Peculiarities of 222Radon and 238Uranium Behavior in Mineral Waters of Highland Terrains
Abstract
1. Introduction
2. Objects and Methods
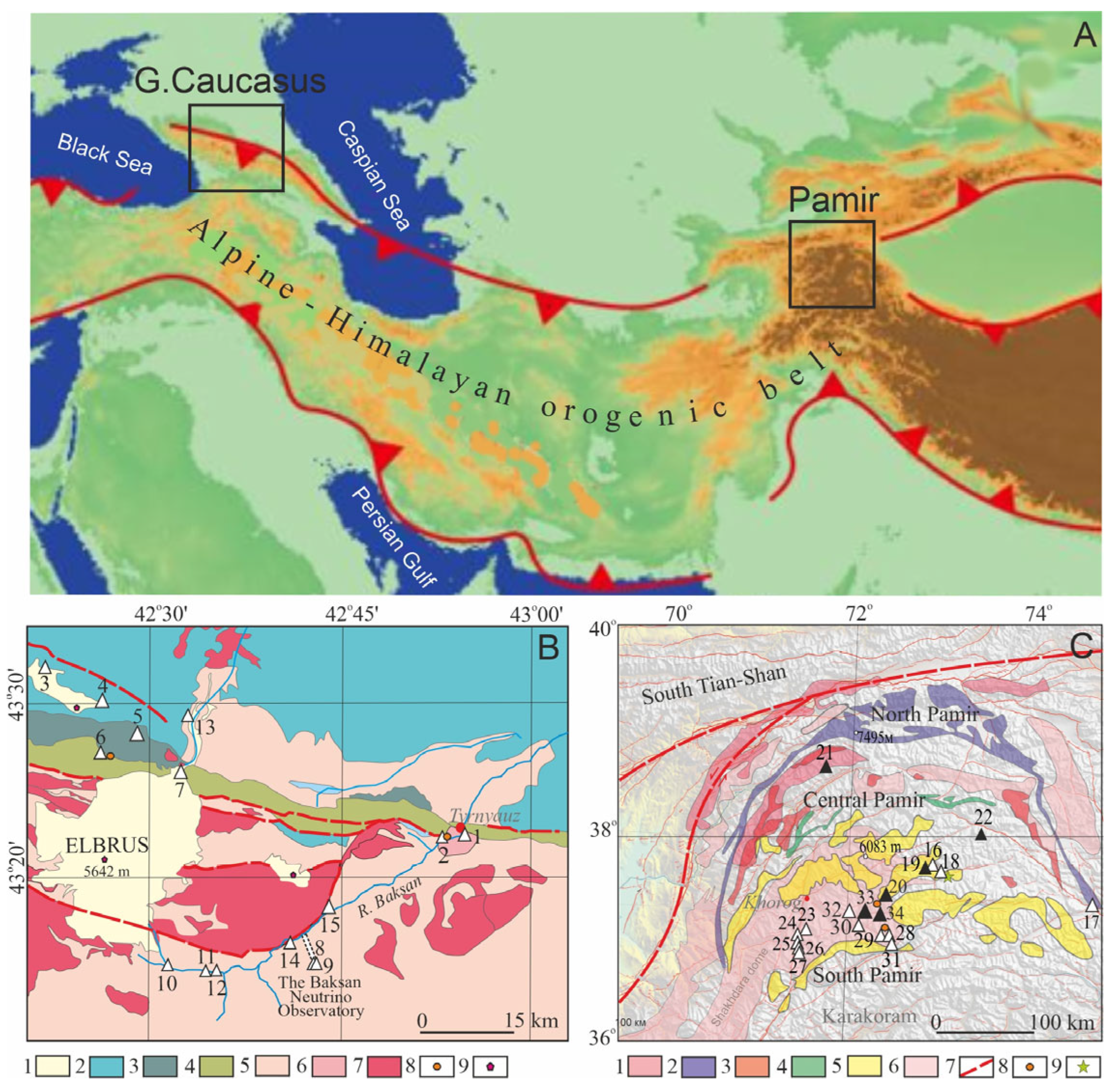
2.1. Study Area
2.2. Methods
3. Results and Discussion
3.1. Geology, Orogeny, and Radionuclides Behavior
3.2. Geochemical Aspects of 238U and 222Rn Migration
3.2.1. Uranium
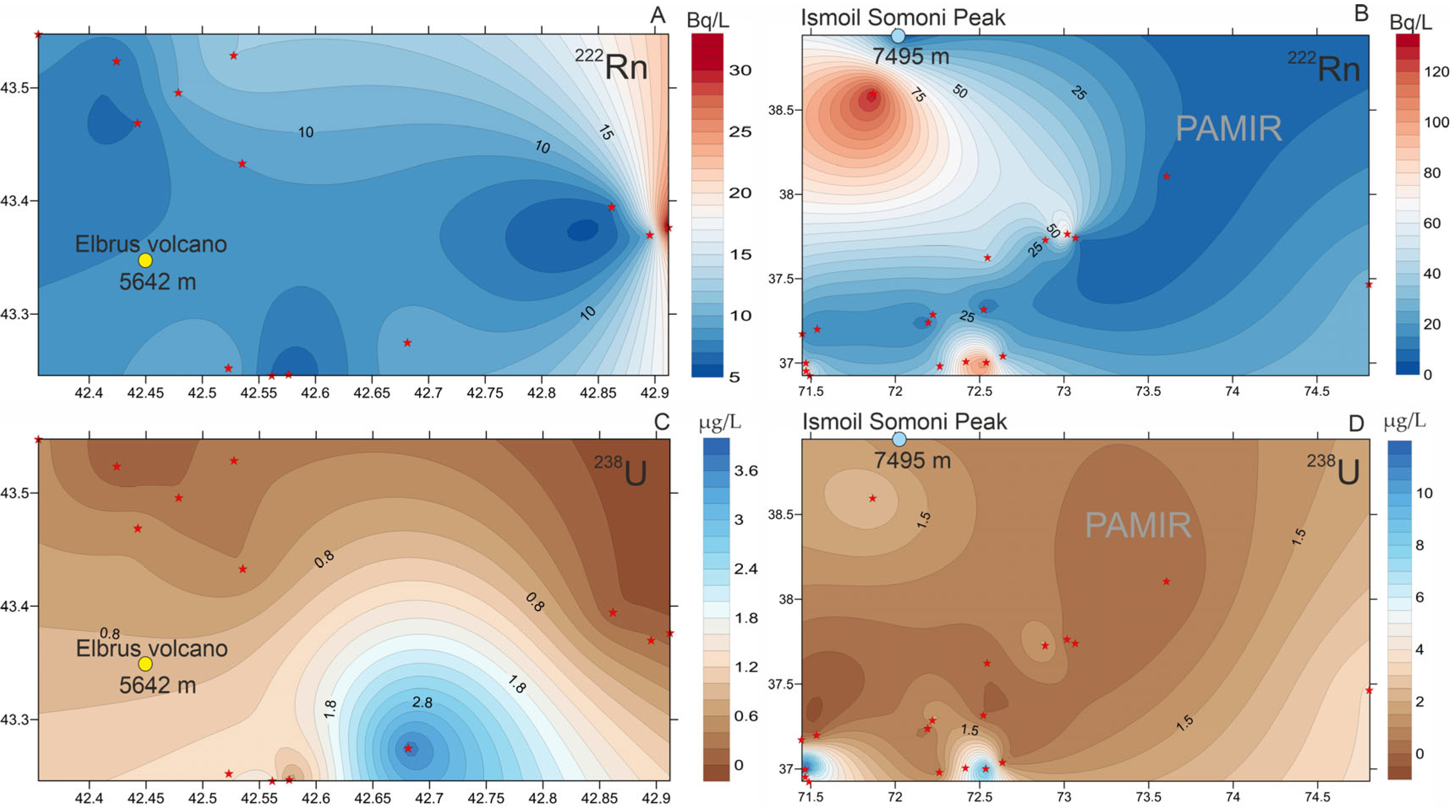
3.2.2. Radon
3.3. Probable Sources of Radionuclides: Insight from Mineral Equilibrium Calculations
3.4. Radionuclides, Stable Isotopes, and Associated Gases
3.5. 3He/4He Ratio and Radon Sources
3.6. Health Risk Assessment from Mineral Water Consumption
4. Conclusions
- 1.
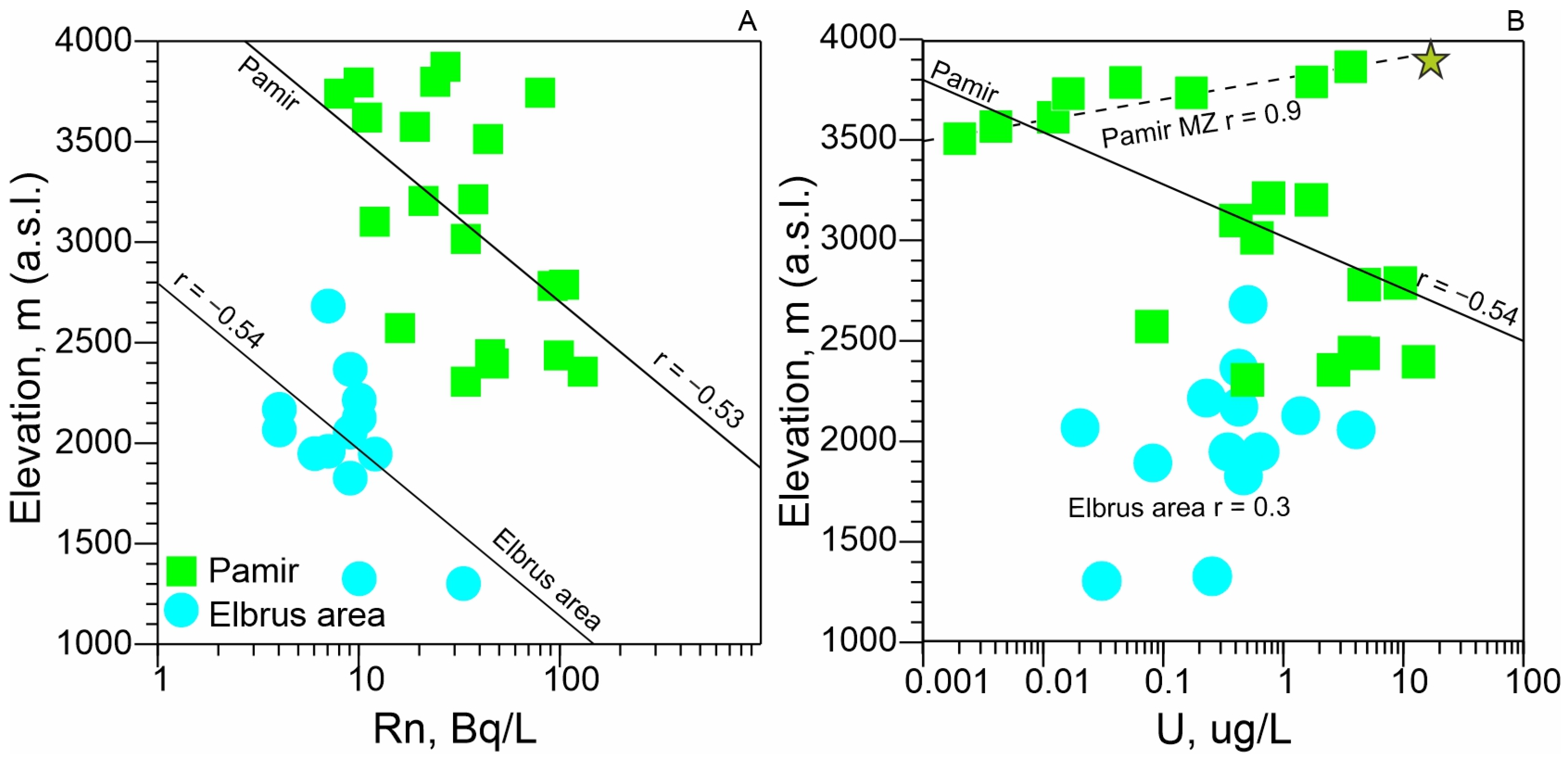
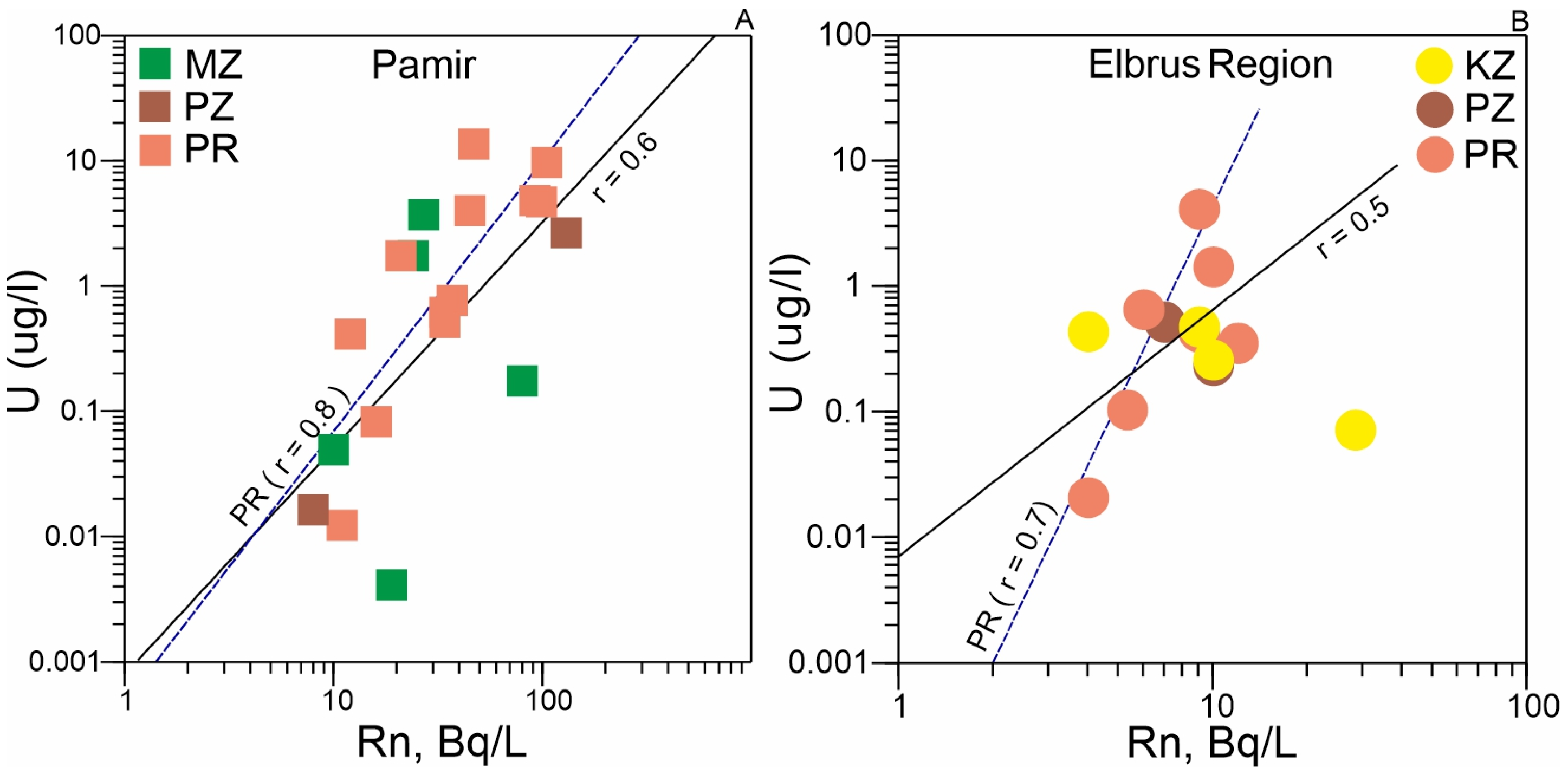
- It has been established that the concentrations of 238U and the activity of 222Rn in the mineral waters of the Pamirs are higher than in the waters of the Elbrus area. In both regions, radon activity shows a strong correlation with uranium concentrations in mineral waters associated with Proterozoic-age rocks, the most ancient geological formations in these areas.
- 2.
- Altitude dependencies of uranium and radon distribution have been established. With increasing altitude, radon activity in groundwater decreases. This can be explained by both the physical properties of 222Rn (high density) and hydrogeological characteristics (low circulation rate exceeding the half-life of radon). On the contrary, the concentration of 238U tends to increase with increasing altitude of the mountain structure, which is associated with oxidizing conditions of the groundwater environment, favorable for its migration.
- 3.
- Thermodynamic calculations revealed key processes influencing uranium and radon incorporation into groundwater. The results demonstrate that: Uranium mobilization is controlled by water-aluminosilicate interactions, which are enhanced in the presence of CO2; Radon activity is governed by both aluminosilicate and evaporite interactions. These processes are particularly pronounced in Pamir waters due to abundant marine-origin rocks. In contrast, the Elbrus region shows generally low radon concentrations due to limited evaporite distribution in its upper geological sections.
- 4.
- The obtained data align well with previous studies, confirming that waters in the Elbrus region exhibit mantle-derived signatures, whereas Pamir waters reflect intracrustal melting processes. Pamir springs show low 3He/4He ratios and elevated 222Rn levels, consistent with a dominant crustal radiogenic contribution. In contrast, Elbrus groundwaters display higher 3He/4He values, indicating mantle input—a feature typical of regions with high heat flow.
- 5.
- It was found that radon activity in high-altitude mineral waters (2300–3792 m above sea level) has been linked to isotopic composition, temperature, and gas content. Such effects had not previously been reported in high-mountain mineral waters. The observed correlations suggest complex physical processes governing radon distribution, which may serve as potential indicator characteristics. However, further research is needed to fully elucidate these mechanisms.The strongest radon–δ18O correlation occurs in CO2-rich Pamir waters, implying that radon is primarily transported by isotopically light (glacial) waters. As the isotopic composition becomes heavier (due to mixing with atmospheric precipitation), radon concentrations decrease.
- 6.
- Health risk assessments of the Pamir and Elbrus mineral waters have shown that neither uranium nor radon negatively impacts human health. Concentrations of 238U do not exceed drinking water limits (15–30 μg/L). Studies confirm that at altitudes above 3000 m, radon activity in groundwater is very low, ensuring safe human exposure levels. However, in intermountain basins at lower altitudes (~2300 m), radon concentrations in water can rise significantly, necessitating further study and monitoring.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quattrini, S.; Pampaloni, B.; Brandi, M.L. Natural mineral waters: Chemical characteristics and health effects. Clin. Cases Miner. Bone Metab. 2016, 13, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Routh, H.B.; Bhowmik, K.R.; Parish, L.C.; Witkowski, J.A. Balneology, mineral water, and spas in historical perspective. Clin. Dermatol. 1996, 14, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Albertini, M.C.; Dachà, M.; Teodori, L.; Conti, M.E. Drinking mineral waters: Biochemical effects and health implications—The state-of-the-art. Int. J. Environ. Health 2007, 1, 153–169. [Google Scholar] [CrossRef]
- Protano, C.; Vitali, M.; De Giorgi, A.; Marotta, D.; Crucianelli, S.; Fontana, M. Balneotherapy using thermal mineral water baths and dermatological diseases: A systematic review. Int. J. Biometeorol. 2024, 68, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.J.; Gomes, C.S.F. Mineral Water: Essential to Life, Health, and Wellness. In Minerals Latu Sensu and Human Health; Gomes, C., Rautureau, M., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Kryshev, A.I.; Sazykina, T.G.; Pavlova, N.N. Issues of establishing the permissible discharge levels of 238U to surface waters taking into account its radiation and toxic effects. Radiat. Hyg. 2020, 13, 41–46. [Google Scholar] [CrossRef]
- Chelnokov, G.; Lavrushin, V.; Ermakov, A.; Guo, Q.; Aidarkozhina, A.; Kharitonova, N.; Bragin, I.; Pavlov, A. Toxic Element Contamination Sources in the Surface and Groundwater of the Elbrus Region: Geochemistry and Health Risks. Water 2024, 16, 701. [Google Scholar] [CrossRef]
- Le Druillennec, T.; Ielsch, G.; Bour, O.; Tarits, C.; Tymen, G.; Alcalde, G.; Aquilina, L. Hydrogeological and geochemical control of the variations of 222Rn concentrations in a hard rock aquifer: Insights into the possible role of fracture-matrix exchanges. Appl. Geochem. 2010, 25, 345–356. [Google Scholar] [CrossRef]
- Fonollosa, E.; Peñalver, A.; Borrull, F.; Aguilar, C. Radon in spring waters in the south of Catalonia. J. Environ. Radioact. 2016, 151, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.-W.; Kim, D.-S.; Kim, M.-S.; Hwang, J.-H.; Choo, C.-O. Hydrogeochemical Characteristics of Uranium and Radon in Groundwater from the Goesan Area of the Ogcheon Metamorphic Belt (OMB), Korea. Sustainability 2021, 13, 11261. [Google Scholar] [CrossRef]
- Rogers, A.S. Physical behavior and geologic control of radon in mountain streams. U.S. Geol. Surv. Bull. 1958, 1052-E, 187–211. [Google Scholar]
- Szabo, Z.; Zapecza, O.S. Geologic and geochemical factors controlling uranium, radium-226, and radon-222 in groundwater, Newark Basin, New Jersey. In Field Studies of Radon in Rocks, Soils. and Water; Gundersen, L.C.S., Wanty, R.B., Eds.; C.K. Smoley: Boca Raton, FL, USA, 1991; pp. 243–265. [Google Scholar]
- Cecil, L.D.; Green, J.R. Radon-222. In Environmental Tracers in Subsurface Hydrology; Cook, P.G., Herczeg, A.L., Eds.; Springer: Boston, MA, USA, 2000. [Google Scholar] [CrossRef]
- Andrews, J.N.; Wood, D.F. Mechanism of radon release in rock matrices and entry into groundwaters. Inst. Min. Metall. Trans. 1972, B81, 198–209. [Google Scholar]
- Wood, W.W.; Kraemer, T.F.; Shapiro, A. Radon (222Rn) in ground water of fractured rocks: A diffusion/ion exchange model. Groundwater 2004, 42, 552–567. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, L.A.; McMahon, P.B.; Rosen, M.R. The Quality of Our Nation’s Waters: Water Quality in Principal Aquifers of the United States, 1991–2010 (Circular 1360); United States Geological Survey: Reston, VA, USA, 2014. [CrossRef]
- Sukanya, S.; Noble, J.; Joseph, S. Application of radon (222Rn) as an environmental tracer in hydrogeological and geological investigations: An overview. Chemosphere 2022, 303 Part 3, 135141. [Google Scholar] [CrossRef] [PubMed]
- Girault, F.; Perrier, F. The Syabru-Bensi hydrothermal system in central Nepal: 2. Modeling and significance of the radon signature. J. Geophys. Res. Solid Earth 2014, 119, 4056–4089. [Google Scholar] [CrossRef]
- Okrostsvaridze, A.; Tormey, D.; Gabrielashvili, R. New Insight into Formation of Tskaltubo Radon-Containing Healing Springs, Georgia, the Caucasus. Int. J. Geol. Earth Sci. 2025, 11, 17–22. [Google Scholar] [CrossRef]
- Cosma, C.; Moldovan, M.; Dicu, T.; Kovacs, T. Radon in water from Transylvania (Romania). Radiat. Meas. 2008, 43, 1423–1428. [Google Scholar] [CrossRef]
- Kozlowska, B.; Walencik, A.; Dorda, J.; Zipper, W. Radon in groundwater and dose estimation for inhabitants in Spas of the Sudety Mountain area. Pol. Appl. Radiat. Isot. 2010, 68, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Chelnokov, G.A.; Lavrushin VYu Bragin, I.V.; Kharitonova, N.A.; Bushkareva KYu Pavlov, A.A.; Chelnokova, B.I. Radon Concentrations in Mineral Waters of the Sikhote-Alin, Primorsky Krai. Russ. J. Pac. Geol. 2023, 17, 284–296. [Google Scholar] [CrossRef]
- Kharitonova, N.A.; Sokolovskaya, M.A.; Baranovskaya, E.I.; Chelnokov, G.A.; Karabtsov, A.A.; Chernoshchekov, L.N.; Bragin, I.V. Trace elements in thermal waters of the northern Tien Shan: Distribution and fate. Mosc. Univ. Bull. Ser. 4 Geol. 2024; 79, 632–648, (In Russian). [Google Scholar] [CrossRef]
- Demonova, A.; Kharitonova, N.; Sardorov, A. Chemical composition of thermal waters of Khoja-Obi-Garm Spa. In Proceedings of the 42 World Congress of the International Society of Medical Hydrology and Climatology, Moscow, Russia, 5–8 June 2017; p. 39. [Google Scholar]
- Khan, F.; Khattak, S.A.; Khan, W.; Ullah, Z.; Alig, F.; Wan, Q. Concentrations of Radon in the Water of Balakot-Bagh Fault Line Region, Lesser Himalayas, North Pakistan. Dokl. Earth Sci. 2024, 517 Pt 1, 1262–1268. [Google Scholar] [CrossRef]
- Chelnokov, G.; Lavrushin, V.; Bragin, I.; Abdullaev, A.; Aidarkozhina, A.; Kharitonova, N. Geochemistry of Thermal and Cold Mineral Water and Gases of the Tien Shan and the Pamir. Water 2022, 14, 838. [Google Scholar] [CrossRef]
- Lavrushin, V.Y. Subsurface Fluids of the Greater Caucasus and Its Surrounding; Transactions of the Geological Institute; Nauka: Moscow, Russia, 2012; p. 348. (In Russian)
- Krainov, S.R.; Petrova, N.G. Minor elements in mineral waters of the Pamir. Geochem. Int. 1962, 4, 366. (In Russian) [Google Scholar]
- Kireeva, T.A.; Salikhov, F.S.; Bychkov, A.Y.; Kharitonova, N.A. Chemical composition of waters and formation conditions of some thermal springs in Tajikistan. Geochemistry 2020, 65, 379–391. [Google Scholar] [CrossRef]
- Soloviev, S.G.; Kryazhev, S.G.; Dvurechenskaya, S.S.; Kryazhev, V.S.; Emkuzhev, M.S.; Bortnikov, N.S. The superlarge Tyrnyauz skarn W-Mo and stockwork Mo(-W) to Au(-Mo, W, Bi, Te) deposit in the Northern Caucasus, Russia: Geology, geochemistry, mineralization, and fluid inclusion characteristics. Ore Geol. Rev. 2021, 138, 2–26. [Google Scholar] [CrossRef]
- Mechie, J.; Yuan, X.; Schurr, B.; Schneider, F.; Sippl, C.; Ratschbacher, L.; Minaev, V.; Gadoev, M.; Oimahmadov, I.; Abdybachaev, U.; et al. Crustal and uppermost mantle velocity structure along a profile across the Pamir and southern Tien Shan as derivedfrom project TIPAGE wide-angle seismic data. Geophys. J. Int. 2012, 188, 385–407. [Google Scholar] [CrossRef]
- Gurbanov, A.G.; Sobisevich, A.L.; Sobisevich, L.E.; Nechaev, Y.N.; Arbuzkin, V.N. Prutski Activity of Elbrus Volcano (North Caucasus). Russ. J. Earth Sci. 2004, 6, 279–291. [Google Scholar] [CrossRef]
- Fletcher, P.J.; Jacobus, F.S. Determination of carbonate and hydrogencarbonate by titration using sequential injection analysis. Anal. Chim. Acta 2003, 485, 187–194. [Google Scholar] [CrossRef]
- Karandashev, V.K.; Leikin, A.Y.; Khvostikov, V.A.; Kutseva, N.K.; Pirogova, S.V. Water Analysis by Inductively Coupled Plasma Mass Spectrometry. Inorg. Mater. 2016, 52, 1391–1404. [Google Scholar] [CrossRef]
- Arnorsson, S.; Bjarnasson, J.O.; Giroud, N.; Gunnarsson, I.; Stefansson, A. Sampling and analysis of geothermal fluids. Geofluids 2006, 6, 203–216. [Google Scholar] [CrossRef]
- Radon Monitot Alfarad+. User’s Manual. Moscow. 2021. Available online: https://ntm.ru/UserFiles/File/document/ION/Aplus/Alpharad_manual.pdf (accessed on 3 July 2025). (In Russian).
- EURATOM Council Directive 2013/51/Euratom Laying Down Requirements for the Protection of the Health of the General Public with Regard to Radioactive Substances in Water Intended for Human Consumption. 2013. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:296:0012:0021:EN:PDF (accessed on 3 July 2025).
- Novikov, D.A.; Kopylova, Y.G.; Pyryaev, A.N.; Maksimova, A.A.; Derkachev, A.S.; Sukhorukova, A.F.; Dultsev, F.F.; Chernykh, A.V.; Khvashchevskaya, A.A.; Kalinkin, P.N.; et al. Radon-rich waters of the Tulinka aquifers, Novosibirsk, Russia. Groundw. Sustain. Dev. 2023, 20, 100886. [Google Scholar] [CrossRef]
- AquaChem. User’s Manual, v.5.11; Schlumberger: Houston, TX, USA, 2011. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. A Computer Program+ for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Denver, CO, USA, 1999; p. 312.
- Girault, F.; Viveiros, F.; Silva, C.; Thapa, S.; Pacheco, J.E.; Adhikari, L.B.; Bhattarai, M.; Koirala, B.P.; Agrinier, P.; France-Lanord, C.; et al. Radon signature of CO2 flux constrains the depth of degassing: Furnas volcano (Azores, Portugal) versus Syabru-Bensi (Nepal Himalayas). Sci. Rep. 2022, 12, 10837. [Google Scholar] [CrossRef] [PubMed]
- Perelman, A.I. Geochemistry of Elements in the Hypergenesis Zone; Nedra: Moscow, Russia, 1972; 288p. (In Russian) [Google Scholar]
- Yun, U.; Kim, M.S.; Jeong, D.H.; Hwang, J.H.; Cho, B.W. Uranium and radon concentrations in groundwater of the Daejeon granite area: Comparison with other granite areas. J. Eng. Geol. 2018, 28, 631–643. [Google Scholar]
- Kharaka, Y.; Hitchon, B.; Hanor, J. Groundwater and Petroleum; The Groundwater Project: Guelph, ON, Canada, 2023; 375p. [Google Scholar] [CrossRef]
- Gaskova, O.L.; Bukaty, M.B. Solubility of uranium dioxide during underground disposal of radioactive wastes and spent nuclear fuel. Chem. Sustain. Dev. 2004, 12, 649–655. [Google Scholar]
- Smedley, P.L.; Kinniburgh, D.G. Uranium in natural waters and the environment: Distribution, speciation and impact. Appl. Geochem. 2023, 148, 105534. [Google Scholar] [CrossRef]
- Schubert, M.; Paschke, A.; Lieberman, E.; Burnett, W. Air-water partitioning of Rn-222 and its dependence on water temperature and Salinity. Environ. Sci. Technol. 2012, 46, 3905–3911. [Google Scholar] [CrossRef] [PubMed]
- Nevinsky, I.; Tsvetkova, T.; Nevinskaya, E. Measurement of radon in ground waters of the Western Caucasus for seismological application. J. Environ. Radioact. 2015, 149, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Demonova, A.Y.; Kharitonova, N.A.; Chelnokov, G.A.; Bragin, I.V.; Tarasenko, I.V. Microcomponent composition of low-mineralized nitrogen thermes of the Gissar Ridge (Pamir-Alay Mountain system). Bull. Tomsk. Polytech. Univ. Geo. Аssets Eng. 2019, 330, 7–20. [Google Scholar]
- Zuddas, P. Water-Rock Interaction Processes Seen through Thermodynamics. Elements 2010, 6, 305–308. [Google Scholar] [CrossRef]
- Wood, W. Hydrogeochemistry—A Journey of Discovery; The Groundwater Project: Guelph, ON, Canada, 2025; 262p. [Google Scholar] [CrossRef]
- Key, R.M.; Guinasso, M.L.; Schink, D.R. Emanation of 222Rn from marine sediments. Mar. Chem. 1979, 7, 221–250. [Google Scholar] [CrossRef]
- Bogomolov, G.V.; Gribik, Y.G. Radioactivity of Groundwater as a Search Criterion for Oil and Gas Potential (Using the Pripyat Trough as an Example) Minsk, Science and Technology. 1982. Available online: http://www.geolib.ru/OilGasGeo/1988/04/Stat/stat10.html (accessed on 4 July 2025). (In Russian).
- Liu, Q.; Tian, L.D.; Wang, J.L.; Wen, R.; Weng, Y.B.; Shen, Y.P.; Vladislav, M.; Kanaev, E. A study of longitudinal and altitudinal variations in surface water stable isotopes in West Pamir, Tajikistan. Atmos. Res. 2014, 153, 10–18. [Google Scholar] [CrossRef]
- Vasil’chuk, Y.; Chizhova, J.; Frolova, N.; Budantseva, N.; Kireeva, M.; Oleynikov, A.; Tokarev, I.; Rets, E.; Vasil’chuk, A. A variation of stable isotope composition of snow with altitude on the Elbrus mountain, Central Caucasus. Geogr. Environ. Sustain. 2020, 13, 172–182. [Google Scholar] [CrossRef]
- Plyusnin, A.M.; Zamana, L.V.; Shvartsev, S.L.; Tokarenko, O.G.; Chernyavskii, M.K. Hydrogeochemical peculiarities of the composition of nitric thermal waters in the Baikal Rift Zone. Russ. Geol. Geophys. 2013, 54, 495–508. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Tian, J.; He, M.; Li, J.; Dong, J.; Li, L.; Li, Z.; Xing, L.; Zheng, G. Constraints on geological structures and dynamics along the Tian Shan-Pamir orogenic belt with helium isotopes in hot springs. Appl. Geochem. 2024, 160, 105859. [Google Scholar] [CrossRef]
- Aydarkozhina, A.S.; Lavrushin, V.Y.; Ermakov, A.V.; Chelnokov, G.A.; Zhang, L. CO2-rich thermal waters of the Neutrino research tunnel (Baksan Neutrino observatory, North Caucasus). Dokl. Earth Sci. 2024, 515, 526–534. [Google Scholar] [CrossRef]
- Sharif-Zade, V.B.; Verkhovskii, A.B.; Loktev, V.A.; Markov, I.M.; Meshik, A.P.; Shukolyukov, Y.A. Isotopes of noble gases in the nitrogen thermal waters and carbon dioxide waters of the southern Pamir. Geokhimiya 1988, 8, 1187–1198. (In Russian) [Google Scholar]
- Polyak, B.G.; Tolstikhin, I.N.; Yakovlev, L.E.; Marty, B.; Cheshko, A.L. Helium isotopes, tectonics and heat flow in the Northern Caucasus. Geochim. Cosmochim. Acta 2000, 64, 1925–1944. [Google Scholar] [CrossRef]
- US EPA (17 September 2013). “Basic Information About Radionuclides in Drinking Water”. Available online: http://water.epa.gov/drink/contaminants/basicinformation/radionuclides.cfm (accessed on 3 July 2025).
- WHO (World Health Organization). Uranium in Drinking Water, Background Document for Development of WHO Guide-Lines for Drinking-Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2004; Volume 1, pp. 197–209. [Google Scholar]
- Federal Center of Hygiene and Epidemiology. Maximum Allowable Concentrations (MACs) of Chemicals in the Water of Water Objects Used for Drinking and Domestic Recreation Purposes; Federal Center of Hygiene and Epidemiology Publisher: Moscow, Russia, 2008; 11p. (In Russian) [Google Scholar]
- NRB 99/2009; Radiation Safety Standards. Federal Center for Hygiene and Epidemiology of Rospotrebnadzor: Moscow, Russia, 2009.
- Kulikov, G.V.; Zhevlakov, A.V.; Bondarenko, S.S. Mineral Therapeutic Waters of the USSR; A Reference Book; Nedra: Moscow, Russia, 1991. (In Russian) [Google Scholar]
- Nuccetelli, C.; Risica, S. Societe Francaise de Radioprotection—SFRP, BP72, 92263 Fontenay-aux-Roses Cedex (France). Radionu-clides in drinking water: The approach of the European Union, the World Health Organization and the US Environmental Protection Agency, from knowledge to action. In Proceedings of the Second European IRPA Congress on Radiation Protection—Radiation Protection, Paris, France, 15–19 May 2006. [Google Scholar]
- Malakootian, M.; Khashi, Z.; Iranmanesh, F.; Rahimi, M. Radon concentration in drinking water in villages nearby Rafsanjan fault and evaluation the annual effective dose. J. Radioanal Nucl.Chem. 2014, 302, 1167–1176. [Google Scholar] [CrossRef]
- Karpenko, E.I.; Sanzharova, N.I.; Spiridonov, S.I.; Serebryakov, I.S. Radioeco-logical situation in the area, where the former uranium mining company “Almaz” was located. Radiat. Risk 2009, 4, 73–81. (In Russian) [Google Scholar]
- Miklyaev, P.S.; Petrova, T.B.; Shchitov, D.V.; Sidyakin, P.A.; Murzabekov, M.A.; Marennyy, A.M.; Nefedov, N.A.; Sapozhnikov, Y.A. The results of long-term simultaneous measurements of radon exhalation rate, radon concentrations in soil gas and groundwater in the fault zone. Appl. Radiat. Isot. 2021, 167, 109460. [Google Scholar] [CrossRef] [PubMed]
- Fillimonova, E.; Kharitonova, N.; Baranovskaya, E.; Maslov, A.; Aseeva, A. Geochemistry and therapeutic properties of Caucasian mineral waters: A review. Environ. Geochem. Health 2022, 44, 2281–2299. [Google Scholar] [CrossRef] [PubMed]



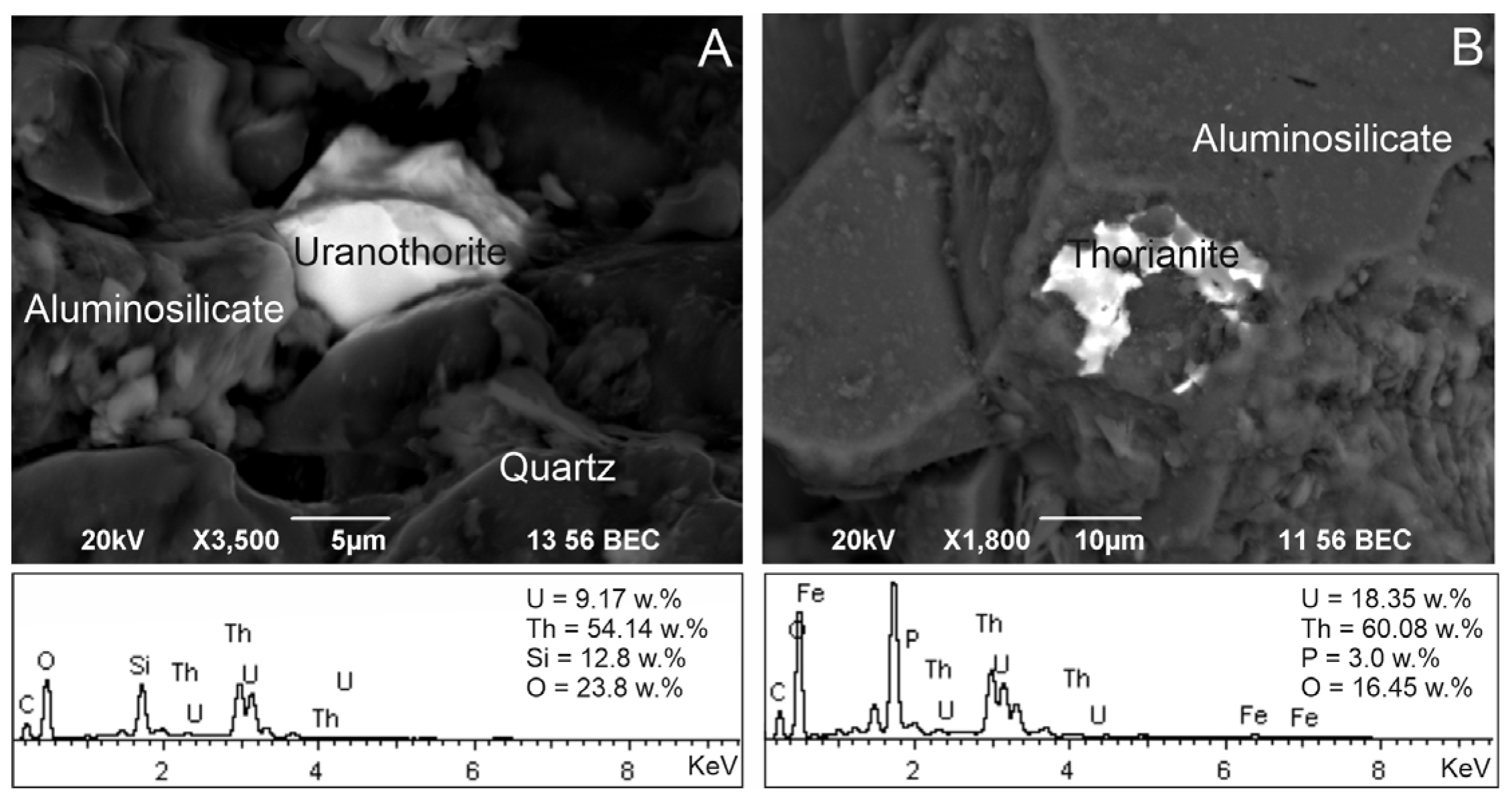
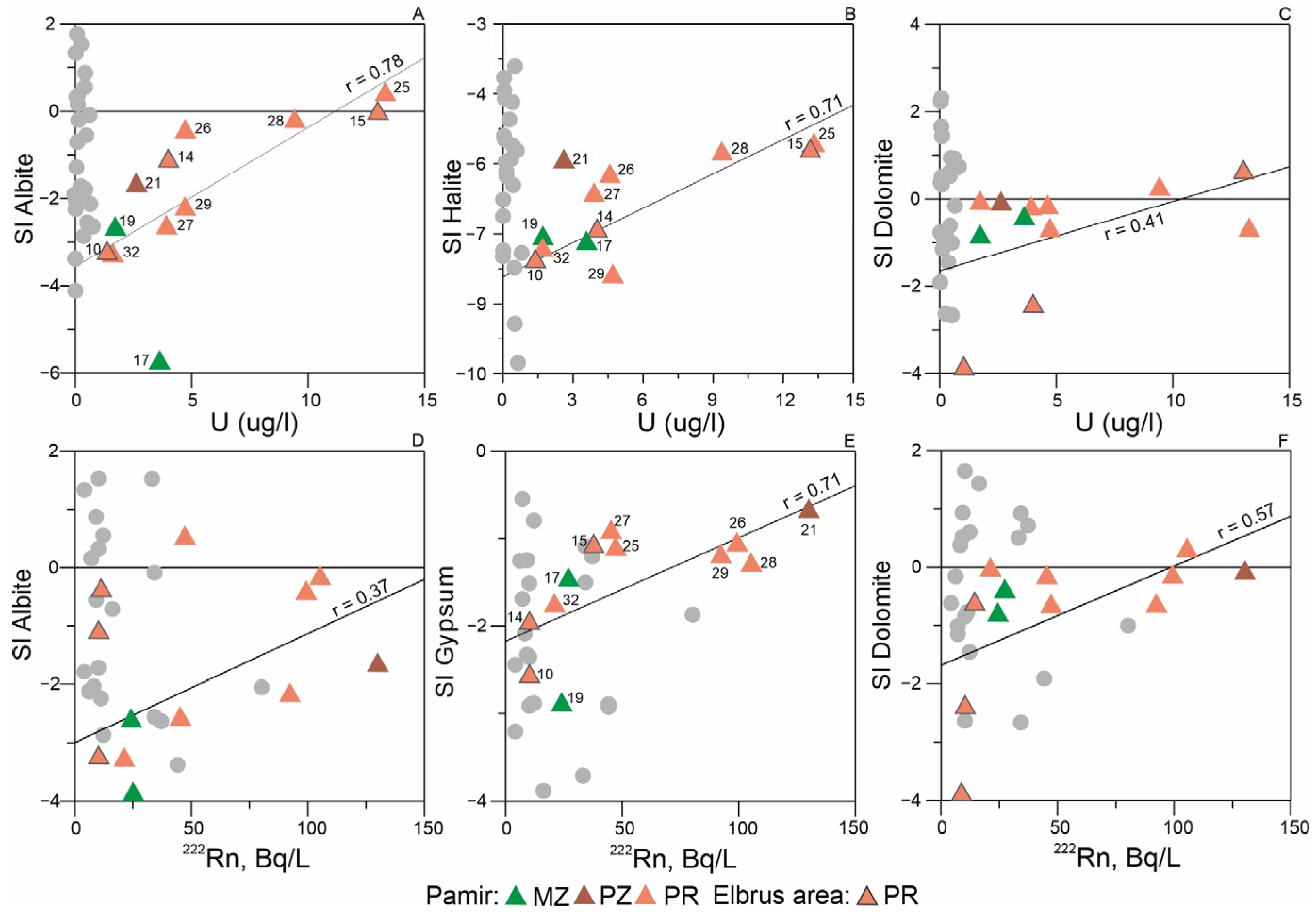
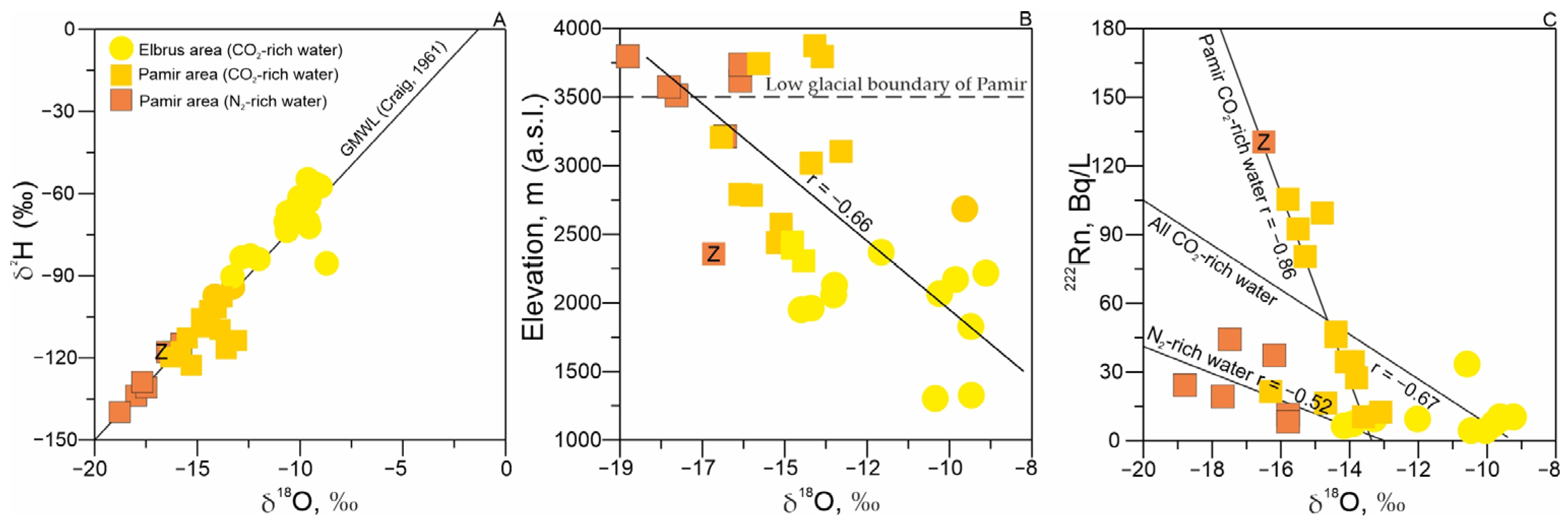

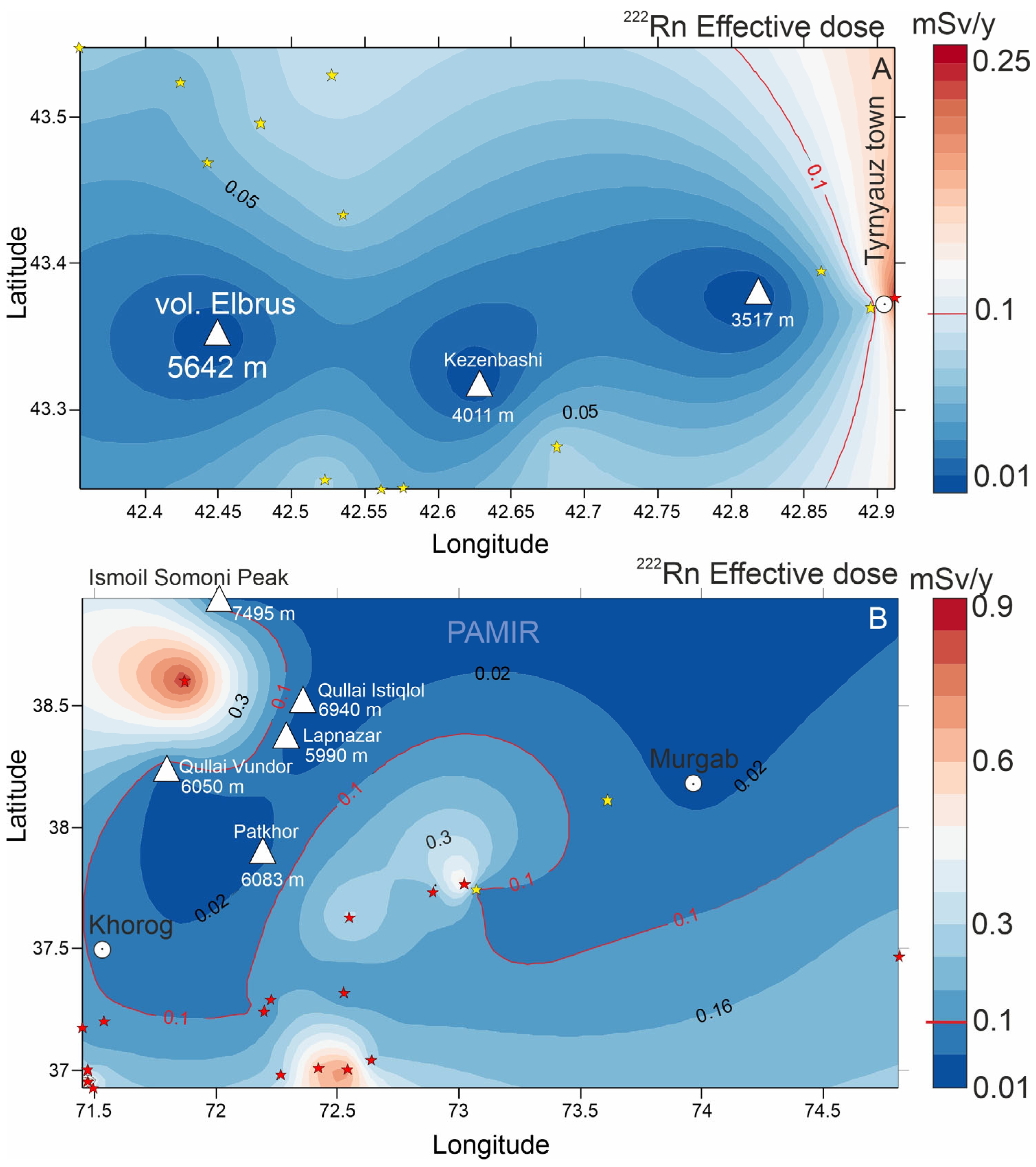
| № | Name | Age | Host Rocks Types | Elevation m. a.s.l | Type | TDS, g/L | T, °C | pH | Eh, mV | 238U | Th | δ18O | δ2H | 222Rn | N2 | CH4 | CO2 | He | δ13C(CO2) | δ13C(CH4) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/L | VSMOW, ‰ | Bq/L | % | ‰, VPDB | ‰, VPDB | |||||||||||||||
| Rock | Water | Associated Gases | ||||||||||||||||||
| Mineral Waters of the Elbrus (№ 1-15) and Pamir (№ 16-34) Areas | ||||||||||||||||||||
| 1 | Tyrnyauz park (well) | KZ | Granites (Eldzurty) | 1296 | Na-HCO3-Cl | 5.4 | 17 | 6.7 | −71 | 0.07 | 0.02 | −10.6 | −72.1 | 33 | 21.4 | 1.22 | 76.7 | 0.032 | −11 | −61.1 |
| 2 | Parametric (well) | 1320 | Na-HCO3 | 5.7 | 17 | 6.4 | −50 | 0.25 | 0.002 | −9.6 | −70.5 | 10 | 8.6 | 0.45 | 90.4 | 0.001 | −7.3 | −22.3 | ||
| 3 | Shaushiib (spring) | Granodiorite, andesite, dacite | 1820 | Na-HCO3-Cl | 10.6 | 10 | 6.4 | 43 | 0.46 | 0.007 | −9.6 | −54.9 | 9 | 0.95 | 0.011 | 98.8 | 0.008 | −5.7 | ||
| 4 | Toxana (low spring) | 2163 | Na-HCO3 | 3.0 | 5.4 | 6.2 | −48 | 0.04 | 0.03 | −10.1 | −61.7 | 7 | 0.45 | 0.012 | 98.9 | 0.001 | −5.8 | −63.1 | ||
| 5 | Ingushli (upper spring) | PZ | Sedimentary and volcanic rocks | 2209 | Na-HCO3-Cl | 1.9 | 6.4 | 5.9 | 54 | 0.22 | 0.009 | −9.2 | −56.7 | 10 | 0.7 | 0.008 | 98.7 | 0.001 | - | |
| 6 | Toxana (upper spring) | 2677 | Ca-HCO3-SO4 | 1.9 | 7.2 | 6.3 | 107 | 0.55 | 0.002 | −9.8 | −62.7 | 7 | 58.3 | 0.13 | 40.6 | 0.048 | −9.5 | −48.3 | ||
| 7 | Djilusu (well) | 2362 | Na-Ca-HCO3 | 3.1 | 22 | 6.4 | −14 | 0.41 | 0.007 | −12.0 | −84.2 | 9 | 0.6 | 0.005 | 98.9 | 0.001 | −5.8 | −18.3 | ||
| 8 | Neutrino tunnel (lake) | PR | Granites, gneisses, crystalline schists | 2060 | Na-HCO3-Cl | 8.5 | 34 | 6.8 | 92 | 0.05 | 0.02 | −8.4 | −83.9 | 7 | 1.8 | 0.39 | 97.1 | 0.001 | −7.3 | −26 |
| 9 | Neutrino tunnel (spring) | 2060 | Na-Cl-HCO3 | 7.3 | 41 | 6.7 | 19 | 0.05 | 0.02 | −10.5 | −66.8 | 6 | 1.6 | 0.39 | 97.1 | 0.001 | −8 | |||
| 10 | Terskol (spring) | 2121 | Ca-HCO3-Cl | 0.3 | 9.6 | 5.8 | 61 | 1.37 | 0.34 | −13.3 | −94.5 | 10 | 0.9 | 0.38 | 97.3 | 0.001 | −7.2 * | |||
| 11 | Poliana Narzanov (well) | 1954 | Ca-Na-HCO3 | 2.3 | 12 | 6.1 | 10 | 1.51 | 0.007 | −13.9 | −98.3 | 7 | 0.9 | 0.001 | 97.8 | 0.003 | −8.3 | |||
| 12 | Badaevka (well) | 1942 | Ca-HCO3 | 1.3 | 17 | 6.7 | 39 | 0.63 | 0.002 | −14.2 | −97.5 | 6 | 59 | 0.15 | 37.7 | 0.009 | −9.2 | |||
| 13 | Ingushli (low spring) | 1940 | Na-HCO3-Cl | 1.5 | 16 | 6.5 | 32 | 0.35 | 0.005 | −7.9 | −50.2 | 12 | 1 | 0.01 | 98.8 | 0.0004 | −4.8 | |||
| 14 | Serebryni Klych (well) | 2050 | Ca-HCO3 | 0.6 | 15 | 6.4 | 0 | 3.74 | 0.001 | −13.3 | −90.5 | 10 | 62.8 * | 0.0002 | 30.1 * | 0.014 | −8.0 * | |||
| 15 | Baksan (upper spring) | 1557 | Ca-HCO3-SO4 | 2.4 | 14 | 6.1 | 35 | 0.13 | 0.0001 | −10.6 | −73.8 | 6 | still | still | still | still | ||||
| 16 | Madjura (spring) | MZ | Granitoids | 3737 | Na-HCO3 | 1.5 | 51 | 6.6 | −140 | 0.17 | 0.006 | −15.3 | −123 | 80 | 40.9 | 3.55 | 53.8 | 0.48 | −8.8 | −27.9 |
| 17 | Kizilrabat (spring) | 3867 | Ca-HCO3 | 1.3 | 39 | 6.3 | 0 | 3.6 | 0.006 | −13.8 | −98 | 27 | 19.6 | 0.16 | 79.7 | 0.021 | −4.4 | |||
| 18 | Yashikul (geyser) | 3788 | Na-HCO3 | 4.0 | 41 | 7.3 | −15 | 0.05 | 0.014 | −13.6 | −117 | 10 | 2 | 0.47 | 97.2 | 0.11 | −5.7 | −29.2 | ||
| 19 | Sasik-Bulak (well) | 3792 | Na-HCO3 | 0.4 | 32 | 8.7 | 27 | 1.7 | 0.007 | −18.8 | −140 | 24 | 90.6 | 0.001 | 8.1 | 0.36 | −16.9 | |||
| 20 | Djelandy (well) | 3568 | Na-SO4 | 0.4 | 79 | 9.4 | −224 | 0.004 | 0.006 | −17.7 | −129 | 44 | 97 | 0.72 | 0.12 | 0.07 | −20.9 | −18.9 | ||
| 21 | Zagitor (spring) | PZ | Igneous and metamorphic rocks | 2349 | Na-Ca-SO4-Cl | 1.8 | 21 | 7.1 | 35 | 2.6 | 0.014 | −16.5 | −117 | 130 | 94.1 | 0.0002 | 3.2 | 0.3 | −12.6 | |
| 22 | Modyan (spring) | 3733 | Na-HCO3 | 1.1 | 63 | 7.5 | 18 | 0.02 | 0.01 | −15.8 | −115 | 8 | 88.3 | 0.74 | 8.1 | 0.12 | −13.9 | −17.6 | ||
| 23 | Garm-Chashma | PR | Gneisses, marbles, migmatites, evaporites | 2566 | Na-HCO3 | 3.3 | 60 | 6.7 | −281 | 0.08 | 0.006 | −14.7 | −109 | 16 | 1.7 | 0.009 | 97.8 | 0.001 | −4.5 | |
| 24 | Sist (spring) | 2300 | Ca-HCO3-SO4 | 0.9 | 11 | 5.9 | 98 | 0.5 | 0.014 | −14.1 | −102 | 34 | still | still | still | still | s | |||
| 25 | Barshor (spring) | 2390 | Ca-Na-HCO3-Cl | 2.9 | 15 | 6.1 | 95 | 13.3 | 0.006 | −14.3 | −104 | 47 | 2 | 0.001 | 97.2 | 0.003 | −5.3 | |||
| 26 | AVJD (low spring) | 2431 | Ca-Na-HCO3 | 1.8 | 31 | 6.4 | 135 | 3.9 | 0.014 | −14.5 | −106 | 99 | still | still | still | still | still | |||
| 27 | AVJD (well) | 2436 | Ca-HCO3 | 1.8 | 35 | 6.2 | 69 | 4.6 | 0.015 | −14.1 | −103 | 45 | still | still | still | still | still | |||
| 28 | Sherigin (spring) | 2783 | Ca-Na-HCO3-Cl | 2.7 | 34 | 6.3 | 0 | 9.4 | 0.010 | −15.8 | −118 | 105 | 0.3 | 0.001 | 99.5 | 0.0001 | −5.2 | |||
| 29 | Vranch (spring) | 2776 | Ca-HCO3 | 1.9 | 19 | 6.1 | 26 | 4.7 | 0.005 | −15.5 | −113 | 92 | 6.7 | 0.013 | 92.9 | 0.053 | −5.8 | |||
| 30 | Sovetbond (spring) | 3096 | Na-HCO3-Cl | 6.1 | 23 | 6.4 | −6 | 0.4 | 0.010 | −13.1 | −114 | 12 | 0.28 | 0.0002 | 99.6 | 0.0009 | −7.9 | |||
| 31 | Sharalai (spring) | 3010 | Na-HCO3 | 2.7 | 44 | 7.1 | 71 | 0.6 | 0.011 | −13.9 | −110 | 34 | 2.2 | 0.09 | 97 | 0.01 | −5.7 | |||
| 32 | Kauk (spring) | 3199 | Ca-HCO3 | 0.5 | 34 | 7.3 | 76 | 1.7 | 0.003 | −16.3 | −120 | 21 | still | still | still | still | still | |||
| 33 | Bibi-Fatima (spring) | 3208 | Ca-SO4-HCO3 | 0.7 | 45 | 7.6 | 83 | 0.75 | 0.005 | −16.2 | −118 | 37 | still | still | still | still | still | |||
| 34 | Naspar (spring) | 3614 | Na-SO4-HCO3 | 0.6 | 22 | 9.4 | −101 | 0.01 | 0.009 | −15.8 | −117 | 11 | 98 | 0.08 | 0.11 | 0.3 | −10.9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelnokov, G.; Lavrushin, V.; Kharitonova, N.; Pavlov, A.; Salikhov, F. Peculiarities of 222Radon and 238Uranium Behavior in Mineral Waters of Highland Terrains. Water 2025, 17, 2211. https://doi.org/10.3390/w17152211
Chelnokov G, Lavrushin V, Kharitonova N, Pavlov A, Salikhov F. Peculiarities of 222Radon and 238Uranium Behavior in Mineral Waters of Highland Terrains. Water. 2025; 17(15):2211. https://doi.org/10.3390/w17152211
Chicago/Turabian StyleChelnokov, George, Vasilii Lavrushin, Natalya Kharitonova, Andrey Pavlov, and Farid Salikhov. 2025. "Peculiarities of 222Radon and 238Uranium Behavior in Mineral Waters of Highland Terrains" Water 17, no. 15: 2211. https://doi.org/10.3390/w17152211
APA StyleChelnokov, G., Lavrushin, V., Kharitonova, N., Pavlov, A., & Salikhov, F. (2025). Peculiarities of 222Radon and 238Uranium Behavior in Mineral Waters of Highland Terrains. Water, 17(15), 2211. https://doi.org/10.3390/w17152211







