Current State of Research on the Three-Dimensional Particle Electrode System for Treating Organic Pollutants from Wastewater Streams: Particle Electrode, Degradation Mechanism, and Synergy Effects
Abstract
1. Introduction
2. Three-Dimensional Electrochemical Process
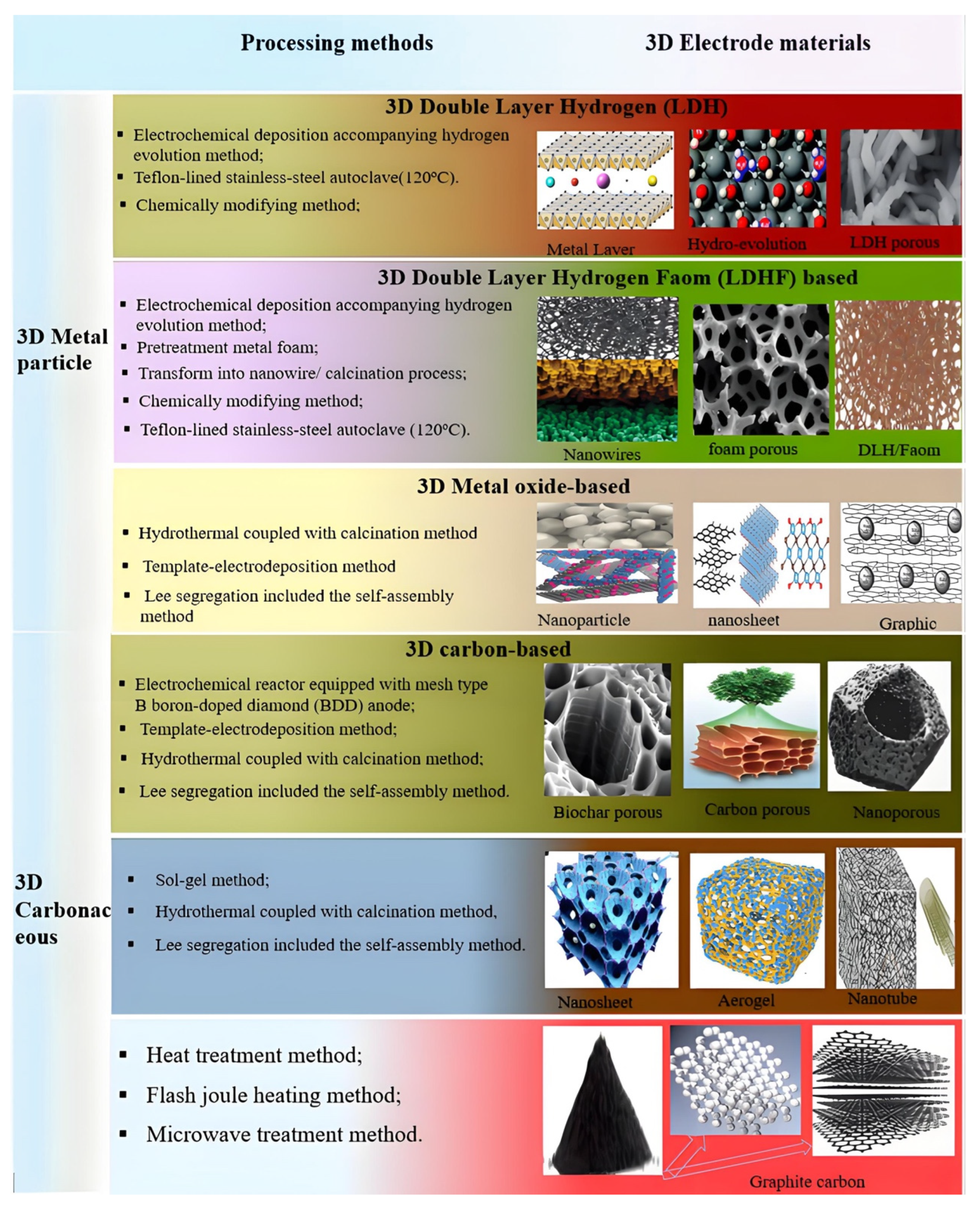
2.1. Three-Dimensional Electrodes
2.1.1. 3D Carbon Aerogel
2.1.2. 3D Activated Carbon-Based Electrodes
2.1.3. 3D Carbon Nanotube/Carbon Fiber Electrodes
2.1.4. 3D Slag-Based Particle Electrodes
2.1.5. 3D Biochar-Based Particle Electrodes
2.1.6. 3D Graphite Particle Electrodes
2.1.7. 3D Metal Electrode
3D Metal Oxide Particle Electrodes
3D Metal Foam-Based Particle Electrodes
2.2. Degradation and Synergistic Effect of 3D Particle Electrodes
3. Mechanisms of 3D Electrochemical Processes
3.1. Three-Dimensional Electrodes
3.1.1. Oxidation and Characteristics of Three-Dimensional Particle Anode Systems
3.1.2. Characteristics and Reduction Process of Three-Dimensional Cathode Systems
3.2. Three-Dimensional Mechanism Removal
3.3. Three-Dimensional Electro-Sorption
3.4. 3D Electro-Oxidation/Electroreduction
3.5. Removal Mechanisms of 3D Electrochemical Particle Electrode Systems
4. Applications of 3D Particle Electrode Systems in Environmental Management
4.1. Dye Wastewater Treatment
4.2. Pharmaceutical Pollutant Treatment
4.3. Wastewater Treatment
5. Conclusions, Perspectives, and Challenges
5.1. Enhanced Organic Pollutant Removal
5.2. Surface Adsorption and Catalytic Degradation Mechanisms
5.3. Electro-Sorption and Charge Interaction Mechanisms
5.4. Generation of Reactive Oxidants
5.5. Synergistic Effects in Binary Oxide-Based Electrode Systems
Supplementary Materials
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| 3D | Three-dimensional |
| POPs | Persistent Organic Pollutants |
| PPCPs | Pharmaceuticals, and personal care products |
| 2D | Two-dimensional |
| STR | Space-time yield |
| CE | Current efficiency |
| 3D-NPC | Nitrogen-doped porous carbon |
| PMS | Peroxymonosulfate |
| AC | Activated carbon |
| GAC | Granular activated carbon |
| AOP | Advanced oxidation process |
| CNTs | Carbon nanotubes |
| 1D | One-dimensional |
| ACF | Activated carbon fibers |
| TOC | Total organic carbon |
| BF | Blast furnace |
| BOF | Basic oxygen furnace |
| VS | Vanadium slag |
| BFS | Blast furnace slag |
| FBA | Furnace Bottom Ash |
| SMX | Sulfamethoxazole |
| BPA | Bisphenol A |
| PP | Propylparaben |
| PR | Piroxicam |
| BET | Brunauer–Emmett–Teller |
| X-ray | X-ray energy spectroscopy |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| BCS | Biochar samples |
| BC | Biochar |
| FMBC | Iron-manganese modified biochar |
| SG | Spent graphite |
| LIBs | Lithium-ion batteries |
| PVC | Polyvinyl chloride |
| SICs | Superionic conductors |
| NF-Fe | Iron-coated nickel foam |
| NF | Nickel foam |
| Cuf | Copper foam |
| 3DE-EF | 3D electro-Fenton |
| FA | Folic acid |
| TC | Tetracycline |
| PFR | Persistent free radicals |
| CV | Crystal violet |
| LDHs | Layered Double Hydroxides |
| BDD | Boron-doped diamond |
| EC | Electrocatalytic |
| MEA | Membrane electrode assembly |
| 3D-BERs | Three-dimensional biofilm electrode reactor |
| DB80 | Direct Blue 80 |
| COD | Chemical oxygen demand |
| GF | Graphite felt |
| OCP | Open circuit potential |
| IBP | Ibuprofen |
| ROS | Reactive oxygen species |
| MB | Methylene blue |
| EPR | Electron paramagnetic resonance |
| CBZ | Carbamazepine |
| 3D-ER | Three-dimensional electrochemical reactor |
| CNT | γ-Fe2O3-carbon nanotube |
| RhB | Rhodamine B |
| CPE | Catalytic particle electrodes |
| AO7 | Acid orange 7 |
| OTC | Oxytetracycline |
| DPW | Board@dopamine-W@WC |
| NOR | Norfloxacin |
| PS | Peroxysulfate |
| CIP | Ciprofloxacin |
| 3D-AER | Three-dimensional aeration electrocatalysis reactor |
| FPE | Flotation-tailings particle electrode |
| TNGA | Titanium dioxide nanotubes/graphene aerogel |
| TCH | Tetracycline hydrochloride |
| ABC | Algal biochar |
| TN | Total nitrogen |
| EIS | Electrochemical impedance spectroscopy |
| PAC | Powdered activated carbon |
| EO | Electrochemical oxidation |
| BPB | Banana peel biochar |
| VTM | Vanadium titanomagnetite |
References
- Ma, J.; Gao, M.; Shi, H.; Ni, J.; Xu, Y.; Wang, Q. Progress in Research and Development of Particle Electrodes for Three-Dimensional Electrochemical Treatment of Wastewater: A Review. Environ. Sci. Pollut. Res. 2021, 28, 47800–47824. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, S.; Zhang, G. Three-dimensional structured electrode for electrocatalytic organic wastewater purification: Design, mechanism and role. J. Hazard. Mater. 2023, 445, 130524. [Google Scholar] [CrossRef] [PubMed]
- Silva Barni, M.F.; Doumic, L.I.; Procaccini, R.A.; Ayude, M.A.; Romeo, H.E. Layered platforms of Ti4O7 as flow-through anodes for intensifying the electro-oxidation of bentazon. J. Environ. Manag. 2020, 263, 110403. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.; Abdelhedi, R.; Brillas, E.; Sirés, I. Comparative electrochemical degradation of the triphenylmethane dye methyl violet with boron-doped diamond and pt anodes. J. Electroanal. Chem. 2009, 627, 41–50. [Google Scholar] [CrossRef]
- Okeke, E.S.; Chukwudozie, K.I.; Nyaruaba, R.; Ita, R.E.; Oladipo, A.; Ejeromedoghene, O.; Atakpa, E.O.; Agu, C.V.; Okoye, C.O. Antibiotic Resistance in Aquaculture and Aquatic Organisms: A Review of Current Nanotechnology Applications for Sustainable Management. Environ. Sci. Pollut. Res. 2022, 29, 69241–69274. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Cheng, J.; Hu, C.; Yang, Z.; Wu, C. Three-dimensional particle electrode system treatment of organic wastewater: A general review based on patents. J. Clean. Prod. 2021, 308, 127324. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Bo, G.; Wu, S.; Luo, L. Degradation of Pollutants in Polluted River Water Using Ti/IrO2–Ta2O5 Coating Electrode and Evaluation of Electrode Characteristics. J. Clean. Prod. 2020, 273, 123019. [Google Scholar] [CrossRef]
- Ramalingam, G.; Perumal, N.; Priya, A.K.; Rajendran, S. A review of graphene-based semiconductors for photocatalytic degradation of pollutants in wastewater. Chemosphere 2022, 300, 134391. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, J.; Du, J.; Hou, C.; Zhou, X.; Chen, J.; Zhang, Y. Non-phytoremediation and phytoremediation technologies of integrated remediation for water and soil heavy metal pollution: A comprehensive review. Sci. Total Environ. 2024, 948, 174237. [Google Scholar] [CrossRef]
- Kumar, R.; Sudhaik, A.; Raizada, P.; Singh, A.; Ahamad, T.; Khan, A.A.P.K.; Rangabhashiyam, S.; Singh, P. Insight of the effect of chitosan modification on the photocatalytic properties of metal-based and metal-free photocatalysts. Biomater. Polym. Horiz. 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Kumar, R.; Sudhaik, A.; Sonu; Nguyen, V.H.; Van Le, Q.; Ahamad, T.; Thakur, S.; Kumar, N.; Hussain, C.M.; Singh, P.; et al. Graphene oxide modified K, P Co-Doped g-C3N4 and CoFe2O4 composite for photocatalytic degradation of antibiotics. J. Taiwan Inst. Chem. Eng. 2023, 150, 105077. [Google Scholar] [CrossRef]
- Kumar, R.; Sudhaik, A.; Sonu; Raizada, P.; Nguyen, V.H.; Van Le, Q.; Ahamad, T.; Thakur, S.; Hussain, C.M.; Singh, P. Integrating K and P Co-Doped g-C3N4 with ZnFe2O4 and graphene oxide for s-scheme-based enhanced adsorption coupled photocatalytic real wastewater treatment. Chemosphere 2023, 337, 139267. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Sudhaik, A.; Sonu; Kumar, R.; Raizada, P.; Khan, A.A.P.; Ahamad, T.; Nguyen, V.H.; Selvasembian, R.; Kaushik, A.l. Recent Advances in Synthesis Methods and Surface Structure Manipulating Strategies of Copper Selenide (Cuse) Nanoparticles for Photocatalytic Environmental and Energy Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2024; Volume 12, ISBN 5656500152. [Google Scholar]
- Zhang, G.; Ding, Y.; Nie, W.; Tang, H. Efficient degradation of drug ibuprofen through catalytic activation of peroxymonosulfate by Fe 3 C embedded on carbon. J. Environ. Sci. 2019, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.L.; Nikbakht Fini, M.; Molnar, P.K.; Muff, J. Synergy of combined adsorption and electrochemical degradation of aqueous organics by granular activated carbon particulate electrodes. Sep. Purif. Technol. 2019, 208, 51–58. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Xi, L.; Yu, Y.; Chen, N.; Sun, S.; Wang, W.; Lange, K.M.; Zhang, B. Single-Atom Au/NiFe Layered double hydroxide electrocatalyst: Probing the origin of activity for oxygen evolution reaction. J. Am. Chem. Soc. 2018, 140, 3876–3879. [Google Scholar] [CrossRef]
- Mühlbauer, A.; Keiner, D.; Gerhards, C.; Caldera, U.; Sterner, M.; Breyer, C. Assessment of technologies and economics for carbon dioxide removal from a portfolio perspective. Int. J. Greenh. Gas Control 2024, 141, 104297. [Google Scholar] [CrossRef]
- Li, Q.; Bi, J.; Yao, Y.; Li, X.; Xu, D. A Novel 3D CoNiCu-LDH@CuO Micro-Flowers on Copper Foam as Efficient electrocatalyst for overall water splitting. Appl. Surf. Sci. 2023, 622, 156874. [Google Scholar] [CrossRef]
- Wang, J.; Lv, G.; Wang, C. A Highly Efficient and Robust Hybrid Structure of CoNiN@NiFe LDH for overall water splitting by accelerating hydrogen evolution kinetics on NiFe LDH. Appl. Surf. Sci. 2021, 570, 151182. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vinayagam, S.; Gnanasekaran, L.; Suresh, R.; Soto-Moscoso, M.; Chen, W.H. Environmental Fate of Aquatic Pollutants and Their Mitigation by Phycoremediation for the Clean and Sustainable Environment: A Review. Environ. Res. 2024, 240, 117460. [Google Scholar] [CrossRef]
- Backhurst, J.R.; Coulson, J.M.; Goodridge, F.; Plimley, R.E.; Fleischmann, M. A Preliminary Investigation of Fluidized Bed Electrodes. J. Electrochem. Soc. 1969, 116, 1600. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, P.; Patidar, R.; Srivastava, V.C.; Lo, S.L.; Štangar, U.L. Catalytic Oxidation of Bisphenol A with Co3+ Rich Spinel Co3O4: Performance Evaluation with Peroxymonosulfate Activation and Mineralization Mechanism. J. Environ. Chem. Eng. 2023, 11, 110023. [Google Scholar] [CrossRef]
- Guan, Z.; Guo, Y.; Li, S.; Feng, S.; Deng, Y.; Ou, X.; Ren, J.; Sun, S.; Liang, J. Decomplexation of heterogeneous catalytic ozonation assisted with heavy metal chelation for advanced treatment of coordination complexes of Ni. Sci. Total Environ. 2020, 732, 139223. [Google Scholar] [CrossRef] [PubMed]
- Moersidik, S.S.; Nugroho, R.; Handayani, M.; Kamilawati; Pratama, M.A. Optimization and reaction kinetics on the removal of nickel and cod from wastewater from electroplating industry using electrocoagulation and advanced oxidation processes. Heliyon 2020, 6, e03319. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An Overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Park, H.; Mameda, N.; Choo, K.H. Catalytic Metal Oxide Nanopowder Composite Ti Mesh for Electrochemical Oxidation of 1,4-Dioxane and Dyes. Chem. Eng. J. 2018, 345, 233–241. [Google Scholar] [CrossRef]
- Sowmiya, S.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V. Granular activated carbon as a particle electrode in three-dimensional electrochemical treatment of reactive black B from aqueous solution. Environ. Prog. Sustain. Energy 2016, 35, 1617. [Google Scholar] [CrossRef]
- Sahu, J.N.; Kapelyushin, Y.; Mishra, D.P.; Ghosh, P.; Sahoo, B.K.; Trofimov, E.; Meikap, B.C. Utilization of ferrous slags as coagulants, filters, adsorbents, neutralizers/stabilizers, catalysts, additives, and bed materials for water and wastewater treatment: A Review. Chemosphere 2023, 325, 138201. [Google Scholar] [CrossRef]
- Li, S.; Jiang, B.; Liu, G.; Shi, C.; Yu, H.; Lin, Y. Recent progress of particle electrode materials in three-dimensional electrode reactor: Synthesis strategy and electrocatalytic applications. Environ. Sci. Pollut. Res. 2023, 31, 11490–11506. [Google Scholar] [CrossRef]
- Piao, M.; Du, H.; Teng, H. An Overview of the recent advances and future prospects of three-dimensional particle electrode systems for treating wastewater. RSC Adv. 2024, 14, 27712–27732. [Google Scholar] [CrossRef]
- Wu, X.; Li, K.; Ying, S.; Liu, L.; Wang, M.; Liao, Y. Three-dimensional graphene materials for UO22+ electrosorption. J. Radioanal Nucl. Chem. 2019, 321, 977–984. [Google Scholar] [CrossRef]
- Mavre, F.; Anand, R.K.; Laws, D.R.; Chow, K.F.; Chang, B.Y.; Crooks, J.A.; Crooks, R.M. Bipolar electrodes: A useful tool for concentration, separation, and detection of analytes in microelectrochemical systems. Anal. Chem. 2010, 82, 8766–8774. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Bui, J.C.; Lees, E.W.; Marin, D.H.; Stovall, T.N.; Chen, L.; Kusoglu, A.; Nielander, A.C.; Jaramillo, T.F.; Boettcher, S.W.; Bell, A.T.; et al. Multi-scale physics of bipolar membranes in electrochemical processes. Nat. Chem. Eng. 2024, 1, 45–60. [Google Scholar] [CrossRef]
- Blommaert, M.A.; Aili, D.; Tufa, R.A.; Li, Q.; Smith, W.A.; Vermaas, D.A. Insights and challenges for applying bipolar membranes in advanced electrochemical energy systems. ACS Energy Lett. 2021, 6, 2539–2548. [Google Scholar] [CrossRef]
- Can, W.; Yao-Kun, H.; Qing, Z.; Min, J. Treatment of secondary effluent using a three-dimensional electrode system: Cod removal, biotoxicity assessment, and disinfection effects. Chem. Eng. J. 2014, 243, 1–6. [Google Scholar] [CrossRef]
- Liu, W.; Dong, Y.; Yang, D.; Zhang, C.; Zhang, L.; Lu, Y.; Jin, Q.; Liu, Z.; Liu, J.; Lin, H. Unraveling the highly efficient synergy of adsorption and degradation for norfloxacin elimination by mo/fe anchored carbon fiber aerogel via peroxydisulfate activation. Chem. Eng. J. 2023, 464, 142667. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Han, L.; Wang, Y.; Cui, W.; Wei, C.; Zhou, X.; Gao, Y.; Lu, L. Research progress in the preparation and application of three-dimensional carbon aerogel materials. Chin. J. Eng. 2023, 46, 447–457. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Yin, S.; Shi, X.; Wang, L.; She, P.; Song, Y.; Liu, Z.; Sun, H. 3D-printed n-doped porous carbon aerogels for efficient flow-through degradation and disinfection of wastewater. Sep. Purif. Technol. 2023, 320, 124116. [Google Scholar] [CrossRef]
- Tan, C.; Yu, M.; Tang, J.; Gao, X.; Yin, Y.; Zhang, Y.; Wang, J.; Gao, X.; Zhang, C.; Zhou, X.; et al. 2D Fin Field-Effect Transistors Integrated with Epitaxial High-k Gate Oxide. Nature 2023, 616, 66–72. [Google Scholar] [CrossRef]
- Yonghui, S.; Siming, L.; Ning, Y.; Wenjin, H. Treatment of Cyanide wastewater dynamic cycle test by three-dimensional electrode system and the reaction process analysis. Environ. Technol. 2021, 42, 1693–1702. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Yaqoob, A.A.; Ahmad, A. Microbial Fuel Cells: Emerging trends in electrochemical applications. Microb. Fuel Cells: Emerg. Trends Electrochem. Appl. 2022, 1–108. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chai, L.; Liu, M.; Shu, Y.; Li, Q.; Wang, Y.; Wang, Q.; Qiu, D. Capacitive deionization of chloride ions by activated carbon using a three-dimensional electrode reactor. Sep. Purif. Technol. 2018, 191, 424–432. [Google Scholar] [CrossRef]
- Khan, M.E. State-of-the-Art developments in carbon-based metal nanocomposites as a catalyst: Photocatalysis. Nanoscale Adv. 2021, 3, 1887–1900. [Google Scholar] [CrossRef]
- Liu, W.; Hu, X.; Sun, Z.; Duan, P. Electrochemical modification of activated carbon fiber as 3-d particle electrodes: Characterization and enhancement for the degradation of m-cresol. Environ. Sci. Pollut. Res. 2019, 26, 16433–16448. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Song, K.; Zhang, Q.; Xu, L.; Yu, Z.; Tang, P.; Pan, Z. Performance of a three-dimensional electrochemical reactor (3der) on bisphenol a degradation. Front. Chem. 2022, 10, 960003. [Google Scholar] [CrossRef]
- Wei, L.; Guo, S.; Yan, G.; Chen, C.; Jiang, X. Electrochemical pretreatment of heavy oil refinery wastewater using a three-dimensional electrode reactor. Electrochim. Acta 2010, 55, 8615–8620. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Wu, D.; Fu, R. Feasibility Study of using carbon aerogel as particle electrodes for decoloration of rbrx dye solution in a three-dimensional electrode reactor. Chem. Eng. J. 2008, 138, 47–54. [Google Scholar] [CrossRef]
- Wang, B.; Kong, W.; Ma, H. Electrochemical Treatment of Paper Mill Wastewater Using Three-Dimensional Electrodes with Ti/Co/SnO2-Sb2O5 Anode. J. Hazard. Mater. 2007, 146, 295–301. [Google Scholar] [CrossRef]
- Wu, X.; Song, X.; Chen, H.; Yu, J. Treatment of phenolic compound wastewater using CuFe2O4 /Al2O3 particle electrodes in a three-dimensional electrochemical oxidation system. Environ. Technol. 2021, 42, 4393–4404. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Shah, K.J.; Chiang, P.C.; Zheng, H. Electrocatalytic oxidation of tetracycline by bi-sn-sb/γ-al2o3 three-dimensional particle electrode. J. Hazard. Mater. 2019, 370, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Zeng, T.; Zhang, Y.; Wan, Q.; Li, Y.; Yang, N. Three-dimensional catalyst systems from expanded graphite and metal nanoparticles for electrocatalytic oxidation of liquid fuels. Nanoscale 2019, 11, 7952–7958. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Yang, Q.; Yao, F.; Zhong, Y.; Ren, W.; Chen, F.; Sun, J.; Ma, Y.; Fu, Z.; Wang, D.; et al. Electrocatalytic hydrodechlorination of 4-chlorophenol on pd supported multi-walled carbon nanotubes particle electrodes. Chem. Eng. J. 2019, 358, 903–911. [Google Scholar] [CrossRef]
- Liu, D.; Roberts, E.P.L.; Martin, A.D.; Holmes, S.M.; Brown, N.W.; Campen, A.K.; De Las Heras, N. Electrochemical regeneration of a graphite adsorbent loaded with acid violet 17 in a spouted bed reactor. Chem. Eng. J. 2016, 304, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; He, X.; Yang, G.; Qi, J.; Zhao, C. Organic pollutants removal performance and enhanced mechanism investigation of surface-modified steel slag particle electrode. Environ. Prog. Sustain. Energy 2018, 38. [Google Scholar] [CrossRef]
- Li, M.; Zhao, F.; Sillanpää, M.; Meng, Y.; Yin, D. Electrochemical degradation of 2-diethylamino-6-methyl-4-hydroxypyrimidine using three-dimensional electrodes reactor with ceramic Particle Electrodes. Sep. Purif. Technol. 2015, 156, 588–595. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, H.; Wang, L.; Chen, Y. Electrochemical removal of nitrate by cu/ti electrode coupled with copper-modified activated carbon particles at a low current density. Environ. Sci. Pollut. Res. 2019, 26, 17567–17576. [Google Scholar] [CrossRef]
- Baptista-Pires, L.; Norra, G.-F.; Radjenovic, J. Graphene-based sponges for electrochemical degradation of persistent organic contaminants. Water Res. 2021, 203, 117492. [Google Scholar] [CrossRef]
- Lin, C.-J.; Zhang, R.; Waisner, S.A.; Nawaz, T.; Center, L.; Gent, D.B.; Johnson, J.L.; Holland, S. Effects of process factors on the performance of electrochemical disinfection for wastewater in a continuous-flow cell reactor. Environ. Sci. Pollut. Res. 2021, 28, 36573–36584. [Google Scholar] [CrossRef]
- Habib, A.; Bhatti, H.N.; Iqbal, M. Metallurgical processing strategies for metals recovery from industrial slags. Zeitschrift fur physikalische Chemie 2020, 234, 201–231. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Manjón Fernández, Á.; Masaguer Torres, V. Hydrometallurgical Processes for the Recovery of Metals from Steel Industry By-Products: A Critical Review. J. Sustain. Metallurgy 2020, 6, 505–540. [Google Scholar] [CrossRef]
- Mercado-Borrayo, B.M.; González-Chávez, J.L.; Ramírez-Zamora, R.M.; Schouwenaars, R. Valorization of metallurgical slag for the treatment of water pollution: An emerging technology for resource conservation and re-utilization. J. Sustain. Metall. 2018, 4, 50–67. [Google Scholar] [CrossRef]
- Huanosta-Gutiérrez, T.; Dantas, R.F.; Ramírez-Zamora, R.M.; Esplugas, S. Evaluation of copper slag to catalyze advanced oxidation processes for the removal of phenol in water. J. Hazard. Mater. 2012, 213–214, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Liu, T.J.; Kang, L.L.; Wang, Y.T.; Li, J.G.; Wang, F.P.; Yu, Q.; Wang, X.M.; Liu, H.; Guo, H.W.; et al. A review of metallurgical slag for efficient wastewater treatment: Pretreatment, performance and mechanism. J. Clean. Prod. 2022, 380, 135076. [Google Scholar] [CrossRef]
- Nengzi, L.; Cao, R.; Qiu, Y.; Meng, L.; Hailai, W.; Li, H.; Qiu, G. Steel Slags for enhanced removal of landfill leachate in a three-dimensional electrochemical oxidation system. Sci. Rep. 2023, 13, 12751. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Petala, A.; Bampos, G.; Frontistis, Z. Using Sawdust Derived biochar as a novel 3d particle electrode for micropollutants degradation. Water 2022, 14, 357. [Google Scholar] [CrossRef]
- Li, S.; Chan, C.Y.; Sharbatmaleki, M.; Trejo, H.; Delagah, S. Engineered Biochar Production and Its Potential Benefits in a Closed-Loop Water-Reuse Agriculture System. Water 2020, 12, 2847. [Google Scholar]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, R.; Li, M.; Yang, S.; Zhang, J.; Yuan, S.; Hou, Y.; Li, C.; Chen, Y. Manganese-nitrogen co-doped biochar (mnn@bc) as particle electrode for three-dimensional (3d) electro-activation of peroxydisulfate: Active sites enhanced radical/non-Radical Oxidation. J. Hazard. Mater. 2023, 459, 132089. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lai, C.; Huang, F.; Cheng, M.; Zeng, G.; Huang, D.; Li, B.; Liu, S.; Zhang, M.M.; Qin, L.; et al. Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: Synergism of bio-char and fe–mn binary oxides. Water Res. 2019, 160, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Xu, L.; Chen, C.; Wang, Z.Y.; Fu, M.L.; Yuan, B. Recovery of graphite from spent lithium-ion batteries and its wastewater treatment application: A review. Sep. Purif. Technol. 2024, 330, 125289. [Google Scholar] [CrossRef]
- Yi, C.; Yang, Y.; Zhang, T.; Wu, X.; Sun, W.; Yi, L. A Green and facile approach for regeneration of graphite from spent lithium ion battery. J. Clean. Prod. 2020, 277, 123585. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-fenton-like system with a TiO2/Graphite Cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef]
- Ning, Z.; Duan, X.; Li, Y.; Zhao, X.; Chang, L. Degradation of polyvinyl chloride microplastics via electrochemical oxidation with a CeO2–PbO2 Anode. J. Clean. Prod. 2023, 432, 139668. [Google Scholar] [CrossRef]
- Kim, K.J.; Balaish, M.; Wadaguchi, M.; Kong, L.; Rupp, J.L.M. Solid-State Li–Metal Batteries: Challenges and horizons of oxide and sulfide solid electrolytes and their interfaces. Adv. Energy Mater. 2021, 11, 1–63. [Google Scholar] [CrossRef]
- Jia, R.; Wang, Y.; Li, A.; Cheng, C. Recent advance on three-dimensional ordered macroporous metal oxide-based photoelectrodes for photoelectrochemical water splitting. Mater. Chem. Front. 2023, 20. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, H.; Wang, H.; Qin, J.; Chen, X.; Lu, Z. 3D Flower-like CuO@NiAl-LDH Microspheres with enhanced removal affinity to organic dyes: Mechanistic insights, dft calculations and toxicity assessment. J. Mater. Chem. A 2023, 11, 22396–22408. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; Lee, K. Nanostructured materials on 3d nickel foam as electrocatalysts for water splitting. Nanoscale 2017, 9, 12231–12247. [Google Scholar] [CrossRef]
- Mohammadi, H.; Bina, B.; Ebrahimi, A. A Novel Three-dimensional electro-fenton system and its application for degradation of anti-inflammatory pharmaceuticals: Modeling and degradation pathways. Process Saf. Environ. Prot. 2018, 117, 200–213. [Google Scholar] [CrossRef]
- Bai, X.; Sun, F.; Ma, L.; Jiang, Z.; Di, H.; Pan, C.; Zhang, F.; Yang, J.; Zhang, H. A Novel NiFe-LDH/AC Three-dimensional particle electrode system and its application for degradation of n-nitrosamines: Condition Optimization and Degradation Mechanism. J. Environ. Chem. Eng. 2024, 12, 112500. [Google Scholar] [CrossRef]
- Yang, Z.; Tuo, Y.; Lu, Q.; Chen, C.; Liu, M.; Liu, B.; Duan, X.; Zhou, Y.; Zhang, J. Hierarchical Cu3P-based nanoarrays on nickel foam as efficient electrocatalysts for overall water splitting. Green Energy Environ. 2022, 7, 236–245. [Google Scholar] [CrossRef]
- Das, M.; Khan, Z.B.; Banerjee, M.; Biswas, A.; Dey, R.S. Three-dimensional nickel and copper-based foam-in-foam architecture as an electrode for efficient water electrolysis. Catal. Today 2023, 424, 113836. [Google Scholar] [CrossRef]
- Ratsoma, M.S.; Poho, B.L.O.; Makgopa, K.; Raju, K.; Modibane, K.D.; Jafta, C.J.; Oyedotun, K.O. Application of nickel foam in electrochemical systems: A review. J. Electron. Mater. 2023, 52, 2264–2291. [Google Scholar] [CrossRef]
- Zheng, Y.; Qiu, S.; Deng, F.; Zhu, Y.; Li, G.; Ma, F. Three-Dimensional electro-fenton system with iron foam as particle electrode for folic acid wastewater pretreatment. Sep. Purif. Technol. 2019, 224, 463–474. [Google Scholar] [CrossRef]
- Liu, W.; Ai, Z.; Zhang, L. Design of a neutral three-dimensional electro-fenton system with foam nickel as particle electrodes for wastewater treatment. J. Hazard. Mater. 2012, 243, 257–264. [Google Scholar] [CrossRef]
- Yang, S.; Feng, Y.; Gao, D.; Wang, X.; Suo, N.; Yu, Y.; Zhang, S. Electrocatalysis degradation of tetracycline in a three-dimensional aeration electrocatalysis reactor (3D-AER) with a flotation-tailings particle electrode (FPE): Physicochemical properties, influencing factors and the degradation mechanism. J. Hazard. Mater. 2021, 407, 124361. [Google Scholar] [CrossRef]
- Yuan, S.-J.; Dai, X.-H. Sewage Sludge-Based Functional Nanomaterials: Development and Applications. Environ. Sci. Nano 2017, 4, 17–26. [Google Scholar] [CrossRef]
- Tang, M.; Lai, L.; Su, C.; Li, C.; Zhang, C.; Guo, S. MoCoB metallic glass microwire catalysts for highly efficient and pH-universal degradation of wastewater. Npj Mater. Degrad. 2023, 7, 73. [Google Scholar] [CrossRef]
- Wu, S.; Hu, Y.H. A Comprehensive Review on Catalysts for Electrocatalytic and Photoelectrocatalytic Degradation of Antibiotics. Chem. Eng. J. 2021, 409, 127739. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, S.; Liu, L.; Xu, J. Graphite Particle Electrodes That enhance the detoxification of municipal solid waste incineration fly ashes in a three-dimensional electrokinetic platform and its mechanisms. Environ. Pollut. 2018, 243, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Y.F.; Zhang, L.; Xu, H.Y. Enhancement strategies for efficient activation of persulfate by heterogeneous cobalt-containing catalysts: A Review. Chemosphere 2021, 291, 132954. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Han, J.; Sun, Y.; Li, Y.; Hu, W.; Zhan, S. Enhanced catalytic degradation by using rgo-ce/wo3 nanosheets modified cf as electro-fenton cathode: Influence factors, reaction mechanism and pathways. J. Hazard. Mater. 2019, 367, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zeng, X.; Zhang, C.; Huang, R.; Ding, W. Enhanced electrocatalytic removal of tetracycline using dual carbon material combined particle electrodes in a three-dimensional electrochemical system: Degradation pathway and mechanism. J. Clean. Prod. 2023, 419, 138257. [Google Scholar] [CrossRef]
- Rheima, A.M.; sabri Abbas, Z.; Kadhim, M.M.; Hashim Mohammed, S.; Yahaia Alhameedi, D.; Rasen, F.A.; jawad al-bayati, A.d.; Ramadan, M.F.; Talib Abed, Z.; Salam Jaber, A.; et al. Aluminum oxide nano porous: Synthesis, properties, and applications. case studies in Chem. Environ. Eng. 2023, 8, 100428. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, K.; Qin, X.; Chen, S.; Yu, H.; Quan, X. Treatment of organic wastewater by a synergic electrocatalysis process with Ti3+ Self-Doped TiO2 nanotube arrays electrode as both cathode and anode. J. Hazard. Mater. 2022, 424, 127747. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Liang, J.; Yue, L.; Li, T.; Luo, Y.; Liu, Q.; Lu, S.; Asiri, A.M.; Gong, Z.; et al. Anodic oxidation for the degradation of organic pollutants: Anode materials, operating conditions and mechanisms. a mini review. Electrochem. Commun. 2021, 123, 106912. [Google Scholar] [CrossRef]
- Thangamani, R.; Vidhya, L.; Varjani, S. Electrochemical technologies for wastewater treatment and resource reclamation. In Microbe Mediated Remediation of Environmental Contaminants; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical Technologies for Wastewater Treatment and Resource Reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Candia-Onfray, C.; Espinoza, N.; Sabino Da Silva, E.B.; Toledo-Neira, C.; Espinoza, L.C.; Santander, R.; García, V.; Salazar, R. Treatment of winery wastewater by anodic oxidation using bdd electrode. Chemosphere 2018, 206, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Banda, G.R.; Santos, G.d.O.S.; Duarte Gonzaga, I.M.; Dória, A.R.; Barrios Eguiluz, K.I. Developments in electrode materials for wastewater treatment. Curr. Opin. Electrochem. 2021, 26, 100663. [Google Scholar] [CrossRef]
- Heckert, E.G.; Seal, S.; Self, W.T. Fenton-like Reaction Catalyzed by the Rare Earth Inner Transition Metal Cerium. Environ. Sci. Technol. 2008, 42, 5014–5019. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Sheng, Y.; Li, C.; Wang, W.; Liu, X. Simultaneous Removal of Nitrate and Sulfate Using an Up-Flow Three-Dimensional Biofilm Electrode Reactor: Performance and Microbial Response. Bioresour. Technol. 2020, 318, 124096. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Xu, J.; Wu, L.; Ni, B.J. Three-Dimensional Biofilm Electrode Reactors (3D-BERs) for Wastewater Treatment. Bioresour. Technol. 2022, 344, 126274. [Google Scholar] [CrossRef]
- Berenguer, R.; Sieben, J.M.; Quijada, C.; Morallón, E. Electrocatalytic degradation of phenol on pt- and ru-doped ti/sno2-sb anodes in an alkaline medium. Appl. Catal. B Environ. 2016, 199, 394–404. [Google Scholar] [CrossRef]
- Huang, H.; Han, C.; Wang, G.; Feng, C. Lignin Combined with polypyrrole as a renewable cathode material for H2O2 generation and its application in the electro-fenton process for azo dye removal. Electrochim. Acta 2018, 259, 637–646. [Google Scholar] [CrossRef]
- Zuo, K.; Garcia-Segura, S.; Cerrón-Calle, G.A.; Chen, F.Y.; Tian, X.; Wang, X.; Huang, X.; Wang, H.; Alvarez, P.J.J.; Lou, J.; et al. Electrified water treatment: Fundamentals and roles of electrode materials. Nat. Rev. Mater. 2023, 8, 472–490. [Google Scholar] [CrossRef]
- Yuan, M.; Lin, H.T.; Boyd-Graber, J. Cold-Start Active Learning through self-supervised language modeling. In Proceedings of the EMNLP 2020 Conference on Empirical Methods in Natural Language Processing, Punta Cana, Dominican Republic, 16–20 November 2020; pp. 7935–7948. [Google Scholar] [CrossRef]
- Li, J.; Hou, L.; Wang, L.; Tang, L. Decoupling analysis between economic growth and air pollution in key regions of Air Pollution Control in China. Sustainability 2021, 13, 6600. [Google Scholar] [CrossRef]
- Sun, Y.; Li, P.; Zheng, H.; Zhao, C.; Xiao, X.; Xu, Y.; Sun, W.; Wu, H.; Ren, M. Electrochemical Treatment of Chloramphenicol Using Ti-Sn/γ-Al2O3 Particle Electrodes with a Three-Dimensional Reactor. Chem. Eng. J. 2017, 308, 1233–1242. [Google Scholar] [CrossRef]
- Mameda, N.; Park, H.; Shah, S.S.A.; Lee, K.; Li, C.W.; Naddeo, V.; Choo, K.H. Highly Robust and Efficient Ti-Based Sb-SnO2 Anode with a Mixed Carbon and Nitrogen Interlayer for Electrochemical 1,4-Dioxane Removal from Water. Chem. Eng. J. 2020, 393, 124794. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water Desalination via Capacitive Deionization: What Is It and What Can We Expect from It? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]
- Chen, R.; Sheehan, T.; Ng, J.L.; Brucks, M.; Su, X. Capacitive Deionization and Electrosorption for Heavy Metal Removal. Environ. Sci. Water Res. Technol. 2020, 6, 258–282. [Google Scholar] [CrossRef]
- GracePavithra, K.; Senthil Kumar, P.; Jaikumar, V.; SundarRajan, P.S. A Review on Three-Dimensional Eletrochemical Systems: Analysis of Influencing Parameters and Cleaner Approach Mechanism for Wastewater. Rev. Environ. Sci. Biotechnol. 2020, 19, 873–896. [Google Scholar] [CrossRef]
- Qin, T.; Yao, B.; Zhou, Y.; Wu, C.; Li, C.; Ye, Z.; Zhi, D.; Lam, S.S. The three-dimensional electrochemical processes for water and wastewater remediations: Mechanisms, affecting parameters, and applications. J. Clean. Prod. 2023, 408, 137105. [Google Scholar] [CrossRef]
- Maarof, H.I.; Daud, W.M.A.W.; Aroua, M.K.D. Recent Trends in removal and recovery of heavy metals from wastewater by electrochemical technologies. Rev. Chem. Eng. 2017, 33, 359–386. [Google Scholar] [CrossRef]
- Norra, G.F.; Radjenovic, J. Removal of persistent organic contaminants from wastewater using a hybrid electrochemical-granular activated carbon (gac) system. J. Hazard. Mater. 2021, 415, 125557. [Google Scholar] [CrossRef]
- Qi, F.; Zeng, Z.; Wen, Q.; Huang, Z. Enhanced organics degradation by three-dimensional (3d) electrochemical activation of persulfate using sulfur-doped carbon particle electrode: The role of thiophene sulfur functional group and specific capacitance. J. Hazard. Mater. 2021, 416, 125810. [Google Scholar] [CrossRef]
- Qiao, J.; Xiong, Y. Electrochemical oxidation technology: A review of its application in high-efficiency treatment of wastewater containing persistent organic pollutants. J. Water Process Eng. 2021, 44, 102308. [Google Scholar] [CrossRef]
- Sinha, A.; Singh, S.P.; Gupta, A.B. Introduction to Persistent Pollutants in Water and Advanced Treatment Technology; Springer Nature: Singapore, 2023; ISBN 9789819920617. [Google Scholar]
- Yang, Y. Recent Advances in the electrochemical oxidation water treatment: Spotlight on byproduct control. Front. Environ. Sci. Eng. 2020, 14, 85. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical advanced oxidation processes for wastewater treatment: Advances in formation and detection of reactive species and mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Du, Y.; Li, Z.; Wang, Y.; Xu, P.; Zhao, H.; Wang, F.; Li, C.; Han, X. Facile synthesis of 3d flower-like ni microspheres with enhanced microwave absorption properties. J. Mater. Chem. C 2018, 6, 9615–9623. [Google Scholar] [CrossRef]
- Ren, Y.; Lu, P.; Qu, G.; Ning, P.; Ren, N.; Wang, J.; Wu, F.; Chen, X.; Wang, Z.; Zhang, T.; et al. Study on the mechanism of rapid degradation of rhodamine b with fe/cu@antimony tailing nano catalytic particle electrode in a three dimensional electrochemical reactor. Water Res. 2023, 244, 120487. [Google Scholar] [CrossRef]
- Di, J.; Jamakanga, R.; Chen, Q.; Li, J.; Gai, X.; Li, Y.; Yang, R.; Ma, Q. Degradation of rhodamine b by activation of peroxymonosulfate using co3o4-rice husk ash composites. Sci. Total Environ. 2021, 784, 147258. [Google Scholar] [CrossRef]
- Zheng, S.; Ding, B.; Qian, X.; Yang, Y.; Mao, L.; Zheng, S.; Zhang, J. High efficiency degradation of tetracycline and rhodamine b using z-type BaTiO3/γ-Bi2O3 heterojunction. Sep. Purif. Technol. 2022, 278, 119666. [Google Scholar] [CrossRef]
- Tan, M.; Zhang, S.; Dong, J.; Huang, J.; Wu, X.; Tang, X.; Wu, D. Removal performance and mechanism for a three-dimensional electrode system treating biochemical effluent of a wastewater treatment plant. Water Sci. Technol. 2023, 87, 1159–1173. [Google Scholar] [CrossRef]
- Liu, P.; Zhong, D.; Xu, Y.; Zhong, N. Nitrogen doped cu/fe@pc derived from metal organic frameworks for activating peroxymonosulfate to degrade rhodamine b. J. Environ. Chem. Eng. 2022, 10, 107595. [Google Scholar] [CrossRef]
- Jing, X.; Wang, X.; Li, X.; Wang, D.; Xu, H.; Yan, W. Progress in the Preparation of Metal Oxide Electrodes for the Electrochemical Treatment of Organic Wastewater: A Short Review. Catalysts 2023, 13, 1096. [Google Scholar] [CrossRef]
- He, W.; Ma, Q.; Wang, J.; Yu, J.; Bao, W.; Ma, H.; Amrane, A. Preparation of novel kaolin-based particle electrodes for treating methyl orange wastewater. Appl. Clay Sci. 2014, 99, 178–186. [Google Scholar] [CrossRef]
- Ren, X.; Fu, H.; Peng, D.; Shen, M.; Tang, P.; Song, K.; Lai, B.; Pan, Z. Intensive treatment of organic wastewater by three-dimensional electrode system within mn-loaded steel slag as catalytic particle electrodes. Molecules 2024, 29, 952. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Cheng, L.; Zhang, Y.; Wang, Q.; Wu, Q.; Xue, Y.; Meng, X. Efficiency and mechanisms of rhodamine b degradation in fenton-like systems Based on Zero-Valent Iron. RSC Adv. 2020, 10, 28509–28515. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Fu, L.; Lu, H.; Zhang, M.; Wang, W.; Hu, B.; Zhou, Y.; Yu, G. Electrochemical Oxidation Degradation of Rhodamine B Dye on Boron-Doped Diamond Electrode: Input Mode of Power Attenuation. J. Clean. Prod. 2023, 401, 136794. [Google Scholar] [CrossRef]
- Li, X.-Y.; Xu, J.; Cheng, J.-P.; Feng, L.; Shi, Y.-F.; Ji, J. TiO2-SiO2/GAC particles for enhanced electrocatalytic removal of acid orange 7 (ao7) dyeing wastewater in a three-dimensional electrochemical reactor. Sep. Purif. Technol. 2017, 187, 303–310. [Google Scholar] [CrossRef]
- Mohammadi, H.; Alinejad, A.; Khajeh, M.; Darvishmotevalli, M.; Moradnia, M.; Tehrani, A.M.; Hosseindost, G.; Zare, M.R.; Mengelizadeh, N. Optimization of the 3D electro-Fenton process in removal of acid orange 10 from aqueous solutions by response surface methodology. J Chem. Technol. Biotechnol. 2019, 94, 3158–3171. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Shah, K.J.; Zheng, H.; Ma, B. Electrochemical degradation of oxytetracycline by ti-sn-sb/γ-al2o3 three-dimensional electrodes. J. Environ. Manag. 2019, 241, 22–31. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Li, F.; Hu, X.; Xie, Z.; Hua, T. Activation of peroxymonosulfate in an electrochemical filter by mnfe2o4-rgo electro-assisted catalytic membrane for the degradation of oxytetracycline. J. Environ. Chem. Eng. 2022, 10, 107008. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Liu, Y.; Tang, C.; Cheng, J.; Zhu, X.; Wang, G.; Xie, J. Effective degradation of norfloxacin by 3d diatomite plate@polydopamine@wc co-catalytic fenton at near-neutral pH. Chem. Eng. J. 2024, 486, 150038. [Google Scholar] [CrossRef]
- Wang, T.; Ta, M.; Guo, J.; Liang, L.E.; Bai, C.; Zhang, J.; Ding, H. Insight into the synergy between rice shell biochar particle electrodes and peroxymonosulfate in a three-dimensional electrochemical reactor for norfloxacin degradation. Sep. Purif. Technol. 2023, 304, 122354. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, Y.; Liang, Y.; Li, H.; Liang, W. Degradation mechanism of norfloxacin in water using persulfate activated by BC@nZVI/Ni. Chem. Eng. J. 2020, 389, 124276. [Google Scholar] [CrossRef]
- Gu, H.; Shui, X.; Zhang, Y.; Zeng, T.; Yang, J.; Wu, Z.; Zhang, X.; Yang, N. Porous carbon scaffolded fe-based alloy nanoparticles for electrochemical quantification of acetaminophen and rutin. Carbon 2024, 221, 118954. [Google Scholar] [CrossRef]
- Lu, C.; Gu, J.; Wei, G.; Ba, J.; Zhang, L.; Li, Z.; Pei, R.; Li, J.; Wei, J. Three-dimensional electro-fenton degradation of ciprofloxacin catalyzed by cuo doped red mud particle electrodes: Influencing factors, possible degradation pathways and energy consumption. J. Environ. Chem. Eng. 2022, 10, 107737. [Google Scholar] [CrossRef]
- Hu, X.; Huang, L.; Sun, T.; Gao, Z.; Qu, Z. TiO2-Loading modification on graphene aerogel particle electrode for electrochemical oxidation of tch wastewater with low electrolyte concentration: Performance and mechanism. J. Electroanal. Chem. 2024, 962, 118268. [Google Scholar] [CrossRef]
- Yu, D.; Pei, Y. Persulfate-Enhanced continuous flow three-dimensional electrode dynamic reactor for treatment of landfill leachate. J. Environ. Manag. 2022, 321, 115890. [Google Scholar] [CrossRef]
- Yu, D.; Pei, Y.; Ji, Z.; He, X.; Yao, Z. A Review on the landfill leachate treatment technologies and application prospects of three-dimensional electrode technology. Chemosphere 2022, 291, 132895. [Google Scholar] [CrossRef]
- Guo, F.; Lou, Y.; Yan, Q.; Xiong, J.; Luo, J.; Shen, C.; Vayenas, D.V. Insight into the Fe–Ni/Biochar Composite Supported Three-Dimensional Electro-Fenton Removal of Electronic Industry Wastewater. J. Environ. Manag. 2023, 325, 116466. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Kou, L.; Gao, X.; Wang, Y.; Guo, Y.; Li, X. Preparation of particle electrodes for landfill leachate treatment by doping Cu2+-modified needle coke with fe-based oxides extracted from iron tailings. Chem. Eng. J. 2024, 481, 148427. [Google Scholar] [CrossRef]
- Yuan, M.; Yan, F.; Chen, Y.; Luo, J.; Li, Z. A Three-dimensional electrochemical oxidation system with α-Fe2O3/PAC as the particle electrode for ammonium nitrogen wastewater treatment. RSC Adv. 2020, 10, 8773–8779. [Google Scholar] [CrossRef]
- Jin, C.; Tang, Q.; Gao, Y.; Xu, H.; Zhang, L.; Sheng, Y. Nitrogen removal from low-c/n-ratio wastewater using a three-dimensional bioelectrical reactor. J. Water Process Eng. 2023, 53, 103835. [Google Scholar] [CrossRef]
- Fang, Y.; Pan, X.; Zhang, K.; Tang, Y.; Xu, X.; Yu, Y.; Zheng, X.; Zhao, M.; Jin, H. Unraveling the Crucial Role of Electrogenerated Cu(III) on the Cu–Sb–Sn/Granular Activated Carbon Three-Dimensional Electrode for Electrocatalytic Oxidation Reactions. ACS EST Water 2025. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Thi, C.H.N.; Tran, T.K.; Nguyen, V.T.; Nguyen, N.H. Comparison of 2d and 3d electrochemical oxidation systems for removal of reactive dyes in water. Desalination Water Treat. 2022, 247, 309–318. [Google Scholar] [CrossRef]
- Haider Kazmi, S.M.; Du, J.; Hassan, A.; Xu, Z.; Faheem, M.; Waseem, O.A.; Yousuf, M. Turning waste into value: Banana peel biochar particle electrode boost methyl violet 2b dye removal in 3d-electrochemical system. Kuwait J. Sci. 2025, 52, 100413. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Wang, K.; Zhang, T.; Sun, Y.; Li, W. Dissolved organic matter (dom) removal from coking wastewater with efficient vanadium-titanium magnetite particle electrodes by 3d/ec/kps system: Optimization, Performance, and Mechanism. J. Clean. Prod. 2021, 322, 128683. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, W.; Hu, X.; Yang, S.; Liang, W. Efficient electrochemical-catalytic reduction of nitrate using co/ac0.9-ab0.1 particle electrode. Sci. Total Environ. 2020, 732, 139245. [Google Scholar] [CrossRef] [PubMed]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Choi, C.H.W.; Shin, J.; Eddy, L.; Granja, V.; Wyss, K.M.; Damasceno, B.; Guo, H.; Gao, G.; Zhao, Y.; Higgs, C.F.; et al. Flash-within-Flash Synthesis of Gram-Scale Solid-State Materials. Nat. Chem. 2024, 16, 1831–1837. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, J.; Hao, Z.; Zhang, Y.; Zhou, X. Novel Three-dimensional electrochemical reactor with p and n-codoped activated carbon for water decontamination: High efficiency and contribution of singlet oxygen. ACS EST Water 2022, 2, 721–729. [Google Scholar] [CrossRef]
- Cho, S.; Kim, C.; Hwang, I. Electrochemical degradation of ibuprofen using an activated-carbon-based continuous-flow three-dimensional electrode reactor (3der). Chemosphere 2020, 259, 127382. [Google Scholar] [CrossRef]



| Particle Electrode | Identification Basis | Name | Electrode | Characteristic | Significant Advantage |
|---|---|---|---|---|---|
| Without a particle electrode | Simple electrolysis reaction | Traditional electrochemical | 2D | Without the particle electrode, the electrochemical processes include electrodes, and under appropriate voltage conditions. | Lower removal efficiency and high energy consumption |
| With a particle electrode | Particle electrode polarizable | Unipolar electrodes | 3D | The typical outcome of a chemical reaction in the cathode and anode chambers is the reduction of metal in the cathode chamber and the oxidation of organic materials in the anode chamber [27]. | One of the most straightforward processes for the production of hydrogen is water electrolysis. The capacity of water electrolysis to generate hydrogen exclusively from renewable energy sources represents a significant advantage. It is essential to reduce the energy consumption, cost, and maintenance requirements of existing electrolytic systems while simultaneously enhancing their efficacy in removing pollutants to promote the adoption of water electrolysis [1]. The rise in hydroxyl radicals was attributable to electrochemical oxidation and electro-Fenton oxidation, which occurred concurrently in the three-dimensional electrochemical oxidation system [28]. |
| Polar electrodes | Electrostatic induction charges the particle electrode, thereby transforming it into a polar particle with positive and negative poles, respectively. Subsequently, reactions occur at the cathode and anode terminals [27,29]. | ||||
| With a particle electrode | Particle electrode filling state | Fixed bed electrodes | The fixed bed electrodes are one of the most straightforward processes for the production of hydrogen in water electrolysis. The capacity to generate hydrogen exclusively from renewable energy sources represents a significant advantage. It is imperative to reduce the energy consumption, cost, and maintenance of existing electrolytic processes simultaneously. Enhancing their efficacy in removing pollutants to promote the adoption of water electrolysis. | ||
| Mobile bed electrodes | Characterize to assess the usage, expense, and upkeep needs of current electrolytic systems while also evaluating their ability to remove contaminants to encourage the use of water electrolysis. | ||||
| With a particle electrode | Current and flow direction of fluid in the reactor | Circulation electrode | The flow of particle electrodes within the reactor enhances the efficiency with which the materials are transmitted. | ||
| Flow-through electrode | The current and the fluid within the reactor both flow in the same direction. | ||||
| With a particle electrode | Reactor shape | Round, ring, and electrodes, etc. | The direction of the reactor fluid flow is perpendicular to the current direction. | ||
| Connection-mode | Single-stage electrode | Due to the ability to adjust cathode positions, circular and annular electrodes are often observed in the same reactor [29]. | |||
| Bipolar electrodes | The power source is connected in parallel to the anode and cathode. The two ends of the electrical supply are affixed to the poles. | ||||
| With a particle electrode | Electromagnetic-type air pump, Peristaltic pump, Aeration micro-pipes…/3D-ER | Monopolar | The primary electrode is separated into an anode and cathode zone. The particles in the anode area extend the main electrode and increase the reaction area by acquiring the same charge distribution as the main electrode [27,29]. | These techniques have the potential to reduce side reactions, enhance the reactor’s overall electrolytic efficiency, promote a uniform potential distribution, and increase time-space productivity. Also, results in stratification and the loss of the catalyst coating on the particle electrodes. An uneven distribution of the feed current and potential may facilitate improved oxidation [30]. | |
| Bipolar | An electric field induces electrostatic induction, which results in the charging of the particle electrodes. At both ends of the charged particles, electrochemical oxidation and reduction reactions occur as the particles transform into independent three-dimensional electrodes on the surface and increase the electrochemical charged particle [31] reaction. These occur at the ends of the bipolar electrode even though there is no direct electrical connection between it and an external power supply [32]. | ||||
| Membrane particle electrode | Monopolar | Monopolar membrane electrochemical anion exchange membrane and the cation exchange membrane both require either poor-performing cathode catalysts or costly catalysts (such as IrO2 at the anode) for acidic cation exchange membrane systems, as well as problems with bicarbonate formation and crossover in anion exchange membrane cells. This is because both membranes electrolyze CO2 and water [33]. | The membrane electrochemical l enhances the performance of the (bi)carbonate electrolyzer. Membranes are appropriate for several uses and allow for the regulation of ion fluxes and concentrations in electrochemical cells. Management of the ions considerable obstacles to the durability, selectivity, and energy efficiency of electrolysis, the chemical engineering opportunities [34]. | ||
| Bipolar | Ion-conductive polymers, known as bipolar membranes, are composed of two fixedly charged layers that are bonded to one another, often with the inclusion of a catalyst layer between them. The cation-exchange layer is constituted by one ionomer layer with fixed negative charges. The anion exchange layer also exhibits fixed positive charges. In electrochemical systems, bipolar membranes can function in two distinct ways: forward bias and reverse bias [34,35]. |
| (a) Removal Performance of 2D and 3D Particle Electrode | |||||||
|---|---|---|---|---|---|---|---|
| Carriers | Main Electrodes | Reactor Type | Catalysts | Pollutants | Reaction Conditions | Removal Efficiency (%) | References |
| TMP | A: titanic C: stainless steel | 2D 3D | Fe | BPA | I = 300 mA C0 = 10 mg L−1 pH = 9 t = 55 min | >98% | [48] |
| GAC | A: titanic C: stainless steel | 2D 3D | Reactive Black B | U = 10 V C0 = 100 mg L−1 pH = 3 t = 60 min | 61.46% 74.77% | [27] | |
| GAC and PCP | 2D 3D | Ceramist particle (PCP) | Heavy oil refinery wastewater | J = 30 mA cm−2 pH not adjusted C0 = 2973 mg L−1 T = 60 °C | 30.8% 45.6% | [49] | |
| AC | A: Ti/SnO2+Sb2O5 DSA C: stainless steel | 2D 3D | Granular carbon aerogels | Reactive brilliant red X-3B | Ed = 20 V pH = 5.1 C0 = 800 mg L−1 Airflow rate = 0.4 L min−1 | 20% 95% | [50] |
| Modified kaolin | A: Ti/SnO2+Sb2O5 C: stainless steel mesh | 2D 3D | Ti/Co/SnO2Sb2O5 | Sodium dodecylbenzene sulfonate | J = 38.1 mA cm−1 C0 = 750 mg L−1 pH = 3 T = 20 °C | 56% 86% | [51] |
| GAC | A: graphite C: stainless steel mesh | 2D 3D | Ti/Co/SnO2Sb2O5 | Paper mill wastewater | J = 167 mA cm−2 C0 = 1357 mg L−1 pH = 11 T = 20 °C | 45% 86% | [51] |
| (b) Lists the Carriers and Catalysts Employed in Wastewater Treatment 3D Electrode Reactors. | |||||||
| Carriers | Catalysts | Pollutants | Main electrodes | Reaction conditions | Removal efficiency (%) | References | |
| Al2O3 | CuFe2O4 | p-nitrophenol (PNP) | A: Ti/RuO2 C: stainless steel | J = 24 mA cm−2 C0 = 150 mg L−1 pH = 10 t = 30 min | 90.69% | [52] | |
| TMP | Pd | BPA | A: titanic C: stainless steel | I = 300 mA C0 = 10 mg L−1 pH = 9 t = 55 min | >98% | [48] | |
| γ-Al2O3 | Bi-Sn-Sb | Tetracycline | i = 0.1 A C0 = 100 mg L−1 pH = 5.9 t = 180 min | 86.0% | [53] | ||
| granular activated carbon | Ti/PbO2 | COD | A: Ti/PbO2 C: stainless steel | i = 7.8 mA t = 30 min | 95% | [36] | |
| Manganese Slag | Cu/Fe | Salicylic acid, Rhodamine B | A: titanium mesh C: carbon fiber | U = 10 V CE = 0.05 M pH = 3 C0 = 0.10 M | 76.9% | [54] | |
| MWCNTs | Pd | 4-Chloropheno | A: Ti C: Ti | J = 4.0 mA cm−2 C0 = 0.2 mM | 100% | [55] | |
| Ni foam | Pd-Fe | Dimetridazole | A: Pt sheets C: Pt sheets | J = 31 mA cm−2 C0 = 50 mg L−1 pH = 3 AFR = 1.0 L min−1 | 96.5% | [56] | |
| Steel Slag | Mn | Rhodamine B | U = 5 V C0 = 5 mg L−1 pH = 6 DPE = 15 g L−1 CE = 0.15 mol L−1 | 100% | [57] | ||
| Ceramic particle | Cu/Zn | Salicylic acid, Rhodamine B | A: lead alloy C: stainless steel | HRT = 150 min U = 15 V pH = 3 C0 = 0.75 g L−1 CB = 30 g L−1 | 83.45% | [58] | |
| AC | Cu | Nitrate | A: Ru/Ir/Ti C: Cu/Ti | U = 5 V C0 = 50 mg L−1 HRT = 3 h | 96.05% | [59] | |
| EO, FT | B-doped Gr | Iopromide | A: B-doped Gr C: N-doped Gr 3D-B-doped | 17 mA cm−2 0.01 M Na2HPO4/NaH2PO4 pH = 7.5 HRT = 3.45 min C0 = 2 μM | 91.3% 84.0% 99.0% 88.0% | [60] | |
| EO, FT | - | Diatrizoate, Triclosan, Diclofenac | A: Ti/SnO2-Sb/PbO2 C: stainless steel | 22 mA cm−2 WF = 3500 L m−2h COD = 230 mg L−1 | 60% | [61] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razack, G.L.; Wang, J.; Zhao, X.; Noel, W.C.; Sun, H.; Pang, J.; Ding, J.; Wang, W.; Yang, X.; Cui, C.; et al. Current State of Research on the Three-Dimensional Particle Electrode System for Treating Organic Pollutants from Wastewater Streams: Particle Electrode, Degradation Mechanism, and Synergy Effects. Water 2025, 17, 2490. https://doi.org/10.3390/w17162490
Razack GL, Wang J, Zhao X, Noel WC, Sun H, Pang J, Ding J, Wang W, Yang X, Cui C, et al. Current State of Research on the Three-Dimensional Particle Electrode System for Treating Organic Pollutants from Wastewater Streams: Particle Electrode, Degradation Mechanism, and Synergy Effects. Water. 2025; 17(16):2490. https://doi.org/10.3390/w17162490
Chicago/Turabian StyleRazack, Guene L., Jiayi Wang, Xian Zhao, Worou Chabi Noel, Hanjun Sun, Jiwei Pang, Jie Ding, Wenshuo Wang, Xiaoyin Yang, Chenhao Cui, and et al. 2025. "Current State of Research on the Three-Dimensional Particle Electrode System for Treating Organic Pollutants from Wastewater Streams: Particle Electrode, Degradation Mechanism, and Synergy Effects" Water 17, no. 16: 2490. https://doi.org/10.3390/w17162490
APA StyleRazack, G. L., Wang, J., Zhao, X., Noel, W. C., Sun, H., Pang, J., Ding, J., Wang, W., Yang, X., Cui, C., Zang, Y., Wang, Y., Luo, G., Ren, N., & Yang, S. (2025). Current State of Research on the Three-Dimensional Particle Electrode System for Treating Organic Pollutants from Wastewater Streams: Particle Electrode, Degradation Mechanism, and Synergy Effects. Water, 17(16), 2490. https://doi.org/10.3390/w17162490








