Microbiome and Chemistry Insights into Two Oligotrophic Karst Water Springs in Slovenia from 2016 and 2023 Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Sites
2.2. Sampling
2.3. Analysis of Physicochemical and Microbiological Water Quality Parameters
2.4. UV-VIS Spectroscopy and Organic Matter Indices
2.5. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) for Quantification of Metals in Water Samples
2.6. Isotopic Analysis
2.7. Discharge and Precipitation
2.8. Filtration of Water Samples for Microbiome Analyses
2.9. DNA Extraction
2.10. Shotgun Metagenomic Sequencing
2.11. Bioinformatic and Statistical Analysis
3. Results and Discussion
3.1. Physicochemical and Microbiological Water Quality Parameters
3.2. Carbonate Weathering and Alkalinity Sources
3.3. Isotopic Composition of Water
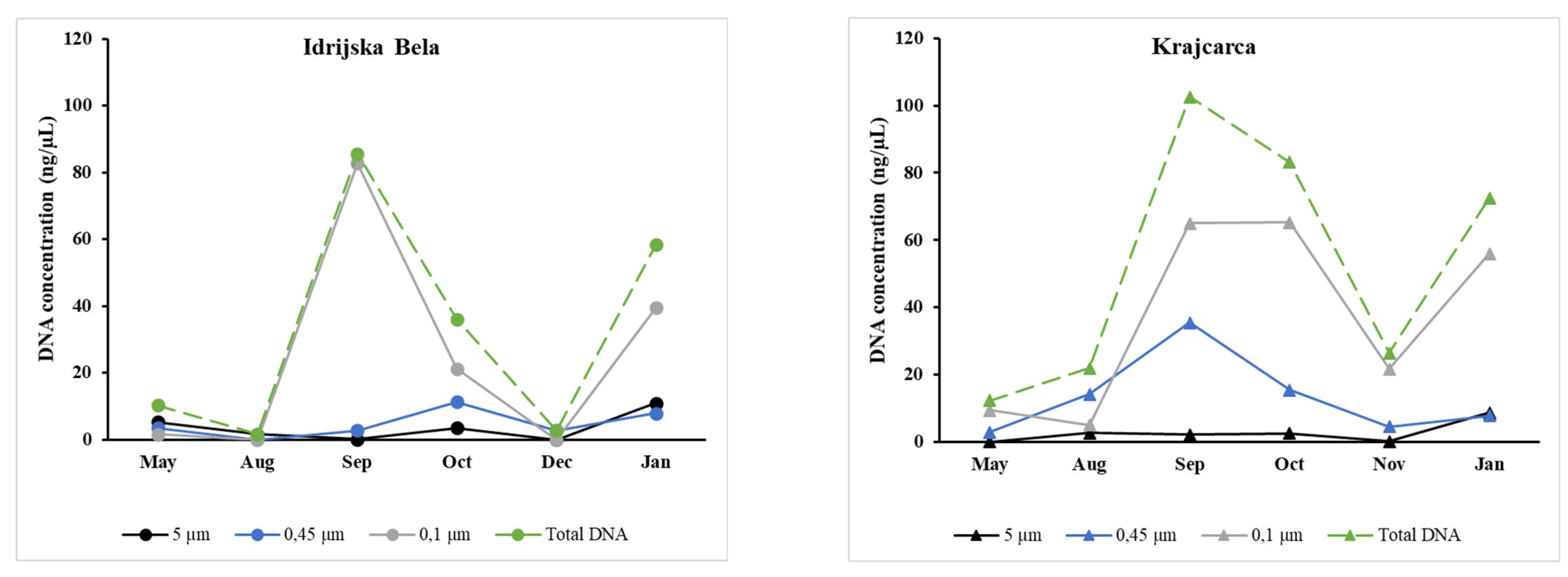
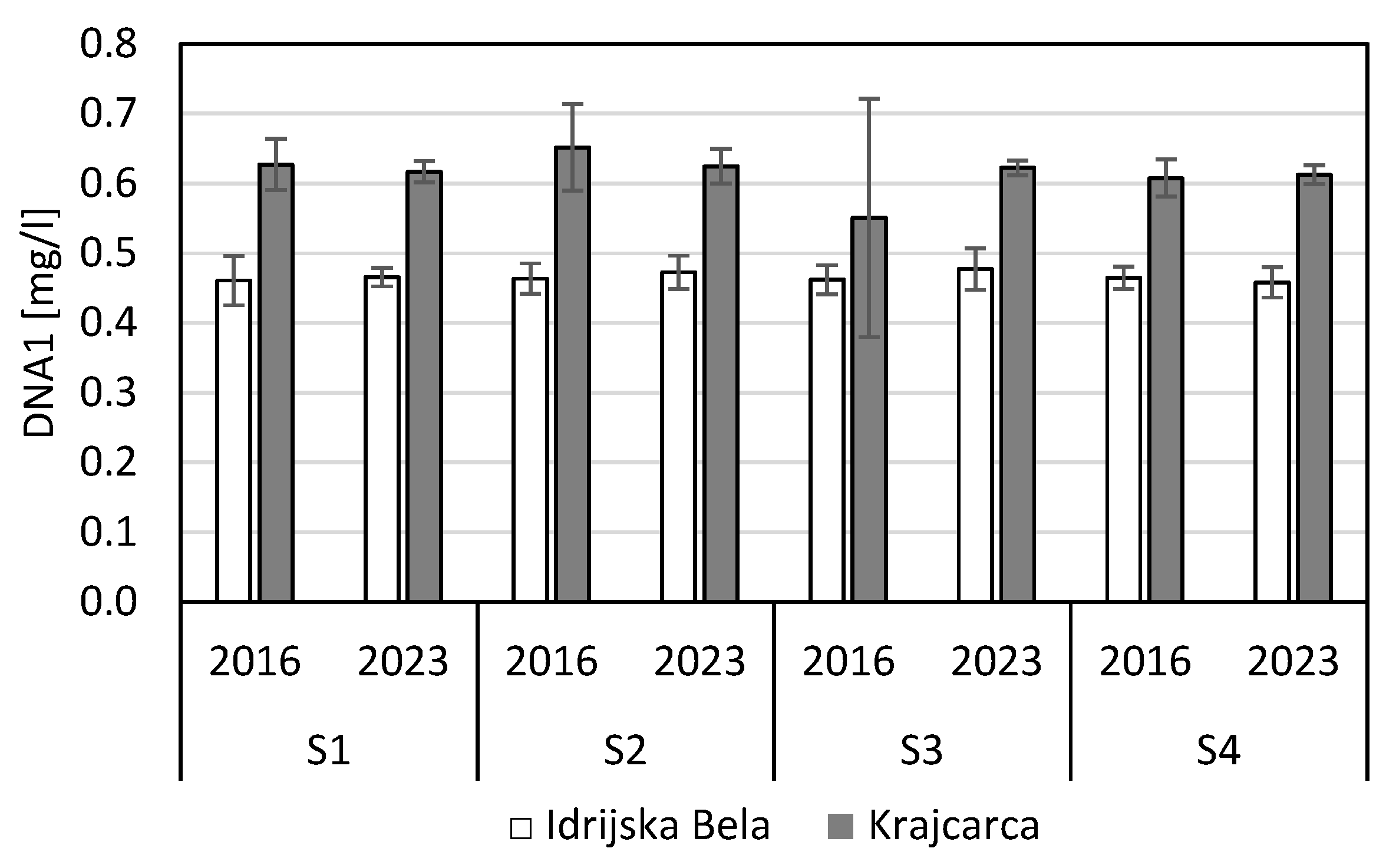
3.4. Microbial Biomass and DNA Concentrations
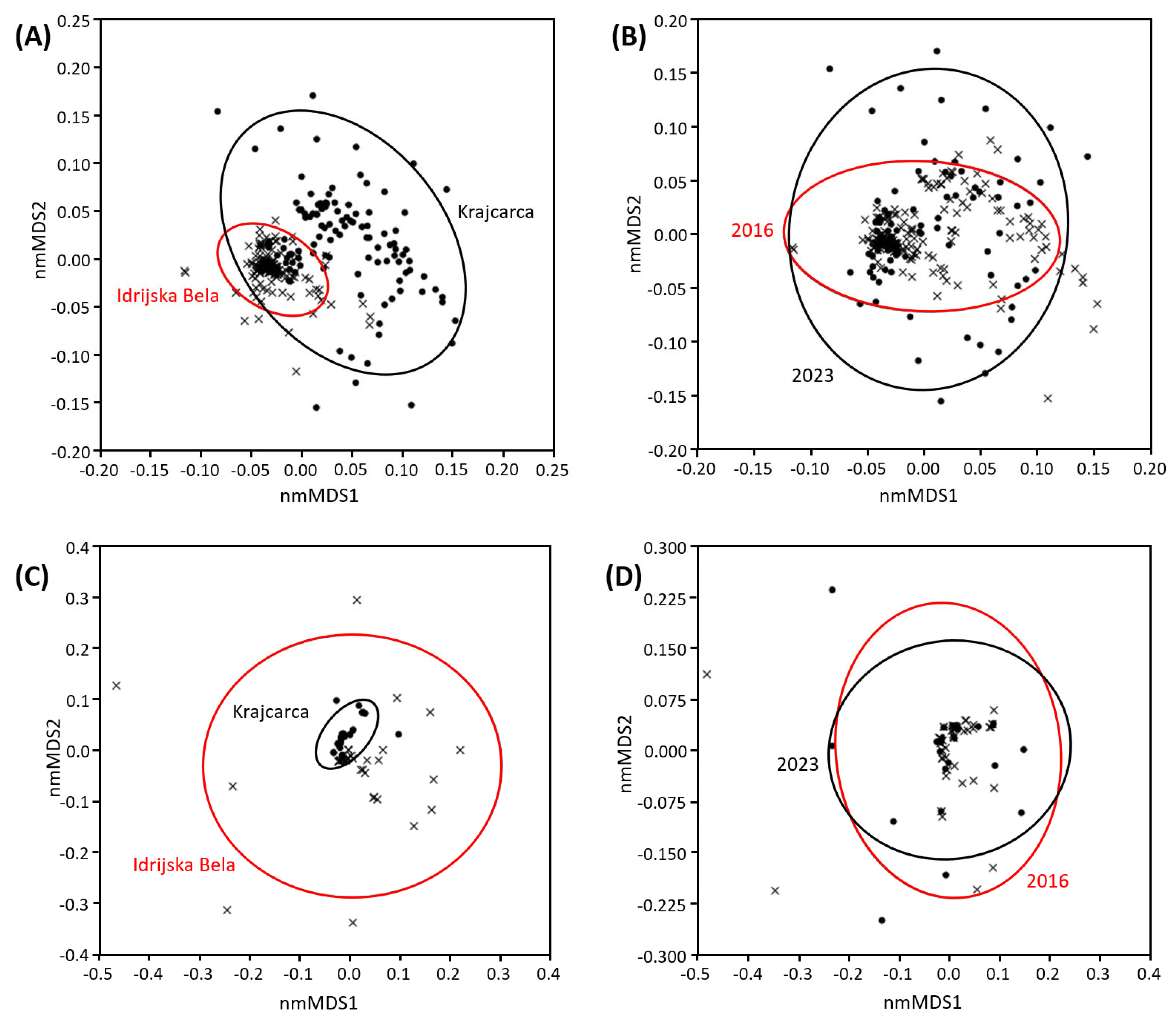
3.5. Differences in Microbiome at Bacterial, Archaeal, and Functional Layers
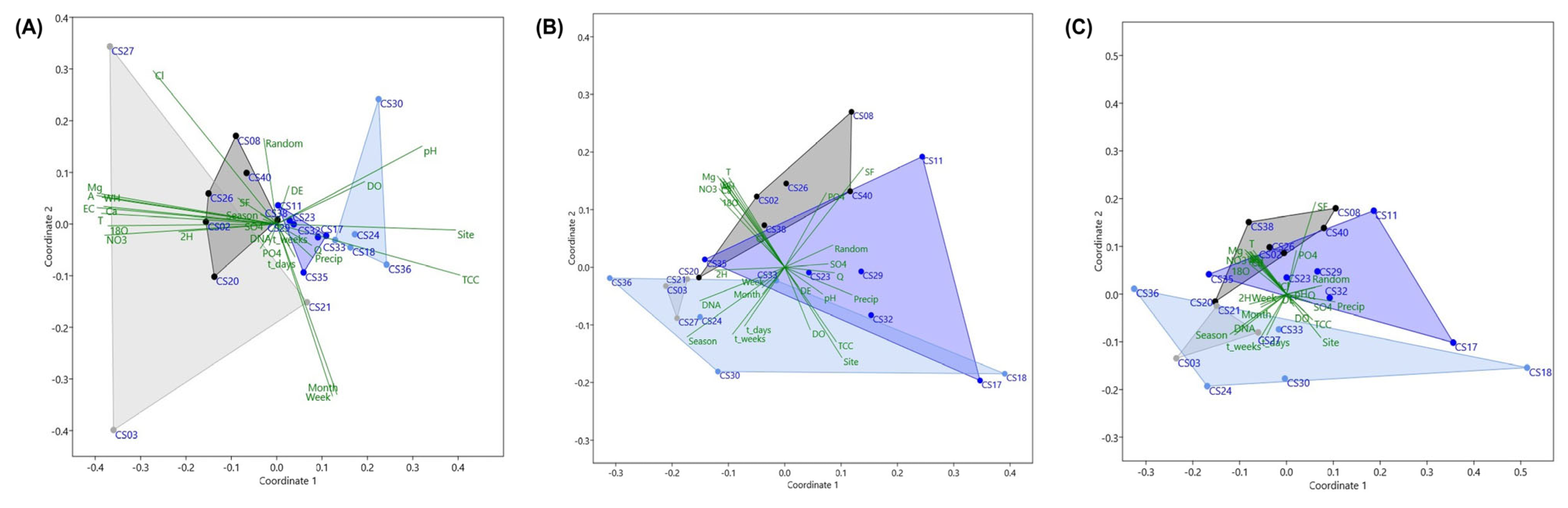
3.6. Influence of Environmental Variables on Bacterial and Archaeal, and Functional Gene Profiles
3.7. Variation Partitioning in Microbial Communities and Functional Potential Explained by Environmental and Temporal Factors
3.8. Comparison of UV-VIS, Indices, and Trace Metal Content Results Between the 2016 and 2023 Seasons
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 16S rRNA | Subunit of ribosomal ribonucleic acid |
| ARSO | Slovenian Environment Agency |
| DOM | Dissolved organic matter |
| eDNA | Environmental DNA |
| IB | Idrijska Bela |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| K | Krajcarca |
| LMWL | Local meteoric water line |
| nmMDS | Non-metric multidimensional scaling |
| PAST | PAleontological Statistics program |
| PERMANOVA | One-way Permutational Multivariate Analysis of Variance |
| RDA | Redundancy analysis |
| SEC | Specific electrical conductivity |
| t-RFLP | Terminal restriction fragment length polymorphism |
| WMS | Whole-metagenome sequencing |
References
- Gleeson, T.; Cuthbert, M.; Ferguson, G.; Perrone, D. Global Groundwater Sustainability, Resources, and Systems in the Anthropocene. Annu. Rev. Earth Planet. Sci. 2020, 48, 431–463. [Google Scholar] [CrossRef]
- Farnleitner, A.H.; Wilhartitz, I.; Ryzinska, G.; Kirschner, A.K.T.; Stadler, H.; Burtscher, M.M.; Hornek, R.; Szewzyk, U.; Herndl, G.; Mach, R.L. Bacterial Dynamics in Spring Water of Alpine Karst Aquifers Indicates the Presence of Stable Autochthonous Microbial Endokarst Communities. Environ. Microbiol. 2005, 7, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Griebler, C.; Lueders, T. Microbial Biodiversity in Groundwater Ecosystems. Freshw. Biol. 2009, 54, 649–677. [Google Scholar] [CrossRef]
- Anantharaman, K.; Brown, C.T.; Hug, L.A.; Sharon, I.; Castelle, C.J.; Probst, A.J.; Thomas, B.C.; Singh, A.; Wilkins, M.J.; Karaoz, U.; et al. Thousands of Microbial Genomes Shed Light on Interconnected Biogeochemical Processes in an Aquifer System. Nat. Commun. 2016, 7, 13219. [Google Scholar] [CrossRef]
- Schwab, V.F.; Herrmann, M.; Roth, V.-N.; Gleixner, G.; Lehmann, R.; Pohnert, G.; Trumbore, S.; Küsel, K.; Totsche, K.U. Functional Diversity of Microbial Communities in Pristine Aquifers Inferred by PLFA- and Sequencing-Based Approaches. Biogeosciences 2017, 14, 2697–2714. [Google Scholar] [CrossRef]
- Xiu, W.; Wu, M.; Nixon, S.L.; Lloyd, J.R.; Bassil, N.M.; Gai, R.; Zhang, T.; Su, Z.; Guo, H. Genome-Resolved Metagenomic Analysis of Groundwater: Insights into Arsenic Mobilization in Biogeochemical Interaction Networks. Environ. Sci. Technol. 2022, 56, 10105–10119. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, T.; Li, Y.; Zheng, X. A Critical Review of the Central Role of Microbial Regulation in the Nitrogen Biogeochemical Process: New Insights for Controlling Groundwater Nitrogen Contamination. J. Environ. Manag. 2023, 328, 116959. [Google Scholar] [CrossRef]
- Zhou, Y.; Kellermann, C.; Griebler, C. Spatio-Temporal Patterns of Microbial Communities in a Hydrologically Dynamic Pristine Aquifer. FEMS Microbiol. Ecol. 2012, 81, 230–242. [Google Scholar] [CrossRef]
- Wilhartitz, I.C.; Kirschner, A.K.T.; Brussaard, C.P.D.; Fischer, U.R.; Wieltschnig, C.; Stadler, H.; Farnleitner, A.H. Dynamics of Natural Prokaryotes, Viruses, and Heterotrophic Nanoflagellates in Alpine Karstic Groundwater. Microbiologyopen 2013, 2, 633–643. [Google Scholar] [CrossRef]
- Savio, D.; Stadler, P.; Reischer, G.H.; Demeter, K.; Linke, R.B.; Blaschke, A.P.; Mach, R.L.; Kirschner, A.K.T.; Stadler, H.; Farnleitner, A.H. Spring Water of an Alpine Karst Aquifer Is Dominated by a Taxonomically Stable but Discharge-Responsive Bacterial Community. Front. Microbiol. 2019, 10, 28. [Google Scholar] [CrossRef]
- Zhong, S.; Zhou, S.; Liu, S.; Wang, J.; Dang, C.; Chen, Q.; Hu, J.; Yang, S.; Deng, C.; Li, W.; et al. May Microbial Ecological Baseline Exist in Continental Groundwater? Microbiome 2023, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Sinreich, M.; Pronk, M.; Kozel, R. Microbiological Monitoring and Classification of Karst Springs. Environ. Earth Sci. 2014, 71, 563–572. [Google Scholar] [CrossRef]
- Hemme, C.L.; Tu, Q.; Shi, Z.; Qin, Y.; Gao, W.; Deng, Y.; Van Nostrand, J.D.; Wu, L.; He, Z.; Chain, P.S.G.; et al. Comparative Metagenomics Reveals Impact of Contaminants on Groundwater Microbiomes. Front. Microbiol. 2015, 6, 1205. [Google Scholar] [CrossRef] [PubMed]
- Hershey, O.S.; Kallmeyer, J.; Wallace, A.; Barton, M.D.; Barton, H.A. High Microbial Diversity Despite Extremely Low Biomass in a Deep Karst Aquifer. Front. Microbiol. 2018, 9, 2823. [Google Scholar] [CrossRef]
- Flynn, T.M.; Sanford, R.A.; Ryu, H.; Bethke, C.M.; Levine, A.D.; Ashbolt, N.J.; Santo Domingo, J.W. Functional Microbial Diversity Explains Groundwater Chemistry in a Pristine Aquifer. BMC Microbiol. 2013, 13, 146. [Google Scholar] [CrossRef]
- Sirisena, K.A.; Daughney, C.J.; Moreau, M.; Sim, D.A.; Lee, C.K.; Cary, S.C.; Ryan, K.G.; Chambers, G.K. Bacterial Bioclusters Relate to Hydrochemistry in New Zealand Groundwater. FEMS Microbiol. Ecol. 2018, 94, fiy170. [Google Scholar] [CrossRef]
- Beyer, A.; Rzanny, M.; Weist, A.; Möller, S.; Burow, K.; Gutmann, F.; Neumann, S.; Lindner, J.; Müsse, S.; Brangsch, H.; et al. Aquifer Community Structure in Dependence of Lithostratigraphy in Groundwater Reservoirs. Environ. Sci. Pollut. Res. 2015, 22, 19342–19351. [Google Scholar] [CrossRef]
- Goldscheider, N.; Hunkeler, D.; Rossi, P. Review: Microbial Biocenoses in Pristine Aquifers and an Assessment of Investigative Methods. Hydrogeol. J. 2006, 14, 926–941. [Google Scholar] [CrossRef]
- Pronk, M.; Goldscheider, N.; Zopfi, J. Microbial Communities in Karst Groundwater and Their Potential Use for Biomonitoring. Hydrogeol. J. 2009, 17, 37–48. [Google Scholar] [CrossRef]
- Vierheilig, J.; Savio, D.; Ley, R.E.; Mach, R.L.; Farnleitner, A.H.; Reischer, G.H. Potential Applications of next Generation DNA Sequencing of 16S RRNA Gene Amplicons in Microbial Water Quality Monitoring. Water Sci. Technol. 2015, 72, 1962–1972. [Google Scholar] [CrossRef]
- Chik, A.H.S.; Emelko, M.B.; Anderson, W.B.; O’Sullivan, K.E.; Savio, D.; Farnleitner, A.H.; Blaschke, A.P.; Schijven, J.F. Evaluation of Groundwater Bacterial Community Composition to Inform Waterborne Pathogen Vulnerability Assessments. Sci. Total Environ. 2020, 743, 140472. [Google Scholar] [CrossRef] [PubMed]
- Demeter, K.; Linke, R.; Ballesté, E.; Reischer, G.; Mayer, R.E.; Vierheilig, J.; Kolm, C.; Stevenson, M.E.; Derx, J.; Kirschner, A.K.T.; et al. Have Genetic Targets for Faecal Pollution Diagnostics and Source Tracking Revolutionized Water Quality Analysis Yet? FEMS Microbiol. Rev. 2023, 47, fuad028. [Google Scholar] [CrossRef] [PubMed]
- Savio, D.; Stadler, P.; Reischer, G.H.; Kirschner, A.K.T.; Demeter, K.; Linke, R.; Blaschke, A.P.; Sommer, R.; Szewzyk, U.; Wilhartitz, I.C.; et al. Opening the Black Box of Spring Water Microbiology from Alpine Karst Aquifers to Support Proactive Drinking Water Resource Management. WIREs Water 2018, 5, e1282. [Google Scholar] [CrossRef] [PubMed]
- Stres, B.; Kronegger, L. Shift in the Paradigm towards Next-Generation Microbiology. FEMS Microbiol. Lett. 2019, 366, fnz159. [Google Scholar] [CrossRef]
- Quince, C.; Walker, A.W.; Simpson, J.T.; Loman, N.J.; Segata, N. Shotgun Metagenomics, from Sampling to Analysis. Nat. Biotechnol. 2017, 35, 833–844. [Google Scholar] [CrossRef]
- Barbosa, F.A.S.; Brait, L.A.S.; Coutinho, F.H.; Ferreira, C.M.; Moreira, E.F.; de Queiroz Salles, L.; Meirelles, P.M. Ecological Landscape Explains Aquifers Microbial Structure. Sci. Total Environ. 2023, 862, 160822. [Google Scholar] [CrossRef]
- Pašić, L.; Kovče, B.; Sket, B.; Herzog-Velikonja, B. Diversity of Microbial Communities Colonizing the Walls of a Karstic Cave in Slovenia. FEMS Microbiol. Ecol. 2009, 71, 50–60. [Google Scholar] [CrossRef]
- Mulec, J.; Skok, S.; Pašić, L. Low Bacterial Diversity and Nitrate Levels in Cores from Deep Boreholes in Pristine Karst. Life 2024, 14, 677. [Google Scholar] [CrossRef]
- Opalički Slabe, M.; Danevčič, T.; Hug, K.; Fillinger, L.; Mandić-Mulec, I.; Griebler, C.; Brancelj, A. Key Drivers of Microbial Abundance, Activity, and Diversity in Karst Spring Waters across an Altitudinal Gradient in Slovenia. Aquat. Microb. Ecol. 2021, 86, 99–114. [Google Scholar] [CrossRef]
- Kanduč, T. Hydrogeochemical Characteristics of the River Idrijca (Slovenia). Geologija 2008, 51, 39–49. [Google Scholar] [CrossRef]
- Kanduč, T.; Kocman, D.; Ogrinc, N. Hydrogeochemical and Stable Isotope Characteristics of the River Idrijca (Slovenia), the Boundary Watershed Between the Adriatic and Black Seas. Aquat. Geochem. 2008, 14, 239–262. [Google Scholar] [CrossRef]
- Baptista-Salazar, C.; Biester, H. The Role of Hydrological Conditions for Riverine Hg Species Transport in the Idrija Mining Area. Environ. Pollut. 2019, 247, 716–724. [Google Scholar] [CrossRef]
- Škerjanec, M.; Atanasova, N.; Žagar, D.; Novak, G. Idrijca and Soča/Isonzo River Discharges Estimation for Modelling Mercury Pollution. Acta Hydrotech. 2024, 37, 153–171. [Google Scholar] [CrossRef]
- Buser, S. Tolmač Lista Gorica: L 33-78: Socialistična Federativna Republika Jugoslavija, Osnovna Geološka Karta, 1:100 000; Zvezni geološki zavod: Beograd, Yugoslavia, 1973. [Google Scholar]
- Mlakar, I.; Čar, J. Geološka Karta Idrijsko-Cerkljanskega Hribovja Med Stopnikom in Rovtami 1:25.000 [Kartografsko Gradivo] = Geological Map of the Idrija—Cerkno Hills Between Stopnik and Rovte 1:25.000; Geološki zavod Slovenije: Ljubljana, Slovenia, 2009. [Google Scholar]
- Čar, J.; Placer, L. The Middle Triassic Structure of the Idrija Region. Geologija 1977, 20, 141–166. [Google Scholar]
- Mlakar, I. Nappe Structure of the Idrija-Žiri Region. Geologija 1969, 12, 5–72. [Google Scholar]
- Čar, J.; Šegina, E. Geologic Structure and Origin of the Zadlog Karst Polje. Geologija 2024, 67, 249–271. [Google Scholar] [CrossRef]
- Gosar, A. Measurements of Tectonic Micro-Displacements within the Idrija Fault Zone in the Učja Valley (W Slovenia). Acta Geogr. Slov. 2020, 60, 79–93. [Google Scholar] [CrossRef]
- Placer, L.; Popit, T.; Rižnar, I. Tectonics and Gravitational Phenomena, Part Two: The Trnovski Gozd-Banjšice-Šentviška Gora Degraded Plain. Geologija 2024, 67, 129–156. [Google Scholar] [CrossRef]
- Janež, J. Karst Springs in the Upper Soča Valley. Geologija 2002, 45, 393–400. [Google Scholar] [CrossRef]
- Jurkovšek, B. Tolmač Listov Beljak in Ponteba: L33-51: L33-52: Socialistična Federativna Republika Jugoslavija, Osnovna Geološka Karta, 1:100 000; Jurkovšek, B., Buser, S., Čakalo, M., Ferjančić, L., Poljak, M., Ramovš, A., Stojanović, B., Toman, M., Eds.; Zvezni Geološki Zavod: Beograd, Yugoslavia, 1987. [Google Scholar]
- Zupan Hajna, N. Explanatory Book to the Map: Geological Structure of the Idrija-Cerkno Hills. Acta Carsologica 2010, 39. The map and book are available from the Geological Survey of Slovenia (http://www.geo-zs.si/). [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Stres, B.; Sul, W.J.; Murovec, B.; Tiedje, J.M. Recently Deglaciated High-Altitude Soils of the Himalaya: Diverse Environments, Heterogenous Bacterial Communities and Long-Range Dust Inputs from the Upper Troposphere. PLoS ONE 2013, 8, e76440. [Google Scholar] [CrossRef]
- Stres, B.; Philippot, L.; Faganeli, J.; Tiedje, J.M. Frequent Freeze—Thaw Cycles Yield Diminished yet Resistant and Responsive Microbial Communities in Two Temperate Soils: A Laboratory Experiment. FEMS Microbiol. Ecol. 2010, 74, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Erratum: Absorption Spectral Slopes and Slope Ratios as Indicators of Molecular Weight, Source, and Photobleaching of Chromophoric Dissolved Organic Matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Repinc, S.K.; Bizjan, B.; Budhiraja, V.; Dular, M.; Gostiša, J.; Brajer Humar, B.; Kaurin, A.; Kržan, A.; Levstek, M.; Arteaga, J.F.M.; et al. Integral Analysis of Hydrodynamic Cavitation Effects on Waste Activated Sludge Characteristics, Potentially Toxic Metals, Microorganisms and Identification of Microplastics. Sci. Total Environ. 2022, 806, 151414. [Google Scholar] [CrossRef]
- Zupanc, M.; Humar, B.B.; Dular, M.; Gostiša, J.; Hočevar, M.; Repinc, S.K.; Krzyk, M.; Novak, L.; Ortar, J.; Pandur, Ž.; et al. The Use of Hydrodynamic Cavitation for Waste-to-Energy Approach to Enhance Methane Production from Waste Activated Sludge. J. Environ. Manag. 2023, 347, 119074. [Google Scholar] [CrossRef]
- Blagojevič, M.; Zupanc, M.; Gostiša, J.; Stres, B.; Šmid, A.; Dular, M.; Slemenik Perše, L.; Gradišar Centa, U.; Bizjan, B.; Rak, G.; et al. The Impact of Radicals on Physicochemical Properties of Waste Activated Sludge during Hydrodynamic Cavitation Treatment. Ultrason. Sonochem. 2025, 115, 107291. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Mulec, J.; Oarga-Mulec, A.; Holko, L.; Pašić, L.; Kopitar, A.N.; Eleršek, T.; Mihevc, A. Microbiota Entrapped in Recently-Formed Ice: Paradana Ice Cave, Slovenia. Sci. Rep. 2021, 11, 1993. [Google Scholar] [CrossRef]
- Meyer, F.; Paarmann, D.; D’Souza, M.; Olson, R.; Glass, E.M.; Kubal, M.; Paczian, T.; Rodriguez, A.; Stevens, R.; Wilke, A.; et al. The Metagenomics RAST Server—A Public Resource for the Automatic Phylogenetic and Functional Analysis of Metagenomes. BMC Bioinform. 2008, 9, 386. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the Spatial Component of Ecological Variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Legendre, P.; Dray, S.; Borcard, D. Variation Partitioning of Species Data Matrices: Estimation and Comparison of Fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef] [PubMed]
- Kanduč, T.; Mori, N.; Kocman, D.; Stibilj, V.; Grassa, F. Hydrogeochemistry of Alpine Springs from North Slovenia: Insights from Stable Isotopes. Chem. Geol. 2012, 300–301, 40–54. [Google Scholar] [CrossRef]
- Menció, A.; Boy, M.; Mas-Pla, J. Analysis of Vulnerability Factors That Control Nitrate Occurrence in Natural Springs (Osona Region, NE Spain). Sci. Total Environ. 2011, 409, 3049–3058. [Google Scholar] [CrossRef]
- Shuster, E.T.; White, W.B. Seasonal Fluctuations in the Chemistry of Lime-Stone Springs: A Possible Means for Characterizing Carbonate Aquifers. J. Hydrol. 1971, 14, 93–128. [Google Scholar] [CrossRef]
- Pronk, M.; Goldscheider, N.; Zopfi, J. Dynamics and Interaction of Organic Carbon, Turbidity and Bacteria in a Karst Aquifer System. Hydrogeol. J. 2006, 14, 473–484. [Google Scholar] [CrossRef]
- Valentine, D.L. Adaptations to Energy Stress Dictate the Ecology and Evolution of the Archaea. Nat. Rev. Microbiol. 2007, 5, 316–323. [Google Scholar] [CrossRef]
- Stan-Lotter, H.; Fendrihan, S. Halophilic Archaea: Life with Desiccation, Radiation and Oligotrophy over Geological Times. Life 2015, 5, 1487–1496. [Google Scholar] [CrossRef]
- Jelen, M.; Mikoš, M.; Bezak, N. Karst Springs in Slovenia: Trend Analysis. Acta Hydrotech. 2020, 33, 1–12. [Google Scholar] [CrossRef]
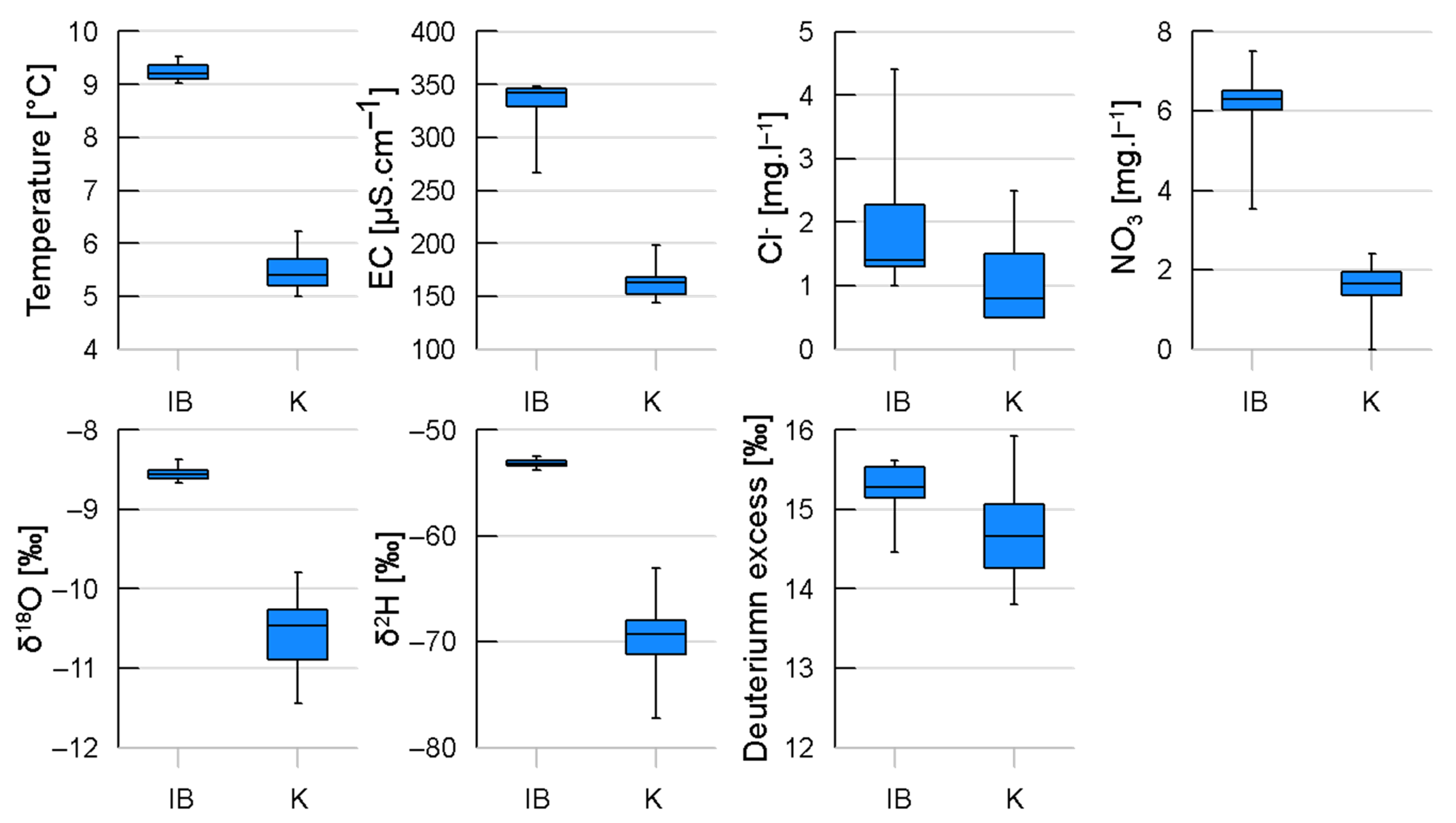
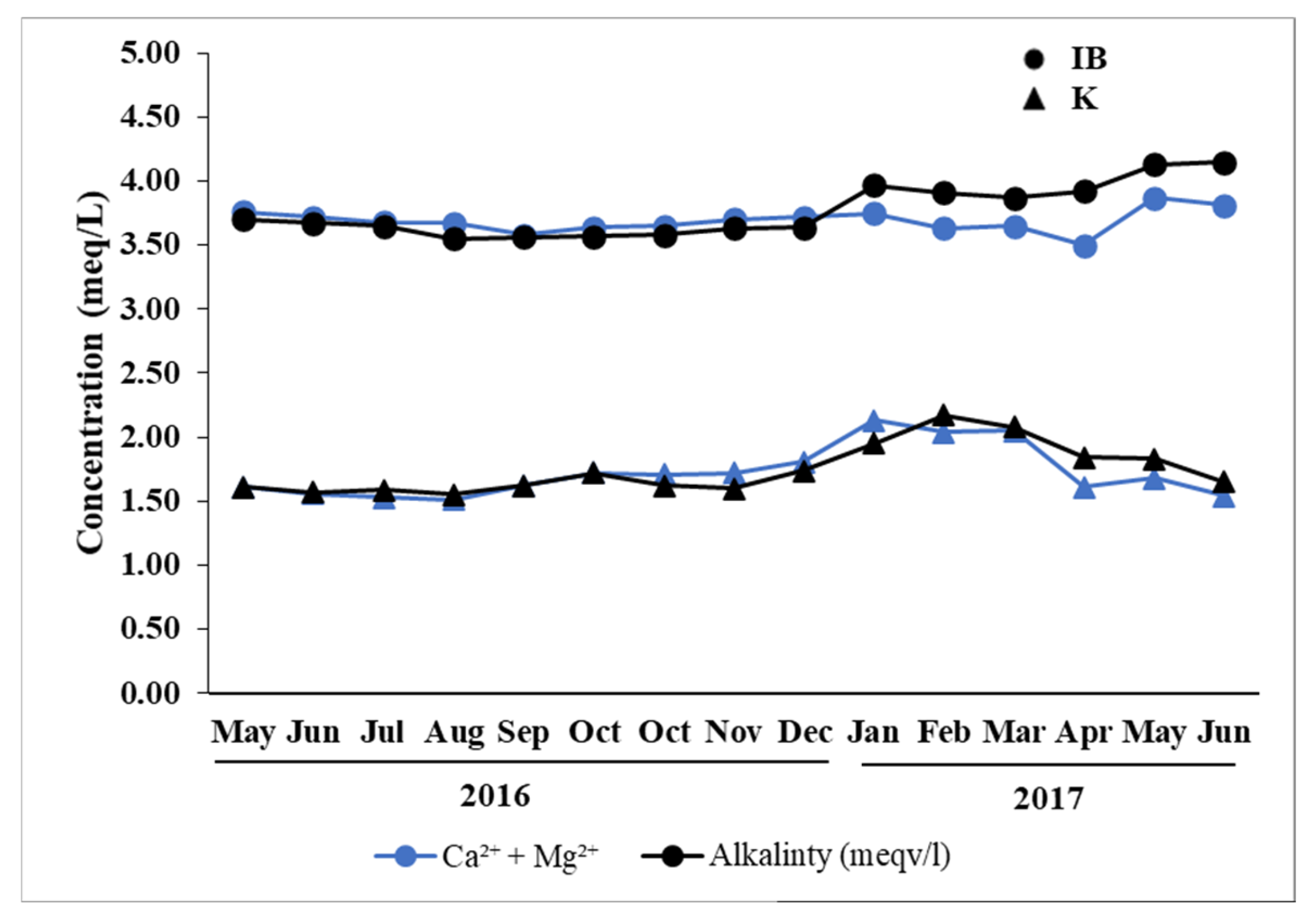
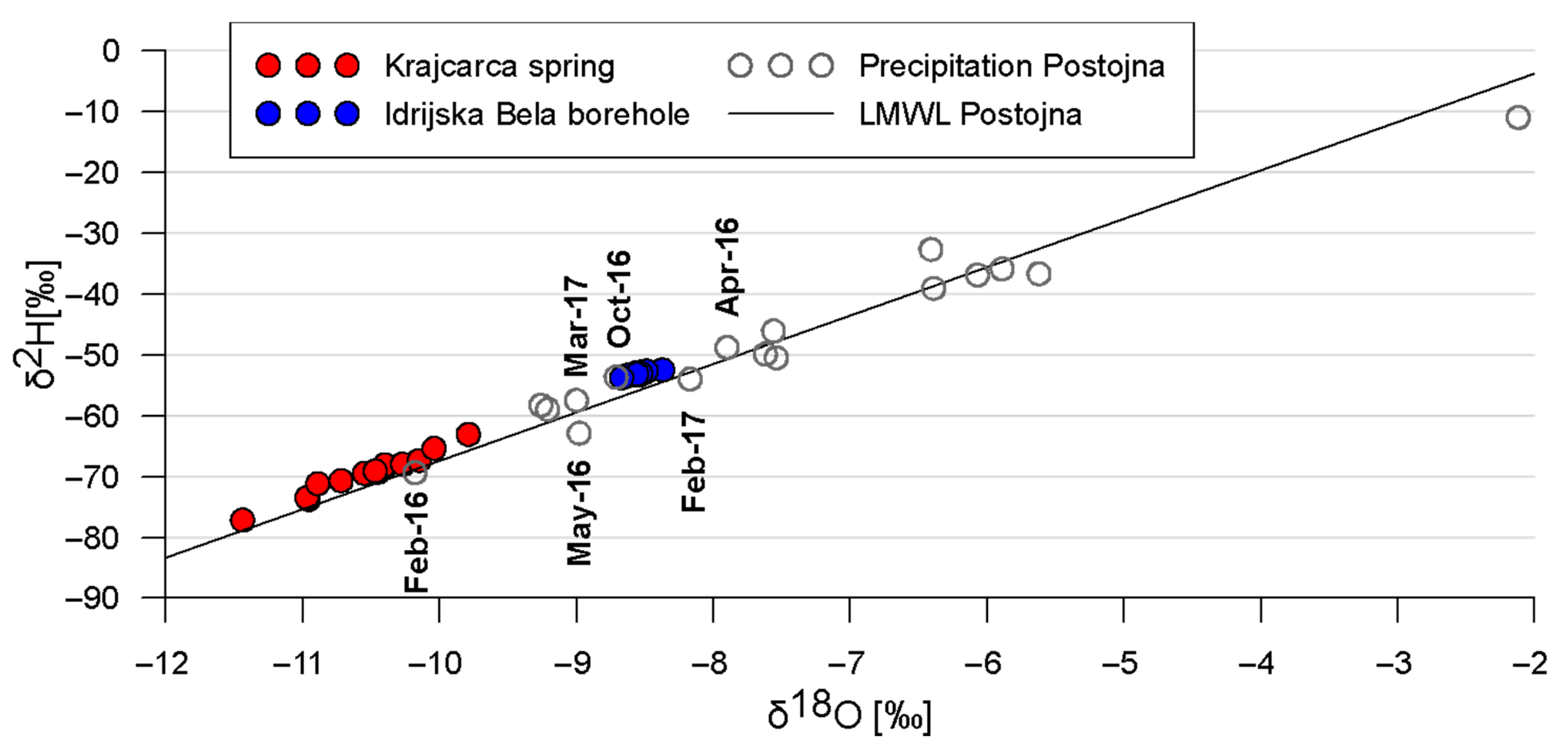
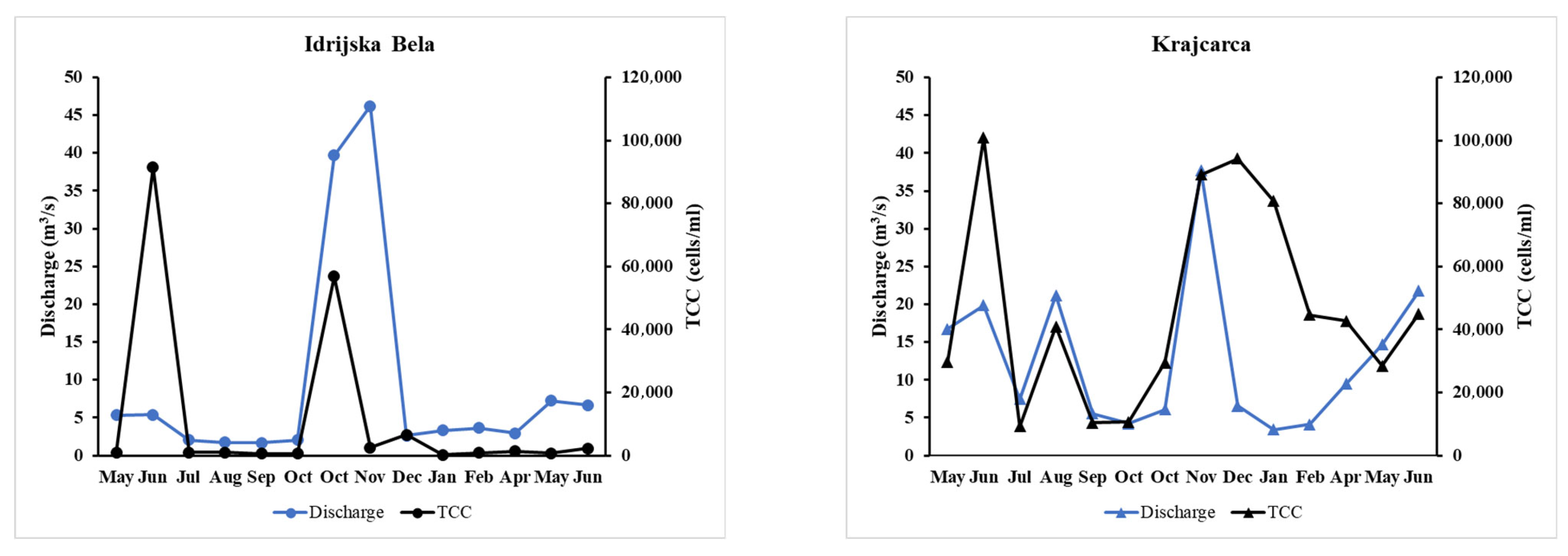

| Parameter (Unit) | IB | K | ||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| Temperature (°C) | 9.2 | 9.0–9.5 | 5.4 | 5.0–6.2 |
| pH | 7.8 | 7.6–8.0 | 8.2 | 7.9–9.0 |
| Electrical conductivity (µS cm−1) | 335 | * (267) 326–348 | 164 | 149–198 |
| Dissolved oxygen (mg L−1) | 11.3 | 10.5–12.9 | 12.0 | 11.7–13.0 |
| Ca (mg L−1) | 43.0 | 38.9–48.7 (64.3) * | 27.7 | 26.1–31.9 |
| Mg (mg L−1) | 18.7 | * (3.5) 16.8–21.3 | 4.2 | 1.7–8.2 |
| Water hardness (CaCO3 mg L−1) | 184.6 | 175.2–193.7 | 86.2 | 75.6–106.6 |
| Alkalinity (CaCO3 mg L−1) | 188.5 | 177.7–207.7 | 87.2 | 77.6–108.6 |
| Cl (mg L−1) | 2.0 | 1.0–4.4 | 1.1 | 0.5–2.5 |
| NO3 (mg L−1) | 6.1 | 3.5–7.5 | 1.6 | 0–2.4 |
| SO42− (mg L−1) | 0.9 | 0–2.6 | 1.0 | 0–3.3 |
| PO43− (mg L−1) | 0.04 | 0–0.02 (0.5) * | 0.01 | 0–0.05 |
| δ18O (‰) | −8.55 | −8.67–−8.37 | −10.53 | −11.44–−9.79 |
| δ2H (‰) | −53.15 | −53.8–−52.5 | −69.6 | −77.2–−63.1 |
| Deuterium excess (‰) | 15.27 | 14.46–15.62 | 14.65 | 13.80–15.92 |
| Community/Function | Level | Adjusted Explained Variation (%) | Key Environmental Drivers |
|---|---|---|---|
| Archaea | Species | 24.2 | * A, WH, Ca2+, Mg2+, SEC, T, Site |
| Genus | 21.2 | Same | |
| Family | 29.4 | Same | |
| Bacteria | Species | 57.0 | Season, SF, Mg2+, WH, A, SEC, NO3, Site, T, DNA, Ca2+, 18O |
| Genus | 67.2 | Same | |
| Family | 70.8 | Same except SF | |
| Functional Potential | Subsystems Function | 46.4 | Mg2+. WH, A, SEC, SF, Season, Site, Ca2+, NO3, T, 18O |
| Subsystems Level 3 | 41.7 | Same | |
| Subsystems Level 2 | 45.0 | Same |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Likar, M.; Blagojevič, M.; Ošlak, M.; Mikoš, M.; Prevoršek, Z.; Holko, L.; Ribič, D.; Likozar, B.; Novak, U.; Murovec, B.; et al. Microbiome and Chemistry Insights into Two Oligotrophic Karst Water Springs in Slovenia from 2016 and 2023 Perspectives. Water 2025, 17, 2402. https://doi.org/10.3390/w17162402
Likar M, Blagojevič M, Ošlak M, Mikoš M, Prevoršek Z, Holko L, Ribič D, Likozar B, Novak U, Murovec B, et al. Microbiome and Chemistry Insights into Two Oligotrophic Karst Water Springs in Slovenia from 2016 and 2023 Perspectives. Water. 2025; 17(16):2402. https://doi.org/10.3390/w17162402
Chicago/Turabian StyleLikar, Mojca, Marko Blagojevič, Maša Ošlak, Matjaž Mikoš, Zala Prevoršek, Ladislav Holko, Dragana Ribič, Blaž Likozar, Uroš Novak, Boštjan Murovec, and et al. 2025. "Microbiome and Chemistry Insights into Two Oligotrophic Karst Water Springs in Slovenia from 2016 and 2023 Perspectives" Water 17, no. 16: 2402. https://doi.org/10.3390/w17162402
APA StyleLikar, M., Blagojevič, M., Ošlak, M., Mikoš, M., Prevoršek, Z., Holko, L., Ribič, D., Likozar, B., Novak, U., Murovec, B., Kolbl Repinc, S., & Stres, B. (2025). Microbiome and Chemistry Insights into Two Oligotrophic Karst Water Springs in Slovenia from 2016 and 2023 Perspectives. Water, 17(16), 2402. https://doi.org/10.3390/w17162402












