Abstract
The escalating issue of water pollution driven by rapid industrialization necessitates the development of advanced remediation technologies. In this study, a novel method for producing chromium (Cr(VI)) ion-imprinted biochar (Cr(VI)-IIP-PEI@NBC) from wheat residue was proposed. After acid-oxidative modifications, polyethyleneimine (PEI) and glutaraldehyde (GA) were employed as the functional monomer and crosslinker, respectively, to enhance the biochar’s selectivity and adsorption capacity. Under optimized conditions (pH 2.0, 55 °C), the adsorbent achieved a maximum Cr(VI) uptake of 212.63 mg/g, which was 2.3 times higher than that of the non-imprinted biochar. The material exhibited exceptional specificity (99.64%) for Cr(VI) and maintained >80% adsorption efficiency after five regeneration cycles, demonstrating excellent reusability. Comprehensive structural characterization via Fourier transform infrared spectroscopy (FT-IR), thermal gravimetric analysis (TGA), Brunner–Emmet–Teller measurements (BET), and Scanning Electron Microscopy (SEM) confirmed successful Cr(VI) imprinting in the biochar and its high thermal stability and mesoporous architecture, elucidating the mechanisms behind its superior performance. This study presents a sustainable and high-performance adsorbent for the efficient treatment of chromium-contaminated wastewater, with significant potential for industrial applications.
1. Introduction
The increase in industrialization has resulted in significant damage to aquatic ecosystems because of the release of insufficiently treated wastewater [1]. Of the different types of environmental contamination, water resource pollution is one of the most critical and pressing issues. Chromium (Cr), classified as a heavy metal pollutant, causes permanent harm to the environment and humans [2]. Chromium found in nature primarily exists in two oxidation states: Cr(III) and Cr(VI). It is important to note that Cr(VI) is much more soluble and mobile in water, where it mainly appears in ionic forms. As a result, Cr(VI) has a higher level of toxicity and potential for environmental harm [3]. It has been noted that mice given drinking water with Cr(VI) showed a decrease in body weight compared to a control group [4]. Additionally, the brain, liver, and kidneys are the main organs affected by Cr(VI)’s toxic effects. Cr(VI) buildup in mice leads to significant damage to the nervous system and brain [5]. The neurotoxic effects linked to Cr(VI) exposure have also been confirmed in zebrafish studies [6]. Treatment methods for chromium-containing wastewater include reduction–precipitation, membrane separation, electrodialysis, and adsorption. Of these, adsorption has gained widespread recognition due to its high efficiency, straightforward operation, and low cost. This approach primarily utilizes high-performing solid adsorbents to remove chromium. Following separation from the water, these materials can often be regenerated, significantly reducing both energy consumption and operational expenses.
Among solid adsorbents, biochar stands out for its remarkable ability to adsorb environmental pollutants, thanks to its naturally porous structure [7]. In particular, biochar made from straw offers a straightforward, affordable, and effective method for removing Cr(VI) from water systems [8,9]. In addition to its main use, biochar made from straw offers a variety of important multifunctional advantages; for example, when biochar is used in agricultural fields, it captures greenhouse gases like CH4, N2O, and CO2 in the soil structure [10]. Research by Spokas et al. [11] also showed that biochar remains stable in soil over the long term, indicating its ability to contribute to ongoing reductions in carbon emissions. When applied to agricultural land, biochar greatly improves the soil quality by boosting fertility, balancing the soil pH, and creating a strong base for healthy crop development [12,13]. Transforming straw into biochar is an eco-friendly, energy-efficient, and sustainable approach; therefore, using modified biochar to absorb various pollutants is a key area of current research.
At present, the main technique for producing biochar from straw is pyrolysis, which involves heating the straw in an environment with no or limited oxygen. When certain high temperatures are achieved, the straw biomass breaks down and reorganizes, producing a carbon-dense solid byproduct known as biochar [14]. Pyrolysis produces both bio-oil and biogas simultaneously, and the ratios of these two products are greatly affected by the composition of the feedstock and the specific parameters of the process [15,16]. Therefore, it is essential to establish the optimal pyrolysis conditions for various kinds of straw biomass in order to enhance biochar production while ensuring its quality [17,18]. Nevertheless, biochar in its original form typically needs to be modified to improve its adsorptive ability and to adapt it for various needs in different water systems [19]. Rao et al. [20] created iron-loaded activated biochar, which showed a Cr(VI) adsorption capacity of 25.68 mg/g and achieved a removal efficiency of 56.00%. Additionally, Anushka et al. [8] utilized a method integrating Cr(VI) reduction, to diminish chromium toxicity, along with adsorption using modified biochar. This approach resulted in a Cr(VI) adsorption capacity of 50.02 mg/g and an overall chromium removal efficiency of 90.00%. Although these techniques provide a basis for using biochar to remediate Cr(VI)-contaminated wastewater, their limited adsorption capacities and lack of selectivity pose challenges for real-world use.
At present, ion-imprinted materials are highly valuable in water treatment. These materials show a strong ability to selectively adsorb particular ions from water. Studies have investigated the creation of imprinted materials utilizing biochar as a base, revealing that they perform much better than their non-imprinted counterparts [21]. Zhang et al. [22] effectively created an imprinted biochar material through surface grafting for the purpose of heavy metal ion adsorption. This material showed specificity for Cd(II) adsorption and demonstrated good reusability, significantly outperforming non-imprinted materials. These results highlight the distinct benefits of materials imprinted with biochar; however, there are obstacles to the creation of Cr(VI)-specific ion-imprinted biochar materials, such as a lack of diverse preparation methods and inadequate material stability. These restrictions greatly hinder the development of highly selective biomass-derived chromium adsorbents.
To tackle these challenges, in this research, we utilize wheat straw as a raw material to examine how different pyrolysis conditions influence the biochar yield and carbon content. Based on our findings, a Cr(VI)-imprinted biochar material called Cr(VI)-IIP-PEI@NBC was created through a series of acid-oxidative modifications, followed by crosslinking with PEI and GA. To our best known, this study presents a facile route to synthesize Cr(VI)-imprinted biochar material as novel adsorbents was provided, as well as a new approach for using biochar to address chromium pollution.
2. Method and Materials
2.1. Materials
Wheat residues (Xuzhou, China). Methanol (Yasheng Chemical Co., Ltd., Wuxi, China). Ethanol (Yasheng Chemical Co., Ltd., Wuxi, China). Decalin (Aladdin Biochemical Technology Co., Ltd., Shanghai, China). Anhydrous sodium sulfite (Aladdin Biochemical Technology Co., Ltd., Shanghai, China) Hydrochloric acid (Aladdin Biochemical Technology Co., Ltd., Shanghai, China). Nitric acid (China National Pharmaceutical Group Chemical Reagent Co., Ltd., Shanghai, China). Hydrogen peroxide (China National Pharmaceutical Group Chemical Reagent Co., Ltd., Shanghai, China). Polyethyleneimine (PEI) (China National Pharmaceutical Group Chemical Reagent Co., Ltd., Shanghai, China). Sulfuric acid (Lingfeng Chemical Reagent Co., Ltd., Shanghai, China). Glutaraldehyde (GA) (Lingfeng Chemical Reagent Co., Ltd., Shanghai, China). Acetic acid (Yuanye Biotechnology Co., Ltd., Shanghai, China). Potassium dichromate (Yuanye Biotechnology Co., Ltd., Shanghai, China).
2.2. Preparation of Straw Biochar
Raw straw was chopped into pieces of consistent length and width, washed thoroughly with deionized water, and then dried in a vacuum oven until it reached a stable weight. The dried pieces were subsequently sealed in an airtight container and kept at room temperature until they were needed.
Equal amounts of straw fragments were placed into quartz boats; the reaction rates were adjusted to between 2 and 10 °C/min; the temperature was increased to various levels (400–600 °C) then maintained for different durations (0.5–2.5 h); and the atmosphere was continuously changed (nitrogen, argon, air) throughout the reaction. Once the reaction ended, the biochar was extracted, ground into a powder, and weighed to determine the yield. The below formula was employed to determine the carbon yield of biochar made from wheat straw:
where W represents the carbon yield of wheat straw biochar; mc denotes the yield of wheat straw biochar in grams; C indicates the carbon content of wheat straw biochar as a percentage; and M refers to the mass of wheat straw in grams.
2.3. Preparation of Chromium Ion-Imprinted Materials
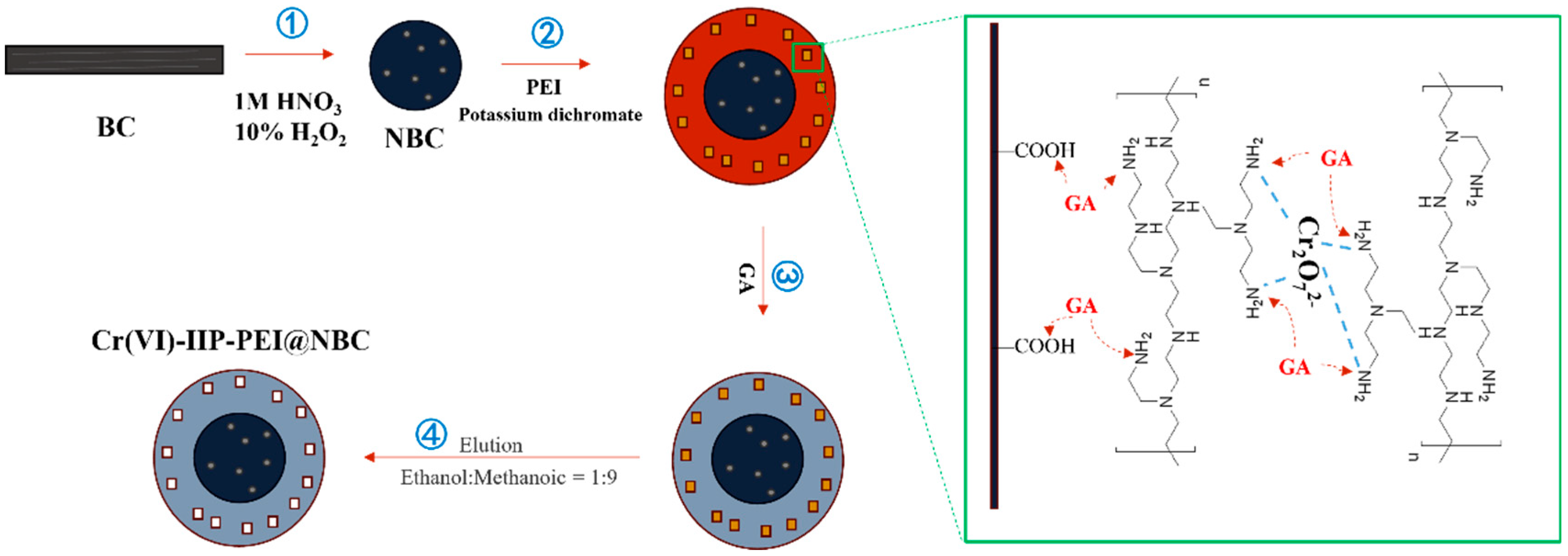
To a mass of 0.5 g of biochar (BC), 5 mL of H2O2 and a 5.00% nitric acid solution were added and mixed in. The mixture was stirred well for 2 h to activate it, and then rinsed with deionized water to produce nitric acid-modified biochar (NBC). To determine the optimal PEI content, a constant quantity of 0.5 g of NBC was combined with varying amounts of PEI (ranging from 0.5 to 2.5 g) and 3 mL of anhydrous ethanol. Next, 3 mL of a 100 mg/L K2Cr2O7 solution was incorporated, mixed thoroughly, and then polymerized in a temperature-controlled water bath at a designated temperature (between 30 and 70 °C) for a specified duration (from 1 to 5 h). Finally, a specific quantity (1 to 5 mL) of GA was introduced for the purpose of crosslinking. The final product was rinsed with an eluent mixture of methanol and acetic acid at a 9:1 ratio for 24 h, replacing the eluent three times. This was then washed with deionized water and dried in a vacuum oven until a constant weight was achieved, resulting in the ion-imprinted polymer Cr(VI)-IIP-PEI@NBC. The process for preparing the non-ionic imprinted material Cr(VI)-NIP-PEI@NBC followed the same steps, except that template ions were not included, see Figure 1.
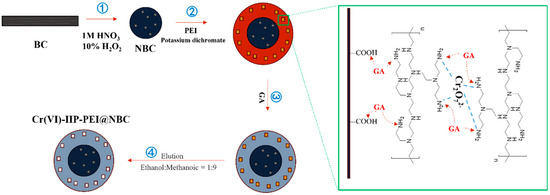
Figure 1.
Schematic diagram of IIP preparation (Cr2O72− as template ion).
The adsorption tests were performed using the prepared ion-imprinted polymer. It was immersed in a potassium dichromate solution with a pH of 6.0 and an initial concentration of 100 mg/L. Then, it was agitated in a temperature-controlled shaker at 25 °C and 200 revolutions per minute for 2 h. Next, ICP-OES was employed to measure the chromium concentration, and the chromium adsorption capacity Qt and removal rate R of the modified biochar for chromium were determined using the formulas provided below:
where Qt refers to the chromium adsorption capacity of modified biochar at a specific time t, measured in mg/g. C0 indicates the initial chromium concentration in mg/L, while Ct denotes the chromium concentration in the solution at time t, also in mg/L. V represents the volume of adsorption in liters, m is the mass of the modified biochar in grams, and R signifies the rate of adsorption or removal.
2.4. Material Characterization and Performance Testing
2.4.1. FT-IR
Cr(VI)-IIP-PEI@NBC was analyzed using FT-IR within a scanning range of 400 to 4000 cm−1.
2.4.2. SEM
Cr(VI)-IIP-PEI@NBC was utilized for vacuum drying, table preparation, and vacuum spraying in the gold treatment process. SEM was employed to examine the surface structure of the materials mentioned above and monitor the alterations in surface morphology throughout the adsorbent preparation process.
2.4.3. TGA
The thermal stability of the Cr(VI)-IIP-PEI@NBC material was assessed during its preparation using TGA in a nitrogen environment, with the highest temperature set at 800 °C.
2.4.4. BET
Characterization was performed using a fully automated physical adsorption device specifically for Cr(VI)-IIP-PEI@NBC. The degassing procedure was conducted at a temperature of 120 °C, using nitrogen as the gas being adsorbed.
2.5. Study on Chromium Adsorption for Cr(VI)-IIP-PEI@NBC
2.5.1. pH Value
A 500 mL solution of Cr(VI) at a concentration of 100 mg/L was created, from which 10 mL portions were dispensed into screw-cap flasks. The pH was modified to 2.0, 4.0, 6.0, 8.0, and 10.0 by using diluted HCl and NaOH solutions. A mass of 0.01 g of Cr(VI)-IIP-PEI@NBC was added and the mixtures were stirred in a constant-temperature shaker set at 25 °C and 200 rpm for a duration of 2 h. The quantities of chromium adsorbed were calculated by measuring the chromium concentrations before and after the adsorption process.
2.5.2. Adsorption Duration and Kinetic Analysis
The absorption system included Cr(VI) at a concentration of 100 mg/L and 0.01 g of Cr(VI)-IIP-PEI@NBC, with the pH adjusted to 6. The mixture was stirred in a temperature-controlled shaker maintained at 25 °C and a rate of 200 rpm for periods of 1, 2, 3, 4, and 5 h, respectively. Finally, 1 mL of the supernatant was taken, filtered, and then diluted to measure the chromium concentration. The kinetics of the adsorption of Cr(VI) onto the Cr(VI)-IIP-PEI@NBC system were examined through the application of pseudo-first-order and pseudo-second-order kinetic models to represent the adsorption process.
The proposed first-order dynamic equation is
The proposed second-order dynamic equation is
where k1 denotes the pseudo-first-order rate constant measured in min−1, while k2 indicates the pseudo-second-order rate constant expressed in g/(mg·min). T refers to the duration of the reaction in minutes, and Qe and Qt signify the amounts of Cr(VI) adsorbed at equilibrium and at time t, respectively, measured in mg/g.
2.5.3. Initial Chromium Concentration and Adsorption Isotherm
Cr(VI) solutions were prepared at concentrations ranging from 100 to 500 mg/L, with the pH maintained at 6.0. Flasks were filled with 10 mL of each solution, to which 0.01 g of Cr(VI)-IIP-PEI@NBC was added, and these were shaken in a shaker set at 25 °C and 200 rpm for two hours. After that, the Cr(VI) concentration was measured by taking 1 mL of the supernatant and diluting as necessary.
The Langmuir adsorption isotherm model is
The Freundlich adsorption isotherm model is
In the above equations, Qe indicates the Cr(VI) adsorption capacity of Cr(VI)-IIP-PEI@NBC at equilibrium, measured in mg/g; KL denotes the Langmuir adsorption equilibrium constant, expressed in L/mg; Ce refers to the chromium concentration in the solution at adsorption equilibrium, measured in mg/L; and Qm signifies the maximum Cr(VI) adsorption capacity of Cr(VI)-IIP-PEI@NBC, also in mg/g.
2.5.4. Adsorption Temperature and Thermodynamics
The absorption system’s content was identical to that described in Section 2.5.3. Initially, the material’s ability to adsorb Cr(VI) was evaluated at temperatures of 15 °C, 25 °C, 35 °C, 45 °C, and 55 °C. Then, the system was oscillated at a speed of 200 r/min for 2 h in a constant-temperature shaker set at 25 °C, 30 °C, or 35 °C. The chromium concentration was measured before and after the adsorption process, and the amount of chromium adsorbed was determined. The changes in free energy (∆G in kJ/mol), enthalpy (∆H in kJ/mol), and entropy (∆S in J/(mol·K)) were determined using the following thermodynamic formulas:
In the above equations, K represents the equilibrium constant, which can be substituted with KL in the Langmuir model. Linear fitting was conducted with lnK and 1/T, allowing for the calculation of the enthalpy change (∆H) and entropy change (∆S) from the slope and intercept. Next, the change in free energy was determined using Equation (9).
2.6. Adsorption Property Selection and Reusability
To create a potassium dichromate solution containing the interfering ions Na+, Mg2+, Ca2+, and K+, NaCl, MgCl2, CaCl2, and KCl were added to a solution of Cr(VI) at a concentration of 100 mg/L. Next, 10 mL from each solution was taken and stored in individual screw-cap bottles, to which 0.01 g of Cr(VI)-IIP-PEI@NBC was added to each bottle. Afterward, the levels of chromium before and after the adsorption process were assessed, and the quantity of chromium that had been adsorbed was determined.
The ability of an adsorbent to regenerate cyclically is an important factor in controlling adsorption costs, as it can be used to evaluate how stable and effective the adsorbent is after multiple uses. In this experiment, 0.01 g of Cr(VI)-IIP-PEI@NBC was added to 10 mL of a potassium dichromate solution with a Cr(VI) concentration of 100 mg/L and a pH of 6.0. The mixture was stirred in a temperature-regulated shaker at 25 °C and 200 rpm for two hours. Afterward, the Cr(VI)-IIP-PEI@NBC was washed, dried, and then placed back into the absorption system. This process was repeated for subsequent cycles.
3. Results and Discussion
3.1. Preparation of Biochar
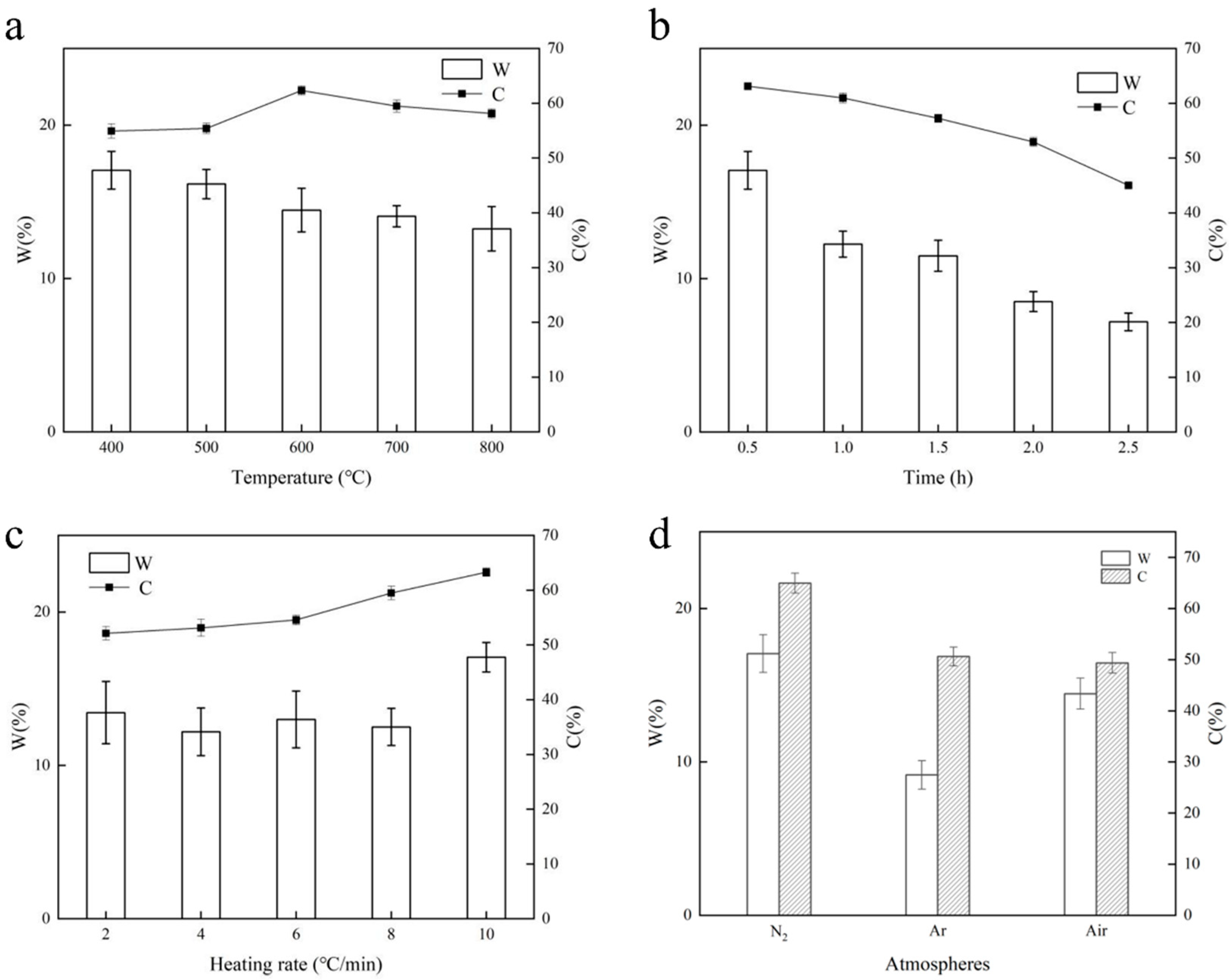
The carbon content serves as a key indicator of biochar’s quality. Along with the carbon content, the carbon yield is also a crucial aspect of biochar production. Figure 2a displays the biochar yields at various temperatures, showing how the biochar yielded from wheat straw decreases steadily with increasing temperature, aligning with the findings of slow thermal cracking observed in other types of straw biochars [23]. This is primarily influenced by the carbon structure and the volatile substances present in biochar. The formation of a biochar structure is influenced by the levels of biomass constituents as well as its pyrolysis properties. Studies indicate that hemicellulose undergoes pyrolysis at temperatures ranging from 180 °C to 300 °C, while cellulose pyrolysis occurs within the range of 280 °C to 380 °C [24]. As a result, the initial carbonization process should be completed prior to reaching 400 °C. Furthermore, research has indicated that there may be a positive relationship between the lignin content and biochar production [25]. As a result, the resulting reduction in yield could be associated with the pyrolysis of lignin and the subsequent carbonization of cellulose. As the temperature rises, the production of volatile matter accelerates, and the reaction time with solid residues decreases, which leads to less buildup of secondary carbon. Additionally, the significant amount of volatile matter in straw typically leads to a reduced carbon yield. The significant release of volatile substances at elevated temperatures could be another key factor contributing to the low yield. Consequently, lowering the pyrolysis temperature appropriately is a useful method for maintaining a good carbon yield. We concluded that the ideal pyrolysis temperature for wheat straw is 600 °C, according to the carbon content curve.
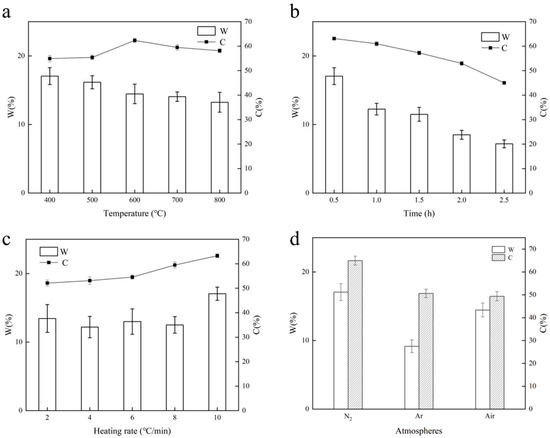
Figure 2.
The influence of preparation conditions on biochar products. (a). Effect of temperature on biochar production. (b). Effect of insulation duration on biochar production. (c). Effect of heating rate on biochar production. (d). Effect of different atmospheres on biochar production.
As shown in Figure 2b, the duration of insulation greatly influences the gradual thermal cracking of biomass: as the duration of insulation increases, both the yield and carbon content of biochar decline considerably. Following 2.5 h of insulation, the carbon yield dropped to 7.20%, which suggests the swift depletion of solid elements. One possible explanation for this is that the primary phase of lignin pyrolysis occurs at 600 °C, which, along with the adequate breakdown of cellulose and hemicellulose, leads to the more pronounced release of volatile substances due to the extended insulation period [25]. As a result, the ideal insulation duration is determined to be 0.5 h.
Figure 2c illustrates how the heating rate influences the yield and carbon content of biochar. The carbon content exhibits a notably positive relationship with the heating rate, a finding that was also observed by Inguanzo et al. [26], who noted that the reactivity of the produced carbonaceous material is influenced by both the pyrolysis temperature and the heating rate of the sample. In the early phase, the pyrolysis temperature plays a more significant role, but as pyrolysis continues, the heating rate increasingly influences reactivity, surpassing the impact of the final pyrolysis temperature. Consequently, 10 °C/min was chosen as the ideal heating rate for producing wheat straw biochar. Our laboratory’s tube furnace has a maximum heating rate of 10 °C/min; hence, our discussion is limited in this regard.
The thermal stability during biomass pyrolysis is primarily influenced by the type of reaction medium employed. Figure 2d illustrates how various atmospheres affect the production of biochar, clearly showing that a nitrogen atmosphere is more advantageous for enhancing the carbon yield and increasing the carbon content compared to argon and air atmospheres. It is worth noting that performing pyrolysis in an argon environment significantly reduces the carbon yield, since, as it is an inert gas, argon does not readily take part in reactions. In contrast, nitrogen and air exhibit more intricate kinetic and thermodynamic behaviors during the biomass pyrolysis process.
3.2. Optimization of Cr(VI)-IIP-PEI@NBC Production Conditions
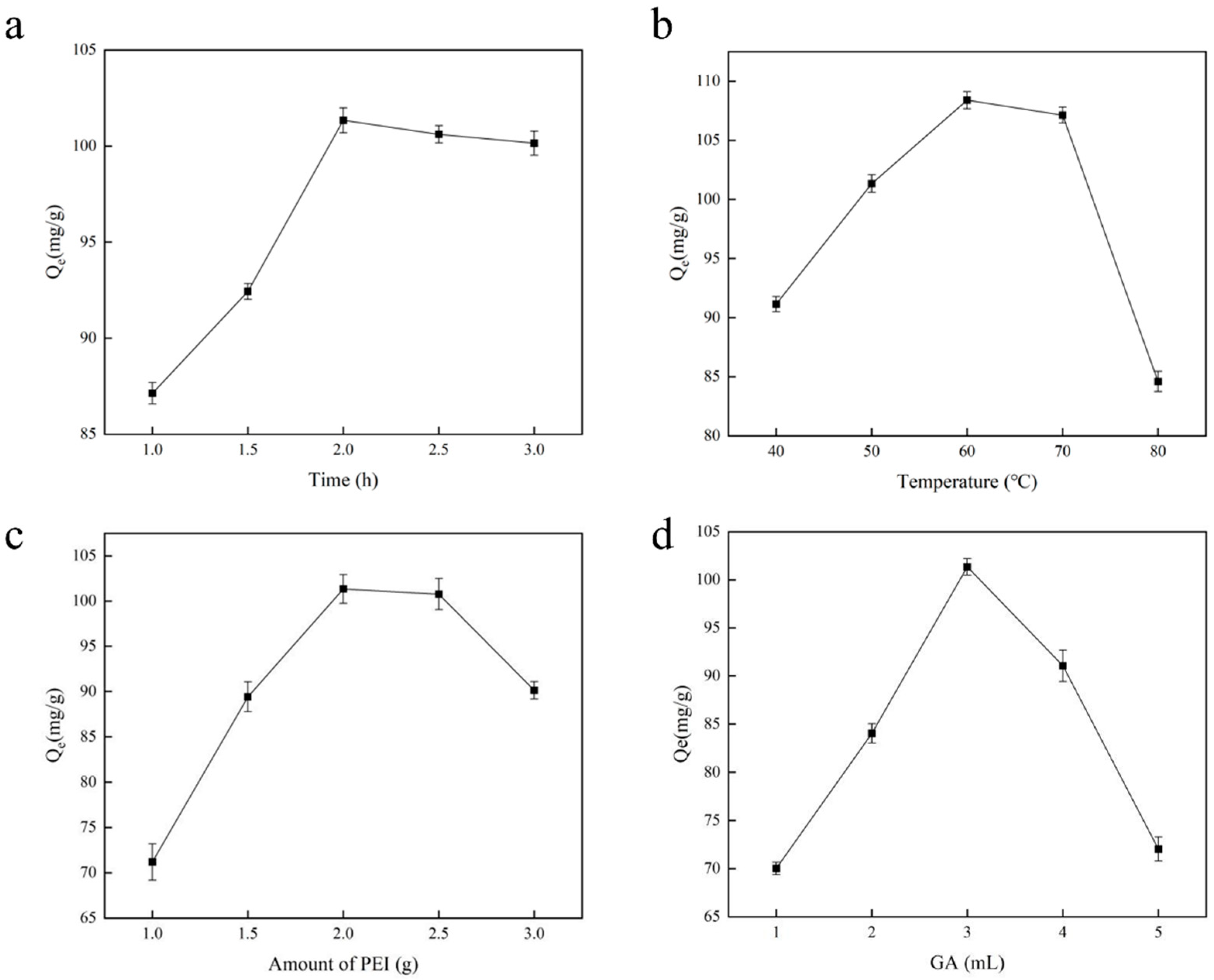
The length of the polymerization reaction influences the polymer’s structural characteristics and the development of imprinting sites during the polymerization process. In this research, we examined the chromium adsorption capability of Cr(VI)-IIP-PEI@NBC by determining the optimal polymerization duration; to achieve this, the reaction duration was set to 1.0, 1.5, 2.0, 2.5, and 3.0 h, with the results shown in Figure 3a. The duration of polymerization impacts the material’s adsorption capabilities by affecting how fully the monomer polymerizes. The graph clearly shows that, once the polymerization duration reaches 2.0 h, the material’s adsorption capacity begins to plateau, suggesting that the cavities in the imprinting material are fully developed. Past this point, the reaction will indeed influence the imprinting sites that have already been established. If the polymerization duration is too short, leading to an incomplete reaction, this results in a low polymer yield and incomplete imprinting sites being formed in the polymer structure. If the polymerization process takes too long, the polymer’s structure will harden, making it easier for the imprinting sites to become embedded, which can negatively impact the adsorption efficiency [27].
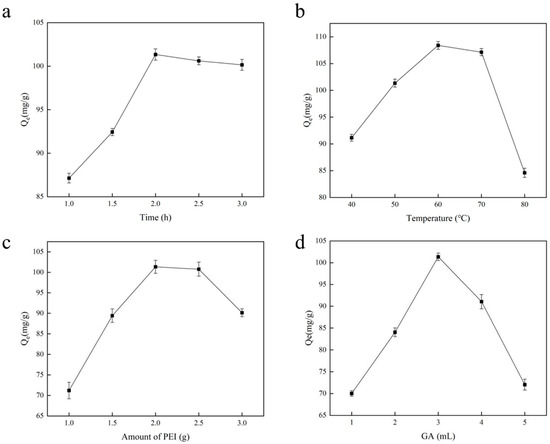
Figure 3.
The influence of Cr(VI)-IIP-PEI@NBC production conditions. (a). Effect of polymerization time on adsorption capacity. (b). Effect of polymerization temperature on adsorption capacity. (c). Effect of PEI content on adsorption capacity. (d). Effect of amount of GA on adsorption capacity.
In heat-induced polymerization reactions, the temperature has a direct impact on both the speed of the polymerization process and the stability and structure of the resulting polymer. Our findings on temperature’s effect on the adsorption capacity are illustrated in Figure 3b, showing that the optimal temperature for polymerization was found to be 60 °C, resulting in an adsorption capacity of 106.33 mg/g. In this system, temperature influences both the rate of polymerization and the adsorption of Cr(VI) onto functional monomers. If the system’s reaction temperature is too low, leading to a low rate of polymerization of functional monomers, this results in the incomplete formation of the imprinting materials. If the system’s temperature is excessively elevated, causing the polymerization of the functional monomers to be overly vigorous, this reduces effective control of the reaction [28]. Therefore, selecting the right temperature for polymerization is essential. Cr(VI) release leads to unstable template cavities, which, on a larger scale, translate to a reduction in adsorption capacity.
The PEI content’s influence on the material’s adsorption capability was evaluated, and it was found that as the amount of PEI increased, the material’s adsorption capability greatly enhanced, peaking at a PEI dosage of 2.0 g (Figure 3c). Nonetheless, when the PEI dosage surpassed 2.0 g, the adsorption capacity started to decline, suggesting that an excessive amount of polymer negatively affects the material’s adsorption performance. The amount of functional monomers used greatly influences the imprinting effect, stability, and smoothness of imprinting material production processes [29]: the inclusion of too many or too few functional monomers can negatively impact the quality of the imprinting materials produced.
Based on the optimal PEI content, we fixed the PEI dosage at 2.0 g and chose various volumes of GA (ranging from 1 to 5 mL) to examine the chromium adsorption capabilities of Cr(VI)-IIP-PEI@NBC; the findings are illustrated in Figure 3d. As the volume of GA added increased, the material’s adsorption performance initially improved before subsequently declining. This phenomenon frequently takes place during the production of imprinting materials. As the GA dosage increases, the material progressively becomes more rigid, significantly impeding ion detection [30,31]. As a result, GA at a dosage of 3 mL was chosen as the crosslinking agent.
3.3. Optimization of Cr(VI)-IIP-PEI@NBC Adsorption Conditions
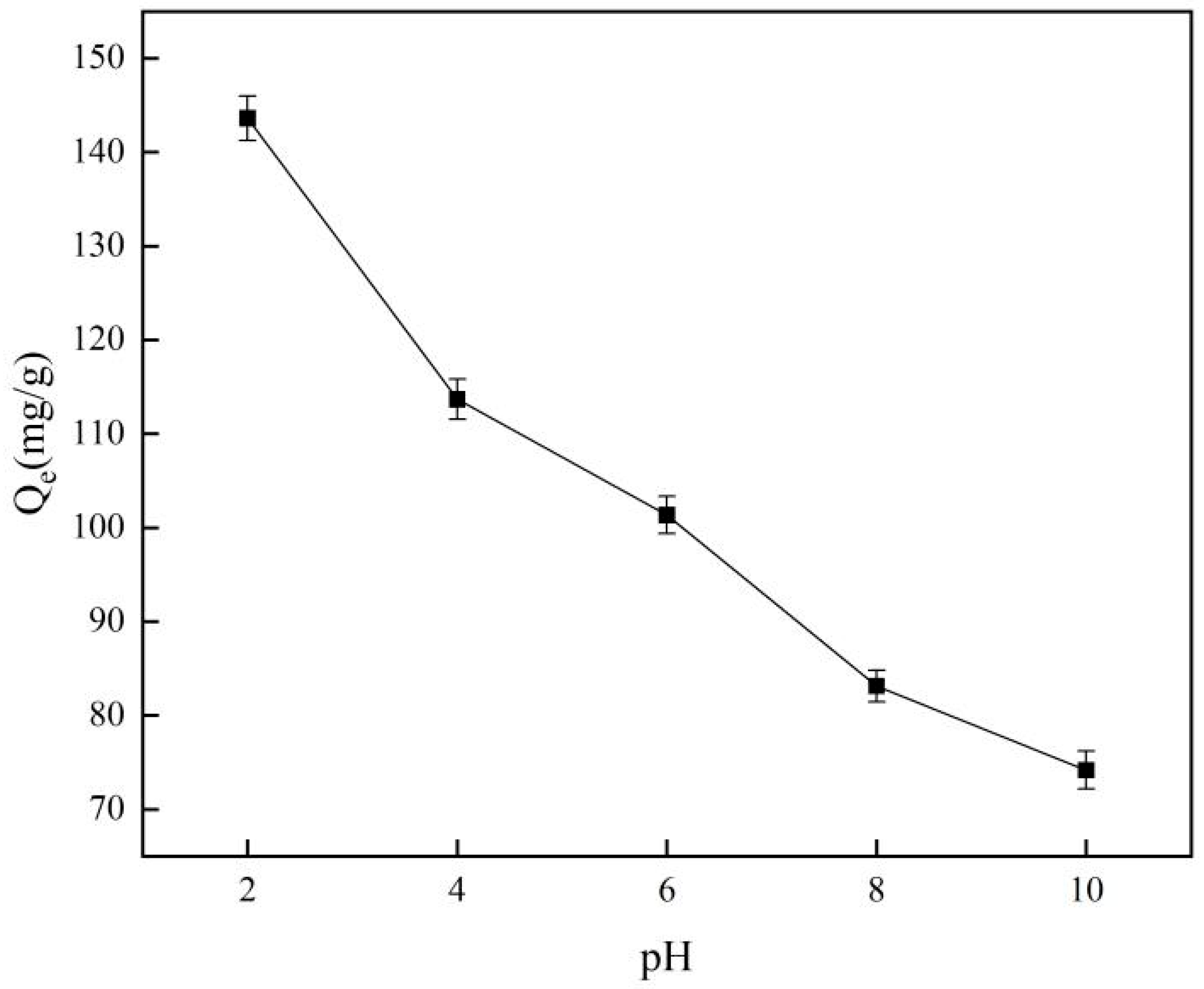
Figure 4 illustrates how pH influences the adsorption capabilities of the material, revealing that the highest adsorption performance occurs at a pH of 2.0. Furthermore, it indicates that alkaline conditions hinder the material’s adsorption of template ions. The characteristics of monomer adsorption indicate that pH levels can influence the state of chromium in a solution, which is critical for ion-imprinted materials [32]. The cavities in ion-imprinted materials exhibit a low affinity for non-template ions. Simultaneously, in alkaline environments, chromium elements will be converted into chromate forms, and this alteration in electrical properties further increases the material’s repulsion to them.
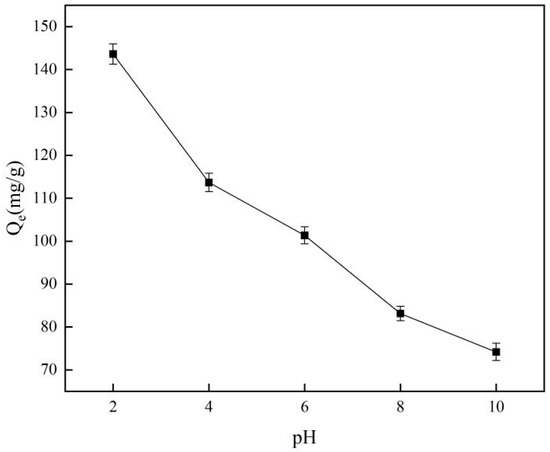
Figure 4.
Effect of pH on adsorption capacity.
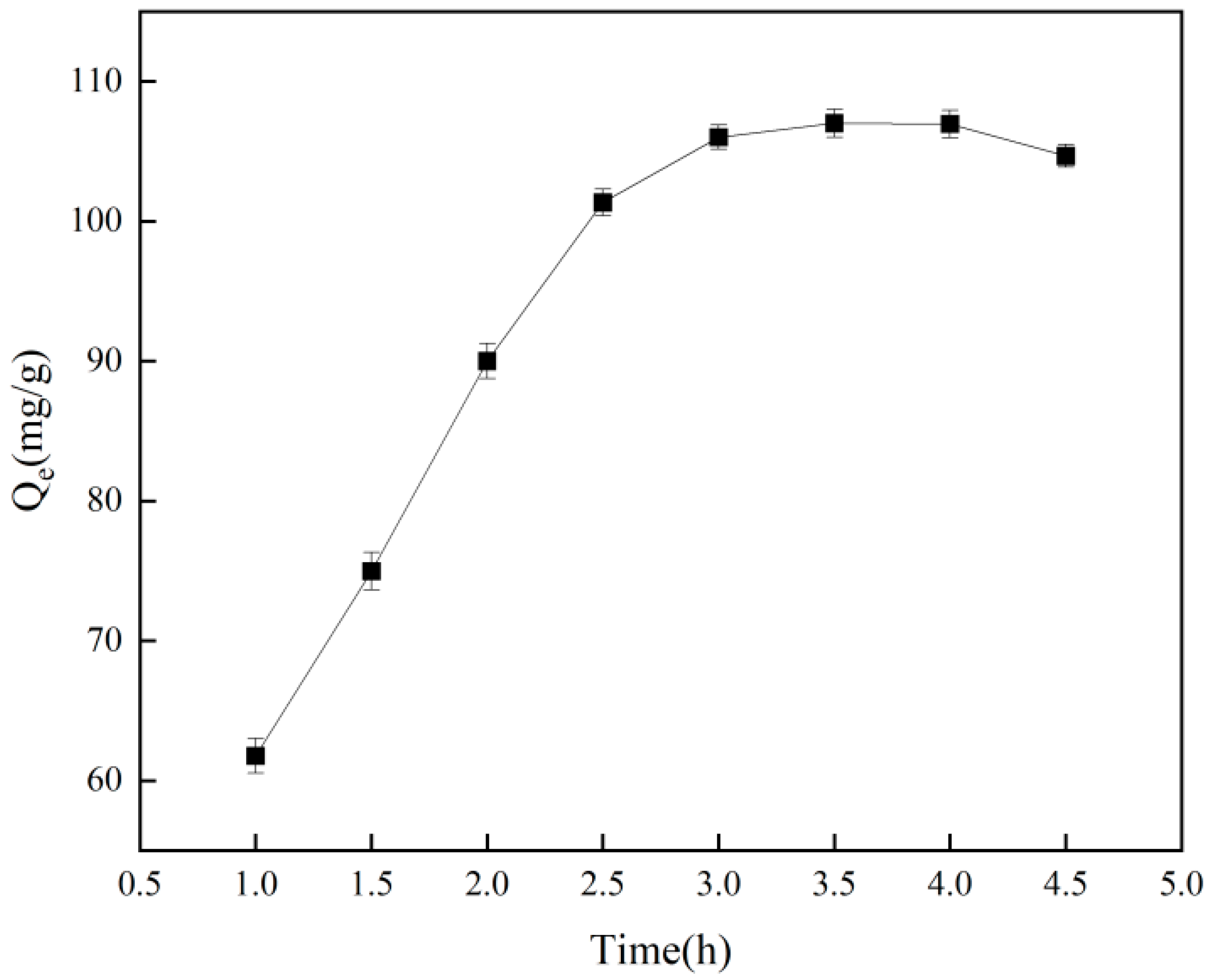
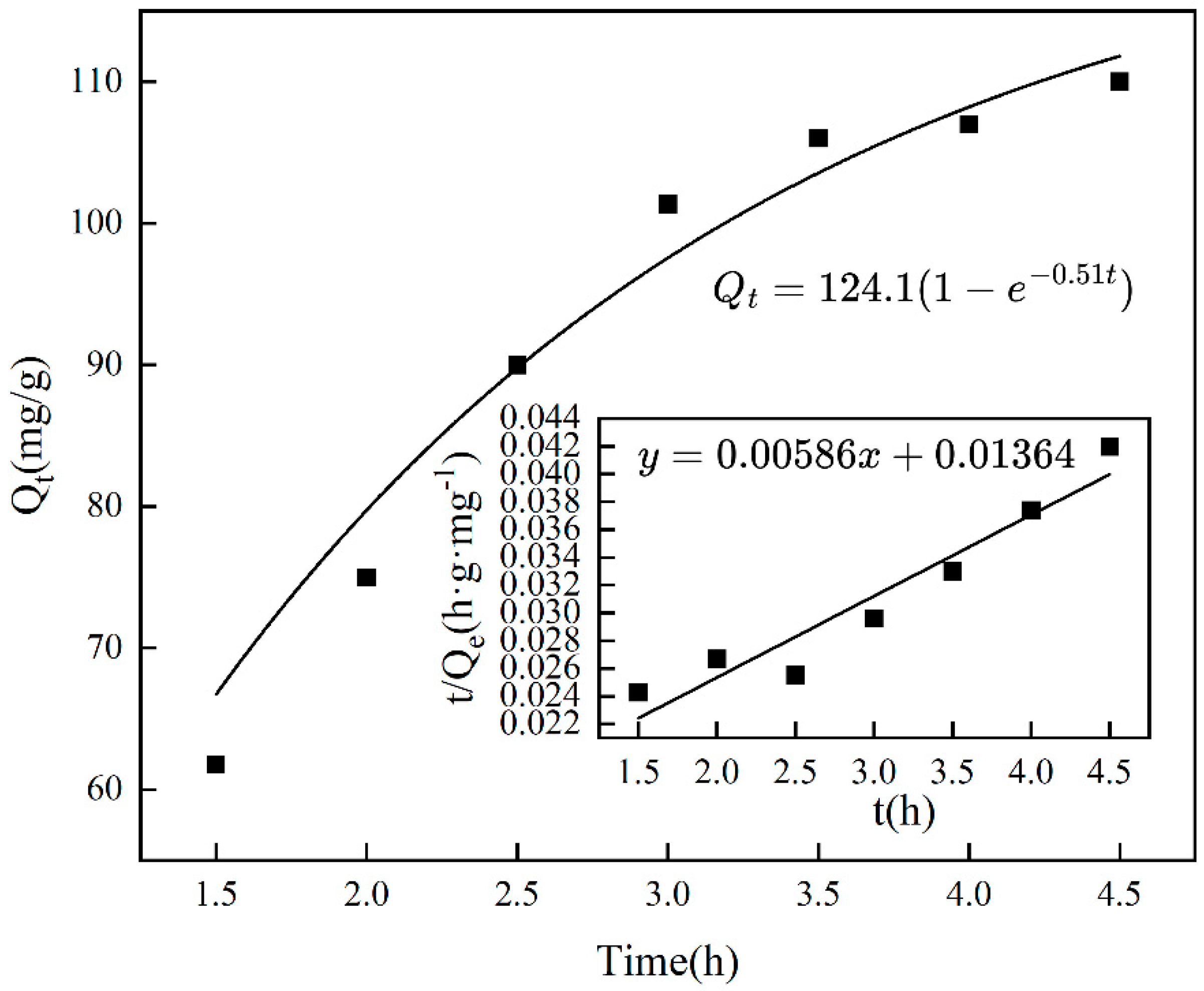
The duration of adsorption influences the adsorption capacity of imprinting materials, as illustrated in Figure 5, which shows that adsorption increases with time and eventually plateaus, achieving equilibrium after 3.5 h. Extending the adsorption time results in a slight decrease in the amount adsorbed, likely due to the occurrence of desorption. Figure 6 and Table 1 indicate that, based on the pseudo-first-order kinetic model, the estimated saturated adsorption capacity of Cr(VI)-IIP-PEI@NBC is 124.1 mg/g, and the equilibrium constant is −0.516 min−1 (R2 = 0.920). In comparison, the pseudo-second-order kinetic simulation did not perform well (R2 = 0.905), indicating a possible increase in physical adsorption processes within the ion-imprinted materials [33].
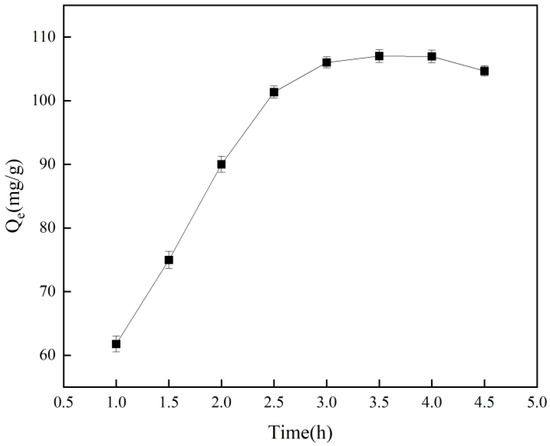
Figure 5.
Effect of adsorption time on adsorption capacity.
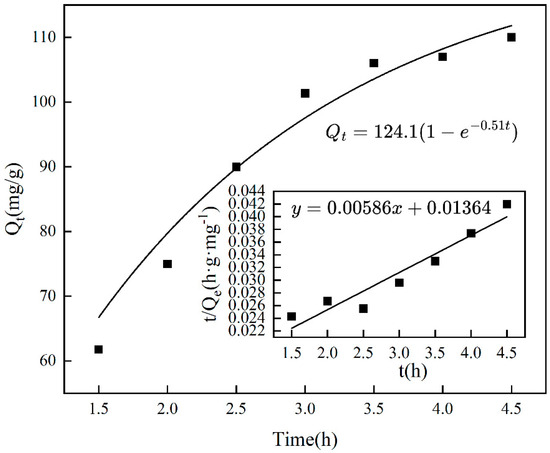
Figure 6.
Kinetic fitting of chromium adsorption by Cr(VI)-IIP-PEI@NBC.

Table 1.
Two adsorption kinetics fitting parameters of Cr(VI)-IIP-PEI@NBC.
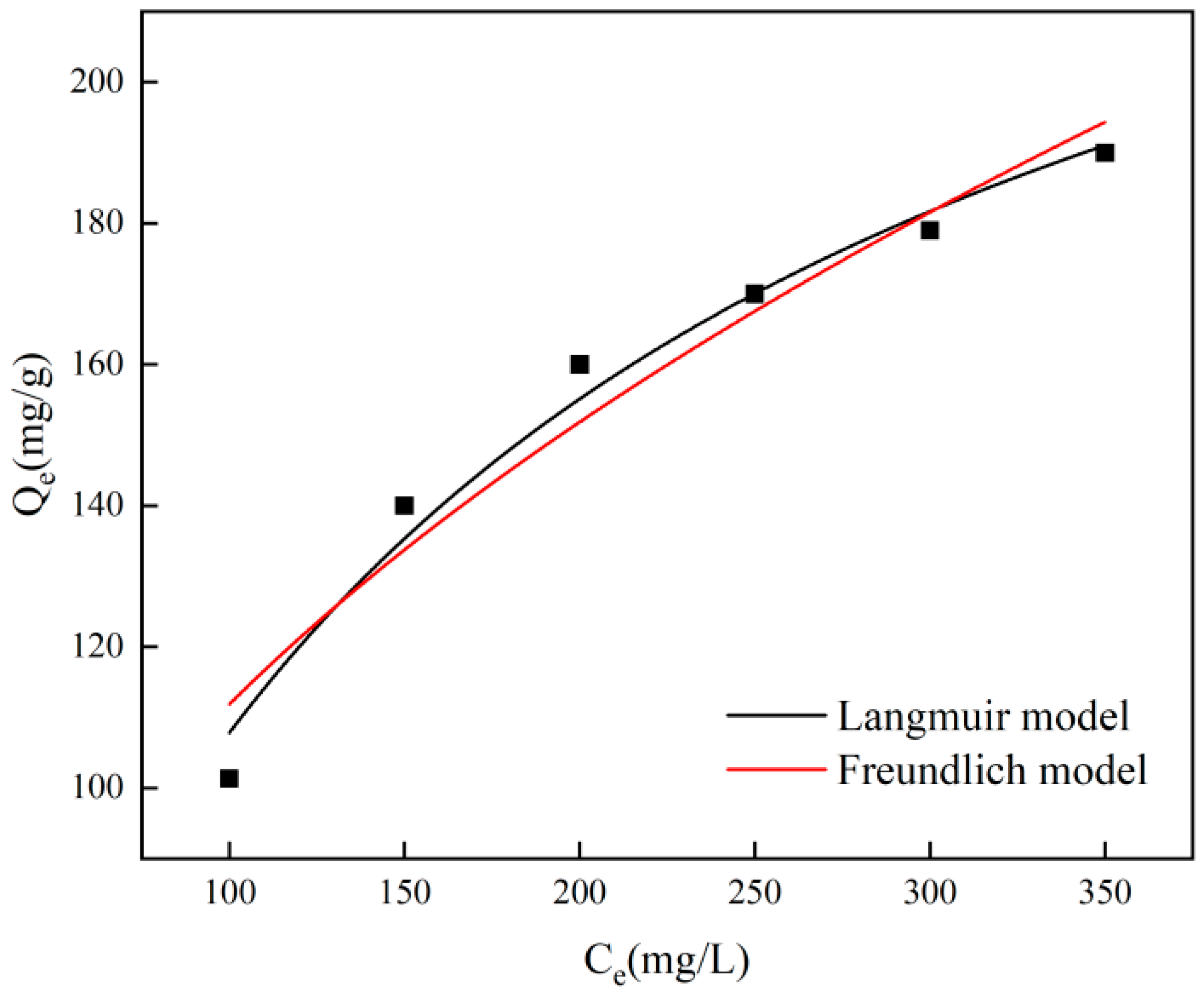
Figure 7 illustrates how the initial chromium concentration impacts adsorption, revealing that the model estimates a maximum adsorption capacity of 276.47 mg/g, accompanied by a Langmuir adsorption equilibrium constant of 0.00639. Furthermore, the adsorption data align closely with the Langmuir isotherm adsorption model simulation, achieving a correlation coefficient of R2 = 0.9813. The Langmuir isotherm model illustrates the consistency of the adsorbent surface and the occurrence of single-layer adsorption among adsorbents, whereas the Freundlich isotherm model characterizes the irregularity of the adsorbent surface and the presence of multi-layer adsorption [34]. To gain a deeper understanding of the adsorption process, the adsorption behavior was examined using the Langmuir and Freundlich models at a temperature of 25 °C. The pertinent findings are displayed in Figure 7 and Table 2, according to which the Langmuir model provides a more precise fit for the adsorbent concerning Cr(VI). This suggests that a single layer of adsorption takes place between the adsorbent and chromium, with the adsorption sites uniformly spread across the surface.
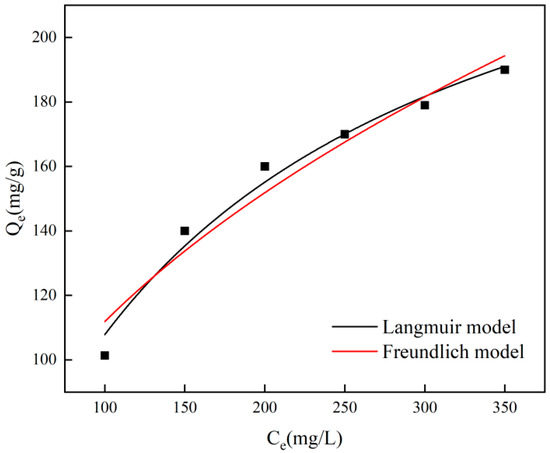
Figure 7.
Langmuir and Freundlich fitting of adsorption isotherms.

Table 2.
Fitting coefficients of the Langmuir and Freundlich models.
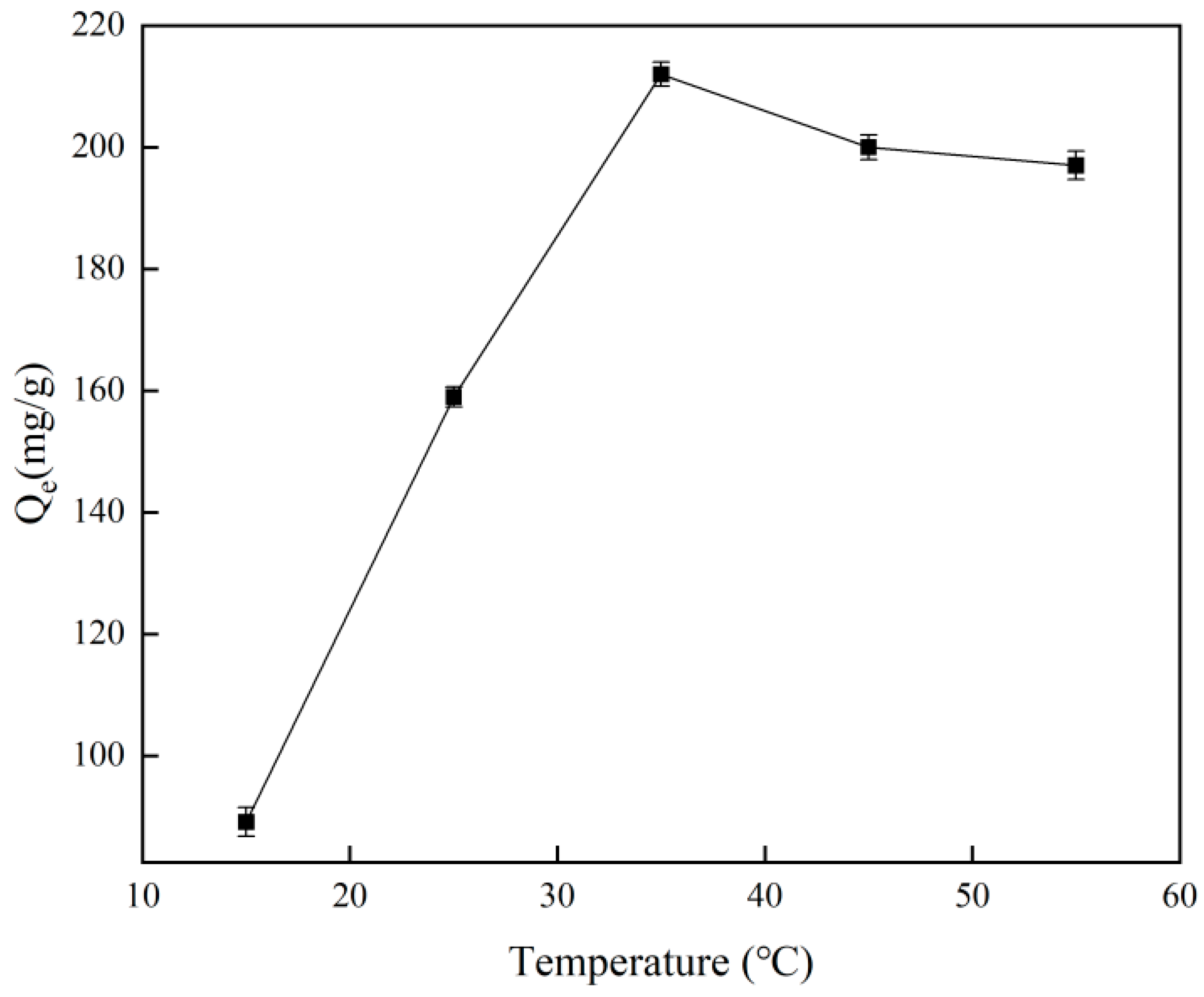
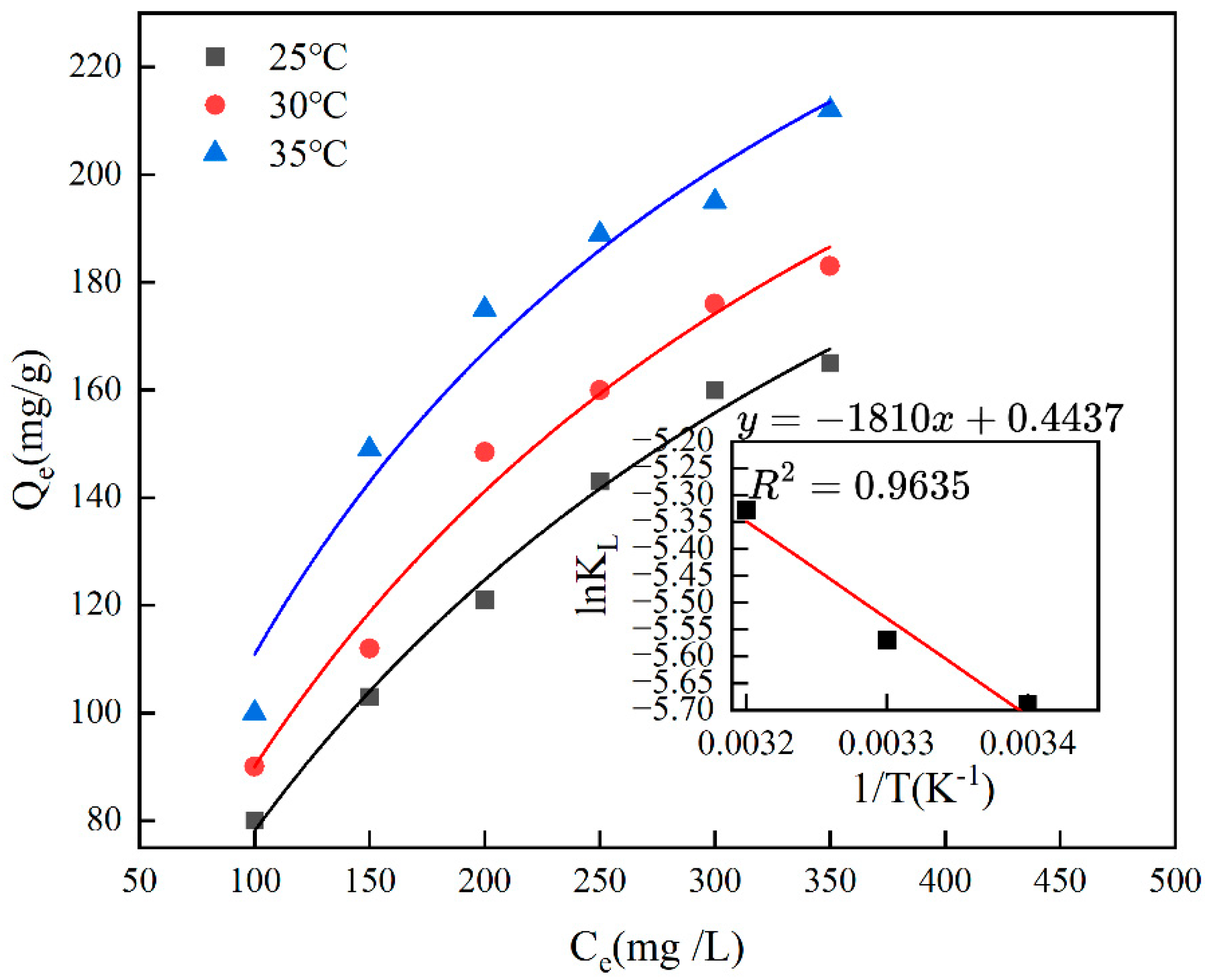
Figure 8 illustrates how temperature affects the adsorption capacity of the material, with the ideal temperature for Cr(VI)-IIP-PEI@NBC adsorption found to be 35 °C. Additionally, when the temperature exceeds 35 °C, the adsorption capacity of Cr(VI)-IIP-PEI@NBC becomes almost constant as the temperature increases, suggesting that the material is not sensitive to temperature within a certain range. The maximum adsorption capacity was ultimately determined to be 212.63 mg/g, which was 2.3 times higher than that of the non-imprinted biochar. Compared to the findings of previous researchers, the adsorption capacity of this material was improved by 3.1-fold [35], see Table 3.
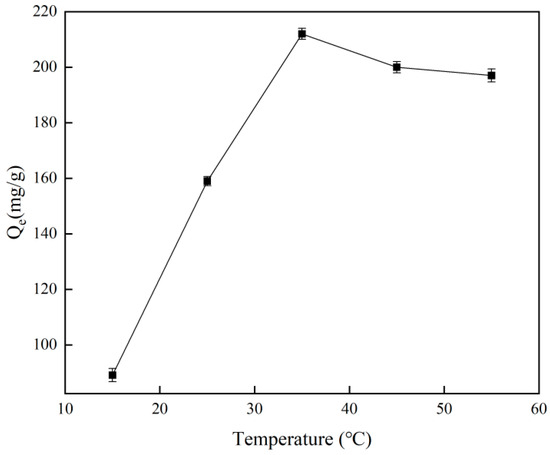
Figure 8.
Effect of temperature on adsorption capacity of Cr(VI)-IIP-PEI@NBC.

Table 3.
The absorption capacity of different materials.
Thermodynamic analysis is employed in adsorption research to elucidate the adsorption process through an energy-based viewpoint. Usually, the thermodynamic parameters for chromium adsorption using Cr(VI)-IIP-PEI@NBC would be determined at constant concentrations. Figure 9 illustrates how temperature affects the adsorption of chromium by Cr(VI)-IIP-PEI@NBC. By applying Formula (9), plotting lnKL against 1/T results in a slope of −ΔH/R and an intercept of ΔS/R. Table 4 presents the ΔG values calculated at various temperatures, showing that ΔG is positive at all three temperatures, which suggests that the adsorption of chromium by Cr(VI)-IIP-PEI@NBC is not spontaneous. As the temperature rises, the ∆G value for adsorption tends to decrease, indicating that at elevated temperatures, the free energy associated with the adsorption process decreases, which enhances the likelihood of the reaction occurring; it also shows that the adsorption process absorbs heat.
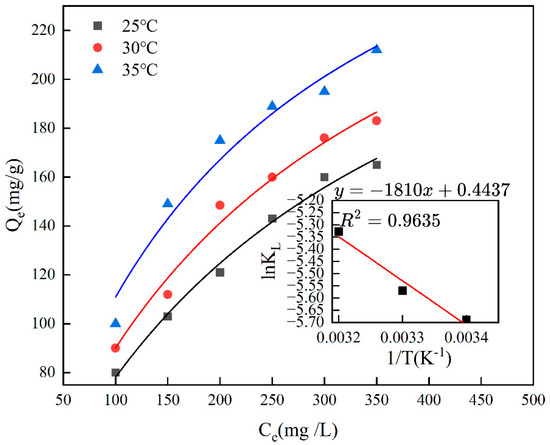
Figure 9.
The isothermal adsorption of Cr(VI)-IIP-PEI@NBC at different temperatures.

Table 4.
Thermodynamic parameters of adsorption.
Thermodynamic experiments on adsorption show that the ability of Cr(VI)-IIP-PEI@NBC to adsorb chromium rises as temperature increases. In general, increasing the temperature facilitates mass transfer and lowers the solution’s viscosity, which in turn improves the rate of chromium ion diffusion. As a result, raising the solution temperature effectively enhances the adsorption of chromium by Cr(VI)-IIP-PEI@NBC.
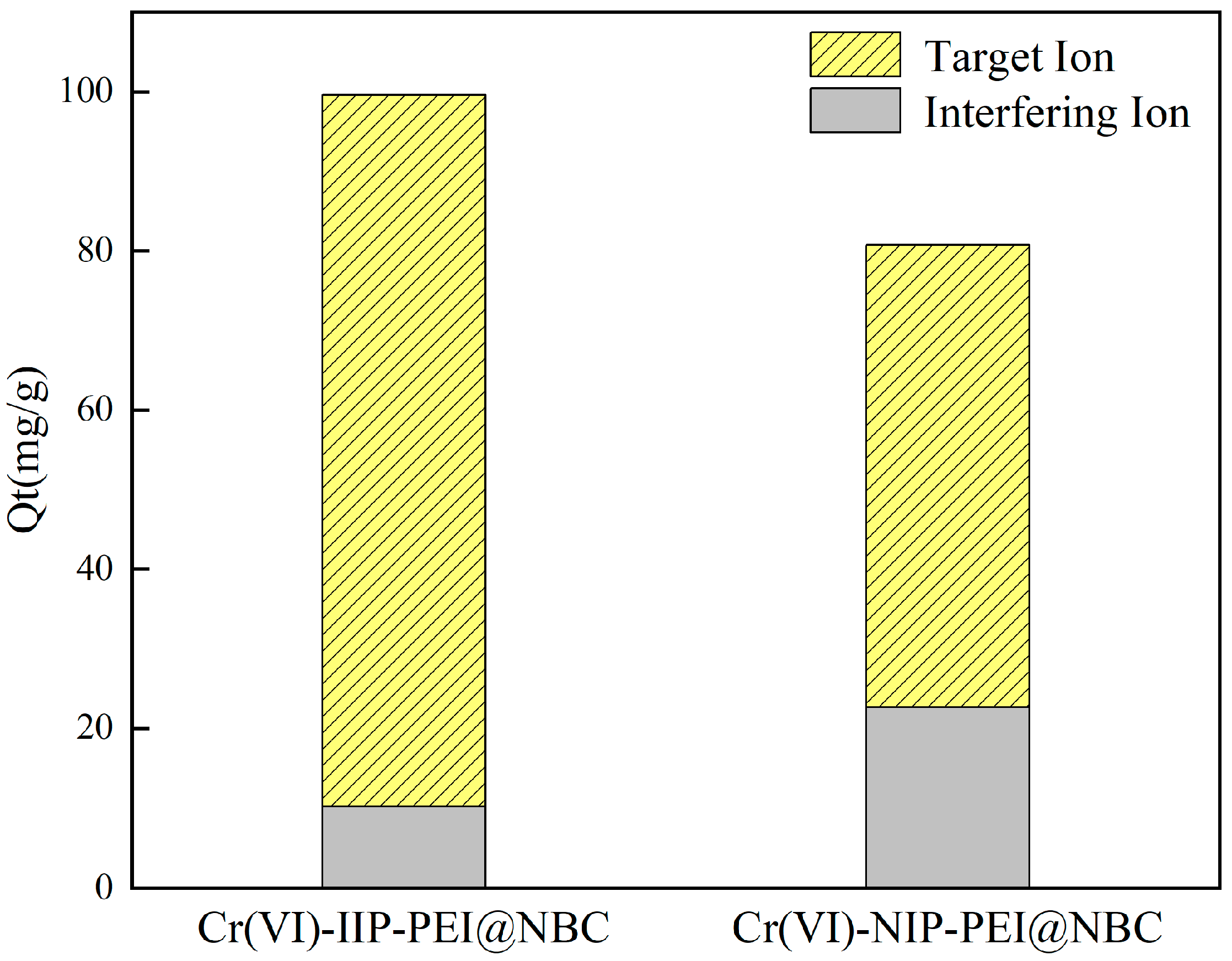
Na+, Mg2+, Ca2+, and K+ were chosen as coexisting ions to study the adsorption selectivity of Cr(VI)-IIP-PEI@NBC and Cr(VI)-NIP-PEI@NBC in relation to various competing ions. The findings presented in Figure 10 indicate that, in general, the selective adsorption of Cr (VI) by Cr(VI)-IIP-PEI@NBC was slightly lower compared to that with the ion solution without interference. However, it demonstrated a high level of resistance to interference from other ions, with its ability to adsorb chromium being significantly greater than that for other competing ions. This indicates that there may be many imprinted cavity sites in the material that are structurally compatible with Cr (VI) ions, allowing for the selective identification and absorption of these ions.
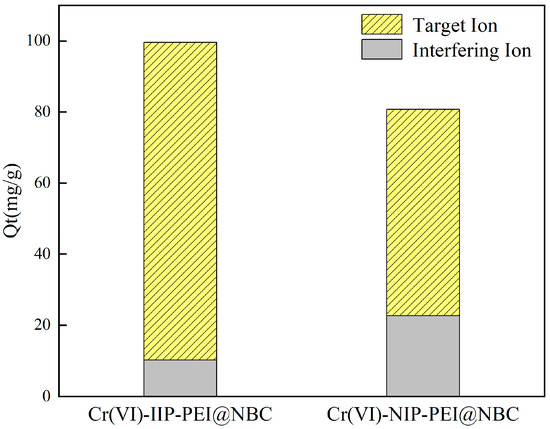
Figure 10.
Adsorption capacity of Cr(VI)-IIP-PEI@NBC and Cr(VI)-NIP-PEI@NBC for chromium and other coexisting ions (Including Na+, K+, Cl−, Ca2+, Mg2+).
In comparison, the non-imprinted material exhibited weak adsorption capabilities for Cr (VI) ions, which could be explained by the non-imprinted material not having any imprinting ions added, resulting in a lack of corresponding imprinting sites and a reduced adsorption efficiency. Furthermore, in comparison to the ion-imprinted material Cr(VI)-IIP-PEI@NBC, the non-imprinted material Cr(VI)-NIP-PEI@NBC showed a greater ability to adsorb Na+. One possible explanation for this is that the non-imprinted material, which does not contain imprinting ions, lacks pore channels that facilitate effective Cr(VI) adsorption. Rather, sodium ions are absorbed via coordination bonding between the amino groups on the surface and the metal ions.
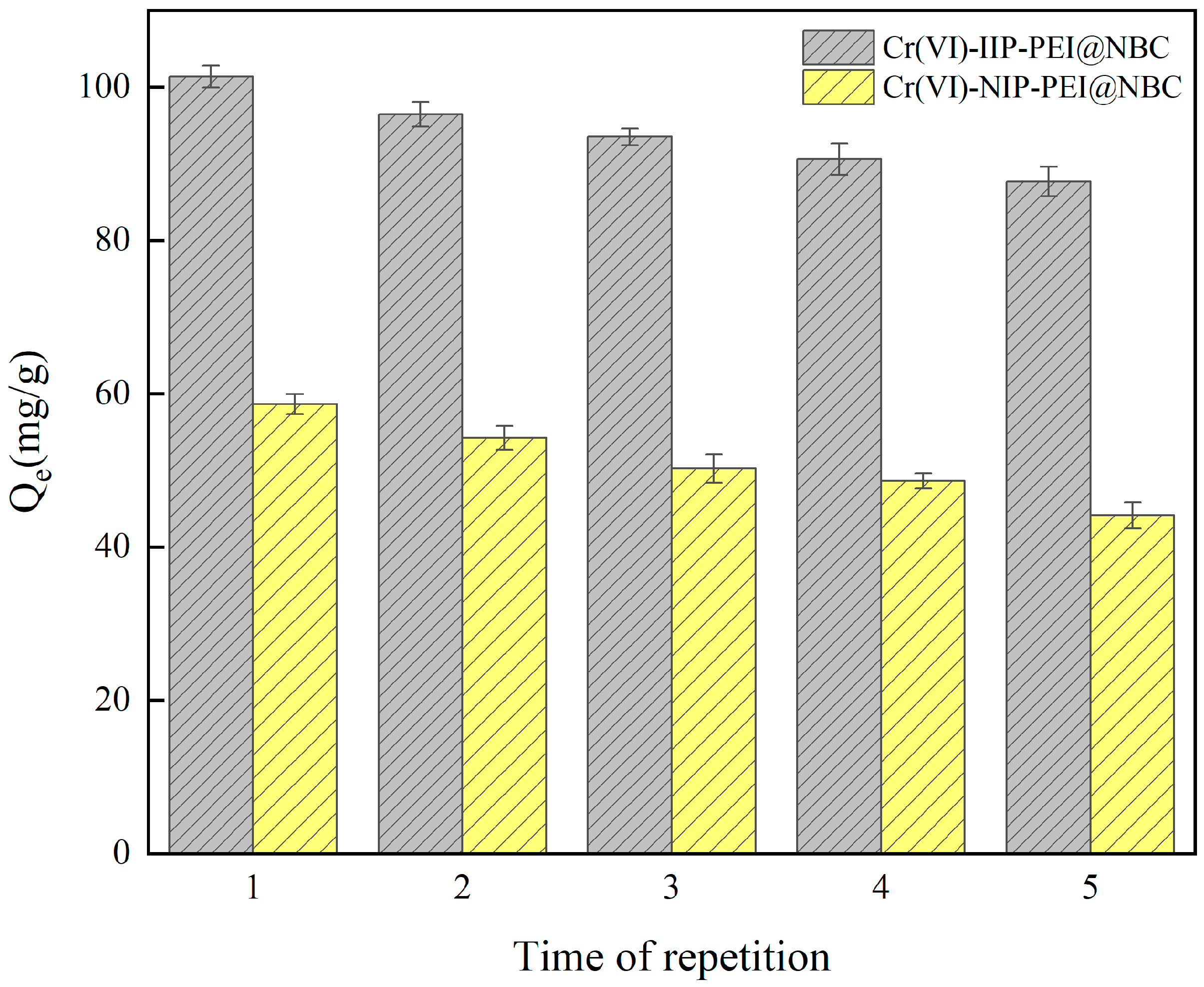
Reusability experiments were carried out to examine how the adsorption capacity of Cr(VI)-IIP-PEI@NBC and Cr(VI)-NIP-PEI@NBC changes after several cycles of adsorption and desorption. As illustrated in Figure 11, the results of five reusability tests indicated that the adsorption of Cr(VI) ions by both materials decreased progressively with each successive adsorption and elution cycle. This could be explained by the continuous cycles of adsorption and desorption potentially deactivating the active imprinting sites on the surface of the material, resulting in a decreased ability to adsorb Cr(VI)-IIP-PEI@NBC. However, the experimental results show that even with this decrease, Cr(VI)-IIP-PEI@NBC maintained an adsorption capacity over 84.00% after five cycles. This demonstrates the outstanding reusability of the synthesized Cr(VI)-IIP-PEI@NBC, proving that it is a viable material for effective adsorption in the treatment of Cr(VI)-contaminated wastewater.
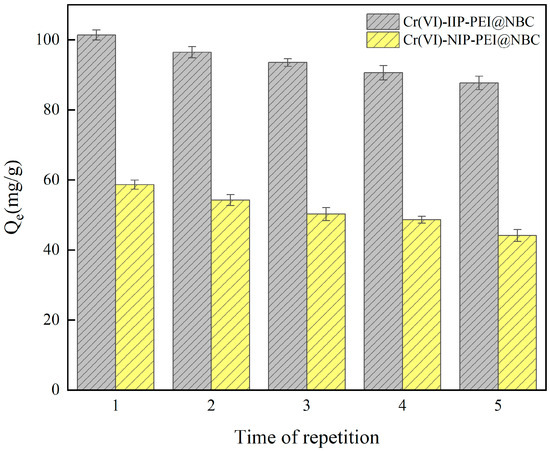
Figure 11.
Reusability of Cr(VI)-IIP-PEI@NBC and Cr(VI)-NIP-PEI@NBC.
Table 5 presents a comparison of various novel Cr(VI) adsorbents. The adsorbent prepared in this study exhibits superior adsorption performance relative to others, which can be attributed to the inherently porous nature of biochar. However, in terms of reusability, although the material retained 84.00% of its adsorption capacity after five cycles, PMGO and PEI/MW@MNHC materials demonstrated more outstanding regeneration performance. The regeneration process requires chemical elution of Cr(VI), which inevitably causes damage to the material’s internal structure and chemical environment. While this study successfully modified the chemical environment on the biochar surface, enabling effective grafting of PEI, the instability of the chemical environment—resulting from the complex surface functional groups of biochar—led to inferior reusability compared to inorganic materials. Overall, Cr(VI)-IIP-PEI@NBC shows high adsorption capacity and considerable practical potential for treating Cr(VI)-containing wastewater.

Table 5.
Cr(VI) adsorbents.
3.4. Material Characterization
3.4.1. SEM
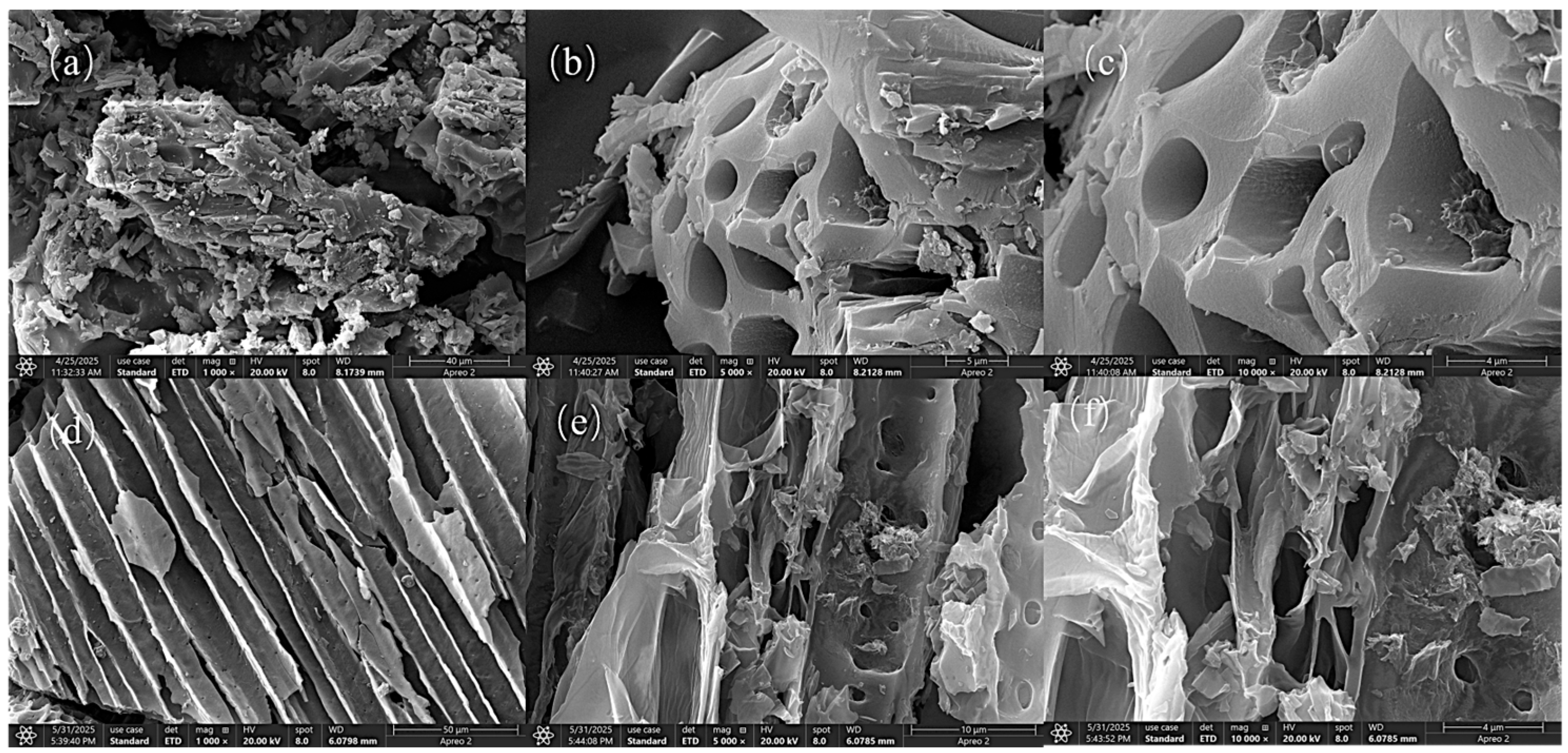
The SEM images presented in Figure 12 illustrate the morphological structure of Cr(VI)-IIP-PEI@NBC at various magnifications. Figure 12a shows a detailed image of Cr(VI)-IIP-PEI@NBC, highlighting its noticeable dispersion and texture. Images (b) and (c) display Cr(VI)-IIP-PEI@NBC at magnifications of 5000× and 10,000×, respectively. At these levels of magnification, clear pore-like structures can be observed on the surface of the imprinted material. These pores increase the material’s surface area and allow for more imprint points to form, which is conducive to adsorption.
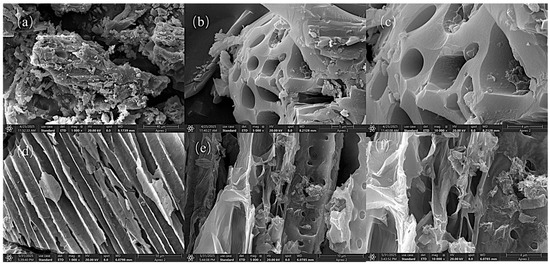
Figure 12.
SEM images of Cr(VI)-IIP-PEI@NBC and NBC. (a). Cr(VI)-IIP-PEI@NBC at magnifications of 1000×. (b). Cr(VI)-IIP-PEI@NBC at magnifications of 5000×. (c). Cr(VI)-IIP-PEI@NBC at magnifications of 10000×. (d). NBC at magnifications of 1000×. (e). NBC at magnifications of 5000×. (f). NBC at magnifications of 10000×.
3.4.2. FT-IR
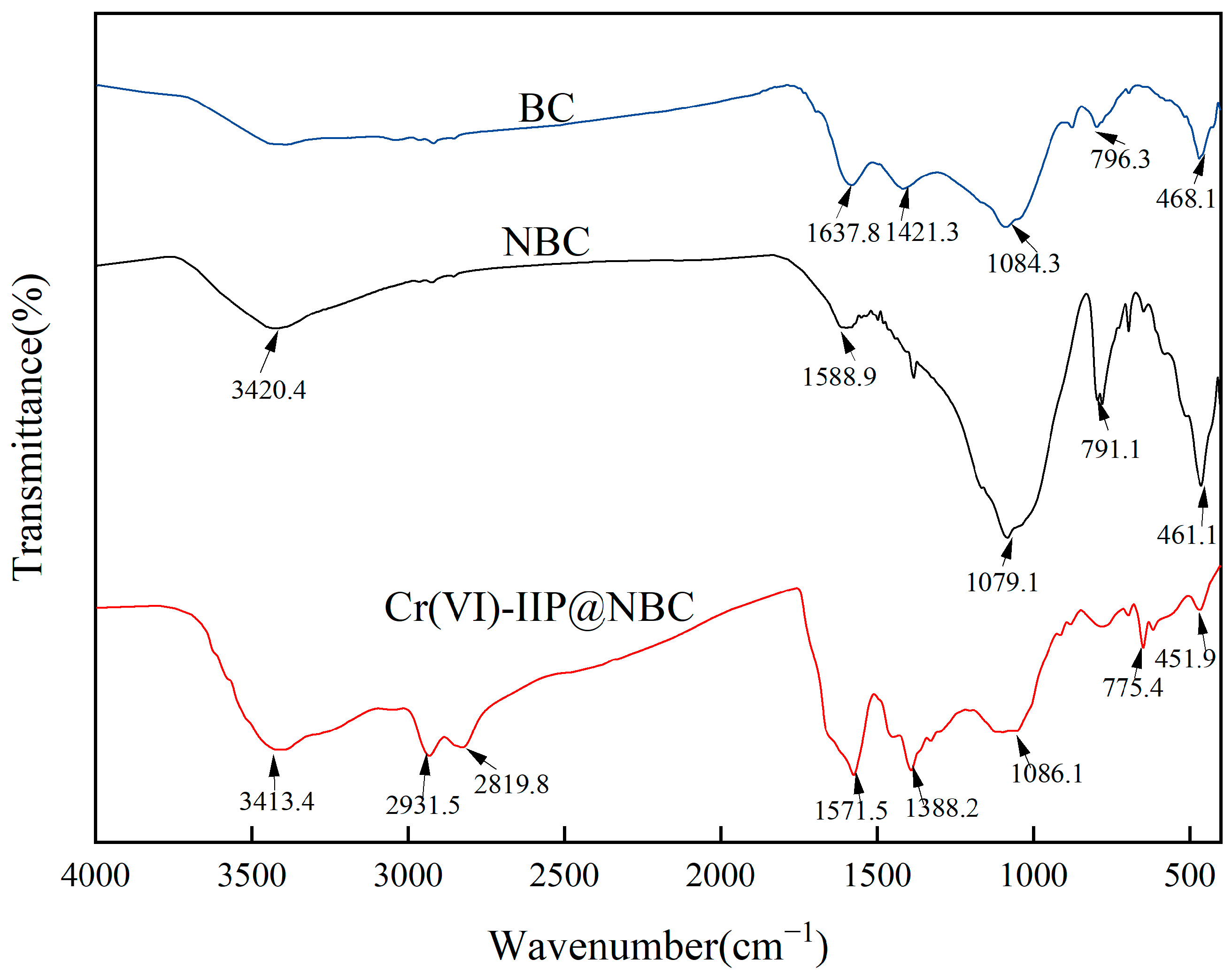
Figure 13 displays the FT-IR spectrum of Cr(VI)-IIP-PEI@NBC; the wavelengths associated with the chemical bonds are as follows [40,41,42]: The wide peak observed at 3413.4 cm−1 is attributed to the stretching vibration of N-H. Meanwhile, the peaks for C-H stretching vibrations from methyl, methylene, and similar groups are located near 2931.5 cm−1 and 2819.8 cm−1 [43]. The peak for the C=O stretching vibration of the carbonyl group is located at 1571.5 cm−1. The peak at 1379.4 cm−1 is associated with the stretching vibration of the C-N bond. The absorption peak of NBC at 1079 cm−1 suggests the existence of hydroxyl groups, and when considered alongside the absorption at 3420 cm−1, this verifies the presence of alcohol hydroxyl groups. The reduced absorption intensity at this location suggests that PEI forms crosslinks with the biochar’s surface hydroxyl groups. This proves that PEI was effectively incorporated into NBC as a functional monomer.
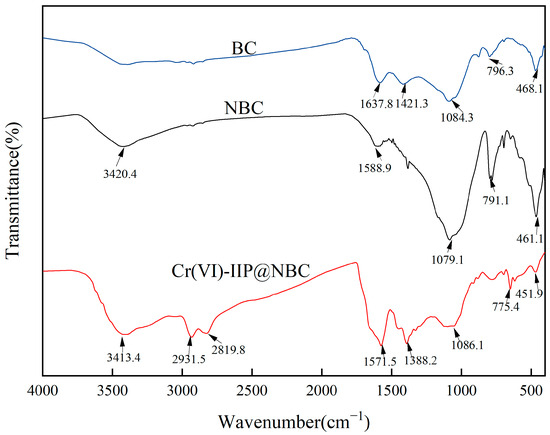
Figure 13.
FT-IR analysis of Cr(VI)-IIP-PEI@NBC.
3.4.3. TGA
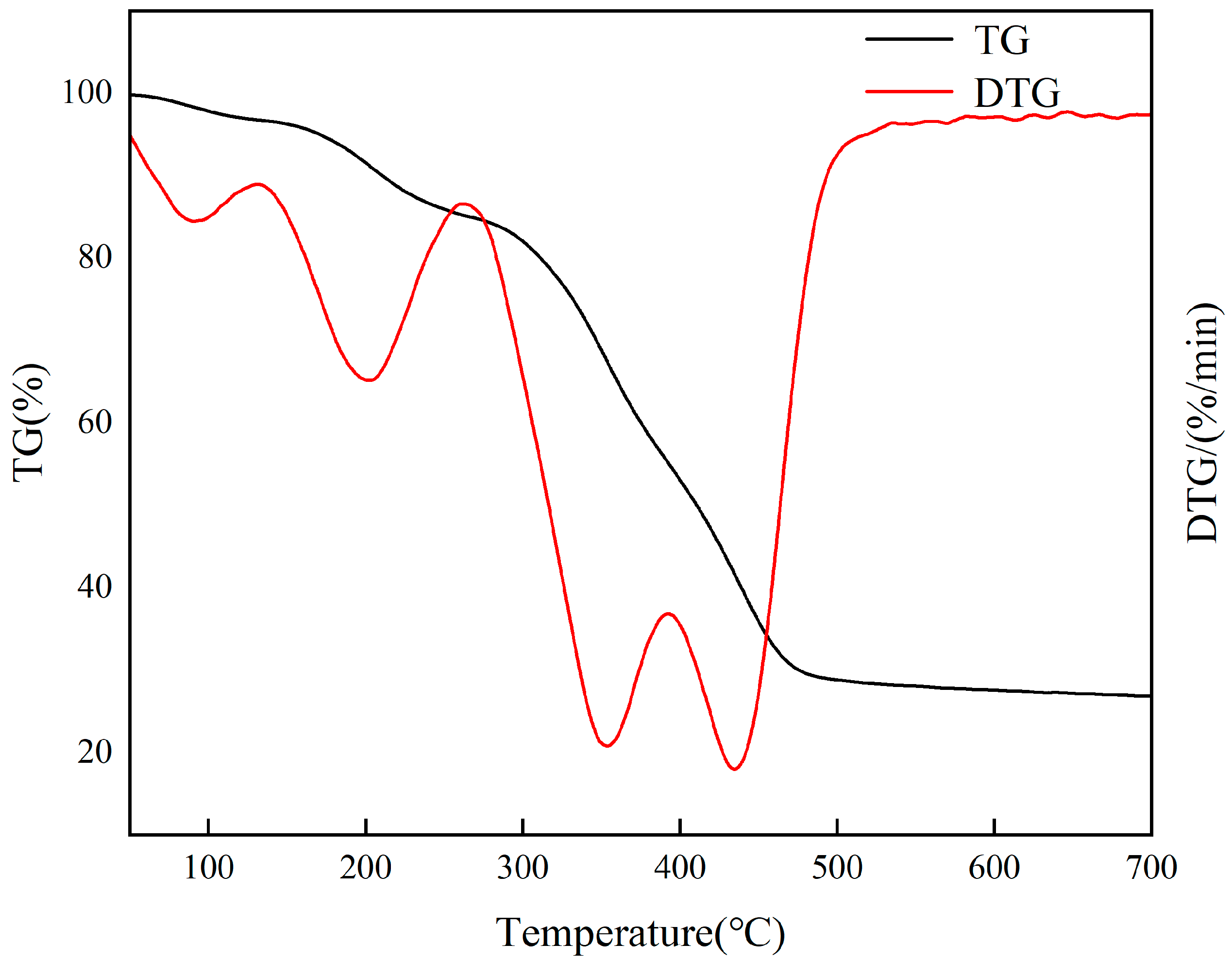
Thermogravimetric analysis is a crucial method for assessing the thermal stability of various materials in a nitrogen atmosphere. Figure 14 illustrates that Cr(VI)-IIP-PEI @NBC experiences three stages of weight loss at temperatures of 0–300 °C, 300–500 °C, and 500–800 °C. Initially, the little free water present in the material evaporates, followed by the decomposition of PEI into oligoethylene polyamine, which gradually vaporizes and leads to a reduction in the material’s weight. The second phase is the primary stage for weight loss. At temperatures between 300 and 400 °C, the PEI crosslinked on the biochar’s surface undergoes pyrolysis, resulting in the formation of various small nitrogen-containing organic compounds that are continuously released as gases. In the last phase, GA and residual PEI undergo continuous pyrolysis at temperatures between 400 and 500 °C, emitting small-molecule compounds until the process stabilizes. In practical adsorption applications, the temperature is typically kept below 100 °C, resulting in approximately 90% of the material being retained. As a result, Cr(VI)-IIP-PEI@NBC exhibits strong thermal stability.
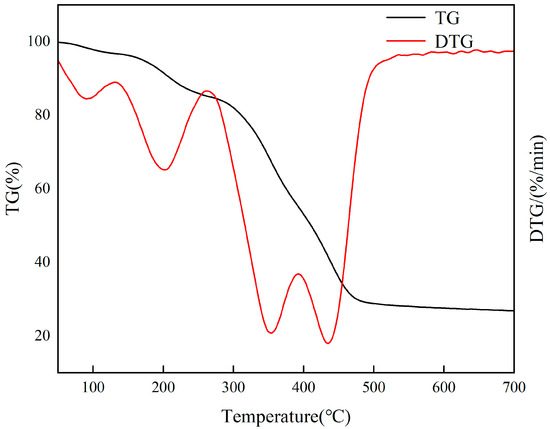
Figure 14.
TGA results for Cr(VI)-IIP-PEI@NBC.
3.4.4. BET
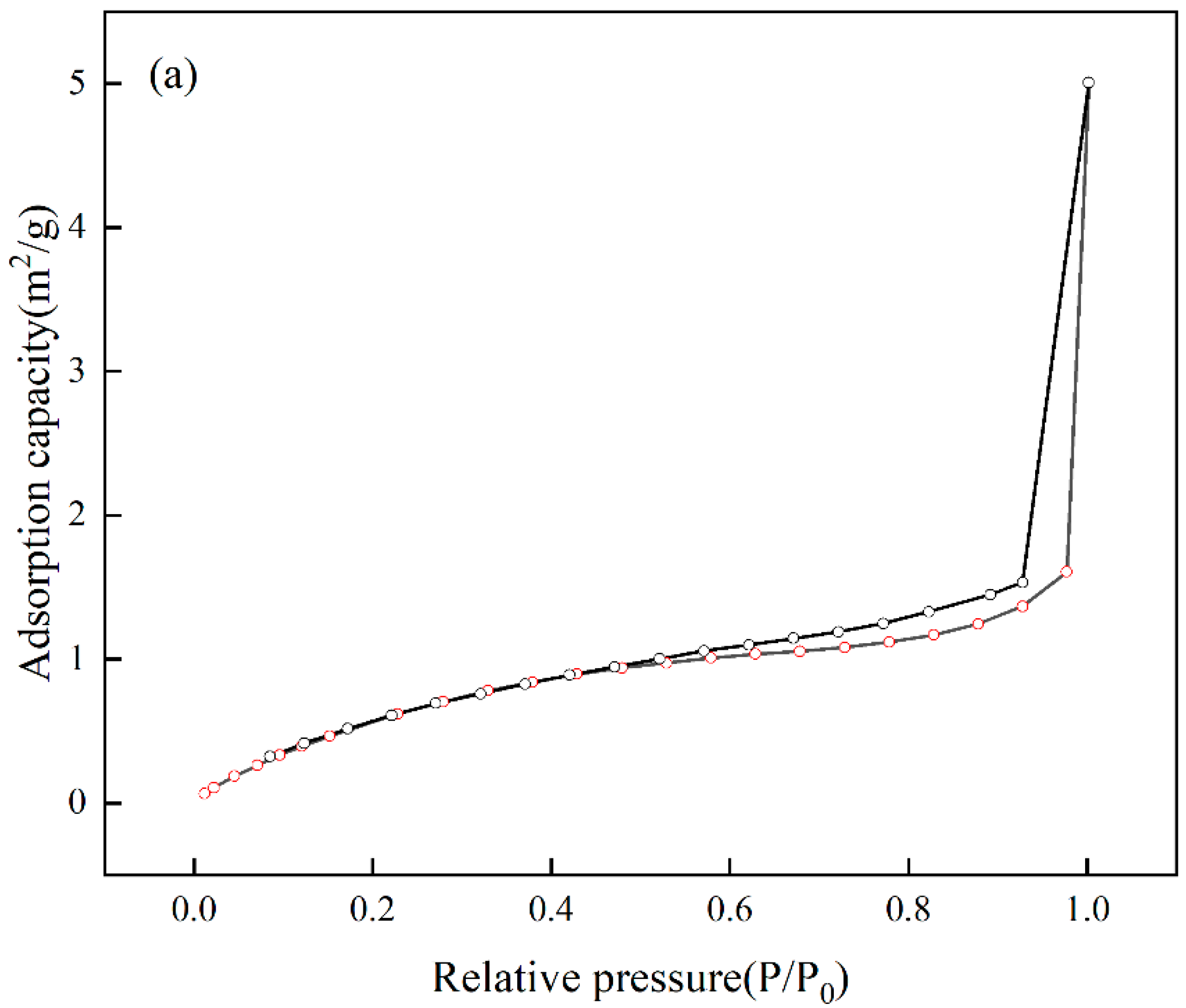
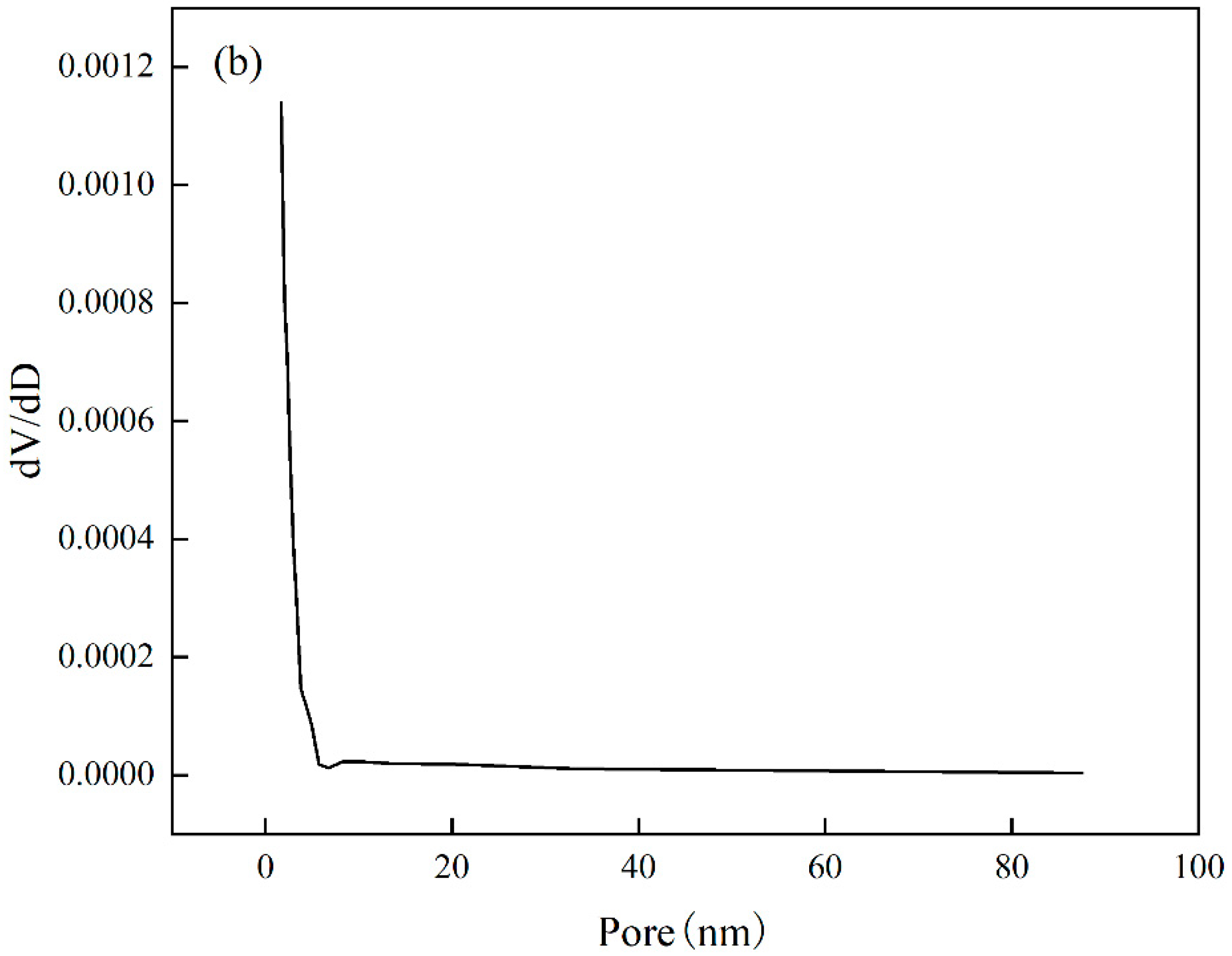
The analysis of the specific surface area and pore size of Cr(VI)-IIP-PEI@NBC is presented in the Figure 15. The adsorption–desorption isotherm displays a standard Type IV curve along with an H3-type hysteresis loop, which is typical of mesoporous materials [44], indicating that Cr(VI)-IIP-PEI@NBC is indeed mesoporous. The surface area is 3.126 m2/g, the pore diameter measures 11.699 nm, and the pore volume is 0.0077 cm3/g. In comparison to NBC, Cr(VI)-IIP-PEI@NBC exhibits larger pore sizes but smaller pore volumes. This change may be due to the ion-imprinting effect of Cr(VI) ions, which creates recognizable mesoporous structures on the surface of Cr(VI)-IIP-PEI@NBC, see Table 6.
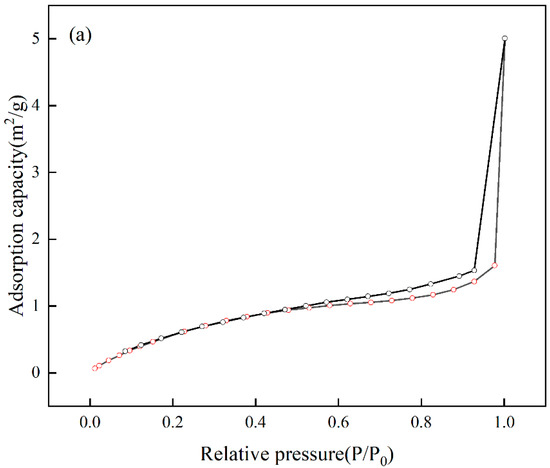
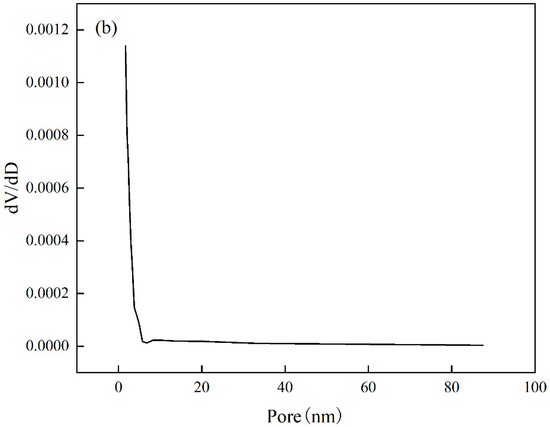
Figure 15.
Nitrogen sorption isotherms (a) and pore sizes (b).

Table 6.
Surface properties.
Due to equipment constraints, this study proposed the adsorption mechanism of Cr(VI)-IIP-PEI@NBC based on comparisons with similar materials. The adsorption process occurs in three main stages. Initially, under low-pH conditions, PEI is protonated [45]. The protonated amino groups then electrostatically attract negatively charged Cr(VI), enriching it around the material [46]. Finally, PEI, as a functional monomer, specifically reacts with Cr(VI), primarily through imine, amind, and protonated amine groups [28,47]. Additionally, lower pH promotes the reduction of Cr(VI) to Cr(III), further enhancing Cr(VI) removal from water [48].
4. Conclusions
In this study, we successfully optimized the pyrolysis of wheat straw under a nitrogen atmosphere using a heating rate of 10 °C/min up to 600 °C with 30 min retention, yielding 17.06% biochar containing 64.96 wt% carbon. The novel chromium ion-imprinted adsorbent Cr(VI)-IIP-PEI@NBC was synthesized through the functionalization of 0.1 g of biochar with 2 g PEI and 5 mL GA at 60 °C for 2 h. The material exhibited an exceptional chromium removal performance with a maximum adsorption capacity of 212.63 mg/g at pH 2.0 and 55 °C, representing a 2.3-fold improvement over non-imprinted controls. The material was comprehensively characterized employing BET and SEM analysis, confirming its mesoporous structure, while TGA demonstrated its thermal stability up to 300 °C. With exceptional selectivity reaching 99.64%, an adsorption efficiency exceeding 80.00% after five regeneration cycles, and pH-dependent adsorption behavior, this high-performing adsorbent material represents a sustainable and scalable solution for industrial wastewater treatment, effectively translating laboratory innovation into practical environmental applications.
Author Contributions
Conceptualization, X.Z.; Methodology, X.Z. and H.J.; Software, T.D. and L.C.; Validation, X.Z. and L.C.; Resources, Z.L. and H.J.; Writing—original draft, X.Z.; Supervision, W.G.; Funding acquisition, W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded The National Natural Science Foundation of China (22078157), and The Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture (XTC2208). And The APC was funded by Xinchi Zong.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, J.; Yang, R. Study on the predictive algorithm of plant restoration under heavy metals. Sci. Program. 2021, 2021, 6193182. [Google Scholar] [CrossRef]
- Chen, Y.G.; He, X.L.S.; Huang, J.H.; Luo, R.; Chen, S.H. Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotoxicol. Environ. Saf. 2021, 219, 112336. [Google Scholar] [CrossRef]
- Liu, P.; Ptacek, J.C.; Blowes, W.D.; Finfrock, Y.Z.; Liu, Y. Characterization of chromium species and distribution during Cr(VI) removal by biochar using confocal micro-X-ray fluorescence redox mapping and X-ray absorption spectroscopy. Environ. Int. 2020, 134, 105216. [Google Scholar] [PubMed]
- Lisbania, V.; Mohammad, S.N.; Edwin, E.; Marta-Lena, A.; Farid, A. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2020, 102, 342–379. [Google Scholar] [CrossRef]
- Gibb, H.; Lees, P.; Pinsky, P.; Rooney, B.C. Lung cancer among workers in chromium chemical production. Am. J. Ind. Med. 2000, 38, 115–126. [Google Scholar] [CrossRef]
- Yan, T.; Xu, Y.; Zhu, Y.; Jiang, P.; Zhang, Z.; Li, L.; Wu, Q. Chromium exposure altered metabolome and microbiome-associated with neurotoxicity in zebrafish. J. Appl. Toxicol. 2023, 43, 1026–1038. [Google Scholar] [CrossRef]
- Shan, R.; Shi, Y.; Gu, J.; Bi, J.; Chen, Y. Aqueous Cr(VI) removal by biochar derived from waste mangosteen shells: Role of pyrolysis and modification on its absorption process. J. Environ. Chem. Eng. 2020, 8, 103885. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Alam, M.S.; Chen, N.; Alessi, D.S.; Ok, Y.S. Removal of hexavalent chromium in aqueous solutions using biochar: Chemical and spectroscopic investigations. Sci. Total Environ. 2018, 625, 1567–1573. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Yin, X. Efficient removal of chromium by a novel biochar-microalga complex: Mechanism and performance. Environ. Technol. Innov. 2023, 31, 103156. [Google Scholar] [CrossRef]
- Adviento-Borbe, M.A.; Arlene, A.; Linquist, B. Assessing fertilizer N placement on CH4 and N2O emissions in irrigated rice systems. Geoderma 2016, 266, 40–45. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2014, 1, 289–303. [Google Scholar] [CrossRef]
- Chen, S.; Han, J.; Zhu, Y.; Zhang, X.; Zheng, C.; Ma, L.; Liu, S.; Yang, Y.; Zou, L.; He, L. Preparation of biochar-based surface molecularly imprinted polymers and evaluation of their selective adsorption and removal of carbaryl from rice and corn. J. Chromatogr. A 2023, 2023, 464210. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cen, B.; Yu, Z.; Qiu, R.; Gao, T.; Long, X. The key role of biochar in amending acidic soil: Reducing soil acidity and improving soil acid buffering capacity. Biochar 2025, 7, 52. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. The biochar dilemma. Soil Res. 2014, 52, 217. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Hui, H.G.; Gikas, P.; Chong, K.K.; Chew, K.W. Challenges and opportunities for biochar to promote circular economy and carbon neutrality. J. Environ. Manag. 2023, 332, 117429. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Balla, P.; Krishna, B.B.; Adhikari, S.; Bhaskar, T. Physiochemical characteristics of bio-char derived from pyrolysis of rice straw under different temperatures. Biomass Convers. Biorefinery 2022, 14, 12775–12783. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Li, H.; Westholm, L.J.; Carvalho, L.; Thorin, E.; Yu, Z.; Yu, X.; Skreiberg, Ø. A critical review on production, modification and utilization of biochar. J. Anal. Appl. Pyrolysis 2022, 161, 105405. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Meca, S.; Zhang, S.; Clarens, F.; Hu, X. Understanding the dependence of biochar properties on different types of biomass. Waste Manag. 2024, 182, 142–163. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, P.; Wang, Y.; Finfrock, Y.Z.; Xie, X.; Su, C.; Liu, N.; Yang, Y.; Xu, Y. Distribution and speciation of iron in Fe-modified biochars and its application in removal of As(V), As(III), Cr(VI), and Hg(II): An X-ray absorption study. J. Hazard. Mater. 2020, 384, 121342. [Google Scholar] [CrossRef]
- He, R.; Yuan, X.; Huang, Z.; Wang, H.; Jiang, L.; Huang, J.; Tan, M.; Li, H. Activated biochar with iron-loading and its application in removing Cr(VI) from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123642. [Google Scholar] [CrossRef]
- Li, Z.; Tian, W.; Chu, M.; Zou, M.; Zhao, J. Molecular imprinting functionalization of magnetic biochar to adsorb sulfamethoxazole: Mechanism, regeneration and targeted adsorption. Process Saf. Environ. Prot. 2023, 171, 238–249. [Google Scholar]
- Zhang, S.; Yang, X.; Le, L.; Ju, M.; Zheng, K. Adsorption Behavior of Selective Recognition Functionalized Biochar to Cd(II) in Wastewater. Materials 2018, 11, 299. [Google Scholar] [CrossRef]
- Wang, H.; Xin, W.; Cui, Y.; Xue, Z.; Ba, Y. Slow pyrolysis polygeneration of bamboo (Phyllostachys pubescens): Product yield prediction and biochar formation mechanism. Bioresour. Technol. 2018, 263, 444–449. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Inguanzo, M.; Menéndez, J.A.; Fuente, E.; Pis, J.J. Reactivity of pyrolyzed sewage sludge in air and CO2. J. Anal. Appl. Pyrolysis 2001, 58–59, 943–954. [Google Scholar] [CrossRef]
- Bojdi, M.K.; Mashhadizadeh, M.H.; Behbahani, M.; Farahani, A.; Davarani, S.S.H.; Bagheri, A. Synthesis, characterization and application of novel lead imprinted polymer nanoparticles as a high selective electrochemical sensor for ultra-trace determination of lead ions in complex matrixes. Electrochim. Acta 2014, 136, 59–65. [Google Scholar] [CrossRef]
- Kong, Z.; Du, Y.; Wei, J.; Zhang, H.; Fan, L. Synthesis of a new ion-imprinted polymer for selective Cr(VI) adsorption from aqueous solutions effectively and rapidly. J. Colloid Interface Sci. 2021, 588, 749–760. [Google Scholar] [CrossRef]
- Jia, J.; Wu, A.H.; Luan, S.J. Synthesis and investigation of the imprinting efficiency of ion imprinted nanoparticles for recognizing copper. Phys. Chem. Chem. Phys. 2014, 16, 16158–61615. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y. Molecular imprinting for anion recognition in aqueous media. Microchim. Acta 2011, 176, 23–47. [Google Scholar] [CrossRef]
- Azizi, A.; Bottaro, C.S. A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J. Chromatogr. A 2020, 1614, 460603. [Google Scholar] [CrossRef]
- Tytlak, A.; Oleszczuk, P.; Dobrowolski, R. Sorption and desorption of Cr(VI) ions from water by biochars in different environmental conditions. Environ. Sci. Pollut. Res. 2015, 22, 5985–5994. [Google Scholar] [CrossRef]
- Chen, S.; Yue, Q.; Gao, B.; Qian, L.; Xing, X. Removal of Cr(VI) from aqueous solution using modified corn stalks, Characteristic, equilibrium, kinetic and thermodynamic study. Chem. Eng. J. 2011, 168, 909–917. [Google Scholar] [CrossRef]
- Bi, S.; Yang, X.; Zhang, B.; Tong, Y.; Zhang, F.; Tian, M. Preparation of polyethylenimine modified molecular imprinting adsorbent with immobilized metal oxides for highly efficient ovalbumin adsorption. Microchem. J. 2022, 180, 107583. [Google Scholar] [CrossRef]
- Nie, D.; Ma, R.; Zhang, Y.; Wang, W.; Nie, G.; Liu, G.; Liu, W.; Zou, D. Efficient removal of Cr(VI) from wastewater by composite adsorptive membrane modified with polyethyleneimine (PEI). Sep. Purif. Technol. 2024, 346, 127410. [Google Scholar] [CrossRef]
- Pattarith, K.; Nugroho, D.; Nanan, S.; Benchawattananon, R. Cellulose Modified with Polyethylenimine (PEI) Using Microwave Methodology for Adsorption of Chromium from Aqueous Solutions. Molecules 2023, 28, 4514. [Google Scholar] [CrossRef]
- Singh, S.; Arputharaj, E.; Dahms, H.U.; Patel, A.K.; Huang, Y.L. Chitosan-based nanocomposites for removal of Cr(VI) and synthetic food colorants from wastewater. Bioresour. Technol. 2022, 351, 127018. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Wang, X.; Dong, Y.; Xin, J.; Ren, J.; An, J.; Jing, R. Construction of recyclable magnetic PEI-modified GO/Fe3O4 adsorbents for high-efficient removal of Cr(VI) ions from aqueous solutions. Solid State Sci. 2024, 154, 107611. [Google Scholar] [CrossRef]
- Qu, J.; Bi, F.; Hu, Q.; Wu, P.; Ding, B.; Tao, Y.; Ma, S.; Qian, C.; Zhang, Y. A novel PEI-grafted N-doping magnetic hydrochar for enhanced scavenging of BPA and Cr(VI) from aqueous phase. Environ. Pollut. 2023, 321, 121142. [Google Scholar] [CrossRef]
- Li, M.; Guo, X.; Wei, Y.; Liu, A.; Liu, X. Adsorption mechanism and structure-performance relationship of chromium ions by biochar. Water Air Soil Pollut. 2020, 231, 517–523. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, P.; Wang, S.; Finfrock, Y.Z.; Ye, Z.; Feng, Y.; Li, X. Iron-modified biochar-based bilayer permeable reactive barrier for Cr(VI) removal. J. Hazard. Mater. 2022, 439, 129636. [Google Scholar] [CrossRef]
- Nakanishi, K.; Ding, L.; Guo, X.; Kanamori, K.; Yang, H. Functionalization of hierarchically porous silica monoliths with polyethyleneimine (PEI) for CO2 adsorption. Microporous Mesoporous Mater. 2017, 245, 51–57. [Google Scholar]
- Choi, K.; Lee, S.; Park, J.O.; Park, J.A.; Cho, S.H.; Lee, S.Y.; Lee, J.H.; Choi, J.W. Chromium removal from aqueous solution by a PEI-silica nanocomposite. Sci. Rep. 2018, 8, 1438. [Google Scholar] [CrossRef]
- Bai, K.; Fan, S.; Chen, Y.; Wang, Y.; Chen, J.; Mai, Z.; Liu, J.; Deng, L.; Xiao, Z. Membrane adsorber with hierarchically porous HKUST-1 immobilized in membrane pores by flowing synthesis. J. Membr. Sci. 2022, 650, 120424. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Chen, X.; Leng, L.; Li, H.; Zeng, G. Facile synthesis of polypyrrole decorated reduced graphene oxide–Fe3O4 magnetic composites and its application for the Cr(VI) removal. Chem. Eng. J. 2015, 262, 597–606. [Google Scholar] [CrossRef]
- Guo, R.; Guo, W.; Pei, H.; Wang, B.; Guo, X.; Liu, N.; Mo, Z. Polypyrrole deposited electrospun PAN/PEI nanofiber membrane designed for high efficient adsorption of chromium ions (VI) in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127183. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Srinivasu, V.V.; Onyango, M.S. Removal of hexavalent chromium from aqueous solution using polypyrrole-polyaniline nanofibers. Chem. Eng. J. 2012, 181–182, 323–333. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Li, Y.; Li, Y.; Yang, R.; Wang, C. Branched polyethyl enimine grafted electrospun polyacrylonitrile fiber membrane: A novel and effective adsorbent for Cr(VI) remediation in wastewater. J. Mater. Chem. A 2017, 5, 1133–1144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).