Effect of Carbon Dioxide on the Growth and Nutrient Uptake of the Microalgae Chlorella sorokiniana from Digestate
Abstract
1. Introduction
2. Materials and Methods
2.1. Initital Chlorella sorokiniana Cultivation
2.2. Anaerobic Digestion Effluent Collection and Processing
2.3. Cultivations with Digestate and Different CO2 Concnetrations
2.4. Microalgae Pre-Treatment for Composition Analysis
2.5. Analytical Measurements
2.5.1. Growth Determination and Nutrient Analysis
2.5.2. Nutrient Analysis
2.5.3. Lipids Determination
2.5.4. Proteins Determination
2.5.5. Carbohydrates Determination
2.5.6. Elemental Analysis
2.5.7. Statistical Analysis
3. Results
3.1. Effect of CO2 Flow Rate on Chlorella sorokiniana Growth
3.2. Effect of CO2 Flow Rate in the Macronutrient and Vital Elements Concentration of Biomass
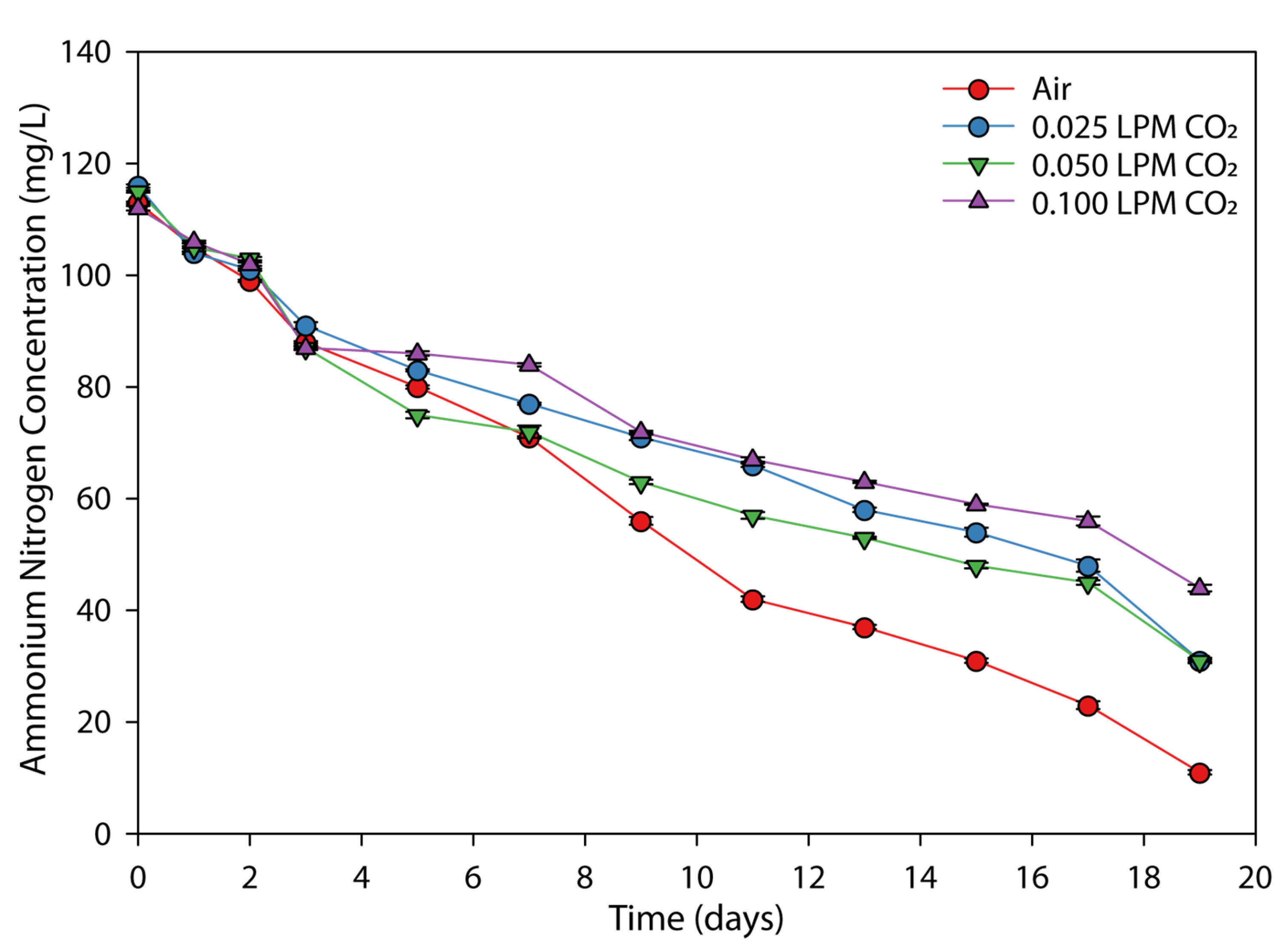
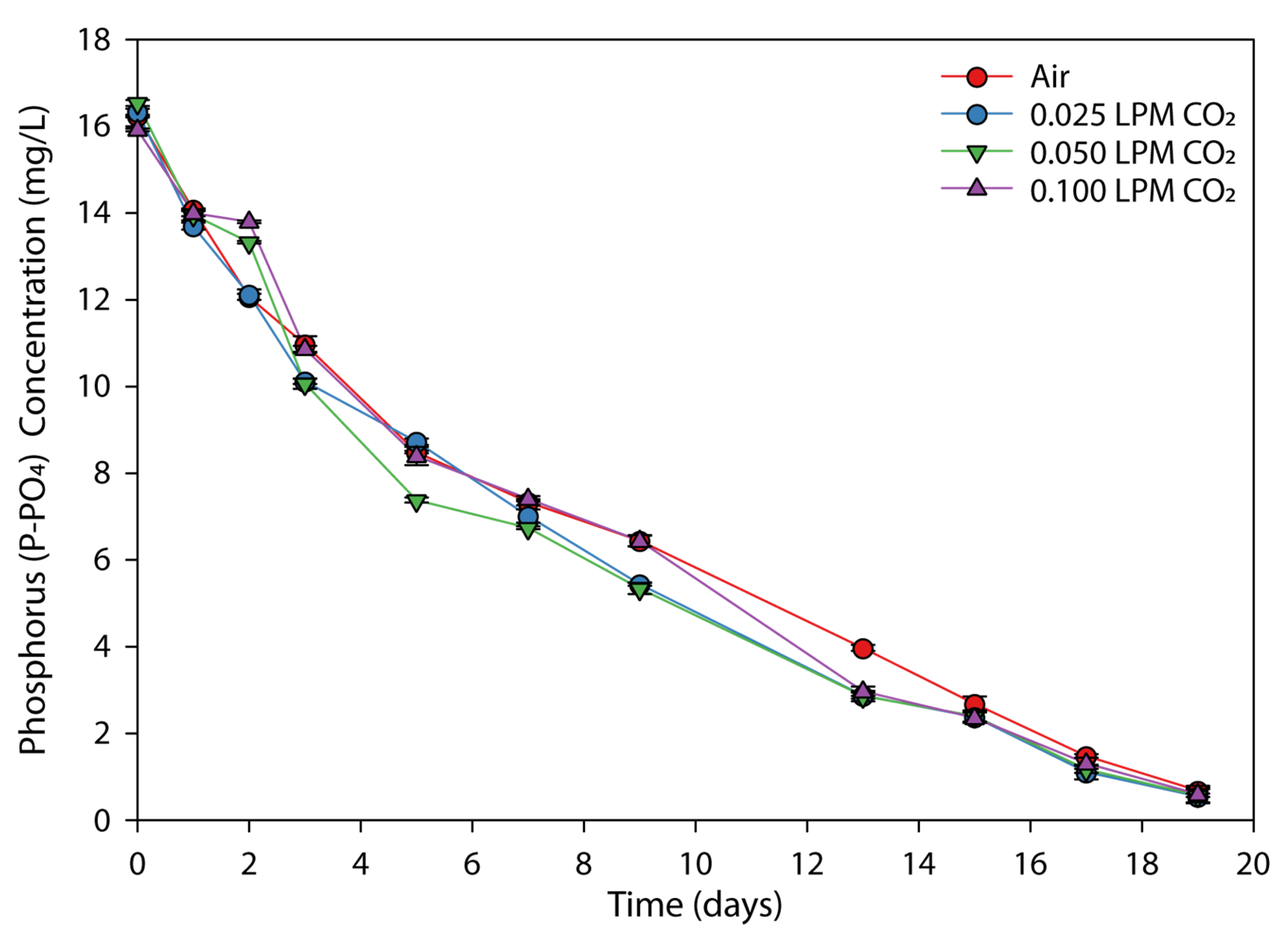
3.3. Effect of CO2 Flow Rate on the Nutrients Removal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hung Anh Le, H.A.L.; Thi Kim Khuyen Vo, T.K.K.V.; Ngoc Nam Trinh, N.N.T.; Duy Khoa Vo, D.K.V. Effects of microalgae on nutrient removal from mariculture wastewater in Can Gio District, Ho Chi Minh City, Vietnam. J. Viet. Env. 2016, 8, 114–120. [Google Scholar] [CrossRef]
- Martinez-Porchas, M.; Martinez-Cordova, L.R.; Lopez-Elias, J.A.; Porchas-Cornejo, M.A. Bioremediation of aquaculture effluents. In Microbial Biodegradation and Bioremediation: Techniques and Case Studies for Environmental Pollution, 2nd ed.; Das, S., Dash, H.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 539–553. [Google Scholar] [CrossRef]
- Vale, A.M.; Ferreira, A.; Pires, C.M.J.; Goncalves, L.A. CO2 Capture Using Microalge. In Advances in Carbon Capture: Methods, Technology and Applications; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 308–405. [Google Scholar] [CrossRef]
- Goh, P.S.; Ahmad, N.A.; Lim, J.W.; Liang, Y.Y.; Kang, H.S.; Ismail, A.F.; Arthanareeswaran, G. Microalgae Enabled Wastewater Remediation and Nutrient Recovery through Membrane Photobioreactors: Recent Achievements and Future Perspective. Membranes 2022, 12, 1094. [Google Scholar] [CrossRef] [PubMed]
- Obaideen, K.; Shehata, N.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Olabi, A.G. The role of wastewater treatment in achieving sustainable development goals (SDGs) and sustainability guideline. Energy Nexus 2022, 7, 100112. [Google Scholar] [CrossRef]
- Abdel-Karim, O.H.; Gheda, S.F.; Ismail, G.A.; Abo-Shady, A.M. Phytochemical Screening and antioxidant activity of Chlorella vulgaris. Delta J. Sci. 2020, 41, 79–91. [Google Scholar] [CrossRef]
- Palikrousis, T.; Banti, D.; Karayiannis, V.; Samaras, P. Nutrient Recovery from Wastewater. In Advances in Sustainable Applications of Microalgae; Pires, J., Esteves, A., Salgado, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 225–259. [Google Scholar]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The potential and challenge of microalgae as promisingfuture food sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- Chowdury, K.H.; Nahar, N.; Deb, U.K. The growth factors involved in microalgae cultivation for biofuel production: A review. Comput. Water Energy, Environ. Eng. 2020, 9, 185–215. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hosano, N.; Hosano, H. Recovering microalgal bioresources: A review of cell disruption methods and extraction technologies. Molecules 2022, 27, 2786. [Google Scholar] [CrossRef]
- Haris, N.; Manan, H.; Jusoh, M.; Khatoon, H.; Katayama, T.; Kasan, N.A. Effect of different salinity on the growth performance and proximate composition of isolated indigenous microalgae species. Aquac. Rep. 2022, 22, 100925. [Google Scholar] [CrossRef]
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Ferrer, I.; Díez-Montero, R. Can microalgae grown in wastewater reduce the use of inorganic fertilizers? J. Environ. Manag. 2022, 323, 116224. [Google Scholar] [CrossRef]
- Yücel, S.; Terzioğlu, P.; Boğoçlu, M.E.; Celikkol, M. Changes in the cell growth, lipid content and lipid profile of chlorella protothecoides under different mediums. Sigma J. Eng. Nat. Sci. 2016, 34, 183–190. [Google Scholar]
- Hanifzadeh, M.M.; Nabati, Z.; Tavakoli, O.; Sarrafzadeh, M.H. Waste to energy from flue gas of industrial plants to biodiesel: Effect of CO2 on microalgae growth. Int. J. Waste Resour. 2017, 7, 2. [Google Scholar]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of nutrients from wastewaters using microalgae. Front. Sustain. Food Syst. 2018, 2, 59. [Google Scholar] [CrossRef]
- Matabanchoy-Mesias, Y.; Rodríguez-Caicedo, Y.A.; Imués-Figueroa, M.A. Population growth of Chlorella sp. in three types of tubular photobioreactors, under laboratory conditions. Aquac. Aquar. Conserv. Legis. 2020, 13, 2094–2106. [Google Scholar]
- Kuo, C.M.; Sun, Y.L.; Lin, C.H.; Lin, C.H.; Wu, H.T.; Lin, C.S. Cultivation and biorefinery of microalgae (Chlorella sp.) for producing biofuels and other byproducts: A review. Sustainability 2021, 13, 13480. [Google Scholar] [CrossRef]
- Ghosh, G.; Daile, S.B.; Chakraborty, S.; Atta, A. Influence of super-optimal light intensity on the acetic acid uptake and microalgal growth in mixotrophic culture of Chlorella sorokiniana in bubble-column photobioreactors. Bioresour. Technol. 2024, 393, 130152. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V. Reorienting waste remediation towards harnessing bioenergy: A Paradigm Shift. In Industrial Wastewater Treatment, Recycling and Reuse; Ranade, V.V.V., Bhandari, M.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 235–281. [Google Scholar]
- Montoya-Vallejo, C.; Guzmán Duque, F.L.; Quintero Díaz, J.C. Biomass and lipid production by the native green microalgae Chlorella sorokiniana in response to nutrients, light intensity, and carbon dioxide: Experimental and modeling approach. Front. Bioeng. Biotechnol. 2023, 11, 1149762. [Google Scholar] [CrossRef] [PubMed]
- Politaeva, N.; Ilin, I.; Velmozhina, K.; Shinkevich, P. Carbon dioxide utilization using Chlorella microalgae. Environments 2023, 10, 109. [Google Scholar] [CrossRef]
- Psachoulia, P.; Schortsianiti, S.N.; Lortou, U.; Gkelis, S.; Chatzidoukas, C.; Samaras, P. Assessment of nutrients recovery capacity and biomass growth of four microalgae species in anaerobic digestion effluent. Water 2022, 14, 221. [Google Scholar] [CrossRef]
- Yew, G.Y.; Chew, K.W.; Malek, M.A.; Ho, Y.C.; Chen, W.H.; Ling, T.C.; Show, P.L. Hybrid liquid biphasic system for cell disruption and simultaneous lipid extraction from microalgae Chlorella sorokiniana CY-1 for biofuel production. Biotechnol. Biofuels 2019, 12, 252. [Google Scholar] [CrossRef]
- Ding, G.T.; Yasin, N.H.M.; Takriff, M.S.; Kamarudin, K.F.; Salihon, J.; Yaakob, Z.; Hakimi, N.I.N.M. Phycoremediation of palm oil mill effluent (POME) and CO2 fixation by locally isolated microalgae: Chlorella sorokiniana UKM2, Coelastrella sp. UKM4 and Chlorella pyrenoidosa UKM7. J. Water Process. Eng. 2020, 35, 101202. [Google Scholar] [CrossRef]
- Onyeaka, H.; Miri, T.; Obileke, K.; Hart, A.; Anumudu, C.; Al-Sharify, Z.T. Minimizing carbon footprint via microalgae as a biological capture. Carbon Capture Sci. Technol. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Iwuozor, K.O.; Ogunfowora, L.A.; Abdulsalam, A.; Iwuchukwu, F.U.; Itabana, B.; Bright, O.C.; Igwegbe, C.A. Regenerative desulphurisation of pyrolysis oil: A paradigm for the circular economy initiative. J. Environ. Chem. Eng. 2021, 9, 106864. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of carbon capture technology: Microalgal biorefinery concept and state-of-the-art. Front. Mar. Sci. 2019, 6, 417505. [Google Scholar] [CrossRef]
- Wirth, R.; Pap, B.; Böjti, T.; Shetty, P.; Lakatos, G.; Bagi, Z.; Kovacs, L.K.; Maróti, G. Chlorella vulgaris and its phycosphere in wastewater: Microalgae-bacteria interactions during nutrient removal. Front. Bioeng. Biotechnol. 2020, 8, 557572. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sirohi, R.; Udayan, A.; Yadav, P.; Raj, A.; Sim, S.J.; Pandey, A. Sustainable microalgal biomass production in food industry wastewater for low-cost biorefinery products: A review. Phytochem. Rev. 2023, 22, 969–991. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae cultivation technologies as an opportunity for bioenergetic system development advantages and limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Lim, J.W.; Lam, M.K.; Ho, Y.C.; Show, P.L. Biorefinery of Chlorella sorokiniana using ultra sonication assisted liquid triphasic flotation system. Bioresour. Technol. 2020, 303, 122931. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Hong, Y.; Gu, W.P. Influence of light quality on Chlorella growth, photosynthetic pigments and high-valued products accumulation in coastal saline-alkali leachate. Water Reuse 2021, 11, 301–311. [Google Scholar] [CrossRef]
- Danquah, M.K.; Gladman, B.; Moheimani, N.; Forde, G.M. Microalgal growth characteristics and subsequent influence on dewatering efficiency. Chem. Eng. J. 2009, 151, 73–78. [Google Scholar] [CrossRef]
- Kim, B.H.; Kang, Z.; Ramanan, R.; Choi, J.E.; Cho, D.H.; Oh, H.M.; Kim, H.S. Nutrient removal and biofuel production in high rate algal pond using real municipal wastewater. J. Microbiol. Biotechnol. 2014, 24, 1123–1132. [Google Scholar] [CrossRef]
- Gu, Z.; Liu, Y.; Zhu, L.; Fan, B.; Li, Y.; Liu, C.; Wang, Y.; Cui, X.; Yu, Z.; Ruan, R.; et al. Hormetic effect of dissolved organic matter from pig manure anaerobic digestion effluents on Chlorella sp.: Physiological and transcriptomic responses. Water Res. 2025, 283, 123877. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.S.; Kim, Y.S.; Yoon, H.S. Characterization of Chlorella sorokiniana and Chlorella vulgaris fatty acid components under a wide range of light intensity and growth temperature for their use as biological resources. Heliyon 2020, 6, e04447. [Google Scholar] [CrossRef] [PubMed]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Do Thi, C.V.; Tran, D.T.; Nguyen, Q.T. Preliminary investigation of CO2 sequestration by Chlorella sorokiniana TH01 in single and sequential photobioreactors. VNU J. Sci. Earth Environ. Sci. 2020, 36, 57–69. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Gabel, B.V.; Sinetova, M.A.; Gabrielian, A.K.; Markelova, A.G.; Shcherbakova, N.V.; Los, D.A. Optimization of CO2 supply for the intensive cultivation of Chlorella sorokiniana IPPAS C-1 in the laboratory and pilot-scale flat-panel photobioreactors. Life 2022, 12, 1469. [Google Scholar] [CrossRef]
- Daliry, S.; Hallajisani, A.; Mohammadi Roshandeh, J.; Nouri, H.; Golzary, A. Investigation of optimal condition for Chlorella vulgaris microalgae growth. Glob. J. Environ. Sci. Manag. 2017, 3, 217–230. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Aditya, L.; Vu, H.P.; Johir, A.H.; Bennar, L.; Ralph, P.; Nghiem, L.D. Nutrient removal by algae-based wastewater treatment. Curr. Pollut. Rep. 2022, 8, 369–383. [Google Scholar] [CrossRef]
- Santos-Ballardo, D.U.; Rossi, S.; Hernández, V.; Gómez, R.V.; del Carmen Rendón-Unceta, M.; Caro-Corrales, J.; Valdez-Ortiz, A. A simple spectrophotometric method for biomass measurement of important microalgae species in aquaculture. Aquaculture 2015, 448, 87–92. [Google Scholar] [CrossRef]
- Al-Ameri, M.; Al-Zuhair, S. Using switchable solvents for enhanced, simultaneous microalgae oil extraction-reaction for biodiesel production. Biochem. Eng. J. 2019, 141, 217–224. [Google Scholar] [CrossRef]
- Palikrousis, L.T.; Manolis, C.; Kalamaras, D.S.; Samaras, P. Effect of Light Intensity on the Growth and Nutrient Uptake of the Microalga Chlorella sorokiniana Cultivated in Biogas Plant Digestate. Water 2024, 16, 2782. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetricmethod for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Khan, T.A.; Liaquat, R.; Khoja, A.H.; Bano, A. Biological carbon capture, growth kinetics and biomass composition of novel microalgal species. Bioresour. Technol. Rep. 2022, 17, 100982. [Google Scholar] [CrossRef]
- Mountourakis, F.; Papazi, A.; Kotzabasis, K. The microalga Chlorella vulgaris as a natural bioenergetic system for effective CO2 mitigation—New perspectives against global warming. Symmetry 2021, 13, 997. [Google Scholar] [CrossRef]
- Li, C.T.; Trigani, K.; Zuñiga, C.; Eng, R.; Chen, E.; Zengler, K.; Betenbaugh, M.J. Examining the impact of carbon dioxide levels and modulation of resulting hydrogen peroxide in Chlorella vulgaris. Algal Res. 2021, 60, 102492. [Google Scholar] [CrossRef]
- Gao, K.; Xue, C.; Yang, M.; Li, L.; Qian, P.; Gao, Z.; Gao, Z.; Deng, X. Optimization of light intensity and photoperiod for growing Chlorella sorokiniana on cooking cocoon wastewater in a bubble-column bioreactor. Algal Res. 2022, 62, 102612. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front. Microbiol. 2016, 7, 183978. [Google Scholar] [CrossRef]
- Cheng, D.; He, Q. Assessment of environmental stresses for enhanced microalgal biofuel production–an overview. Front. Energy Res. 2014, 2, 26. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Ortíz-Sánchez, E.; Guillén-Garcés, R.A.; Morales-Arrieta, S.; Ugochukwu Okoye, P.; Olvera-Vargas, H.; Sebastian, P.J.; Arias, D.M. Cultivation of carbohydrate-rich microalgae with great settling properties using cooling tower wastewater. Environ. Sci. Pollut. Res. 2024, 31, 38999–39014. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.M.; Gislerød, H.R. The growth of Chlorella sorokiniana as influenced by CO2, light, and flue gases. J. Appl. Phycol. 2016, 28, 813–820. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Y.F.; Du, J. Elevated CO2 improves lipid accumulation by increasing carbon metabolism in Chlorella sorokiniana. Plant Biotechnol. J. 2016, 14, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Beigbeder, J.B.; Lavoie, J.M. Effect of photoperiods and CO2 concentrations on the cultivation of carbohydrate-rich P. kessleri microalgae for the sustainable production of bioethanol. J. CO2 Util. 2022, 58, 101934. [Google Scholar] [CrossRef]
- Patil, L.; Kaliwal, B. Effect of CO2 concentration on growth and biochemical composition of newly isolated indigenous microalga Scenedesmus bajacalifornicus BBKLP-07. Appl. Biochem. Biotechnol. 2017, 182, 335–348. [Google Scholar] [CrossRef]
- Varshney, P.; Beardall, J.; Bhattacharya, S.; Wangikar, P.P. Isolation and biochemical characterisation of two thermophilic green algal species- Asterarcys quadricellulare and Chlorella sorokiniana, which are tolerant to high levels of carbon dioxide and nitric oxide. Algal Res. 2018, 30, 28–37. [Google Scholar] [CrossRef]
- Cecchin, M.; Paloschi, M.; Busnardo, G.; Cazzaniga, S.; Cuine, S.; Li-Beisson, Y.; Wobbe, L.; Ballottari, M. CO2 supply modulates lipid remodelling, photosynthetic and respiratory activities in Chlorella species. Plant Cell Environ. 2021, 44, 2987–3001. [Google Scholar] [CrossRef]
- Li, G.; Xiao, W.; Yang, T.; Lyu, T. Optimization and process effect for microalgae carbon dioxide fixation technology applications based on carbon capture: A comprehensive review. J. Carbon Res. 2023, 9, 35. [Google Scholar] [CrossRef]
- Gerotto, C.; Norici, A.; Giordano, M. Toward enhanced fixation of CO2 in aquatic biomass: Focus on microalgae. Front. Energy Res. 2020, 8, 213. [Google Scholar] [CrossRef]
- Mota, M.F.S.; Souza, M.F.; Bon, E.P.; Rodrigues, M.A.; Freitas, S.P. Colorimetric protein determination in microalgae (Chlorophyta): Association of milling and SDS treatment for total protein extraction. J. Phycol. 2018, 54, 577–580. [Google Scholar] [CrossRef]
- Safaee, M.; Abdolalian, S.; Moghaddam, S.Y. Optimization of chlorella sorokiniana growth via response surface methodology. In Proceedings of the Third International Conference on New Research and Achievements in Science, Engineering and Technologies, Berlin, Germany, 6 January 2024. [Google Scholar]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour. Ind. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Khalid, A.A.H.; Yaakob, Z.; Abdullah, S.R.S.; Takriff, M.S. Analysis of the elemental composition and uptake mechanism of Chlorella sorokiniana for nutrient removal in agricultural wastewater under optimized response surface methodology (RSM) conditions. J. Clean. Prod. 2019, 210, 673–686. [Google Scholar] [CrossRef]
- Yu, H.; Kim, J.; Rhee, C.; Shin, J.; Shin, S.G.; Lee, C. Effects of different pH control strategies on microalgae cultivation and nutrient removal from anaerobic digestion effluent. Microorganisms 2022, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Gay, S.W.; Knowlton, K.F. Ammonia Emissions and Animal Agriculture; Publication 442-110; Virginia Cooperative Extension, Biological Systems Engineering: Blacksburg, VA, USA, 2005; Available online: https://p2infohouse.org/ref/43/42959.pdf (accessed on 4 September 2025).
- González, C.; Marciniak, J.; Villaverde, S.; Leon, C.; García, P.A.; Munoz, R. Efficient nutrient removal from swine manure in a tubular biofilm photo-bioreactor using algae-bacteria consortia. Water Sci. Technol. 2008, 58, 95–102. [Google Scholar] [CrossRef]
- Mulbry, W.; Kondrad, S.; Pizarro, C.; Kebede-Westhead, E. Treatment of dairy manure effluent using freshwater algae: Algal productivity and recovery of manure nutrients using pilot-scale algal turf scrubbers. Bioresour. Technol. 2008, 99, 8137–8142. [Google Scholar] [CrossRef]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef]
- Yan, H.; Lu, R.; Liu, Y.; Cui, X.; Wang, Y.; Yu, Z.; Zhang, Q. Development of microalgae-bacteria symbiosis system for enhanced treatment of biogas slurry. Bioresour. Technol. 2022, 354, 127187. [Google Scholar] [CrossRef]




| Parameters | Before Dilution | After Dilution |
|---|---|---|
| COD (mg L−1) | 6523 ± 34.00 | 481 ± 3.00 |
| TN (mg L−1) | 2097.6 ± 12.50 | 155.7 ± 2.10 |
| N-NH4 (mg L−1) | 1719 ± 12.24 | 127.3 ± 1.80 |
| N-NO3 (mg L−1) | 63.2 ± 3.31 | 5.0 ± 0.30 |
| N-NO2 (mg L−1) | 3.18 ± 0.12 | <0.60 |
| P-PO4 (mg L−1) | 19.8 ± 0.37 | 1.53 ± 0.20 |
| pH | 8.31 ± 0.20 | 8.11 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palikrousis, T.L.; Kalamaras, S.D.; Samaras, P. Effect of Carbon Dioxide on the Growth and Nutrient Uptake of the Microalgae Chlorella sorokiniana from Digestate. Water 2025, 17, 2674. https://doi.org/10.3390/w17182674
Palikrousis TL, Kalamaras SD, Samaras P. Effect of Carbon Dioxide on the Growth and Nutrient Uptake of the Microalgae Chlorella sorokiniana from Digestate. Water. 2025; 17(18):2674. https://doi.org/10.3390/w17182674
Chicago/Turabian StylePalikrousis, Thomas L., Sotirios D. Kalamaras, and Petros Samaras. 2025. "Effect of Carbon Dioxide on the Growth and Nutrient Uptake of the Microalgae Chlorella sorokiniana from Digestate" Water 17, no. 18: 2674. https://doi.org/10.3390/w17182674
APA StylePalikrousis, T. L., Kalamaras, S. D., & Samaras, P. (2025). Effect of Carbon Dioxide on the Growth and Nutrient Uptake of the Microalgae Chlorella sorokiniana from Digestate. Water, 17(18), 2674. https://doi.org/10.3390/w17182674







