Assessment of Stock Enhancement Efficacy for Hypophthalmichthys molitrix and Aristichthys nobilis in the Xixi of Jiulong River Basin
Abstract
1. Introduction
2. Materials and Methods
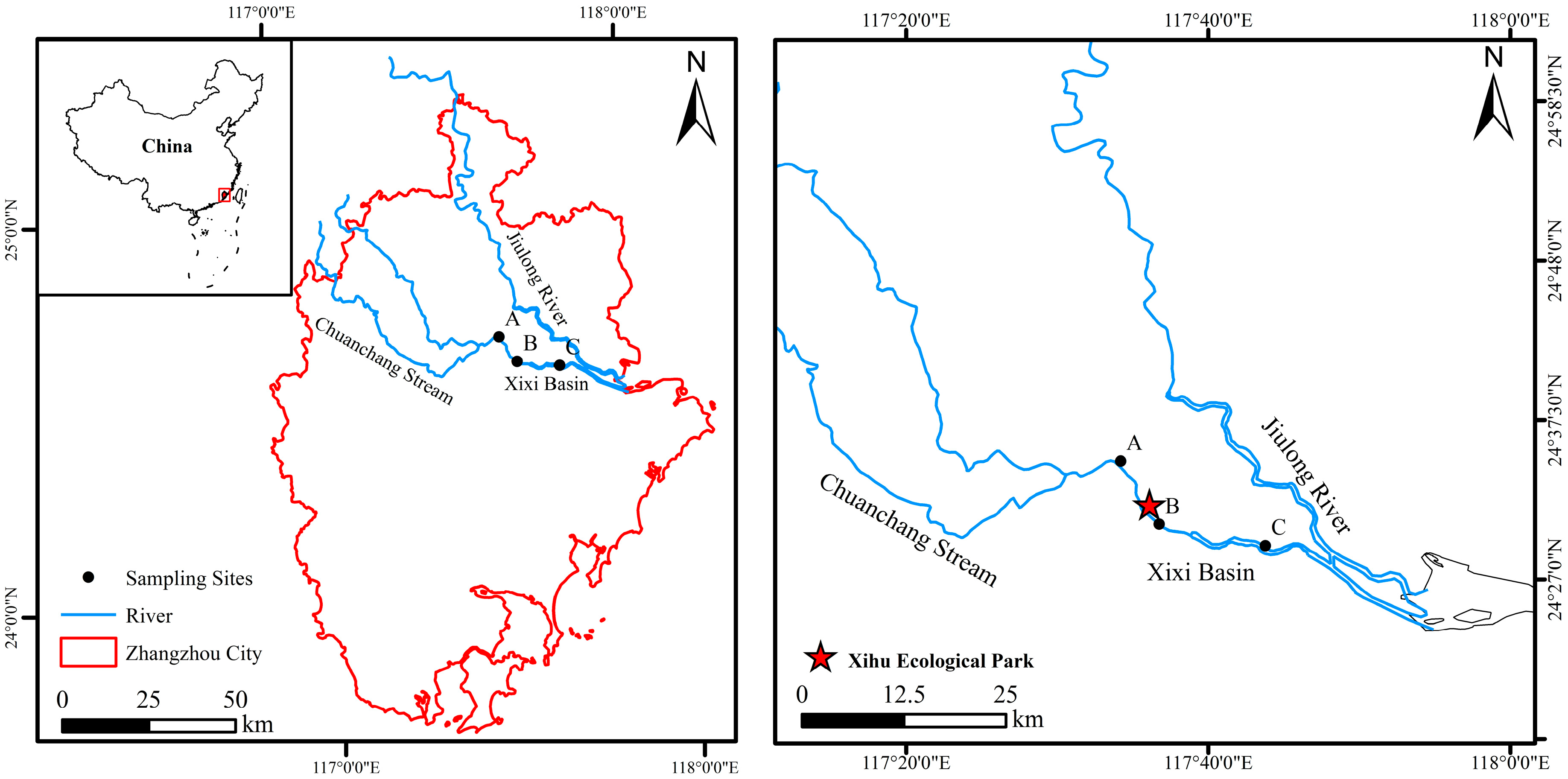
2.1. Study Area and Sampling Stations
2.2. Traditional Resource Investigation Methods
2.3. eDNA Analysis Method
2.4. Data Analysis
2.4.1. Estimation of Growth and Death Parameters
2.4.2. Biomass Analysis
3. Results
3.1. Population Composition
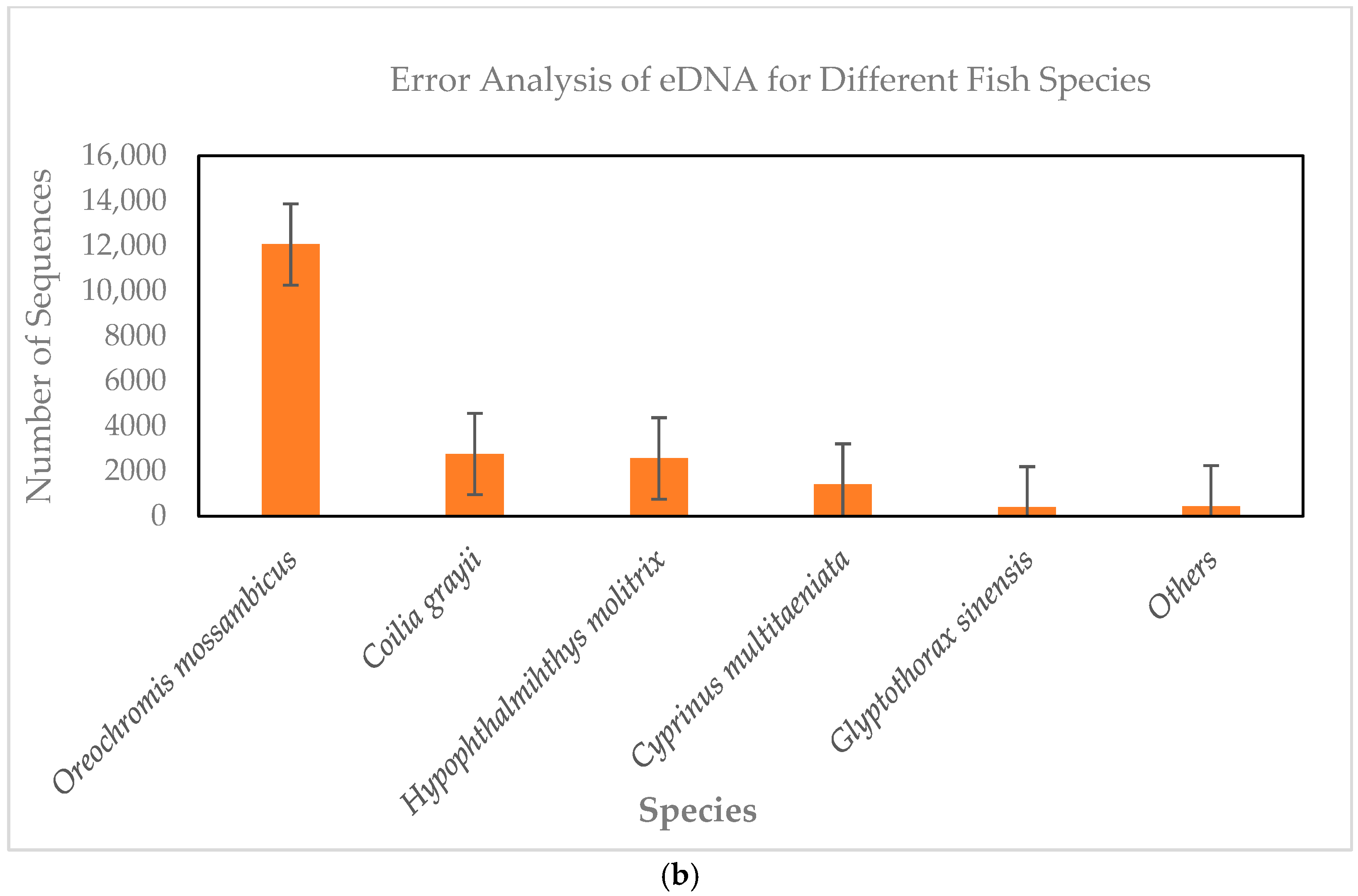
3.2. eDNA Results
3.3. Survival Rate and Standing Stock Resource Calculations
4. Discussion
4.1. Efficacy Assessment of H. molitrix and A. nobilis Stock Enhancement
4.2. The Differences Between eDNA and Traditional Assessment Methods
4.3. Management Suggestions
- Implement synchronized control over stocking scale and germplasm quality by establishing dedicated germplasm resource bases to ensure high-quality fingerlings. Optimal stocking scales should be selected to maximize post-release survival rates, with appropriate size classes identified to enhance survival rates and economic returns [66].
- Optimize release timing and regional strategies through comprehensive consideration of species’ breeding seasons, water temperature, food resource availability, stocking objectives, predators, and habitat conditions [67]. For instance, selecting fast-growth seasons for releases improves fishery output, while avoiding summer (excessive heat) and winter (extreme cold) periods that compromise transportation and survival [68].
- Establish sustained monitoring and evaluation mechanisms involving multidimensional assessments. Implement long-term ecological monitoring and data analysis for stock enhancement in the Xixi River basin, incorporating novel technologies (eDNA, microsatellite markers) for resource evaluation [69].
- Strengthen surveillance and control of invasive species. This survey detected dominant non-native species including Coptodon zillii, Cichlasoma managuense, Sarotherodon galilaeus, an Pterygoplichthys pardalis, which impact native species composition. Long-term management through physical removal, biological control, and natural predator introduction should be implemented.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, R.W. Qiantang River (Lanxi-Fuyang section). Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2023. (In Chinese). [Google Scholar]
- Zhang, L.Z.; He, R.J. Research progress on hydrobios enhancement releasing devices. Mar. Fish. 2024, 6, 1–14. (In Chinese) [Google Scholar]
- Huang, L.M.; Wang, J.Q.; Shih, Y.J.; Li, J.; Chu, T.J. Revealing the effectiveness of fisheries policy: A biological observation of species Johnius belengerii in Xiamen Bay. J. Mar. Sci. Eng. 2022, 10, 732. [Google Scholar] [CrossRef]
- Zhang, C.L.; Xu, B.D.; Xue, Y.; Ren, Y.P. Fisheries stock enhancement assessment: Progress and prospect. J. Fish. China 2022, 46, 1509–1524. (In Chinese) [Google Scholar]
- Luo, G.; Zhuang, P.; Zhao, F.; Feng, G.P.; Wang, Q.N.; Han, F. Development status, existing issues and countermeasure in the selection of suitable species for stock enhancement. Mar. Fish. 2016, 38, 551–560. (In Chinese) [Google Scholar]
- Richardson, L.E.; Lenfant, P.; Clarke, L.J.; Fontcuberta, A.; Gudefin, A.; Lecaillon, G.; Le Vay, L.; Radford, A.N.; Simpson, S.D. Examining current best-practices for the use of wild post-larvae capture, culture, and release for fisheries enhancement. Front. Mar. Sci. 2023, 9, 1058497. [Google Scholar] [CrossRef]
- Wang, J.Q.; Shih, Y.J.; Huang, L.M.; Li, J.; Li, W.W.; Shih, C.H.; Chu, T.J. Evaluating the effects related to restocking and stock replenishment of Penaeus penicillatus in the Xiamen Bay, China. J. Mar. Sci. Eng. 2021, 9, 1122. [Google Scholar] [CrossRef]
- Lorenzen, K. Fish population regulation beyond “Stock and Recruitment”: The role of density-dependent growth in the recruited stock. Bull. Mar. Sci. 2008, 83, 181–196. [Google Scholar]
- Li, L.P.; Huang, S.L. A study on management of stock enhancement in China. J. Shanghai Ocean Univ. 2011, 20, 765–772. (In Chinese) [Google Scholar] [CrossRef]
- Du, C.Z.; Jin, G.F.; Wu, L.; Que, P.Z.; Lu, X.Y.; Lu, H.H.; Sun, C.; Gu, Z.X. Evaluation of the proliferation and release effect of Schizothorax in high-altitude areas based on labeling technology. Environ. Ecol. 2025, 7, 46–52. (In Chinese) [Google Scholar]
- Zhu, C.W.; Zhang, H.; Ben, C.K.; Yuan, J.M.; Hu, H.S.; Xiao, Y.Y. Study on marked release and effect evaluation of Larimichthys Crocea in coastal areas of Jiangsu Province. J. Aquacult. 2023, 44, 1–9. (In Chinese) [Google Scholar]
- Zhang, S.; Lin, L.; Wang, X. Optimization of a marine fish release strategy: A case study of black sea bream Acanthopagrus Schlegelii in the Zhanjiang estuary, Northern South China Sea. Front. Environ. Sci. 2021, 9, 779544. [Google Scholar] [CrossRef]
- Ben, C.K.; Zhang, H.; Yuan, J.M.; Hu, H.S.; Xiao, Y.Y.; Zu, K.W.; Zhu, C.W. Status of Cynoglossus semilaevis resources after enhancement and release in Jiangsu coastal waters. J. Aquacult. 2023, 44, 9–15. (In Chinese) [Google Scholar]
- Zhang, C.; Zhang, M.Y.; Zheng, C.F.; Zhang, X.Y.; Chen, C.; Shao, X.B.; Zhao, W.H.; Liu, W.C. Otolith strontium marking of juvenile Liza haematocheila. Chin. J. Ecol. 2024, 43, 975–981. (In Chinese) [Google Scholar]
- Riley, W.D.; Ibbotson, A.T.; Beaumont, W.R.C.; Rycroft, P.; Cook, A.C. A portable, cost effective, pass-through system to detect downstream migrating salmonids marked with 12mm passive integrated transponder tags. Fish. Res. 2010, 101, 203–206. [Google Scholar] [CrossRef]
- Tang, R.; Lu, H.J.; Fu, M.; Su, S.Q.; Yao, W.Z. Study on double fluorescence making of Spinibarbus sinensis juvenile. Acta Hydrobiol. Sin. 2021, 45, 29–38. (In Chinese) [Google Scholar]
- Yuan, J.; Wen, J.; Kong, Q.; Zhou, X. Study on fish species diversity in the Pingzhai Reservoir based on environmental DNA technology. Fishes 2024, 9, 382. [Google Scholar] [CrossRef]
- Liu, S.M.; Yu, Q.; Chen, R.Y.; Hu, W.H.; Yan, X.J.; Han, Q.G.; Xu, D.D.; Zhu, Q.H. Comparison of genetic diversity between Hatchery-Reared and wild rock bream (Oplegnathus fasciatus) based on microsatellite markers and mitochondrial COI sequences. Aquacult. Res. 2024, 2024, 5570764. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Willerslev, E. Environmental DNA -an emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Shu, L.; Ludwig, A.; Peng, Z. Standards for methods utilizing environmental DNA for detection of fish species. Genes 2020, 11, 296. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Miaud, C.; Pompanon, F.; Taberlet, P. Species detection using environmental DNA from water samples. Biol. Lett. 2008, 4, 423–425. [Google Scholar] [CrossRef]
- Stoeckle, M.Y.; Adolf, J.; Charlop-Powers, Z.; Dunton, K.J.; Hinks, G.; VanMorter, S.M. Trawl and eDNA assessment of marine fIsh diversity, seasonality, and relative abundance in Coastal New Jersey, USA. ICES J. Mar. Sci. 2021, 78, 293–304. [Google Scholar] [CrossRef]
- Wang, X.Y.; Lu, G.Q.; Zhao, L.L.; Du, X.Q.; Gao, T.X. Assessment of fishery resources using environmental DNA: The large yellow croaker (Larimichthys crocea) in the East China Sea. Fish. Res. 2021, 235, 105813. [Google Scholar] [CrossRef]
- Osathanunkul, M.; Suwannapoom, C. Sustainable fisheries management through reliable restocking and stock enhancement evaluation with environmental DNA. Sci. Rep. 2023, 13, 11297. [Google Scholar] [CrossRef]
- Tang, S.K.; Liu, Y.S.; Wang, H.; Li, D.M.; Zhang, T.Q.; Sun, J.Y.; Xu, F.; Wang, Z.H. Application of environmental DNA in monitoring of fish resources in Shaobo Lake. Fish Sci. 2022, 41, 1007–1016. (In Chinese) [Google Scholar]
- Huang, F.; Zhang, R.; Lv, Z.; Xiang, Y.; Min, W.; Wang, X.; Liu, W.; Wang, W.; Zeng, S. Environmental DNA was utilized to assess fish diversity and community structure in the Qingshui River. Fishes 2025, 10, 165. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhou, X.X.; Li, Q.G.; Zhang, J.M.; Shen, Y.J. Spatial patterns of fish diversity in the Chongqing section of the Yangtze River basin based on eDNA. J. Fish. China 2025, 49, 103–118. (In Chinese) [Google Scholar]
- Tang, J.; Dai, Y.; Li, M.; Tian, L.; Lou, B.; Huang, F.; Xie, Z.; Dai, Y.; He, W. Environmental DNA reveals ecologically relevant temporal and spatial variation of fish community in silver carp- and bighead carp-dominant drinking water reservoirs. Water 2025, 17, 1057. [Google Scholar] [CrossRef]
- Liang, X.; Yang, X.; Sha, N.; Wang, J.; Qiu, G.; Chang, M. Application of eDNA metabarcoding technology to monitor the health of aquatic ecosystems. Water 2025, 17, 1109. [Google Scholar] [CrossRef]
- Alenzi, A.M. Evaluation of fish biodiversity in estuaries through environmental DNA metabarcoding: A comprehensive review. Fishes 2024, 9, 422. [Google Scholar] [CrossRef]
- Wu, X.H. Investigation and evaluation of water environment quality in Xixi River of Jiulong River in Zhangzhou. EAD 2021, 33, 92–96. (In Chinese) [Google Scholar]
- Xie, S.N. Jiulongjiang water quality analysis and pollution prevention and control suggestion of Jiulongjiang. Sci. Technol. Inf. 2022, 20, 114–116. (In Chinese) [Google Scholar]
- Wang, J.Q.; Li, J.; Shih, Y.J.; Huang, L.M.; Wang, X.R.; Chu, T.J. Sustainability perspective of Minjiang estuary coastal fisheries management—estimation of fish richness. Water 2023, 15, 2648. [Google Scholar] [CrossRef]
- Huang, L.M.; Xu, H.Q.; Yu, J.Y.; Chen, Y.H.; Wang, J.Q.; Ji, F.F.; Li, J.; Cai, J.D.; Chu, T.J. The ecological niches and interspecific associations of the dominant fishes in the Xiamen Seas, China. Fishes 2024, 9, 354. [Google Scholar] [CrossRef]
- Fang, D.-a.; Sun, H.; Peng, Y.; Kuang, Z.; Zhou, Y.; Xu, D. Living status and perspective of the silver carp (Hypophthalmichthys molitrix) in the lower reach of the Yangtze River: Insights from population distribution, age structure, and habitat preference analyses. Fishes 2022, 7, 254. [Google Scholar] [CrossRef]
- Wu, Y.X.; Gong, H.B.; Yi, P.P.; Fu, X.J.; Yu, J.X.; Yu, Z.J.; Meng, Z.H.; Huang, B.; Wang, S.B. Difference in the trophic structure of fish communities in the ecological-stocking area of Hypophthalwvichthys molitrix and H. nobilis and the closed-fishing area in Shangyoujiang Reservoir. Freshwater. Fish. 2024, 54, 13–23. (In Chinese) [Google Scholar]
- Luo, X.Z.; Shi, H.; Sha, H.; Li, X.H.; Liang, H.W.; Zou, G.W.; Cui, F. Correlation analysis of the microsatellite markers with growth traits of silver carp. Freshwater. Fish. 2022, 52, 17–25. (In Chinese) [Google Scholar]
- Dai, P.; Han, F.; Yin, S.H.; Hang, H.B. Research progress on growth characteristics of Hypophthalmichthys molitrix and Hypophthalmichthys nobilis. J. Aquacult. 2021, 42, 31–35. (In Chinese) [Google Scholar]
- Wang, J.; Lei, C.X.; Liang, X.; Xue, S.W.; Xiong, Y.; Wang, W.Y.; Zhang, J.B. Characteristics of filter feeding organs and digestive tissues, and feeding strategies of Hypophthalmichthys molitrix and Aristichys nobilis. J. Yunnan Agric. Univ. (Nat. Sci). 2023, 38, 942–947. (In Chinese) [Google Scholar]
- Yu, J.; Chen, J.; Deng, X.; Wu, Z.; Yu, Z.; Xu, J.; Su, H.; Liu, J.; Wang, L.; Wu, Y.; et al. Trophic patterns of bighead carp and silver carp follow the seasonality of resource availability. Water 2019, 11, 1429. [Google Scholar] [CrossRef]
- Liu, M.; Lin, J.; Peng, Q.; Yu, L.; Chen, D.; Liu, S.; Duan, X. Relationship between the distribution of broodstock and vorticity of spawning grounds of four major Chinese carps in the middle reaches of the Yangtze River during ecological operation of the Three Gorges Dam. Water 2018, 10, 1487. [Google Scholar] [CrossRef]
- Duan, X.B.; Chen, D.Q.; Li, Z.H.; Wang, K.; Huan, M.G.; Liu, S.P. Current status of spawning grounds of fishes with pelagic eggs in the middle reaches of the Yangtze River after impoundment of the Three Gorges Reservoir. J. Fish. Sci. China 2008, 4, 523–532. (In Chinese) [Google Scholar]
- Ban, X.J.; Fan, B.; Liu, H.; Yu, L.X.; Lin, J.Q.; Xia, J.H.; Zhang, D. Relationship between fish spawning behavior and eco-hydrological indicators: A case study of the four major Chinese carps in the Yangtze River. J. Hydroecology 2024, 45, 67–74. (In Chinese) [Google Scholar]
- SC/T 9249-2019; Technical Specification for Freshwater Fishery Resources Survey in River. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2019.
- Ji, F.F. Establishment of eDNA Technology and Application in Diversity Assessment of Three Kinds of Aquatic Organisms in Typical Lakes and Reservoirs. Masters’ Thesis, Huazhong Agricultural University, Wuhan, China, 2022. (In Chinese). [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Gayanilo, F.C.; Sparre, P.; Pauly, D. FISAT II—FAO-ICLARM Stock Assessment Tools II: User’s Guide; Computerized Information Series: Fisheries; FAO: Rome, Italy, 2005. [Google Scholar]
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L.K. Food habits of albacore, bluefin tuna, and bonito in Californian waters. Calif. Fish Game. 1971, 152, 1–105. [Google Scholar]
- Liu, Z.M.; Feng, M.Q.; Yang, R.J.; Liu, G.G.; Shi, X.S.; Wu, W.P. Community structure of plankton and the feeding habits of silver carp and bighead silver carp, as well as the assessment of fish productivity in Zhangze Reservoir. Xi’an Ligong Daxue Xuebao 2024, 40, 03. (In Chinese) [Google Scholar]
- Xin, Y.; Xing, X.D.; Guo, Y.; Qin, C.X.; Yu, G.; Ma, Z.H.; Wang, X.Q. Study on biomass evaluation method of Lutjanus erythropterus based on eDNA technology. Mar. Fish. 2024, 46, 275–285. (In Chinese) [Google Scholar]
- Wu, Y.S.; Tang, Y.K.; Li, J.L.; Liu, K.; Li, H.X.; Wang, Q.; Yu, J.H.; Xu, P. The application of environmental DNA in the monitoring of the Yangtze finless porpoise, Neophocaena phocaenoides asaeorientalis. J. Fish. Sci. China 2019, 26, 124–132. (In Chinese) [Google Scholar] [CrossRef]
- Meng, H.; Lin, Y.; Zhong, W.; Zhao, Z.; Shen, L.; Ling, Z.; Zhao, K.; Xu, S. Fish biomonitoring and ecological assessment in the Dianchi Lake basin based on environmental DNA. Water 2023, 15, 399. [Google Scholar] [CrossRef]
- Hao, L.; Gu, K.; Zhou, Y.; An, J.; Hu, W.; Wu, Z.; Shao, J.; Pan, J.; He, G.; Liu, Q.; et al. Comparing diversity and structure of freshwater fish assemblages using environmental DNA and gillnetting methods: A case study of a large deep reservoir in East China. Ecol. Indic. 2024, 166, 112538. [Google Scholar] [CrossRef]
- Zhu, Y.D. (Ed.) Fujian Yulei Zhi [Fujian Fish Gazetteer]; Fujian Science and Technology Press: Fuzhou, China, 1984–1985; Volumes 1–2. (In Chinese) [Google Scholar]
- He, Y.; Zhao, X.; Shi, C.; Peng, K.; Wang, Z.; Jiang, Z. Fish community monitoring in floodplain lakes: eDNA metabarcoding and traditional sampling revealed inconsistent fish community composition. Ecol. Indic. 2024, 166, 112467. [Google Scholar] [CrossRef]
- Li, W.-P.; Liu, Z.F.; Guo, T.; Chen, H.; Xie, X. Using optimal environmental DNA method to improve the fish diversity survey—from laboratory to aquatic life reserve. Water 2021, 13, 1468. [Google Scholar] [CrossRef]
- Qu, J.Q.; Hao, T.F.; Li, Y.G.; Liu, L.M.; Hao, D.S.; Jia, C.X.; Zhang, Q.J. Analysis of fish community structure and ecological niche changes in Miyun Reservoir based on environmental DNA technology. J. Dalian Ocean Univ. 2024, 39, 298–307. (In Chinese) [Google Scholar]
- Li, Z.; Zhao, W.; Jiang, Y.; Wen, Y.; Li, M.; Liu, L.; Zou, K. New insights into biologic interpretation of bioinformatic pipelines for fish eDNA metabarcoding: A case study in Pearl River Estuary. J. Environ. Manag. 2024, 368, 122136. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Y.; Yoshizawa, S.; Iwasaki, W.; Li, Y.; Xian, W.; Zhang, H. Seasonal variation and assessment of fish resources in the Yangtze Estuary based on environmental DNA. Water 2020, 12, 2874. [Google Scholar] [CrossRef]
- Gu, S.Y.; Chen, K.; Jin, X.W.; Li, W.P.; Chen, X.F.; Xiong, J.; Tang, M.Z.; Jiang, C.Q.; Xiong, J.; Li, T.; et al. Development, applications, and standardization of Environment DNA monitoring technology for aquatic organisms. Acta Hydrobiol. Sin. 2024, 48, 1443–1458. (In Chinese) [Google Scholar]
- Coble, A.A.; Flinders, C.A.; Homyack, J.A.; Penaluna, B.E.; Cronn, R.C.; Weitemier, K. eDNA as a tool for identifying freshwater species in sustainable forestry: A critical review and potential future applications. Sci. Total Environ. 2019, 649, 1157–1170. [Google Scholar] [CrossRef]
- Trebitz, A.S.; Hoffman, J.C.; Darling, J.A.; Pilgrim, E.M.; Kelly, J.R.; Brown, E.A.; Chadderton, W.L.; Egan, S.P.; Grey, E.K.; Hashsham, S.A.; et al. Early detection monitoring for aquatic non-indigenous species: Optimizing surveillance, incorporating advanced technologies, and identifying research needs. J. Environ. Manag. 2017, 202, 299–310. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Shao, F.; Song, H.; Song, N.; Zhang, X.; Zhao, L. Exploring seasonal variations in fish communities: A study of the Yellow River Estuary and its adjacent waters using eDNA and trawl surveys. Fishes 2024, 9, 192. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Q.; Zhu, S.; Li, Y.; Li, X.; Li, J. Do changes in prey community in the environment affect the feeding selectivity of silver Carp (Hypophthalmichthys molitrix) in the Pearl River, China? Sustainability 2022, 14, 11175. [Google Scholar] [CrossRef]
- Li, W.; Liu, S.W.; Ye, S.W.; Lin, M.L.; Yuan, J.; Li, Z.J.; Zhang, T.L. Research progress on technique and effect evaluation of mandarin fish stock enhancement in large water bodies. J. Fish. Sci. China 2021, 28, 808–818. (In Chinese) [Google Scholar]
- Zhuang, P.; Zhao, F.; Luo, G.; Zhang, T.; Shi, X.T.; Feng, G.P.; Wang, S.K. Progress and problem on the stock enhancement of aquatic biological resources. Acta Hydrobiol. Sin. 2025, 49, 43–54. (In Chinese) [Google Scholar]
- Liang, Z.L.; Gui, Z.W.; Jiang, H.S.; Chao, Z.Z.; Wang, L.G. Habitat characteristics and optimization of proliferation and releasing points for rare and endemic fish resources in the upper reaches of the Yangtze River. J. Yangtze River Sci. Res. Inst. 2024, 45, 54–56. (In Chinese) [Google Scholar]
- Zhang, M.; Zhao, Y.; Shan, B.; Liu, Y.; Yang, C.; Wang, L.; Liu, M.; Xie, Q.; Li, Y.; Zou, J.; et al. Microsatellite-marker-based evaluation of stock enhancement for kuruma prawn Penaeus japonicus in Beibu Gulf, South China Sea. Fishes 2023, 8, 568. [Google Scholar] [CrossRef]



| Species | Station A | Station B | Station C |
|---|---|---|---|
| Pleuronectiformes | |||
| Paralichthyidae | |||
| Tephrinectes sinensis | + | ||
| Clupeiformes | |||
| Engraulidae | |||
| Coilia grayii | + | + | + |
| Clupeidae | |||
| Konosirus punctatus | + | + | + |
| Clupanodon thrissa | + | + | + |
| Elopiformes | |||
| Megalopidae | |||
| Megalops cyprinoides | + | ||
| Cypriniformes | |||
| Cyprinidae | |||
| Hemiculter leucisculus | + | + | + |
| Ctenopharyngodon idella | + | + | |
| Squaliobarbus curriculus | + | + | |
| Spinibarbus hollandi | + | ||
| Sinibrama macrops | + | ||
| Cyprinus carpiovar.specularis | + | ||
| Carassius auratus | + | + | |
| Cyprinus carpio | + | + | + |
| Hypophthalmihthys molitrix | + | + | + |
| Cirrhinus molitorella | + | + | |
| Cirrhinus mrigala | + | ||
| Megalobrama terminalis | + | + | |
| Culter alburnus | + | + | + |
| Mylopharyngodon piceus | + | + | |
| Chanodichthys dabryi | + | + | + |
| Osteochilus salsburyi | + | + | |
| Aristichthys nobilis | + | + | + |
| Cobitidae | |||
| Paramisgurnus dabryanus | + | ||
| Perciformes | |||
| Clariidae | |||
| Clarias fuscus | + | ||
| Theraponidae | |||
| Therapon oxyrhynchus | + | ||
| Gerreidae | |||
| Gerres filamentosus | + | ||
| Cichlidae | |||
| Sarotherodon galilaeus | + | + | + |
| Oreochromis sp. | + | + | |
| Cichlasoma managuense | + | + | |
| Oreochromis niloticus | + | + | + |
| Coptodon zillii | + | + | + |
| Anabantidae | |||
| Anabas testudineus | + | ||
| Eleotridae | |||
| Eleotris oxycephala | + | + | |
| Gobiidae | |||
| Glossogobiuss giuris | + | + | + |
| Mugilogobius | + | ||
| Siluriformes | |||
| Bagridae | |||
| Pelteobaggrus nitidus | + | ||
| Tachysurus fulvidraco | + | + | |
| Amblycipitidae | |||
| Ictalurus punctatus | + | + | |
| Loricariidae | |||
| Pterygoplichthys pardalis | + | + | + |
| Tetraodontiformes | |||
| Tetraodontidae | |||
| Takifugu ocellatus | + | + | |
| Mugiliformes | |||
| Mugilidae | |||
| Mugil cephalus | + | + | + |
| Survey Dates | Station A | Station B | Station C | Total |
|---|---|---|---|---|
| 2023/10 | 117 | 110 | 90 | 317 |
| 2023/12 | 147 | 39 | 85 | 271 |
| 2024/2 | 74 | 180 | 159 | 413 |
| 2024/4 | 183 | 120 | 90 | 394 |
| 2024/6 | 374 | 186 | 266 | 826 |
| 2024/8 | 274 | 290 | 280 | 844 |
| 2024/10 | 202 | 133 | 94 | 429 |
| Total | 1371 | 1058 | 1064 | 3494 |
| Species | W | N | F | IRI |
|---|---|---|---|---|
| Hypophthalmihthys molitrix | 0.67044 | 0.27619 | 1.00 | 946.63 |
| Coptodon zillii | 0.03008 | 0.12822 | 1.00 | 158.30 |
| Cichlasoma managuense | 0.01622 | 0.14797 | 0.86 | 140.74 |
| Sarotherodon galilaeus | 0.03997 | 0.09101 | 1.00 | 130.98 |
| Cyprinus carpio | 0.08439 | 0.02776 | 1.00 | 112.15 |
| Coilia grayii | 0.00940 | 0.09130 | 1.00 | 100.70 |
| Oreochromis niloticus | 0.02514 | 0.04980 | 1.00 | 74.94 |
| Aristichthys nobilis | 0.04865 | 0.02261 | 1.00 | 71.26 |
| Hemiculter leucisculus | 0.00940 | 0.05810 | 0.86 | 57.85 |
| Pterygoplichthys pardalis | 0.01282 | 0.01631 | 1.00 | 29.14 |
| Konosirus punctatus | 0.00677 | 0.01717 | 0.86 | 20.53 |
| Culter alburnus | 0.00445 | 0.01460 | 0.86 | 16.33 |
| Clupanodon thrissa | 0.00509 | 0.01030 | 0.86 | 13.20 |
| Chanodichthys dabryi | 0.00137 | 0.00572 | 0.86 | 6.08 |
| Glossogobiuss giuris | 0.00075 | 0.00744 | 0.71 | 5.85 |
| Carassius auratus | 0.00253 | 0.00372 | 0.86 | 5.36 |
| Squaliobarbus curriculus | 0.00562 | 0.00544 | 0.43 | 4.74 |
| Ctenopharyngodon idella | 0.00560 | 0.00200 | 0.43 | 3.26 |
| Mugil cephalus | 0.00377 | 0.00172 | 0.57 | 3.13 |
| Clarias fuscus | 0.00493 | 0.00143 | 0.43 | 2.73 |
| Takifugu ocellatus | 0.00119 | 0.00515 | 0.43 | 2.72 |
| Cirrhinus molitorella | 0.00114 | 0.00114 | 0.43 | 0.98 |
| Tachysurus fulvidraco | 0.00035 | 0.00172 | 0.43 | 0.89 |
| Osteochilus salsburyi | 0.00124 | 0.00172 | 0.29 | 0.84 |
| Gerres filamentosus | 0.00041 | 0.00200 | 0.29 | 0.69 |
| Ictalurus punctatus | 0.00275 | 0.00057 | 0.14 | 0.47 |
| Mylopharyngodon piceus | 0.00213 | 0.00086 | 0.14 | 0.43 |
| Oreochromis sp. | 0.00046 | 0.00114 | 0.14 | 0.23 |
| Pelteobaggrus nitidus | 0.00015 | 0.00143 | 0.14 | 0.23 |
| Mugilogobius | 0.00008 | 0.00057 | 0.29 | 0.19 |
| Therapon oxyrhynchus | 0.00010 | 0.00114 | 0.14 | 0.18 |
| Megalops cyprinoides | 0.00073 | 0.00029 | 0.14 | 0.15 |
| Eleotris oxycephala | 0.00014 | 0.00086 | 0.14 | 0.14 |
| Megalobrama terminalis | 0.00026 | 0.00057 | 0.14 | 0.12 |
| Spinibarbus hollandi | 0.00054 | 0.00029 | 0.14 | 0.12 |
| Cyprinus carpiovar.specularis | 0.00049 | 0.00029 | 0.14 | 0.11 |
| Cirrhinus mrigala | 0.00018 | 0.00029 | 0.14 | 0.07 |
| Anabas testudineus | 0.00010 | 0.00029 | 0.14 | 0.06 |
| Tephrinectes sinensis | 0.00008 | 0.00029 | 0.14 | 0.05 |
| Paramisgurnus dabryanus | 0.00003 | 0.00029 | 0.14 | 0.05 |
| Sinibrama macrops | 0.00002 | 0.00029 | 0.14 | 0.04 |
| Species | Station A | Station B | Station C | Ci (%) |
|---|---|---|---|---|
| Hypophthalmichthys molitrix | + | + | + | 38.7167% |
| Oreochromis mossambicus | + | + | + | 34.2677% |
| Coreoperca whiteheadi | + | + | + | 16.0247% |
| Mugil cephalus | + | + | + | 3.1629% |
| Coilia grayii | + | + | + | 2.5585% |
| Cyprinus multitaeniata | + | + | + | 1.3063% |
| Mylopharyngodon piceus | + | + | + | 1.2881% |
| Chelon haematocheilus | + | + | 0.6391% | |
| Hemibagrus wyckioides | + | + | 0.5400% | |
| Glyptothorax sinensis | + | 0.3824% | ||
| Squaliobarbus curriculus | + | + | 0.2632% | |
| Pseudolaubuca sinensis | + | 0.2474% | ||
| Konosirus punctatus | + | + | 0.1897% | |
| Sinocyclocheilus anshuiensis | + | + | + | 0.1072% |
| Elopichthys bambusa | + | + | 0.0698% | |
| Cyprinus carpio | + | + | + | 0.0439% |
| Spinibarbus denticulatus yunnanensis | + | + | 0.0315% | |
| Pseudobagrus medianalis | + | + | 0.0247% | |
| Lateolabrax japonicus | + | + | 0.0182% | |
| Metzia formosae | + | + | + | 0.0130% |
| Channa striata | + | + | 0.0124% | |
| Acrossocheilus monticola | + | + | + | 0.0102% |
| Perccottus glehni | + | + | 0.0080% | |
| Barbodes semifasciolatus | + | + | + | 0.0074% |
| Neolissochilus hexagonolepis | + | + | + | 0.0074% |
| Mastacembelus marmatus | + | + | 0.0074% | |
| Aphyocypris kikuchii | + | + | + | 0.0071% |
| Carassius auratus | + | + | 0.0068% | |
| Microphysogobio fukiensis | + | 0.0059% | ||
| Microphysogobio brevirostris | + | + | + | 0.0056% |
| Neolissochilus benasi | + | + | + | 0.0056% |
| Psilorhynchus homaloptera | + | + | + | 0.0046% |
| Sarcocheilichthys sinensis | + | + | + | 0.0046% |
| Acipenser ruthenus | + | 0.0043% | ||
| Puntius sophore | + | + | + | 0.0040% |
| Garra micropulvinus | + | + | 0.0031% |
| Species | Total Mortality Coefficients (Z) | Total Mortality Rates (%) | Natural Mortality Coefficients (M) | Natural Mortality Rates (%) | Fishing Mortality Coefficients (F) | Fishing Mortality Rates (%) |
|---|---|---|---|---|---|---|
| H. molitrix | 0.91 | 59.75% | 0.64 | 47.15% | 0.27 | 12.60% |
| A. nobilis | 0.73 | 51.81% | 0.51 | 39.96% | 0.22 | 11.85% |
| Species | Total Mortality Coefficients (Z) | Xixi River Basin | |||||
|---|---|---|---|---|---|---|---|
| H. molitrix (×104 Ind.) | Survivors (Ind.) | Contribution Rate (%) | A. nobilis (×104 Ind.) | Survivors (Ind.) | Contribution Rate (%) | ||
| 2017/2 | 23 | 17.25 | 5.75 | ||||
| 2018/12 | 100 | 75 | 69,435 | 40.3 | 25 | 27,710 | 48.2 |
| 2019/11 | 80 | 60 | 329,842 | 91.5 | 20 | 133,831 | 90.0 |
| 2020/2 | 20 | 15 | 374,284 | 64.5 | 5 | 160,876 | 59.9 |
| 2021/11 | 10 | 7.5 | 211,037 | 28.6 | 2.5 | 101,622 | 23.7 |
| 2022/1 | 30 | 22.5 | 115,137 | 26.2 | 7.5 | 61,020 | 19.7 |
| 2023/9 | 10 | 7.5 | 136,913 | 66.2 | 2.5 | 65,550 | 55.1 |
| 2024 | 85,299 | 35.4 | 68.25 | 43,637 | 27.6 | ||
| Total/average | 273 | 204.75 | 50.4 | 46.3 | |||
| Years | H. molitrix | A. nobilis | ||
|---|---|---|---|---|
| Number Stocked (Ind.) | Value Estimate (CNY) | Number Stocked (ind.) | Value Estimate (CNY) | |
| 2018 | 750,000 | 1,871,420 | 250,000 | 1,287,008 |
| 2019 | 600,000 | 1,490,000 | 200,000 | 1,025,000 |
| 2020 | 150,000 | 372,000 | 50,000 | 255,000 |
| 2021 | 75,000 | 195,500 | 25,000 | 130,000 |
| 2022 | 225,000 | 560,800 | 75,000 | 374,000 |
| 2023 | 75,000 | 347,175 | 25,000 | 221,680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chu, T.-J.; Zeng, Q.-M.; Wang, J.-Q.; Huang, L.-M.; Liu, K.; Ji, F.-F.; Guo, S.-P.; Shih, Y.-J. Assessment of Stock Enhancement Efficacy for Hypophthalmichthys molitrix and Aristichthys nobilis in the Xixi of Jiulong River Basin. Water 2025, 17, 2667. https://doi.org/10.3390/w17182667
Li H, Chu T-J, Zeng Q-M, Wang J-Q, Huang L-M, Liu K, Ji F-F, Guo S-P, Shih Y-J. Assessment of Stock Enhancement Efficacy for Hypophthalmichthys molitrix and Aristichthys nobilis in the Xixi of Jiulong River Basin. Water. 2025; 17(18):2667. https://doi.org/10.3390/w17182667
Chicago/Turabian StyleLi, Hong, Ta-Jen Chu, Qing-Min Zeng, Jia-Qiao Wang, Liang-Min Huang, Kai Liu, Fen-Fen Ji, Shao-Peng Guo, and Yi-Jia Shih. 2025. "Assessment of Stock Enhancement Efficacy for Hypophthalmichthys molitrix and Aristichthys nobilis in the Xixi of Jiulong River Basin" Water 17, no. 18: 2667. https://doi.org/10.3390/w17182667
APA StyleLi, H., Chu, T.-J., Zeng, Q.-M., Wang, J.-Q., Huang, L.-M., Liu, K., Ji, F.-F., Guo, S.-P., & Shih, Y.-J. (2025). Assessment of Stock Enhancement Efficacy for Hypophthalmichthys molitrix and Aristichthys nobilis in the Xixi of Jiulong River Basin. Water, 17(18), 2667. https://doi.org/10.3390/w17182667







