Abstract
This study provides a comprehensive six-year assessment (2018–2023) of heavy metal contamination in the Romanian Black Sea sector, integrating data from seawater, surface sediments, and benthic mollusks. Sampling was conducted across a broad spatial gradient, including transitional, coastal, shelf, and offshore waters beyond 200 m depth. Concentrations of six potentially toxic metals, including cadmium (Cd), lead (Pb), nickel (Ni), chromium (Cr), copper (Cu), and cobalt (Co), were measured to evaluate regional variability, potential sources, and ecological implications. Results indicate some exceedances of regulatory thresholds for Cd and Pb in transitional and coastal waters, associated with Danube River input and coastal pressures. Seabed substrate analysis revealed widespread enrichment in Ni, moderate levels of Cr, and sporadic Cd elevation in Danube-influenced areas, along with localized hotspots of Cu and Pb near port and industrial zones. Biological uptake patterns in mollusks (bivalves Mytilus galloprovincialis and Anadara inequivalvis and gastropod Rapana venosa) highlighted Cd among key metals of concern, with elevated Bioconcentration Factor (BCF) and Biota–Sediment Accumulation Factor (BAF). Offshore waters generally exhibited lower pollution levels. However, isolated exceedances, such as Cr outliers recorded in 2022, suggest that deep-sea inputs from atmospheric or maritime sources may be both episodic in nature and underrecognized due to limited monitoring coverage. The combined use of water, sediment, and biota data emphasize the strength of multi-matrix approaches in marine pollution evaluation, revealing persistent nearshore pressures and less predictable offshore anomalies. These findings contribute to a more complete understanding of heavy metal distribution in the northwestern Black Sea and provide a scientific basis for improving long-term environmental monitoring and risk management strategies in the region.
1. Introduction
Heavy metal (HM) environmental loading remains a major threat to marine ecosystems and human health due to its persistence, toxicity, and capacity to accumulate in food webs [1]. These pollutants originate from various contributors, including industrial discharge, agricultural runoff, atmospheric deposition, and maritime traffic. However, it is important to emphasize that HMs are also naturally present in marine environments as part of the Earth’s geochemical background. Their occurrence in seawater, sediments, and biota can reflect both natural sources, such as weathering of continental rocks, riverine transport of mineral particles, or volcanic and hydrothermal inputs and anthropogenic activities. Therefore, evaluating HM contamination requires careful consideration of background levels to distinguish between lithogenic and pollution-related enrichment. Coastal and semi-enclosed seas are particularly susceptible to contamination due to limited water exchange and high anthropogenic pressure [2,3,4,5].
The Black Sea, bordered by heavily industrialized and densely populated regions, exemplifies these vulnerabilities [4,6,7,8,9]. The Romanian coastline, although only 244 km in length, is significantly affected by multiple stressors: riverine input from the Danube in the north, and industrial effluents, port operations, and urban runoff in the south [5,10]. Seasonal tourism and shoreline modifications further amplify localized pollution. Due to the basin’s stratified hydrodynamics and semi-enclosed nature, contaminants are retained and redistributed, particularly within surface waters and sediments [11,12,13].
Although HM burden has been documented in various parts of the Black Sea region [14,15,16,17,18], comprehensive and long-term data for offshore waters remain scarce, particularly beyond the scope of routine regional surveillance programs. While some research has explored deeper marine areas [19,20,21], the availability of consistent and spatially extensive data from offshore environments across the region remains limited, in spite of their growing importance in the current context of intensified maritime activity, environmental change, and recent geopolitical pressures in the Black Sea basin [22,23,24]. Moreover, uncertainties persist in distinguishing the relative contributions of riverine inputs versus atmospheric deposition or other inputs, as well as in assessing the bioavailability and accumulation patterns of these metals in benthic organisms [25].
A portion of the data collected during the 2020–2022 period was previously included in a regional assessment [26], which focused on multiple contaminant groups and applied the technical framework of the Marine Strategy Framework Directive (MSFD) to assess Good Environmental Status (GES) [27]. That study emphasized regulatory thresholds and indicator-based evaluations. In contrast, the present paper offers a more detailed investigation focused exclusively on geographic patterns and ecosystem implications of HMs, extending the time frame to 2018–2023. It employs a multi-matrix approach (upper water layer, seabed substrate, and benthic biota), integrates seabed substrate granulometry, and applies bioconcentration and bioaccumulation indices (BCF and BAF) to assess contaminant behavior and ecological relevance. This work complements the MSFD-based analysis by providing new insights into regional variation, geochemical drivers, and biological uptake processes in the Romanian Black Sea (RBS) sector.
This study aims to provide a comprehensive evaluation of HM contamination in the RBS sector through an integrated, multi-matrix approach encompassing seawater, sediments, and benthic mollusks, over a period of six years (2018–2023). By analyzing key trace metals: cadmium (Cd), lead (Pb), nickel (Ni), chromium (Cr), copper (Cu), and cobalt (Co), across a gradient from transitional zones to offshore waters, we seek to (i) quantify metal levels and variability across environmental compartments; (ii) investigate spatial patterns and their potential links to natural and human-driven sources; and (iii) assess the extent of retention in representative benthic species to better understand the ecological implications of contaminant exposure.
While national routine surveillance efforts during 2018–2023 have focused on coastal and shelf zones up to 100 m depth, this study offers the opportunity to additionally assess HM presence in deeper marine waters beyond 200 m. These offshore stations, surveyed during research cruises in 2020–2022, provide valuable information into trace metal behavior under more stable hydrodynamic regimes and comparatively reduced direct man-made pressure. The findings presented herein contribute to a more complete understanding of pollution dynamics along the RBS sector and support the development of targeted, evidence-based strategies for marine environmental monitoring and protection [28].
2. Materials and Methods
2.1. Study Area
The investigation was carried out in the Romanian Exclusive Economic Zone (EEZ) of the northwestern Black Sea, an area subject to complex hydrodynamic and human-induced influences affecting pollutant behavior. Fieldwork was conducted during eleven oceanographic campaigns between 2018 and 2023 and was performed in the warm season (summer–autumn) using the research vessels Steaua de mare 1 and Mare Nigrum.
Sampling network covered the marine reporting units (MRU) [27,29] along the Romanian Black Sea (RBS) coast: transitional (T), coastal (C), shelf (S), and offshore (O) zones. Transitional waters, located in the northern part of the littoral and extending to the 29 m isobath, are influenced by the Danube River. Southern coastal waters, also up to the 29 m isobath, are shaped by local littoral dynamics. Shelf waters span from 30 m to 200 m depth, while offshore areas extend beyond the 200 m isobath and are characterized by deeper, more stable marine conditions (Table A1).
A total of 298 surface water and 277 sediment samples were collected. Sediment sampling sites generally overlapped with water sampling stations (Figure 1a), except for those located at depths exceeding 150 m, where seabed substrate collection was not possible due to logistical constraints. Additionally, 48 mollusk samples (Mytilus galloprovincialis, Rapana venosa, and Anadara inequivalvis) were collected from transitional, coastal, and shelf zones during the 2018–2023 period (Figure 1b).
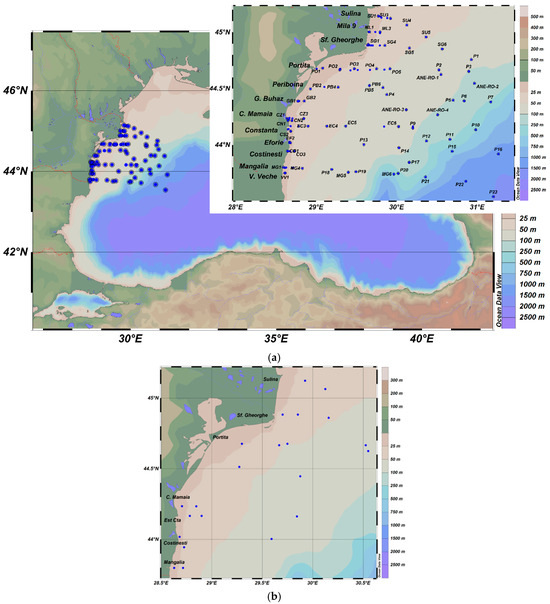
Figure 1.
Locations of water and sediment (a) and mollusk (b) sampling stations of the Romanian Black Sea sector, 2018–2023.
The RBS sector, encompassing 244 km of coastline (~6% of the total basin), is subject to diverse and overlapping environmental pressures. In the north, the influence of the Danube River dominates, while the southern coastal zone is shaped by dense urbanization, industrial activity, and maritime infrastructure [5,10,30,31]. Additional freshwater inputs from rivers such as the Dniester, Dnieper, and Southern Bug directly affect the northwestern marine environment. Other river systems, like the Don, influence the adjacent Sea of Azov, which is connected to the Black Sea via the Kerch Strait. Key RBS pressures include wastewater discharge, port operations (Constanta, Mangalia, Midia), seasonal tourism, oil and gas extraction, etc. [5,32]. Pollution in this region arises from multiple drivers: shipping, dredging, shoreline modification, and land-based runoff, all contributing to contaminant accumulation and habitat disturbance [10]. Though some improvements in wastewater treatment infrastructure have been reported since 2018 [5], the cumulative impact of nutrient loading, marine litter, and pollutants input remain considerable [4,33,34,35]. The semi-enclosed nature of the area, combined with limited water exchange, facilitates pollutant retention and redistribution, particularly in coastal and shelf waters. These features make the region a representative case study for assessing pollution trends and environmental threats in the broader Black Sea context [20,34,35].
2.2. Sample Collection
Surface water samples were collected from the 0–1 m layer using Niskin bottles. These samples, representative of the most reactive water layer influenced by surface inputs, were immediately cooled to 4–8 °C for preservation prior to analysis [36]. Sediments were sampled using a Van Veen grab, targeting the upper 2–5 cm layer to capture recent depositional inputs [37]. The sampler’s design minimized disturbance, preserving the surface integrity. Bivalve and gastropod specimens (Mytilus galloprovincialis, Anadara inequivalvis, and Rapana venosa) were obtained using a biological dredge. Composite samples comprising 5–10 adults individuals of each species per station were prepared [38]. Samples were stored frozen. In the laboratory, mollusk soft tissues and sediment samples were freeze-dried to remove moisture. Sediments were sieved to exclude coarse fraction particles > 2 mm and homogenized prior to pollutant analysis.
2.3. Analytical Techniques
2.3.1. Heavy Metals (HMs)
HMs in water were determined from unfiltered samples acidified to pH ~2 using ultrapure nitric acid (HNO3). For sediments and biota, 0.05–0.5 g of freeze-dried material was digested with 10 mL concentrated HNO3 in sealed Teflon vessels on a hot plate at 120 °C. Digests were diluted to 100 mL with ultrapure water (18.2 MΩ·cm; Millipore, Burlington, MA, USA). Elemental analysis was performed using a High-Resolution Continuum Source Atomic Absorption Spectrometer (HR-CS AAS, ContrAA 800 G; Analytik Jena, Jena, Germany). Calibration standards were prepared from Merck solutions within the following ranges: 0–50 μg/L (Cu, Ni, Cr), 0–25 μg/L (Pb), and 0–10 μg/L (Cd, Co). Each sample was analyzed in triplicate, and mean values were reported. Detection limits ranged from 0.001 to 0.01 μg/L depending on the element [39]. Final results were expressed as μg/L for seawater and μg/g dry weight (dw) for sediments. For biota, metal concentrations were expressed in μg/g wet weight (ww) when compared to EU Maximum Allowable Concentrations (MACs) [40] and μg/kg wet weight (ww) when calculating BCFs, as both are based on wet weight to reflect seafood as consumed. For BAF calculations, tissue concentrations were converted to dry weight (dw) to match sediment values, which are conventionally reported on a dry weight basis.
2.3.2. Granulometry
Seabed substrate grain size analysis was performed on 53 samples collected in 2021, using two complementary methods selected according to the coarseness of the sample. For fine-grained sediments, granulometric analysis was conducted using a laser diffraction technique. Samples were suspended in distilled water and analyzed using an Analysette 22 Nanotec laser particle size analyzer. The suspension was circulated through a measuring cell equipped with dual lenses, and a laser beam was directed through the sample. The angle and intensity of laser diffraction were recorded by a detector, and the accompanying software calculated the particle size distribution based on the observed diffraction patterns. Results were expressed as the relative volume percentage for each defined particle size class.
For coarse-grained samples, a dry mechanical sieving method was employed. Samples were first dried to constant mass, then subjected to sieving using a vibratory sieve shaker fitted with a set of 20 sieves, ranging from 6.3 mm to 63 µm mesh size. The mass retained on each sieve was recorded, and the grain size distribution was computed from the cumulative weight percentages.
The resulting granulometric data from both methods were processed using the GRADISTAT Software Version 9.1 (October 2001), a grain-size analysis program developed by Blott (2001) [41], which calculates statistical parameters such as mean grain size, sorting, and percent contributions of gravel, sand, silt, and clay fractions, following standard Folk and Ward (1957) procedures [42]. Sediment textural classes (e.g., Mud, Sandy Mud, and Muddy Sand) were assigned based on the relative proportions of gravel (>2 mm), sand (63 µm–2 mm), and mud (<63 µm, i.e., silt + clay).
2.4. Data Processing
The data was interpreted using MS Excel 365, Statistica (TIBCO Software Version 14.0.1.25) [43], and Ocean Data View (ODV) version 5.1.7 [44]. Distribution maps were created using the weighted averaging gridding method in ODV.
Descriptive statistics, including mean, median, minimum, maximum, 25th and 75th percentiles, standard deviation, and coefficient of variation (CV), were calculated using MS Excel 365 and Statistica (TIBCO Software, version 14.0.1.25). Boxplots present medians, interquartile ranges, whiskers (minimum–maximum), and outliers. For comparative assessment, HM concentrations in seawater were evaluated against the Maximum Allowable Concentration–Environmental Quality Standards (MAC–EQS) defined in Directive 2013/39/EU [45] and, for Cu, national legislation [46]. Sediment quality was assessed using Effect Range–Low (ERL) values proposed by Long et al. (1995) [47] and adopted in NOAA and OSPAR frameworks [48], representing conservative thresholds below which adverse effects on benthic fauna are expected to be infrequent. For biota, measured concentrations were compared to the Maximum Allowable Concentrations (MACs) established under Commission Regulation (EU) 2023/915 [40]. Pearson correlation coefficients (r) were calculated in MS Excel 365 to assess relationships among HM concentrations and sediment texture variables, with statistical significance evaluated at p < 0.05.
Principal Component Analysis (PCA) was plotted using AI-assisted tools (OpenAI ChatGPT-4 Plus, 2025), while variable selection and interpretation of the components were independently performed and validated by the authors.
To assess the accumulation of HMs in marine organisms, two indices were calculated: the Bioconcentration Factor (BCF) and the Biota–Sediment Accumulation Factor (BAF). These metrics reflect contaminant uptake from water and sediment, respectively, and are commonly applied in ecological risk assessment.
The BCF was computed as the ratio between metal concentration in soft tissues Cbiota (µg/kg wet weight) and its corresponding concentration in seawater Cwater (µg/L) following REACH Regulation (EC No 1907/2006) [49] and ECHA guidance [50]. Classification thresholds were used to distinguish between non-bioaccumulative (BCF < 1000), bioaccumulative (1000 ≤ BCF ≤ 5000), and very bioaccumulative substances (BCF > 5000).
The BAF was calculated by comparing metal values in biota Cbiota (µg/g dry weight) and surface sediments Csediments (µg/g dry weight), accounting for both direct exposure and trophic uptake. While no formal REACH thresholds exist for BAF, a value >1.0 is generally considered indicative of significant sediment contribution, based on USEPA guidance [51].
Both indices were applied across all sampled bivalves and gastropods to evaluate interspecies differences and identify metals with pronounced accumulation potential from distinct environmental compartments.
3. Results
3.1. Seawater
A total of 298 upper water layer samples collected between 2018 and 2023 revealed notable heterogeneity in HM concentrations across RBS waters (Table 1). The average levels occurred as follows: Cu > Ni ≈ Cr > Pb > Cd. Cu exhibited the highest mean value (10.80 µg/L), with a wide range spanning from 0.14 to 51.56 µg/L. Ni and Cr had similar mean values (7.16 and 7.22 µg/L, respectively), but Ni was distinguished by a higher standard deviation (11.44 µg/L) and coefficient of variation (159.6%), indicating significant variability across stations. Pb had a lower mean value (5.68 µg/L) but also showed raised fluctuations (CV = 136.1%), suggesting sporadic high amounts. Cd, while present at the lowest average concentration (0.46 µg/L), exhibited the highest relative variation (CV = 160.8%), indicative of both sporadic increased values and many measurements near the detection limit, similar with Pb. These statistical patterns, including wide concentration ranges and high coefficients of variation, especially for Ni, Pb, and Cd, highlight the influence of localized or episodic pollution events within the study area.

Table 1.
Descriptive statistics of HM concentration (µg/L) in Romanian Black Sea waters, 2018–2023.
The compliance of measured HM with Environmental Quality Standards (EQS) was assessed based on the comparison of obtained values against the MAC-EQS thresholds defined in Directive 2013/39/EU [45] or national legislation (for Cu) [46]. The analysis revealed that, although the majority of samples complied with regulatory limits, some exceedances were recorded (Figure 2). These individual exceedances, even when sporadic, indicate episodic inputs or localized sources that may temporarily elevate amounts beyond acceptable environmental thresholds. In line with MSFD Descriptor 8 evaluation principles, such instances represent potential risks to ecosystem health, even if the median or percentile values remain within regulatory limits.
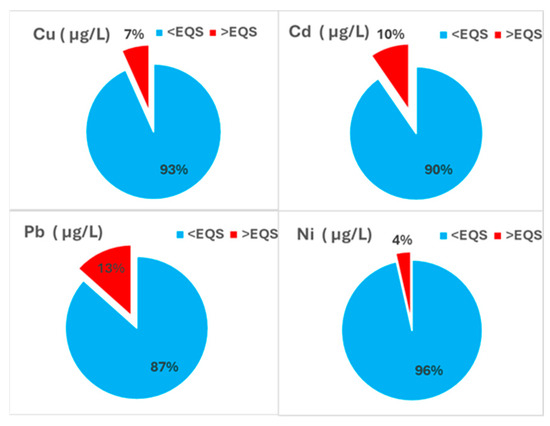
Figure 2.
HM concentrations in seawater (2018–2023) compared to MAC-EQS thresholds.
The geographic patterns of HMs (Figure 3), together with their statistical profiles across MRUs (Figure 4), highlight consistent gradients alongside atypical distribution patterns for specific elements. Pb, Cd, and Ni exhibited their highest values in transitional waters of the northern sector, under the strong influence of the Danube River. These areas displayed increased medians, broad interquartile ranges, and frequent outliers, some exceeding EQS, reflecting the combined impact of fluvial discharge and localized inputs. A general decline was observed from transitional through coastal and shelf areas toward offshore waters for most metals. However, Cu and Ni also showed enrichment in shelf waters of the central and southern sectors, linked to maritime traffic, offshore installations, and seabed substrate resuspension. These trends are reflected in Figure 4 through wider fluctuations and outliers in the shelf reporting unit. Cr displayed a distinct spatial pattern compared to the other metals. While moderate amounts were recorded across most areas, unusually high values for Cr were detected in offshore waters during the 2022 sampling campaign. This pattern deviates from the typical nearshore enrichment observed for Pb, Cd, and Ni.
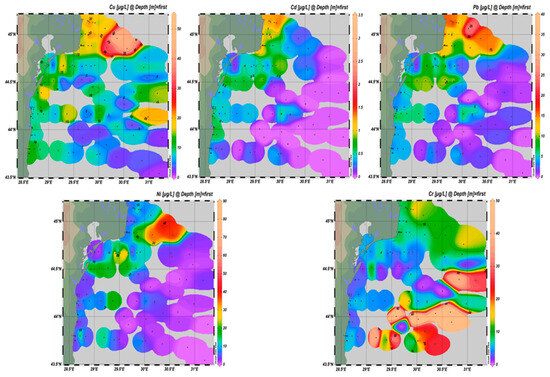
Figure 3.
HM distribution in surface seawater from Romanian Black Sea, bathymetric strip 10–1500 m, 2018–2023.
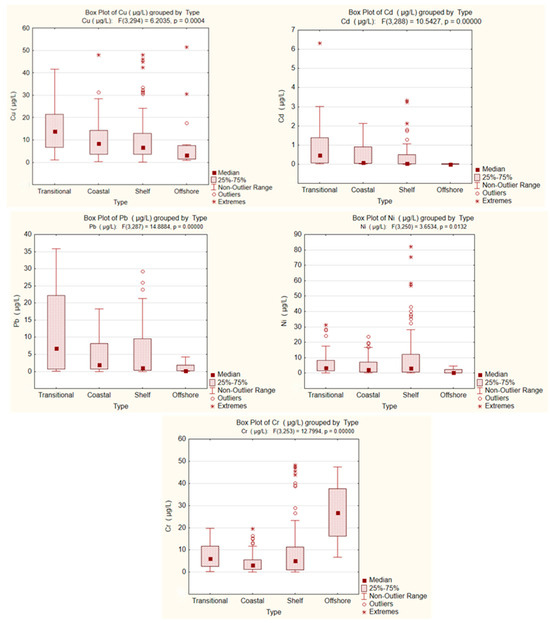
Figure 4.
Boxplots of Cu, Cd, Pb, Ni, and Cr concentrations in seawater across marine reporting units (MRUs): transitional, coastal, shelf, and offshore, 2018–2023.
Offshore stations beyond 200 m depth were sampled during 2020–2022 cruises, providing a rare dataset for deep-sea HM presence in Romanian waters. In general, levels of Pb, Cd, and Ni were lower in offshore waters compared to transitional and coastal zones, consistent with their predominant land-based origins. However, isolated cases of offshore outliers were recorded, suggesting possible episodic inputs from atmospheric deposition, maritime activity, or long-range transport. Overall, the integration of interpolated maps and statistical dispersion reveals regional patterns shaped by both regional pressures and metal-specific behavior. While Pb, Cd, and Ni conform to expected coastal enrichment profiles, the offshore anomalies in Cr and localized shelf peaks in Cu and Ni highlight the importance of temporal resolution and spatially differentiated observation strategies, including offshore areas not typically prioritized for pollution investigation.
As water concentrations are inherently variable and influenced by short-term inputs and hydrodynamic conditions, long-term trends are more reliably reflected in sediment records.
3.2. Sediments
A total of 277 samples collected between 2018 and 2023 revealed considerable heterogeneity in HMs along the RBS sector (Table 2). The average values were as follows: Ni > Cr > Cu > Pb > Co > Cd. While this order partly aligns with crustal abundance patterns, the elevated Ni concentration, despite its lower typical crustal abundance compared to Cr, suggests a combination of lithogenic origin and localized enrichment. Ni displayed the highest mean concentration (40.20 µg/g), followed by Cr (25.54 µg/g) and Cu (22.75 µg/g). Cd had the lowest average value (0.29 µg/g) but exhibited a high coefficient of variation (CV = 149.9%). Pb and Co also showed variability (CV = 83.1% and 58.8%, respectively), suggesting non-uniform patterns likely shaped by localized inputs or mixed natural and anthropogenic contributors. The wide ranges between minimum and maximum values, especially for Ni (0.24–126.81 µg/g) and Cu (1.47–123.90 µg/g), highlight the influence of both natural and human-driven factors.

Table 2.
Descriptive statistics of HM concentrations (µg/g) in RBS sediments, 2018–2023.
In the absence of legally binding sediment quality standards for trace metals at the European level, this study employed the Effect Range–Low (ERL) values proposed by Long et al. (1995) as conservative screening benchmarks to assess potential ecological risk [47,52,53]. ERLs are empirically derived from large datasets linking measured contaminant concentrations to observed biological responses in field and laboratory studies. Specifically, they represent the 10th percentile concentration of toxicants in sediment samples associated with adverse effects on benthic organisms, meaning that at or below this level, harmful biological effects are expected to occur rarely (in fewer than 10% of cases). These thresholds are widely used in regional monitoring frameworks (e.g., NOAA, OSPAR) and enable comparative assessments across spatial and temporal datasets [48,54]. While ERLs do not account for natural geochemical variation or sediment texture, they are well-established within international monitoring programs and support consistency with prior regional assessments [20,26,29,55,56]. Their conservative nature ensures that areas warranting closer scrutiny for potential ecological stress are flagged.
The HMs measured during the study period were evaluated against ERL thresholds to assess potential ecological concern (Figure 5). While most average values remained below ERL levels, exceedances were recorded for Ni, Cu, and Pb. Notably, Ni exceeded the ERL (20.9 µg/g) in over 75% of samples, while Cu and Pb surpassed their respective thresholds (34.0 and 46.7 µg/g) in 21% and 4% of samples. These exceedances indicate a potential for biological effects, especially in sediments with higher fine fraction content, in which metal mobility and bioavailability are enhanced. However, Ni exceedances do not necessarily reflect widespread anthropogenic input. Previous studies [57] have reported high background levels of Ni in the northwestern Black Sea seabed substrate, linked to natural geochemical conditions. These works attribute the pattern to the regional mineralogical composition, in which Ni shows strong correlations with Fe2O3 and total organic carbon (TOC), indicating a predominantly natural, lithogenic origin [56,57,58]. Consequently, Ni ERL exceedances should be interpreted with caution as they may reflect natural enrichment rather than recent human-driven inputs, depending on the area.
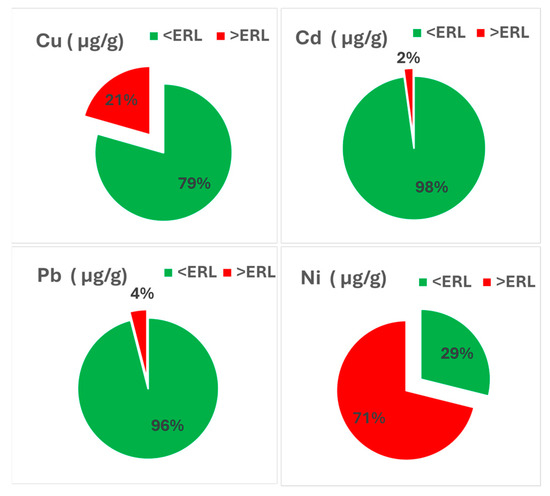
Figure 5.
HM concentrations in sediments (2018–2023) compared to ERL thresholds.
The occurrence of HMs in sediments (Figure 6), together with their statistical profiles across MRUs (Figure 7), reveals distinct regional trends. Ni and Cr showed widespread enrichment across the shelf, with increased amounts in both the northern sector, under the influence of fluvial-derived inputs, and the southern coastal and shelf zone, particularly near port infrastructure. These patterns are confirmed by the raised medians and broad interquartile ranges observed in shelf sediments in Figure 7, suggesting a combination of natural accumulation and anthropogenic contributions.
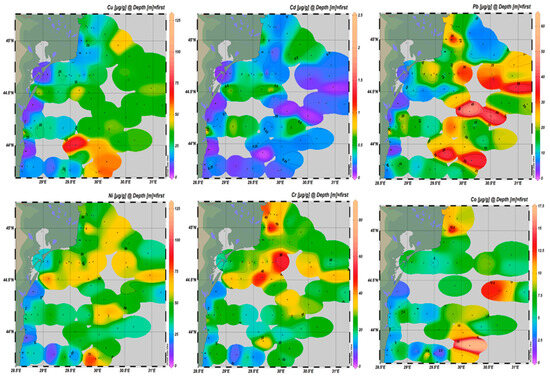
Figure 6.
HM distribution in sediments from Romanian Black Sea, bathymetric strip 10–150 m, 2018–2023.
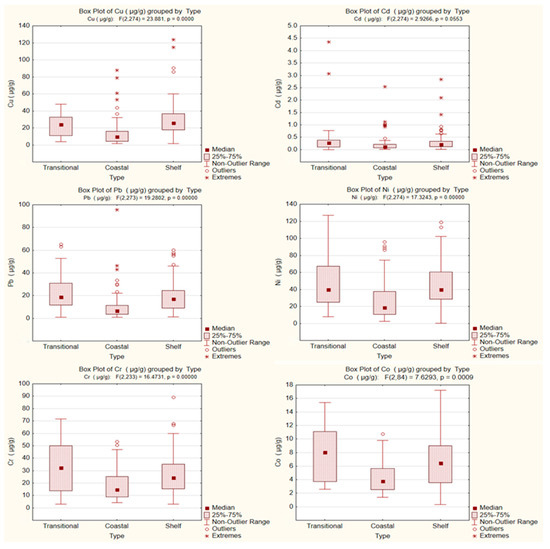
Figure 7.
Boxplots of Cu, Cd, Pb, Ni, Cr, and Co concentrations in sediments across marine reporting units (MRUs) (transitional, coastal, shelf), 2018–2023.
Cu displayed more localized hotspots, especially in southern shelf, consistent with the influence of maritime activity and urban-industrial discharges. The high variability and frequent outliers for Cu in shelf areas further support this interpretation. In contrast, Pb and Cd were most enriched in the northern part of the study area, indicating a dominant riverine input. This is reflected in the distribution maps as well as the higher medians and upper-range values recorded in the transitional area in Figure 7. Co, although measured in fewer samples (since 2021), also showed moderate values in both transitional and shelf areas, though with less pronounced gradients.
Together, Figure 6 and Figure 7 highlight a pattern in which transitional areas are primarily enriched in Pb and Cd, shelf seabed substrate shows higher concentrations of Ni, Cr, and Cu, and coastal zones exhibit intermediate or variable values depending on their proximity to local pollution sources. This zonation reflects the combined effects of source-specific inputs, hydrodynamic regimes, and sediment deposition processes.
This spatial structure is shaped not only by source inputs but also by sediment properties that influence metal retention. To explore this further, the pattern of the fine fraction (silt + clay) is presented in Figure 8. Since trace metals tend to bind preferentially to fine particles due to their high surface area and sorption capacity, understanding the spread of mud content is essential for interpreting metal accumulation and potential bioavailability [59]. Figure 8, along with granulometric data from Table A2, shows that mud-rich sediments dominate in transitional and northern shelf areas, where silt and clay fractions often exceed 70%. These fine materials are primarily delivered by multiple freshwater contributors discharging into the northwestern Black Sea, including the Danube River and associated distributaries and channels [60].
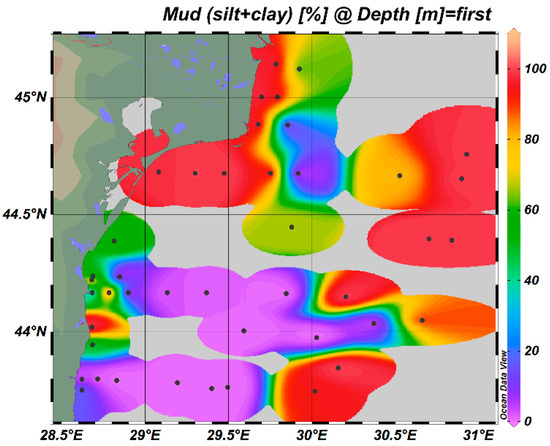
Figure 8.
Fine fraction (silt + clay) (%) distribution in sediments from RBS, bathymetric strip 10–150 m, 2021.
In contrast, seabed substrate from the southern coastal zone and parts of the central shelf are more texturally heterogeneous, containing greater proportions of sand and sandy-silt, as a result of stronger hydrodynamic forcing and sediment redistribution. A positive association was observed between mud content and concentrations of metals such as Ni, Cr, and Cd, consistent with their known affinity for fine-grained, organic-rich sediments. This relationship is further supported by pronounced levels of total organic carbon (TOC) in mud-dominated areas [61], which enhance trace metal retention through sorption and complexation mechanisms.
Even so, several stations with relatively low mud content also exhibited high metal accumulation, especially in urban-adjacent or industrial zones, indicating human-induced inputs that may override grain-size influence. In addition, low-energy depositional environments, such as harbors and semi-enclosed coastal inlets, function as efficient sinks for both fine particles and associated contaminants due to restricted circulation and sediment trapping. These spatial patterns, further explored through multivariate analyses, highlight the importance of jointly considering seabed substrate texture, fluvial supply, organic matter content, and local pollution sources when evaluating trace metal in coastal and shelf environments.
The Pearson correlation matrix (Figure 9) revealed strong and statistically significant associations among several HMs. For instance, high correlation coefficients were observed between Ni and Co (r = 0.88), Pb and Ni (r = 0.81), and Co and Pb (r = 0.78), suggesting a high degree of co-occurrence and potential geochemical similarity. These relationships may reflect both natural lithogenic contributions and overlapping anthropogenic drivers such as industrial discharges, shipping-related inputs, or urban runoff. Moderate correlations between Cu and the other metals (e.g., Cu–Pb, r = 0.56) may indicate more localized or variable sources. With regard to granulometric texture, the fine fraction (silt + clay) showed weak to moderate positive correlations with several metals: Cr (r = 0.42), Co (r = 0.38), Ni (r = 0.37), and Cd (r = 0.36). These trends are consistent with the known affinity of trace metals for fine-grained particles. However, relatively modest correlation values also suggest that factors beyond grain size, such as direct human-driven inputs or localized hydrodynamic conditions, play an important role in shaping metal distributions.
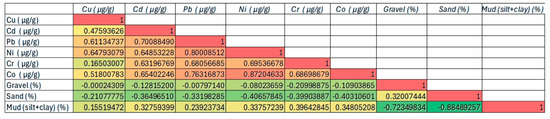
Figure 9.
Correlation matrix of HMs and sediment texture (mud, sand, gravel) from RBS sediments, bathymetric strip 10–150 m, 2021 (n = 53). Cell colors represent the magnitude and direction of correlation coefficients: warm colors (red to orange) indicate positive correlations of increasing strength, cool colors (light green to dark green) indicate negative correlations of increasing strength, and yellow shades indicate weak or near-zero correlations.
The PCA biplot (Figure 10) highlights the spread of samples based on grain size and total HM content. Gravel (%) was excluded from the PCA since HMs were analyzed in the <2 mm fraction. The first principal component (PC1), which explains 70.3% of total variance, separates samples primarily by mud (%) and Total HM, both of which load positively, while sand (%) loads negatively, consistent with expected geochemical patterns. Most shelf and transitional stations cluster along the positive PC1 axis, where fine-grained sediments are associated with increased metal levels. However, a group of anomalous samples appears in the upper-left quadrant, characterized by low mud content (≤0.5%) but high Total HM values. These include stations PO5, P14, P13, and EC3, all of which have mud fractions between 0.2% and 0.5% but exhibit some of the highest Total HM amount in the dataset. These outcomes indicate a decoupling between grain size and trace metal enrichment, pointing toward localized anthropogenic input. Potential contributors may include port activities, offshore industry, dredging operations, maritime traffic, and sand extraction. In such dynamic environments, even coarse sediments may accumulate metals through oxide coatings, biofilm binding, or recent deposition of fine contaminated particles [62]. These anomalous patterns underscore the importance of incorporating both geochemical affinity and site-specific pollution drivers when interpreting seabed substrate data in marine surveillance frameworks.
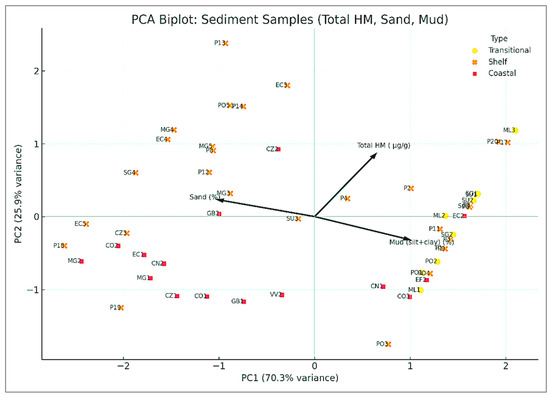
Figure 10.
Principal Component Analysis (PCA) biplot showing the distribution of sediment samples from the RBS coast based on Total HM (Cu, Cd, Pb, Ni, Cr, Co) (µg/g), Sand (%), and Mud (silt+clay) (%), bathymetric strip 10–150 m, 2021 (n = 53).
3.3. Biota
Table 3 presents descriptive statistics for HM concentrations in benthic mollusks, pooled across the three studied species: the bivalves Mytilus galloprovincialis and Anadara inaequivalvis, and the gastropod Rapana venosa. The data indicates a wide range of values for all metals, with particularly high variability (CV > 100%) observed for Cd and Pb, suggesting the influence of localized events or differences in metal bioavailability. Cu and Ni also exhibited broad concentration ranges, reflecting variable environmental interaction and uptake potential. These values represent aggregated data across species and stations; detailed species- and site-specific information will be presented in the context of bioconcentration (BCF) and bioaccumulation (BAF) analyses.

Table 3.
Descriptive statistics of HM concentration (µg/g ww) in Romanian Black Sea mollusks, 2018–2023.
Regulatory compliance was assessed by comparing individual HM measured in mollusk tissues to the Maximum Allowable Concentrations (MAC) set by EU Regulation No. 2023/915 [40] (Figure 11). The majority of samples complied with MAC thresholds for Cd and Pb. However, exceedances were recorded in some cases, particularly for Cd, indicating potential interaction to contaminated sediments or waterborne sources. While Cu and Ni are not currently regulated under food safety legislation, their presence at elevated levels in some samples suggests a need for further attention in environmental and ecotoxicological investigations.
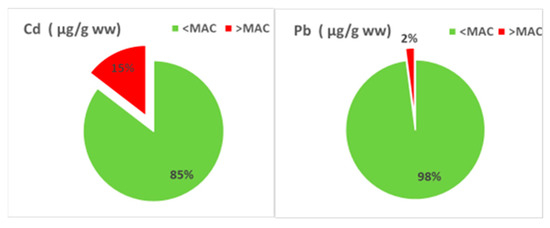
Figure 11.
HMs concentrations in mollusks (2018–2023) compared to MACs thresholds.
The occurrence of HM in mollusk soft tissues is illustrated in Figure 12. Increased levels of Cd, Pb, and Ni were generally observed in mollusks collected from the northern and central coastal sectors, which are influenced by riverine discharges, port activity, and industrial inputs. In contrast, lower values were typically recorded in the southern coastal zone and shelf areas, where the influence of direct anthropogenic sources is reduced and environmental dilution is more effective. Localized peaks in Cu were also observed in some nearshore zones, particularly near harbors and shipping lanes. While species-specific uptake capacity influences the observed levels, the maps present an integrated view across all taxa and stations, providing a first approximation of spatial exposure patterns. Species- and site-specific trends will be examined further through the BCF and BAF analyses.
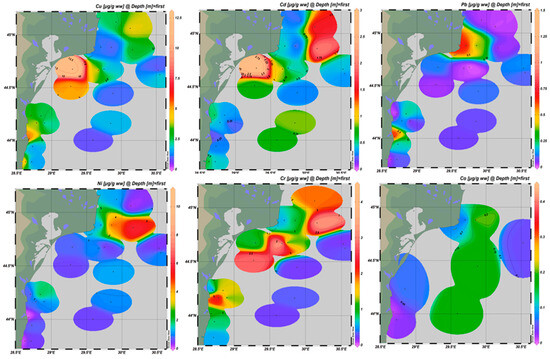
Figure 12.
HM distribution in mollusks from Romanian Black Sea, bathymetric strip 10–150 m, 2018–2023.
The bioconcentration factors (BCFs) presented in Table A3 demonstrate distinct trends according to metal type, species, and sampling area. Cd exhibited the highest BCF values across all species, frequently exceeding 5000 and classified as very bioaccumulative. Ni was generally within the non-bioaccumulative and bioaccumulative range (1000–5000), particularly in M. galloprovincialis and A. inaequivalvis. Pb was predominantly non-bioaccumulative across species; however, a few elevated and very bioaccumulative values were recorded in Mytilus and, to a lesser extent, in Anadara. Cr showed the lowest BCF values overall, with most values <1000, indicating non-bioaccumulative tendencies. These results align with known differences in metal solubility, biological uptake pathways, and bioavailability in marine systems.
Among species, M. galloprovincialis exhibited the highest overall BCFs, particularly for Cd, and, to a lesser extent, for Ni and Cr. This is attributed to its epifaunal position and high filtration capacity, which ensures continuous interaction with suspended particles in the water column. A. inaequivalvis also showed elevated BCFs for Cd and Ni, consistent with its suspension-feeding behavior and interface contact with overlying waters. R. venosa displayed consistently lower BCFs across all metals except Cd, in line with its carnivorous trophic level and reduced direct exposure to the water column.
The highest BCF values were recorded in organisms from transitional and coastal areas, in agreement with increased metal levels in nearshore surface waters influenced by riverine and other human-driven inputs. Shelf areas typically exhibited lower BCFs. However, in some shelf stations (e.g., SG4, EC3, P4), extremely high BCF values (≥105) were recorded. These high ratios could result not from unusually high tissue values, but from very low water metal levels, sometimes near detection limits, and should not be interpreted as evidence of exceptional bioaccumulation. It is also important to note that HMs were measured only in the surface water layer, which may not fully reflect conditions near the seabed, where benthic organisms reside and sinking particles accumulate. Accordingly, the BCF values presented should be interpreted as approximate indicators of metal uptake from the water column.
Figure 13 provides a comparative overview of BCF classifications across species and metals, reinforcing the trends observed in Table A3. M. galloprovincialis displays the highest proportion of very bioaccumulative values, particularly for Cd and, to a lesser extent, Ni, consistent with its strong filtration capacity and continuous interaction with the water column. A. inaequivalvis also shows a substantial proportion of bioaccumulative and very bioaccumulative values for Cd and Ni, reflecting its suspension-feeding behavior near the sediment–water interface. In contrast, R. venosa exhibits a more restricted assimilation profile, but still shows significant bioaccumulative BCFs for Cd, likely driven by trophic transfer. Cr exhibited moderate bioaccumulation in M. galloprovincialis and R. venosa, with some BCF values exceeding the 1000 threshold, while remaining largely non-bioaccumulative in Anadara. Cu showed consistently low BCF values across all species, indicating a non-bioaccumulative profile under the studied conditions, with only a few borderline values in M. galloprovincialis. Pb was generally non-bioaccumulative across species, although a few elevated values were observed in Mytilus and Anadara.
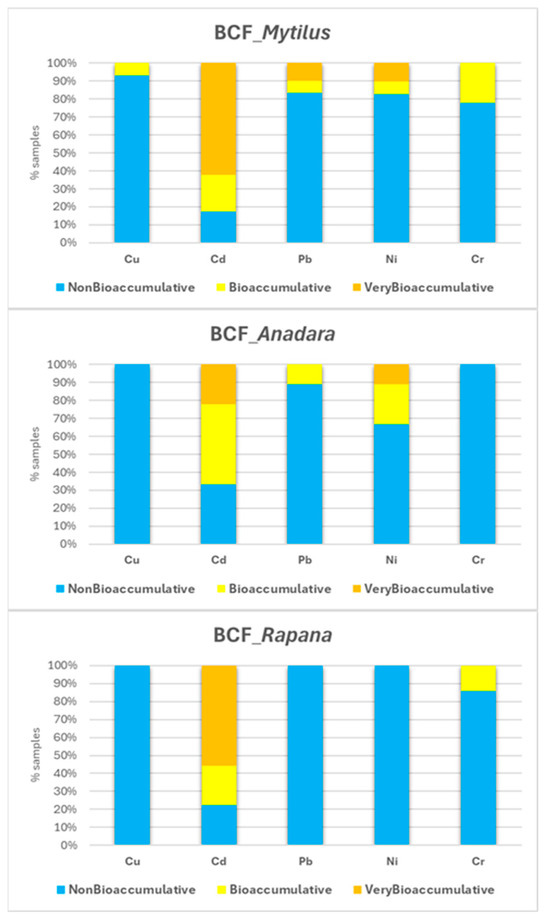
Figure 13.
Proportion of non-bioaccumulative, bioaccumulative, and very bioaccumulative values for each HM, presented by species.
The BAF values calculated for HMs in M. galloprovincialis, A. inaequivalvis, and R. venosa (Table A4) revealed metal-, species-, and area-specific accumulation patterns. Based on the conventional threshold (BAF > 1), the results show that Cd was the most consistently bioaccumulated element, with raised values observed across all species and areas. Cu also showed a tendency to accumulate in tissues, particularly in Rapana, and, to a lesser extent, in Mytilus, while Cr and Ni exhibited variable behavior, with accumulation more evident in Mytilus (Cr) and Anandara (Ni). In contrast, Pb generally displayed lower BAF values, suggesting limited assimilation from sediment contribution.
Species differences were evident in BAF profiles. While Cd was consistently bioaccumulated across all three mollusk species, the highest values were generally observed in Mytilus galloprovincialis. This widespread accumulation reflects Cd’s strong bioavailability and the organisms’ exposure to sediment-associated sources. While generally non-bioaccumulative for other metals, Mytilus also displayed sporadic bioaccumulative values for Cu and Cr, indicating occasional sediment uptake. Anadara inaequivalvis, a suspension-feeding bivalve partially embedded in the seabed, showed elevated BAFs for Cd and Ni, consistent with sediment exposure pathways. Though Rapana venosa displayed lower BAFs overall, it also demonstrated notable accumulation of Cd and sporadic bioaccumulative values for Cu, likely via trophic transfer from contaminated prey and occasional sediment interaction.
Higher BAF values were typically recorded in transitional and coastal areas, whereas shelf areas generally showed lower bioaccumulation, although Cd and Cu remained bioaccumulative in some shelf samples, indicating their persistence and bioavailability even in less contaminated environments.
Figure 14 summarizes the BAF classifications by species and metal, showing the proportion of samples classified as bioaccumulative (BAF >1) or non-bioaccumulative (BAF < 1). The figure highlights that Cd is bioaccumulated across all species, underscoring its consistent uptake from sediment-associated burden. Cu also shows substantial bioaccumulation, particularly in R. venosa, indicating possible dietary and sediment contact pathways. Ni is bioaccumulated to a lesser extent, with bioaccumulative values observed primarily in A. inaequivalvis. Cr is predominantly non-bioaccumulative but shows some bioaccumulative values in Mytilus. Pb remains mostly non-bioaccumulative in all species. These results reinforce the high bioavailability of HMs, especially Cd, in the seabed substrate compartment and the importance of species-specific accumulation profiles.
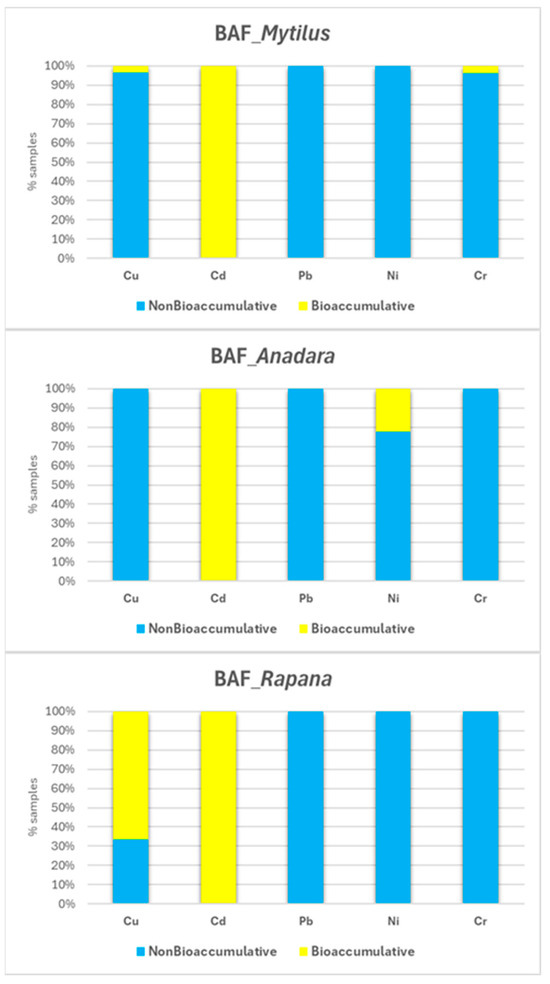
Figure 14.
Proportion of bioaccumulative (BAF > 1) and non-bioaccumulative (BAF < 1) values for each HM, presented by species.
These BAF results complement the BCF analysis and highlight the relevance of sediment-associated exposure, particularly for infaunal and epifaunal benthic species, such as A. inaequivalvis, M. galloprovincialis, and R. venosa, which interact with contaminated sediments through burial, surface contact, and trophic transfer, respectively. They also emphasize the importance of considering both direct and indirect uptake routes in the assessment of metal assimilation in marine organisms.
4. Discussion
4.1. HMs in Seawater
The distribution of HMs in the RBS waters during the 2018–2023 evaluation period revealed notable spatial and temporal heterogeneity, shaped by both point and diffuse sources. Cu consistently exhibited the highest mean values among the analyzed elements, with values exceeding 50 µg/L at specific locations, particularly in coastal and shelf waters. This pattern is likely driven by a combination of urban runoff, port activities, and maritime traffic, factors known to contribute significantly to pollutant loading in coastal environments [63]. Although Cu levels generally remained below regulatory thresholds, its increased presence in shelf waters of the central and southern sectors underscores the influence of marine transport corridors and anthropogenic pressures associated with port infrastructures [64].
Ni and Pb displayed a clear north-to-south gradient, with the highest levels detected in transitional waters under the strong influence of the Danube River. These transitional zones exhibited frequent outliers and wide interquartile ranges, suggesting episodic discharges or sediment resuspension during high-flow events. The variation in Pb concentrations (CV > 130%) points to localized spikes, potentially linked to port discharges, legacy pollution from urban-industrial zones, or shipborne emissions [65]. Cd, although present at the lowest mean levels, also showed high relative variability, including exceedances of MAC-EQS thresholds at some stations. This indicates that Cd, while typically low in baseline conditions, may reach ecologically concerning levels [66] during specific pollution events or under certain hydrodynamic conditions.
Cr demonstrated a distinct behavior compared to the other metals. While its average values were moderate, a cluster of offshore samples collected in 2022 showed unusually high Cr values, diverging from the expected nearshore enrichment pattern. This episodic offshore anomaly likely reflects non-terrestrial inputs, such as atmospheric deposition or intensified maritime activity [67,68], possibly in the context of the recent geopolitical disruptions in the region [22]. The lack of similar observations in previous years supports the interpretation of a transient, possibly transport-related event; however, continued surveillance of offshore waters is needed to assess recurrence and trends.
From a regulatory standpoint, the majority of the results complied with European MAC-EQS thresholds; however, occasional exceedances were recorded for some metals. These exceedances, even when sporadic, are ecologically relevant as they may correspond to short-term pulses of bioavailable metals, especially in dynamic nearshore environments with high biological productivity. The MSFD Descriptor 8 framework emphasizes the need to consider such episodes as even brief periods of intensified loads can impact sensitive life stages of marine organisms or lead to sediment accumulation.
A recent study assessed surface water chemical status across the Lower Danube, Danube Delta, and adjacent RBS coastal zone, focusing, among other pollutants, on nine metals (As, Cd, Cr, Cu, Hg, Mn, Ni, Pb, Zn) [69]. In coastal areas, Pb and Cd levels occasionally exceeded MAC-EQS thresholds set by Directive 2013/39/EU. These exceedances were attributed to strong riverine inputs from the Danube, which acts as a major transport pathway for metals originating from industrial effluents, mining runoff, urban wastewater, and agricultural drainage throughout its multi-national catchment. Although dilution at the river–sea interface reduces overall burden, the study confirmed that the Danube remains the dominant source of metal and other pollutants to the Romanian coastal zone [69]. These observations support our observations of increased Pb and Cd in transitional waters and emphasize the importance of upstream controls and cross-border pollution management for achieving regulatory compliance in marine waters.
Overall, the aqueous environment HM data reveal a distinct gradient: nearshore areas, particularly those influenced by riverine discharge and urban-industrial activity, exhibit elevated and more variable values, while offshore regions are typically less contaminated but not exempt from episodic metal enrichment. These patterns highlight the importance of maintaining broad spatial monitoring coverage, particularly in offshore areas that are less covered by national surveillance programs. The identification of offshore anomalies underscores the need to include deep-sea stations in future investigations and to consider atmospheric and maritime drivers in integrated marine pollution strategies.
Preisner et al. (2021) [70] highlighted the dominant role of riverine and atmospheric inputs in delivering HMs to the Polish coastal waters of the Baltic Sea. Their assessment of Cd, Pb, and Hg loads into three major marine basins demonstrated that atmospheric deposition and river inflows remain the primary input pathways for HM in semi-enclosed basins [70]. These observations reinforce the importance of transboundary riverine contributions and diffuse atmospheric contributions as key vectors for seawater chemical burden in regional seas, consistent with observed patterns in the northwestern Black Sea, where Danube inflow [34,69] and atmospheric fallout [71] also govern nearshore metal enrichment.
A comparable study conducted along the eastern Egyptian Mediterranean coast [72] assessed variations of HMs (Cd, Co, Cu, Zn, Ni, Mn, and Fe) in both surface and bottom water. Metal amounts were generally higher in bottom waters, particularly in sectors influenced by industrial discharges and port activities. Among the metals, Zn and Cu were most abundant, with Cu reaching up to 15 μg/L in some surface samples. Significant regional heterogeneity was observed for Cd, Co, Zn, and Mn in bottom waters, while seasonal variation affected Zn, Cd, and Mn levels more markedly in summer than winter [72]. Despite localized enrichment, most values remained below regulatory thresholds for marine waters. These findings reinforce the role of benthic fluxes and coastal anthropogenic inputs in shaping nearshore metal profiles and offer valuable regional comparison points for the Black Sea context.
Recent research of upper water layer quality along the Turkish Black Sea coast by Atabay et al. (2024) [73] provides relevant regional context to support our outcomes. Based on over 380 samples collected between 2021 and 2024, their study identified high concentrations of Cu, Ni, Cr, and Cd that were especially evident in coastal areas influenced by riverine inputs, due to upstream industrial activities, and human-driven pressures, like deep-sea outfalls releasing effluents. These patterns closely parallel the gradients observed in our study, where Pb, Cd, and Ni reached maximum values in transitional waters influenced by the Danube. While Pb and Cr generally exhibited lower values, both studies recorded sporadic peaks in specific coastal zones, indicating the influence of localized or episodic inputs. Source attribution analysis linked Ni, Cr, and Co to industrial sectors, such as metallurgy and surface treatment, while Cd, As, and Pb were associated with emissions from fertilizer and Cu-processing facilities [73]. These similarities reinforce the broader regional picture of HM presence in the Black Sea surface waters and highlight the relevance of industrial coastal pressures and riverine transport as key drivers of observed fluctuations.
4.2. HMs in Sediments
The sedimentary record provides a more stable and integrated reflection of HM pollution than water column measurements, offering insights into both chronic pollution and depositional processes [74]. Over the six-year investigation period, sediment samples from the RBS sector revealed significant heterogeneity in HM patterns, both across regions and between elements. Ni emerged as the most enriched metal overall, frequently exceeding the Effect Range Low (ERL) guideline value of 20.9 µg/g, with exceedances recorded in over 75% of samples. While such levels suggest a potential for adverse effects on benthic fauna, the interpretation must consider the local geochemical context. Numerous studies have shown that Ni in northwestern Black Sea seabed substrate often originates from natural lithogenic sources due to the region’s characteristic mineral composition and high background levels associated with Fe- and Mn-rich particles [57,58,75].
In contrast, Cu and Pb exhibited more localized exceedances of their respective ERL thresholds (34 µg/g and 46.7 µg/g), particularly in shelf and coastal areas adjacent to major ports such as Constanța, Midia, and Mangalia. These hotspots likely reflect anthropogenic inputs from maritime traffic, industrial discharge, and port maintenance operations such as dredging and ballast water release [32,35]. The observed patterns of Cu and Pb were supported by both boxplot analysis and interpolated maps, which revealed raised medians and outlier values concentrated in areas with intense human activity. Cd, while generally below ERL values, showed considerable variability and elevated values in transitional zones near the Danube discharge, pointing to riverine contributions and possible historical accumulation.
The relationship between granulometric texture and metal accumulation was further elucidated through granulometric and multivariate analyses. Fine-grained deposits (silt + clay) dominated the northern transitional and shelf zones, corresponding with higher concentrations of Ni, Cr, and Cd, metals known for their affinity to organic-rich, fine particles. These findings align with established sediment–metal interaction mechanisms, where small particles provide greater surface area and sorptive capacity for trace metals [76]. The positive correlation between mud content and HM levels, although moderate, supports the role of grain size and associated organic matter in metal retention. However, this relationship was not uniform across all samples. Principal Component Analysis (PCA) revealed several stations with anomalously high metal amounts despite low fine fraction content. These outliers, located near urban or industrial centers, suggest that direct human-driven inputs can override natural geochemical controls, allowing contaminants to bind to coarser particles via oxide coatings or organic films [62].
The use of ERL values as precautionary thresholds adds ecological relevance to the sediment evaluation. While ERLs do not distinguish between natural and human-induced enrichment, they offer a valuable screening tool to identify areas at potential risk [47,53]. In this study, ERL exceedances were most pronounced for Ni, followed by Cu and Pb, indicating a gradient of concern. The match between ERL exceedances, texture, and known pollution drivers reinforces the utility of integrating chemical and physical parameters in risk assessment.
Furthermore, the presence of high HMs in coarse or mixed sediments, particularly in shelf zones subject to dredging or offshore industry, points to seabed disturbance and resuspension as key processes in pollutant redistribution [77]. Ports and industrial areas, characterized by lower hydrodynamic energy and higher sedimentation rates, may function as semi-permanent sinks for both fine particles and associated contaminants [78]. In these environments, episodic events such as storms, anchoring, or dredging can remobilize legacy pollutants, increasing their bioavailability and ecological risks.
In summary, the sediment results evinced the dual influence of natural geochemistry and human-driven pressure on HM patterns in the RBS. Transitional and shelf areas are the most impacted, with ERL exceedances highlighting ecological concern, particularly for Ni. The grain size–metal relationship is generally robust but may be disrupted by localized loadings. These findings call for a seabed substrate-focused monitoring strategy that incorporates granulometric, geochemical, and source apportionment analyses to better distinguish between natural enrichment and pollution-driven metal inputs.
Robledo Ardila et al. (2024) [79] investigated HM (Cd, Pb, Cu, Zn, Ni, Cr, As) in surface coastal sediments across the western and central Mediterranean (Spain, Italy, Tunisia, and Greece). Their results revealed moderate to high regional variation, with increased Cu, Zn, and Pb levels particularly near urbanized or industrial coastal zones, such as ports and river estuaries. Exceedances of quality guidelines (e.g., ERL/ERM) were frequent for Pb and Cu, especially in Spanish and Tunisian sectors [79]. The study also confirmed that fine-grained deposits rich in organic matter favored metal accumulation, patterns consistent with our findings in the RBS, where mud-rich shelf area similarly acts as an effective sink for trace metals. These results underscore the combined role of anthropogenic drivers and substrate characteristics in shaping dispersion patterns along semi-enclosed European seas.
Another recent study [80] assessed HMs in coastal sediments off Samsun, Türkiye, where the Kızılırmak and Yeşilırmak rivers discharge into the Black Sea. Their study revealed high levels of Ni, Cr, and Cu, particularly near river mouths, and highlighted the combined influence of natural geological inputs and human-driven contributions, such as agricultural runoff, domestic wastewater, and industrial effluents. Fine-grained deposits and pronounced organic matter content were closely linked to higher metal accumulation [80]. These patterns align with our observations in the RBS shelf, where river-derived inputs and mud-rich sediments similarly drive spatial variability in Ni, Cr, and Cu. The study reinforces the role of major river systems as long-lasting contributors to substrate burden in Black Sea coastal zones.
A comparison with recent assessments from the Bulgarian Black Sea coast shows both similarities and contrasts in contamination patterns. Doncheva et al., (2020) [81] applied multiple pollution indices (Igeo, EF, CF, PLI, ERM-Q) to evaluate metal contamination at 22 coastal stations. Their results indicated that the northern Bulgarian zone, considered under Danube influence (e.g., Krapets, Rusalka, Kaliakra), was moderately enriched in Pb and Zn, while “hot spot” areas such as Varna and Burgas bays showed elevated levels of Ni, Cu, and Pb, associated with historical mining, industrial, and port-related activities. Most stations exhibited low to moderate degrees of contamination, with mean Cu and Ni concentrations below ERL values, though localized exceedances of ERLs were reported for Pb, Cu, and Ni at specific sites (e.g., Rosenets and Burgas). Despite these exceedances, the ERM-Q and PEL-Q values generally indicated low to medium-low ecotoxicological risk, consistent with a 25–30% probability of toxicity for benthic fauna in those zones [81]. These findings align with our observations of spatially variable contamination patterns and support the identification of river-influenced and nearshore urban-industrial areas as key pressure zones across the western Black Sea coast.
Long-term monitoring data (2003–2018) from bays in the Sevastopol region (Crimean Peninsula) show that the spatial distribution of HMs in bottom sediments is closely linked to the physico-chemical properties of sediments, including organic carbon (Corg), CaCO3 content, and grain size [82]. Notably, strong positive correlations were observed between Fe and Corg/silt in Sevastopol Bay (r = 0.7), Cu and silt in Kazachya Bay (r = 0.7), and Ni and fine fraction in Balaklava Bay (r = 0.8). Additionally, Sr content correlated strongly with CaCO3 across all sites (r = 0.8) [82]. These findings highlight the influence of sediment composition and hydrodynamic isolation (i.e., limited water exchange with the open sea) on metal accumulation patterns. The study supports the notion that fine sediments rich in organic matter serve as important sinks for HMs in semi-enclosed coastal environments.
Additional insight into regional sediment contamination is provided by Konstantinova et al. (2023) [83], who investigated HM distribution in the Don River delta and the Azov Sea. Their results revealed elevated concentrations of Zn (up to 190 mg/kg), Ni (up to 62 mg/kg), and Cr (up to 91 mg/kg), particularly in Taganrog Bay and the eastern Don delta, where anthropogenic pressures such as urban-industrial discharge, port activity, and agricultural runoff were prominent. Using geochemical indices, the study identified moderate to strong contamination levels at several stations, with Zn and Ni contributing most significantly to ecological risk. In contrast, the central and western Azov Sea exhibited lower concentrations [83]. These findings emphasize the spatial heterogeneity of contamination within shallow coastal basins and the dominant role of riverine and localized human inputs in shaping sediment quality.
In Baltic Sea sediments, Shahabi-Ghahfarokhi et al. (2021) [84] reported that Cd, Pb, and Zn concentrations peaked in the 1970s–1980s, reflecting historical pollution pressure, but have generally declined since due to regulatory actions. Despite this, surface sediment levels remained above pre-industrial baselines, particularly in the central and western Baltic Sea. Metal accumulation was strongly modulated by redox conditions, with Cd and Zn enriched in anoxic, fine-grained deposits, while Pb displayed complex behavior related to both redox-driven sulfide binding and historical industrial inputs. The authors emphasized that metal substrate occurrence was also influenced by regional grain size patterns and organic matter content [84].
4.3. HM in Biota
The analysis of HM accumulation in mollusk tissues across the RBS sector revealed variable yet significant patterns, reflecting both environmental exposure and species-specific uptake mechanisms. Among the six metals analyzed, Cd was consistently identified as the most bioaccumulative element, based on both bioconcentration factors (BCF) and bioaccumulation factors (BAF). For several samples, particularly for M. galloprovincialis, BCF values exceeded the threshold of 5000, classifying them as very bioaccumulative. These findings are ecologically relevant, as Cd is known to accumulate in soft tissues of marine organisms and can pose risks to consumers [85].
Cd-elevated BCF and BAF values recorded across all three studied mollusk species were particularly evident in organisms collected from transitional and coastal waters, where sediment and water concentrations of Cd were also higher. Cd’s high mobility in marine systems, combined with its affinity for organic matter and fine particles, facilitates its bioavailability to benthic and filter-feeding organisms.
Cu showed species-specific retention patterns. Rapana venosa exhibited the highest Cu BAF values, corresponding to generally higher tissue Cu levels. This likely reflects a combination of trophic transfer from prey and sediment-associated exposure, consistent with its carnivorous, benthic lifestyle. In contrast, M. galloprovincialis and A. inaequivalvis, both suspension feeders, displayed predominantly non-bioaccumulative BCF and BAF Cu values, with only a few isolated cases in Mytilus slightly exceeding the threshold.
Ni demonstrated moderate bioaccumulation, with BCF values falling within the 1000–5000 range observed particularly in A. inaequivalvis and M. galloprovincialis. Pb was predominantly non-bioaccumulative across species, with only a few isolated BCF values exceeding 1000 in Mytilus, and consistently low BAFs. Cr showed low bioaccumulation overall, but a few samples from Mytilus and Rapana exceeded the bioaccumulative threshold. These trends are consistent with Cr’s lower solubility and tendency to associate with particulate matter, limiting its overall bioavailability to benthic organisms.
Species-specific retention patterns reflected differences in feeding strategy and ecological niche. M. galloprovincialis, a high-capacity filter feeder with continuous exposure to the water column, exhibited the highest overall BCF values, particularly for Cd, with additional elevated values for Ni and Cr. Isolated high BCFs for Pb were also observed. In terms of sediment-associated uptake, Mytilus showed consistently elevated BAFs for Cd, while BAF values for Cu and Cr exceeded 1 in only a few cases. A. inaequivalvis, a suspension feeder partially embedded in the sediment, displayed moderate BCFs and frequent BAF values > 1 for Cd and Ni, indicating greater sediment-associated accumulation. R. venosa, though exhibiting lower BCFs overall, accumulated significant amounts of Cd and Cu through BAF, likely due to trophic transfer and sediment contact. These patterns emphasize the importance of ecological traits, such as feeding mode and habitat position, in shaping metal retention pathways across species.
Organisms from transitional and coastal areas consistently exhibited higher bioaccumulation indices, reflecting increased metal levels observed in surrounding water and sediment. However, certain shelf stations, despite generally lower pollution levels, displayed unexpectedly high bioconcentration factors (BCFs) in bivalves, frequently exceeded 104, and in some cases, reached or surpassed 105. As explained in Section 3.3, such high BCFs could reflect low seawater concentrations rather than exceptional metal uptake. Nonetheless, such inflated ratios, while not indicative of acute pollution, may still reflect a metal’s persistence and the organism’s efficiency in scavenging low-concentration contaminants from the environment.
Similar research showed that BCF values are highly sensitive to ambient levels and must be interpreted within their environmental and physiological context. Nakhlé et al. (2006) [86] reported BCFs for Cd, Pb, and Hg in Brachidontes variabilis (a bivalve) and Patella sp. (a gastropod limpet) along the Lebanese coast. In mussels, BCFs ranged from 8.3 × 103 to 3.4 × 104 for Cd, 7.5 × 103 to 8.0 × 103 for Pb, and 3.0 × 104 to 3.2 × 104 for Hg. Limpets showed even higher values for Cd (1.7 × 104 to 7.4 × 104), and relatively stable values for Hg around 1.0 × 104, while Pb ranged from 2.5 × 103 to 6.0 × 103 [86]. High BCFs may occur in low-exposure settings due to active uptake or slow elimination. These mechanisms are well-documented across marine invertebrates, particularly filter-feeding mollusks [87]. Also, BCFs in field conditions often exceed laboratory values as they integrate not only direct waterborne uptake but also dietary or particulate-bound routes, which are particularly relevant for grazers like limpets [88].
Together, these results highlight the importance of interpreting BCFs not as standalone indicators of contamination, but as integrative metrics shaped by metal speciation, uptake route, species physiology, and ambient conditions. High BCFs in low-metal environments should be seen as signals of bioavailability and ecological integration, rather than indicators of direct toxicity or anthropogenic pressure.
Similar outcomes have been reported across different oceanographic and environmental contexts. Zhang et al. (2023) [89] reported a wide range of bioaccumulation values across metals and marine taxa in Haizhou Bay, highlighting both waterborne and sediment-derived uptake. BCF reached up to ~100,000 for Cu in bivalves, ~30,000 for Hg in cephalopods, and in gastropods. Other elements, such as Zn, Ni, Pb, and Cr, exhibited more moderate BCFs, mostly below 500. These values underscore the capacity of benthic and filter-feeding organisms to accumulate metals from seawater, even in low-levels environments where dissolved concentrations are near detection limits. In the same study, sediment-based accumulation (BAF) recorded high values for Zn (~40,000) and Cu (~15,000) in bivalves, As (~12,000) in gastropods, and Hg (>300,000) in crustaceans [89]. In contrast, BAFs for Cr, Ni, and Pb were below 500 in most cases. This sediment-associated pattern aligns with our observations in Black Sea benthic fauna, where BAF values were raised in stations with fine deposits and higher organic matter content.
Previous studies on M. galloprovincialis from the RBS coastal area reported that mussels accumulated Cd, Cu, Cr, and Ni to levels classified as bioaccumulative (BCF > 1000), and in some cases very bioaccumulative (BCF > 5000), particularly in mussels collected from port areas along the Romanian coast [90]. In contrast, Pb remained non-bioaccumulative in most samples. Their BAF values further support this classification: Cd and Ni were bioaccumulated consistently, and Cu showed moderate bioaccumulation, while Cr and Pb were not bioaccumulated. Moreover, the study found seasonal variations, with the highest BCF/BAF values observed in spring, likely reflecting increased metabolic activity during spawning and enhanced contaminant uptake in polluted sites [90]. These findings complement our observations, reinforcing the role of M. galloprovincialis as a sensitive bioindicator and highlighting the interplay between environmental chemical burden and species-specific uptake dynamics.
Together, these data confirm that benthic mollusks are critical bioindicators, integrating both dissolved and particulate burden over time. Importantly, very high BCF or BAF values do not always imply toxic risk, but they do signal sustained bioavailability and long-term integration of trace contaminants in marine systems. The detection of metals at “very bioaccumulative” BCF levels underscores the need for multi-matrix evaluation and careful ratio interpretation, especially in coastal and shelf areas with transient low ambient values but ongoing pollutant input [86,89].
From an environmental health perspective, the presence of bioaccumulative metals in commercially important mollusks highlights the need for sustained surveillance under MSFD Descriptor 9 [26,91,92]. Although most samples complied with EU food safety regulations for Cd and Pb, the occurrence of exceedances, particularly for Cd, points to potential episodic risks to seafood quality and consumer health. The co-occurrence of sedimentary chemical burden and assimilation further validates the inclusion of benthic biota in integrated pollution monitoring.
In conclusion, the biota results reveal that Cd is the most ecologically relevant contaminant in terms of bioaccumulation potential across the RBS shelf and nearshore waters. Its consistent accumulation across all studied mollusk species reflects both high environmental availability and strong biological uptake. Filter-feeding bivalves such as M. galloprovincialis and A. inaequivalvis serve as effective bioindicators of sediment- and water-associated Cd exposure. While Cu and Ni occasionally exhibited elevated bioaccumulation indices, these patterns were more variable and species-specific, highlighting the importance of ecological traits and exposure pathways in shaping metal retention in benthic fauna. These outcomes reinforce the importance of combining seabed substrate, water, and biota results to evaluate the trophic transfer and ecosystem hazard of toxic metals in marine systems.
4.4. Integrated Synthesis of Contamination Patterns Across Matrices
The integration of seawater, sediment, and biota datasets reveals a complex but coherent picture of HM contamination in the RBS sector, shaped by the interplay of terrestrial inputs, marine transport, sediment dynamics, and biological uptake processes. While each matrix offers distinct information about chemical status, when taken together, they provide a more nuanced understanding of pollutant sources, pathways, and ecological implications.
In this study, the highest values of metals in the aqueous environment were recorded in transitional and coastal zones, reflecting the influence of the Danube River, urban runoff, and port activities. These nearshore areas, with reduced dilution capacity and higher sedimentation rates, act as primary receptors for land-based pollutants. In contrast, offshore waters exhibited lower concentrations for most metals, although episodic anomalies point to the potential role of atmospheric inputs and maritime traffic even in deep-sea environments. These patterns are consistent with previous investigations in the northwestern Black Sea, which also identified pronounced anthropogenic pressures in transitional and coastal zones due to land-based inputs, particularly from the Danube and port activities [4,6,26,34,35,55,93].
A recent study confirms the role of the Danube River as a major source of potentially toxic metals, especially Cd and Pb, in the northwestern Black Sea [17]. Although the Danube Delta exhibits a partial buffering capacity by retaining a fraction of these contaminants, high levels were still recorded in downstream waters and fish tissues, indicating continued pollutant transfer toward the marine environment. These findings indicate that the Black Sea remains a vulnerable receiving basin for upstream and riverine inputs of hazardous substances. The weak correlation between environmental burden and tissue retention observed in fish further underscores the complexity of metal dynamics in transitional systems and the need to assess bioavailability and uptake pathways beyond simple concentration metrics [17]. Such studies reinforce the importance of integrated catchment-to-coast observations to better understand and mitigate contaminant fluxes into the Black Sea.
Sediments, acting as long-term reservoirs of pollutants, showed both spatial consistency and localized enrichment. Ni dominated seabed loads across all zones, though its exceedance of screening values often reflects natural geochemical enrichment rather than pollution per se [57]. In contrast, Cu and Pb showed localized hotspots in shelf and port-adjacent areas, strongly suggesting human-driven contributions. The co-occurrence of elevated Cu and Pb with high fine fraction content (silt + clay) further emphasizes the role of grain size and organic matter in enhancing metal retention. However, the presence of high values even in sandy deposits near urban-industrial areas indicates that direct pollutant inputs and sediment resuspension can override these natural controls.
The patterns observed in mollusk tissues correspond closely with environmental metal availability in both water and sediment. High bioconcentration and bioaccumulation factors for some metals across species and sampling zones point to their sustained bioavailability, despite differences in environmental partitioning. The alignment between seabed substrate hotspots and increased BAF values underscores the importance of sediment-bound exposure in benthic bioaccumulation. The highest Cd values in mollusks coincided with both high sediment and measurable water column presence, suggesting dual uptake pathways through both dissolved and particulate routes.
Linking the three compartments reveals important contaminant behaviors. Cd, for instance, showed moderate levels in water, variable presence in seabed substrate, and consistently high bioaccumulation, indicating its strong biological uptake potential even at low environmental loadings. Pb and Cr, while variable in water and substrate, showed limited transfer to biota, possibly due to its tendency to bind strongly to particles and reduced bioavailability in oxidized marine sediments. These compartment-specific behaviors are in line with previous studies in other marine systems, which similarly reported high Cd assimilation despite relatively low ambient levels, as well as limited trophic transfer of other metals due to their strong particle affinity and low bioavailability in well-oxygenated environments [94,95].
Moreover, the observed decoupling between water and sediment for some elements highlights the importance of considering metal speciation, environmental conditions (e.g., redox potential, pH), and seasonal hydrodynamics when interpreting contaminant mobility and risk [96]. Similarly, the occasional lack of correlation between substrate levels and tissue burden suggests that biological factors, such as species-specific uptake, feeding strategy, and metabolic regulation, must be factored into ecological risk assessments [97].
The integrated matrix approach also underscores the importance of wide resolution in monitoring design. Some shelf stations, traditionally perceived as less impacted, revealed high metal loads and biota uptake, pointing to localized anthropogenic pressures such as shipping, offshore industry, and dredging. These results support the inclusion of shelf and offshore zones in routine surveillance efforts and evince the importance of temporal continuity to capture episodic events, which is particularly relevant in the current Black Sea context, where intensified maritime traffic, shifting trade routes, and regional geopolitical instability are placing additional pressures on the marine environment [22,23,24].
Overall, the outcomes emphasize that no single matrix can fully capture the complexity of marine metal pollution. Water provides insight into recent and dynamic events and drivers, sediments reflect longer-term accumulation and depositional history, and biota serve as integrators of biologically available fractions. When assessed together, these matrices offer a powerful framework to understand source–pathway–receptor relationships and to inform adaptive, ecosystem-based management strategies.
4.5. Implications and Future Research
The integrated evaluation of HM across seawater, sediments, and benthic biota in the RBS sector reveals the continued presence and ecological relevance of both diffuse and point-sources in this semi-enclosed marine system. The findings confirm that nearshore and shelf zones remain particularly vulnerable to multiple human-driven pressures, while offshore waters, though less impacted, are not immune to episodic events, highlighting the need for basin-wide vigilance.
One of the key environmental implications of this study is the evidence of sustained bioavailability and trophic transfer potential of HMs, especially Cd, which was consistently detected in mollusk tissues at levels suggesting significant ecological uptake. The identification of shelf and offshore hotspots, including areas previously considered reference or low-risk zones, challenges the assumption that contamination is confined to the immediate vicinity of urban or riverine inputs. This has direct relevance for ecosystem health, fisheries management, and food safety considerations. These observations align with previous studies in the RBS which report accumulation of HM and other pollutants in mollusks and fish, with some values exceeding food safety thresholds. Such evidence highlights potential human health risks from seafood consumption and reinforces the need for continued monitoring and toxicological assessment [85,98,99,100].
From a monitoring and management perspective, the study supports the value of multi-matrix approaches in marine pollution evaluation. Reliance on a single matrix, such as water or seabed substrate alone, risks underestimating anthropogenic impacts, particularly when dealing with metals that are either episodically introduced or strongly retained in specific compartments [1]. The use of species-specific bioaccumulation indicators proved effective in highlighting localized exposure scenarios and potential ecotoxicological concerns [101].
However, the study has limitations that warrant attention. Sediment and biota sampling in offshore areas was constrained by logistical challenges, particularly at depths exceeding 150–200 m, where sample collection was not feasible (only seawater was collected). This limited the ability to fully characterize deep-sea trends. Additionally, the lack of vertical water column profiles and seasonal data restricts the interpretation of dynamic processes such as stratification, resuspension, or water–sediment exchange, which may influence the bioavailability and redistribution of metals. The absence of winter-season data may restrict the interpretation of seasonal variability in HM dynamics. Previous studies in the region have shown that certain metals, such as Cu, Cr, and Pb, can exhibit elevated concentrations during winter, likely due to increased runoff, vertical mixing, and reduced biological uptake [73]. As our sampling was conducted during summer–autumn periods, further research is needed to capture inter-seasonal patterns and fully assess the temporal behavior of metal in the Black Sea.
Another constraint relates to the absence of concurrent measurements of TOC and redox conditions in seabed substrate, which are critical variables influencing metal mobility and speciation [62,102]. While granulometric data were used as proxies for binding capacity, direct evaluation of geochemical conditions would improve source discrimination and risk interpretation. Also, we acknowledge that ERL thresholds, while widely used, may not fully account for natural background variability or local sediment geochemistry. In future work, the use of complementary contamination indices such as the Geoaccumulation Index (Igeo) or Enrichment Factor (EF), which incorporate reference baselines and sediment properties, could offer additional insight into the distinction between natural and anthropogenic contributions, as proposed in recent studies [103,104]. Furthermore, although this study applied ERL values as conservative screening benchmarks to identify potential ecological concerns in sediments, we recognize the benefit of more integrated environmental risk assessment tools. Future research could incorporate approaches such as the Potential Ecological Risk Index (PERI) [105,106] and Sediment Quality Guideline Quotients (SQG-Q) [107], which account for both contaminant levels and toxicological relevance. These frameworks would enhance the interpretation of ecological risk, particularly in areas affected by multi-metal contamination.
Future research should therefore prioritize several directions. First, expanding temporal resolution, especially to include seasonal campaigns, would help capture short-term pollution events and biological responses. Second, greater emphasis should be placed on offshore and deep-water stations, particularly in the context of increasing maritime activity and changing regional circulation patterns. Third, the inclusion of additional ecological indicators, such as bioassays, enzymatic biomarkers, or multi-trophic species comparisons, could provide more comprehensive insights into the sublethal and long-term effects of metal burden [108]. However, the integration of such components into routine monitoring is often hindered by financial and logistical limitations and typically relies on national research initiatives or international and regional programs.
Finally, coupling geochemical analyses with modeling approaches could help predict contaminant behavior under varying hydrodynamic and climatic scenarios, improving early warning systems and mitigation strategies [109]. As coastal and marine pressures in the Black Sea region intensify due to human activity and climate-driven changes, such integrated and adaptive surveillance frameworks will be essential to safeguard ecological integrity and maintain sustainable use of marine resources.
5. Conclusions
This multi-matrix investigation of heavy metal contamination in the Romanian Black Sea sector over a six-year period offers an integrated perspective on the regional dynamics, environmental behavior, and biological accumulation of trace metals. The findings highlight persistent patterns in seawater, sediments, and biota, with a clear distinction between the nearshore areas, dominated by land-based inputs, such as riverine discharge, urban runoff, and port activity, and offshore areas, where episodic signals suggest atmospheric deposition and maritime sources.
Cd emerged among the most ecologically relevant metals based on combined environmental presence and biological uptake across compartments. While sedimentary Ni levels were consistently elevated, interpretation requires caution due to natural geochemical enrichment in the region. The alignment of seabed substrate enrichment, marine water concentrations, and mollusk tissue burdens highlights localized hotspots of concern, especially in shelf and coastal zones, where environmental exposure and biological uptake converge.
The study also underscores the value of integrated monitoring approaches that include chemical, and biological data. Bivalve species such as M. galloprovincialis and A. inaequivalvis proved to be effective bioindicators, capturing the bioavailable fraction of metals and complementing sediment and water measurements. These results demonstrate that HM pollution, while spatially heterogeneous, remains a pressing issue for the ecological functioning of the Romanian marine zone.
At the same time, several limitations were identified, including gaps in offshore sediment coverage, the absence of vertical water column data, and missing geochemical parameters, such as organic matter content. Addressing these limitations in future studies will improve source attribution, enhance ecological risk assessments, and support evidence-based environmental management.
Overall, this work contributes valuable data and interpretations to the regional understanding of HM behavior in coastal and shelf marine systems. It reinforces the need for sustained, extensive, and multidisciplinary monitoring frameworks to capture both chronic and emerging pollution trends in the northwestern Black Sea.
Author Contributions
Conceptualization, A.O. and D.M.; methodology, A.O. and D.M.; validation, A.O., D.M., G.R. and R.D.N.; formal analysis, A.O., D.M., G.R. and R.D.N.; investigation, A.O., D.M., G.R. and R.D.N.; resources, A.O., D.M., G.R. and R.D.N.; data curation, A.O. and D.M.; writing—original draft preparation, A.O., D.M., G.R. and R.D.N.; writing—review and editing, A.O. and D.M.; visualization, A.O., D.M., G.R. and R.D.N.; supervision, A.O. and D.M.; project administration, A.O. and D.M.; funding acquisition, A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript is a result of the “Study on the Black Sea Integrated Marine Ecosystem Monitoring Programme according to the requirements of the Marine Strategy Framework Directive (2008/56/EC)”, MEWF Contract nr. 50/21 April 2023, and the Administrative Capacity Operational Program 2014–2020, priority axis IP12/2018 under project code MySMIS 127598/SIPOCA 608. “Improving the capacity of the central public authority in the field of marine environment protection in terms of monitoring, evaluation, planning, implementation and reporting of requirements set out in the Framework Directive Marine Strategy and for integrated coastal zone management”.
Institutional Review Board Statement
Sampling and handling of benthic mollusks (Mytilus galloprovincialis, Anadara inaequivalvis, Rapana venosa) was conducted in accordance with Romanian national regulations and institutional guidelines for marine environmental research. These species are not listed as endangered or protected under national or EU legislation, and no specific ethical approval was required for their collection. Sampling was performed by trained personnel during authorized oceanographic surveys within the Romanian Exclusive Economic Zone.
Data Availability Statement
The data belong to the National Institute for Marine Research and Development “Grigore Antipa” (NIMRD) and can be accessed upon request to http://www.nodc.ro/data_policy_nimrd.php (accessed on 10 July 2025).
Acknowledgments
During the preparation of this study, the authors used OpenAI’s ChatGPT-4 Plus (2025) for the purposes of assisting with PCA computation and the generation of biplot visualization based on sediment heavy metal and grain size data. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Stations network, 2018–2023 (T, transitional; C, coastal; S, shelf; O, offshore) (W, water; S, sediment; B, biota).
Table A1.
Stations network, 2018–2023 (T, transitional; C, coastal; S, shelf; O, offshore) (W, water; S, sediment; B, biota).
| Transect | Station Code | Type | Longitude [Degrees_East] | Latitude [Degrees_North] | Bot.Depth [m] | Observations | W | S | B |
|---|---|---|---|---|---|---|---|---|---|
| Sulina | SU1 | T | 29.7717 | 45.1439 | 10 | Regular monitoring network | x | x | |
| Sulina | SU2 | T | 29.7934 | 45.1411 | 20 | Regular monitoring network | x | x | |
| Mila9 | ML1 | T | 29.6517 | 45.0033 | 10 | Regular monitoring network | x | x | |
| Mila9 | ML2 | T | 29.7333 | 45.0033 | 20 | Regular monitoring network | x | x | |
| Mila9 | ML3 | T | 29.7928 | 44.9925 | 30 | Regular monitoring network | x | x | |
| Sf. Gheorghe | SG1 | T | 29.6364 | 44.8836 | 10 | Regular monitoring network | x | x | |
| Sf. Gheorghe | SG2 | T | 29.6783 | 44.8836 | 20 | Regular monitoring network | x | x | |
| Sf. Gheorghe | SG3 | T | 29.7017 | 44.8836 | 30 | Regular monitoring network | x | x | x |
| Portita | PO1 | T | 29.0067 | 44.6767 | 10 | Regular monitoring network | x | x | |
| Portita | PO2 | T | 29.2992 | 44.6767 | 20 | Regular monitoring network | x | x | x |
| Periboina | PB2 | T | 28.9301 | 44.4980 | 20 | Project-based (2019) | x | x | |
| Gura Buhaz | GB1 | C | 28.7890 | 44.3897 | 5 | Regular monitoring network | x | x | |
| Gura Buhaz | GB2 | C | 28.8530 | 44.3897 | 20 | Regular monitoring network | x | x | |
| Cazino Mamaia | CZ1 | C | 28.6394 | 44.2358 | 5 | Regular monitoring network | x | x | |
| Cazino Mamaia | CZ2 | C | 28.7061 | 44.2350 | 20 | Regular monitoring network | x | x | x |
| Constanta Nord | CN1 | C | 28.6500 | 44.2167 | 10 | Regular monitoring network | x | x | |
| Constanta Nord | CN2 | C | 28.7003 | 44.2167 | 20 | Regular monitoring network | x | x | |
| Est Constanta | EC1 | C | 28.6833 | 44.1667 | 14 | Regular monitoring network | x | x | |
| Est Constanta | EC2 | C | 28.7833 | 44.1667 | 28 | Regular monitoring network | x | x | x |
| Constanta Sud | CS1 | C | 28.6489 | 44.1383 | 10 | Regular monitoring network | x | x | |
| Constanta Sud | CS2 | C | 28.6850 | 44.1217 | 20 | Regular monitoring network | x | x | |
| Eforie Sud | EF1 | C | 28.6662 | 44.0186 | 10 | Regular monitoring network | x | x | x |
| Eforie Sud | EF2 | C | 28.6816 | 44.0187 | 20 | Regular monitoring network | x | x | x |
| Costinesti | CO1 | C | 28.6442 | 43.9450 | 10 | Regular monitoring network | x | x | |
| Costinesti | CO2 | C | 28.6739 | 43.9450 | 20 | Regular monitoring network | x | x | |
| Costinesti | CO3 | C | 28.7267 | 43.9450 | 30 | Regular monitoring network | x | x | x |
| Mangalia | MG1 | C | 28.5947 | 43.8003 | 10 | Regular monitoring network | x | x | |
| Mangalia | MG2 | C | 28.6278 | 43.7989 | 20 | Regular monitoring network | x | x | x |
| VamaVeche | VV1 | C | 28.6131 | 43.7511 | 10 | Regular monitoring network | x | x | |
| VamaVeche | VV2 | C | 28.6211 | 43.7511 | 20 | Regular monitoring network | x | x | |
| Sulina | SU3 | S | 29.9242 | 45.1228 | 30 | Regular monitoring network | x | x | x |
| Sulina | SU4 | S | 30.1252 | 45.0642 | 40 | Project-based (2019) | x | x | x |
| Sulina | SU5 | S | 30.3641 | 44.9582 | 50 | Project-based (2019) | x | x | |
| Sf. Gheorghe | SG4 | S | 29.8529 | 44.8836 | 40 | Regular monitoring network | x | x | x |
| Sf. Gheorghe | SG5 | S | 30.1580 | 44.8603 | 50 | Project-based (2019) | x | x | x |
| Sf. Gheorghe | SG6 | S | 30.5647 | 44.8521 | 60 | Project-based (2019) | x | x | |
| Portita | PO3 | S | 29.4742 | 44.6767 | 30 | Regular monitoring network | x | x | |
| Portita | PO4 | S | 29.7500 | 44.6767 | 50 | Regular monitoring network | x | x | x |
| Portita | PO5 | S | 29.9167 | 44.6767 | 57 | Regular monitoring network | x | x | x |
| Portita | PO6 | S | 30.5246 | 44.6665 | 70 | Regular monitoring network | x | x | |
| Periboina | PB3 | S | 29.1024 | 44.5145 | 30 | Project-based (2019) | x | x | |
| Periboina | PB4 | S | 29.2735 | 44.5130 | 37 | Project-based (2019) | x | x | x |
| Periboina | PB5 | S | 29.6596 | 44.5270 | 50 | Project-based (2019) | x | x | |
| Periboina | PB6 | S | 29.8296 | 44.5091 | 60 | Project-based (2019) | x | x | |
| Cazino Mamaia | CZ3 | S | 28.8472 | 44.2347 | 30 | Regular monitoring network | x | x | x |
| Est Constanta | EC3 | S | 28.9000 | 44.1667 | 36 | Regular monitoring network | x | x | x |
| Est Constanta | EC4 | S | 29.1333 | 44.1667 | 47 | Regular monitoring network | x | x | |
| Est Constanta | EC5 | S | 29.3667 | 44.1667 | 54 | Regular monitoring network | x | x | |
| Est Constanta | EC6 | S | 30.0239 | 44.1631 | 70 | Regular monitoring network | x | x | |
| Est Constanta | EC7 | S | 30.2007 | 44.1634 | 90 | Regular monitoring network | x | x | |
| Mangalia | MG3 | S | 28.7156 | 43.7986 | 39 | Regular monitoring network | x | x | x |
| Mangalia | MG4 | S | 28.8290 | 43.7921 | 53 | Regular monitoring network | x | x | |
| Mangalia | MG5 | S | 29.3983 | 43.7573 | 70 | Regular monitoring network | x | x | |
| Mangalia | MG6 | S | 29.9644 | 43.7405 | 100 | Regular monitoring network | x | x | |
| P1 | P1 | S | 30.9263 | 44.7579 | 81 | Project-based (2020–2022) | x | x | |
| P2 | P2 | S | 30.5246 | 44.6665 | 72 | Project-based (2020–2022) | x | x | x |
| P3 | P3 | S | 30.8951 | 44.6538 | 91 | Project-based (2020–2022) | x | x | |
| P4 | P4 | S | 29.8771 | 44.4470 | 65 | Project-based (2020–2022) | x | x | x |
| P5 | P5 | S | 30.7003 | 44.3966 | 100 | Project-based (2020–2022) | x | x | |
| P6 | P6 | S | 30.8370 | 44.3902 | 125 | Project-based (2020–2022) | x | x | |
| P7 | P7 | O | 31.1673 | 44.3821 | 481 | Project-based (2020–2022) | x | ||
| P8 | P8 | S | 29.8440 | 44.1630 | 69 | Project-based (2020–2022) | x | x | x |
| P9 | P9 | S | 30.2007 | 44.1490 | 94 | Project-based (2020–2022) | x | x | |
| P10 | P10 | O | 30.9806 | 44.1351 | 499 | Project-based (2020–2022) | x | ||
| P11 | P11 | S | 30.6604 | 44.0490 | 120 | Project-based (2020–2022) | x | x | |
| P12 | P12 | S | 30.3692 | 44.0353 | 140 | Project-based (2020–2022) | x | x | |
| P13 | P13 | S | 29.5922 | 44.0036 | 67 | Project-based (2020–2022) | x | x | x |
| P14 | P14 | S | 30.0277 | 43.9746 | 80 | Project-based (2020–2022) | x | x | |
| P15 | P15 | O | 30.6888 | 43.9433 | 526 | Project-based (2020–2022) | x | ||
| P16 | P16 | O | 31.2623 | 43.9197 | 1223 | Project-based (2020–2022) | x | ||
| P17 | P17 | S | 30.1565 | 43.8453 | 102 | Project-based (2020–2022) | x | x | |
| P18 | P18 | S | 29.1935 | 43.7824 | 60 | Project-based (2020–2022) | x | x | |
| P19 | P19 | S | 29.4941 | 43.7621 | 71 | Project-based (2020–2022) | x | x | |
| P20 | P20 | S | 30.0177 | 43.7466 | 111 | Project-based (2020–2022) | x | x | |
| P21 | P21 | O | 30.3579 | 43.7156 | 504 | Project-based (2020–2022) | x | ||
| P22 | P22 | O | 30.8578 | 43.6769 | 1388 | Project-based (2020–2022) | x | ||
| P23 | P23 | O | 31.1995 | 43.5409 | 1565 | Project-based (2020–2022) | x | ||
| ANE-RO-1 | RO-1 | S | 30.5490 | 44.6253 | 78 | Project-based (2019) | x | x | x |
| ANE-RO-2 | RO-2 | S | 30.9641 | 44.5468 | 106 | Project-based (2019) | x | x | |
| ANE-RO-3 | RO-3 | S | 30.1173 | 44.3124 | 76 | Project-based (2019) | x | x | |
| ANE-RO-4 | RO-4 | S | 30.5072 | 44.2679 | 103 | Project-based (2019) | x | x | |
| ANE-RO-5 | RO-5 | S | 29.6778 | 43.9162 | 67 | Project-based (2019) | x | x | |
| ANE-RO-6 | RO-6 | S | 30.1526 | 43.8430 | 103 | Project-based (2019) | x | x |
Appendix B

Table A2.
Grainsize composition of sediments, 2021 (T, transitional; C, coastal; S, shelf).
Table A2.
Grainsize composition of sediments, 2021 (T, transitional; C, coastal; S, shelf).
| Station Code | Type | Bot. Depth [m] | Gravel (%) | Total G (%) | Sand (%) | Total S (%) | Silt (%) | Clay (%) | Total Mud (%) | Textural Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VCG | CG | MG | FG | VFG | VCS | CS | MS | FS | VFS | VCS | CS | MS | FS | VFS | C | |||||||
| SU1 | T | 10 | 5 | 25 | 28 | 22 | 21 | 100 | Mud | |||||||||||||
| SU2 | T | 20 | 3 | 14 | 27 | 25 | 16 | 15 | 100 | Mud | ||||||||||||
| SU3 | T | 30 | 1 | 41 | 42 | 21 | 18 | 10 | 9 | 58 | Sandy Mud | |||||||||||
| ML1 | T | 10 | 2 | 19 | 27 | 21 | 16 | 15 | 100 | Mud | ||||||||||||
| ML2 | T | 20 | 1 | 1 | 10 | 14 | 22 | 18 | 13 | 12 | 90 | Sandy Mud | ||||||||||
| ML3 | T | 30 | 2 | 25 | 27 | 19 | 14 | 12 | 100 | Mud | ||||||||||||
| SG1 | T | 10 | 7 | 10 | 24 | 24 | 17 | 18 | 100 | Mud | ||||||||||||
| SG2 | T | 20 | 11 | 29 | 27 | 17 | 16 | 100 | Mud | |||||||||||||
| SG3 | T | 30 | 2 | 8 | 31 | 27 | 16 | 15 | 100 | Mud | ||||||||||||
| SG4 | S | 40 | 17 | 17 | 21 | 25 | 15 | 16 | 6 | 83 | 1 | 1 | Gravelly Sand | |||||||||
| PO1 | T | 10 | 2 | 2 | 20 | 22 | 22 | 15 | 10 | 9 | 98 | Mud | ||||||||||
| PO2 | T | 20 | 19 | 27 | 23 | 17 | 14 | 100 | Mud | |||||||||||||
| PO3 | S | 30 | 17 | 30 | 23 | 16 | 13 | 100 | Mud | |||||||||||||
| PO4 | S | 50 | 9 | 30 | 27 | 19 | 14 | 100 | Mud | |||||||||||||
| PO5 | S | 57 | 8 | 33 | 42 | 28 | 16 | 8 | 4 | 2 | 58 | Sandy gravel | ||||||||||
| GB1 | C | 10 | 14 | 29 | 43 | 1 | 18 | 17 | 10 | 11 | 57 | Sandy Mud | ||||||||||
| GB2 | C | 20 | 40 | 21 | 61 | 14 | 7 | 6 | 6 | 3 | 36 | 3 | 3 | Sandy Gravel | ||||||||
| CZ1 | C | 10 | 14 | 47 | 61 | 2 | 1 | 11 | 10 | 6 | 9 | 39 | Muddy Sand | |||||||||
| CZ2 | C | 20 | 7 | 51 | 57 | 9 | 1 | 11 | 10 | 6 | 6 | 43 | Muddy Sand | |||||||||
| CZ3 | S | 30 | 8 | 21 | 29 | 21 | 13 | 14 | 20 | 3 | 71 | 0 | Gravelly sand | |||||||||
| CN1 | C | 10 | 10 | 10 | 27 | 13 | 20 | 14 | 9 | 8 | 90 | Mud | ||||||||||
| CN2 | C | 20 | 5 | 64 | 69 | 1 | 0 | 11 | 8 | 6 | 5 | 31 | Muddy Sand | |||||||||
| EC1 | C | 14 | 25 | 51 | 76 | 4 | 1 | 7 | 6 | 4 | 5 | 25 | Muddy Sand | |||||||||
| EC2 | C | 28 | 1 | 22 | 29 | 21 | 15 | 12 | 100 | Mud | ||||||||||||
| EC3 | S | 36 | 33 | 29 | 62 | 17 | 10 | 5 | 4 | 2 | 38 | Sandy gravel | ||||||||||
| EC4 | S | 47 | 3 | 16 | 19 | 32 | 19 | 12 | 10 | 5 | 79 | 2 | 2 | Gravelly sand | ||||||||
| EC5 | S | 54 | 7 | 7 | 13 | 18 | 31 | 27 | 3 | 92 | 1 | 1 | Gravelly sand | |||||||||
| EF2 | C | 20 | 4 | 17 | 28 | 22 | 15 | 13 | 100 | Mud | ||||||||||||
| CO1 | C | 10 | 0 | 35 | 19 | 53 | 1 | 14 | 14 | 8 | 10 | 47 | Muddy Sand | |||||||||
| CO2 | C | 20 | 15 | 11 | 27 | 9 | 5 | 11 | 32 | 15 | 72 | 2 | 2 | Gravelly sand | ||||||||
| CO3 | C | 30 | 5 | 20 | 23 | 19 | 17 | 13 | 96 | Mud | ||||||||||||
| MG1 | C | 10 | 45 | 5 | 50 | 6 | 7 | 13 | 11 | 12 | 49 | 1 | 1 | Sandy Gravel | ||||||||
| MG2 | C | 20 | 3 | 7 | 10 | 6 | 2 | 2 | 50 | 27 | 86 | 4 | 4 | Gravelly Sand | ||||||||
| MG3 | S | 39 | 31 | 34 | 65 | 17 | 8 | 5 | 3 | 2 | 35 | 1 | 1 | Sandy Gravel | ||||||||
| MG4 | S | 53 | 2 | 21 | 23 | 33 | 18 | 12 | 8 | 5 | 77 | Gravelly Sand | ||||||||||
| MG5 | S | 70 | 7 | 37 | 45 | 29 | 16 | 7 | 3 | 1 | 55 | Sandy Gravel | ||||||||||
| VV2 | C | 20 | 49 | 31 | 80 | 3 | 6 | 5 | 3 | 2 | 2 | 20 | Muddy Gravel | |||||||||
| P1 | S | 81 | 0 | 0 | 0 | 1 | 3 | 13 | 24 | 14 | 19 | 26 | 99 | Mud | ||||||||
| P2 | S | 72 | 1 | 2 | 3 | 5 | 9 | 2 | 0 | 0 | 16 | 2 | 16 | 18 | 20 | 26 | 81 | Sandy Mud | ||||
| P3 | S | 91 | 19 | 29 | 17 | 17 | 18 | 100 | Mud | |||||||||||||
| P4 | S | 65 | 2 | 2 | 17 | 15 | 0 | 0 | 0 | 31 | 0 | 2 | 20 | 19 | 14 | 13 | 67 | Sandy Mud | ||||
| P5 | S | 100 | 1 | 28 | 33 | 15 | 11 | 12 | 100 | Mud | ||||||||||||
| P6 | S | 125 | 22 | 37 | 18 | 11 | 12 | 100 | Mud | |||||||||||||
| P8 | S | 69 | 14 | 32 | 46 | 26 | 15 | 7 | 4 | 1 | 54 | 0 | Sandy Gravel | |||||||||
| P9 | S | 94 | 1 | 22 | 27 | 18 | 17 | 15 | 100 | Mud | ||||||||||||
| P11 | S | 120 | 4 | 6 | 10 | 19 | 20 | 11 | 17 | 23 | 90 | Gravelly mud | ||||||||||
| P12 | S | 140 | 15 | 32 | 46 | 27 | 14 | 6 | 3 | 2 | 51 | 3 | 3 | Sandy Gravel | ||||||||
| P13 | S | 67 | 2 | 21 | 23 | 34 | 23 | 12 | 5 | 2 | 77 | 1 | 1 | Gravelly Sand | ||||||||
| P14 | S | 80 | 13 | 34 | 48 | 27 | 15 | 6 | 3 | 1 | 52 | Sandy Gravel | ||||||||||
| P17 | S | 102 | 1 | 25 | 34 | 16 | 12 | 13 | 100 | Mud | ||||||||||||
| P18 | S | 60 | 1 | 4 | 5 | 11 | 12 | 20 | 49 | 2 | 95 | Gravelly sand | ||||||||||
| P19 | S | 71 | 12 | 35 | 47 | 27 | 16 | 7 | 3 | 1 | 53 | Sandy gravel | ||||||||||
| P20 | S | 111 | 1 | 5 | 6 | 0 | 0 | 0 | 0 | 0 | 11 | 21 | 14 | 20 | 28 | 94 | Gravelly mud | |||||

Table A3.
Bioconcentration factors (BCF) of HMs in benthic mollusks across different marine areas, 2018–2023.
Table A3.
Bioconcentration factors (BCF) of HMs in benthic mollusks across different marine areas, 2018–2023.
| Transitional Area | ||||||||||
| Species | Rapana | Anadara | Mytilus | Anadara | Mytilus | |||||
| Year | 2018 | 2018 | 2023 | 2023 | 2023 | |||||
| Station Code | PO2 | PO2 | SG3 | SG3 | SG3 | |||||
| Cu | 600.42 | 178.20 | 345.22 | 94.93 | 67.98 | |||||
| Cd | 3276.18 | 1168.76 | 4803.48 | 14,518.26 | 5220.87 | |||||
| Pb | 9.23 | 0.41 | 195.84 | 28.35 | 702.04 | |||||
| Ni | 57.33 | 141.45 | 950.80 | 2817.48 | 286.70 | |||||
| Cr | 109.03 | 46.97 | 1392.02 | 320.91 | 215.63 | |||||
| Coastal Area | ||||||||||
| Species | Anadara | Rapana | Rapana | Mytilus | Anadara | Rapana | Mytilus | |||
| Year | 2018 | 2020 | 2018 | 2018 | 2018 | 2019 | 2019 | |||
| Station Code | CZ2 | CZ2 | EC2 | EC2 | EC2 | EC2 | EC2 | |||
| Cu | 196.51 | 759.76 | 257.01 | 121.62 | 78.52 | 337.95 | 156.88 | |||
| Cd | 702.26 | 7347.85 | 627.87 | 177.48 | 793.39 | 8373.91 | 8791.30 | |||
| Pb | 0.39 | 0.76 | 21.67 | 1.89 | 2.73 | 9.71 | 27.43 | |||
| Ni | 123.47 | 16.03 | 12.50 | 111.17 | 55.47 | 253.06 | 500.00 | |||
| Cr | 67.90 | No data | 13.96 | 5.81 | 16.04 | 1673.24 | 3087.32 | |||
| Species | Rapana | Rapana | Mytilus | Mytilus | Mytilus | Mytilus | Rapana | |||
| Year | 2021 | 2021 | 2019 | 2020 | 2022 | 2023 | 2020 | |||
| Station Code | EF2 | EF2 | CO3 | CO3 | CO3 | CO3 | MG2 | |||
| Cu | 685.10 | 904.28 | 196.35 | 201.07 | 198.53 | 544.63 | 729.32 | |||
| Cd | 5868.24 | 6959.46 | 155.17 | 5494.51 | 2351.32 | 4735.38 | 10,525.37 | |||
| Pb | 9.66 | 766.50 | 0.18 | 19.64 | 44.99 | 1241.06 | 11.47 | |||
| Ni | 11.59 | 3.64 | No data | 651.27 | 319.93 | 35,500.00 | 11.14 | |||
| Cr | 168.90 | 341.59 | 251.20 | No data | 79.80 | 1427.66 | No data | |||
| Shelf Area | ||||||||||
| Species | Mytilus | Mytilus | Mytilus | Anadara | Mytilus | Anadara | Mytilus | |||
| Year | 2020 | 2019 | 2021 | 2021 | 2022 | 2022 | 2019 | |||
| Station Code | SU3 | SU4 | SG4 | SG4 | SG4 | SG4 | SG5 | |||
| Cu | 222.14 | 219.84 | 329.03 | 679.36 | 564.49 | 967.04 | 84.06 | |||
| Cd | 9043.37 | 6408.00 | 9839.51 | 2127.16 | 5449.91 | 3894.35 | 5605.56 | |||
| Pb | 39.98 | 0.74 | >105 | >105 | 344.65 | 143.81 | 2.48 | |||
| Ni | 747.39 | 22.33 | 25,356.61 | >105 | 9.25 | 25.62 | 162.86 | |||
| Cr | No data | 157.76 | 315.56 | 286.03 | 561.52 | 606.31 | 468.83 | |||
| Species | Mytilus | Mytilus | Mytilus | Mytilus | Rapana | Anadara | Mytilus | Anadara | Rapana | Anadara |
| Year | 2019 | 2019 | 2019 | 2019 | 2020 | 2021 | 2021 | 2021 | 2021 | 2023 |
| Station Code | PO4 | PO5 | PB4 | CZ3 | CZ3 | CZ3 | CZ3 | CZ3 | CZ3 | CZ3 |
| Cu | 100.64 | 1470.06 | 1323.83 | 58.84 | 649.70 | 137.47 | 77.16 | 107.88 | 460.83 | 250.21 |
| Cd | 420.54 | 1723.81 | 1135.48 | 136.76 | 4691.85 | 978.85 | 1513.36 | 12,427.52 | 629.79 | 26,772.60 |
| Pb | 0.17 | 2.38 | 3.41 | 0.25 | 15.23 | 97.21 | 976.62 | 13.93 | 73.34 | 218.28 |
| Ni | 6.11 | 26.21 | 35.41 | 694.67 | 232.52 | 1106.57 | 157.75 | 1.88 | 15.53 | 414.39 |
| Cr | 2602.35 | 4067.35 | 3182.02 | 331.77 | 0.00 | 165.76 | 646.98 | 236.92 | 313.80 | 656.62 |
| Species | Mytilus | Mytilus | Mytilus | Mytilus | Mytilus | Mytilus | ||||
| Year | 2019 | 2019 | 2020 | 2023 | 2021 | 2021 | ||||
| Station Code | EC3 | MG3 | MG3 | MG3 | P2 | P2 | ||||
| Cu | 200.94 | 147.36 | 360.47 | 275.26 | 293.24 | 236.93 | ||||
| Cd | >105 | 22,860.00 | 14,176.47 | 13,956.63 | 71,977.01 | 25,172.41 | ||||
| Pb | 4.44 | 22.73 | 14.79 | >105 | 402.81 | 168.57 | ||||
| Ni | 37.82 | 58.53 | 2917.49 | 389.56 | 146.90 | 62.32 | ||||
| Cr | 578.70 | 240.22 | No data | 317.34 | 293.24 | 466.80 | ||||
| Species | Mytilus | Mytilus | Mytilus | Mytilus | Mytilus | Mytilus | ||||
| Year | 2021 | 2022 | 2022 | 2022 | 2021 | 2019 | ||||
| Station Code | P4 | P13 | P13 | P13 | P13 | ANE-RO-1 | ||||
| Cu | 359.69 | 311.58 | 223.82 | 112.94 | 265.18 | 355.05 | ||||
| Cd | >105 | 59,942.86 | 64,200.00 | 30,671.43 | 75,450.00 | 716.26 | ||||
| Pb | 5488.32 | 38.85 | 46.67 | 16.43 | 904.35 | 1043.24 | ||||
| Ni | 3270.65 | 540.32 | 277.95 | 196.43 | 5544.69 | 848.28 | ||||
| Cr | 440.80 | 6.86 | 9.71 | 6.81 | 283.08 | 233.91 | ||||
Note: Blue represents non-bioaccumulative; yellow represents bioaccumulative; orange represents very bioaccumulative.

Table A4.
Bioaccumulation factors (BAF) of HMs in benthic mollusks across different marine areas, 2018–2023.
Table A4.
Bioaccumulation factors (BAF) of HMs in benthic mollusks across different marine areas, 2018–2023.
| Transitional Area | ||||||||||
| Species | Rapana | Anadara | Mytilus | Anadara | Mytilus | |||||
| Year | 2018 | 2018 | 2023 | 2023 | 2023 | |||||
| Station Code | PO2 | PO2 | SG3 | SG3 | SG3 | |||||
| Cu | 1.66 | 0.49 | 0.97 | 0.27 | 0.19 | |||||
| Cd | 47.96 | 17.11 | 5.43 | 16.40 | 5.90 | |||||
| Pb | 0.02 | 0.001 | 0.11 | 0.02 | 0.39 | |||||
| Ni | 0.04 | 0.09 | 0.20 | 0.60 | 0.06 | |||||
| Cr | No data | No data | 0.44 | 0.10 | 0.07 | |||||
| Coastal Area | ||||||||||
| Species | Anadara | Rapana | Rapana | Mytilus | Anadara | Rapana | Mytilus | |||
| Year | 2018 | 2020 | 2018 | 2018 | 2018 | 2019 | 2019 | |||
| Station Code | CZ2 | CZ2 | EC2 | EC2 | EC2 | EC2 | EC2 | |||
| Cu | 0.72 | 3.43 | 0.57 | 0.27 | 0.18 | 0.96 | 0.44 | |||
| Cd | 26.07 | 17.96 | 8.59 | 2.43 | 10.86 | 5.23 | 5.49 | |||
| Pb | 0.003 | 0.0003 | 0.13 | 0.01 | 0.02 | 0.03 | 0.07 | |||
| Ni | 0.12 | 0.01 | 0.02 | 0.15 | 0.07 | 0.24 | 0.48 | |||
| Cr | No data | No data | 0.06 | 0.02 | 0.06 | 1.15 | 2.13 | |||
| Species | Rapana | Rapana | Mytilus | Mytilus | Mytilus | Mytilus | Rapana | |||
| Year | 2021 | 2021 | 2019 | 2020 | 2022 | 2023 | 2020 | |||
| Station Code | EF2 | EF2 | CO3 | CO3 | CO3 | CO3 | MG2 | |||
| Cu | 1.55 | 2.04 | 0.99 | 0.53 | 0.32 | 0.32 | 2.046 | |||
| Cd | 5.17 | 6.13 | 10.04 | 3.38 | 8.16 | 7.63 | 40.754 | |||
| Pb | 0.001 | 0.36 | 0.001 | 0.01 | 0.03 | 0.22 | 0.003 | |||
| Ni | 0.01 | 0.001 | 0.11 | 0.11 | 0.06 | 0.12 | 0.006 | |||
| Cr | 0.10 | 0.21 | 0.46 | No data | 0.09 | 0.18 | No data | |||
| Shelf Area | ||||||||||
| Species | Mytilus | Mytilus | Mytilus | Anadara | Mytilus | Anadara | Mytilus | |||
| Year | 2020 | 2019 | 2021 | 2021 | 2022 | 2022 | 2019 | |||
| Station Code | SU3 | SU4 | SG4 | SG4 | SG4 | SG4 | SG5 | |||
| Cu | 0.87 | 0.70 | 0.18 | 0.38 | 0.28 | 0.47 | 0.48 | |||
| Cd | 9.40 | 22.19 | 5.69 | 1.23 | 6.86 | 4.90 | 19.63 | |||
| Pb | 0.01 | 0.02 | 0.39 | 0.11 | 0.03 | 0.01 | 0.02 | |||
| Ni | 0.14 | 0.02 | 0.13 | 1.60 | 0.22 | 0.60 | 0.48 | |||
| Cr | No data | 0.47 | 0.20 | 0.19 | 0.21 | 0.23 | 0.60 | |||
| Species | Mytilus | Mytilus | Mytilus | Mytilus | Rapana | Anadara | Mytilus | Anadara | Rapana | Anadara |
| Year | 2019 | 2019 | 2019 | 2019 | 2020 | 2021 | 2021 | 2021 | 2021 | 2023 |
| Station Code | PO4 | PO5 | PB4 | CZ3 | CZ3 | CZ3 | CZ3 | CZ3 | CZ3 | CZ3 |
| Cu | 0.289 | 1.001 | 0.99 | 0.933 | 0.677 | 0.968 | 0.544 | 0.760 | 3.246 | 0.450 |
| Cd | 3.610 | 96.964 | 7.44 | 8.253 | 2.744 | 4.864 | 7.520 | 61.751 | 3.129 | 25.702 |
| Pb | 0.001 | 0.005 | 0.02 | 0.005 | 0.002 | 0.039 | 0.389 | 0.006 | 0.029 | 0.029 |
| Ni | 0.029 | 0.107 | 0.05 | 0.357 | 0.081 | 2.073 | 0.296 | 0.004 | 0.029 | 0.078 |
| Cr | 0.095 | 0.299 | 0.45 | 0.591 | No data | 0.178 | 0.694 | 0.254 | 0.337 | 0.163 |
| Species | Mytilus | Mytilus | Mytilus | Mytilus | Mytilus | Mytilus | ||||
| Year | 2019 | 2019 | 2020 | 2023 | 2021 | 2021 | ||||
| Station Code | EC3 | MG3 | MG3 | MG3 | P2 | P2 | ||||
| Cu | 0.170 | 0.688 | 0.372 | 0.215 | 0.076 | 0.061 | ||||
| Cd | 7.673 | 3.594 | 3.661 | 7.177 | 11.182 | 3.911 | ||||
| Pb | 0.001 | 0.007 | 0.014 | 0.059 | 0.027 | 0.011 | ||||
| Ni | 0.079 | 0.141 | 0.043 | 0.043 | 0.070 | 0.030 | ||||
| Cr | 0.168 | 0.163 | No data | 0.073 | 0.027 | 0.043 | ||||
| Species | Mytilus | Mytilus | Mytilus | Mytilus | Mytilus | Mytilus | ||||
| Year | 2021 | 2022 | 2022 | 2022 | 2021 | 2019 | ||||
| Station Code | P4 | P13 | P13 | P13 | P13 | ANE-RO-1 | ||||
| Cu | 0.12 | 0.335 | 0.241 | 0.121 | 0.042 | 0.37 | ||||
| Cd | 8.04 | 22.367 | 23.955 | 11.445 | 13.315 | 30.29 | ||||
| Pb | 0.02 | 0.027 | 0.032 | 0.011 | 0.016 | 0.01 | ||||
| Ni | 0.18 | 0.102 | 0.052 | 0.037 | 0.092 | 0.12 | ||||
| Cr | 0.04 | 0.119 | 0.169 | 0.119 | 0.037 | 0.04 | ||||
Note: Blue represents Non-bioaccumulative; yellow represents Bioaccumulative.
References
- El-Sharkawy, M.; Alotaibi, M.O.; Li, J.; Du, D.; Mahmoud, E. Heavy Metal Pollution in Coastal Environments: Ecological Implications and Management Strategies: A Review. Sustainability 2025, 17, 701. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, K.; Chu, F.; Ge, Q.; Xu, D.; Han, X.; Ye, L. Sources and Spatial Variations of Heavy Metals in Offshore Sediments of the Western Pearl River Estuary. Mar. Pollut. Bull. 2023, 188, 114599. [Google Scholar] [CrossRef]
- Krutov, A. (Ed.) BSC State of the Environment of the Black Sea (2009–2014/5); Publications of the Commission on the Protection of the Black Sea Against Pollution (BSC): Istanbul, Turkey, 2019. [Google Scholar]
- Lazar, L.; Vlas, O.; Pantea, E.; Boicenco, L.; Marin, O.; Abaza, V.; Filimon, A.; Bisinicu, E. Black Sea Eutrophication Comparative Analysis of Intensity between Coastal and Offshore Waters. Sustainability 2024, 16, 5146. [Google Scholar] [CrossRef]
- Lazar, L.; Spanu, A.; Boicenco, L.; Oros, A.; Damir, N.; Bisinicu, E.; Abaza, V.; Filimon, A.; Harcota, G.; Marin, O.; et al. Methodology for Prioritizing Marine Environmental Pressures under Various Management Scenarios in the Black Sea. Front. Mar. Sci. 2024, 11, 1388877. [Google Scholar] [CrossRef]
- Cozzi, S.; Ibáñez, C.; Lazar, L.; Raimbault, P.; Giani, M. Flow Regime and Nutrient-Loading Trends from the Largest South European Watersheds: Implications for the Productivity of Mediterranean and Black Sea’s Coastal Areas. Water 2018, 11, 1. [Google Scholar] [CrossRef]
- Daskalov, G.M.; Boicenco, L.; Grishin, A.N.; Lazar, L.; Mihneva, V.; Shlyakhov, V.A.; Zengin, M. Architecture of Collapse: Regime Shift and Recovery in an Hierarchically Structured Marine Ecosystem. Glob. Change Biol. 2017, 23, 1486–1498. [Google Scholar] [CrossRef]
- Bișinicu, E.; Lazăr, L.; Timofte, F. Dynamics of Zooplankton along the Romanian Black Sea Coastline: Temporal Variation, Community Structure, and Environmental Drivers. Diversity 2023, 15, 1024. [Google Scholar] [CrossRef]
- Bișinicu, E.; Boicenco, L.; Pantea, E.; Timofte, F.; Lazăr, L.; Vlas, O. Qualitative Model of the Causal Interactions between Phytoplankton, Zooplankton, and Environmental Factors in the Romanian Black Sea. Phycology 2024, 4, 168–189. [Google Scholar] [CrossRef]
- Bisinicu, E.; Abaza, V.; Boicenco, L.; Adrian, F.; Harcota, G.-E.; Marin, O.; Oros, A.; Pantea, E.; Spinu, A.; Timofte, F.; et al. Spatial Cumulative Assessment of Impact Risk-Implementing Ecosystem-Based Management for Enhanced Sustainability and Biodiversity in the Black Sea. Sustainability 2024, 16, 4449. [Google Scholar] [CrossRef]
- Kudelsky, A.V. Global Geoenvironmental Problems: Black Sea Basin. Water Resour. 2011, 38, 849–858. [Google Scholar] [CrossRef]
- Orhon, D. Evaluation of the Impact from the Black Sea on the Pollution of the Marmara Sea. Water Sci. Technol. 1995, 32, 191–198. [Google Scholar] [CrossRef]
- Strokal, M.; Strokal, V.; Kroeze, C. The Future of the Black Sea: More Pollution in over Half of the Rivers. Ambio 2023, 52, 339–356. [Google Scholar] [CrossRef]
- Bat, L.; Öztekin, A.; Şahin, F.; Arıcı, E.; Özsandıkçı, U. An Overview of the Black Sea Pollution in Turkey. Mediterr. Fish. Aquac. Res. 2018, 1, 66–86. [Google Scholar]
- Bat, L.; Ozkan, E.Y.; Buyukisik, H.B.; Oztekin, H.C. Assessment of Metal Pollution in Sediments along Sinop Peninsula of the Black Sea. Int. J. Mar. Sci. 2017, 7, 205–213. [Google Scholar] [CrossRef]
- Ulger, S.; Higano, Y. A Preliminary Study to Improve the Water Quality in the Black Sea: Turkish Black Sea Basin. Stud. Reg. Sci. 2001, 32, 353–367. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Cristea, D.S.; Petrea, S.-M.; Mogodan, A.; Nicoara, M.; Plavan, G.; Baltag, E.S.; Jijie, R.; Strungaru, S.-A. Preliminary Investigation of Lower Danube Pollution Caused by Potentially Toxic Metals. Chemosphere 2021, 264, 128496. [Google Scholar] [CrossRef] [PubMed]
- Bucşe, A.; Pârvulescu, O.C.; Vasiliu, D.; Rădulescu, F.; Lupaşcu, N.; Ispas, B.A. Spatial Distribution of Trace Elements and Potential Contamination Sources for Surface Sediments of the North-Western Black Sea, Romania. Front. Mar. Sci. 2024, 10, 1310164. [Google Scholar] [CrossRef]
- Oros, A.; Coatu, V.; Secrieru, D.; Tiganus, D.; Vasiliu, D.; Atabay, H.; Beken, C.; Tolun, L.; Moncheva, S.; Bat, L. Results of the Assessment of the Western Black Sea Contamination Status in the Frame of the MISIS Joint Cruise. Cercet. Mar. 2016, 46, 61–81. [Google Scholar]
- Lazar, L.; Boicenco, L.; Todorova, V.; Denga, Y.; Atabay, H.; Kurt, G.; Marian, P.; Tonay, M.; Abaza, V.; Bisinicu, E.; et al. ANEMONE Deliverable 2.3 “Black Sea State of Environment Based on ANEMONE Joint Cruise”; Lazar, L., Ed.; Editura CD Press: București, Romania, 2021. [Google Scholar]
- Alygizakis, N.; Giannakopoulos, T.; Τhomaidis, N.S.; Slobodnik, J. Detecting the Sources of Chemicals in the Black Sea Using Non-Target Screening and Deep Learning Convolutional Neural Networks. Sci. Total Environ. 2022, 847, 157554. [Google Scholar] [CrossRef]
- Shchiptsov, O.A.; Goncharov, O.Y. European Research Project on the State of Pollution in the Black Sea «Black Sea SIERRA»: Mission and Participation of Ukrainian Oceanographers. Geofizicheskiy Zhurnal 2023, 45, 162–171. [Google Scholar] [CrossRef]
- Jiang, D.; Khokhlov, V.; Tuchkovenko, Y.; Kushnir, D.; Ovcharuk, V.; Spyrakos, E.; Stanica, A.; Slabakova, V.; Tyler, A. The Biogeochemical Response of the North-Western Black Sea to the Kakhovka Dam Breach. Commun. Earth Environ. 2025, 6, 185. [Google Scholar] [CrossRef]
- Bisinicu, E.; Lazar, L. Assessing the Black Sea Mesozooplankton Community Following the Nova Kakhovka Dam Breach. J. Mar. Sci. Eng. 2025, 13, 67. [Google Scholar] [CrossRef]
- Cyriac, M.; Gireeshkumar, T.R.; Furtado, C.M.; Fathin, K.P.F.; Shameem, K.; Shaik, A.; Vignesh, E.R.; Nair, M.; Kocherla, M.; Balachandran, K.K. Distribution, Contamination Status and Bioavailability of Trace Metals in Surface Sediments along the Southwest Coast of India. Mar. Pollut. Bull. 2021, 164, 112042. [Google Scholar] [CrossRef] [PubMed]
- Oros, A.; Coatu, V.; Damir, N.; Danilov, D.; Ristea, E. Recent Findings on the Pollution Levels in the Romanian Black Sea Ecosystem: Implications for Achieving Good Environmental Status (GES) Under the Marine Strategy Framework Directive (Directive 2008/56/EC). Sustainability 2024, 16, 9785. [Google Scholar] [CrossRef]
- European Union. European Parliament Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). OJL 2008, 164, 19–40. [Google Scholar]
- Tornero, V.; Hanke, G. Chemical Contaminants Entering the Marine Environment from Sea-Based Sources: A Review with a Focus on European Seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef]
- Boicenco, L.; Abaza, V.; Anton, E.; Bisinicu, E.; Buga, L.; Coatu, V.; Damir, N.; Diaconeasa, D.; Dumitrache, C.; Filimon, A.; et al. Studiu Privind Elaborarea Raportului Privind Starea Ecologică a Ecosistemului Marin Marea Neagră Conform Cerintelor Art. 17 Ale Directivei Cadru Strategia Pentru Mediul Marin (2008/56/EC). 2018. Available online: https://www.mmediu.ro/app/webroot/uploads/files/Studiu_raport_MSFD-2024.pdf (accessed on 20 July 2025).
- Panin, N.; Jipa, D. Danube River Sediment Input and Its Interaction with the North-Western Black Sea. Estuar. Coast. Shelf Sci. 2002, 54, 551–562. [Google Scholar] [CrossRef]
- Panin, N.; Jipa, D.C.; Gomoiu, M.T.; Secrieru, D. Importance of Sedimentary Processes in Environmental Changes: Lower River Danube—Danube Delta—Western Black Sea System. In Environmental Degradation of the Black Sea: Challenges and Remedies; Springer: Dordrecht, The Netherlands, 1999; pp. 23–41. [Google Scholar]
- Catianis, I.; Ungureanu, C.; Magagnini, L.; Ulazzi, E.; Campisi, T.; Stanica, A. Environmental Impact of the Midia Port-Black Sea (Romania), on the Coastal Sediment Quality. Open Geosci. 2016, 8, 174–194. [Google Scholar] [CrossRef]
- Marin, D.; Ciucă, A.-M.; Filimon, A.; Stoica, E. Marine Litter and Plastic Detection on Black Sea Beaches by Using Unmanned Aerial Vehicle (UAV). Ovidius Univ. Ann. Chem. 2024, 35, 43–50. [Google Scholar] [CrossRef]
- Lazar, L.; Boicenco, L.; Moncheva, S.; Denga, Y.; Atabay, H.; Abaza, V.; Bisinicu, E.; Valentina, C.; Adrian, F.; George, H.; et al. ANEMONE Deliverable 2.1, 2021. “Impact of the Rivers on the Black Sea Ecosystem”; Lazar, L., Ed.; Editura CD Press: București, Romania, 2021; ISBN 978-606-528-528-6. [Google Scholar]
- Lazar, L.; Boicenco, L.; Denga, Y.; Tolun, L.; Kurt, G.; Bisinicu, E.; Valentina, C.; Adrian, F.; Marin, O.; Oros, A.; et al. ANEMONE Deliverable 2.2 “Anthropogenic Pressures and Impacts on the Black Sea Coastal Ecosystem”; Lazar, L., Ed.; Editura CD Press: București, Romania, 2021; ISBN 978-606-528-529-3. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. (Eds.) Methods of Seawater Analysis, 3rd ed.; Willey-VCH: Weinheim, Germany, 1999; ISBN 978-3-527-61399-1. [Google Scholar]
- UNEP; IOC; IAEA UNEP. Manual for the Geochemical Analysis of Marine Sediments and Suspended Particulate Matter; Reference Methods for Marine Pollution Studies No. 63; United Nations Environment Programme: Nairobi, Kenya, 1995. [Google Scholar]
- UNEP; FAO; IOC; IAEA. Guidelines for Monitoring Chemical Contaminants in the Sea Using Marine Organisms; References Methods for Marine Pollution Studies No 6; United Nations Environment Programme: Nairobi, Kenya, 1993. [Google Scholar]
- IAEA-MEL. Training Manual on the Measurement of Heavy Metals in Environmental Samples; IAEA-MEL: Monaco-Ville, Monaco, 1999. [Google Scholar]
- European Union. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. OJL 2023, 119, 103–157. [Google Scholar]
- Blott, S.J.; Pye, K. GRADISTAT: A Grain Size Distribution and Statistics Package for the Analysis of Unconsolidated Sediments. Earth Surf. Process Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Folk, R.L.; Ward, W.C. Brazos River Bar [Texas]; a Study in the Significance of Grain Size Parameters. J. Sediment. Res. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- TIBCO Statistica Inc. TIBCO Software, Version 14.0.1.25; Palo Alto Inc.: Santa Clara, CA, USA, 2023.
- Schlitzer, R. Ocean Data View. Available online: https://odv.awi.de (accessed on 11 July 2024).
- European Union. European Parliament Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. OJL 2013, 226, 1–17. [Google Scholar]
- Order No. 161/2006 for the Approval of the Regulation on the Classification of Surface Water Quality for Determining the Ecological Status of Water Bodies. In Official Gazette of Romania, Bucharest, Romania; Government of Romania: Bucharest, Romania, 2006; Volume 511. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/72574 (accessed on 20 July 2025). (In Romanian)
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of Adverse Biological Effects within Ranges of Chemical Concentrations in Marine and Estuarine Sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- 2019 Updated Audit Trail of OSPAR EACs and Other Assessment Criteria Used to Distinguish above and below Thresholds; 2020. Available online: https://www.ospar.org/documents?v=43066 (accessed on 20 July 2025).
- European Parliament Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC; 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20250623 (accessed on 20 July 2025).
- European Chemicals Agency (ECHA) Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.11: PBT/VPvB Assessment, Version 4.0, ECHA-23-H-10-EN 2023. Available online: https://echa.europa.eu/guidance-documents/guidance-on-information-requirements-and-chemical-safety-assessment (accessed on 20 July 2025).
- U.S. Environmental Protection Agency (USEPA) Bioaccumulation Testing and Interpretation for the Purpose of Sediment Quality Assessment: Status and Needs. EPA-823-R-00-001. 2000. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/20003TM1.PDF?Dockey=20003TM1.PDF (accessed on 20 July 2025).
- Long, E.; Morgan, L. The Potential for Biological Effects of Sediment-Sorbed Contaminants Tested in the National Status and Trends Program. NOAA Tech. Memo. NOS OMA 1991, 52. Available online: https://repository.library.noaa.gov/view/noaa/1750 (accessed on 20 July 2025).
- Long, E.R.; Field, L.J.; MacDonald, D.D. Predicting Toxicity in Marine Sediments with Numerical Sediment Quality Guidelines. Environ. Toxicol. Chem. 1998, 17, 714–727. [Google Scholar] [CrossRef]
- Buchman, M.F. Screening Quick Reference Tables (SQuiRTs). 2008. Available online: https://semspub.epa.gov/work/03/2171359.pdf (accessed on 20 July 2025).
- Oros, A. Monitoring and Assessment of Heavy Metals in the Romanian Black Sea Ecosystem during 2006-2018, in the Context of Marine Strategy Framework Directive (MSFD) 2008/56/EC Implementation. Cercet. Mar.-Rech. Mar. 2019, 49, 8–33. Available online: https://www.marine-research-journal.org/index.php/cmrm/article/view/140/105 (accessed on 20 July 2025).
- Ristea, E.; Pârvulescu, O.C.; Lavric, V.; Oros, A. Assessment of Heavy Metal Contamination of Seawater and Sediments Along the Romanian Black Sea Coast: Spatial Distribution and Environmental Implications. Sustainability 2025, 17, 2586. [Google Scholar] [CrossRef]
- Secrieru, D.; Secrieru, A. Heavy Metal Enrichment of Man-Made Origin of Superficial Sediment on the Continental Shelf of the North-Western Black Sea. Estuar. Coast. Shelf Sci. 2002, 54, 513–526. [Google Scholar] [CrossRef]
- Vignati, D.A.L.; Secrieru, D.; Bogatova, Y.I.; Dominik, J.; Céréghino, R.; Berlinsky, N.A.; Oaie, G.; Szobotka, S.; Stanica, A. Trace Element Contamination in the Arms of the Danube Delta (Romania/Ukraine): Current State of Knowledge and Future Needs. J. Environ. Manag. 2013, 125, 169–178. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Gao, J.; Yuan, Y.-Q.; Ma, J.; Yu, S. Relationship between Heavy Metal Contents and Clay Mineral Properties in Surface Sediments: Implications for Metal Pollution Assessment. Cont. Shelf Res. 2016, 124, 125–133. [Google Scholar] [CrossRef]
- Constantinescu, A.M.; Tyler, A.N.; Stanica, A.; Spyrakos, E.; Hunter, P.D.; Catianis, I.; Panin, N. A Century of Human Interventions on Sediment Flux Variations in the Danube-Black Sea Transition Zone. Front. Mar. Sci. 2023, 10, 1068065. [Google Scholar] [CrossRef]
- Lazar, L.; Gomoiu, M.T.; Boicenco, L.; Vasiliu, D. Total Organic Carbon (TOC) of the Surface Layer Sediments Covering the Seafloor of the Romanian Black Sea Coast. Geoecomarina 2012, 18, 121–132. [Google Scholar]
- Zhang, C.; Yu, Z.; Zeng, G.; Jiang, M.; Yang, Z.; Cui, F.; Zhu, M.; Shen, L.; Hu, L. Effects of Sediment Geochemical Properties on Heavy Metal Bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The Pollution Conveyed by Urban Runoff: A Review of Sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef]
- Byrnes, T.A.; Dunn, R.J.K. Boating- and Shipping-Related Environmental Impacts and Example Management Measures: A Review. J. Mar. Sci. Eng. 2020, 8, 908. [Google Scholar] [CrossRef]
- Frank, J.J.; Poulakos, A.G.; Tornero-Velez, R.; Xue, J. Systematic Review and Meta-Analyses of Lead (Pb) Concentrations in Environmental Media (Soil, Dust, Water, Food, and Air) Reported in the United States from 1996 to 2016. Sci. Total Environ. 2019, 694, 133489. [Google Scholar] [CrossRef]
- Faucher, K.; Fichet, D.; Miramand, P.; Lagardère, J.-P. Impact of Chronic Cadmium Exposure at Environmental Dose on Escape Behaviour in Sea Bass (Dicentrarchus labrax L.; Teleostei, Moronidae). Environ. Pollut. 2008, 151, 148–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thiagarajan, V.; Nah, T.; Xin, X. Impacts of Atmospheric Particulate Matter Deposition on Phytoplankton: A Review. Sci. Total Environ. 2024, 950, 175280. [Google Scholar] [CrossRef] [PubMed]
- Janssen, D.J.; Gilliard, D.; Rickli, J.; Nasemann, P.; Koschinsky, A.; Hassler, C.S.; Bowie, A.R.; Ellwood, M.J.; Kleint, C.; Jaccard, S.L. Chromium Stable Isotope Distributions in the Southwest Pacific Ocean and Constraints on Hydrothermal Input from the Kermadec Arc. Geochim. Cosmochim. Acta 2023, 342, 31–44. [Google Scholar] [CrossRef]
- Ene, A.; Teodorof, L.; Chiţescu, C.L.; Burada, A.; Despina, C.; Bahrim, G.E.; Vasile, A.M.; Seceleanu-Odor, D.; Enachi, E. Surface Water Contaminants (Metals, Nutrients, Pharmaceutics, Endocrine Disruptors, Bacteria) in the Danube River and Black Sea Basins, SE Romania. Appl. Sci. 2025, 15, 5009. [Google Scholar] [CrossRef]
- Preisner, M.; Smol, M.; Szołdrowska, D. A Toxic-Free Environment Ambition in the Light of the Polish Baltic Sea Coastal Zone Pollution by Heavy Metals. Desalination Water Treat. 2021, 232, 225–235. [Google Scholar] [CrossRef]
- Theodosi, C.; Stavrakakis, S.; Koulaki, F.; Stavrakaki, I.; Moncheva, S.; Papathanasiou, E.; Sanchez-Vidal, A.; Koçak, M.; Mihalopoulos, N. The Significance of Atmospheric Inputs of Major and Trace Metals to the Black Sea. J. Mar. Syst. 2013, 109–110, 94–102. [Google Scholar] [CrossRef]
- Abdel Ghani, S.; Hamdona, S.; Shakweer, L.; El Saharty, A. Spatial Distribution and Pollution Assessment of Heavy Metals in Surface and Bottom Water along the Eastern Part of the Egyptian Mediterranean Coast. Mar. Pollut. Bull. 2023, 197, 115713. [Google Scholar] [CrossRef]
- Atabay, H.; Aslan, E.; Tan, İ.; Tolun, L.; Çağlar Balkıs, N. Spatial and Temporal Assessment of Heavy Metal Contamination in the Black Sea Surface Waters. Int. J. Environ. Geoinform. 2024, 11, 17–28. [Google Scholar] [CrossRef]
- Yottiam, A.; Chaikaew, P.; Kulsawat, W.; Srithongouthai, S. Application of Novel Background Criteria for Assessing Metal Contamination in Sediments of the Inner Gulf of Thailand. Chemosphere 2025, 375, 144235. [Google Scholar] [CrossRef]
- Oaie, G.; Secrieru, D.; Bondar, C.; Szobotkat, S.; Duţu, L.; Stănescu, L.; Opreanu, G.; Duţu, F.; Pojar, L.; Manta, T. Lower Danube River: Characterization of Sediments and Pollutants. Geo-Eco-Marina 2015, 21, 19–34. [Google Scholar]
- Buyang, S.; Yi, Q.; Cui, H.; Wan, K.; Zhang, S. Distribution and Adsorption of Metals on Different Particle Size Fractions of Sediments in a Hydrodynamically Disturbed Canal. Sci. Total Environ. 2019, 670, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S.; Goh, B.P.L.; Chou, L.M. Environmental Impact of Heavy Metals from Dredged and Resuspended Sediments on Phytoplankton and Bacteria Assessed in in Situ Mesocosms. Ecotoxicol. Environ. Saf. 2004, 59, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Mali, M.; Malcangio, D.; Dell’ Anna, M.M.; Damiani, L.; Mastrorilli, P. Influence of Hydrodynamic Features in the Transport and Fate of Hazard Contaminants within Touristic Ports. Case Study: Torre a Mare (Italy). Heliyon 2018, 4, e00494. [Google Scholar] [CrossRef]
- Robledo Ardila, P.A.; Álvarez-Alonso, R.; Árcega-Cabrera, F.; Durán Valsero, J.J.; Morales García, R.; Lamas-Cosío, E.; Oceguera-Vargas, I.; DelValls, A. Assessment and Review of Heavy Metals Pollution in Sediments of the Mediterranean Sea. Appl. Sci. 2024, 14, 1435. [Google Scholar] [CrossRef]
- Varol, M.; Ustaoğlu, F.; Tokatlı, C. Metal Pollution, Eco-Health Risks and Source Apportionment in Coastal Sediments of Samsun, Türkiye: A Receiving Zone for the Kızılırmak and Yeşilırmak Rivers. Environ. Res. 2025, 282, 122113. [Google Scholar] [CrossRef]
- Doncheva, V.; Hristova, O.; Slavova, K. Metal Pollution Assessment in Sediments of the Bulgarian Black Sea Coastal Zone. Ecol. Balk. 2020, 12, 179–189. [Google Scholar]
- Kotelyanets, E.A.; Sovga, E.E.; Konovalov, S.K. Spatial Distribution of Heavy Metals in the Bottom Sediments of Bays of the Sevastopol Region. Lomonosov Geogr. J. 2024, 79, 68–79. [Google Scholar] [CrossRef]
- Konstantinova, E.; Minkina, T.; Nevidomskaya, D.; Bauer, T.; Zamulina, I.; Latsynnik, E.; Dudnikova, T.; Yadav, R.K.; Burachevskaya, M.; Mandzhieva, S. Pollution and Ecological Risk Assessment of Potentially Toxic Elements in Sediments Along the Fluvial-to-Marine Transition Zone of the Don River. Water 2024, 16, 3200. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarokhi, S.; Josefsson, S.; Apler, A.; Kalbitz, K.; Åström, M.; Ketzer, M. Baltic Sea Sediments Record Anthropogenic Loads of Cd, Pb, and Zn. Environ. Sci. Pollut. Res. 2021, 28, 6162–6175. [Google Scholar] [CrossRef] [PubMed]
- Oros, A.; Galatchi, M. Long-Term Heavy Metal Bioaccumulation in Sprat (Sprattus Sprattus) from the Romanian Black Sea: Ecological and Human Health Risks in the Context of Sustainable Fisheries. Fishes 2025, 10, 178. [Google Scholar] [CrossRef]
- Nakhlé, K.F.; Cossa, D.; Khalaf, G.; Beliaeff, B. Brachidontes Variabilis and Patella Sp. as Quantitative Biological Indicators for Cadmium, Lead and Mercury in the Lebanese Coastal Waters. Environ. Pollut. 2006, 142, 73–82. [Google Scholar] [CrossRef] [PubMed]
- McGeer, J.C.; Brix, K.V.; Skeaff, J.M.; DeForest, D.K.; Brigham, S.I.; Adams, W.J.; Green, A. Inverse Relationship between Bioconcentration Factor and Exposure Concentration for Metals: Implications for Hazard Assessment of Metals in the Aquatic Environment. Environ. Toxicol. Chem. 2003, 22, 1017–1037. [Google Scholar] [CrossRef]
- Gagnon, C.; Fisher, N.S. Bioavailability of Sediment-Bound Methyl and Inorganic Mercury to a Marine Bivalve. Environ. Sci. Technol. 1997, 31, 993–998. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, K.; Gao, S.; Liang, B.; Lu, J.; Fu, G. Bioaccumulation of Heavy Metals in the Water, Sediment, and Organisms from The Sea Ranching Areas of Haizhou Bay in China. Water 2023, 15, 2218. [Google Scholar] [CrossRef]
- Pantea, E.-D.; Oros, A.; Rosioru, D.M.; Rosoiu, N. Condition Index of Mussel Mytilus Galloprovincialis (Lamarck, 1819) as a Physiological Indicator of Heavy Metals Contamination. Ann. Acad. Rom. Sci. Ser. Biol. Sci. 2020, 9, 20–36. [Google Scholar] [CrossRef]
- Long, R. The Marine Strategy Framework Directive: A New European Approach to the Regulation of the Marine Environment, Marine Natural Resources and Marine Ecological Services. J. Energy Nat. Resour. Law 2011, 29, 1–44. [Google Scholar] [CrossRef]
- Golumbeanu, M.; Oros, A.; Nenciu, M.; Zavatarelli, M.; Drago, A. Contribution of Environmental Indices in Meeting the Objectives and Principles of the Marine Strategy Framework Directive (MSFD). J. Environ. Prot. Ecol. 2014, 15, 1130–1138. [Google Scholar]
- Bisinicu, E.; Harcota, G.; Lazar, L. Interactions between Environmental Factors and the Mesozooplankton Community from the Romanian Black Sea Waters. Turk. J. Zool. 2023, 47, 202–215. [Google Scholar] [CrossRef]
- Rainbow, P.S.; Kriefman, S.; Smith, B.D.; Luoma, S.N. Have the Bioavailabilities of Trace Metals to a Suite of Biomonitors Changed over Three Decades in SW England Estuaries Historically Affected by Mining? Sci. Total Environ. 2011, 409, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ward, D.; Williams, J.; Fisher, N. Metal Bioaccumulation by Estuarine Food Webs in New England, USA. J. Mar. Sci. Eng. 2016, 4, 41. [Google Scholar] [CrossRef]
- Hauser-Davis, R.A.; Wosnick, N. Climate Change Implications for Metal and Metalloid Dynamics in Aquatic Ecosystems and Its Context within the Decade of Ocean Sciences. Water 2022, 14, 2415. [Google Scholar] [CrossRef]
- Abdallah, M.A.M. Bioaccumulation of Heavy Metals in Mollusca Species and Assessment of Potential Risks to Human Health. Bull. Environ. Contam. Toxicol. 2013, 90, 552–557. [Google Scholar] [CrossRef]
- Damir, N.; Coatu, V.; Danilov, D.; Lazăr, L.; Oros, A. From Waters to Fish: A Multi-Faceted Analysis of Contaminants’ Pollution Sources, Distribution Patterns, and Ecological and Human Health Consequences. Fishes 2024, 9, 274. [Google Scholar] [CrossRef]
- Oros, A.; Pantea, E.-D.; Ristea, E. Heavy Metal Concentrations in Wild Mussels Mytilus Galloprovincialis (Lamarck, 1819) during 2001–2023 and Potential Risks for Consumers: A Study on the Romanian Black Sea Coast. Sci 2024, 6, 45. [Google Scholar] [CrossRef]
- Galaţchi, M.; Oros, A.; Coatu, V.; Costache, M.; Coprean, D.; Galaţchi, L.-D. Pollutant Bioaccumulation in Anchovy (Engraulis Encrasicolus) Tissue, Fish Species of Commercial Interest at the Romanian Black Sea Coast. Ovidius Univ. Ann. Chem. 2017, 28, 11–17. [Google Scholar] [CrossRef]
- Phaenark, C.; Phankamolsil, Y.; Sawangproh, W. Ecological and Health Implications of Heavy Metal Bioaccumulation in Thai Fauna: A Systematic Review. Ecotoxicol. Environ. Saf. 2024, 285, 117086. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.; Tao, Z.; Li, Y.; Magni, P.; Zhang, L.; Zheng, X.; Pan, K. The Importance of Organic Matter in Controlling the Metal Variability and Mobility in Seagrass Sediments. Environ. Pollut. 2025, 366, 125542. [Google Scholar] [CrossRef]
- Nawrot, N.; Wojciechowska, E.; Mohsin, M.; Kuittinen, S.; Pappinen, A.; Rezania, S. Trace Metal Contamination of Bottom Sediments: A Review of Assessment Measures and Geochemical Background Determination Methods. Minerals 2021, 11, 872. [Google Scholar] [CrossRef]
- Birch, G.F. A Review and Critical Assessment of Sedimentary Metal Indices Used in Determining the Magnitude of Anthropogenic Change in Coastal Environments. Sci. Total Environ. 2023, 854, 158129. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control.a Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Shen, Z.; Niu, J.; Tang, Z. Distribution and Speciation of Heavy Metals in Sediments from the Mainstream, Tributaries, and Lakes of the Yangtze River Catchment of Wuhan, China. J. Hazard. Mater. 2009, 166, 1186–1194. [Google Scholar] [CrossRef]
- Long, E.R.; MacDonald, D.D.; Severn, C.G.; Hong, C.B. Classifying Probabilities of Acute Toxicity in Marine Sediments with Empirically Derived Sediment Quality Guidelines. Environ. Toxicol. Chem. 2000, 19, 2598–2601. [Google Scholar] [CrossRef]
- El-SiKaily, A.; Shabaka, S. Biomarkers in Aquatic Systems: Advancements, Applications and Future Directions. Egypt. J. Aquat. Res. 2024, 50, 169–182. [Google Scholar] [CrossRef]
- Kolovoyiannis, V.; Mazioti, A.A.; Potiris, M.; Mamoutos, I.; Majamäki, E.; Hänninen, R.; Krasakopoulou, E.; Tragou, E.; Zervakis, V.; Sofiev, M.; et al. Modelling the Impact of Present and Future Maritime Transport on Marine Pollution at an Environmentally Sensitive Coastal Ecosystem (Saronikos Gulf, Eastern Mediterranean). Mar. Pollut. Bull. 2025, 219, 118335. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).