Distribution and Environmental Implications of GDGTs in Sediments from Three Asian Mangrove Wetlands
Abstract
1. Introduction
2. Materials and Methods
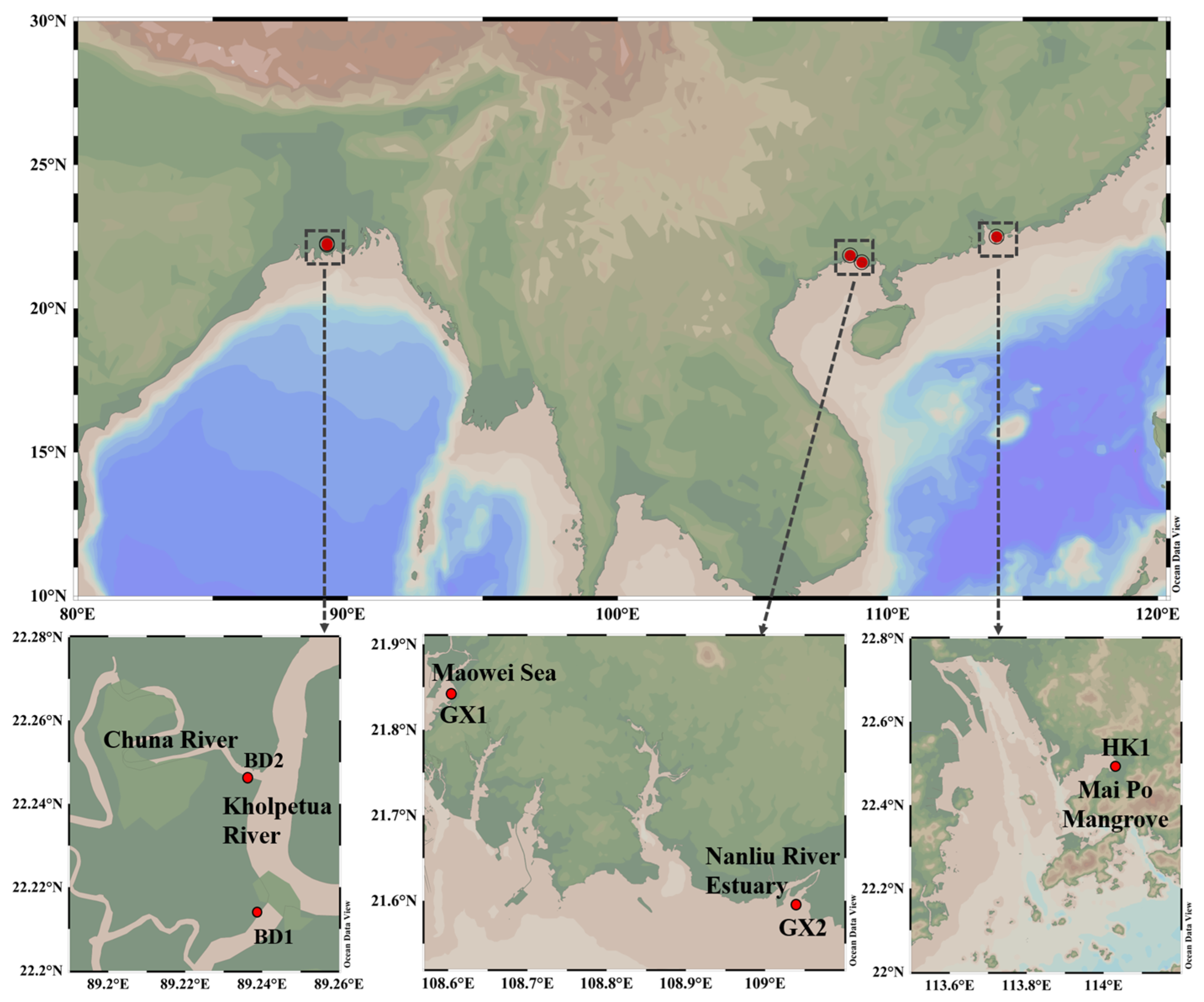
2.1. Study Area and Sampling
2.2. Environmental Parameter Measurement
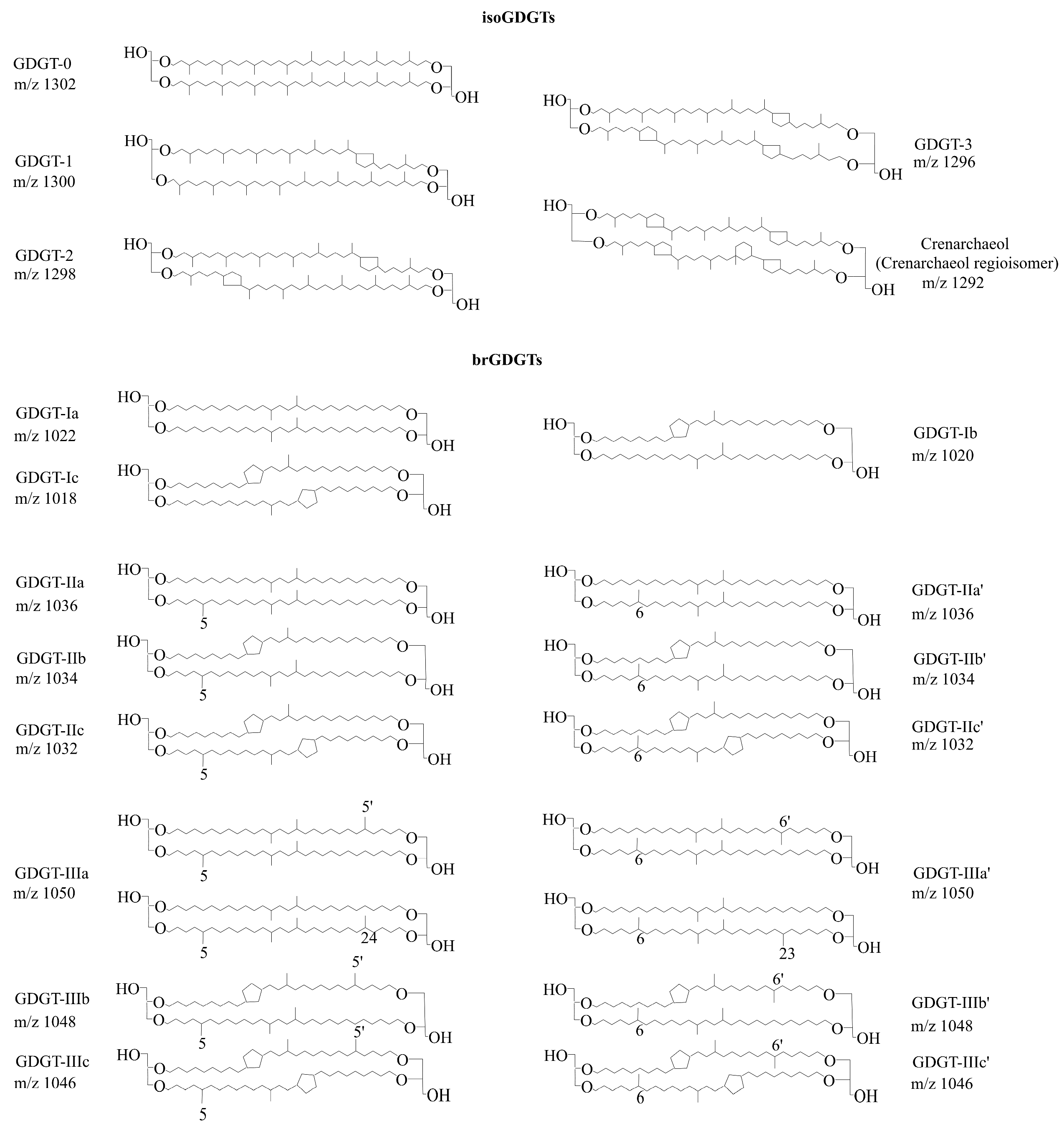
2.3. Extraction and Analysis of GDGTs
2.4. GDGTs-Derived Indices
2.5. DNA Extraction and Sequencing
2.6. Statistical Analysis
3. Results
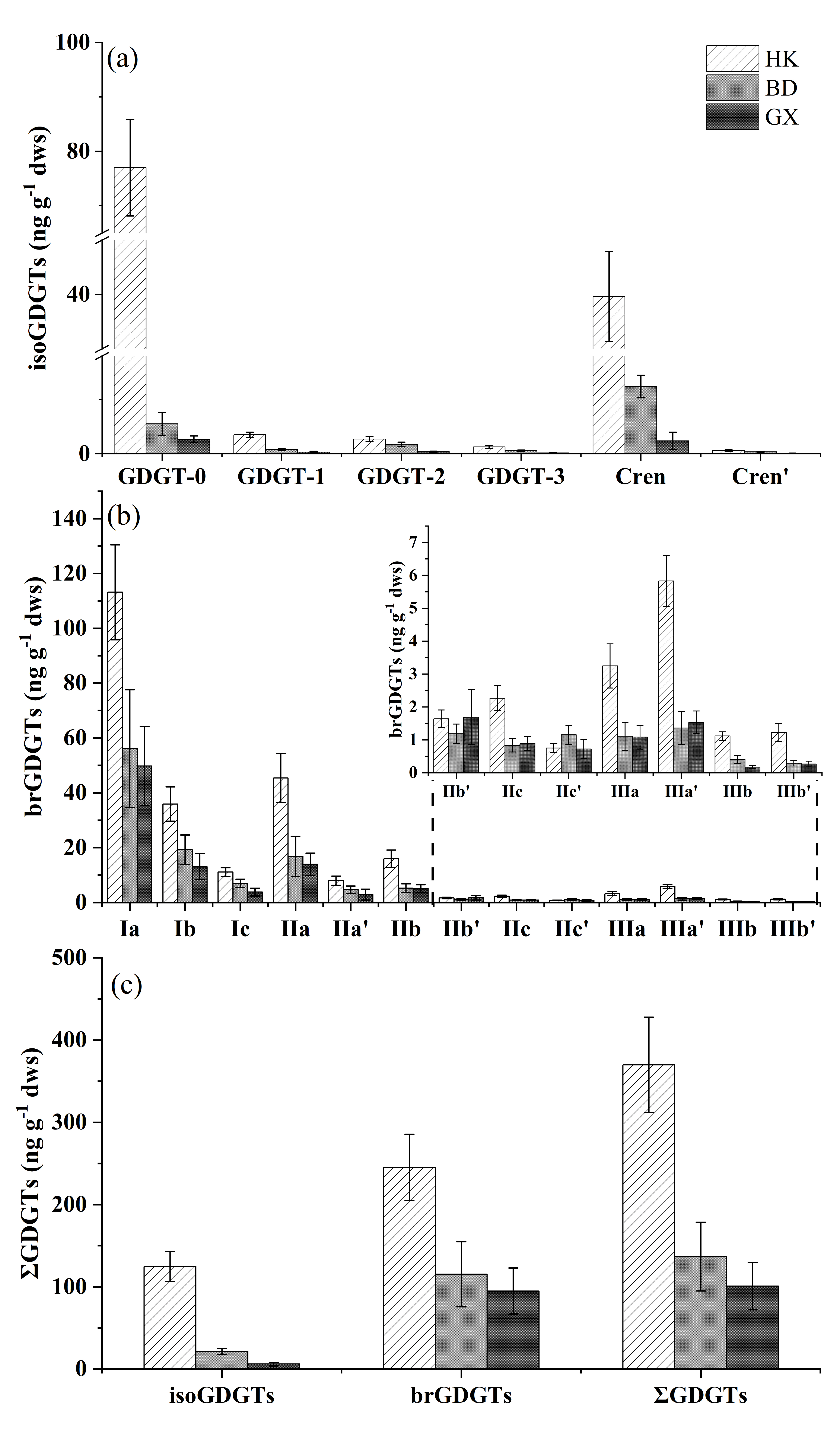
3.1. Concentration and Composition of GDGTs in Surface Sediments
3.2. Depth Distributions of TOC and GDGTs in Core Sediments
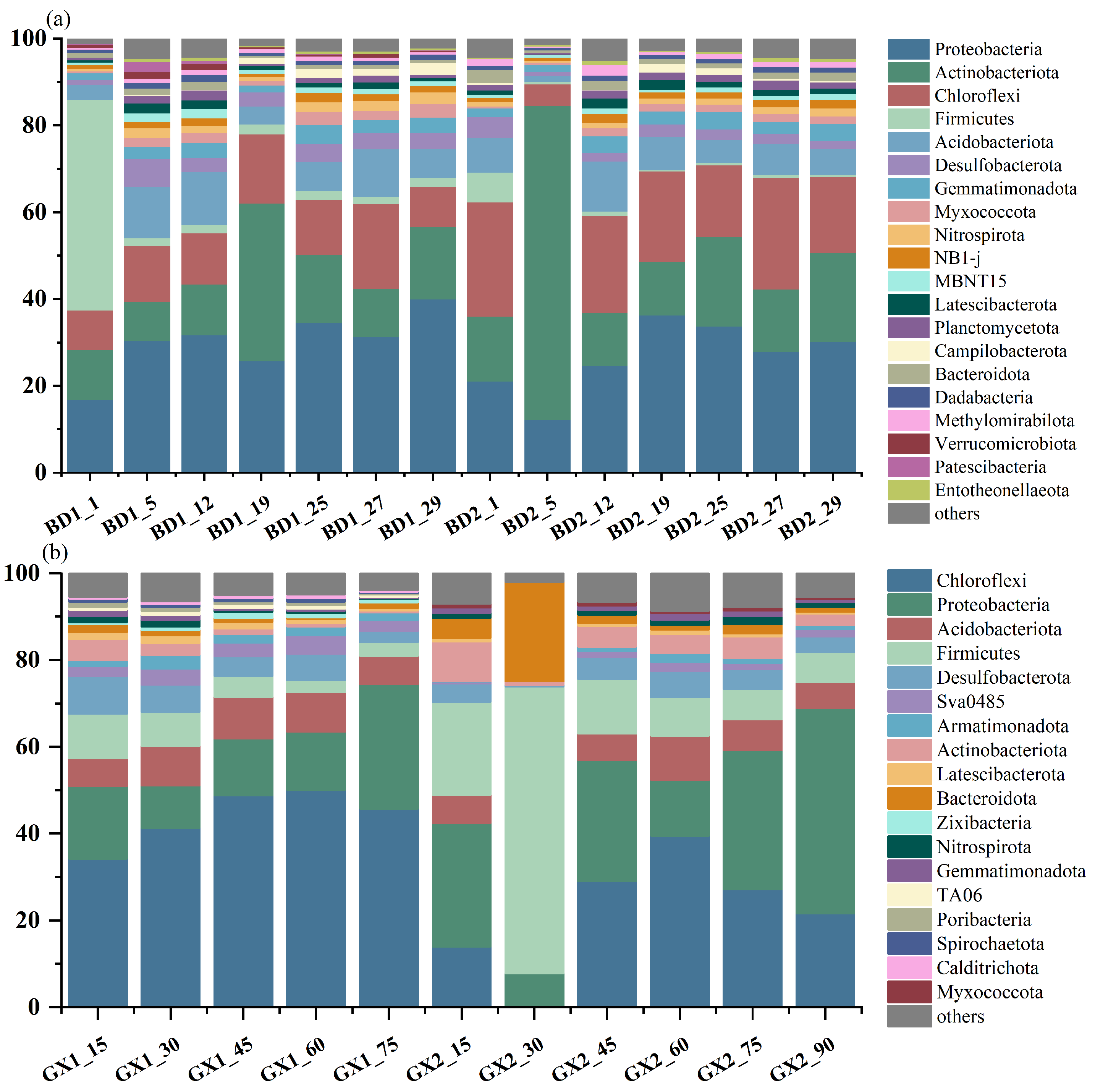
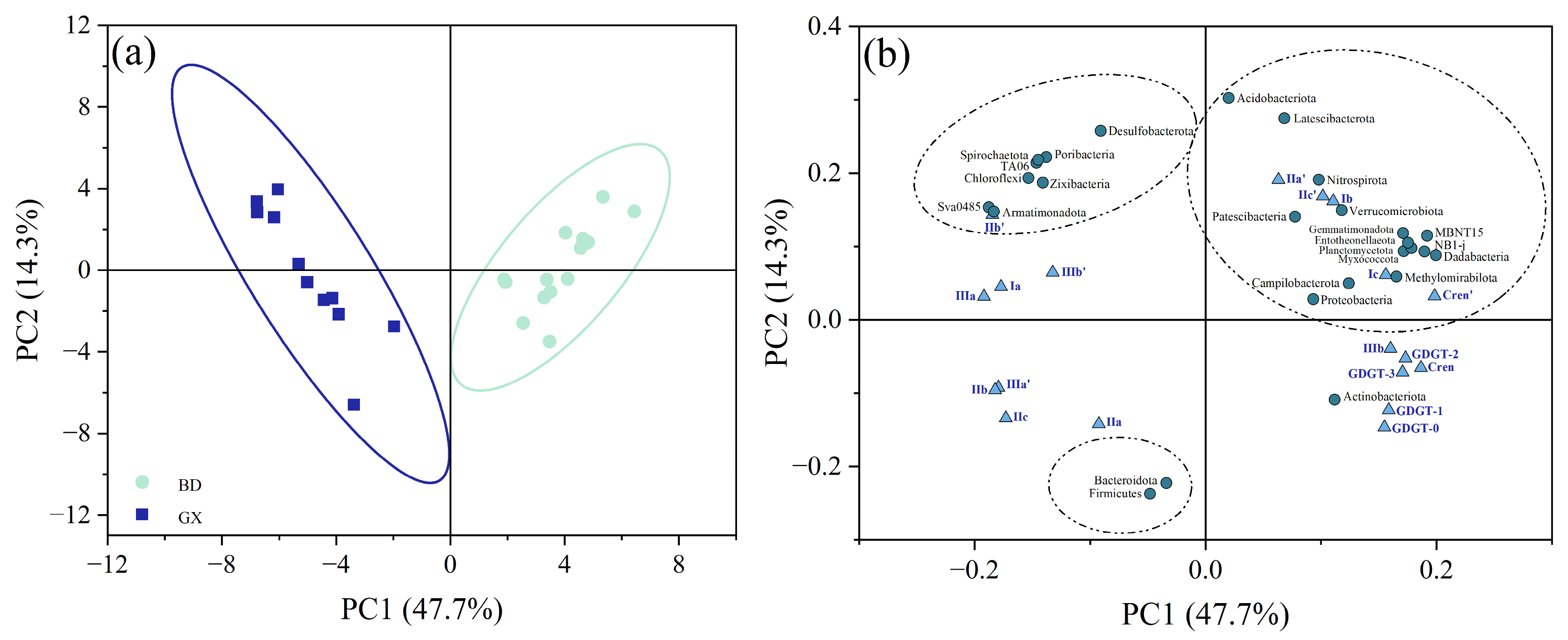
3.3. Bacterial Communities in Mangrove Sediments
3.4. pH and Temperature
4. Discussion
4.1. Source of GDGTs in Sediments from Three Mangrove Wetlands
4.2. Heterogenous Distributions of GDGTs in Three Mangrove Wetlands
4.3. GDGTs-Based Temperature and pH Indices in Mangrove Sediments
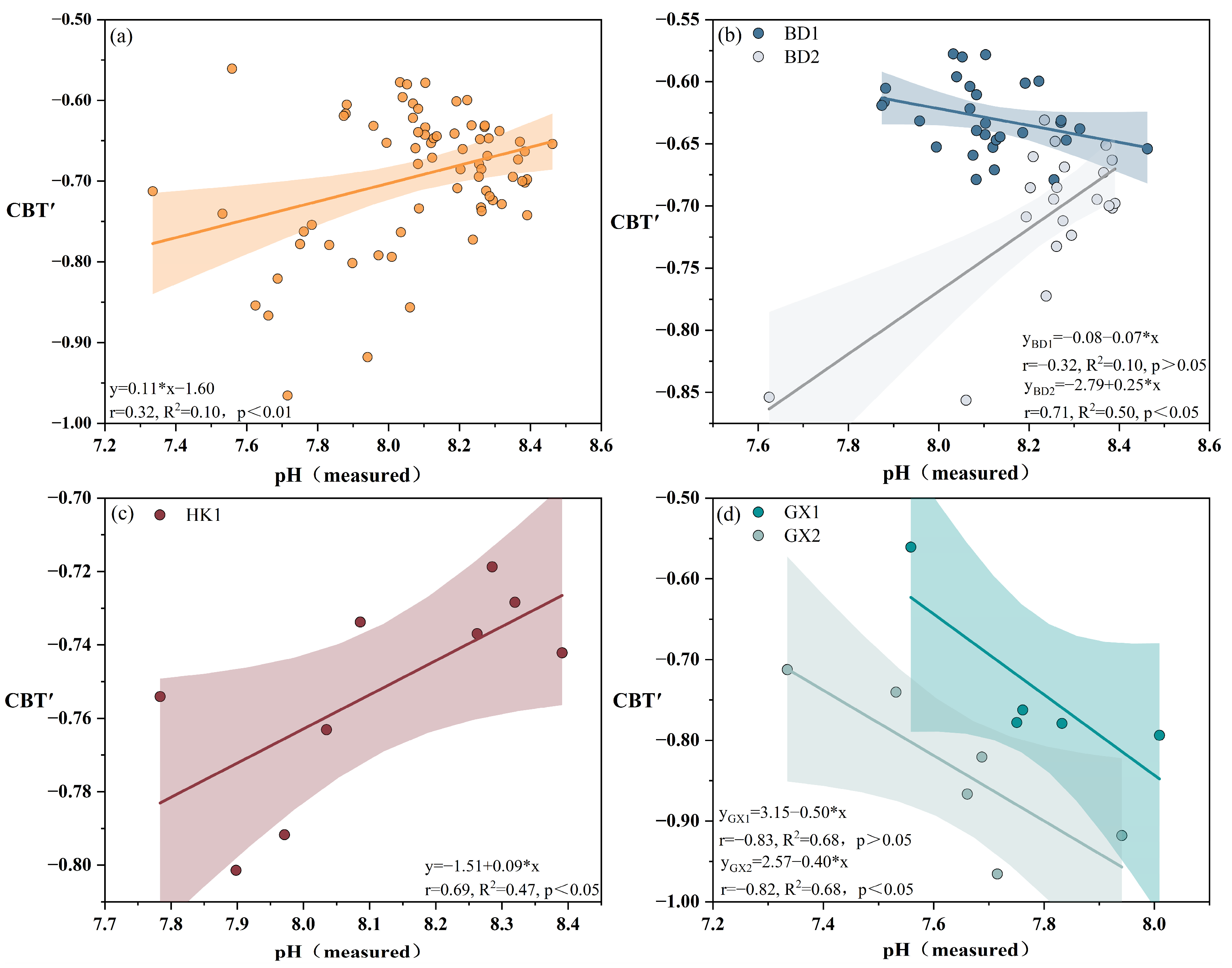
4.3.1. pH Reconstruction Based on CBT’
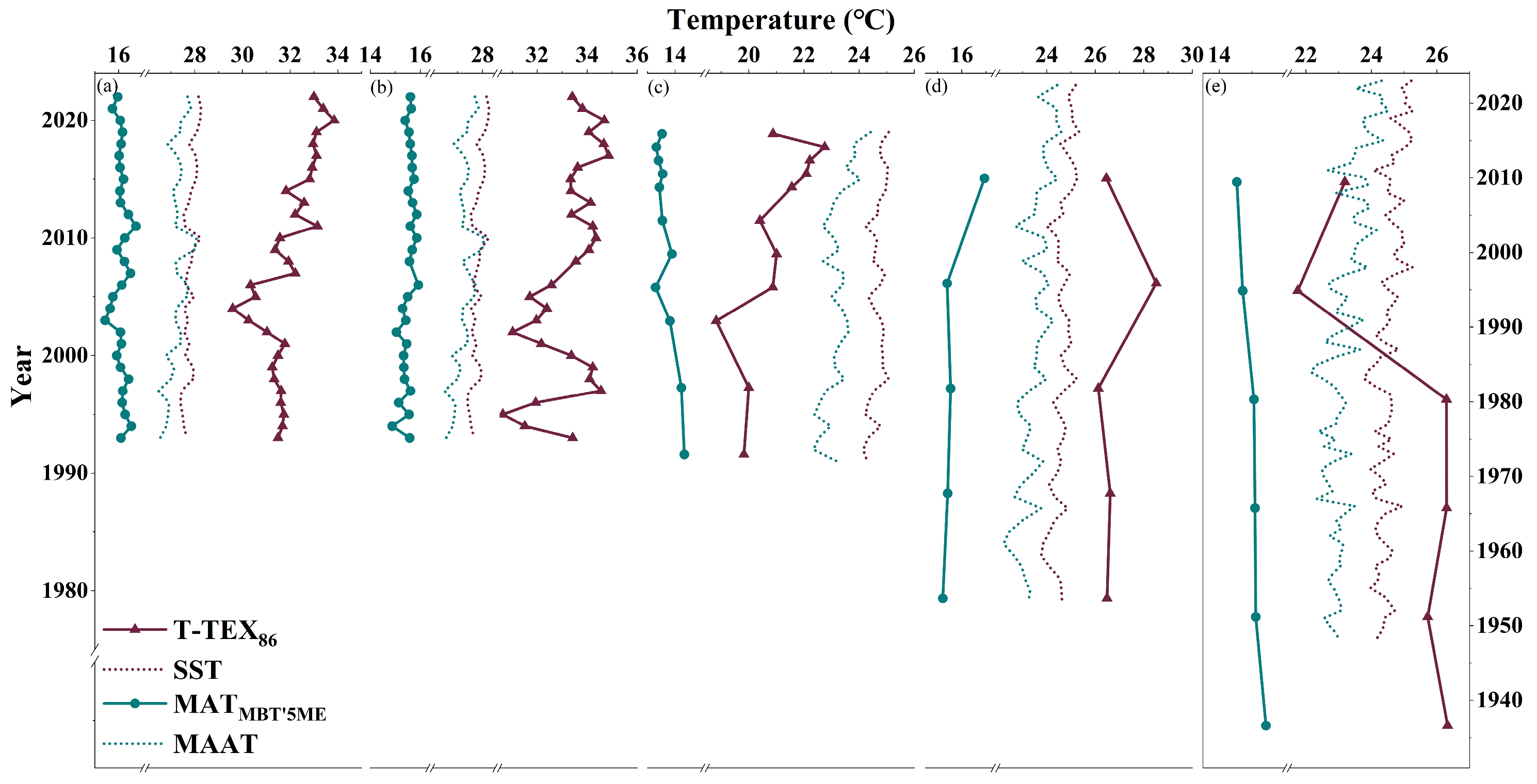
4.3.2. Temperature Based on MBT’5ME
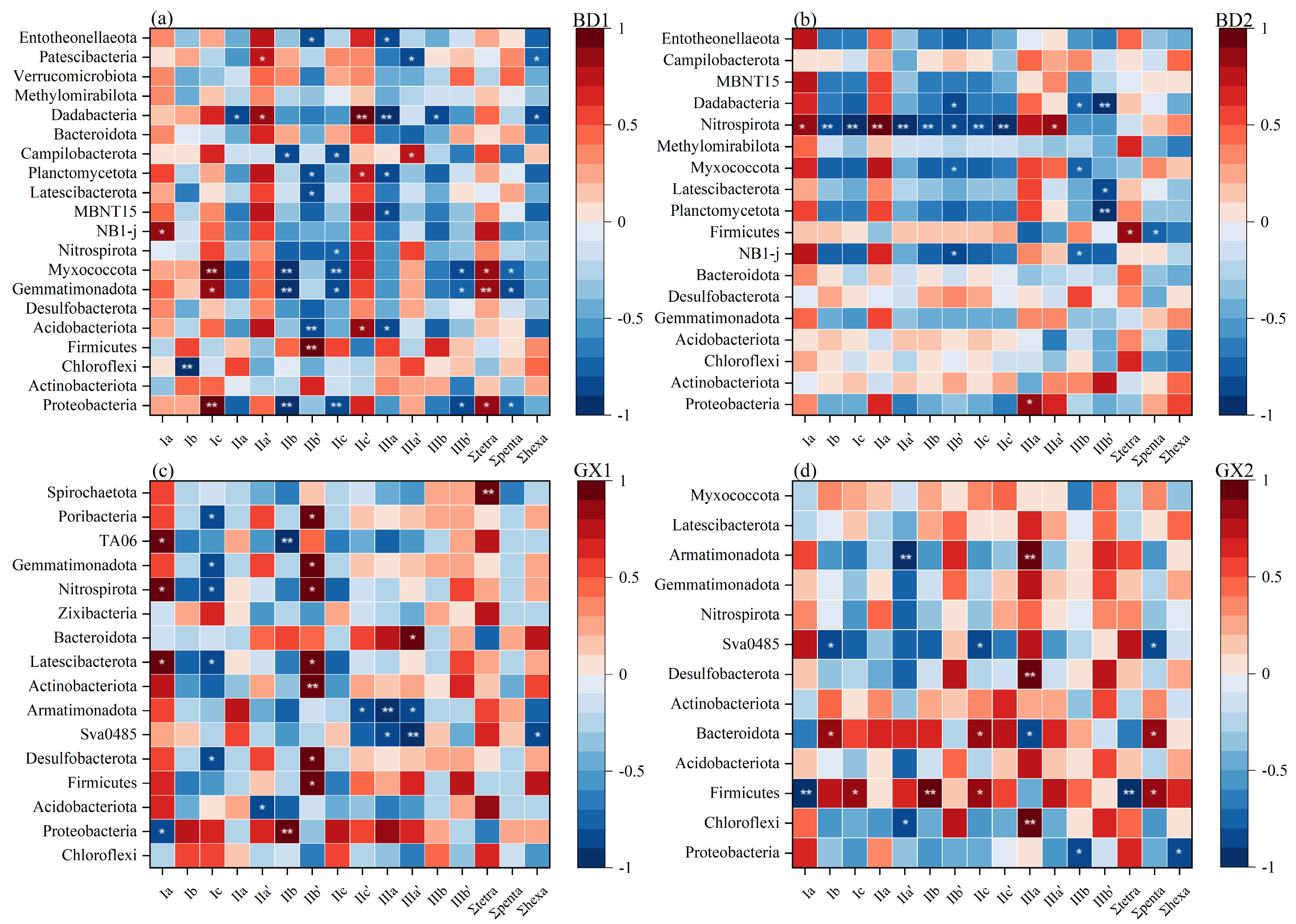
4.4. Effect of Bacterial Community on brGDGTs in Mangrove Sediments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schouten, S.; Hopmans, E.C.; Sinninghe Damsté, J.S. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review. Org. Geochem. 2013, 54, 19–61. [Google Scholar] [CrossRef]
- Lloyd, C.T.; Iwig, D.F.; Wang, B.; Cossu, M.; Metcalf, W.W.; Boal, A.K.; Booker, S.J. Discovery, structure and mechanism of a tetraether lipid synthase. Nature 2022, 609, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Weijers, J.W.H.; Schouten, S.; van den Donker, J.C.; Hopmans, E.C.; Sinninghe Damsté, J.S. Environmental controls on bacterial tetraether membrane lipid distribution in soils. Geochim. Cosmochim. Acta 2007, 71, 703–713. [Google Scholar] [CrossRef]
- Kim, J.-H.; van der Meer, J.; Schouten, S.; Helmke, P.; Willmott, V.; Sangiorgi, F.; Koç, N.; Hopmans, E.C.; Sinninghe Damsté, J.S. New indices and calibrations derived from the distribution of crenarchaeal isoprenoid tetraether lipids: Implications for past sea surface temperature reconstructions. Geochim. Cosmochim. Acta 2010, 74, 4639–4654. [Google Scholar] [CrossRef]
- Boyd, E.S.; Pearson, A.; Pi, Y.; Li, W.-J.; Zhang, Y.G.; He, L.; Zhang, C.L.; Geesey, G.G. Temperature and pH controls on glycerol dibiphytanyl glycerol tetraether lipid composition in the hyperthermophilic crenarchaeon Acidilobus sulfurireducens. Extremophiles 2011, 15, 59–65. [Google Scholar] [CrossRef]
- De Jonge, C.; Guo, J.; Hällberg, P.; Griepentrog, M.; Rifai, H.; Richter, A.; Ramirez, E.; Zhang, X.; Smittenberg, R.H.; Peterse, F.; et al. The impact of soil chemistry, moisture and temperature on branched and isoprenoid GDGTs in soils: A study using six globally distributed elevation transects. Org. Geochem. 2024, 187, 104706. [Google Scholar] [CrossRef]
- Lattaud, J.; Gondwe, M.J.; Griepentrog, M.; Helfter, C.; De Jonge, C. Soil chemistry effect on GDGT abundances and their proxies in soils of the Okavango Delta. Org. Geochem. 2024, 195, 104847. [Google Scholar] [CrossRef]
- Schouten, S.; Hopmans, E.C.; Schefuß, E.; Sinninghe Damsté, J.S. Distributional variations in marine crenarchaeotal membrane lipids: A new tool for reconstructing ancient sea water temperatures? Earth Planet. Sci. Lett. 2002, 204, 265–274. [Google Scholar] [CrossRef]
- Peterse, F.; van der Meer, J.; Schouten, S.; Weijers, J.W.H.; Fierer, N.; Jackson, R.B.; Kim, J.-H.; Sinninghe Damsté, J.S. Revised calibration of the MBT–CBT paleotemperature proxy based on branched tetraether membrane lipids in surface soils. Geochim. Cosmochim. Acta 2012, 96, 215–229. [Google Scholar] [CrossRef]
- Hopmans, E.C.; Weijers, J.W.H.; Schefuß, E.; Herfort, L.; Sinninghe Damsté, J.S.; Schouten, S. A novel proxy for terrestrial organic matter in sediments based on branched and isoprenoid tetraether lipids. Earth Planet. Sci. Lett. 2004, 224, 107–116. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Zhou, S.; Hu, L.; Yang, H.; Xu, Y. Ubiquitous production of branched glycerol dialkyl glycerol tetraethers (brGDGTs) in global marine environments: A new source indicator for brGDGTs. Biogeosciences 2016, 13, 5883–5894. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhang, C.L.; Liu, X.-L.; Li, L.; Hinrichs, K.-U.; Noakes, J.E. Methane Index: A tetraether archaeal lipid biomarker indicator for detecting the instability of marine gas hydrates. Earth Planet. Sci. Lett. 2011, 307, 525–534. [Google Scholar] [CrossRef]
- Kim, B.; Zhang, Y.G. Methane Index: Towards a quantitative archaeal lipid biomarker proxy for reconstructing marine sedimentary methane fluxes. Geochim. Cosmochim. Acta 2023, 354, 74–87. [Google Scholar] [CrossRef]
- Yang, H.; Pancost, R.D.; Dang, X.; Zhou, X.; Evershed, R.P.; Xiao, G.; Tang, C.; Gao, L.; Guo, Z.; Xie, S. Correlations between microbial tetraether lipids and environmental variables in Chinese soils: Optimizing the paleo-reconstructions in semi-arid and arid regions. Geochim. Cosmochim. Acta 2014, 126, 49–69. [Google Scholar] [CrossRef]
- Dang, X.; Yang, H.; Naafs, B.D.A.; Pancost, R.D.; Xie, S. Evidence of moisture control on the methylation of branched glycerol dialkyl glycerol tetraethers in semi-arid and arid soils. Geochim. Cosmochim. Acta 2016, 189, 24–36. [Google Scholar] [CrossRef]
- Zell, C.; Kim, J.-H.; Hollander, D.; Lorenzoni, L.; Baker, P.; Silva, C.G.; Nittrouer, C.; Sinninghe Damsté, J.S. Sources and distributions of branched and isoprenoid tetraether lipids on the Amazon shelf and fan: Implications for the use of GDGT-based proxies in marine sediments. Geochim. Cosmochim. Acta 2014, 139, 293–312. [Google Scholar] [CrossRef]
- Umoh, U.U.; Li, L.; Wang, J.; Kauluma, N.; Asuquo, F.E.; Akpan, E.R. Glycerol dialkyl glycerol tetraether signatures in tropical mesotidal estuary sediments of Qua Iboe River, Gulf of Guinea. Org. Geochem. 2022, 170, 104461. [Google Scholar] [CrossRef]
- Baxter, A.J.; van Bree, L.G.J.; Peterse, F.; Hopmans, E.C.; Villanueva, L.; Verschuren, D.; Sinninghe Damsté, J.S. Seasonal and multi-annual variation in the abundance of isoprenoid GDGT membrane lipids and their producers in the water column of a meromictic equatorial crater lake (Lake Chala, East Africa). Quat. Sci. Rev. 2021, 273, 107263. [Google Scholar] [CrossRef]
- Tierney, J.E.; Russell, J.M. Distributions of branched GDGTs in a tropical lake system: Implications for lacustrine application of the MBT/CBT paleoproxy. Org. Geochem. 2009, 40, 1032–1036. [Google Scholar] [CrossRef]
- Powers, L.; Werne, J.P.; Vanderwoude, A.J.; Sinninghe Damsté, J.S.; Hopmans, E.C.; Schouten, S. Applicability and calibration of the TEX86 paleothermometer in lakes. Org. Geochem. 2010, 41, 404–413. [Google Scholar] [CrossRef]
- Zheng, Y.; Pancost, R.D.; Naafs, B.D.A.; Li, Q.; Liu, Z.; Yang, H. Transition from a warm and dry to a cold and wet climate in NE China across the Holocene. Earth Planet. Sci. Lett. 2018, 493, 36–46. [Google Scholar] [CrossRef]
- Baker, A.; Blyth, A.J.; Jex, C.N.; McDonald, J.A.; Woltering, M.; Khan, S.J. Glycerol dialkyl glycerol tetraethers (GDGT) distributions from soil to cave: Refining the speleothem paleothermometer. Org. Geochem. 2019, 136, 103890. [Google Scholar] [CrossRef]
- Zech, R.; Gao, L.; Tarozo, R.; Huang, Y. Branched glycerol dialkyl glycerol tetraethers in Pleistocene loess-paleosol sequences: Three case studies. Org. Geochem. 2012, 53, 38–44. [Google Scholar] [CrossRef]
- Pan, A.; Yang, Q.; Zhou, H.; Ji, F.; Wang, H.; Pancost, R.D. A diagnostic GDGT signature for the impact of hydrothermal activity on surface deposits at the Southwest Indian Ridge. Org. Geochem. 2016, 99, 90–101. [Google Scholar] [CrossRef]
- Zhang, T.; He, W.; Liang, Q.; Zheng, F.; Xiao, X.; Zeng, Z.; Zhou, J.; Yao, W.; Chen, H.; Zhu, Y.; et al. Lipidomic diversity and proxy implications of archaea from cold seep sediments of the South China Sea. Front. Microbiol. 2023, 14, 1241958. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Kathiresan, K.; MubarakAli, D.; Kayalvizhi, K.; Rajendran, N.; Hemalatha, S.; Chen, J. Soil-microbial communities indexing from mangroves rhizosphere and barren sandy habitats. Physiol. Mol. Plant Pathol. 2018, 104, 58–68. [Google Scholar] [CrossRef]
- Wei, P.; Lei, A.; Zhou, H.; Hu, Z.; Wong, Y.; Tam, N.F.Y.; Lu, Q. Comparison of microbial community structure and function in sediment between natural regenerated and original mangrove forests in a National Nature Mangrove Reserve, South China. Mar. Pollut. Bull. 2021, 163, 111955. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dai, Z.; Yuan, R.; Guo, Z.; Xi, H.; He, Z.; Wei, M. Effects of Salinity on Assembly Characteristics and Function of Microbial Communities in the Phyllosphere and Rhizosphere of Salt-Tolerant Avicennia marina Mangrove Species. Microbiol. Spectr. 2023, 11, e03000-22. [Google Scholar] [CrossRef]
- Huguet, C.; Hopmans, E.C.; Febo-Ayala, W.; Thompson, D.H.; Sinninghe Damsté, J.S.; Schouten, S. An improved method to determine the absolute abundance of glycerol dibiphytanyl glycerol tetraether lipids. Org. Geochem. 2006, 37, 1036–1041. [Google Scholar] [CrossRef]
- Kim, J.-H.; Schouten, S.; Hopmans, E.C.; Donner, B.; Sinninghe Damsté, J.S. Global sediment core-top calibration of the TEX86 paleothermometer in the ocean. Geochim. Cosmochim. Acta 2008, 72, 1154–1173. [Google Scholar] [CrossRef]
- De Jonge, C.; Hopmans, E.C.; Zell, C.I.; Kim, J.-H.; Schouten, S.; Sinninghe Damsté, J.S. Occurrence and abundance of 6-methyl branched glycerol dialkyl glycerol tetraethers in soils: Implications for palaeoclimate reconstruction. Geochim. Cosmochim. Acta 2014, 141, 97–112. [Google Scholar] [CrossRef]
- Blaga, C.I.; Reichart, G.-J.; Heiri, O.; Sinninghe Damsté, J.S. Tetraether membrane lipid distributions in water-column particulate matter and sediments: A study of 47 European lakes along a north–south transect. J. Paleolimnol. 2008, 41, 523–540. [Google Scholar] [CrossRef]
- Dai, T.; Wen, D.; Bates, C.T.; Wu, L.; Guo, X.; Liu, S.; Su, Y.; Lei, J.; Zhou, J.; Yang, Y. Nutrient supply controls the linkage between species abundance and ecological interactions in marine bacterial communities. Nat. Commun. 2022, 13, 175. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- STACKEBRANDT, E.; GOEBEL, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.; Liu, X.; Xu, C.; Song, J.; Li, X.; Yuan, H.; Li, N.; Wang, D.; Yuan, H.; Ye, S. The origins and implications of glycerol ether lipids in China coastal wetland sediments. Sci. Rep. 2019, 9, 18529. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Liu, Y.; Zhang, X.; Shi, L.; Xu, Y. Predominance of hexamethylated 6-methyl branched glycerol dialkyl glycerol tetraethers in the Mariana Trench: Source and environmental implication. Biogeosciences 2020, 17, 2135–2148. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, W.; Wu, J.; Han, L.; Zhang, H.; Xu, Y. Source, composition, and distributional pattern of branched tetraethers in sediments of northern Chinese marginal seas. Org. Geochem. 2021, 157, 104244. [Google Scholar] [CrossRef]
- Raberg, J.H.; Miller, G.H.; Geirsdóttir, Á.; Sepúlveda, J. Near-universal trends in brGDGT lipid distributions in nature. Am. Assoc. Adv. Sci. 2022, 8, eabm7625. [Google Scholar] [CrossRef]
- Hällberg, P.L.; Schenk, F.; Jarne-Bueno, G.; Schankat, Y.; Zhang, Q.; Rifai, H.; Phua, M.; Smittenberg, R.H. Branched GDGT source shift identification allows improved reconstruction of an 8,000-year warming trend on Sumatra. Org. Geochem. 2023, 186, 104702. [Google Scholar] [CrossRef]
- Mei, X.; Wang, Z.; Xiong, W.; Zhang, Y.; Dou, Y.; Huang, L.; Mi, B.; Wang, Z.; Cong, J. Glycerol dialkyl glycerol tetraethers in surficial marine sediments across shelf-to-basin depth transects in the East China Sea: Their sources and potential as sea water temperature proxies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 636, 111992. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; He, Y.; Zhou, A.; Zhao, H.; Liu, H.; Cao, Y.; Hu, J.; Meng, B.; Jiang, J.; et al. Salinity-controlled isomerization of lacustrine brGDGTs impacts the associated MBT5ME’ terrestrial temperature index. Geochim. Cosmochim. Acta 2021, 305, 33–48. [Google Scholar] [CrossRef]
- Davtian, N.; Ménot, G.; Bard, E.; Poulenard, J.; Podwojewski, P. Consideration of soil types for the calibration of molecular proxies for soil pH and temperature using global soil datasets and Vietnamese soil profiles. Org. Geochem. 2016, 101, 140–153. [Google Scholar] [CrossRef]
- Lipp, J.S.; Morono, Y.; Inagaki, F.; Hinrichs, K.-U. Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature 2008, 454, 991–994. [Google Scholar] [CrossRef]
- Rossel, P.E.; Elvert, M.; Ramette, A.; Boetius, A.; Hinrichs, K.-U. Factors controlling the distribution of anaerobic methanotrophic communities in marine environments: Evidence from intact polar membrane lipids. Geochim. Cosmochim. Acta 2011, 75, 164–184. [Google Scholar] [CrossRef]
- Naafs, B.D.A.; Inglis, G.N.; Zheng, Y.; Amesbury, M.J.; Biester, H.; Bindler, R.; Blewett, J.; Burrows, M.A.; del Castillo Torres, D.; Chambers, F.M.; et al. Introducing global peat-specific temperature and pH calibrations based on brGDGT bacterial lipids. Geochim. Cosmochim. Acta 2017, 208, 285–301. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, F.; Yang, H.; Yang, W.; Wu, R.; Liu, X.; Liang, H.; Chen, H.; Pei, H.; Zhang, C.; et al. The production of diverse brGDGTs by an Acidobacterium providing a physiological basis for paleoclimate proxies. Geochim. Cosmochim. Acta 2022, 337, 155–165. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Li, J.; Meng, X.; Liu, L.; Li, C. Pollen Assemblage and sedimentary environment evolution in the coastal zone in Qinzhou Bay, Guangxi Province. Quat. Res. 2010, 30, 598–608, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Yue, Y. Response of mangrove swamp sedimentation rate to climate change. J. Trop. Oceanogr. 2017, 36, 40–47. [Google Scholar] [CrossRef]
- Lei, Y.; Yan, H.; Zhong, C.; Feng, P.; Jiang, S. Regime shift recorded by sediments from the Futian mangrove ecosystem in the Shenzhen Bay. Acta Ecol. Sin. 2020, 40, 8539–8548. [Google Scholar] [CrossRef]
- Rogers, K.G.; Goodbred, S.L.; Mondal, D.R. Monsoon sedimentation on the ‘abandoned’ tide-influenced Ganges—Brahmaputra delta plain. Estuar. Coast. Shelf Sci. 2013, 131, 297–309. [Google Scholar] [CrossRef]
- Weijers, J.W.H.; Schouten, S.; Spaargaren, O.C.; Sinninghe Damsté, J.S. Occurrence and distribution of tetraether membrane lipids in soils: Implications for the use of the TEX86 proxy and the BIT index. Org. Geochem. 2006, 37, 1680–1693. [Google Scholar] [CrossRef]
- Knight, J.M.; Griffin, L.; Dale, P.E.R.; Sheaves, M. Short-term dissolved oxygen patterns in sub-tropical mangroves. Estuar. Coast. Shelf Sci. 2013, 131, 290–296. [Google Scholar] [CrossRef]
- Mattone, C.; Sheaves, M. Patterns, drivers and implications of dissolved oxygen dynamics in tropical mangrove forests. Estuar. Coast. Shelf Sci. 2017, 197, 205–213. [Google Scholar] [CrossRef]
- Qin, W.; Carlson, L.T.; Armbrust, E.V.; Devol, A.H.; Moffett, J.W.; Stahl, D.A.; Ingalls, A.E. Confounding effects of oxygen and temperature on the TEX86 signature of marine Thaumarchaeota. Proc. Natl. Acad. Sci. USA 2015, 112, 10979–10984. [Google Scholar] [CrossRef]
- Halamka, T.A.; Raberg, J.H.; McFarlin, J.M.; Younkin, A.D.; Mulligan, C.; Liu, X.L.; Kopf, S.H. Production of diverse brGDGTs by Acidobacterium Solibacter usitatus in response to temperature, pH, and O2 provides a culturing perspective on brGDGT proxies and biosynthesis. Geobiology 2023, 21, 102–118. [Google Scholar] [CrossRef]
- Naafs, B.D.A.; Oliveira, A.S.F.; Mulholland, A.J. Molecular dynamics simulations support the hypothesis that the brGDGT paleothermometer is based on homeoviscous adaptation. Geochim. Cosmochim. Acta 2021, 312, 44–56. [Google Scholar] [CrossRef]
- Sinninghe Damsté, J.S.; Rijpstra, W.I.; Hopmans, E.C.; Weijers, J.W.; Foesel, B.U.; Overmann, J.; Dedysh, S.N. 13,16-Dimethyl octacosanedioic acid (iso-diabolic acid), a common membrane-spanning lipid of Acidobacteria subdivisions 1 and 3. Appl. Environ. Microbiol. 2011, 77, 4147–4154. [Google Scholar] [CrossRef] [PubMed]
- Sinninghe Damsté, J.S.; Rijpstra, W.I.C.; Foesel, B.U.; Huber, K.J.; Overmann, J.; Nakagawa, S.; Kim, J.J.; Dunfield, P.F.; Dedysh, S.N.; Villanueva, L. An overview of the occurrence of ether- and ester-linked iso-diabolic acid membrane lipids in microbial cultures of the Acidobacteria: Implications for brGDGT paleoproxies for temperature and pH. Org. Geochem. 2018, 124, 63–76. [Google Scholar] [CrossRef]
- Guo, J.; Ma, T.; Liu, N.; Zhang, X.; Hu, H.; Ma, W.; Wang, Z.; Feng, X.; Peterse, F. Soil pH and aridity influence distributions of branched tetraether lipids in grassland soils along an aridity transect. Org. Geochem. 2022, 164, 104347. [Google Scholar] [CrossRef]



| Symbol | Definition | Corresponding Equations |
|---|---|---|
| Summation operator, representing the sum of relative abundances of the listed GDGT compounds (e.g., ) | (2) | |
| Sample size, referring to the number of samples used for establishing regression calibration models. | (4), (7), (9) | |
| Coefficient of determination, an indicator of the goodness of fit for regression models. A value closer to 1 indicates a better fit between the independent and dependent variables. | (4), (7), (9) | |
| Root mean square error, reflecting the average deviation between the predicted values (from calibration models) and observed values. A smaller value indicates higher prediction accuracy. | (4), (7), (9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, Y.; Li, X.; Abdul Baki, M.; Saha, S.; Zhou, J.; Xu, Y. Distribution and Environmental Implications of GDGTs in Sediments from Three Asian Mangrove Wetlands. Water 2025, 17, 2677. https://doi.org/10.3390/w17182677
Li Q, Wang Y, Li X, Abdul Baki M, Saha S, Zhou J, Xu Y. Distribution and Environmental Implications of GDGTs in Sediments from Three Asian Mangrove Wetlands. Water. 2025; 17(18):2677. https://doi.org/10.3390/w17182677
Chicago/Turabian StyleLi, Qiunan, Yasong Wang, Xinxin Li, Mohammad Abdul Baki, Shilpi Saha, Jiaodi Zhou, and Yunping Xu. 2025. "Distribution and Environmental Implications of GDGTs in Sediments from Three Asian Mangrove Wetlands" Water 17, no. 18: 2677. https://doi.org/10.3390/w17182677
APA StyleLi, Q., Wang, Y., Li, X., Abdul Baki, M., Saha, S., Zhou, J., & Xu, Y. (2025). Distribution and Environmental Implications of GDGTs in Sediments from Three Asian Mangrove Wetlands. Water, 17(18), 2677. https://doi.org/10.3390/w17182677






