Synthesis and Characterization of Rosa Canina-Fe3O4/Chitosan Nanocomposite and Treatment of Safranin O Dye from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
2.3. Preparation of m-ECH-RC Nanocomposite
2.4. Characterization Studies
2.5. Adsorption Studies
3. Results
3.1. Characterization of m-ECH-RC Nanocomposite
3.1.1. m-ECH-RC Nanocomposite BET Analysis
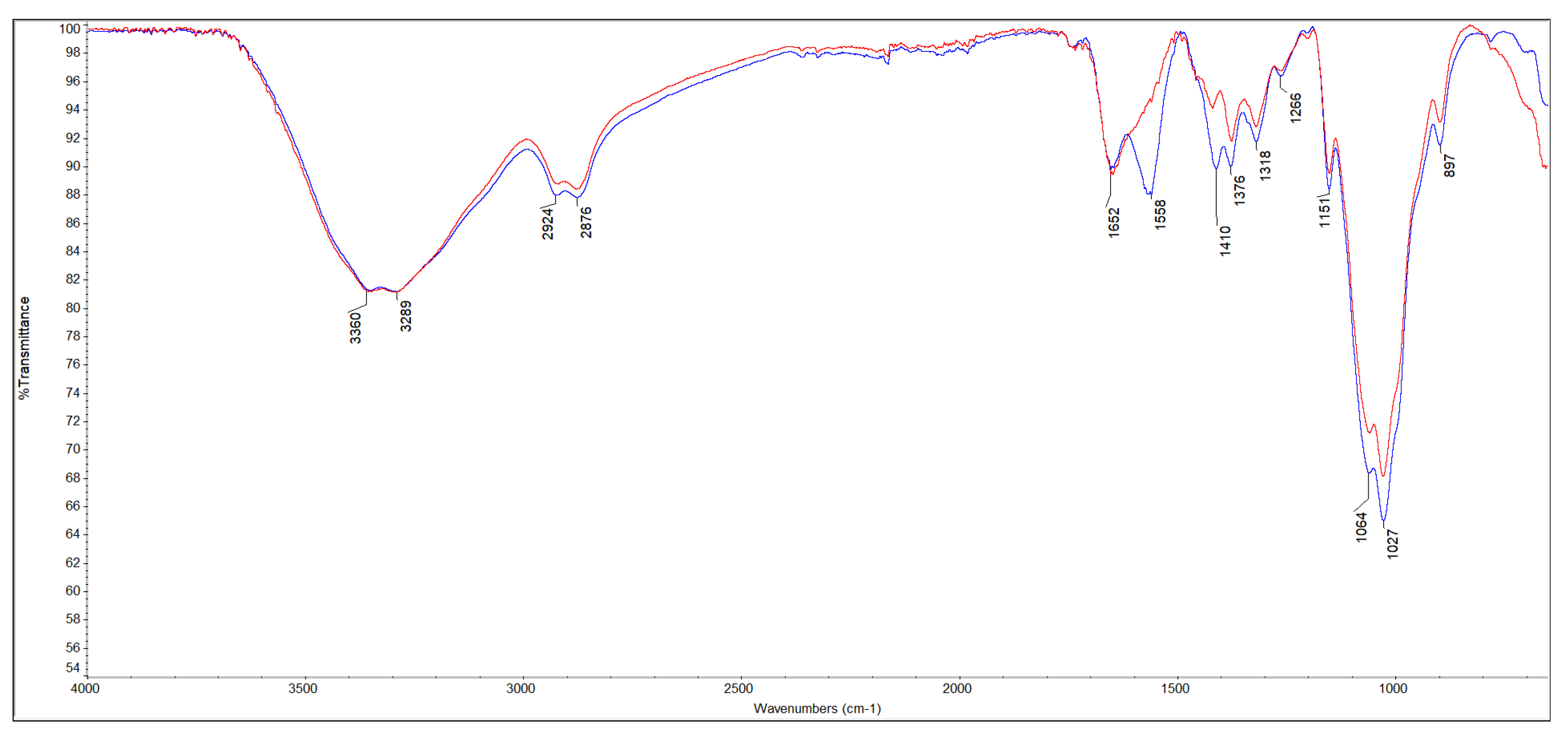
3.1.2. m-ECH-RC Nanocomposite FT-IR Analysis
3.1.3. m-ECH-RC Nanocomposite SEM Analysis
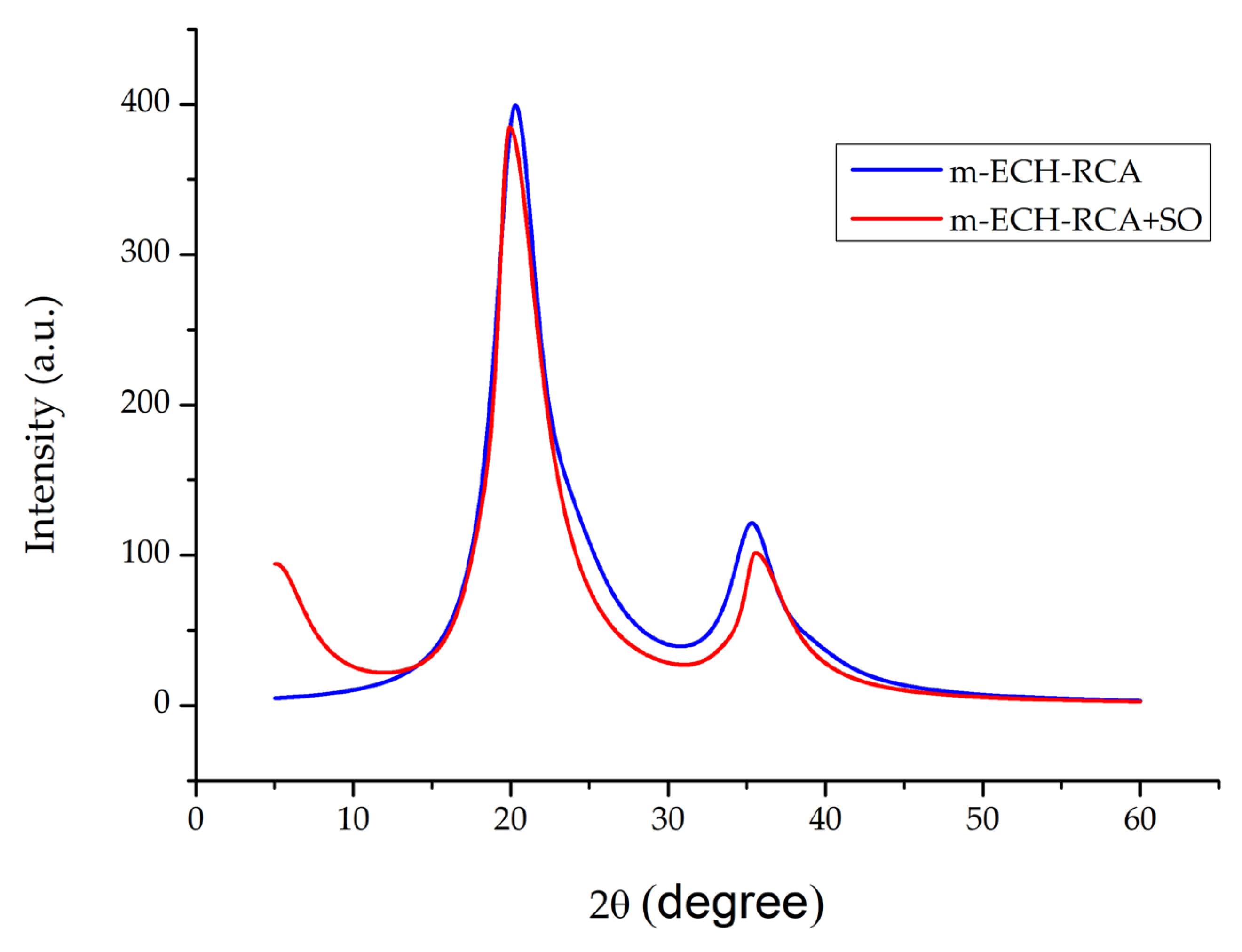
3.1.4. m-ECH-RC Nanocomposite XRD Analysis
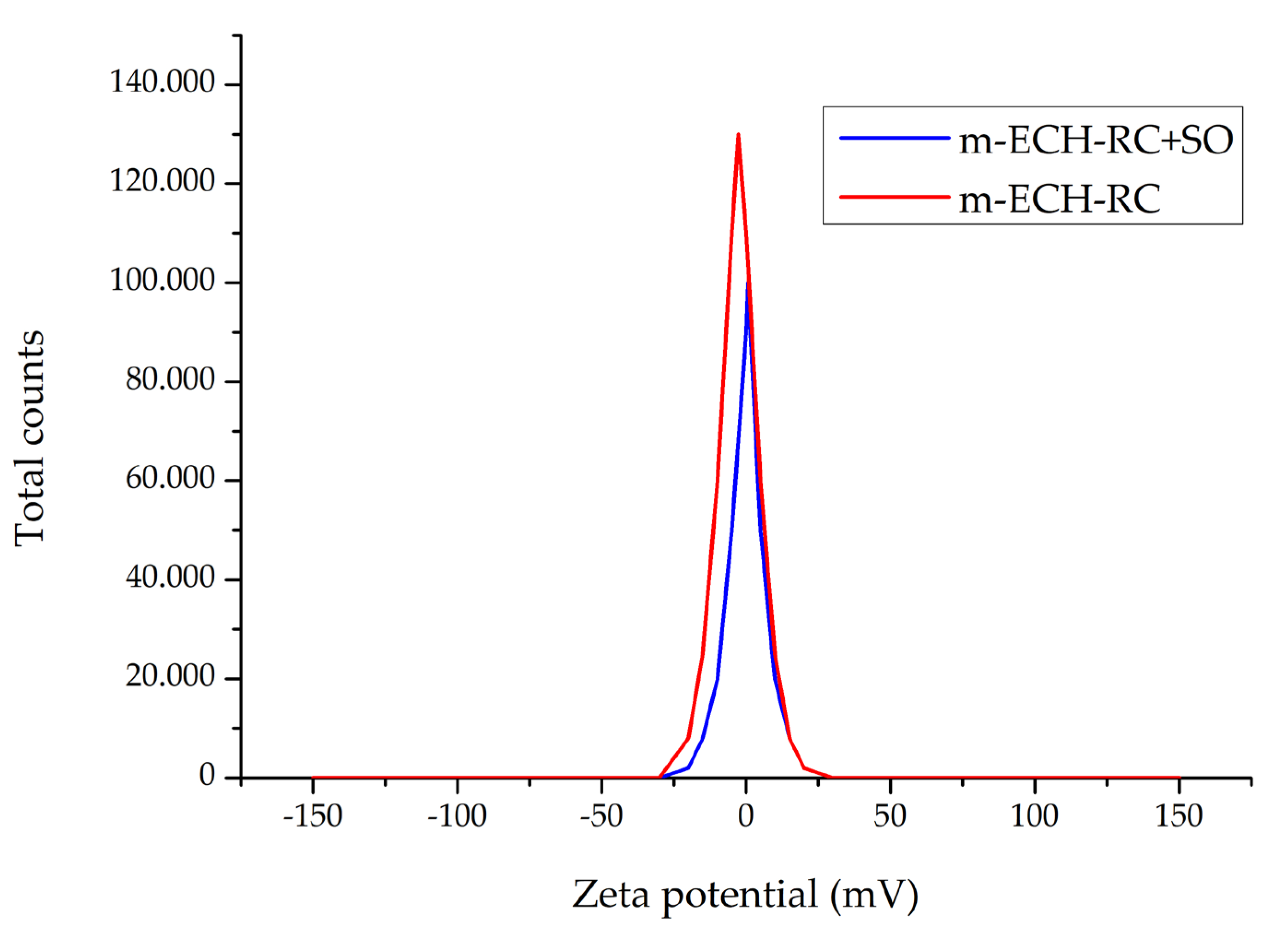
3.1.5. Zeta Potential
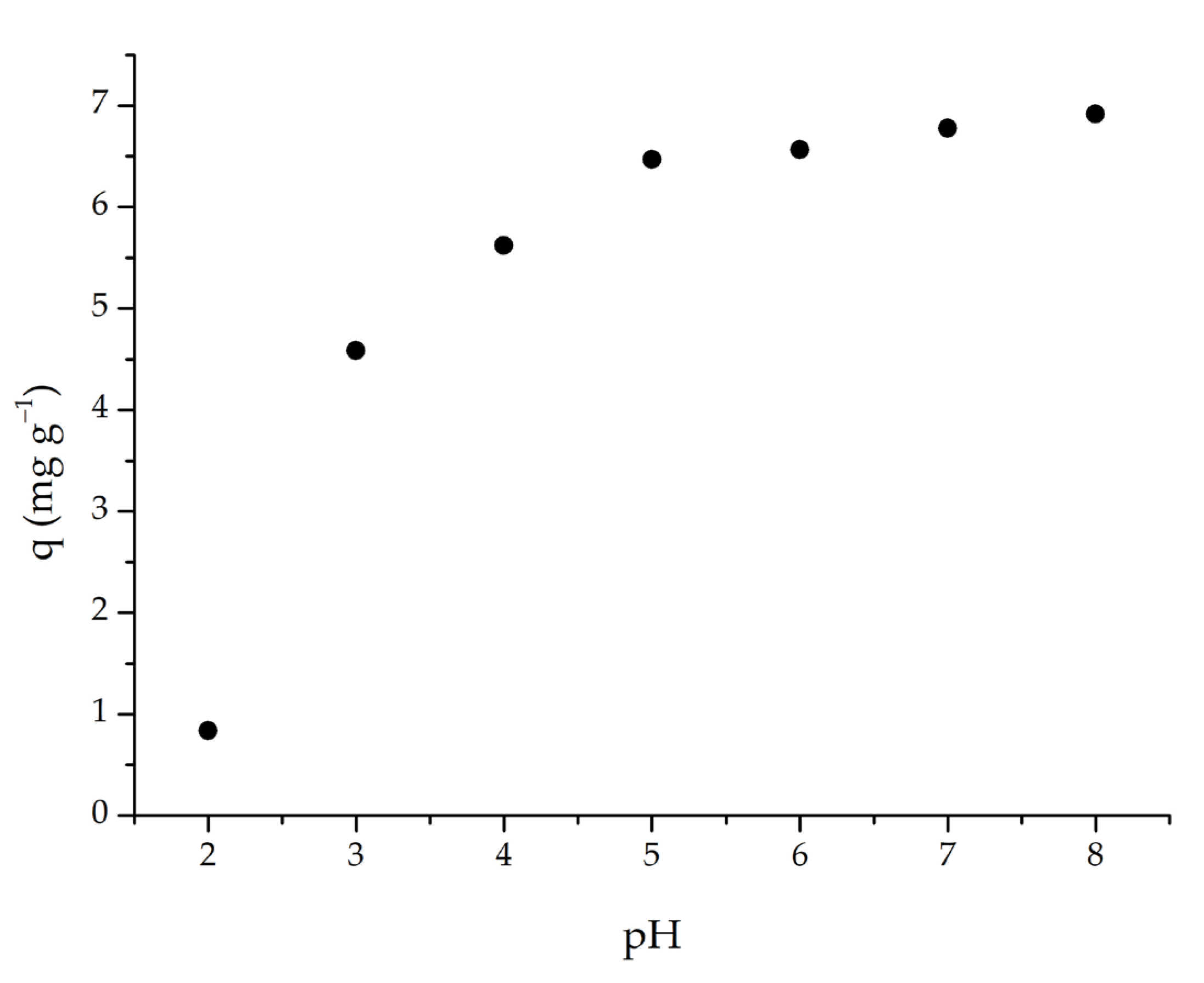
3.2. Batch System Adsorption Studies
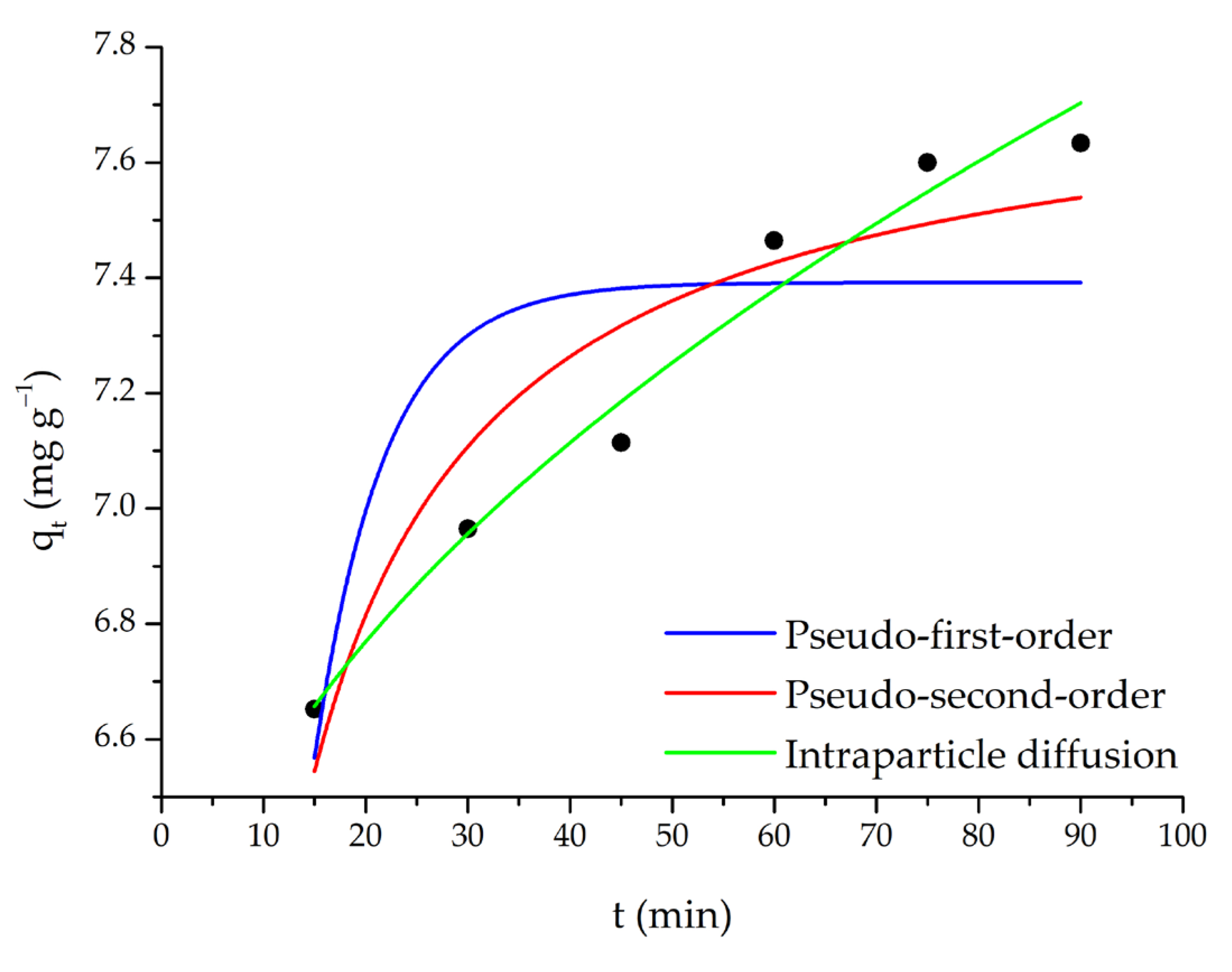
3.3. Kinetic Calculations
3.4. Isotherm Calculations
3.5. Real Wastewater Application
3.6. Desorption and Reusability
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| m-ECH-RC | Magnetic epichlorohydrin Rosa canina |
| SO | Safranin O |
| RC | Rosa canina |
| BET | Brunauer–Emmett–Teller |
| FT-IR | Fourier transform infrared spectroscopy |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
References
- Wu, J.; Yao, J.; Zhu, X.; Zuo, D.; Zhang, H.; Jiang, S.; Li, H. Synthesis and characterization of zwitterionic hydrogels with Ag@gC3N4 for adsorption-photocatalytic removal of methylene blue and methyl orange dyes. Adsorption 2025, 31, 14. [Google Scholar] [CrossRef]
- Liu, R.; Ji, W.; Wang, W.; Li, Y.; Yin, L.; Song, Y.; He, G. Innovative amorphous calcium carbonate for superior anionic dye adsorption towards near-zero discharge. Sep. Purif. Technol. 2025, 361, 131349. [Google Scholar] [CrossRef]
- Jassim, N.J.; Younis, F.H.; Alshamkhani, M.T. Adsorption of Safranin-O Dye onto Almond Shell Sustainable Activated Carbon: Identifying Key Process Factors and Their Effects. Eng. Rep. 2025, 7, e13121. [Google Scholar] [CrossRef]
- Hettige, A.I.; Mowjood, M.I.M. Reduction of colour in treated wastewater from textile ındustry using sawdusts as bio-sorbents. Trop. Agric. Res. 2015, 26, 666–676. [Google Scholar] [CrossRef]
- Niinimaki, K.; Peters, G.; Dahlbo, H.; Perry, P.; Rissanen, T.; Gwilt, A. The environmental price of fast fashion. Nat. Rev. Earth Environ. 2020, 1, 189–200. [Google Scholar] [CrossRef]
- Su, X.; Wang, X.; Ge, Z.; Bao, Z.; Lin, L.; Chen, Y.; Pillai, S.C. KOH-activated biochar and chitosan composites for efficient adsorption of industrial dye pollutants. Chem. Eng. J. 2024, 486, 150387. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Zhao, J.; Li, W.; Shi, Y.; Zheng, X.; Feng, Q.; Low, S.C.; Liu, Z. Biomimetic heat-localized and solar absorption-enhanced hollow structural nanofibrous membrane for clean water production from saline water and dye wastewater. J. Colloid Interface Sci. 2025, 678, 1112–1124. [Google Scholar] [CrossRef]
- Deng, X.; Wu, W.; Tian, S.; He, Y.; Wang, S.; Zheng, B.; Tang, L. Composite adsorbents of aminated chitosan@ ZIF-8 MOF for simultaneous efficient removal of Cu (II) and Congo Red: Batch experiments and DFT calculations. Chem. Eng. J. 2024, 479, 147634. [Google Scholar] [CrossRef]
- Rao, R.; Huang, Y.; Zhang, H.; Hu, C.; Dong, X.; Fang, W.; Ling, Q. A simple melamine-assisted cellulose pyrolysis synthesis of magnetic and mesoporous N-doped carbon composites with excellent adsorption of Congo red. Sep. Purif. Technol. 2024, 347, 127678. [Google Scholar] [CrossRef]
- Hussain, N.A.; Jasim, L.S. Synthesis and characterization of kappa (κ)-carrageenan-grafted poly (acrylic acid-co-itaconic acid)/ multi-walled carbon nanotube (κC-g-poly (AAC-co-IA)/MWCNT) composite for removing safranin-o dye from aqueous solution. Process Saf. Environ. Prot. 2025, 195, 106828. [Google Scholar] [CrossRef]
- Ozdemir, N.C.; Saleh, M.; Bilici, Z.; Arslan, H.; Dizge, N. Preparation of leonardite powder-embedded calcium alginate beads and adsorption of Safranin-O dye. Water Pract. Technol. 2023, 18, 1711–1726. [Google Scholar] [CrossRef]
- Phuong, D.T.M.; Loc, N.X. Rice Straw Biochar and Magnetic Rice Straw Biochar form Safranin O Adsorption from Aqueous Solution. Water 2022, 14, 186. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P.A. review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, H.R.; Sulaiman, N.M.N.; Hashim, N.A.; Hassan, C.R.C.; Ramli, M.R. Synthetic reactive dye wastewater treatment by using nano-membrane filtration. Desalin. Water Treat. 2015, 55, 86–95. [Google Scholar] [CrossRef]
- Yang, Z.; Asoh, T.A.; Uyama, H. Removal of cationic or anionic dyes from water using ion exchange cellulose monoliths as adsorbents. Bull. Chem. Soc. Jpn. 2019, 92, 1453–1461. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Asgher, M. Biosorption of reactive dyes: A review. Water Air Soil Pollut. 2012, 223, 2417–2435. [Google Scholar] [CrossRef]
- Sanroman, M.A.; Pazos, M.; Ricart, M.T.; Cameselle, C. Electrochemical decolourisation of structurally different dyes. Chemosphere 2004, 57, 233–239. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, C.; Zeng, X.; Shen, G.; Ye, Q.; Fu, H. Redox-copolymer-enhanced electrochemical catalysis membrane for efficient water decontamination. Chem. Eng. J. 2025, 505, 159383. [Google Scholar] [CrossRef]
- Ali, A.; Akram, A.; Siddique, A.B.; Amin, H.M.A.; Zohra, L.; Abbas, A.; Sher, M.; Hussain, M.A.; Haseeb, M.T.; Imran, M. A model batch and column study for Cd(II) uptake using citric acid cross-linked Salvia spinosa hydrogel: Optimization through Box-Behnken design. J. Ind. Eng. Chem. 2025, 151, 746–761. [Google Scholar] [CrossRef]
- Farid-ul-Haq, M.; Ali, A.; Hussain, M.A.; Abbas, A.; Kausar, F.; Amin, H.M.A.; Sher, M.; Hussain, S.Z.; Hussain, I. Chemical modification of a polysaccharide from Artemisia vulgaris engenders a supersorbent for the removal of Cd2+ from spiked high-hardness groundwater. Desalin. Water Treat. 2021, 212, 129–142. [Google Scholar] [CrossRef]
- Gharbi, A.H.; Laouini, S.E.; Hemmami, H.; Bouafia, A.; Gherbi, M.T.; Amor, I.B.; Hasan, G.G.; Abdullah, M.M.S.; Trzepiecinski, T.; Abdullah, J.A.A. Eco-friendly synthesis of Al2O3 nanoparticles: Comprehensive characterization properties, mechanics, and photocatalytic dye adsorption study. Coatings 2024, 14, 848. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Chang, H.; Xie, F.; Wu, R.; Zhou, S.; Zhu, X.; Liang, H.; Zhu, J. Triboelectric pulse promotes self-cleaning, catalysis ofnano-confined domain lamellar membranes for effectivewater decontamination. Adv. Funct. Mater. 2025, 35, 2418565. [Google Scholar] [CrossRef]
- Tzvetkov, G.; Kaneva, N.; Spassov, T. Room-temperature fabrication of core-shell nano-ZnO/pollen grain biocomposite for adsorptive removal of organic dye from water. Appl. Surf. Sci. 2017, 400, 481–491. [Google Scholar] [CrossRef]
- Tunali Akar, S.; Balk, Y.; Sayin, F.; Akar, T. Magnetically functionalized alunite as a recyclable and ecofriendly adsorbent for efficient removal of Pb2+. J. Water Process Eng. 2022, 48, 102867. [Google Scholar] [CrossRef]
- Mohamad, N.; Salleha, N.M.; Mahmud, H.N.M.E. Synthesis, characterization and analysis of conducting polymercomposites toward dye adsorption. Int. J. Polym. Anal. Charact. 2024, 29, 127–145. [Google Scholar] [CrossRef]
- Xin, Y.; Bai, Y.; Wu, X.; Zhang, D.; Ao, W.; Fang, M.; Huang, Z.; Yao, Y. Adsorption performance of modified graphite from synthetic dyes solutions. Materials 2024, 29, 4349. [Google Scholar] [CrossRef]
- Alam, Z.; Bari, N.; Kawsari, S. Statistical optimization of Methylene Blue dye removal from a synthetic textile wastewater using indigenous adsorbents. Environ. Sustain. Indic. 2022, 14, 100176. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Ghosh, S.; Islam, S.; Nice, S.; Islam, K.R.; Netema, B.N.; Rahman, S.; Habib, A.; Zaman, S.; Ghosh, G.C.; et al. Removal of hazardous textile dye from simulated wastewater by municipal organic solid waste charcoal using machine learning approaches: Kinetics, isotherm, and thermodynamics. Heliyon 2023, 9, e18856. [Google Scholar] [CrossRef]
- Chandra, T.C.; Mima, M.M.; Sudaryanto, Y.; Ismadji, S. Adsorption of basic dye onto activated carbon prepared from durian shell: Studies of adsorption equilibrium and kinetics. Chem. Eng. J. 2007, 127, 121–129. [Google Scholar] [CrossRef]
- Mirizadeh, S.; Solisio, C.; Converti, A.; Casazza, A.A. Efficient removal of tetracycline, ciprofloxacin, and amoxicillin by novel magnetic chitosan/microalgae biocomposites. Sep. Purif. Technol. 2024, 329, 125115. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Hanafiah, M.A.K.M.; Yong, S.S. Adsorption of humic acid from aqueous solutions on crosslinked chitosan–epichlorohydrin beads: Kinetics and isotherm studies. Colloids Surf. B Biointerfaces 2008, 65, 18–24. [Google Scholar] [CrossRef]
- Rashtbari, Y.; Arfaeinia, H.; Ahmadi, S.; Asl, F.B.; Afshin, S.; Poureshgh, Y.; Fazlzadeh, M. Potential of using green adsorbent of humic acid removal from aqueous solutions: Equilibrium, kinetics, thermodynamic and regeneration studies. J. Environ. Anal. Chem. 2020, 102, 5373–5390. [Google Scholar] [CrossRef]
- Salem, M.A.; Salem, I.A.; Zaki, H.M.; El-Sawy, A.M. Elimination of Safranin-O and a binary mixture of Safranin-O and methylene blue from water by adsorption on magnetite/Ag nanocomposite. Egypt. J. Pet. 2022, 31, 39–49. [Google Scholar] [CrossRef]
- Acelas, N.İ.; Lopera, S.M.; Porras, J.; Torres-Palma, R.A. Evaluating the removal of the antibiotic cephalexin from aqueous solutions using an adsorbent obtained from palm oil fiber. Molecules 2021, 26, 3340. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, N.; Hekmati, M.; Karmakar, B.; Veisi, H. Green synthesis of gold nanoparticles (Au NPs) using Rosa canina fruit extractand evaluation of its catalytic activity in the degradation of organic dye pollutants of water. Inorg. Chem. Commun. 2022, 139, 109351. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y. Methyl orange uptake from aqueous solutions using chitosan-modified with epichlorohydrin and copper (II) Schiff base. Int. J. Biol. Macromol. 2025, 310, 142408. [Google Scholar] [CrossRef]
- Ouerghemmi, S.; Sebei, H.; Siracusa, L.; Ruberto, G.; Saija, A.; Cimino, F.; Cristani, M. Comparative study of phenolic composition and antioxidant activity of leaf extracts from three wild Rosa species grown in different Tunisia regions: Rosa canina L., Rosa moschata Herrm. and Rosa sempervirens L. Ind. Crops Prod. 2016, 94, 167–177. [Google Scholar] [CrossRef]
- Ieri, F.; Innocenti, M.; Possieri, L.; Gallori, S.; Mulinacci, N. Phenolic composition of “bud extracts” of Ribes nigrum L., Rosa canina L. and Tilia tomentosa M. J. Pharm. Biomed. Anal. 2015, 115, 1–9. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Lou, T. Preparation of fibrous chitosan/sodium alginate composite foams for the adsorption of cationic and anionic dyes. J. Hazard. Mater. 2021, 403, 124054. [Google Scholar] [CrossRef]
- Jafari, H.; Mahdavinia, G.R.; Kazemi, B.; Ehrlich, H.; Joseph, Y.; Rahimi-Nasrabadi, M. Highly efficient sunitinib release from pH-responsive mHPMC@Chitosan core-shell nanoparticles. Carbohydr. Polym. 2021, 258, 117719. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Han, H.; Wang, X.; Zhou, L.; Yi, Z.; Fu, Z.; Wu, X.; Li, G.; Zeng, L. Optical and magnetic properties of small-size core–shell Fe3O4@C nanoparticles. Mater. Today Chem. 2021, 22, 100556. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Shi, G.; Yan, Y.; Wang, Q.; Yu, Z.; Yan, L.; Yu, Y. Adsorption of chlorinated hydrocarbons by different kinds of soils: Kinetics, influencing factors, mechanism. J. Hazard. Mater. 2025, 18, 100638. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Liu, F.; Yuan, Y.; Wu, H.; Li, A. Effects of ionic strength on removal of toxic pollutants from aqueous media with multifarious adsorbents: A review. Sci. Total Environ. 2019, 646, 265–279. [Google Scholar] [CrossRef]
- Li, X.; Zeng, L.; Zhu, L.; Jiang, H.; Liu, C.; Dai, Y. Strong adsorption of tetracycline on carbon blacks: An in-depth study of the adsorption mechanism. J. Water Process Eng. 2025, 70, 106784. [Google Scholar] [CrossRef]







| Analysis | Before Adsorption | After Adsorption |
|---|---|---|
| BET surface area (m2 g−1) | 4.92 | ~0 |
| Average pore radius (Å) | 29.07 | 486.39 |
| Total pore volume (cc g−1) | 0.28 | 7.15 10−3 |
| Nanocomposite | υ N−H/O−H | υ C−H | υ C=N υ C=C υ N−Hb* | υ C−Hb* | υ C−O |
|---|---|---|---|---|---|
| m-ECH-RC | 3360 | 2924 | 1652 | 1410 | 1064 |
| 3289 | 2876 | 1558 | 1376 | 1027 | |
| m-ECH-RC+SO | 3354 | 2923 | 1646 | 1455 | 1061 |
| 3288 | 2877 | 1592 | 1374 | 1026 |
| Pseudo-first-order | Kads1 (min−1) | qe (mg g−1) | R2 | χ2 |
| 0.146 | 7.39 | 0.613 | 0.0745 | |
| Pseudo-second-order | Kads2 (g mg−1 min−1) | qe (mg g−1) | R2 | χ2 |
| 0.0456 | 7.78 | 0.878 | 0.0236 | |
| Intraparticle diffusion | Kp (mg g−1 min−1/2) | C (mg g−1) | R2 | χ2 |
| 0.186 | 5.94 | 0.974 | 0.00499 |
| Langmuir | KL (L mg−1) | qmax (mg g−1) | R2 | χ² | |
| 0.432 | 12.6 | 0.977 | 0.369 | ||
| Freundlich | KF (L mg−1) | n | R2 | χ² | |
| 4.03 | 3.77 | 0.719 | 4.51 | ||
| D-R | qmax (mg g−1) | β (L mg−1) | ε (kJ mol−1) | R2 | χ² |
| 10.4 | 2.24 | 0.456 | 0.896 | 1.66 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceylan, T.; Satır, İ.T.; Akmeşe, B. Synthesis and Characterization of Rosa Canina-Fe3O4/Chitosan Nanocomposite and Treatment of Safranin O Dye from Wastewater. Water 2025, 17, 2894. https://doi.org/10.3390/w17192894
Ceylan T, Satır İT, Akmeşe B. Synthesis and Characterization of Rosa Canina-Fe3O4/Chitosan Nanocomposite and Treatment of Safranin O Dye from Wastewater. Water. 2025; 17(19):2894. https://doi.org/10.3390/w17192894
Chicago/Turabian StyleCeylan, Tugba, İlknur Tosun Satır, and Bediha Akmeşe. 2025. "Synthesis and Characterization of Rosa Canina-Fe3O4/Chitosan Nanocomposite and Treatment of Safranin O Dye from Wastewater" Water 17, no. 19: 2894. https://doi.org/10.3390/w17192894
APA StyleCeylan, T., Satır, İ. T., & Akmeşe, B. (2025). Synthesis and Characterization of Rosa Canina-Fe3O4/Chitosan Nanocomposite and Treatment of Safranin O Dye from Wastewater. Water, 17(19), 2894. https://doi.org/10.3390/w17192894






