Impacts of Reclaimed River-Water Recharge on Groundwater of a Multi-Layered Aquifer System: Combining Hydrochemical Analysis and End-Member Mixing Approaches
Abstract
1. Introduction
2. Materials and Methods
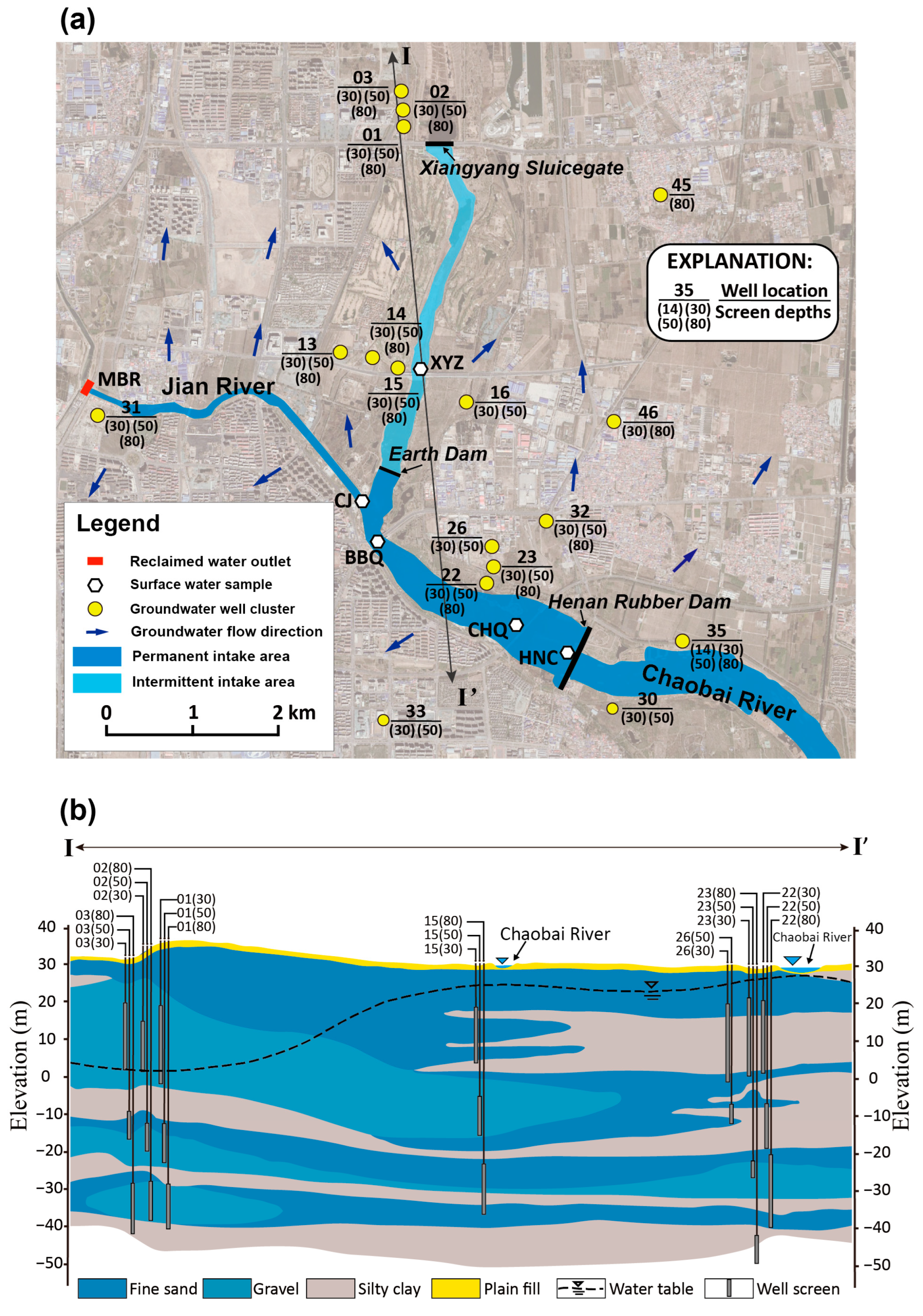
2.1. Site Description and Hydrogeology
2.2. Sample Collection and Analysis
2.3. Data Analysis Methods
3. Results
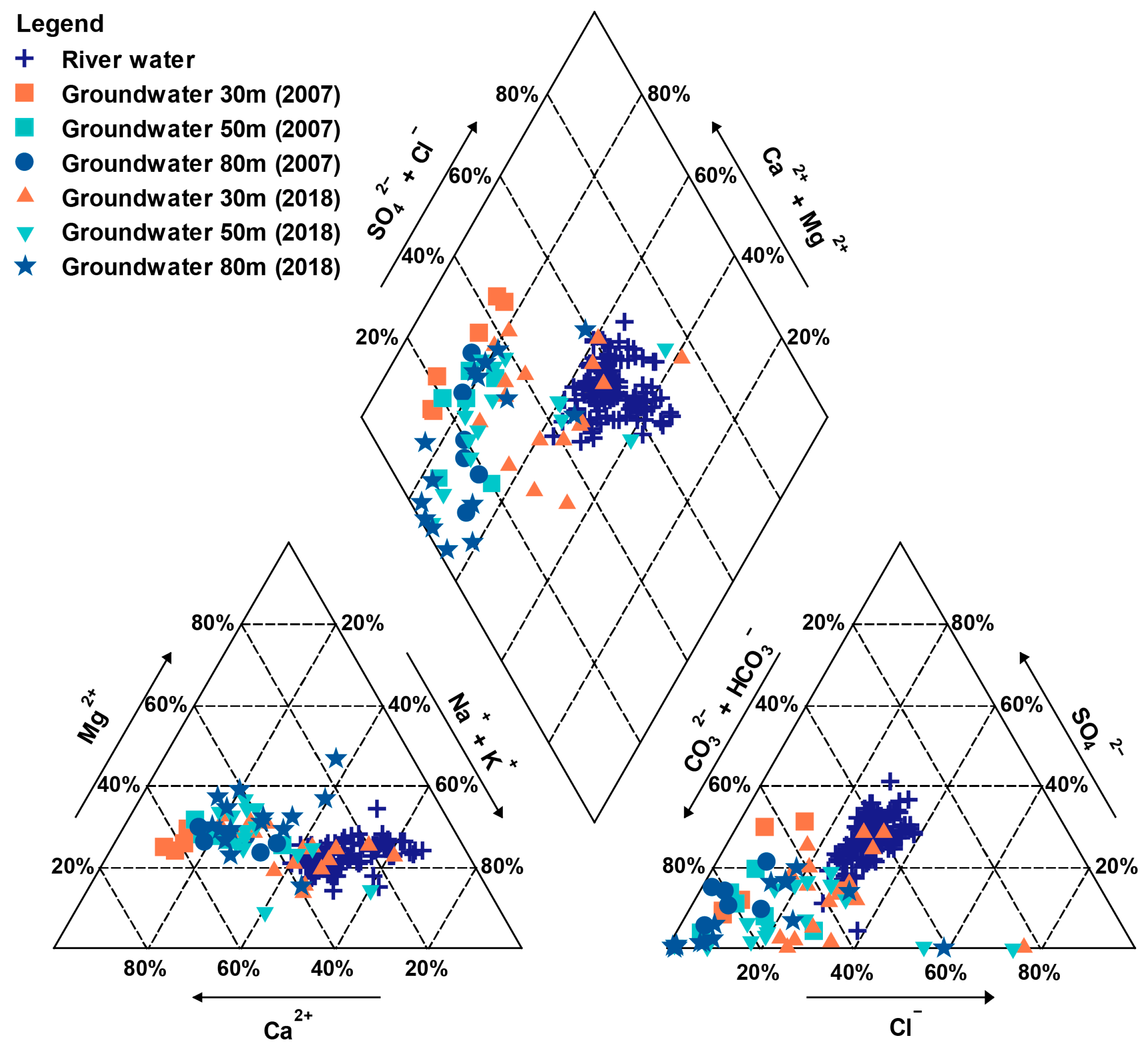
3.1. Hydrochemical Characteristics
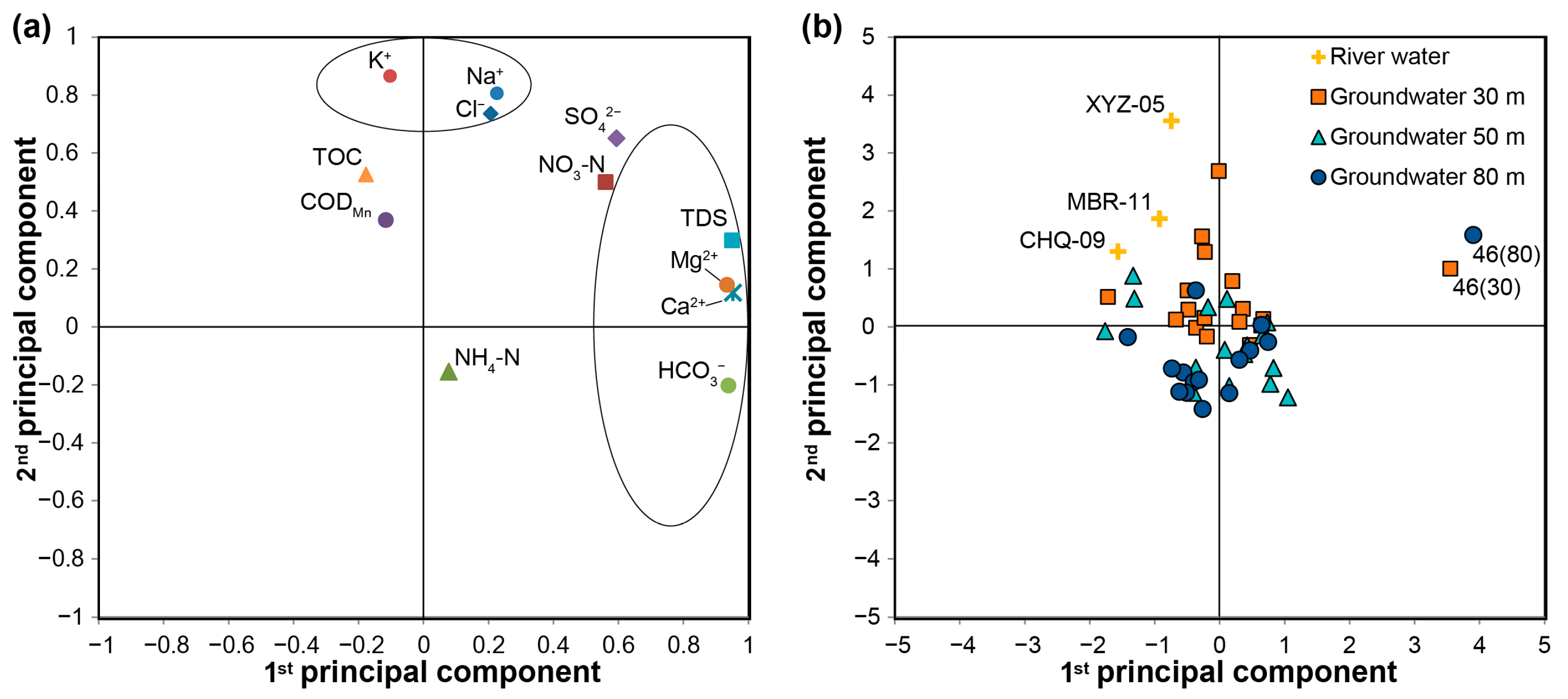
3.2. Principal Component Analysis
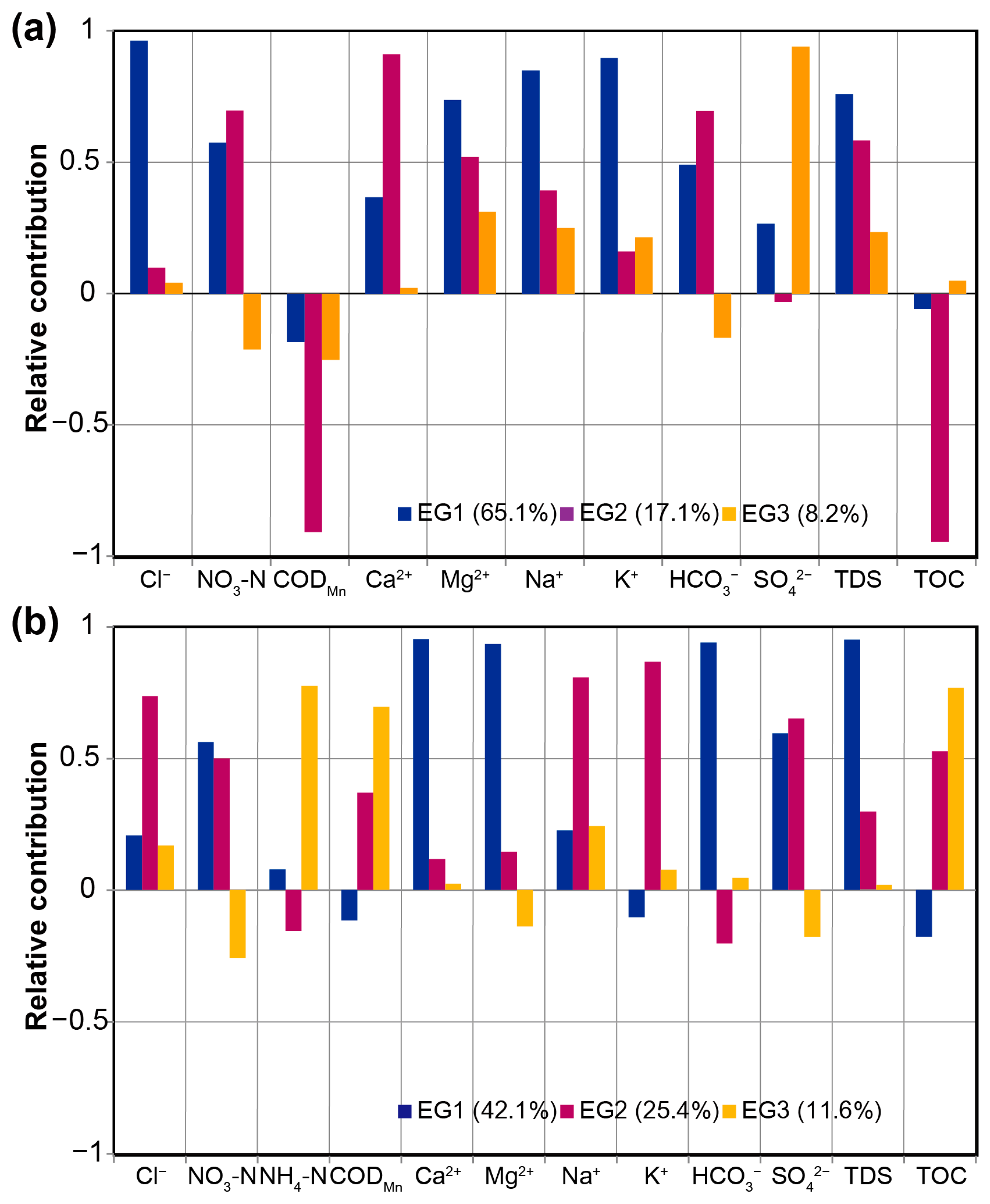
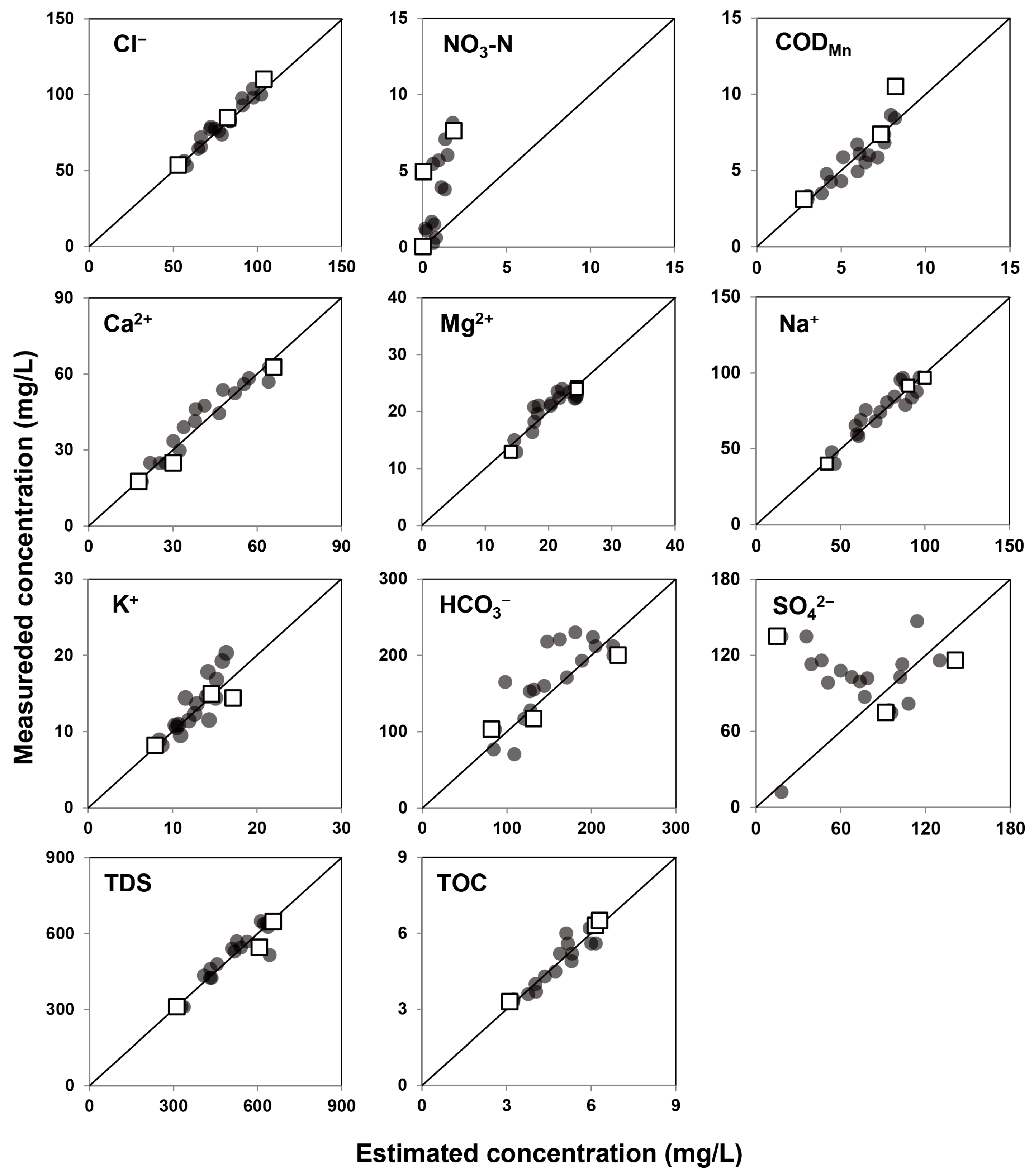
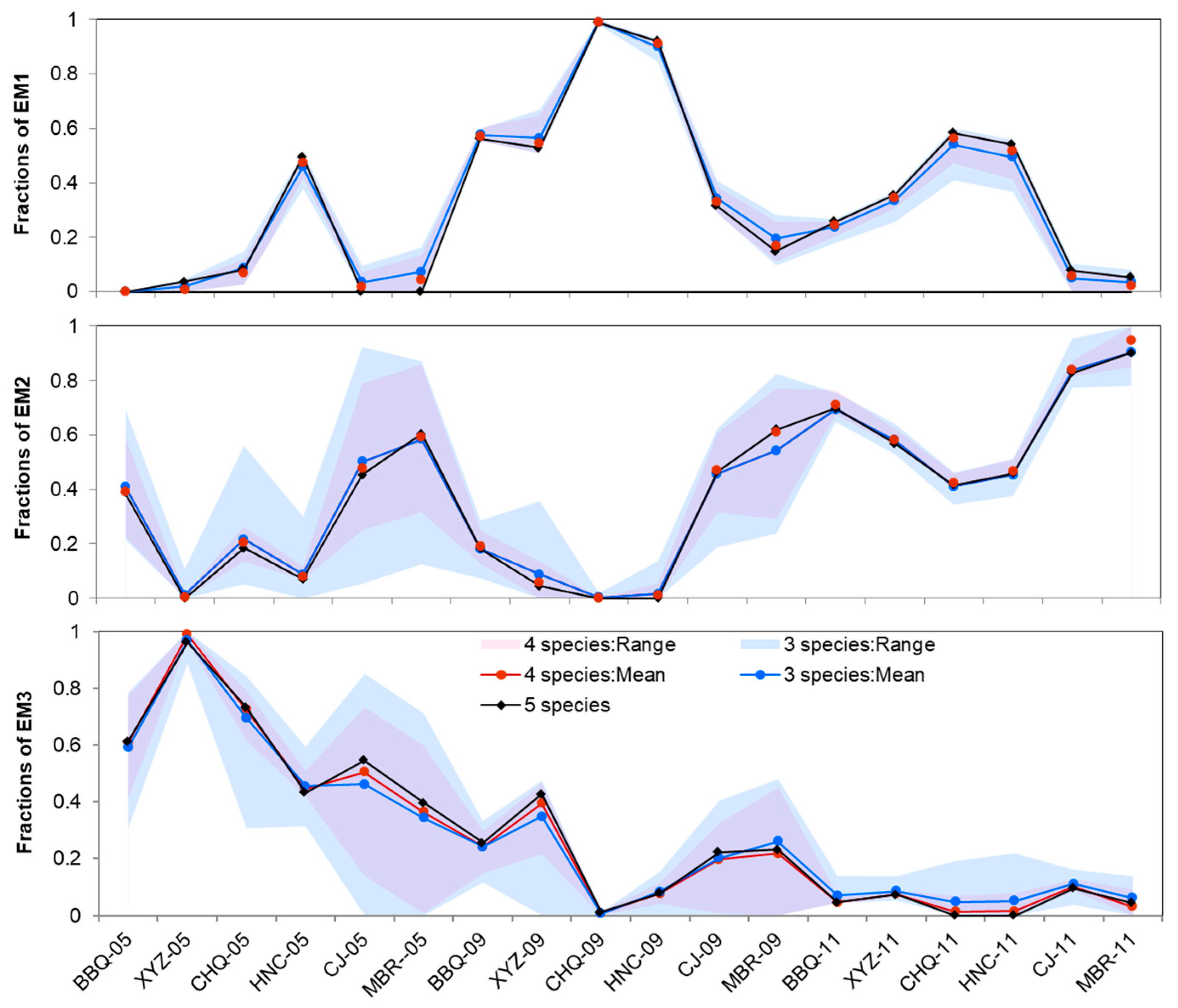
3.3. EMMA for River Water and Groundwater
4. Discussion
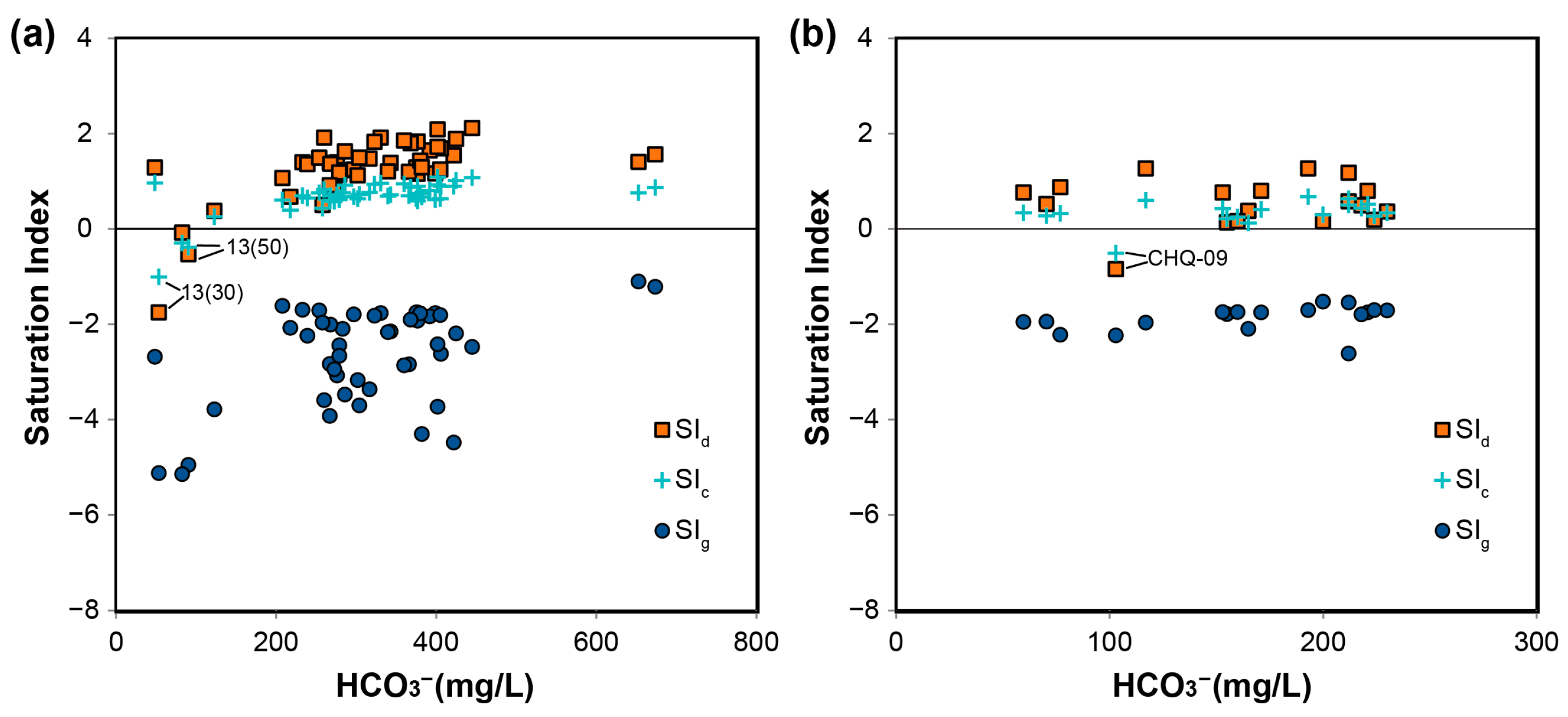
4.1. Processes Controlling Hydrochemical Variations
4.2. Qualitative Evaluation of Mixing Ratio
4.2.1. River Water EMMA
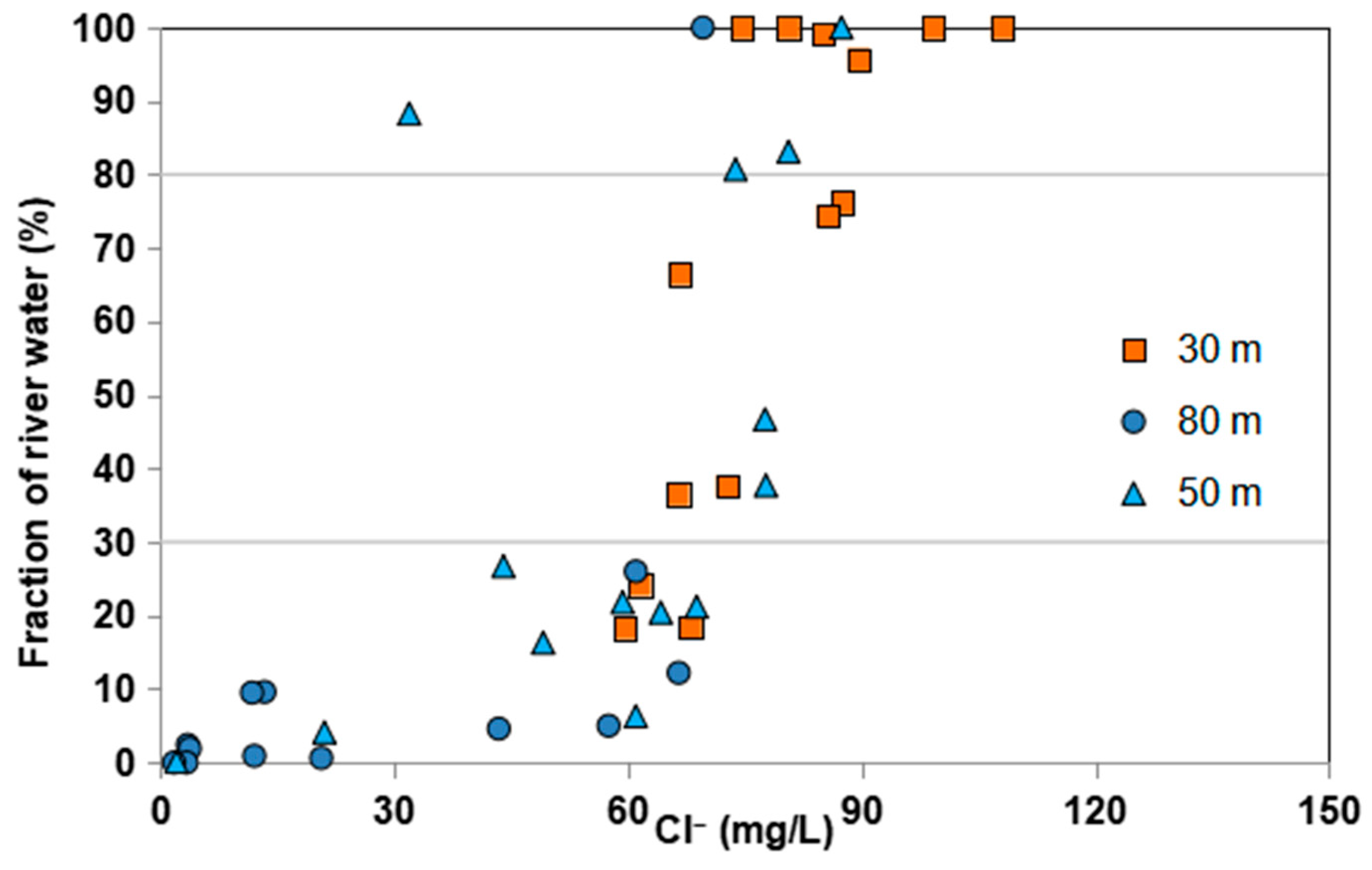
4.2.2. Ground Water EMMA
4.3. Implications for Impacts of Reclaimed River-Water Recharge on Groundwater of Multi-Layered Aquifer Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crook, J.; MacDonald, J.A.; Trussell, R.R. Potable use of reclaimed water. J. AWWA 1999, 91, 40–49. [Google Scholar] [CrossRef]
- Anderson, J. The environmental benefits of water recycling and reuse. Water Supply 2003, 3, 1–10. [Google Scholar] [CrossRef]
- Dillon, P.; Stuyfzand, P.; Grischek, T.; Lluria, M.; Pyne, R.D.G.; Jain, R.C.; Bear, J.; Schwarz, J.; Wang, W.; Fernandez, E.; et al. Sixty years of global progress in managed aquifer recharge. Hydrogeol. J. 2019, 27, 1–30. [Google Scholar] [CrossRef]
- Gilabert-Alarcón, C.; Daesslé, L.W.; Salgado-Méndez, S.O.; Pérez-Flores, M.A.; Knöller, K.; Kretzschmar, T.G.; Stumpp, C. Effects of reclaimed water discharge in the Maneadero coastal aquifer, Baja California, Mexico. Appl. Geochem. 2018, 92, 121–139. [Google Scholar] [CrossRef]
- Bekele, E.; Zhang, Y.; Donn, M.; McFarlane, D. Inferring groundwater dynamics in a coastal aquifer near wastewater infiltration ponds and shallow wetlands (Kwinana, Western Australia) using combined hydrochemical, isotopic and statistical approaches. J. Hydrol. 2019, 568, 1055–1070. [Google Scholar] [CrossRef]
- Narr, C.F.; Singh, H.; Mayer, P.; Keeley, A.; Faulkner, B.; Beak, D.; Forshay, K.J. Quantifying the effects of surface conveyance of treated wastewater effluent on groundwater, surface water, and nutrient dynamics in a large river floodplain. Ecol. Eng. 2019, 129, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bourg, A.C.M.; Bertin, C. Biogeochemical processes during the infiltration of river water into an alluvial aquifer. Environ. Sci. Technol. 1993, 27, 661–666. [Google Scholar] [CrossRef]
- Irmscher, R.; Teermann, I. Riverbank filtration for drinking water supply—A proven method, perfect to face today’s challenges. Water Supply 2002, 2, 1–8. [Google Scholar] [CrossRef]
- Missimer, T.M.; Drewes, J.E.; Amy, G.; Maliva, R.G.; Keller, S. Restoration of Wadi Aquifers by Artificial Recharge with Treated Waste Water. Groundwater 2012, 50, 514–527. [Google Scholar] [CrossRef]
- Ortuño, F.; Molinero, J.; Garrido, T.; Custodio, E. Seawater injection barrier recharge with advanced reclaimed water at Llobregat delta aquifer (Spain). Water Sci. Technol. 2012, 66, 2083–2089. [Google Scholar] [CrossRef]
- Chen, W.; Lu, S.; Jiao, W.; Wang, M.; Chang, A.C. Reclaimed water: A safe irrigation water source? Environ. Dev. 2013, 8, 74–83. [Google Scholar] [CrossRef]
- Szabo, D.; Coggan, T.L.; Robson, T.C.; Currell, M.; Clarke, B.O. Investigating recycled water use as a diffuse source of per- and polyfluoroalkyl substances (PFASs) to groundwater in Melbourne, Australia. Sci. Total Environ. 2018, 644, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, X.; Li, B.; Ma, Y.; Zhang, Y.; Yang, L.; Bu, H.; Holm, P.E. Temporal variation in groundwater hydrochemistry driven by natural and anthropogenic processes at a reclaimed water irrigation region. Hydrol. Res. 2018, 49, 1652–1668. [Google Scholar] [CrossRef]
- Toze, S.; Bekele, E.; Page, D.; Sidhu, J.; Shackleton, M. Use of static Quantitative Microbial Risk Assessment to determine pathogen risks in an unconfined carbonate aquifer used for Managed Aquifer Recharge. Water Res. 2010, 44, 1038–1049. [Google Scholar] [CrossRef]
- Asano, T.; Cotruvo, J.A. Groundwater recharge with reclaimed municipal wastewater: Health and regulatory considerations. Water Res. 2004, 38, 1941–1951. [Google Scholar] [CrossRef]
- Wu, W.; Liao, R.; Hu, Y.; Wang, H.; Liu, H.; Yin, S. Quantitative assessment of groundwater pollution risk in reclaimed water irrigation areas of northern China. Environ. Pollut. 2020, 261, 114173. [Google Scholar] [CrossRef]
- Zheng, F.; Liu, L.; Li, B.; Yang, Y.; Guo, M. Effects of Reclaimed Water Use for Scenic Water on Groundwater Environment in a Multilayered Aquifer System beneath the Chaobai River, Beijing, China: Case Study. J. Hydrol. Eng. 2015, 20, B5014003. [Google Scholar] [CrossRef]
- Li, J.; Fu, J.; Zhang, H.; Li, Z.; Ma, Y.; Wu, M.; Liu, X. Spatial and seasonal variations of occurrences and concentrations of endocrine disrupting chemicals in unconfined and confined aquifers recharged by reclaimed water: A field study along the Chaobai River, Beijing. Sci. Total Environ. 2013, 450–451, 162–168. [Google Scholar] [CrossRef]
- Li, C.; Li, B.; Bi, E. Characteristics of hydrochemistry and nitrogen behavior under long-term managed aquifer recharge with reclaimed water: A case study in north China. Sci. Total Environ. 2019, 668, 1030–1037. [Google Scholar] [CrossRef]
- He, Z.; Han, D.; Song, X.; Yang, L.; Zhang, Y.; Ma, Y.; Bu, H.; Li, B.; Yang, S. Variations of Groundwater Dynamics in Alluvial Aquifers with Reclaimed Water Restoring the Overlying River, Beijing, China. Water 2021, 13, 806. [Google Scholar] [CrossRef]
- Rueedi, J.; Cronin, A.A.; Morris, B.L. Estimation of sewer leakage to urban groundwater using depth-specific hydrochemistry. Water Environ. J. 2009, 23, 134–144. [Google Scholar] [CrossRef]
- Yang, L.; He, J.; Liu, Y.; Wang, J.; Jiang, L.; Wang, G. Characteristics of change in water quality along reclaimed water intake area of the Chaobai River in Beijing, China. J. Environ. Sci. 2016, 50, 93–102. [Google Scholar] [CrossRef]
- McCance, W.; Jones, O.A.H.; Edwards, M.; Surapaneni, A.; Chadalavada, S.; Currell, M. Contaminants of Emerging Concern as novel groundwater tracers for delineating wastewater impacts in urban and peri-urban areas. Water Res. 2018, 146, 118–133. [Google Scholar] [CrossRef]
- Moeck, C.; Radny, D.; Popp, A.; Brennwald, M.; Stoll, S.; Auckenthaler, A.; Berg, M.; Schirmer, M. Characterization of a managed aquifer recharge system using multiple tracers. Sci. Total Environ. 2017, 609, 701–714. [Google Scholar] [CrossRef]
- Helena, B.; Pardo, R.; Vega, M.; Barrado, E.; Fernandez, J.M.; Fernandez, L. Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res. 2000, 34, 807–816. [Google Scholar] [CrossRef]
- Scheiber, L.; Cendón, D.I.; Iverach, C.P.; Hankin, S.I.; Vázquez-Suñé, E.; Kelly, B.F.J. Hydrochemical apportioning of irrigation groundwater sources in an alluvial aquifer. Sci. Total Environ. 2020, 744, 140506. [Google Scholar] [CrossRef]
- Aji, K.; Tang, C.; Song, X.; Kondoh, A.; Sakura, Y.; Yu, J.; Kaneko, S. Characteristics of chemistry and stable isotopes in groundwater of Chaobai and Yongding River basin, North China Plain. Hydrol. Process 2008, 22, 63–72. [Google Scholar] [CrossRef]
- Yu, Y.; Song, X.; Zhang, Y.; Zheng, F. Assessment of Water Quality Using Chemometrics and Multivariate Statistics: A Case Study in Chaobai River Replenished by Reclaimed Water, North China. Water 2020, 12, 2551. [Google Scholar] [CrossRef]
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Carrera, J.; Vázquez-Suñé, E.; Castillo, O.; Sánchez-Vila, X. A methodology to compute mixing ratios with uncertain end-members. Water Resour. Res. 2004, 40, 1–11. [Google Scholar] [CrossRef]
- Tubau, I.; Vàzquez-Suñé, E.; Jurado, A.; Carrera, J. Using EMMA and MIX analysis to assess mixing ratios and to identify hydrochemical reactions in groundwater. Sci. Total Environ. 2014, 470–471, 1120–1131. [Google Scholar] [CrossRef]
- Behrouj-Peely, A.; Mohammadi, Z.; Scheiber, L.; Vázquez-Suñé, E. An integrated approach to estimate the mixing ratios in a karst system under different hydrogeological conditions. J. Hydrol. Reg. Stud. 2020, 30, 100693. [Google Scholar] [CrossRef]
- Christophersen, N.; Hooper, R.P. Multivariate analysis of stream water chemical data: The use of principal components analysis for the end-member mixing problem. Water Resour. Res. 1992, 28, 99–107. [Google Scholar] [CrossRef]
- Pan, X.; Sun, B.; Zhang, S.; Li, G.; Tian, Z.; Guo, Z.; Yu, H.; Yang, Z. Ion sources and seasonal recharge characteristics of groundwater around Dali Lake in semi-arid region of Inner Mongolia Plateau, China. J. Geochemical Explor. 2025, 269, 107612. [Google Scholar] [CrossRef]
- Zheng, F. Case Study on Effects Of reclaimed Water Use for Scenic Water on Groundwater environment in Chaobai River; China University of Geosciences: Beijing, China, 2012. [Google Scholar]
- Yuan, R.; Wang, M.; Wang, S.; Song, X. Water transfer imposes hydrochemical impacts on groundwater by altering the interaction of groundwater and surface water. J. Hydrol. 2020, 583, 124617. [Google Scholar] [CrossRef]
- Rajmohan, N.; Masoud, M.H.Z.; Niyazi, B.A.M. Impact of evaporation on groundwater salinity in the arid coastal aquifer, Western Saudi Arabia. Catena 2021, 196, 104864. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science (80-) 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Mostaza-Colado, D.; Carreño-Conde, F.; Rasines-Ladero, R.; Iepure, S. Hydrogeochemical characterization of a shallow alluvial aquifer: 1 baseline for groundwater quality assessment and resource management. Sci. Total Environ. 2018, 639, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, W.M.; Carrillo-Rivera, J.J.; Cardona, A. Geochemical evolution of groundwater beneath Mexico City. J. Hydrol. 2002, 258, 1–24. [Google Scholar] [CrossRef]
- Huang, T.; Pang, Z.; Li, J.; Xiang, Y.; Zhao, Z. Mapping groundwater renewability using age data in the Baiyang alluvial fan, NW China. Hydrogeol J. 2017, 25, 743–755. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.H.; Thao, N.T.; Batsaikhan, B.; Yun, S.T. Hydrochemical assessment of freshening saline groundwater using multiple end-members mixing modeling: A study of Red River delta aquifer, Vietnam. J. Hydrol. 2017, 549, 703–714. [Google Scholar] [CrossRef]
- Li, S.; Bian, R.; Li, B.; Huang, J.; Qi, W.; Liu, H. Hyporheic zone geochemistry of a multi-aquifer system used for managed aquifer recharge in Beijing, China. Appl. Geochem. 2021, 131, 105032. [Google Scholar] [CrossRef]
- Martinez, J.L.; Raiber, M.; Cendón, D.I. Using 3D geological modelling and geochemical mixing models to characterise alluvial aquifer recharge sources in the upper Condamine River catchment, Queensland, Australia. Sci. Total Environ. 2017, 574, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Roques, C.; Aquilina, L.; Boisson, A.; Vergnaud-Ayraud, V.; Labasque, T.; Longuevergne, L.; Laurencelle, M.; Dufresne, A.; De Dreuzy, J.R.; Pauwels, H.; et al. Autotrophic denitrification supported by biotite dissolution in crystalline aquifers: (2) transient mixing and denitrification dynamic during long-term pumping. Sci. Total Environ. 2018, 619–620, 491–503. [Google Scholar] [CrossRef]
- Pan, W.; Huang, Q.; Huang, G. Nitrogen and Organics Removal during Riverbank Filtration along a Reclaimed Water Restored River in Beijing, China. Water 2018, 10, 491. [Google Scholar] [CrossRef]
- Armengol, S.; Ajami, H.; Acero Triana, J.S.; OSickman, J.; Ortega, L. Isogeochemical Characterization of Mountain System Recharge Processes in the Sierra Nevada, California. Water. Resour. Res. 2024, 60, e2023WR035719. [Google Scholar] [CrossRef]
- Scheiber, L.; Jurado, A.; Pujades, E.; Criollo, R.; Suñé, E.V. Applied multivariate statistical analysis as a tool for assessing groundwater reactions in the Niebla-Posadas aquifer, Spain. Hydrogeol. J. 2023, 31, 521–536. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, H.; Huang, K.; Pan, Y.; Peng, Y.; He, X.; Wang, S.; Wan, J. Epikarst Controls of Runoff Composition in Subterranean Stream After Rainstorm Events. Hydrol. Process 2024, 38, e15305. [Google Scholar] [CrossRef]
- Pelizardi, F.; Bea, S.A.; Carrera, J.; Vives, L. Identifying geochemical processes using End Member Mixing Analysis to decouple chemical components for mixing ratio calculations. J. Hydrol. 2017, 550, 144–156. [Google Scholar] [CrossRef]
- Popp, A.L.; Scheidegger, A.; Moeck, C.; Brennwald, M.S.; Kipfer, R. Integrating Bayesian Groundwater Mixing Modeling With On-Site Helium Analysis to Identify Unknown Water Sources. Water Resour. Res. 2019, 55, 10602–10615. [Google Scholar] [CrossRef]
- Xia, Q.; He, J.; Li, B.; He, B.; Huang, J.; Guo, M.; Luo, D. Hydrochemical evolution characteristics and genesis of groundwater under long-term infiltration (2007—2018) of reclaimed water in Chaobai River, Beijing. Water Res. 2022, 226, 119222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Bu, H.; Song, X.; Zhang, Y. A multivariate analysis of the spatial variations of water quality during high-flow period in the Chaobai River (Beijing, China) restored by reclaimed water. Water Supply 2021, 21, 3168–3179. [Google Scholar] [CrossRef]
- Yeh, T.C.J.; Liu, S. Hydraulic tomography: Development of a new aquifer test method. Water Resour. Res. 2000, 36, 2095. [Google Scholar] [CrossRef]



| Parameters | Groundwater (n = 48) | River Water (n = 18) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Minimum | Maximum | Standard Deviation | Mean | Median | Minimum | Maximum | Standard Deviation | |
| Ca2+ (mg/L) | 59.9 | 58.3 | 10.8 | 188.0 | 33.1 | 40.7 | 43.0 | 17.6 | 62.6 | 14.3 |
| Mg2+ (mg/L) | 24.4 | 21.6 | 2.7 | 67.0 | 12.0 | 20.9 | 21.9 | 12.9 | 24.3 | 3.2 |
| K+ (mg/L) | 2.5 | 2.0 | 0.4 | 11.8 | 2.0 | 13.3 | 13.0 | 8.2 | 20.3 | 3.4 |
| Na+ (mg/L) | 51.8 | 46.4 | 20.6 | 96.4 | 20.2 | 75.3 | 77.3 | 40.1 | 97.2 | 16.1 |
| HCO3− (mg/L) | 314.9 | 310.5 | 48.6 | 674.0 | 120.4 | 167.2 | 168.0 | 70.5 | 230.0 | 49.7 |
| Cl− (mg/L) | 61.0 | 66.5 | 1.7 | 151.0 | 33.8 | 79.9 | 78.2 | 53.1 | 104.0 | 14.3 |
| SO42− (mg/L) | 42.2 | 20.3 | 0.1 | 225.0 | 48.2 | 101.2 | 103.0 | 12.2 | 147.0 | 28.8 |
| NO3-N (mg/L) | 1.2 | 0.0 | 0.0 | 16.1 | 2.7 | 3.3 | 2.7 | 0.0 | 8.2 | 2.8 |
| NH4-N (mg/L) | 0.4 | 0.1 | 0.0 | 3.0 | 0.7 | 0.1 | 0.1 | 0.0 | 0.5 | 0.1 |
| pH | 8.0 | 8.1 | 7.3 | 9.6 | 0.3 | 8.3 | 8.3 | 7.7 | 9.0 | 0.3 |
| COD (mg/L) | 2.2 | 1.5 | 0.3 | 15.2 | 2.4 | 5.9 | 5.9 | 3.1 | 10.5 | 1.9 |
| TOC (mg/L) | 2.1 | 1.8 | 0.5 | 6.1 | 1.2 | 5.0 | 5.2 | 3.3 | 6.5 | 1.1 |
| TDS (mg/L) | 563.3 | 555.2 | 172.7 | 1373.0 | 217.1 | 517.9 | 535.2 | 310.9 | 649.4 | 104.3 |
| Parameters | Initial Concentrations | Estimated End-Members | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 11 Species | 5 Species | ||||||||
| EM1 | EM2 | EM3 | EM1 | EM2 | EM3 | EM1 | EM2 | EM3 | |
| Cl− (mg/L) | 53.1 | 82.4 | 104.0 | 53.5 | 84.8 | 110.1 | 52.3 | 83.1 | 106.3 |
| NO3-N (mg/L) | 0.03 | 7.62 | 4.93 | 0.03 | 1.86 | 0.06 | - | - | - |
| CODMn (mg/L) | 10.5 | 3.1 | 7.4 | 8.2 | 2.8 | 7.4 | - | - | - |
| Ca2+ (mg/L) | 17.6 | 62.6 | 24.8 | 17.9 | 65.9 | 30.2 | 17.7 | 68.2 | 26.9 |
| Mg2+ (mg/L) | 12.9 | 24.3 | 24.0 | 14.1 | 24.5 | 24.5 | 13.7 | 24.9 | 23.2 |
| Na+ (mg/L) | 40.1 | 91.5 | 96.8 | 41.9 | 90.2 | 99.9 | 40.9 | 91.4 | 92.2 |
| K+ (mg/L) | 8.17 | 14.9 | 14.4 | 8.0 | 14.6 | 17.2 | - | - | - |
| HCO3− (mg/L) | 103 | 200 | 117 | 81.8 | 231.6 | 131.8 | - | - | - |
| SO42− (mg/L) | 74.9 | 135.0 | 116.0 | 91.8 | 15.0 | 141.1 | - | - | - |
| TDS (mg/L) | 310.9 | 646.6 | 545.9 | 312.6 | 656.2 | 606.9 | 302.2 | 663.2 | 558.8 |
| TOC (mg/L) | 6.3 | 3.3 | 6.5 | 6.2 | 3.1 | 6.3 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Song, X.; Yang, L.; Wang, S. Impacts of Reclaimed River-Water Recharge on Groundwater of a Multi-Layered Aquifer System: Combining Hydrochemical Analysis and End-Member Mixing Approaches. Water 2025, 17, 2575. https://doi.org/10.3390/w17172575
Zhao Z, Song X, Yang L, Wang S. Impacts of Reclaimed River-Water Recharge on Groundwater of a Multi-Layered Aquifer System: Combining Hydrochemical Analysis and End-Member Mixing Approaches. Water. 2025; 17(17):2575. https://doi.org/10.3390/w17172575
Chicago/Turabian StyleZhao, Zhanfeng, Xianfang Song, Lihu Yang, and Shuyuan Wang. 2025. "Impacts of Reclaimed River-Water Recharge on Groundwater of a Multi-Layered Aquifer System: Combining Hydrochemical Analysis and End-Member Mixing Approaches" Water 17, no. 17: 2575. https://doi.org/10.3390/w17172575
APA StyleZhao, Z., Song, X., Yang, L., & Wang, S. (2025). Impacts of Reclaimed River-Water Recharge on Groundwater of a Multi-Layered Aquifer System: Combining Hydrochemical Analysis and End-Member Mixing Approaches. Water, 17(17), 2575. https://doi.org/10.3390/w17172575








