technol. 2012, 46, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Bach, L.T.; Van Dinh, P.; Prudente, M.; Aguja, S.; Phay, N.; Nakata, H. Ubiquitous detection of artificial sweeteners and iodinated X-ray contrast media in aquatic environmental and wastewater treatment plant samples from Vietnam, the Philippines, and Myanmar. Arch. Environ. Contam. Toxicol. 2016, 70, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Kormos, J.L.; Schulz, M.; Kohler, H.-P.E.; Ternes, T.A. Biotransformation of selected iodinated X-ray contrast media and characterization of microbial transformation pathways. Environ. Sci. Technol. 2010, 44, 4498–4507. [Google Scholar] [CrossRef] [PubMed]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; Garcia-Jares, C.; Rodriguez, I.; Gomez, M.; Ternes, T.A. Behavior of pharmaceuticals, cosmetics, and hormones in sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, X.; Hu, X.; Yin, D. Distribution and relevance of iodinated X-ray contrast media and iodinated trihalomethanes in an aquatic environment. Chemosphere 2017, 184, 253–260. [Google Scholar] [CrossRef]
- Putschew, A.; Schittko, S.; Jekel, M. Quantification of triiodinated benzene derivatives and X-ray contrast media in water samples by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 2001, 930, 127–134. [Google Scholar] [CrossRef]
- Seitz, W.; Weber, W.H.; Jiang, J.-Q.; Lloyd, B.J.; Maier, M.; Maier, D.; Schulz, W. Monitoring of iodinated X-ray contrast media in surface water. Chemosphere 2006, 64, 1318–1324. [Google Scholar] [CrossRef]
- Singh, R.R.; Rajnarayanan, R.; Aga, D.S. Binding of iodinated contrast media (ICM) and their transformation products with hormone receptors: Are ICM the new EDCs? Sci. Total Environ. 2019, 692, 32–36. [Google Scholar] [CrossRef]
- Kim, S.J.; Grossberg, J.A.; Nogueira, R.G.; Haussen, D.C. Hyperacute unilateral contrast-induced parotiditis during cerebral angiography. Radiol. Case Rep. 2018, 13, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, X.; Zheng, X.; Zhi, H.; Tang, G.; Ke, Y.; Liu, B.; Ma, H. Comparing the toxicity of iodinated X-ray contrast media on eukaryote- and prokaryote-based quantified microarray assays. Ecotoxicol. Environ. Saf. 2022, 240, 113678. [Google Scholar] [CrossRef] [PubMed]
- Arnnok, P.; Singh, R.R.; Burakham, R.; Pérez-Fuentetaja, A.; Aga, D.S. Selective uptake and bioaccumulation of antidepressants in fish from effluent-impacted Niagara River. Environ. Sci. Technol. 2017, 51, 10652–10662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Liu, H.; Yang, X.; Watson, P.; Yang, F. Disinfection byproducts of iopamidol, iohexol, diatrizoate, and their distinct acute toxicity on Scenedesmus sp., Daphnia magna, and Danio rerio. Chemosphere 2023, 333, 138885. [Google Scholar] [CrossRef] [PubMed]
- MacKeown, H.; von Gunten, U.; Criquet, J. Iodide sources in the aquatic environment and its fate during oxidative water treatment—A critical review. Water Res. 2022, 217, 118417. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Z.; Dong, H.; Goodman, B.A.; Qiang, Z. Formation of iodotrihalomethanes, iodo-acetic acids, and iodo-acetamides during chloramination of iodide-containing waters: Factors influencing formation and reaction pathways. J. Hazard. Mater. 2017, 321, 28–36. [Google Scholar] [CrossRef]
- Yuan, S.; Jiang, X.; Xia, X.; Zhang, H.; Zheng, S. Detection, occurrence and fate of 22 psychiatric pharmaceuticals in psychiatric hospital and municipal wastewater treatment plants in Beijing, China. Chemosphere 2013, 90, 2520–2525. [Google Scholar] [CrossRef]
- Al Qarni, H.; Collier, P.; O’Keeffe, J.; Akunna, J. Investigating the removal of some pharmaceutical compounds in hospital wastewater treatment plants operating in Saudi Arabia. Environ. Sci. Pollut. Res. 2016, 23, 13003–13014. [Google Scholar] [CrossRef]
- Clara, M.; Kreuzinger, N.; Strenn, B.; Gans, O.; Martinez, E.; Kroiss, H. The solids retention time—A suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J. Hydrol. 2010, 389, 416–428. [Google Scholar] [CrossRef]
- Bhandari, G.; Chaudhary, P.; Gangola, S.; Gupta, S.; Gupta, A.; Rafatullah, M.; Chen, S. A review on hospital wastewater treatment technologies: Current management practices and future prospects. J. Water Process Eng. 2023, 56, 104516. [Google Scholar] [CrossRef]
- Oppenheimer, J.; Stephenson, R.; Burbano, A.; Liu, L. Characterizing the passage of personal care products through wastewater treatment processes. Water Environ. Res. 2007, 79, 2564–2577. [Google Scholar] [CrossRef]
- Ajala, O.J.; Tijani, J.O.; Salau, R.B.; Abdulkareem, A.S.; Aremu, O.S. A review of emerging micro-pollutants in hospital wastewater: Environmental fate and remediation options. Results Eng. 2022, 16, 100671. [Google Scholar] [CrossRef]
- Drug Bank. Available online: https://go.drugbank.com/ (accessed on 8 August 2024).
- Sengar, A.; Vijayanandan, A. Fate and removal of iodinated X-ray contrast media in membrane bioreactors: Microbial dynamics and effects of different operational parameters. Sci. Total Environ. 2023, 869, 161827. [Google Scholar] [CrossRef]
- Żabczyński, S.; Buczyński, R.; Ziembińska, A. X-ray contrast media removal in membrane bioreactors. In Proceedings of the Low Carbon Earth Summit 2011: BIT’s 1st World Congress of Environmental Biotechnology, Dalian, China, 19–26 October 2011. [Google Scholar]
- James, S.N.; Sengar, A.; Vijayanandan, A. Investigating the biodegradability of iodinated X-ray contrast media in simultaneous nitrification and denitrification system. J. Hazard. Mater. 2023, 452, 131196. [Google Scholar] [CrossRef]
- Jeong, J.; Jung, J.; Cooper, W.J.; Song, W. Degradation mechanisms and kinetic studies for the treatment of X-ray contrast media compounds by advanced oxidation/reduction processes. Water Res. 2010, 44, 4391–4398. [Google Scholar] [CrossRef]
- Hapeshi, R.; Lambrianides, A.; Koutsoftas, P.; Kastanos, E.; Michael, C.; Fatta-Kassinos, D. Investigating the fate of iodinated X-ray contrast media iohexol and diatrizoate during microbial degradation in an MBBR system treating urban wastewater. Environ. Sci. Pollut. Res. 2013, 20, 3592–3606. [Google Scholar] [CrossRef]
- Haiß, A.; Kümmerer, K. Biodegradability of the X-ray contrast compound diatrizoic acid, identification of aerobic degradation products and effects against sewage sludge micro-organisms. Chemosphere 2006, 62, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Stüber, J.; Herrmann, N.; McDowell, D.; Ried, A.; Kampmann, M.; Teiser, B. Ozonation: A tool for removal of pharmaceuticals, contrast media, and musk fragrances from wastewater? Water Res. 2003, 37, 1976–1982. [Google Scholar] [CrossRef]
- Borowska, E.; Felis, E.; Żabczyński, S. Degradation of iodinated contrast media in aquatic environment by means of UV, UV/TiO2 process, and by activated sludge. Water Air Soil Pollut. 2015, 226, 151. [Google Scholar] [CrossRef] [PubMed]




| Compound and Properties | Chemical Structure |
| Iohexol (C19H26I3N3O9) MW: 821.14 CAS:-No: 66108-95-0, non ionic Osmolality: 322–844 mOsm/kg H2O | 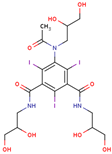 |
| Diatrizoate (C11H9I3N2O4) MW: 613.9136 CAS:-No: 117-96-4, ionic Osmolality: 1500–2000 mOsm/kg H2O | 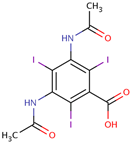 |
| Iodipamide (C20H14I6N2O6) MW: 1139.7618 CAS:-No: 606-17-7, ionic Osmolality: 664 mOsm/kg H2O |  |
| Parameter | Unit | MBR A | MBR B | MBR C |
|---|---|---|---|---|
| Reactor volume | L | 45 | 45 | 45 |
| Hydraulic retention time (HRT) | D | 2.8 | 2.8 | 2.8 |
| Sludge age | D | 40 | 70 | 100 |
| Temperature | °C | 19.5–26.1 | 19.6–26.0 | 19.7–25.9 |
| pH | 8.0–9.0 | 8.0–9.0 | 8.0–9.0 | |
| Dissolved oxygen | mgO2/L | <1.00 | <1.00 | <0.76 |
| Parameter | Unit | MBR A | MBR B | MBR C | MBR A | MBR B | MBR C | MBR A | MBR B | MBR C |
|---|---|---|---|---|---|---|---|---|---|---|
| First Period | Second Period | Third Period | ||||||||
| Sludge concentration | g/L | 3.2–5.2 | 4.2–5.4 | 4.6–5.6 | 2.3–3.3 | 3.5–4.3 | 4.1–5.3 | 1.9–2.7 | 3.1–4.3 | 4.2–5.0 |
| Sludge organic loading rate | gCOD/gMLSS d | 0.04–0.08 | 0.04–0.07 | 0.04–0.07 | 0.12–0.16 | 0.08–0.10 | 0.06–0.09 | 0.14–0.21 | 0.08–0.15 | 0.08–0.10 |
| Sludge NH4+-N loading rate | gNH4+-N/gMLSS d | 0.003–0.004 | 0.003–0.005 | 0.003–0.005 | 0.006–0.009 | 0.004–0.006 | 0.003–0.005 | 0.007–0.011 | 0.004–0.008 | 0.004–0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopka, J.; Kalka, J.; Żabczyński, S. Degradation of X-Ray Contrast Media in Anaerobic Membrane Bioreactors. Water 2025, 17, 188. https://doi.org/10.3390/w17020188
Konopka J, Kalka J, Żabczyński S. Degradation of X-Ray Contrast Media in Anaerobic Membrane Bioreactors. Water. 2025; 17(2):188. https://doi.org/10.3390/w17020188
Chicago/Turabian StyleKonopka, Jakub, Joanna Kalka, and Sebastian Żabczyński. 2025. "Degradation of X-Ray Contrast Media in Anaerobic Membrane Bioreactors" Water 17, no. 2: 188. https://doi.org/10.3390/w17020188
APA StyleKonopka, J., Kalka, J., & Żabczyński, S. (2025). Degradation of X-Ray Contrast Media in Anaerobic Membrane Bioreactors. Water, 17(2), 188. https://doi.org/10.3390/w17020188





