Advanced Treatment of High-Concentration Ammonia–Nitrogen Wastewater by Pantothenic Acid-Enhanced Photosynthetic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
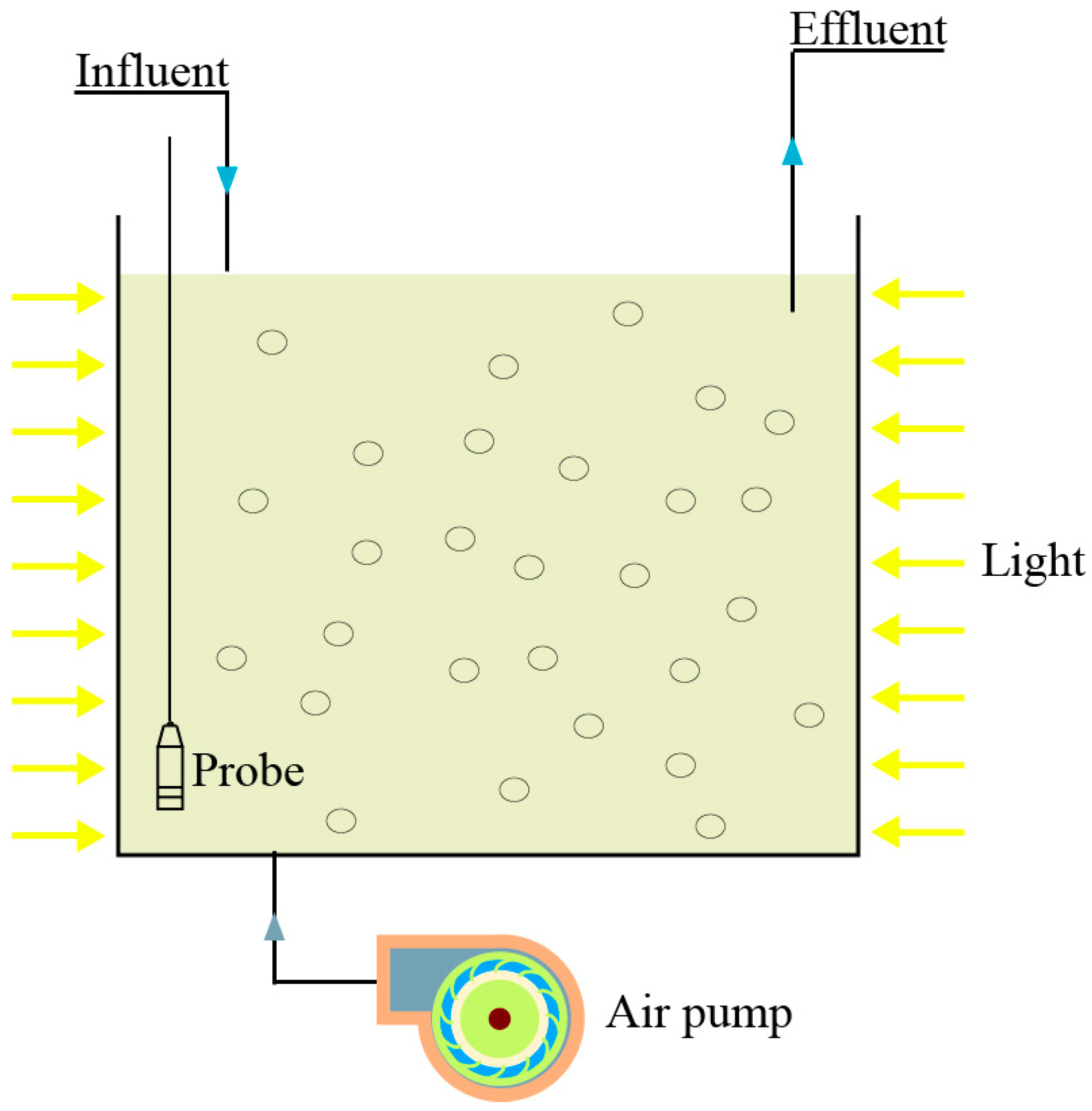
2.2. Experimental Methods
2.3. Analytical Methods
3. Results and Discussions
3.1. Effects of Different Pantothenic Acid Concentrations on PSB
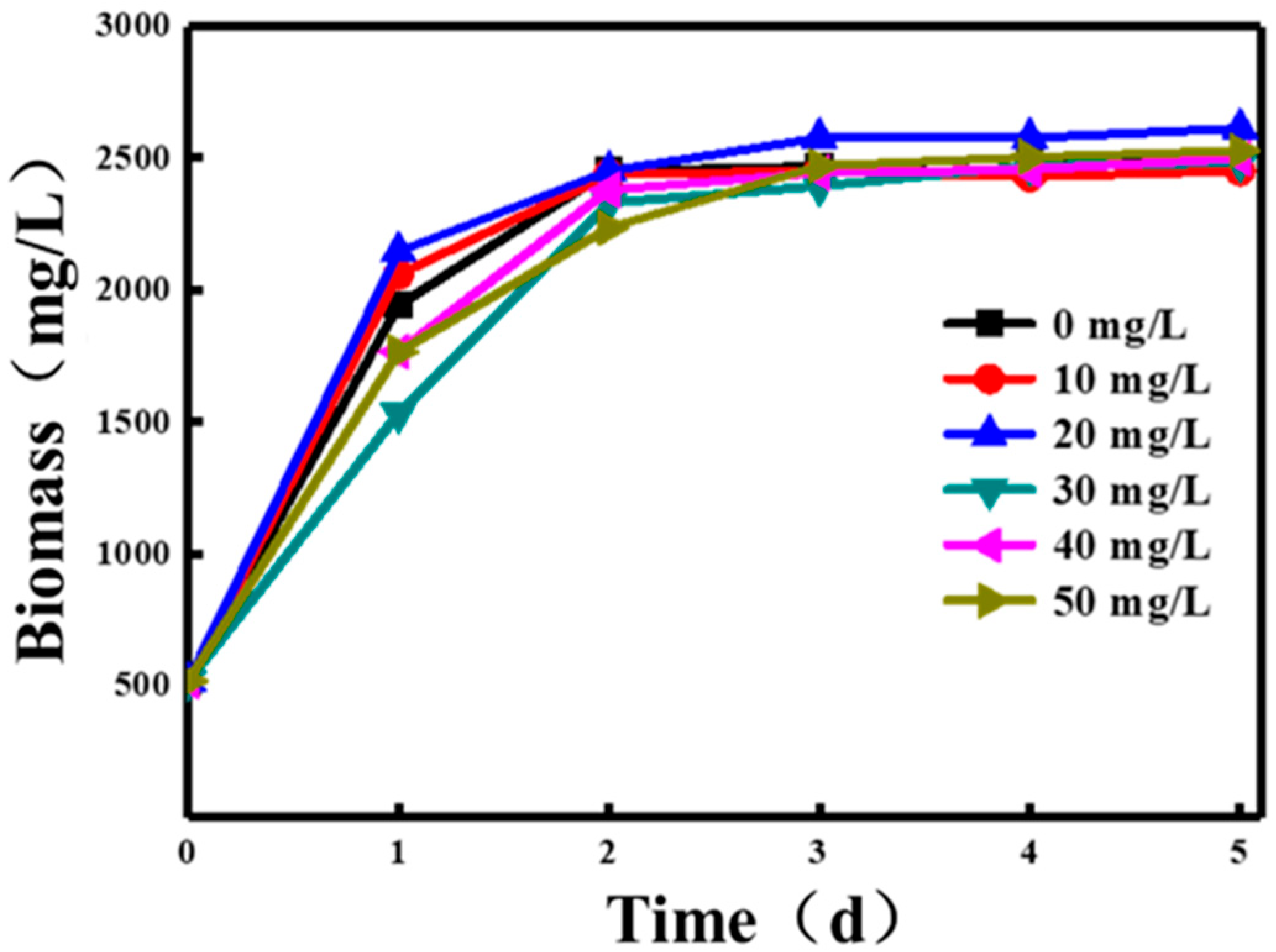
3.1.1. Effects of Pantothenic Acid Concentration on Photosynthetic Bacterial Biomass
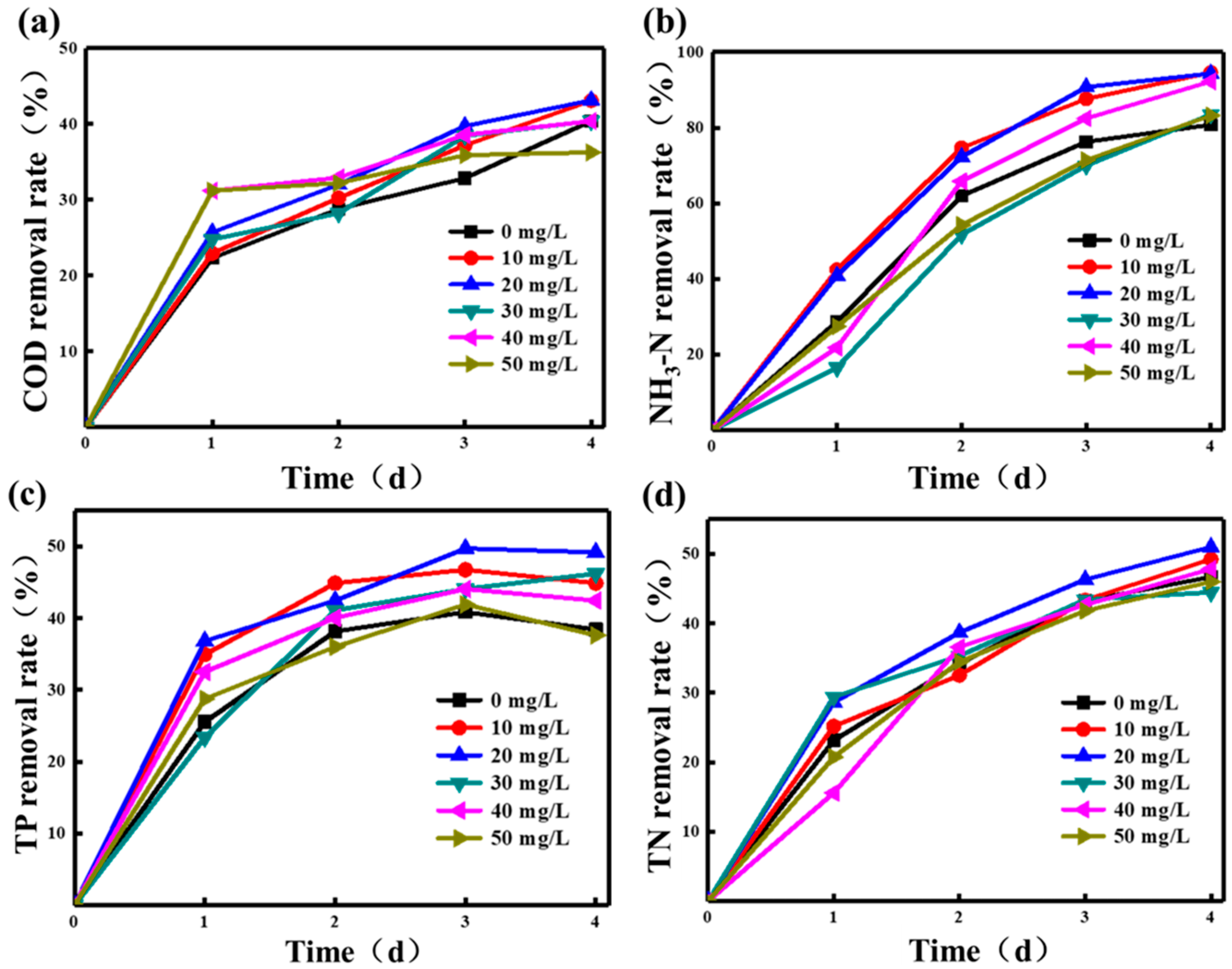
3.1.2. Effects of Pantothenic Acid Addition on Wastewater Treatment
3.2. Effects of Different Cultivation Methods on Photosynthetic Bacterial Growth
3.3. Effects of Different Cultivation Method Ratios on PSB
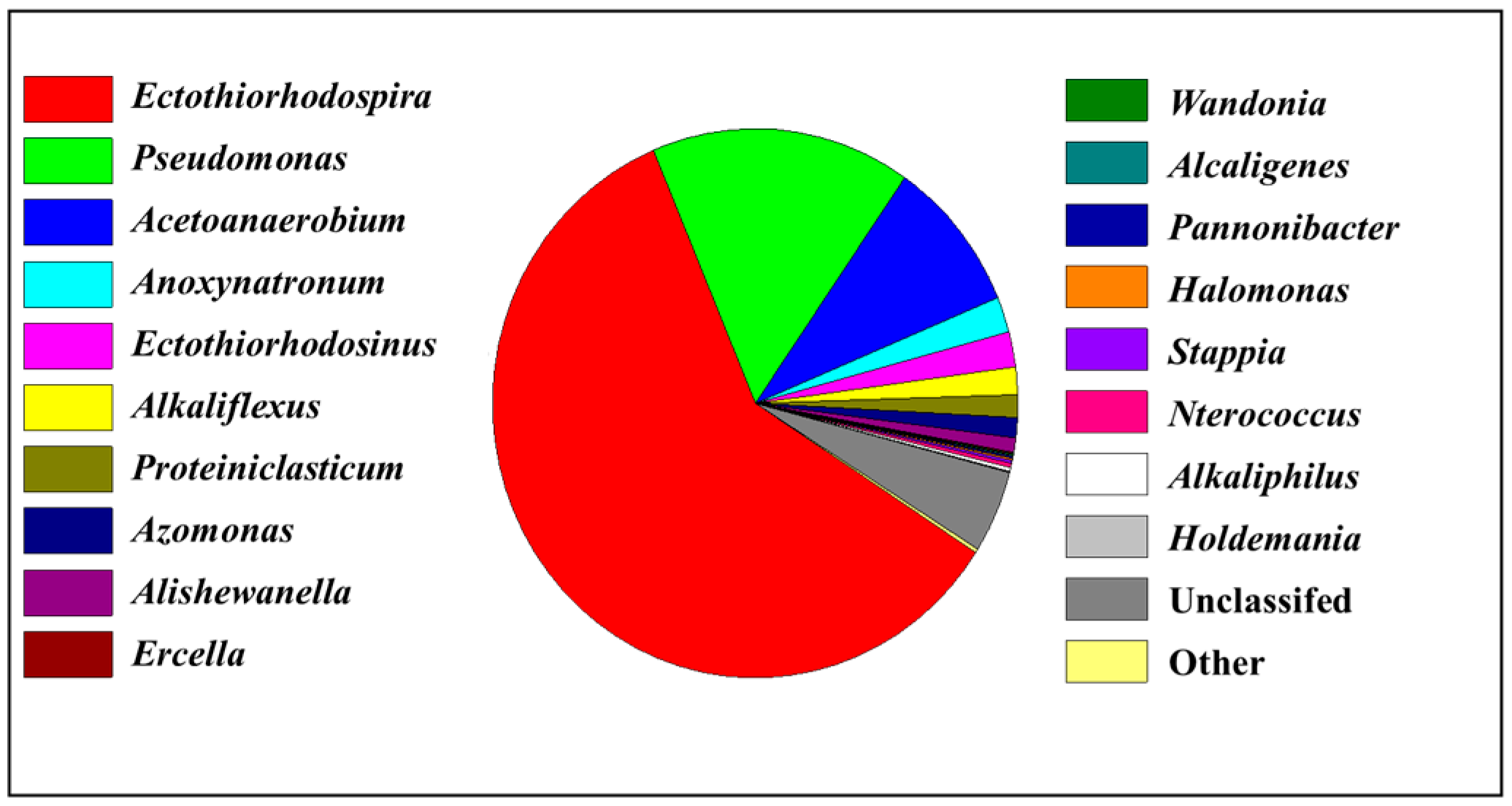
4. Comparison of Biomass Accumulation Among Different PSB
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, G.; Zhang, J.; Liu, H.; Liu, S.; Lv, L.; Liang, J. Treatment of high salinity organic wastewater by photosynthetic bacteria to produce value-added products: Performance, community and metabolic characteristics. J. Water Process Eng. 2024, 64, 105593. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Z.; Hu, M.; Zhao, J.; Zhou, P.; Zhang, Y.; Zheng, X.; Zhang, Y.; Hu, Z.T.; Pan, Z. Cometabolic biodegradation system employed subculturing photosynthetic bacteria: A new degradation pathway of 4-chlorophenol in hypersaline wastewater. Bioresour. Technol. 2022, 361, 127670. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, P.; Zhang, R.; Chang, J.; Chen, L.; Zhang, G.; Wang, A. Bioconversion of volatile fatty acids from organic wastes to produce high-value products by photosynthetic bacteria: A review. Environ. Res. 2024, 242, 117796. [Google Scholar] [CrossRef]
- Ji, J.; Pan, J.; Guo, K.; Liu, J.; Liu, Y.; Wu, F.; Yin, F.; Zhang, W. Improving anaerobic digestion performance of cassava residues through utilization of photosynthetic bacteria under varying light intensities. J. Environ. Chem. Eng. 2025, 13, 115883. [Google Scholar] [CrossRef]
- Zhi, R.; Yang, A.; Zhang, G.; Zhu, Y.; Meng, F.; Li, X. Effects of light-dark cycles on photosynthetic bacteria wastewater treatment and valuable substances production. Bioresour. Technol. 2019, 274, 496–501. [Google Scholar] [CrossRef]
- Deng, Z.; Sun, C.; Ma, G.; Zhang, X.; Guo, H.; Zhang, T.; Zhou, X.; Zhang, Y.; Hu, Y.; Li, D.; et al. Anaerobic treatment of refractory industrial organic wastewater: A review of bioenergy recovery and low-carbon nitrogen removal toward carbon neutrality. J. Water Process Eng. 2025, 72, 107515. [Google Scholar] [CrossRef]
- Feng, Y.; Pang, Y.; Xu, Y.; Zhang, B.; Guan, X.; Qiao, X.; Lu, X. Resource recovery and safe discharge of food wastewater based on membrane treatment technology: A case of mixed processing wastewater from potato and sweet potato. J. Food Eng. 2025, 399, 112626. [Google Scholar] [CrossRef]
- Bhambhani, A.; Jovanovic, O.; Kjerstadius, H.; Trapani, D.D.; Mannina, G.; van der Hoek, J.P.; Kapelan, Z. A novel water-food-energy framework for a comprehensive assessment of resource recovery from wastewater treatment plants. J. Clean. Prod. 2025, 489, 144716. [Google Scholar] [CrossRef]
- Liu, S.; Shen, X.; Daigger, G.T.; Zhang, G.; Kang, J.; Song, G.; Li, G.; Yang, G. Mechanism regulation, production and potential of high value substances in the wastewater treatment by immobilized photosynthetic bacteria: A review. J. Water Process Eng. 2024, 58, 104770. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Daigger, G.T.; Huang, J.; Song, G. Material biosynthesis, mechanism regulation and resource recycling of biomass and high-value substances from wastewater treatment by photosynthetic bacteria: A review. Sci. Total Environ. 2022, 820, 153200. [Google Scholar] [CrossRef] [PubMed]
- Deseure, J.; Obeid, J.; Willison, J.C.; Magnin, J.P. Reliable determination of the growth and hydrogen production parameters of the photosynthetic bacterium Rhodobacter capsulatus in fed batch culture using a combination of the Gompertz function and the Luedeking-Piret model. Heliyon 2021, 7, e07394. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, P.; Zhang, G. Enhancement of cell production in photosynthetic bacteria wastewater treatment by low-strength ultrasound. Bioresour. Technol. 2014, 161, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Chaiyarat, A.; Saejung, C. Photosynthetic bacteria with iron oxide nanoparticles as catalyst for cooking oil removal and valuable products recovery with heavy metal co-contamination. Waste Manag. 2022, 140, 81–89. [Google Scholar] [CrossRef]
- Wu, P.; Xu, X. Fe2+ enhancing biomass production and soybean wastewater treatment of photosynthetic bacteria through regulation of aerobic respiration. Environ. Technol. Innov. 2022, 28, 102701. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Q.; Meng, Q.; Fan, Z.; He, J.; Liu, T.; Shen, C.; Zhang, G. Anoxic oscillating MBR for photosynthetic bacteria harvesting and high salinity wastewater treatment. Bioresour. Technol. 2017, 224, 69–77. [Google Scholar] [CrossRef]
- Saejung, C.; Chanthakhot, T. Single-phase and two-phase cultivations using different light regimes to improve production of valuable substances in the anoxygenic photosynthetic bacterium Rhodopseudomonas faecalis PA2. Bioresour. Technol. 2021, 328, 124855. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, X.-Q.; Wu, P.; Jiang, W.-D.; Liu, Y.; Tang, J.-Y.; Zhang, R.-N.; Zhang, L.; Mi, H.-F.; Feng, L. The role of pantothenic acid in alleviating hypoxia-induced liver injury of sub-adult grass carp (Ctenopharyngodon idellus): Possible mechanisms and implication. Aquaculture 2024, 590, 741083. [Google Scholar] [CrossRef]
- Zou, S.; Liu, J.; Zhao, K.; Zhu, X.; Zhang, B.; Liu, Z.; Zheng, Y. Metabolic engineering of Escherichia coli for enhanced production of D-pantothenic acid. Bioresour. Technol. 2024, 412, 131352. [Google Scholar] [CrossRef] [PubMed]
- Bedani, R.; Cucick, A.C.C.; Albuquerque, M.A.C.; LeBlanc, J.G.; Saad, S.M.I. B-Group Vitamins as Potential Prebiotic Candidates: Their Effects on the Human Gut Microbiome. J. Nutr. 2024, 154, 341–353. [Google Scholar] [CrossRef]
- Qin, L.; Li, H.; Tan, Y.; Yan, X.; Tao, P.; Fan, Z.; Li, T.; Tan, J.; Wang, Y.; Jin, L. Utilizing a Novel Halotolerant Bordetella Bacterium Combined with Co-Metabolites to Boost the Degradation of P-Nitrophenol in High-Salinity Wastewater. Water 2024, 16, 3360. [Google Scholar] [CrossRef]
- Zhi, R.; Cao, K.; Zhang, G.; Zhu, J.; Xian, G. Zero excess sludge wastewater treatment with value-added substances recovery using photosynthetic bacteria. J. Clean. Prod. 2020, 250, 119581. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Du, Z.; Liu, S.; Zhang, J.; Lu, H.; Lv, L.; Liang, J.; Tian, Y. Production of polyhydroxybutyrate with photosynthetic bacteria treating artificial kitchen waste: Effect of light intensity. J. Environ. Chem. Eng. 2024, 12, 114774. [Google Scholar] [CrossRef]
- Tiang, M.F.; Hanipa, M.A.F.; Mahmod, S.S.; Zainuddin, M.T.; Lutfi, A.A.I.; Jahim, J.M.; Takriff, M.S.; Reungsang, A.; Wu, S.Y.; Abdul, P.M. Impact of light spectra on photo-fermentative biohydrogen production by Rhodobacter sphaeroides KKU-PS1. Bioresour. Technol. 2024, 394, 130222. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Sun, S.; Shao, C.; Qiu, Q.; Kong, C.; Qiu, L. Engineered stable partial nitrification/endogenous partial denitrification-anammox process for enhanced nitrogen removal from low carbon-to-nitrogen ratio wastewater. Bioresour. Technol. 2025, 428, 132466. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Li, X.; Lu, Z.; Yang, Y.; Liu, W. Heterotrophic nitrification-aerobic denitrification (HNAD) in marine aquaculture wastewater treatment: Nitrogen removal performance, mechanism and microbial community characteristics. J. Water Process Eng. 2025, 70, 107006. [Google Scholar] [CrossRef]
- Aktan, C.K.; Dinler, V.; Mesum, B.N.; Caliskan, S.N.; Ahmad, J.A.; Sahinkaya, E. A new approach for treating ammonia-rich wastewater deficient in organic matter: A tandem process for nitrification/nitritation in MBBR followed by sulfur-based denitrifying column reactor. Chem. Eng. J. 2025, 513, 163061. [Google Scholar] [CrossRef]
- Lai, Y.C.; Liang, C.M.; Hsu, S.C.; Hsieh, P.H.; Hung, C.H. Polyphosphate metabolism by purple non-sulfur bacteria and its possible application on photo-microbial fuel cell. J. Biosci. Bioeng. 2017, 123, 722–730. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, A.; Meng, F.; Zhang, G. Additives for photosynthetic bacteria wastewater treatment: Latest developments and future prospects. Bioresour. Technol. Rep. 2019, 7, 100229. [Google Scholar] [CrossRef]
- He, R.; Sun, J.; Bai, X.; Lin, Q.; Yuan, Y.; Zhang, Y.; Dai, K.; Xu, Z. A novel alginate-embedded magnetic biochar-anoxygenic photosynthetic bacteria composite microspheres for multipollutant removal: Mechanisms of photo-bioelectrochemical enhancement and excellent reusability performance. Environ. Res. 2024, 247, 118158. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Wang, H. Enhancement of photosynthetic bacteria biomass production and wastewater treatment efficiency by zero-valent iron nanoparticles. J. Biosci. Bioeng. 2020, 130, 306–310. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Q.; Yang, X.; Zhang, S.; Cao, W.; Ma, Y.; Wei, W.; Guo, L. Enhanced photo-fermentative hydrogen production by constructing Rhodobacter capsulatus-ZnO/ZnS hybrid system. Bioresour. Technol. 2024, 414, 131632. [Google Scholar] [CrossRef]
- YAttia, A.; Samer, M.; Moselhy, M.A.; Arisha, A.H.; Abdelqader, A.A.; Abdelsalam, E.M. Influence of laser photoactivated graphitic carbon nitride nanosheets and nickel nanoparticles on purple non-sulfur bacteria for biohydrogen production from biomass. J. Clean. Prod. 2021, 299, 126898. [Google Scholar] [CrossRef]
- Sun, C.; Yu, Q.; Zhao, Z.; Zhang, Y. Effects of electrical stimulation on carbon sequestration of photosynthetic bacteria: Response of the photosynthetic unit to electrical stimulation. J. Clean. Prod. 2024, 458, 142524. [Google Scholar] [CrossRef]
- Lu, H.; He, S.; Zhang, G.; Gao, F.; Zhao, R. Periodic oxygen supplementation drives efficient metabolism for enhancing valuable bioresource production in photosynthetic bacteria wastewater treatment. Bioresour. Technol. 2022, 347, 126678. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, R.; Wang, C.; Zhang, G.; Chen, C.; Li, B.; Han, T. Exploration of flashing light interaction effect on improving biomass, protein, and pigments production in photosynthetic bacteria wastewater treatment. J. Clean. Prod. 2022, 348, 131304. [Google Scholar] [CrossRef]

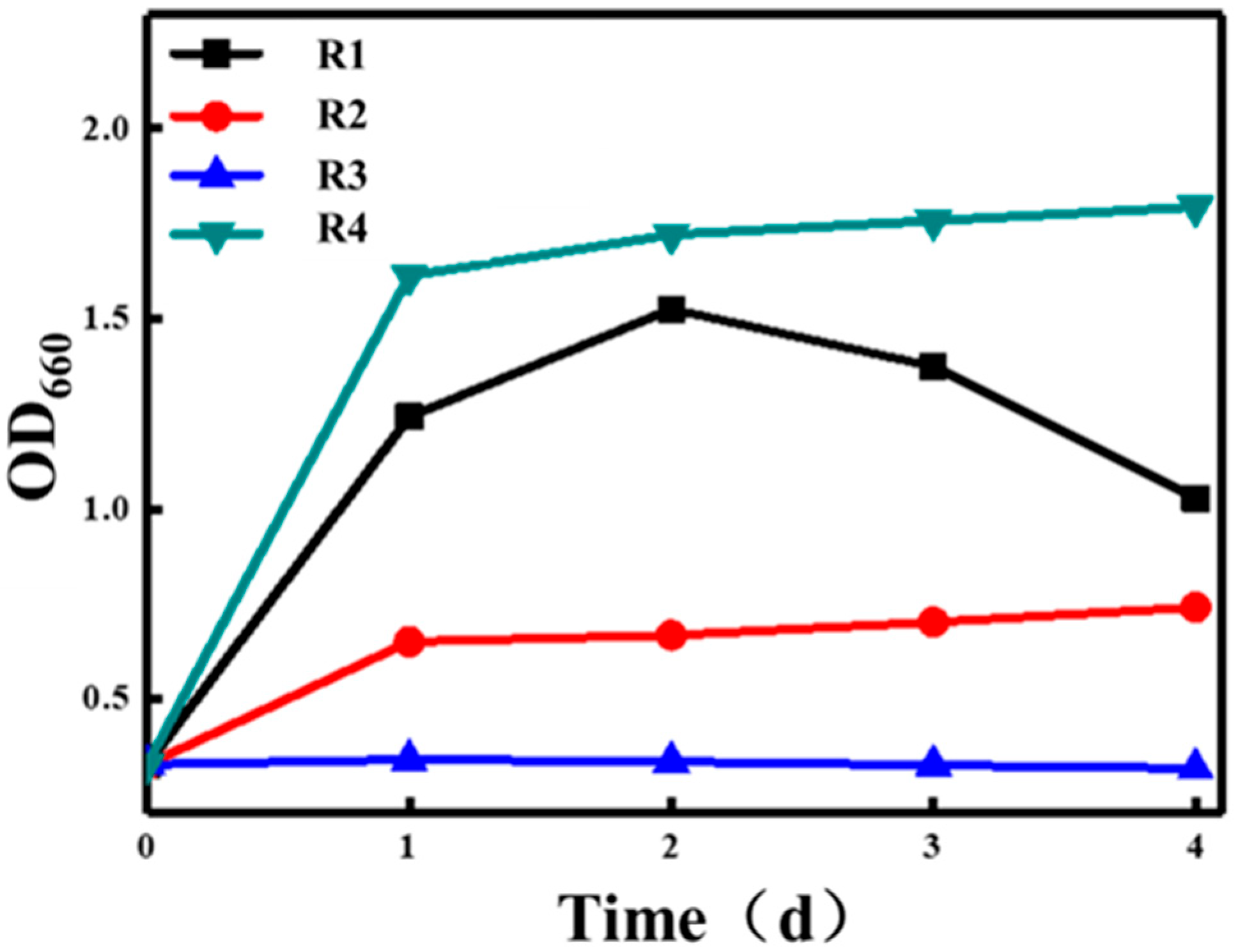
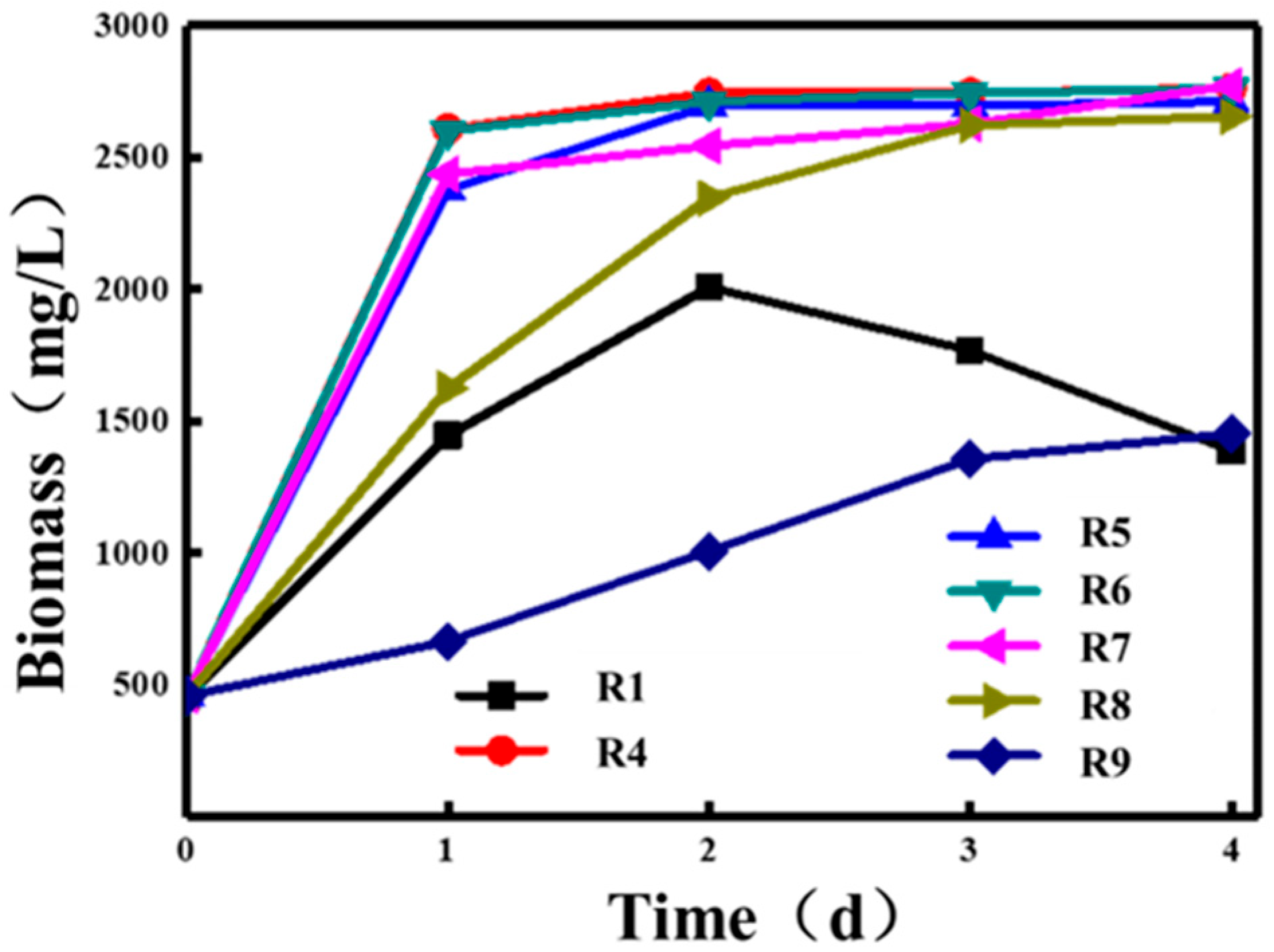

| Experiment Conditions | Groups |
|---|---|
| Dark aeration | R1 |
| Light aeration | R2 |
| Dark | R3 |
| Light | R4 |
| 4 h dark aeration + 20 h light | R5 |
| 8 h dark aeration + 16 h light | R6 |
| 12 h dark aeration + 12 h light | R7 |
| 16 h dark aeration + 8 h light | R8 |
| 20 h dark aeration + 4 h light | R9 |
| 12 h dark + 12 h light | R10 |
| Additives | Photosynthetic Bacteria (Dominant Strain) | Wastewater | Condition | Performance | Ref. |
|---|---|---|---|---|---|
| Fe3O4 nanoparticles (1 g/L) | Rhodopseudomonas faecalis PA2 | Oil wastewater (1% cooking oil) | Anoxygenic–light, 24 h | The biomass was elevated by 2.28 times, while the protein production rose by 1.7 times. | [13] |
| Zero-valent iron nanoparticles (20 mg/L) | Ectothiorhodospira | Sugar wastewater (COD 6000 mg/L, TN 423 mg/L) | Anoxygenic–light, 24 h | The biomass and COD removal rate were enhanced by 122% and 164.3%, respectively. | [30] |
| Fe2+ (20 mg/L) | Rhodobacter sphaeroides | Soybean wastewater (BOD 4370 mg/L, COD 9940 mg/L, TN 590 mg/L) | Aerobic–dark | The biomass production achieved 5000 mg/L. The maximum COD removal rate was 95%, and the HRT was decreased to 24 h. | [14] |
| Alginate-embedded magnetic biochar | Rhodopseudomonas palustris | Secondary effluent (TOC 22 mg/L, TN 50 mg/L, Sulfadiazine 1 mg/L) | Anoxygenic–light, 24 h | The removal rates of sulfadiazine and NH3-N were enhanced by 21.23% and 38%, respectively. | [29] |
| ZnO/ZnS30 (100 mg/L) | Rhodobacter capsulatus | MedA medium (Photo-fermentative) | Anoxygenic–light, 24 h | The hydrogen production increased by 30%. | [31] |
| Graphitic carbon nitride nanosheets (16.5 mg/L) | Purple non-sulfur bacteria | Acetate yeast extract basil medium | Anoxygenic–light, 24 h | Biohydrogen production was four times that of the control group, exceeding 15,000 mL. | [32] |
| Low-intensity ultrasound | Rhodopseudomonas | Artificial wastewater (NaAC 3 g/L, TN 212 mg/L) | Micro-aerobic and natural light, 0.3 W/cm2 with 40 kHz frequency | The cell yield and biomass production rose by 110% and 93%, respectively. | [12] |
| Electrical stimulation | Rhodopseudomonas palustris | Van Niel’s yeast agar medium | Anoxygenic-light, periodic power-on/power-off mode | The CO2 removal rate increased by 10%, and the activity of the key CO2 fixation enzyme Rubisco was enhanced by 16%. | [33] |
| Periodic oxygen supplementation | Ectothiorhodospira | Sugar wastewater (COD 4455 mg/L, TN 272 mg/L, TP 45 mg/L) | Periodic oxygen supplementation with oxygen off/on for every 12 h | The biomass concentration achieved 1338.5 mg/L, while the removal rates for COD and NH3-N were 91.4% and 78.6%, respectively. | [34] |
| Flashing light | Rhodopseudomonas palustris | Sugar wastewater (COD 3100-3500 mg/L, NH4+-N 197-223 mg/L, TP 130-139 mg/L) | Ia 88 μmol/m2/s, I0 147 μmol/m2/s, F 10 Hz, φ 0.6 | The concentrations of biomass and protein achieved 2815.2 mg/L and 1944.3 mg/L, respectively. These values were 23.2–31.1% and 27.4–56.4% higher than the continuous light groups. | [35] |
| Pantothenic acid (20 mg/L) | Rhodospirillum, Pseudomonas | High-concentration organic wastewater | Aerobic–light, 24 h | The biomass was 2500 mg/L. The removal rates for COD, NH3-N, TN, and TP were increased to 71.4%, 95.3%, 57.1%, and 74.7%, respectively. | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Z.; Li, H.; Bao, H.; Chen, Z.; Tan, Y.; Qin, L.; Li, T. Advanced Treatment of High-Concentration Ammonia–Nitrogen Wastewater by Pantothenic Acid-Enhanced Photosynthetic Bacteria. Water 2025, 17, 2166. https://doi.org/10.3390/w17142166
Bao Z, Li H, Bao H, Chen Z, Tan Y, Qin L, Li T. Advanced Treatment of High-Concentration Ammonia–Nitrogen Wastewater by Pantothenic Acid-Enhanced Photosynthetic Bacteria. Water. 2025; 17(14):2166. https://doi.org/10.3390/w17142166
Chicago/Turabian StyleBao, Zhisong, Haorui Li, Huajun Bao, Zhihe Chen, Yingyu Tan, Lei Qin, and Tiejun Li. 2025. "Advanced Treatment of High-Concentration Ammonia–Nitrogen Wastewater by Pantothenic Acid-Enhanced Photosynthetic Bacteria" Water 17, no. 14: 2166. https://doi.org/10.3390/w17142166
APA StyleBao, Z., Li, H., Bao, H., Chen, Z., Tan, Y., Qin, L., & Li, T. (2025). Advanced Treatment of High-Concentration Ammonia–Nitrogen Wastewater by Pantothenic Acid-Enhanced Photosynthetic Bacteria. Water, 17(14), 2166. https://doi.org/10.3390/w17142166






