Scale and Reasons for Changes in Chemical Composition of Waters During the Spring Freshet on Kolyma River, Arctic Siberia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
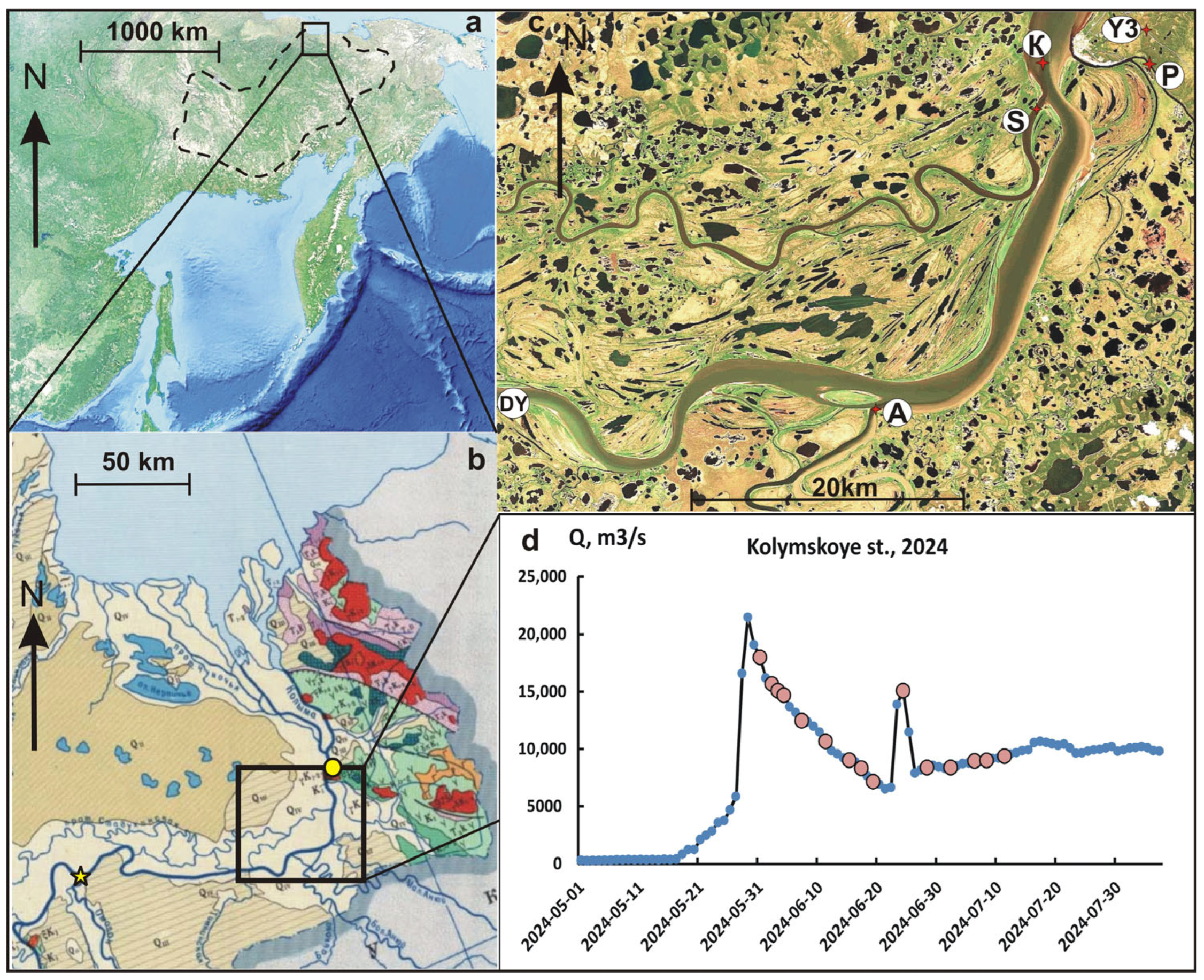
2.2. Sampling, Processing, and Analysis
3. Results
3.1. General Hydrochemical Parameters
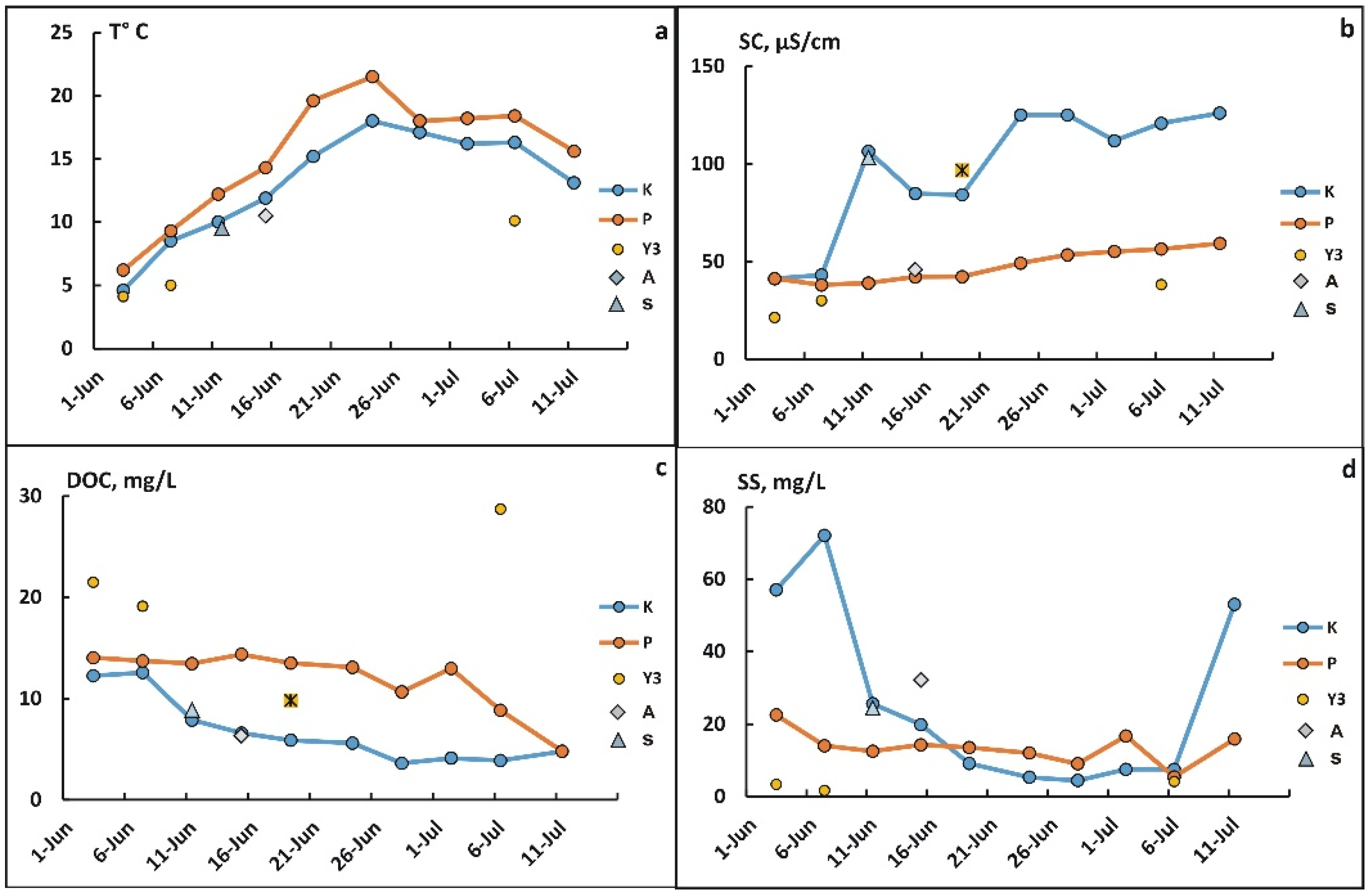
3.2. Content of Coarse Colloids and Suspended Matter According to DLS Intensity Data
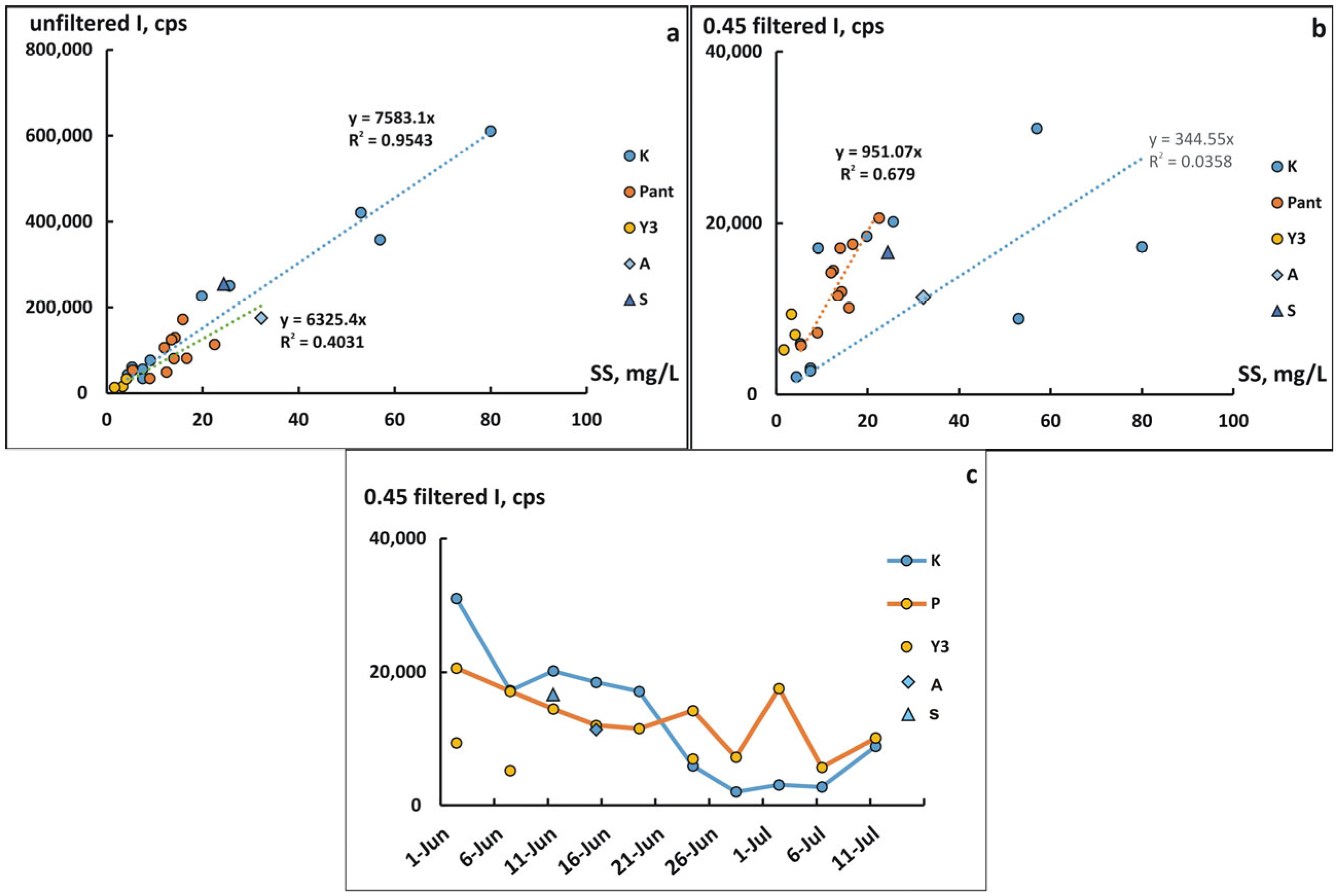
3.3. Concentration of Elements in 0.45 µm Filtrates Obtained by Different Methods
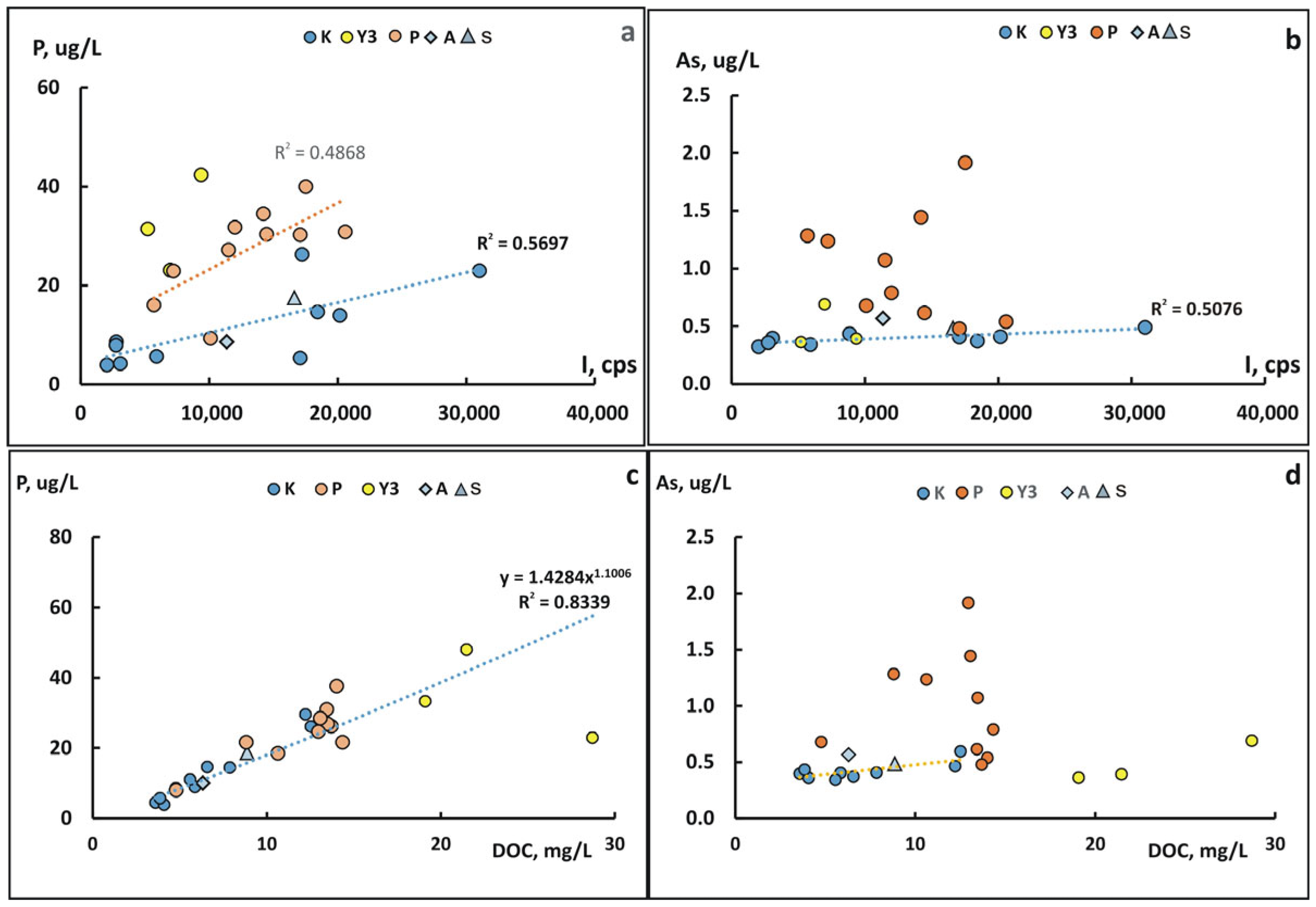
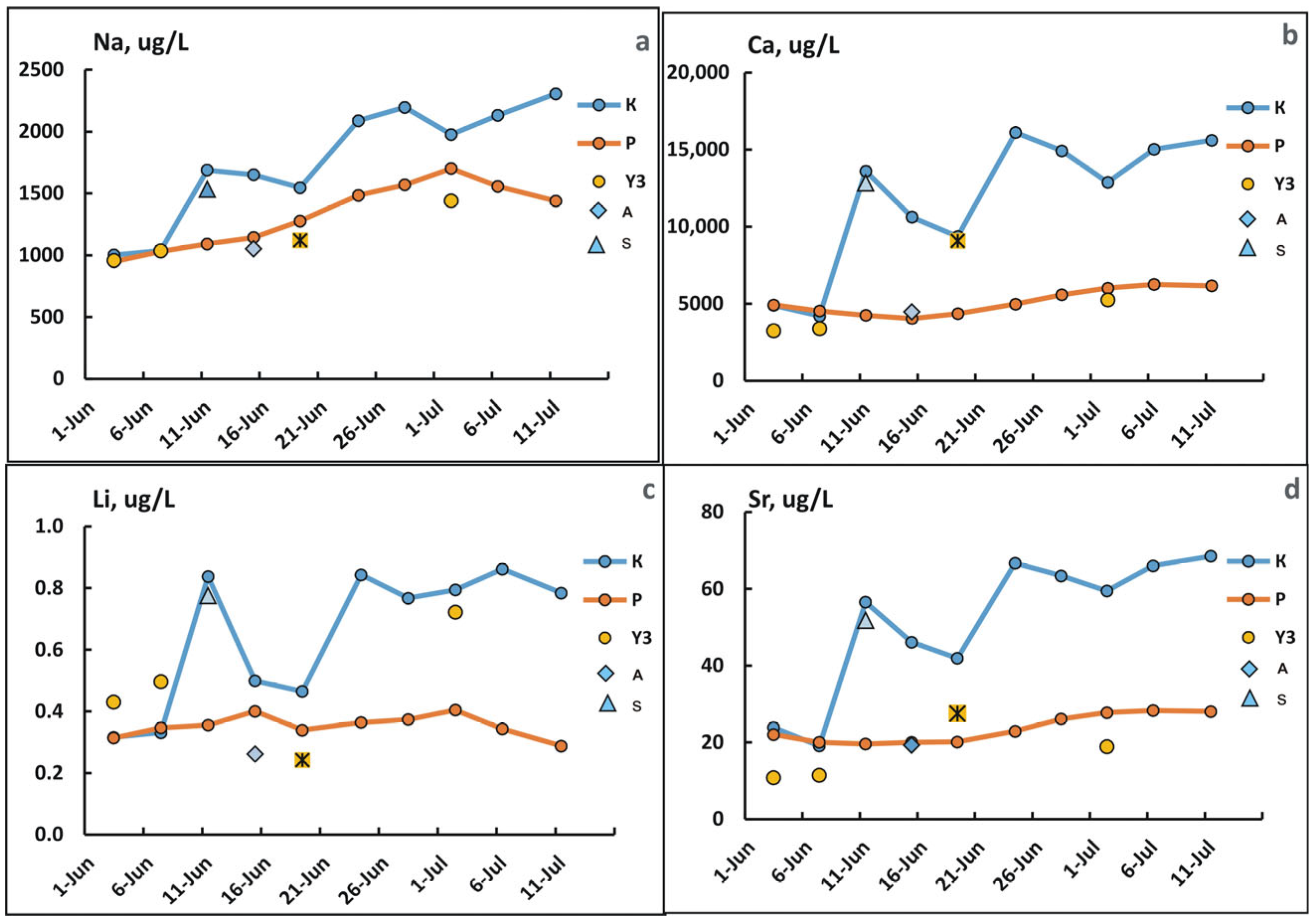
3.4. Changes in the Concentration of Chemical Elements in Fractions <0.45 µm During Freshet
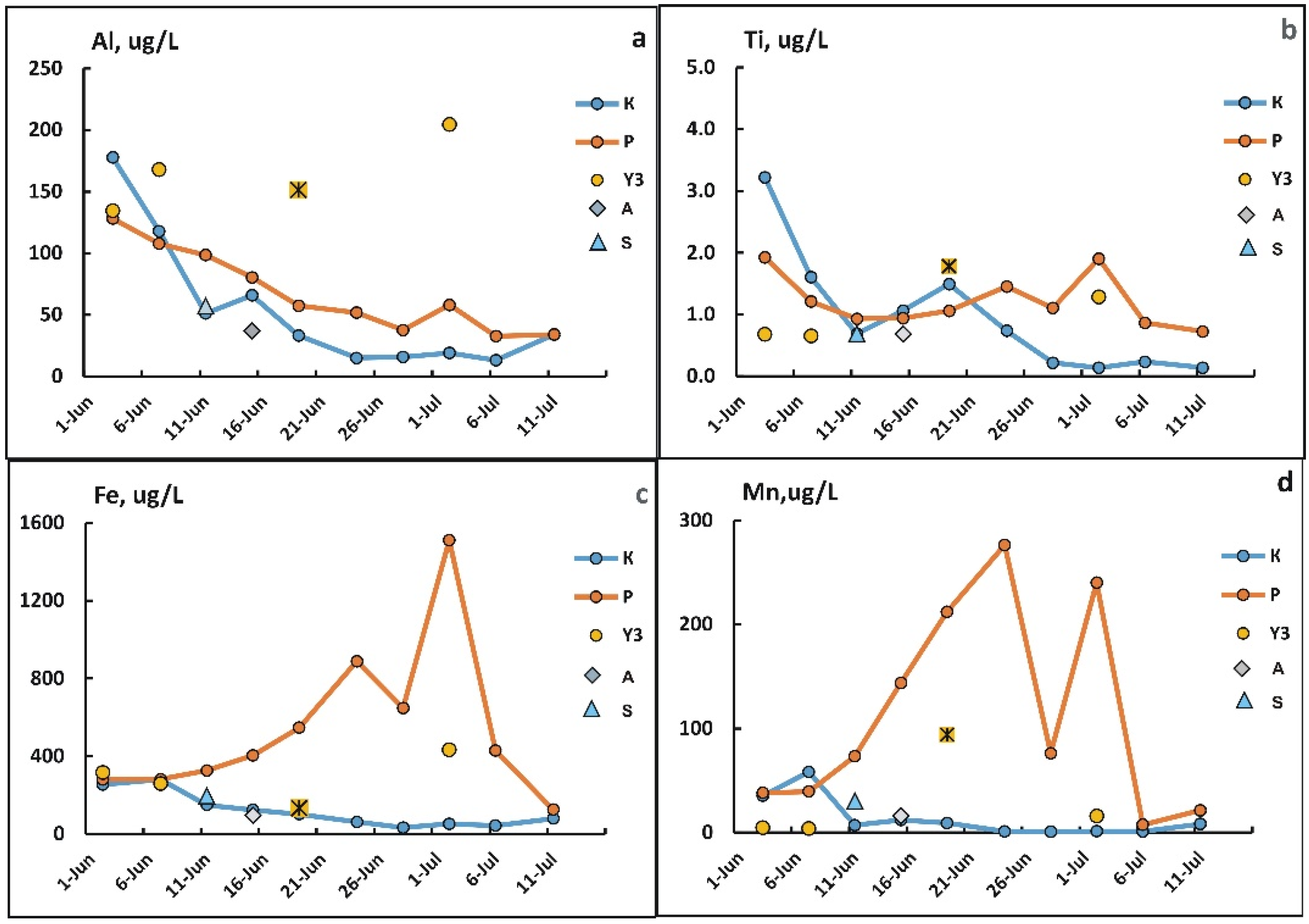
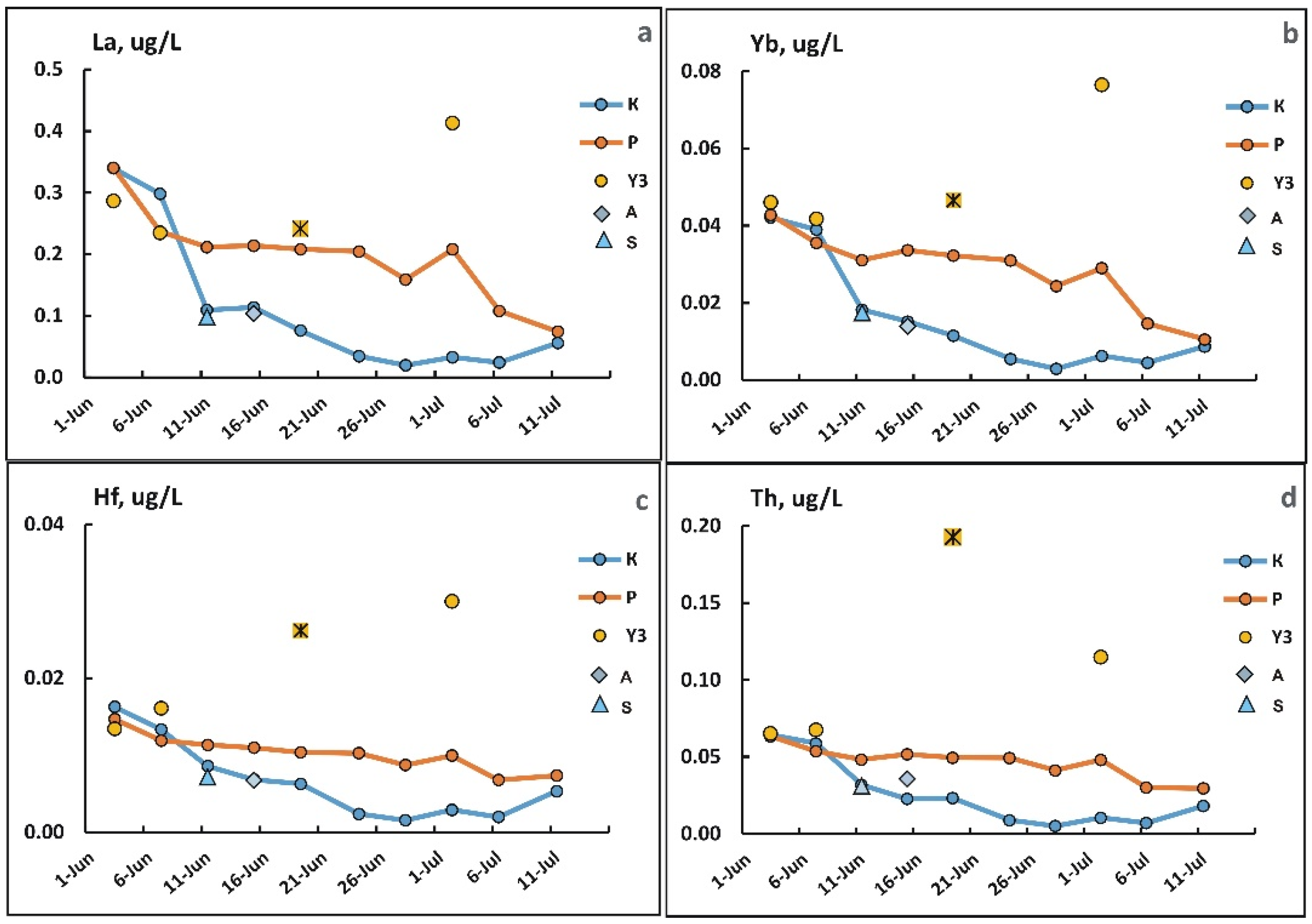
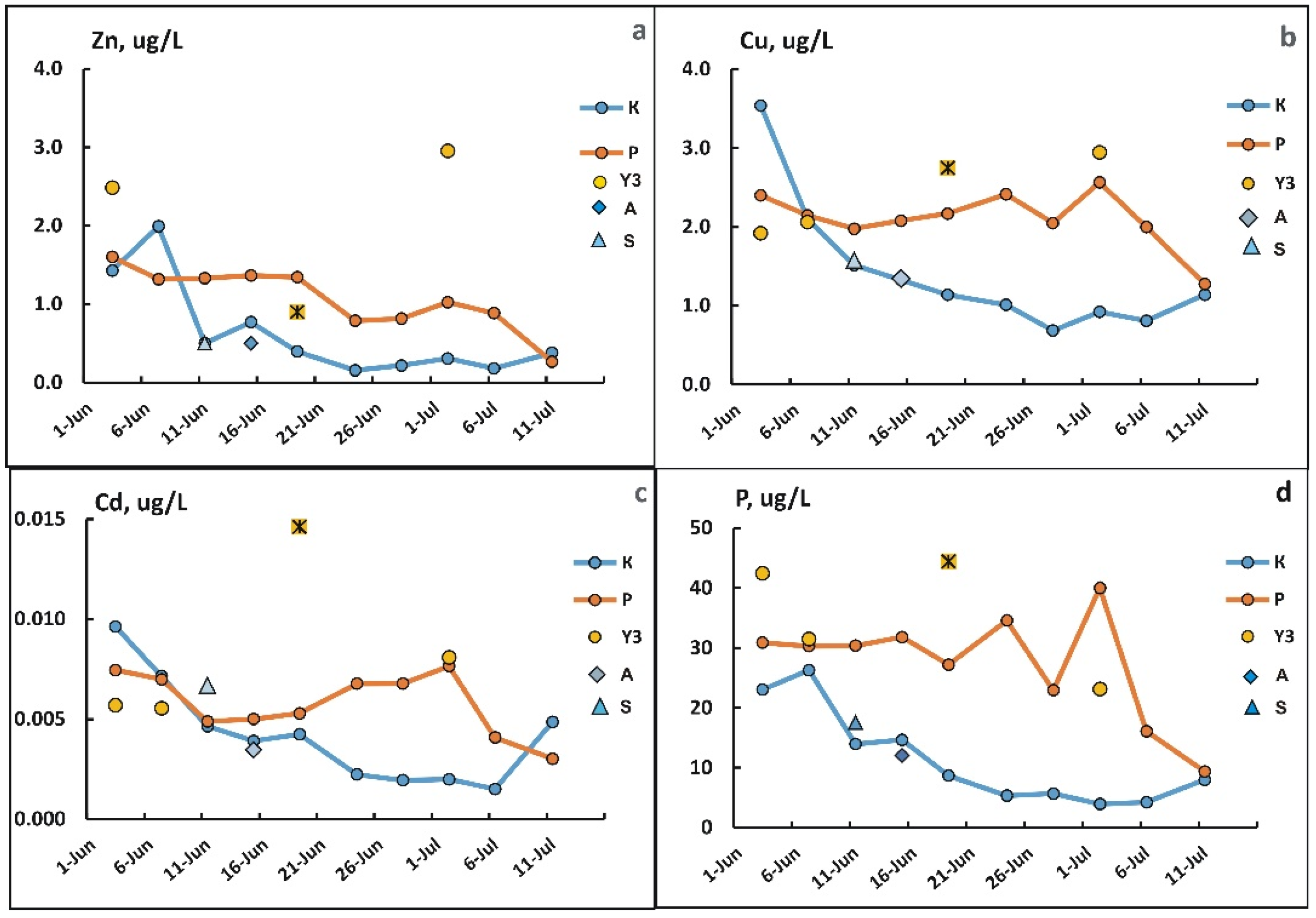
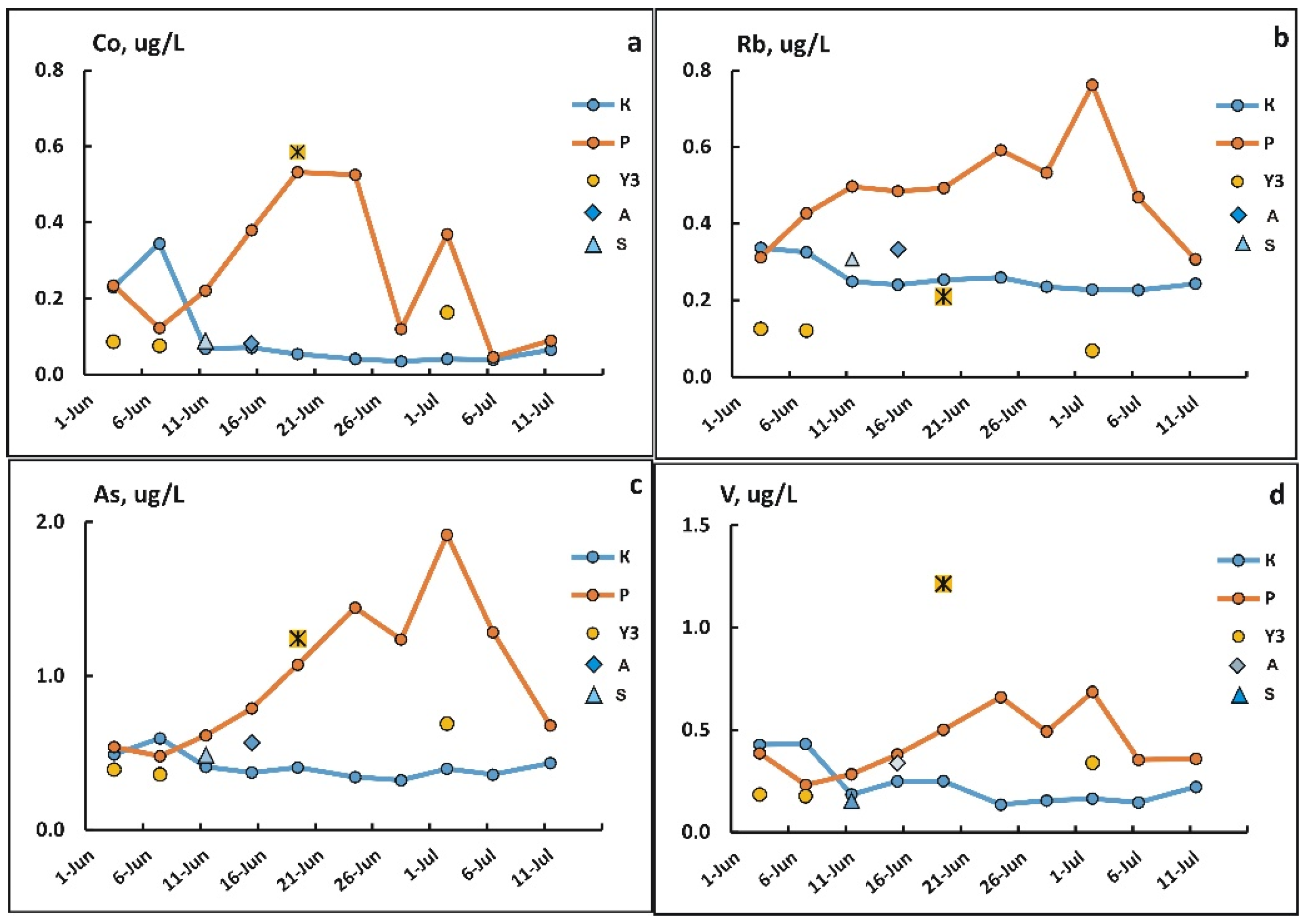
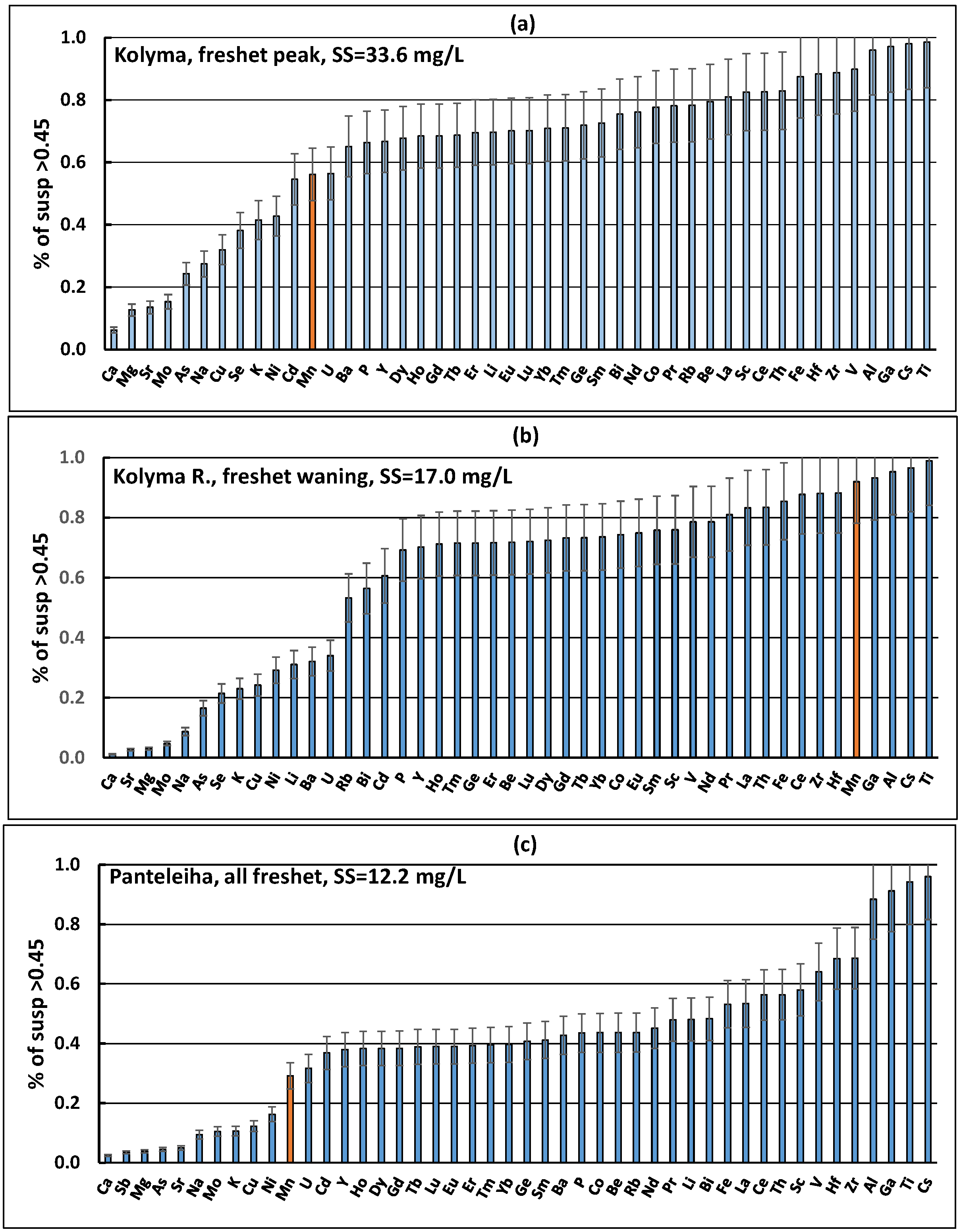
3.5. Changes in Concentrations of Elements in the Suspended Solids
4. Discussion
4.1. Features of the 2024 Freshet Compared to the Water Regimes of 2014 and 2015
4.2. Causes of Changes in the Chemical Composition of Waters in the Lower Reaches of the Kolyma River During Freshet
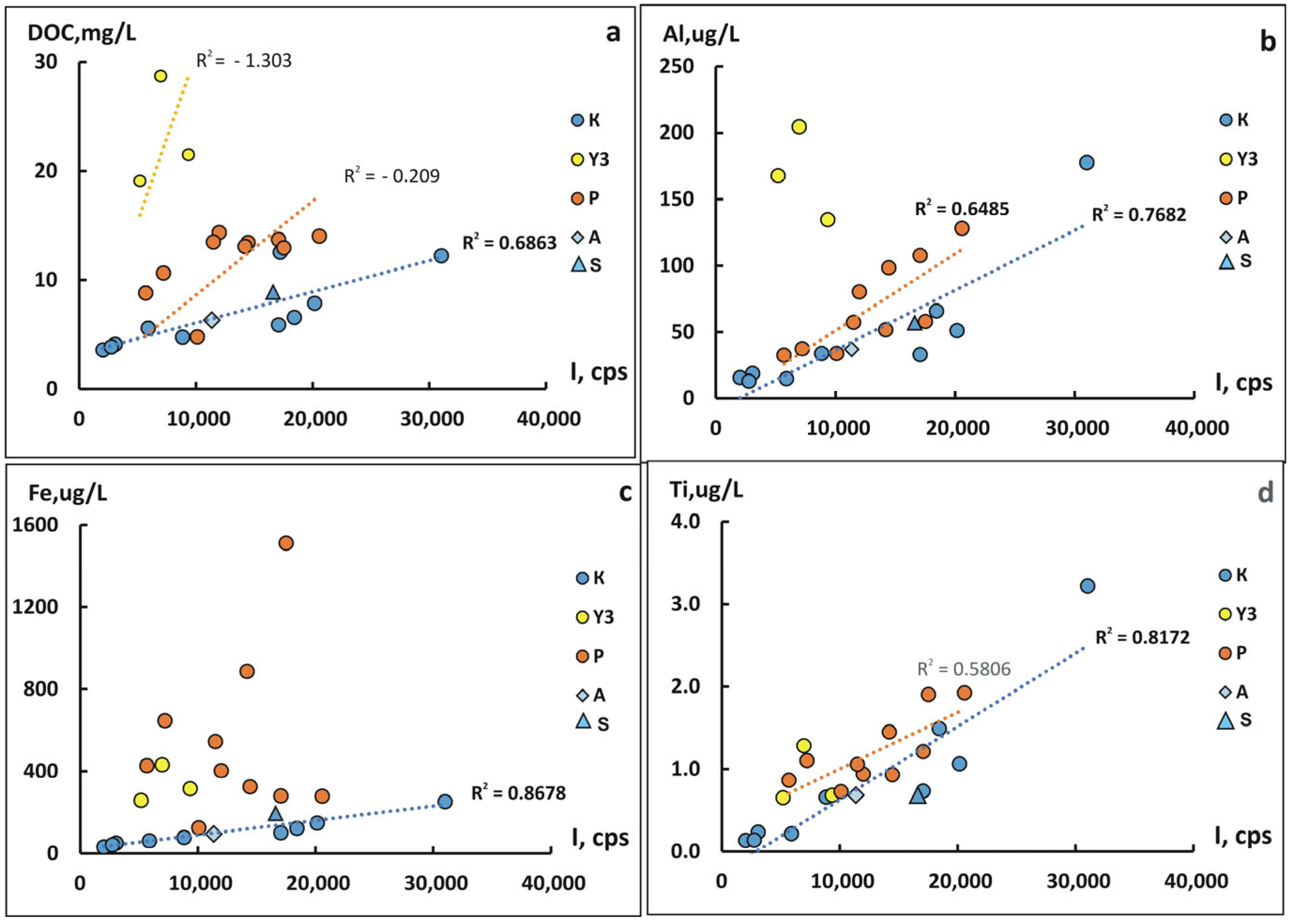
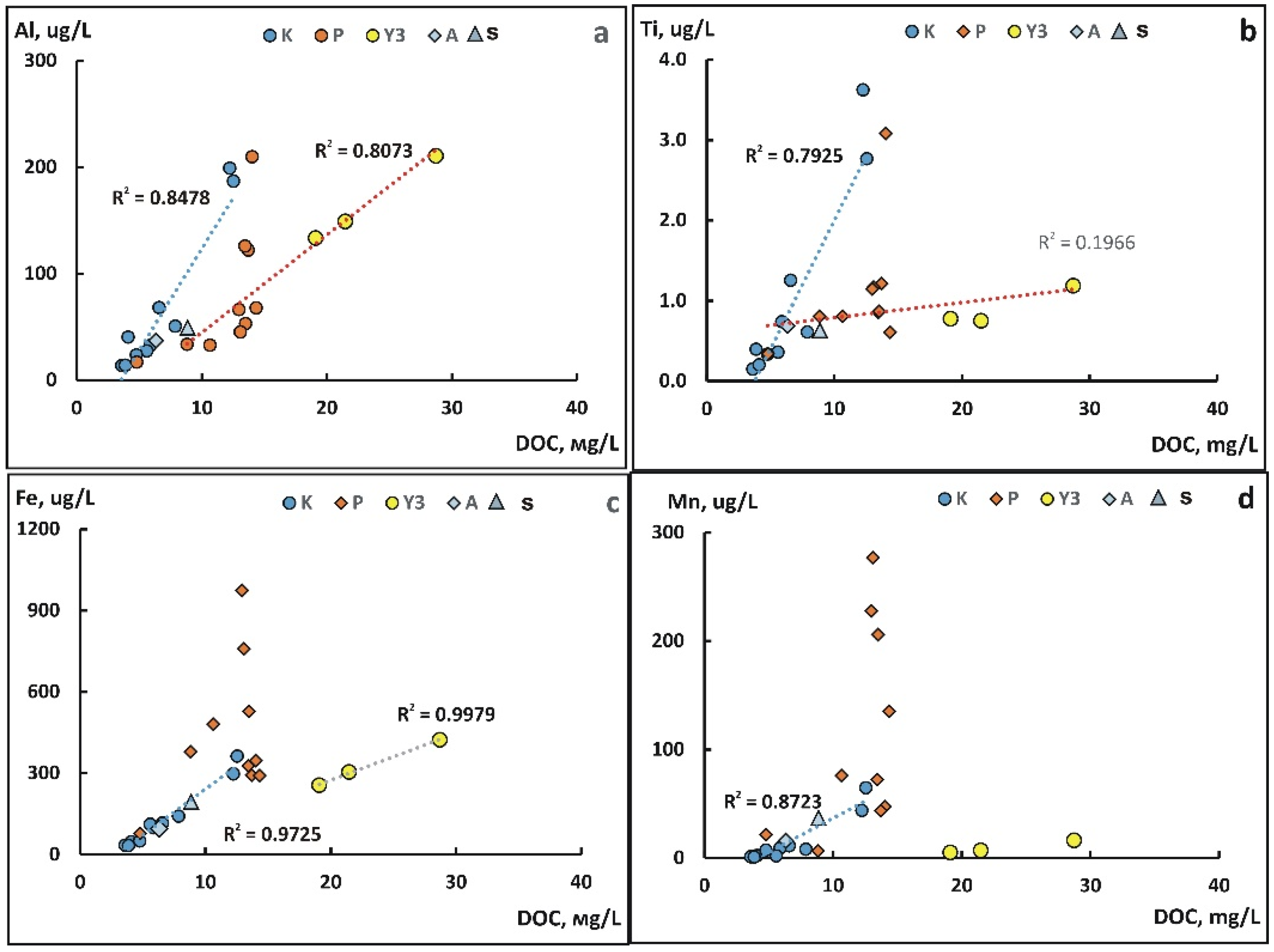
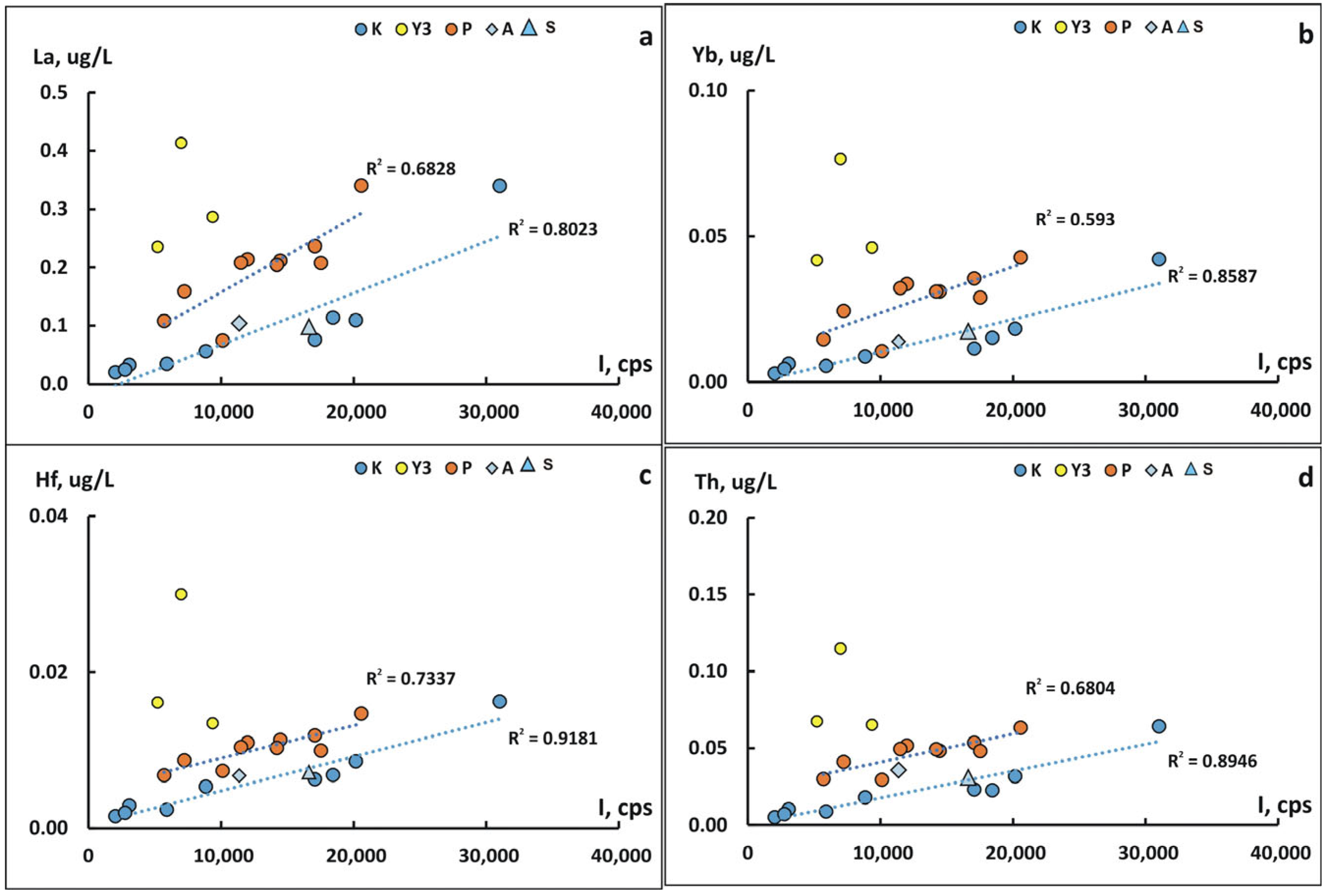
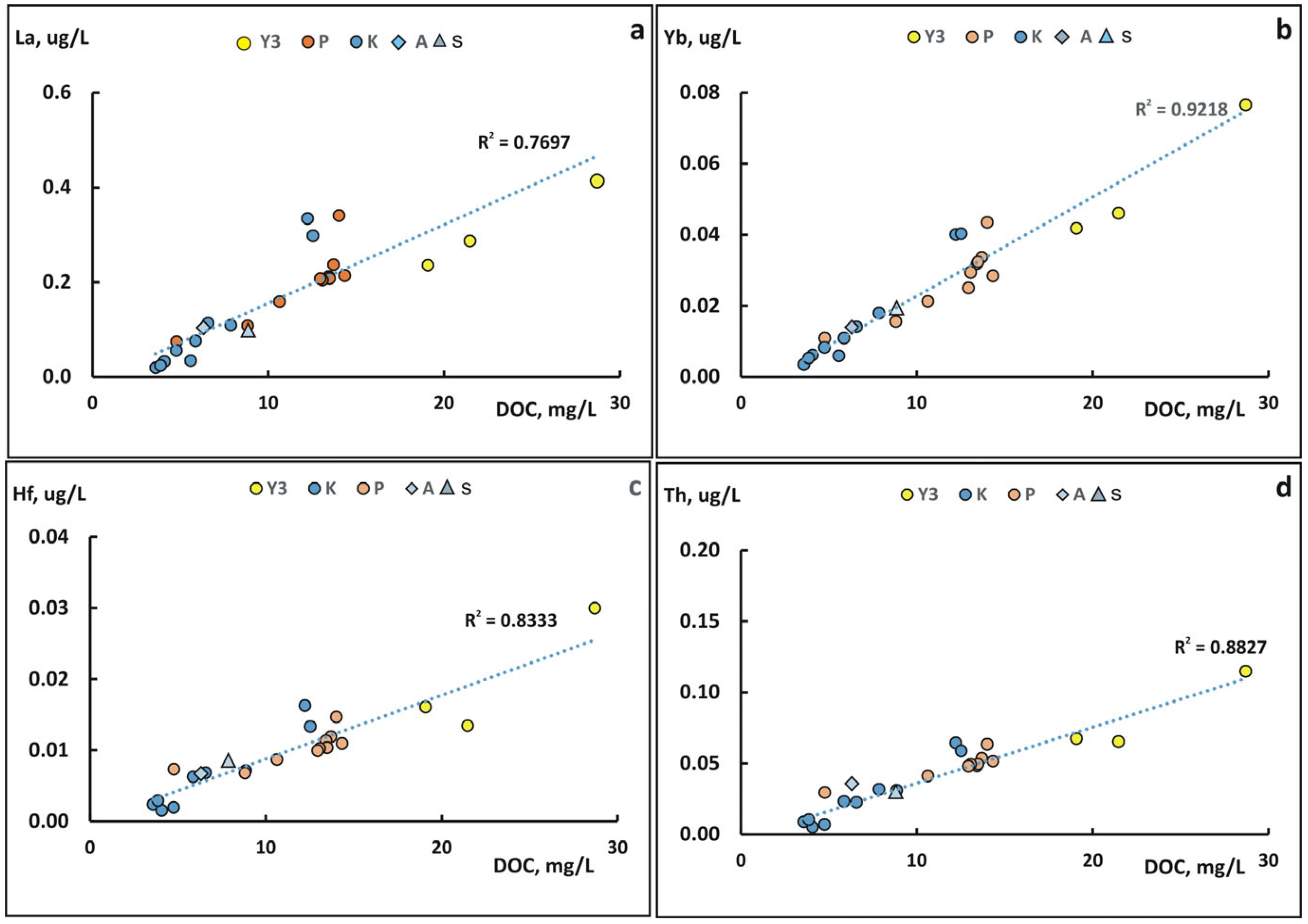
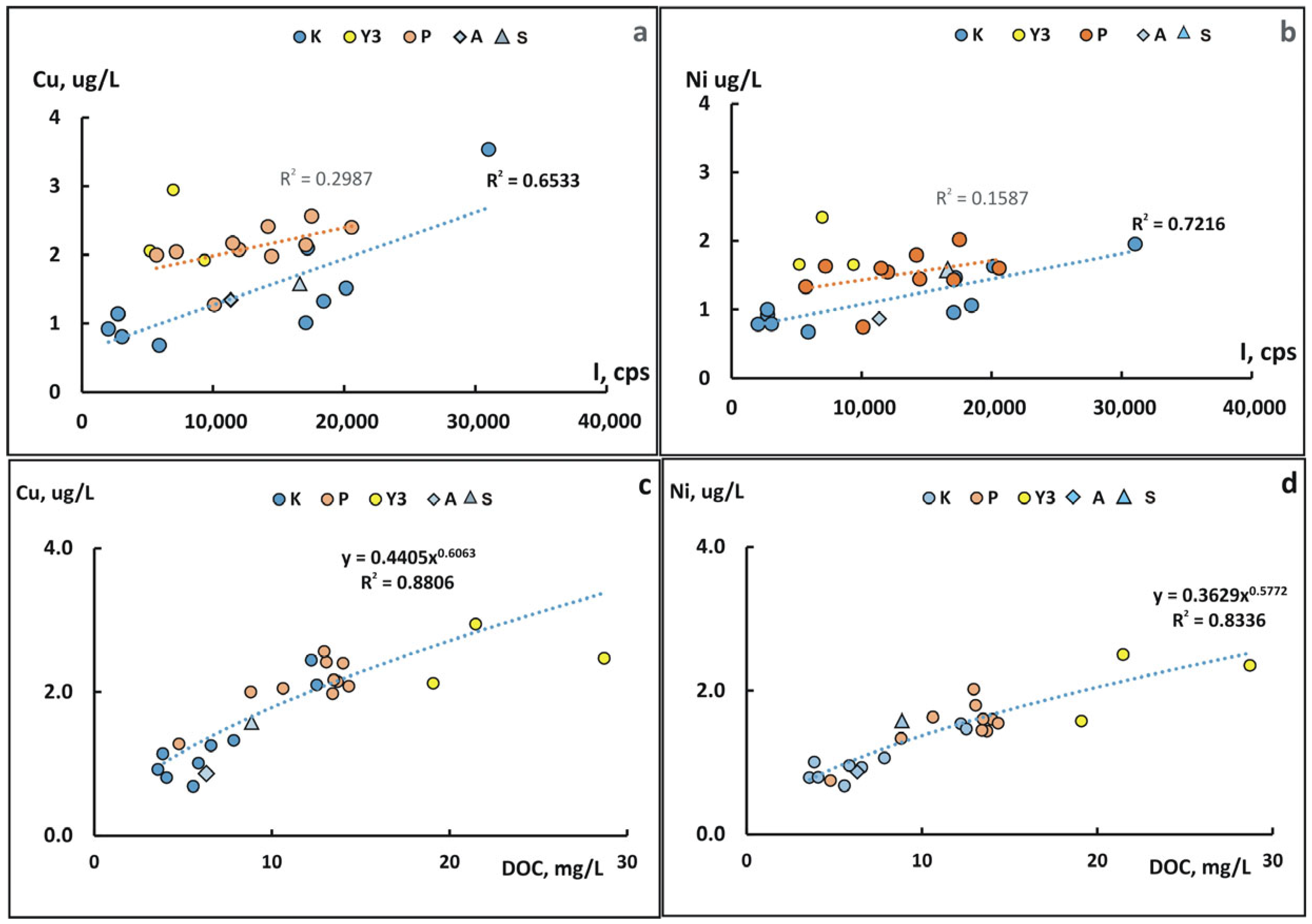
4.3. The Role of Suspended Forms in the Content of Chemical Elements in the Kolyma River During Freshet
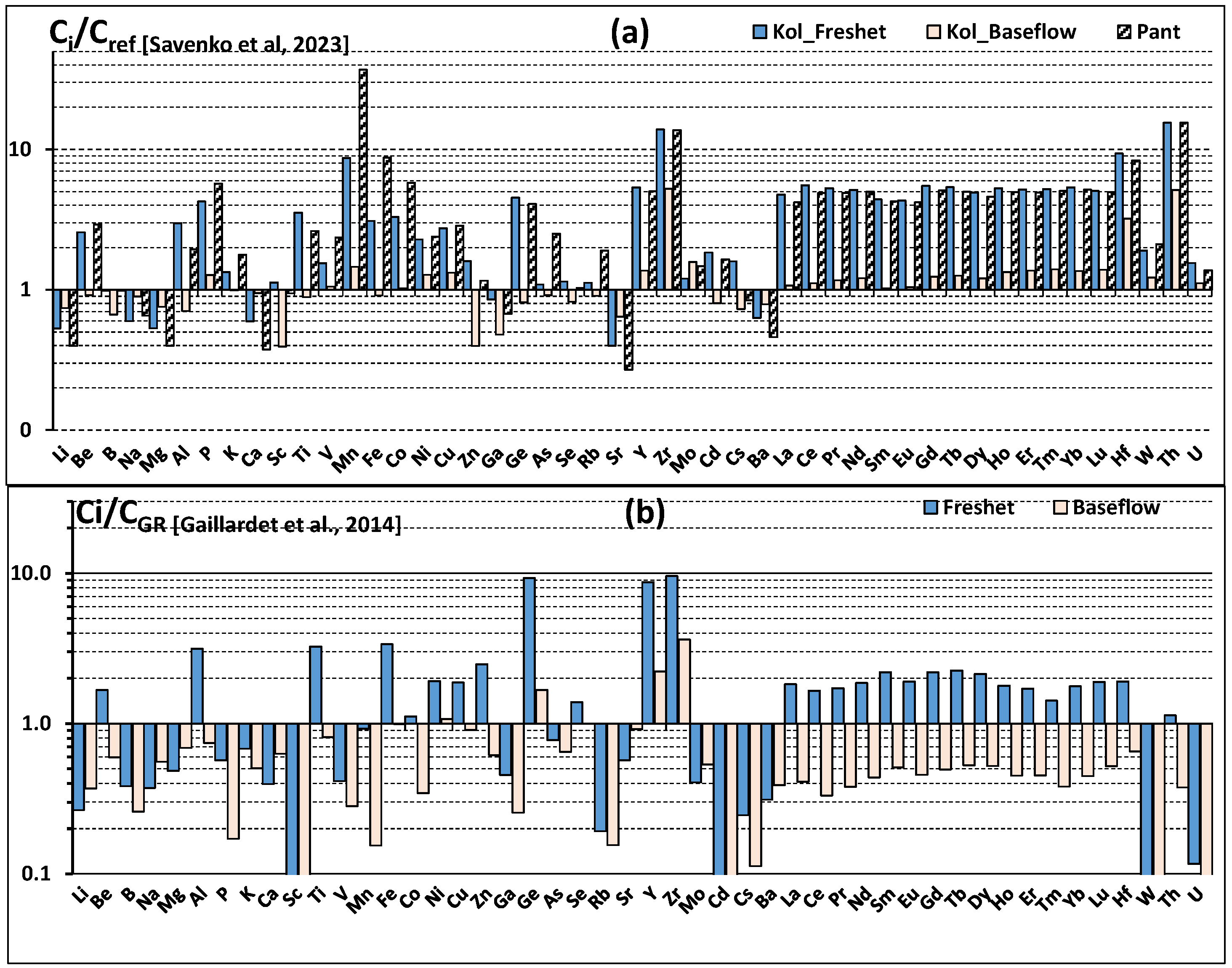
4.4. Comparison of the Chemical Composition of the Lower Reaches of the Kolyma with Other Rivers
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PGI FEBRAS | Pacific Geographical Institute Far Eastern Branch Russian Academy of Sciences |
| FEGI FEBRAS | Far Eastern Geological Institute Far Eastern Branch Russian Academy of Sciences |
| DLS | Dynamic Light Scattering |
References
- Finlay, J.; Neff, J.; Zimov, S.; Davydova, A.; Davydov, S. Snow-melt dominance of dissolved organic carbon in high-latitude watersheds: Implications for characterization and flux of river DOC. Geophys. Res. Lett. 2006, 33, L10401. [Google Scholar] [CrossRef]
- Holmes, R.M.; McClelland, J.W.; Peterson, B.J.; Tank, S.E.; Bulygina, E.; Eglinton, T.I.; Gordeev, V.V.; Gurtovaya, T.Y.; Raymond, P.A.; Repeta, D.J.; et al. Seasonal and annual fluxes of nutrients and organic matter from large rivers to the Arctic Ocean and surrounding seas. Estuaries Coasts 2012, 35, 369–382. [Google Scholar] [CrossRef]
- McClelland, J.W.; Holmes, R.M.; Raymond, P.A.; Striegl, R.G.; Zhulidov, A.V.; Zimov, S.A.; Zimov, N.; Tank, S.E.; Spencer, R.G.M.; Staples, R.; et al. Particulate organic carbon and nitrogen export from major Arctic rivers. Glob. Biogeochem. Cycles 2016, 30, 629–643. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Manasypov, R.M.; Loiko, S.; Krickov, I.A.; Kopysov, S.G.; Kolesnichenko, L.G.; Vorobyev, S.N.; Kirpotin, S.N. Trace element transport in western Siberia rivers across a permafrost gradient. Biogeosciences 2016, 13, 1877–1900. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Manasypov, R.M.; Chupakov, A.V.; Kopysov, S.G. Element transport in the Taz River, western Siberia. Chem. Geol. 2022, 614, 121180. [Google Scholar] [CrossRef]
- Stolpe, B.; Guo, L.; Shiller, A.M. Binding and transport of rare earth elements by organic and iron-rich nanocolloids in Alaskan rivers, as revealed by field-flow fractionation and ICP-MS. Geochim. Cosmochim. Acta 2013, 106, 446–462. [Google Scholar] [CrossRef]
- Savenko, A.V.; Savenko, V.S. Trace Element Composition of the Dissolved Matter Runoff of the Russian Arctic Rivers. Water 2024, 16, 565. [Google Scholar] [CrossRef]
- Rember, R.D.; Trefry, J.H. Increased concentrations of dissolved trace metals and organic carbon during snowmelt in rivers of the Alaskan Arctic. Geochim. Cosmochim. Acta 2004, 68, 477–489. [Google Scholar] [CrossRef]
- Chupakov, A.V.; Pokrovsky, O.S.; Moreva, O.Y.; Shirokova, L.S.; Neverova, N.V.; Chupakova, A.A.; Kotova, E.I.; Vorobyeva, T.Y. High resolution multi-annual riverine fluxes of organic carbon, nutrient and trace element from the largest European Arctic river, Severnaya Dvina. Chem. Geol. 2020, 538, 119491. [Google Scholar] [CrossRef]
- Chupakov, A.V.; Pokrovsky, O.S.; Moreva, O.Y.; Kotova, E.I.; Vorobyeva, T.Y.; Shirokova, L.S. Export of organic carbon, nutrients and metals by the mid-sized Pechora River to the Arctic Ocean. Chem. Geol. 2023, 632, 121524. [Google Scholar] [CrossRef]
- Savenko, A.V.; Savenko, V.S.; Efimov, V.A.; Pokrovsky, O.S. Trace element composition in the waters of the Kolyma mouth section. Dokl. Earth Sci. 2023, 509, 272–275. [Google Scholar] [CrossRef]
- Gordeev, V.V.; Pokrovsky, O.S.; Zhulidov, A.V.; Filippov, A.S.; Gurtovaya, T.Y.; Holmes, R.M.; Kosmenko, L.S.; McClelland, J.W.; Peterson, B.J.; Tank, S.E. Dissolved Major and Trace Elements in the Largest Eurasian Arctic Rivers: Ob, Yenisey, Lena, and Kolyma. Water 2024, 16, 316. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Viers, J.; Shirokova, L.S.; Shevchenko, V.P.; Filipov, A.S.; Dupré, B. Dissolved, suspended, and colloidal fluxes of organic carbon, major and trace elements in the Severnaya Dvina River and its tributary. Chem. Geol. 2010, 273, 136–149. [Google Scholar] [CrossRef]
- Krickov, I.V.; Lim, A.G.; Manasypov, R.M.; Loiko, S.V.; Vorobyev, S.N.; Shevchenko, V.P.; Dara, O.M.; Gordeev, V.V.; Pokrovsky, O.S. Major and trace elements in suspended matter of western Siberian rivers: First assessment across permafrost zones and landscape parameters of watersheds. Geochim. Cosmochim. Acta 2020, 269, 429–450. [Google Scholar] [CrossRef]
- Magritsky, D.V.; Frolova, N.I.; Agafonova, S.A.; Efimov, V.A.; Vasilenko, A.N.; Sazonov, A.A.; Efimova, L.E. Hydrological conditions at the mouth of the Kolyma river in summer 2019. Vestn. Mosc. State Univ. Geogr. 2022, 1, 134–151. [Google Scholar]
- Guo, L.; Cai, Y.; Belzile, C.; Macdonald, R. Sources and export fluxes of inorganic and organic carbon and nutrient species from the seasonally ice-covered Yukon river. Biogeochemistry 2012, 107, 187–206. [Google Scholar] [CrossRef]
- Bagard, M.-L.; Chabaux, F.; Pokrovsky, O.S.; Viers, J.; Prokushkin, A.S.; Stille, P.; Rihs, S.; Dupre, B. Seasonal variability of element fluxes in two Central Siberian rivers draining high latitude permafrost dominated areas. Geochim. Cosmochim. Acta 2011, 75, 3335–3357. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Lim, A.G.; Krickov, I.V.; Korets, M.A.; Shirokova, L.S.; Vorobyev, S.N. Hydrochemistry of Medium-Size Pristine Rivers in Boreal and Subarctic Zone: Disentangling Effect of Landscape Parameters across a Permafrost, Climate, and Vegetation Gradient. Water 2022, 14, 2250. [Google Scholar] [CrossRef]
- Fedorov-Davydov, D.G.; Davydov, S.P.; Davydova, A.I.; Shmelev, D.G.; Ostroymov, V.E.; Kholodov, A.L.; Sorokovikov, V.A. The temperature regime of soils in Northern Yakutia. Earth’s Cryosphere 2018, 22, 12–19. [Google Scholar]
- Romanovsky, V.E.; Drozdov, D.S.; Oberman, N.G.; Malkova, G.V.; Kholodov, A.L.; Marchenko, S.S.; Moskalenko, N.G.; Sergeev, D.O.; Ukraintseva, N.G.; Abramov, A.A.; et al. Thermal state of permafrost in Russia. Permafr. Periglac. Process. 2010, 21, 136–155. [Google Scholar] [CrossRef]
- Glotov, V.Y.; Glotova, L.P.; Ushakov, M.V. Anomalous changes of Kolyma-river runoff condition in winter period. Earth’s Cryosphere 2011, 1, 52–60. (In Russian) [Google Scholar]
- Shiklomanov, A.I.; Holmes, R.M.; McClelland, J.W.; Tank, S.E.; Spencer, R.G.M. Arctic Great Rivers Observatory. Discharge Dataset, Version 20240813. Available online: https://arcticgreatrivers.org/ (accessed on 15 October 2024).
- Ilina, S.M.; Lapitskiy, S.A.; Alekhin, Y.V.; Viers, J.; Benedetti, M.; Pokrovsky, O.S. Speciation, Size Fractionation and Transport of Trace Elements in the Continuum Soil Water–Mire–Humic Lake–River–Large Oligotrophic Lake of a Subarctic Watershed. Aquat. Geochem. 2016, 22, 65–95. [Google Scholar] [CrossRef]
- Shulkin, V.M. Application of Dynamic Light Scattering for Assessing the Content of Large Colloidal and Suspended Particles in River Waters. Dokl. Earth Sci. 2025, 521, 8. [Google Scholar] [CrossRef]
- Filella, M.; Zhang, J.; Newman, M.E.; Buffle, J. Analytical applications of photon correlation spectroscopy for size distribution measurements of natural colloidal suspensions: Capabilities and limitations. Colloids Surf. A Physicochem. Eng. Asp. 1997, 120, 27–46. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Buffle, J.; Leppard, G.G. Characterization of aquatic colloids and macromolecules. 1. Structure and behavior of colloidal material. Environ. Sci. Technol. 1995, 9, 2169–2175. [Google Scholar] [CrossRef]
- Gustafsson, C.; Gschwend, P.M. Aquatic colloids: Concepts, definitions, and current challenges. Limnol. Oceanogr. 1997, 43, 519–528. [Google Scholar] [CrossRef]
- Gaillardet, J.; Viers, J.; Dupre, B. Trace Elements in River Waters. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7, pp. 195–235. [Google Scholar]
- Krickov, I.V.; Lim, A.G.; Shevchenko, V.P.; Starodymova, D.P.; Dara, O.M.; Kolesnichenko, Y.; Zinchenko, D.O.; Vorobyev, S.N.; Pokrovsky, O.S. Seasonal Variations of Mineralogical and Chemical Composition of Particulate Matter in a Large Boreal River and Its Tributaries. Water 2023, 15, 633. [Google Scholar] [CrossRef]
- Viers, J.; Dupré, B.; Gaillardet, J. Chemical composition of suspended sediments in World Rivers: New insights from a new database. Sci. Total Environ. 2009, 407, 853–863. [Google Scholar] [CrossRef]
- Shevchenko, V.P.; Pokrovsky, O.S.; Vorobyev, S.N.; Krickov, I.V.; Manasypov, R.M.; Politova, N.V.; Kopysov, S.G.; Dara, O.M.; Auda, Y.; Shirokova, L.S.; et al. Impact of snow deposition on major and trace element concentrations and elementary fluxes in surface waters of the Western Siberian Lowland across a 1700 km latitudinal gradient. Hydrol. Earth Syst. Sci. 2017, 21, 5725–5746. [Google Scholar] [CrossRef]
- Welp, L.R.; Randerson, J.T.; Finlay, J.C.; Davydov, S.P.; Zimova, G.M.; Davydova, A.I.; Zimov, S.A. A high-resolution time series of oxygen isotopes from the Kolyma River: Implications for the seasonal dynamics of discharge and basin-scale water use. Geophys. Res. Lett. 2005, 32, L14401. [Google Scholar] [CrossRef]
- Stolpe, B.; Guo, L.; Shiller, A.M.; Aiken, G.R. Abundance, size distributions and trace-element binding of organic and iron-rich nanocolloids in Alaskan rivers, as revealed by field-flow fractionation and ICP-MS. Geochim. Cosmochim. Acta 2013, 105, 221–239. [Google Scholar] [CrossRef]
- Shulkin, V.M.; Bogdanova, N.N.; Elovskiy, E.V. Effect of Filter Clogging on the Determination of Concentrations of Chemical Elements Migrating in River Water as Components of True Solutions or in Colloidal Forms. Water Resour. 2022, 49, 122–133. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J. Iron colloids/organic matter associated transport of major and trace elements in small boreal rivers and their estuaries (NW Russia). Chem. Geol. 2002, 190, 141–179. [Google Scholar] [CrossRef]
- Ingri, J.; Widerlund, A.; Land, M.; Gustafsson, O.; Andersson, P.; Ohlander, B. Temporal variations in the fractionation of the rare earth elements in a boreal river; the role of colloidal particles. Chem. Geol. 2000, 166, 23–45. [Google Scholar] [CrossRef]
- Dahlqvist, A.K.; Ingri, J.; Larsson, T.; Stolpe, B.; Turner, D. Temporal variations of colloidal carrier phases and associated trace elements in a boreal river. Geochim. Cosmochim. Acta 2007, 71, 5339–5354. [Google Scholar] [CrossRef]
- Gordeev, V.V.; Lisitzin, A.P. Geochemical interaction between the freshwater and marine hydrospheres. Russ. Geol. Geophys. 2014, 55, 561–581. [Google Scholar] [CrossRef]
- Hirst, C.; Andersson, P.S.; Shaw, S.; Burke, I.T.; Kutscher, L.; Murphy, M.J.; Maximov, T.; Pokrovsky, O.S.; Morth, C.M.; Porcelli, D. Characterisation of Fe-bearing particles and colloids in the Lena River basin, NE Russia. Geochim. Cosmochim. Acta 2017, 213, 553–573. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupre, B.; Lourval, P.; Allegre, C.J. Global silicate weathering and CO consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]

| Element | Kolyma, 1–15.06, n = 7 | Kolyma, 16.06–11.07, n = 7 | Panteleikha n = 8 | [11] | [12] | [29] |
|---|---|---|---|---|---|---|
| Li | 0.49 ± 0.23 | 0.68 ± 0.23 | 0.37 ± 0.03 | 0.92 | - | 1.8 |
| Be | 0.015 ± 0.006 | 0.005 ± 0.002 | 0.017 ± 0.003 | 0.0058 | - | 0.009 |
| B | 3.9 ± 0.9 | 2.65 ± 0.35 | 3.92 ± 0.41 | 3.98 | 1.94 | 10.2 |
| Na | 1270 ± 340 | 1899 ± 446 | 1389 ± 317 | 2120 | 1690 | 3400 |
| Mg | 2210 ± 986 | 3147 ± 922 | 1659 ± 256 | 4160 | 2400 | 4560 |
| Al | 100.7 ± 45.9 | 23.8 ± 10.4 | 65.4 ± 27.5 | 33.8 | 51.5 | 32 |
| P | 21.7 ± 8.1 | 6.5 ± 2.3 | 29 ± 7 | 5.1 | - | 38 |
| K | 682 ± 104 | 505 ± 55 | 904 ± 108 | 510 | 480 | 1000 |
| Ca | 7936 ± 4224 | 12,621 ± 4268 | 4995 ± 854 | 13,300 | 9690 | 20,000 |
| Sc | 0.095 ± 0.031 | 0.034 ± 0.014 | 0.08 ± 0.02 | 0.085 | 0.4 | 0.07 |
| Ti | 1.60 ± 0.82 | 0.40 ± 0.28 | 1.18 ± 0.35 | 0.45 | 0.67 | 0.49 |
| V | 0.29 ± 0.12 | 0.20 ± 0.07 | 0.45. ± 0.17 | 0.19 | 0.33 | 0.71 |
| Mn | 31 ± 17 | 5.3 ± 5.9 | 133 ± 100 | 3.6 | 4.5 | 34 |
| Fe | 222 ± 67 | 65.5 ± 26.4 | 628 ± 406 | 71.9 | 31 | 66 |
| Co | 0.17 ± 0.11 | 0.05 ± 0.02 | 0.289 ± 0.19 | 0.05 | 0.04 | 0.15 |
| Ni | 1.53 ± 0.27 | 0.86 ± 0.11 | 1.60 ± 0.22 | 0.67 | 1.15 | 0.80 |
| Cu | 2.08 ± 0.75 | 1.01 ± 0.22 | 2.17 ± 0.21 | 0.76 | 1.45 | 1.48 |
| Zn | 1.49 ± 0.5 | 0.37 ± 0.15 | 1.08 ± 0.4 | 0.93 | 0.33 | 0.60 |
| Ga | 0.014 ± 0.004 | 0.008 ± 0.002 | 0.011 ± 0.002 | 0.016 | 0.016 | 0.03 |
| Ge | 0.063 ± 0.03 | 0.011 ± 0.05 | 0.057 ± 0.02 | 0.014 | 0.011 | 0.007 |
| As | 0.48 ± 0.08 | 0.40 ± 0.08 | 1.10 ± 0.47 | 0.44 | 0.58 | 0.62 |
| Se | 0.097 ± 0.018 | 0.07 ± 0.01 | 0.088 ± 0.012 | 0.085 | - | 0.07 |
| Rb | 0.31 ± 0.06 | 0.25 ± 0.04 | 0.53 ± 0.11 | 0.28 | 0.19 | 1.63 |
| Sr | 34.2 ± 16.5 | 55.0 ± 18.2 | 23.1 ± 3.7 | 85.6 | 55 | 60 |
| Y | 0.349 ± 0.141 | 0.089 ± 0.044 | 0.327 ± 0.071 | 0.065 | 0.096 | 0.04 |
| Zr | 0.37 ± 0.12 | 0.14 ± 0.08 | 0.37 ± 0.05 | 0.027 | 0.13 | 0.04 |
| Mo | 0.17 ± 0.087 | 0.22 ± 0.12 | 0.21 ± 0.18 | 0.14 | 0.13 | 0.42 |
| Cd | 0.007 ± 0.002 | 0.003 ± 0.001 | 0.006 ± 0.001 | 0.001 | 0.0056 | 0.080 |
| Cs | 0.003 ± 0.001 | 0.001 ± 0.001 | 0.001 ± 0.0005 | 0.0017 | - | 0.011 |
| Ba | 7.2 ± 2.2 | 8.9 ± 2.3 | 5.2 ± 0.5 | 11.4 | 7.2 | 23 |
| La | 0.219 ± 0.111 | 0.049 ± 0.031 | 0.193 ± 0.041 | 0.046 | 0.049 | 0.120 |
| Ce | 0.433 ± 0.235 | 0.087 ± 0.064 | 0.381 ± 0.096 | 0.078 | 0.096 | 0.262 |
| Pr | 0.069 ± 0.034 | 0.015 ± 0.009 | 0.064 ± 0.014 | 0.013 | 0.016 | 0.040 |
| Nd | 0.283 ± 0.136 | 0.067 ± 0.038 | 0.275 ± 0.061 | 0.055 | 0.077 | 0.152 |
| Sm | 0.079 ± 0.036 | 0.018 ± 0.010 | 0.077 ± 0.018 | 0.018 | 0.021 | 0.036 |
| Eu | 0.019 ± 0.008 | 0.004 ± 0.003 | 0.018 ± 0.004 | 0.0043 | 0.006 | 0.010 |
| Gd | 0.088 ± 0.039 | 0.020 ± 0.011 | 0.082 ± 0.019 | 0.016 | 0.027 | 0.040 |
| Tb | 0.012 ± 0.005 | 0.003 ± 0.001 | 0.011 ± 0.003 | 0.0023 | 0.003 | 0.0055 |
| Dy | 0.064 ± 0.026 | 0.016 ± 0.008 | 0.060 ± 0.012 | 0.013 | 0.021 | 0.03 |
| Ho | 0.013 ± 0.005 | 0.003 ± 0.001 | 0.012 ± 0.003 | 0.0024 | 0.004 | 0.007 |
| Er | 0.034 ± 0.014 | 0.009 ± 0.004 | 0.032 ± 0.007 | 0.0066 | 0.012 | 0.02 |
| Tm | 0.005 ± 0.002 | 0.001 ± 0.001 | 0.005 ± 0.001 | 0.001 | - | 0.0033 |
| Yb | 0.030 ± 0.013 | 0.008 ± 0.004 | 0.029 ± 0.007 | 0.0056 | 0.010 | 0.017 |
| Lu | 0.005 ± 0.002 | 0.001 ± 0.001 | 0.004 ± 0.001 | 0.0009 | 0.0015 | 0.0024 |
| Hf | 0.011 ± 0.004 | 0.004 ± 0.002 | 0.010 ± 0.002 | 0.0012 | 0.0065 | 0.0059 |
| W | 0.004 ± 0.001 | 0.002 ± 0.001 | 0.004 ± 0.001 | 0.0019 | 0.005 | 0.10 |
| Th | 0.046 ± 0.017 | 0.015 ± 0.011 | 0.046 ± 0.008 | 0.003 | 0.025 | 0.041 |
| U | 0.043 ± 0.009 | 0.031 ± 0.010 | 0.039 ± 0.004 | 0.028 | 0.050 | 0.37 |
| Element | Kolyma, 1.06–11.07, n = 14 | Panteleikha n = 8 | [14] | [30] | [31] |
|---|---|---|---|---|---|
| Li | 42.6 ± 10.2 | 29.5 ± 5.9 | 10.2 | 25.20 | 35.0 |
| Be | 1.5 ± 0.2 | 1.1 ± 0.1 | - | 1.93 | 1.7 |
| Na * | 1.53 ± 0.18 | 1.13 ± 0.29 | 0.38 | 0.46 | 0.82 |
| Mg * | 0.76 ± 0.12 | 0.52 ± 0.11 | 0.41 | 0.67 | 1.44 |
| Al * | 5.61 ± 1.02 | 4.09 ± 0.8 | 2.41 | 4.32 | 8.63 |
| P | 1210 ± 247 | 1905 ± 418 | 4187 | 4060 | 1000 |
| K * | 1.40 ± 0.19 | 0.89 ± 0.19 | 0.63 | 1.38 | 2.15 |
| Ca * | 1.22 ± 0.19 | 0.97 ± 0.24 | 0.60 | 3.77 | 2.60 |
| Sc | 11.8 ± 2.0 | 9.4 ± 1.4 | - | - | 14 |
| Ti | 3191 ± 555 | 1761 ± 438 | 8237 | 2210 | 3900 |
| V | 92 ± 18 | 67 ± 10 | 56.6 | 159 | 120 |
| Mn | 1290 ± 733 | 3411 ± 2729 | 5829 | 4560 | 1150 |
| Fe * | 4.05 ± 0.46 | 5.50 ± 1.50 | 6.78 | 6.04 | 5.03 |
| Co | 14.1 ± 2.1 | 14.8 ± 3.9 | 21.8 | 18.60 | 19.0 |
| Ni | 36 ± 8 | 26 ± 3 | 23.1 | 58.00 | 50 |
| Cu | 28 ± 3 | 26 ± 3 | 14.0 | 45.50 | 45 |
| Zn | 96 ± 13 | 79 ± 14 | 82.1 | 156.00 | 130 |
| Ga | 15.4 ± 2.3 | 10.0 ± 1.7 | 6.01 | 16.80 | 20 |
| Ge | 3.2 ± 0.4 | 3.2 ± 0.6 | 0.30 | 1.19 | 1.40 |
| As | 5.4 ± 1.8 | 4.2 ± 2.0 | 13.9 | 55.60 | 14.0 |
| Se | 1.8 ± 0.3 | 1.8 ± 0.3 | - | - | 1.50 |
| Rb | 40 ± 14 | 35 ± 6 | 23 | 19.70 | 77 |
| Sr | 131 ± 14 | 95 ± 21 | 115 | 252.00 | 150 |
| Y | 17.0 ± 3.0 | 16.8 ± 2.0 | 8.75 | 7.70 | 25.0 |
| Zr | 89.4 ± 10.2 | 70.4 ± 11.3 | 34.5 | 79 | 150 |
| Mo | 0.99 ± 0.07 | 1.1 ± 0.31 | 0.45 | 1.31 | 1.80 |
| Cd | 0.29 ± 0.03 | 0.3 ± 0.09 | 0.31 | 0.62 | 0.50 |
| Cs | 4.2 ± 1.2 | 3.1 ± 0.5 | 1.39 | 1.21 | 5.20 |
| Ba | 500 ± 57 | 336 ± 46 | 329 | 402 | 500 |
| La | 20.8 ± 4.0 | 18.9 ± 3.2 | 12.2 | 12.9 | 32 |
| Ce | 44.9 ± 6.8 | 40.9 ± 2.9 | 25.5 | 31.70 | 68 |
| Pr | 5.4 ± 0.8 | 5.0 ± 0.6 | 2.9 | 3.76 | 7.7 |
| Nd | 20.4 ± 2.8 | 19.1 ± 2.4 | 11.3 | 15.40 | 29 |
| Sm | 4.74 ± 0.6 | 4.52 ± 0.53 | 2.24 | 3.21 | 5.80 |
| Eu | 1.02 ± 0.13 | 0.97 ± 0.11 | 0.52 | 0.73 | 1.40 |
| Gd | 4.37 ± 0.62 | 4.26 ± 0.49 | 2.20 | 2.95 | 5.60 |
| Tb | 0.64 ± 0.09 | 0.61 ± 0.06 | 0.30 | 0.41 | 0.79 |
| Dy | 3.28 ± 0.44 | 3.14 ± 0.28 | 1.73 | 2.30 | 4.50 |
| Ho | 0.66 ± 0.08 | 0.62 ± 0.05 | 0.33 | 0.42 | 0.90 |
| Er | 1.90 ± 0.23 | 1.77 ± 0.13 | 0.97 | 1.19 | 2.60 |
| Tm | 0.27 ± 0.03 | 0.25 ± 0.02 | 0.14 | 0.16 | 0.38 |
| Yb | 1.76 ± 0.19 | 1.59 ± 0.10 | 0.91 | 1.03 | 2.50 |
| Lu | 0.26 ± 0.03 | 0.24 ± 0.02 | 0.13 | 0.15 | 0.40 |
| Hf | 2.42 ± 0.27 | 1.88 ± 0.31 | 4.57 | 2.95 | 4.40 |
| W | 0.87 ± 0.19 | 0.61 ± 0.15 | 1.04 | 2.74 | 1.40 |
| Th | 6.14 ± 1.14 | 5.14 ± 0.49 | 2.75 | 3.90 | 10.0 |
| U | 1.72 ± 0.24 | 1.51 ± 0.11 | 0.68 | 2.33 | 2.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shulkin, V.; Davydov, S.; Davydova, A.; Lutsenko, T.; Elovskiy, E. Scale and Reasons for Changes in Chemical Composition of Waters During the Spring Freshet on Kolyma River, Arctic Siberia. Water 2025, 17, 2400. https://doi.org/10.3390/w17162400
Shulkin V, Davydov S, Davydova A, Lutsenko T, Elovskiy E. Scale and Reasons for Changes in Chemical Composition of Waters During the Spring Freshet on Kolyma River, Arctic Siberia. Water. 2025; 17(16):2400. https://doi.org/10.3390/w17162400
Chicago/Turabian StyleShulkin, Vladimir, Sergei Davydov, Anna Davydova, Tatiana Lutsenko, and Eugeniy Elovskiy. 2025. "Scale and Reasons for Changes in Chemical Composition of Waters During the Spring Freshet on Kolyma River, Arctic Siberia" Water 17, no. 16: 2400. https://doi.org/10.3390/w17162400
APA StyleShulkin, V., Davydov, S., Davydova, A., Lutsenko, T., & Elovskiy, E. (2025). Scale and Reasons for Changes in Chemical Composition of Waters During the Spring Freshet on Kolyma River, Arctic Siberia. Water, 17(16), 2400. https://doi.org/10.3390/w17162400








