From Raw Water to Pipeline Water: Correlation Analysis of Dynamic Changes in Water Quality Parameters and Microbial Community Succession
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Physicochemical Parameter Analysis and Instrumentation
2.3. Biomass Analysis
2.4. DNA Extraction and Sequencing
2.5. Construction of Correlation Analysis Heatmaps
2.6. Statistics Analysis
3. Results
3.1. Diurnal, Monthly, and Annual Variation Characteristics of Water Quality
3.1.1. Diurnal Variation Characteristics of Water Quality
3.1.2. Intramonthly Variation Characteristics of Water Quality
3.1.3. Annual Variation Characteristics of Water Quality
3.1.4. Association Characteristics of Water Quality Variations
3.2. Correlation Characteristics Between Pipeline Network Water and Other Process Waters
3.2.1. Correlation Between Pipeline Network Water and Raw Water Quality
3.2.2. Correlation Between Pipeline Network Water and Effluent Quality
3.2.3. Continuity Analysis of Water Quality Correlation from Raw Water to Effluents to Pipeline Network Water
3.3. Microbial Community Succession from Raw Water to Effluents to Pipeline Network Water
4. Discussion
4.1. Diurnal, Monthly, and Annual Variation Characteristics of Water Quality
4.1.1. Analysis of Diurnal, Monthly, and Annual Water Quality Characteristics
4.1.2. Correlation Analysis of Water Quality Characteristics
4.2. Continuity Analysis of Water Quality Correlations Across Treatment Stages
4.2.1. Correlation Between Pipeline Water and Raw Water
4.2.2. Correlation Between Pipeline Water and Effluents
4.2.3. Continuity Analysis of Water Quality Correlation from Raw Water to Effluents to Pipeline Network Water
4.3. Microbial Community Succession from Raw Water to Effluents to Pipeline Network Water
5. Conclusions
- (1)
- Diurnal fluctuations in pipeline water quality parameters were primarily caused by biofilm detachment and particle resuspension due to high shear stress from unsteady hydraulic conditions during peak water consumption periods. The significant negative correlation between biomass and free chlorine at monthly scales indicated that insufficient disinfectant residuals were the main driver of microbial resurgence. Annual water quality characteristics exhibited seasonal differences: low winter temperatures reduced pH through humic substance input, while high summer temperatures accelerated disinfectant decomposition and biofilm proliferation, leading to dramatic microbial biomass increases.
- (2)
- From raw water to pipeline water, metal–organic composite correlations weakened (Δr = 0.26 ± 0.19), while turbidity–sulfate and metal–biomass interactions strengthened (Δr = 0.44 ± 0.29), indicating that water treatment processes reshaped the synergistic mechanisms among water quality parameters.
- (3)
- The core factors influencing microbial community succession from source to treated to pipeline water were water nutrient status and disinfection pressure. Pseudomonas and other genera in pipe wall biofilms resisted disinfectants, while Phreatobacter dominated planktonic communities via oligotrophic adaptability, reflecting the decisive role of environmental conditions in shaping microbial community structure.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DWDS | Drinking water distribution systems |
| TCC | Total cell counts |
| ICC | Intact cell concentration |
| TOC | Total organic carbon |
| WHO | World Health Organization |
| THMs | Trihalomethanes |
| TCM | Trichloromethane |
References
- Zhang, X.; Guo, Q.; Shen, X.; Yu, S.; Qiu, G. Water Quality, Agriculture and Food Safety in China: Current Situation, Trends, Interdependencies, and Management. J. Integr. Agric. 2015, 14, 2365–2379. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, M.; Cheng, J.; Jiang, C. Characteristics and Impact Evaluation of Hydrological and Water Quality Changes in the Northern Plain of Cixi, Eastern China, from 2010 to 2022. Water 2024, 16, 489. [Google Scholar] [CrossRef]
- Luo, H.; Nong, X.; Xia, H.; Liu, H.; Zhong, L.; Feng, Y.; Zhou, W.; Lu, Y. Integrating Water Quality Index (WQI) and Multivariate Statistics for Regional Surface Water Quality Evaluation: Key Parameter Identification and Human Health Risk Assessment. Water 2024, 16, 3412. [Google Scholar] [CrossRef]
- Olatunde, K.; Patton, S.K.; Cameron, L.; Stankus, T.; Milaham, P.J. Factors Affecting the Quality of Drinking Water in the United States of America: A Ten-Year Systematic Review. Am. J. Water Resour. 2022, 10, 24–34. [Google Scholar] [CrossRef]
- Perveen, S. Amar-Ul-Haque Drinking Water Quality Monitoring, Assessment and Management in Pakistan: A Review. Heliyon 2023, 9, e13872. [Google Scholar] [CrossRef]
- Price, J.I.; Heberling, M.T. The Effects of Source Water Quality on Drinking Water Treatment Costs: A Review and Synthesis of Empirical Literature. Ecol. Econ. 2018, 151, 195–209. [Google Scholar] [CrossRef]
- Rahman, S.; Khan, A.A.; Brake, K.; Tobin, A. Deriving Water Quality Index Using Site-Specific Water Quality Parameter Guidelines for Real-Time Water Quality Stations. Water Qual. Res. J. 2024, 59, 347–367. [Google Scholar] [CrossRef]
- Xu, W.; Duan, L.; Wen, X.; Li, H.; Li, D.; Zhang, Y.; Zhang, H. Effects of Seasonal Variation on Water Quality Parameters and Eutrophication in Lake Yangzong. Water 2022, 14, 2732. [Google Scholar] [CrossRef]
- Riyadh, A.; Peleato, N.M. Natural Organic Matter Character in Drinking Water Distribution Systems: A Review of Impacts on Water Quality and Characterization Techniques. Water 2024, 16, 446. [Google Scholar] [CrossRef]
- Zlatanović, L.; van der Hoek, J.P.; Vreeburg, J.H.G. An Experimental Study on the Influence of Water Stagnation and Temperature Change on Water Quality in a Full-Scale Domestic Drinking Water System. Water Res. 2017, 123, 761–772. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Li, W.; Cai, X.; Liu, X.; Liao, H. Multi-Scale Impacts of Land Use Change on River Water Quality in the Xinxian River, Yangtze River Basin. Water 2025, 17, 1541. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhou, L. Correlation Analysis between Landscape Metrics and Water Quality under Multiple Scales. Int. J. Environ. Res. Public Health 2018, 15, 1606. [Google Scholar] [CrossRef] [PubMed]
- Moeinzadeh, H.; Yong, K.-T.; Withana, A. A Critical Analysis of Parameter Choices in Water Quality Assessment. Water Res. 2024, 258, 121777. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, M.J.; Laxander, M.; Miettinen, I.T.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. The Effects of Changing Water Flow Velocity on the Formation of Biofilms and Water Quality in Pilot Distribution System Consisting of Copper or Polyethylene Pipes. Water Res. 2006, 40, 2151–2160. [Google Scholar] [CrossRef]
- GB/T 11892-1989; Water Quality-Determination of Permanganate Index. National Environmental Protection Bureau Standards Press of China: Beijing, China, 1990.
- GB/T 5750.4-2006; Standard Examination Methods for Drinking Water—Part 4: Organoleptic and Physical Indices. Standardization Administration of China Standards Press of China: Beijing, China, 2006.
- Aisopou, A.; Stoianov, I.; Graham, N.J.D. In-Pipe Water Quality Monitoring in Water Supply Systems under Steady and Unsteady State Flow Conditions: A Quantitative Assessment. Water Res. 2012, 46, 235–246. [Google Scholar] [CrossRef]
- Juncosa, R.; Cereijo, J.L.; Vázquez, R. Physicochemical Parameters in the Generation of Turbidity Episodes in a Water Supply Distribution System. Water 2022, 14, 3383. [Google Scholar] [CrossRef]
- Novak Babič, M.; Gunde-Cimerman, N. Water-Transmitted Fungi Are Involved in Degradation of Concrete Drinking Water Storage Tanks. Microorganisms 2021, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Calero Preciado, C.; Husband, S.; Boxall, J.; del Olmo, G.; Soria-Carrasco, V.; Maeng, S.K.; Douterelo, I. Intermittent Water Supply Impacts on Distribution System Biofilms and Water Quality. Water Res. 2021, 201, 117372. [Google Scholar] [CrossRef]
- Agudelo-Vera, C.; Blokker, M.; Pieterse-Quirijns, I. Early Warning Systems to Predict Temperature in the Drinking Water Distribution Network. Procedia Eng. 2014, 70, 23–30. [Google Scholar] [CrossRef]
- Manuel, C.M.; Nunes, O.C.; Melo, L.F. Dynamics of Drinking Water Biofilm in Flow/Non-Flow Conditions. Water Res. 2007, 41, 551–562. [Google Scholar] [CrossRef]
- Douterelo, I.; Sharpe, R.L.; Boxall, J.B. Influence of Hydraulic Regimes on Bacterial Community Structure and Composition in an Experimental Drinking Water Distribution System. Water Res. 2013, 47, 503–516. [Google Scholar] [CrossRef]
- Kumpel, E.; Nelson, K.L. Intermittent Water Supply: Prevalence, Practice, and Microbial Water Quality. Environ. Sci. Technol. 2016, 50, 542–553. [Google Scholar] [CrossRef]
- Hu, D.; Zeng, J.; Hu, Y.; Fei, X.; Xiao, X.; Feng, M.; Yu, X. A Survey on Heavy Metal Concentrations in Residential Neighborhoods: The Influence of Secondary Water Supply Systems. J. Environ. Sci. 2022, 117, 37–45. [Google Scholar] [CrossRef]
- Hu, D.; Hong, H.; Rong, B.; Wei, Y.; Zeng, J.; Zhu, J.; Bai, L.; Guo, F.; Yu, X. A Comprehensive Investigation of the Microbial Risk of Secondary Water Supply Systems in Residential Neighborhoods in a Large City. Water Res. 2021, 205, 117690. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.J.; Niu, Z.B.; Chen, C.; Lu, P.P.; Tang, F. Effect of Chloramine Residual on Iron Release in Drinking Water Distribution Systems. Water Supply 2009, 9, 349–355. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Shirokova, L.S. Diurnal Variations of Dissolved and Colloidal Organic Carbon and Trace Metals in a Boreal Lake during Summer Bloom. Water Res. 2013, 47, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Kimbell, L.K.; Wang, Y.; McNamara, P.J. The Impact of Metal Pipe Materials, Corrosion Products, and Corrosion Inhibitors on Antibiotic Resistance in Drinking Water Distribution Systems. Appl. Microbiol. Biotechnol. 2020, 104, 7673–7688. [Google Scholar] [CrossRef] [PubMed]
- Urishev, B.; Artikbekova, F.; Kuvvatov, D.; Nosirov, F.; Kuvatov, U. Trajectory of Sediment Deposition at the Bottom of Water Intake Structures of Pumping Stations. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1030, 012137. [Google Scholar] [CrossRef]
- MacKeown, H.; Adusei Gyamfi, J.; Schoutteten, K.V.K.M.; Dumoulin, D.; Verdickt, L.; Ouddane, B.; Criquet, J. Formation and Removal of Disinfection By-Products in a Full Scale Drinking Water Treatment Plant. Sci. Total Environ. 2020, 704, 135280. [Google Scholar] [CrossRef]
- Valdivia-Garcia, M.; Weir, P.; Frogbrook, Z.; Graham, D.W.; Werner, D. Climatic, Geographic and Operational Determinants of Trihalomethanes (THMs) in Drinking Water Systems. Sci. Rep. 2016, 6, 35027. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Park, B.H.; Lee, J.H.; Kang, J.W. Measurement and Correlation of Solubility of Water in Carbon Dioxide-Rich Phase. Fluid Phase Equilibria 2012, 328, 9–12. [Google Scholar] [CrossRef]
- Li, K.; Cao, X.; Zhou, S.; Li, L. Spatial and Temporal Distribution Characteristics of pCO2 and CO2 Evasion in Karst Rivers under the Influence of Urbanization. Environ. Sci. Pollut. Res. 2023, 30, 53920–53937. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Q.; Wang, B.; Hou, F.; Pang, H.; Guo, Y.; Zhang, L.; Peng, Y. Temperature-Based Strategy for Enhanced Nitrogen Removal in Mainstream via Selectively Strengthening Anammox or Denitrification. npj Clean Water 2025, 8, 23. [Google Scholar] [CrossRef]
- Meyneng, M.; Lemonnier, H.; Le Gendre, R.; Plougoulen, G.; Antypas, F.; Ansquer, D.; Serghine, J.; Schmitt, S.; Siano, R. Subtropical Coastal Microbiome Variations Due to Massive River Runoff after a Cyclonic Event. Environ. Microbiome 2024, 19, 10. [Google Scholar] [CrossRef]
- Delair, Z.; Schoeman, M.; Reyneke, B.; Singh, A.; Barnard, T.G. Assessing the Impact of Escherichia Coli on Recreational Water Safety Using Quantitative Microbial Risk Assessment. J. Water Health 2024, 22, 1781–1793. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Khatami, S.; Deng, Y.; Tien, M.; Hatcher, P.G. Formation of Water-Soluble Organic Matter through Fungal Degradation of Lignin. Org. Geochem. 2019, 135, 64–70. [Google Scholar] [CrossRef]
- Saarinen, T.; Vuori, K.-M.; Alasaarela, E.; Kløve, B. Long-Term Trends and Variation of Acidity, CODMn and Colour in Coastal Rivers of Western Finland in Relation to Climate and Hydrology. Sci. Total Environ. 2010, 408, 5019–5027. [Google Scholar] [CrossRef]
- Xi, H.; Zhou, X.; Arslan, M.; Luo, Z.; Wei, J.; Wu, Z.; Gamal El-Din, M. Heterotrophic Nitrification and Aerobic Denitrification Process: Promising but a Long Way to Go in the Wastewater Treatment. Sci. Total Environ. 2022, 805, 150212. [Google Scholar] [CrossRef]
- Sparrow, L.A.; Uren, N.C. Manganese Oxidation and Reduction in Soils: Effects of Temperature, Water Potential, pH and Their Interactions. Soil Res. 2014, 52, 483–494. [Google Scholar] [CrossRef]
- Paufler, S.; Grischek, T.; Benso, M.R.; Seidel, N.; Fischer, T. The Impact of River Discharge and Water Temperature on Manganese Release from the Riverbed during Riverbank Filtration: A Case Study from Dresden, Germany. Water 2018, 10, 1476. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, Y.; Dai, Z.; Ren, A.; Fang, J.; Li, X.; van der Meer, W.; Medema, G.; Rose, J.B.; Liu, G. Building Water Quality Deterioration during Water Supply Restoration after Interruption: Influences of Premise Plumbing Configuration. Water Res. 2023, 241, 120149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, X.; Zhang, T.; Cen, C.; Mao, R.; Pan, R. Pilot Investigation on Biostability of Drinking Water Distribution Systems under Water Source Switching. Appl. Microbiol. Biotechnol. 2022, 106, 5273–5286. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, K.; Cen, C.; Zhou, X.; Xu, J.; Wu, J.; Wu, X. Characteristics of Biostability of Drinking Water in Aged Pipes after Water Source Switching: ATP Evaluation, Biofilms Niches and Microbial Community Transition. Environ. Pollut. 2021, 271, 116293. [Google Scholar] [CrossRef]
- Liu, J.; Shentu, H.; Chen, H.; Ye, P.; Xu, B.; Zhang, Y.; Bastani, H.; Peng, H.; Chen, L.; Zhang, T. Change Regularity of Water Quality Parameters in Leakage Flow Conditions and Their Relationship with Iron Release. Water Res. 2017, 124, 353–362. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Huang, T.; Yan, M.; Liu, K.; Miao, Y.; He, H.; Li, S.; Sekar, R. Combined Effects of Seasonality and Stagnation on Tap Water Quality: Changes in Chemical Parameters, Metabolic Activity and Co-Existence in Bacterial Community. J. Hazard. Mater. 2021, 403, 124018. [Google Scholar] [CrossRef]
- Sun, H.; Shi, B.; Yang, F.; Wang, D. Effects of Sulfate on Heavy Metal Release from Iron Corrosion Scales in Drinking Water Distribution System. Water Res. 2017, 114, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, B.; Bai, Y.; Sun, H.; Lytle, D.A.; Wang, D. Effect of Sulfate on the Transformation of Corrosion Scale Composition and Bacterial Community in Cast Iron Water Distribution Pipes. Water Res. 2014, 59, 46–57. [Google Scholar] [CrossRef]
- Kumari, M.; Tripathi, S.; Pathak, V.; Tripathi, B.D. Chemometric Characterization of River Water Quality. Environ. Monit. Assess. 2013, 185, 3081–3092. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Singh, V.P.; He, Y.; Bai, X. An Improved Index for Water Quality Evaluation in an Estuary Region: A Case Study in the Eastern Pearl River Delta, China. Water Policy 2019, 21, 310–325. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Zhang, X.; Zheng, S.; Zhang, L.; Guo, Z.; Liu, J.; Tian, J.; Zhang, Y.; Zeng, X. Geochemical Characteristics of the Paleogene and Neogene Saline Lacustrine Source Rocks in the Western Qaidam Basin, Northwestern China. Energy Fuels 2016, 30, 4537–4549. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, B.; Yue, Q. Coagulation Performance and Residual Aluminum Speciation of Al2(SO4)3 and Polyaluminum Chloride (PAC) in Yellow River Water Treatment. Chem. Eng. J. 2010, 165, 122–132. [Google Scholar] [CrossRef]
- Tahraoui, H.; Toumi, S.; Boudoukhani, M.; Touzout, N.; Sid, A.N.E.H.; Amrane, A.; Belhadj, A.-E.; Hadjadj, M.; Laichi, Y.; Aboumustapha, M.; et al. Evaluating the Effectiveness of Coagulation–Flocculation Treatment Using Aluminum Sulfate on a Polluted Surface Water Source: A Year-Long Study. Water 2024, 16, 400. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, Y.; Gu, Y.; Zhou, X.; Tian, B.; Zhang, A.; Yang, Z.; Chen, S.; Ma, J.; Ding, M.; et al. Dual Zn5−NiS4 Sites in a Redox-Active Metal–Organic Framework Enables Efficient Cascade Catalysis for Nitrate-to-Ammonia Conversion. Angew. Chem. Int. Ed. 2025, 64, e202418272. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Wang, Y.; Zhu, Y.; Zhu, Z.; Mo, S.; Zhou, X.; Zhang, Y. Performance and Mechanism of Co and Mn Loaded on Fe-Metal-Organic Framework Catalysts with Different Morphologies for Simultaneous Degradation of Acetone and NO by Photothermal Coupling. Toxics 2024, 12, 524. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, P. Preparation and Characterization of Polyaluminum Titanium Silicate and Its Performance in the Treatment of Low-Turbidity Water. Processes 2018, 6, 125. [Google Scholar] [CrossRef]
- Kitjanukit, S.; Takamatsu, K.; Okibe, N. Natural Attenuation of Mn(II) in Metal Refinery Wastewater: Microbial Community Structure Analysis and Isolation of a New Mn(II)-Oxidizing Bacterium Pseudomonas sp. SK3. Water 2019, 11, 507. [Google Scholar] [CrossRef]
- Cudowski, A.; Pietryczuk, A. Biochemical Response of Rhodotorula mucilaginosa and Cladosporium herbarum Isolated from Aquatic Environment on Iron(III) Ions. Sci. Rep. 2019, 9, 19492. [Google Scholar] [CrossRef]
- Appenzeller, B.M.R.; Yañez, C.; Jorand, F.; Block, J.-C. Advantage Provided by Iron for Escherichia Coli Growth and Cultivability in Drinking Water. Appl. Environ. Microbiol. 2005, 71, 5621–5623. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, J.E.; Birrer, S.C.; Dafforn, K.A.; Mitrovic, S.M.; Crane, S.L.; Johnston, E.L.; Wemheuer, F.; Navarro, A.; Harrison, A.J.; Turner, I.L.; et al. Wastewater Effluents Cause Microbial Community Shifts and Change Trophic Status. Water Res. 2021, 200, 117206. [Google Scholar] [CrossRef]
- Wang, B.; Hu, K.; Li, C.; Zhang, Y.; Hu, C.; Liu, Z.; Ding, J.; Chen, L.; Zhang, W.; Fang, J.; et al. Geographic Distribution of Bacterial Communities of Inland Waters in China. Environ. Res. 2024, 249, 118337. [Google Scholar] [CrossRef]
- Perrin, Y.; Bouchon, D.; Delafont, V.; Moulin, L.; Héchard, Y. Microbiome of Drinking Water: A Full-Scale Spatio-Temporal Study to Monitor Water Quality in the Paris Distribution System. Water Res. 2019, 149, 375–385. [Google Scholar] [CrossRef]
- Ma, X.; Li, G.; Chen, R.; Yu, Y.; Tao, H.; Zhang, G.; Shi, B. Revealing the Changes of Bacterial Community from Water Source to Consumers Tap: A Full-Scale Investigation in Eastern City of China. J. Environ. Sci. 2020, 87, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Cui, H.; Wang, W.; Liu, Y.; Cheng, Z.; Wang, C.; Yang, M.; Qu, L.; Li, Y.; Cai, Y.; et al. Bioremediation of Complex Organic Pollutants by Engineered Vibrio Natriegens. Nature 2025, 642, 1024–1033. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Q.; Chen, X.; Zhu, Y.; Yuan, C.; Zhang, C. The Influence Mechanism of DO on the Microbial Community and Carbon Source Metabolism in Two Solid Carbon Source Systems. Environ. Res. 2022, 206, 112410. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, A.; Silva, J.; Teixeira, P. Acinetobacter spp. in Food and Drinking Water—A Review. Food Microbiol. 2021, 95, 103675. [Google Scholar] [CrossRef] [PubMed]
- Holinger, E.P.; Ross, K.A.; Robertson, C.E.; Stevens, M.J.; Harris, J.K.; Pace, N.R. Molecular Analysis of Point-of-Use Municipal Drinking Water Microbiology. Water Res. 2014, 49, 225–235. [Google Scholar] [CrossRef]
- Vaz-Moreira, I.; Nunes, O.C.; Manaia, C.M. Diversity and Antibiotic Resistance in Pseudomonas Spp. from Drinking Water. Sci. Total Environ. 2012, 426, 366–374. [Google Scholar] [CrossRef]
- Sala-Comorera, L.; Blanch, A.R.; Vilaró, C.; Galofré, B.; García-Aljaro, C. Pseudomonas-Related Populations Associated with Reverse Osmosis in Drinking Water Treatment. J. Environ. Manag. 2016, 182, 335–341. [Google Scholar] [CrossRef]
- Afonso, A.C.; Gomes, I.B.; Saavedra, M.J.; Simões, L.C.; Simões, M. Drinking-Water Isolated Delftia acidovorans Selectively Coaggregates with Partner Bacteria and Facilitates Multispecies Biofilm Development. Sci. Total Environ. 2023, 875, 162646. [Google Scholar] [CrossRef]
- Ren, Y.-X.; Yang, L.; Liang, X. The Characteristics of a Novel Heterotrophic Nitrifying and Aerobic Denitrifying Bacterium, Acinetobacter junii YB. Bioresour. Technol. 2014, 171, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Katayanagi, N.; Agbisit, R.; Llorca, L.; Hosen, Y.; Asakawa, S. Influence of Alternate Wetting and Drying Water-Saving Irrigation Practice on the Dynamics of Gallionella-Related Iron-Oxidizing Bacterial Community in Paddy Field Soil. Soil Biol. Biochem. 2021, 152, 108064. [Google Scholar] [CrossRef]
- Cao, Q.; Li, X.; Xie, Z.; Li, C.; Huang, S.; Zhu, B.; Li, D.; Liu, X. Compartmentation of Microbial Communities in Structure and Function for Methane Oxidation Coupled to Nitrification–Denitrification. Bioresour. Technol. 2021, 341, 125761. [Google Scholar] [CrossRef] [PubMed]
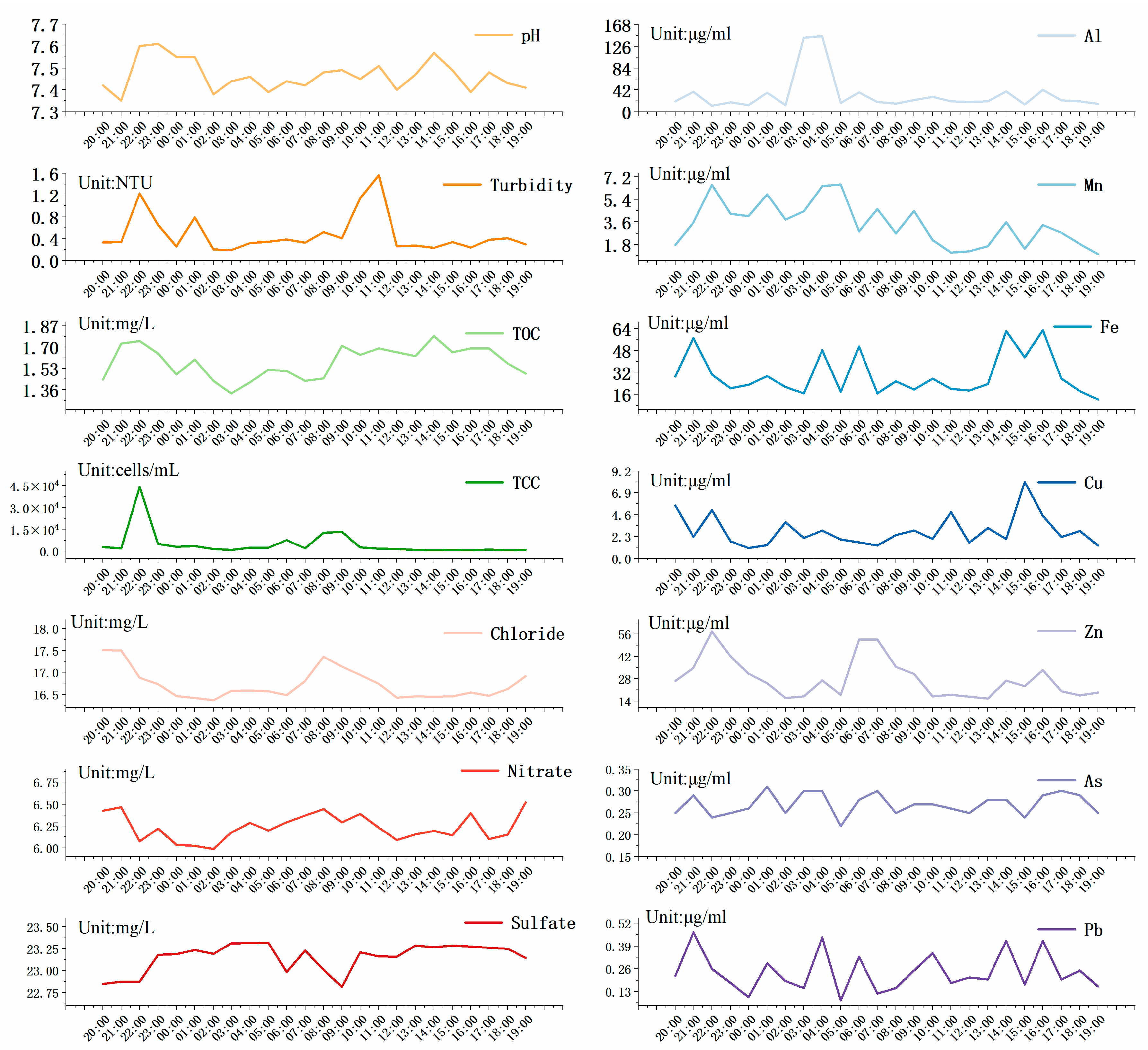

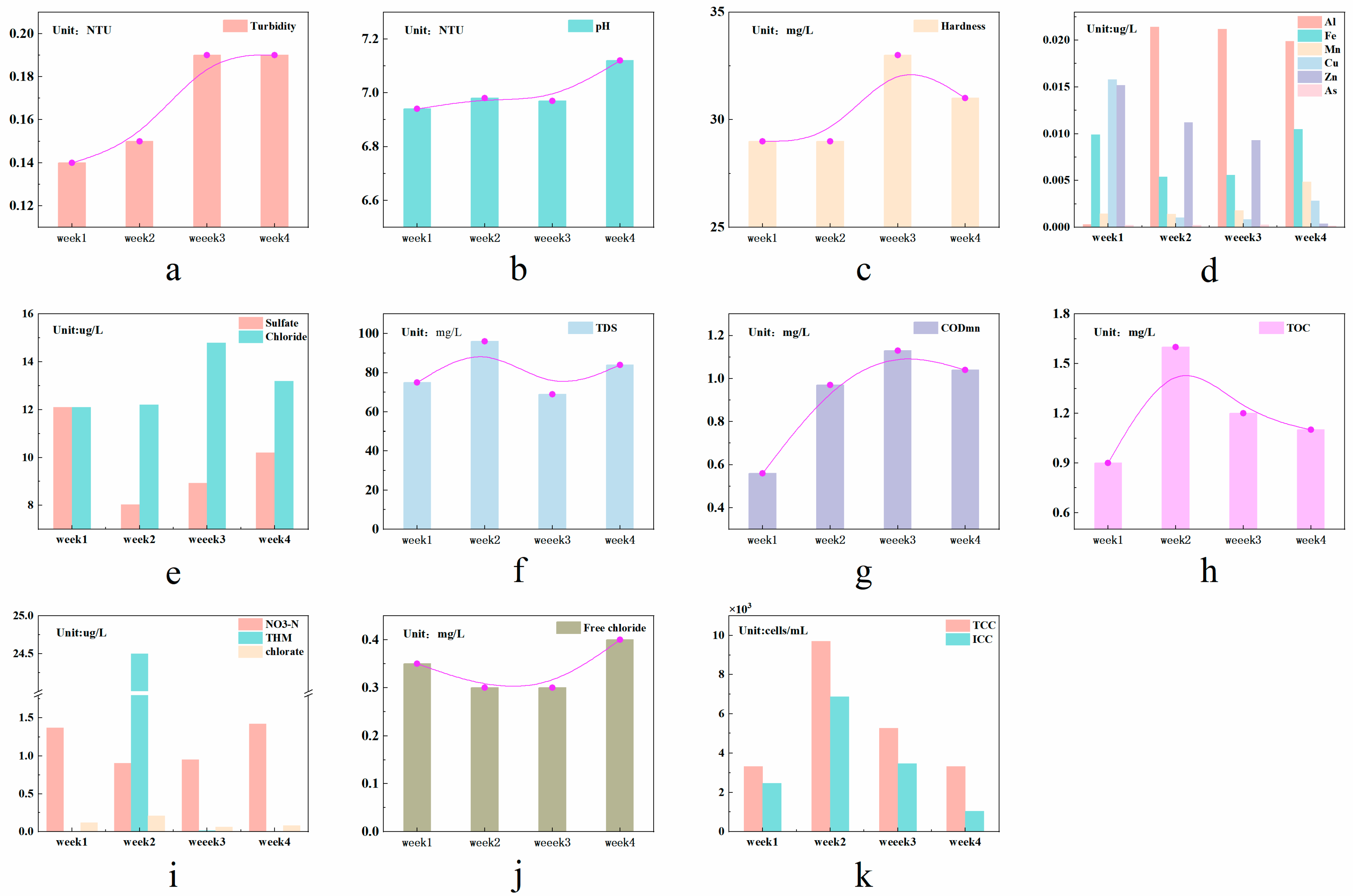
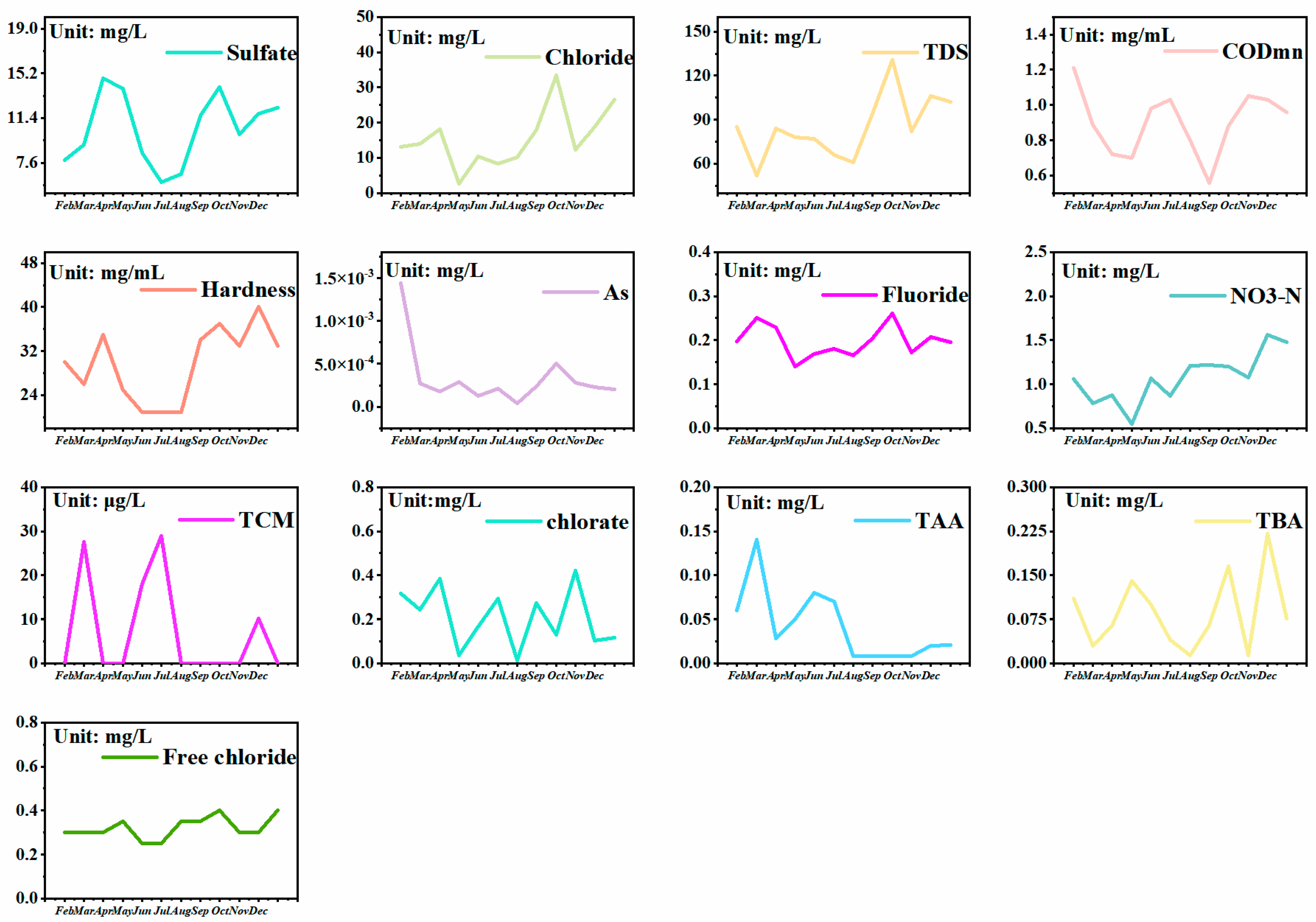
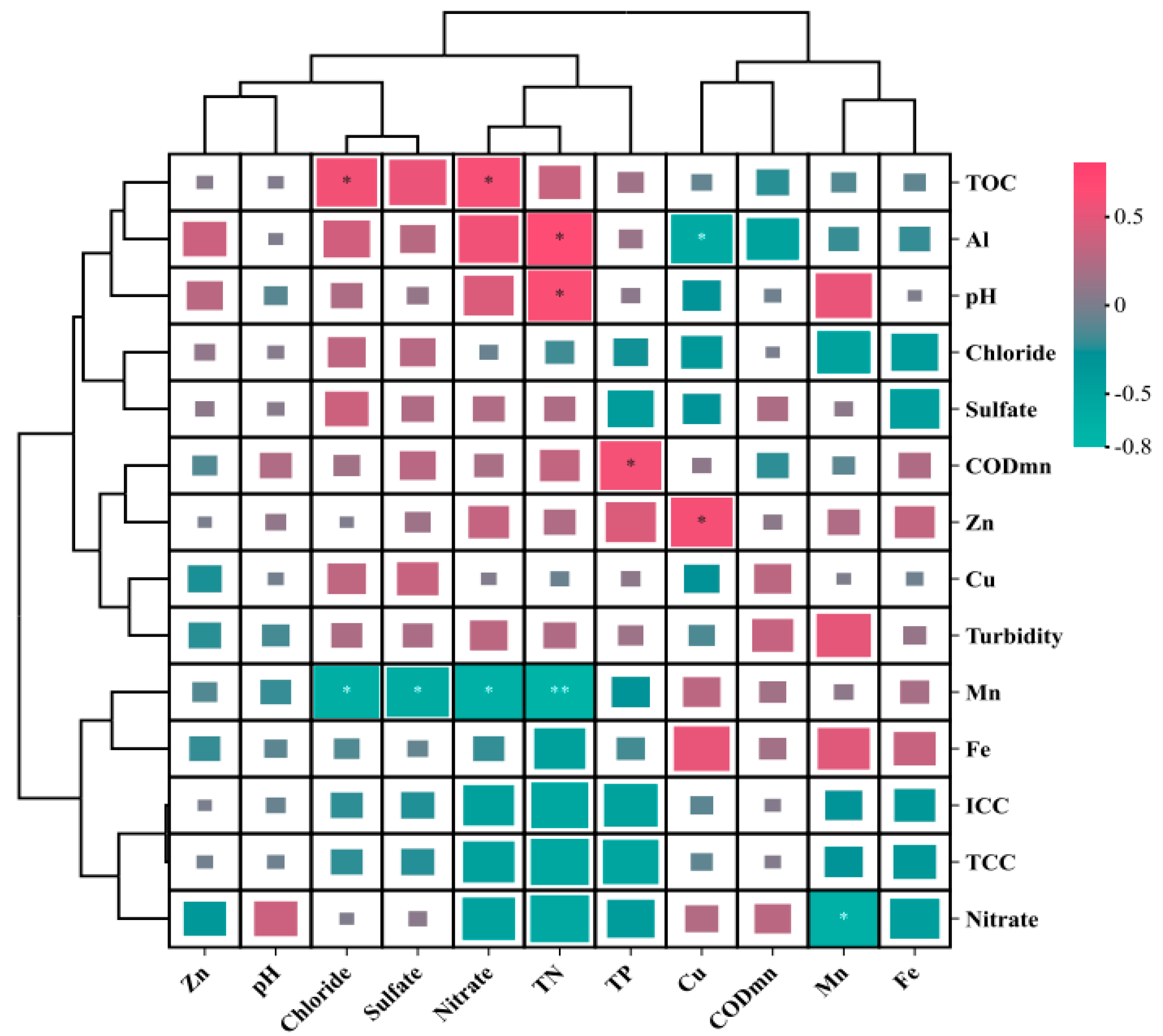

| Units | Daily Variation Characteristics | Monthly Variation Characteristics | Annual Variation Characteristics | |
|---|---|---|---|---|
| TCC | 103 cells/mL | 4.7 ± 9.1 | 5.4 ± 3.0 | 16.2 ± 12.8 |
| ICC | 103 cells/mL | 3.4 ± 8.0 | 3.5 ± 2.5 | 10.1 ± 8.8 |
| Al | μg/L | 33 ± 35.56 | 15.7 ± 10.29 | 25.9 ± 18.59 |
| Fe | μg/L | 3.43 ± 1.75 | 7.85 ± 2.73 | 7.3 ± 5.79 |
| Mn | μg/L | 30.1 ± 15.24 | 2.39 ± 1.65 | 16.62 ± 7.9 |
| Cu | μg/L | 2.9 ± 1.67 | 5.14 ± 7.16 | 2.27 ± 3.74 |
| Zn | μg/L | 28.13 ± 12.48 | 9.03 ± 6.25 | 11.23 ± 10.19 |
| pH | 7.47 ± 0.07 | 7 ± 0.08 | 7.08 ± 0.16 | |
| Sulfate | mg/L | 13.15 ± 0.16 | 9.82 ± 1.76 | 10.53 ± 2.98 |
| Chloride | mg/L | 16.72 ± 0.34 | 13.08 ± 1.25 | 15.54 ± 8.3 |
| Nitrate | mg/L | 1.24 ± 0.15 | 1.16 ± 0.27 | 1.08 ± 0.29 |
| TOC | mg/L | 1.58 ± 0.13 | 1.2 ± 0.29 | 1.33 ± 0.31 |
| Similarity | Water Quality Indicators | Daily Variation Characteristics | Monthly Variation Characteristics | Annual Variation Characteristics |
|---|---|---|---|---|
| high | TCC | 1.89 | 0.56 | 2.13 |
| ICC | 2.34 | 0.72 | 2.2 | |
| middle | Al | 1.08 | 0.66 | 0.72 |
| Fe | 0.51 | 0.35 | 0.48 | |
| Mn | 0.51 | 0.69 | 0.79 | |
| Cu | 0.57 | 1.39 | 1.64 | |
| Zn | 0.44 | 0.69 | 0.91 | |
| low | pH | 0.01 | 0.01 | 0.02 |
| Sulfate | 0.01 | 0.18 | 0.28 | |
| Chloride | 0.02 | 0.1 | 0.53 | |
| Nitrate | 0.02 | 0.23 | 0.26 | |
| TOC | 0.08 | 0.25 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Li, W.; Song, X.; Zhou, Y. From Raw Water to Pipeline Water: Correlation Analysis of Dynamic Changes in Water Quality Parameters and Microbial Community Succession. Water 2025, 17, 2555. https://doi.org/10.3390/w17172555
Jiang X, Li W, Song X, Zhou Y. From Raw Water to Pipeline Water: Correlation Analysis of Dynamic Changes in Water Quality Parameters and Microbial Community Succession. Water. 2025; 17(17):2555. https://doi.org/10.3390/w17172555
Chicago/Turabian StyleJiang, Xiaolong, Weiying Li, Xin Song, and Yu Zhou. 2025. "From Raw Water to Pipeline Water: Correlation Analysis of Dynamic Changes in Water Quality Parameters and Microbial Community Succession" Water 17, no. 17: 2555. https://doi.org/10.3390/w17172555
APA StyleJiang, X., Li, W., Song, X., & Zhou, Y. (2025). From Raw Water to Pipeline Water: Correlation Analysis of Dynamic Changes in Water Quality Parameters and Microbial Community Succession. Water, 17(17), 2555. https://doi.org/10.3390/w17172555







