Photocatalytic Degradation of Microplastics in Aquatic Environments: Materials, Mechanisms, Practical Challenges, and Future Perspectives
Abstract
1. Introduction
2. Performance of Photocatalysts for the Degradation of MPs
2.1. Titanium Dioxide
2.2. Zinc Oxide
| Material | Reactor Type | Reaction Condition | Degradation Efficiency | Reference |
|---|---|---|---|---|
| ZnO nanorods | Batch Reactor | Visible Light Irradiation | High efficiency for LDPE degradation | [66] |
| ZnO-TiO2 composite | Batch Reactor | UV Light | Enhanced degradation due to synergistic effects | [61] |
| ZnO/Graphene oxide | Photocatalytic Reactor | Visible Light | Improved efficiency due to increased surface area | [67] |
| Self-Doped ZnO microrods | Batch Reactor | UV Light | High-temperature stability and effective degradation | [68] |
2.3. Photocatalysts Other than TiO2 and ZnO
| Material | Reactor Type | Reaction Condition | Degradation Efficiency | Reference |
|---|---|---|---|---|
| GO | Batch and continuous flow systems | Visible light irradiation | High efficiency in degradation of low-density polyethylene (LDPE) | [8] |
| WO3 | Batch and continuous flow systems | Visible light irradiation at ~pH 6–7 | 60–90% for MPs (PS, PE) over extended periods (12–24 h) | [81] |
| Pt/ZnO | Photocatalytic reactor | A 50 W dichroic halogen lamp operating in atmospheric conditions, providing illumination in the visible spectrum (60 to 70 klux). | The VI and the CI increased by 15% and 13% for the LDPE fragments. | [66] |
| BiOCl-X | Photocatalytic reactor | Under the illumination of 250 W Xe lamp (λ > 420 nm) for 5 h. | 5.38% for Polyethene MPs | [73] |
| Fe3O4-PVP@ZIF-67 | Photocatalytic reactor | The catalyst concentration is 0.15 g·L−1, PMS concentration is 0.3 mM, and without pH adjustment for 60 min | 99.8% BPF removal | [79] |
| g-C3N4/PDI@ NH2-MIL-53(Fe) | Photocatalytic reactor | 30 min in the presence of H2O2 and visible LED light (420 < λ < 800 nm) | Maximum efficiency of 100% (10 min) for bisphenol A (BPA) | [8] |
2.4. Self-Propelled Photocatalytic Micromotors
3. Mechanisms of Photocatalytic Degradation of MPs
- (1)
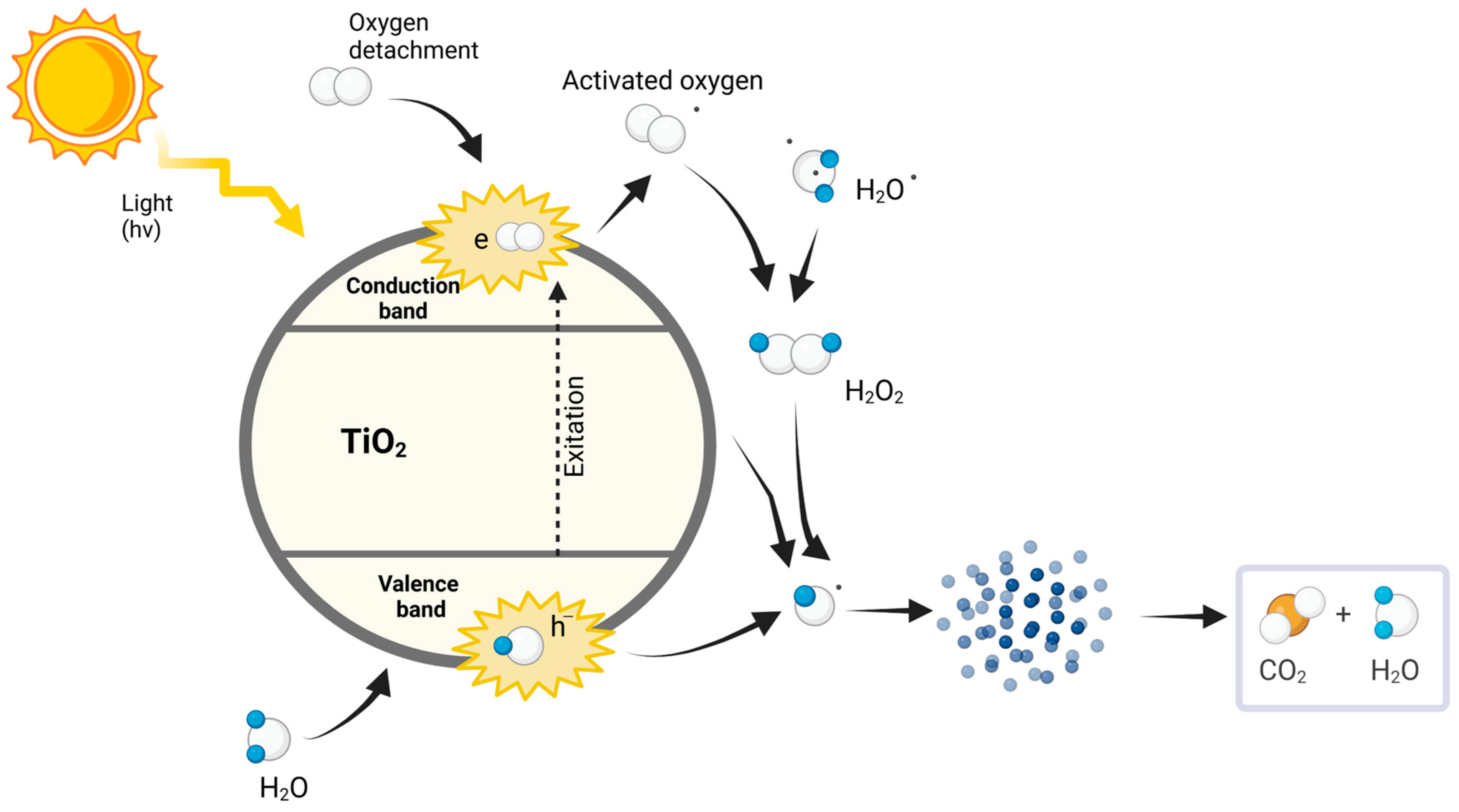
- Photocatalytic oxidation and scission of polymer chains: The initial step of the photocatalytic degradation of MPs is achieved through the oxidation of polymer chains induced by ROS produced during the photocatalytic process. When exposed to UV light, photocatalysts, such as titanium dioxide (TiO2) or ZnO, create electron-hole pairs, which can react with water and oxygen to form hydroxyl radicals (⋅OH) and superoxide radicals (O2−⋅) [18,98]. These radicals are highly reactive and initiate the oxidation of the polymer chains, resulting in scission, which splits the long-chain polymers into smaller fragments [99]. At the solid–solid interface between photocatalyst and MP, this process is highly efficient as it favors charge separation and avoids charge carrier recombination [18].
- (2)
- Formation of intermediate degradation products: As the polymer chains are cleaved, various intermediate degradation products are formed, including oligomers and monomers. These intermediates can vary in size and chemical structure, depending on the type of MP and the specific conditions of the photocatalytic process [100]. As an example, recent studies have demonstrated that the fragmentation of high-density polyethylene (HDPE) MPs has been accompanied by the generation of oligomers, which, under prolonged photocatalytic action, were converted into even smaller monomers [76]. The formation of these intermediates provides insight into the efficiency of the degradation process and the potential for complete mineralization, making their identification and characterization critical.
- (3)
- Complete mineralization into CO2, H2O, and non-toxic residues: The final step in the photocatalytic degradation pathway is the complete mineralization of the degradation products into harmless byproducts such as CO2 and H2O. This process involves further oxidation of the intermediates, which can be facilitated by the continued presence of ROS [101,102]. For instance, under optimal conditions, polystyrene photocatalytic degradation can achieve complete mineralization, where the residual products are completely converted to gaseous CO2 and liquid H2O, and leaving no toxic residues [103]. The mineralization efficiency can be influenced by various factors, including the type of photocatalyst used, the intensity of light, and the presence of additional oxidants [11].
4. Factors Affecting Photocatalytic Degradation of MPs
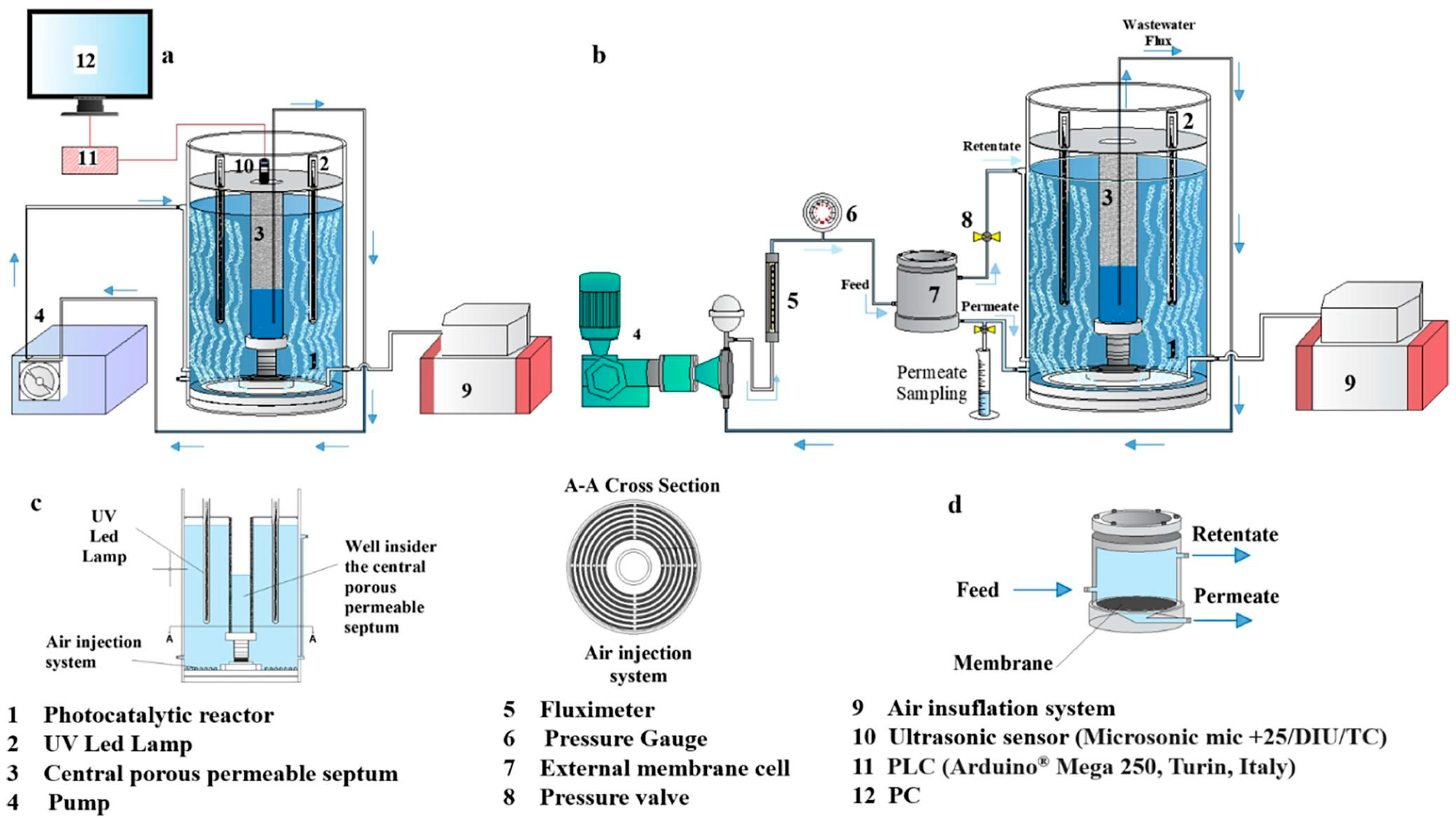
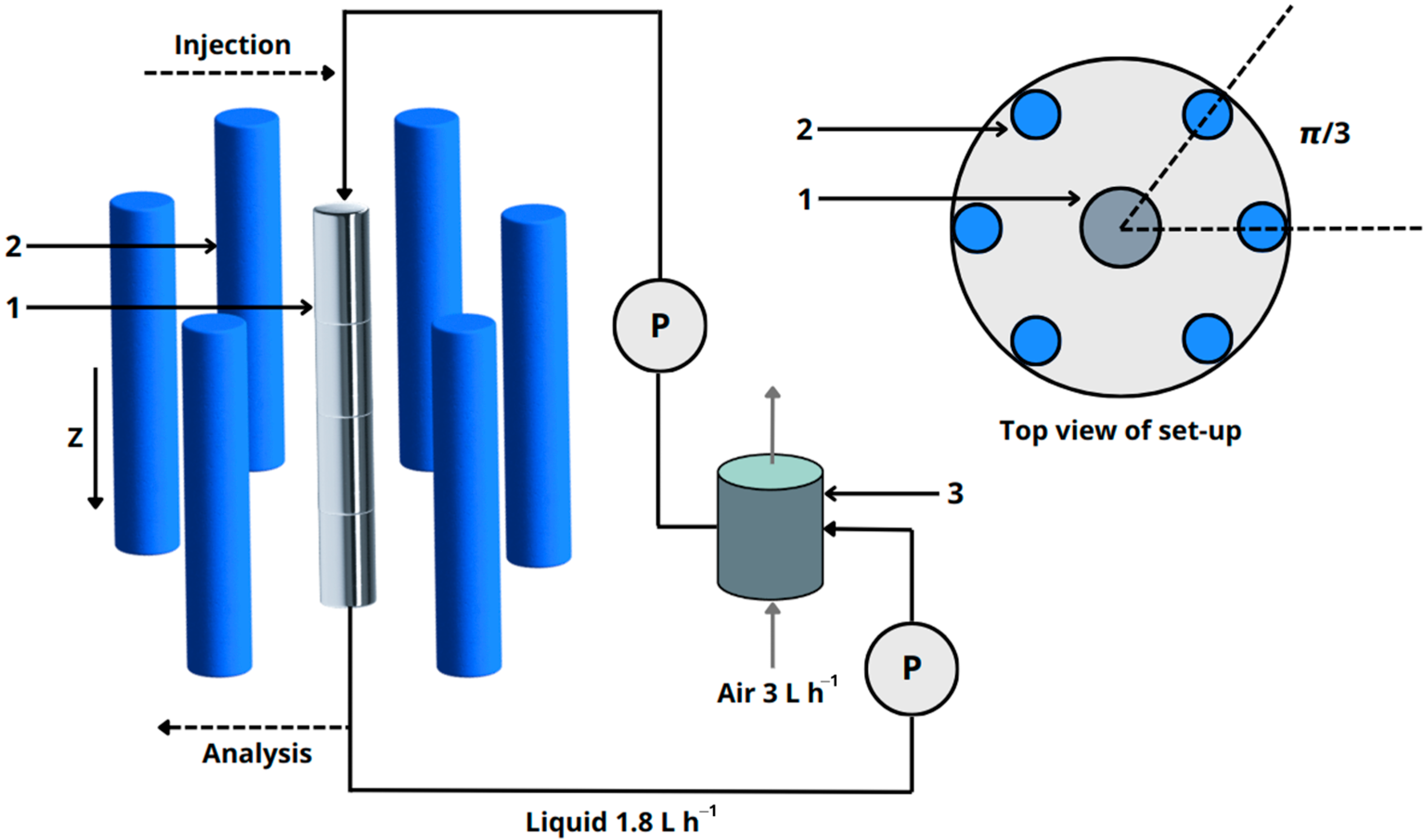
5. Photocatalytic Reactor Designs for Microplastic Degradation
- (1)
- Immobilizing photocatalysts onto support materials can facilitate easy recovery after the degradation process. This approach not only simplifies the separation of the catalyst from the reaction medium but also enhances the stability and reusability of the photocatalysts [135]. For instance, immobilized TiO2 photocatalysts have shown promising results in terms of reusability without significant loss of activity [135].
- (2)
- The development of magnetic photocatalysts allows for easy separation using external magnetic fields. This method has been successfully applied to various photocatalytic systems, enabling efficient recovery and reuse of the catalyst [134].
- (3)
- Enhancing the properties of photocatalysts, such as their surface area and porosity, can improve their performance and longevity. For example, the introduction of oxygen vacancies on TiO2 surfaces has been shown to enhance photocatalytic activity and stability, contributing to better reusability [136].
- (4)
- Implementing these optimization strategies can lead to significant improvements in the efficiency of photocatalytic degradation of MPs. By enhancing light penetration, photocatalytic systems can achieve higher degradation rates, while effective catalyst recovery and reusability can reduce operational costs and environmental impact.
6. Current Challenges and Limitations of Photocatalytic Degradation of MPs
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Sinitsyna, O.O.; Yeremin, G.B.; Turbinskii, V.V.; Pushkareva, M.V.; Shiryaeva, M.A.; Markova, O.L.; Borisova, D.S. Microplastics Pollution in Water is a Threat for Human Health and the Environment (Literature Review). Health Risk Anal. 2023, 172–179. [Google Scholar] [CrossRef]
- Colson, B.C.; Michel, A.P.M. Flow-Through Quantification of Microplastics Using Impedance Spectroscopy. ACS Sens. 2021, 6, 238–244. [Google Scholar] [CrossRef]
- An, X.; Wang, Y.; Adnan, M.; Li, W.; Zhang, Y. Natural Factors of Microplastics Distribution and Migration in Water: A Review. Water 2024, 16, 1595. [Google Scholar] [CrossRef]
- Bexeitova, K.; Zhantikeyev, U.; Yeszhan, Y.; Sapargali, I.; Kudaibergenov, K.; Toshtay, K.; Mikhalovsky, S.; Amrousse, R.; Berndtsson, R.; Azat, S. Evaluating Microplastic Detection Techniques in Human-Impacted Water Systems: A Mini-Review. ES Energy Environ. 2024, 25, 1233. [Google Scholar] [CrossRef]
- Bexeitova, K.; Baimenov, A.; Varol, E.A.; Kudaibergenov, K.; Zhantikeyev, U.; Sailaukhanuly, Y.; Toshtay, K.; Tauanov, Z.; Azat, S.; Berndtsson, R. Microplastics in Freshwater Systems: A Review of Classification, Sources, and Environmental Impacts. Chem. Eng. J. Adv. 2024, 20, 100649. [Google Scholar] [CrossRef]
- Kumar, R. Metal Oxides-Based Nano/Microstructures for Photodegradation of Microplastics. Adv. Sustain. Syst. 2023, 7, 2300033. [Google Scholar] [CrossRef]
- Sura, A.; Nain, S. Visible Light Driven Degradation of BPA and LDPE Microplastic Films Using GO/SCN Nanocomposite. RSC Adv. 2024, 14, 35336–35347. [Google Scholar] [CrossRef]
- Hamd, W.; Daher, E.A.; Tofa, T.S.; Dutta, J. Recent Advances in Photocatalytic Removal of Microplastics: Mechanisms, Kinetic Degradation, and Reactor Design. Front. Mar. Sci. 2022, 9, 885614. [Google Scholar] [CrossRef]
- Sacco, N.A.; Zoppas, F.M.; Devard, A.; del Pilar González Muñoz, M.; García, G.; Marchesini, F.A. Recent Advances in Microplastics Removal from Water with Special Attention Given to Photocatalytic Degradation: Review of Scientific Research. Microplastics 2023, 2, 278–303. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Ariza-Tarazona, M.C.; Cedillo-González, E.I.; Siligardi, C.; Simmchen, J. Combining Photocatalytic Collection and Degradation of Microplastics Using Self-Asymmetric Pac-Man TiO2. Nanoscale 2023, 15, 14774–14781. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, A.; Corti, A.; Erba, F.; Modugno, F.; La Nasa, J.; Bianchi, S.; Castelvetro, V. The Hidden Microplastics: New Insights and Figures from the Thorough Separation and Characterization of Microplastics and of Their Degradation Byproducts in Coastal Sediments. Environ. Sci. Technol. 2018, 52, 5634–5643. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, Y.; Xu, W.; Zhu, J. Photodegradation of Microplastics through Nanomaterials: Insights into Photocatalysts Modification and Detailed Mechanisms. Materials 2024, 17, 2755. [Google Scholar] [CrossRef]
- Mayorga-Burrezo, P.; Mayorga-Martinez, C.C.; Pumera, M. Photocatalysis Dramatically Influences Motion of Magnetic Microrobots: Application to Removal of Microplastics and Dyes. J. Colloid Interface Sci. 2023, 643, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Beladi-Mousavi, S.M.; Hermanová, S.; Ying, Y.; Plutnar, J.; Pumera, M. A Maze in Plastic Wastes: Autonomous Motile Photocatalytic Microrobots against Microplastics. ACS Appl. Mater. Interfaces 2021, 13, 25102–25110. [Google Scholar] [CrossRef]
- Sutradhar, M. A Review of Microplastics Pollution and Its Remediation Methods: Current Scenario and Future Aspects. Arch. Agric. Environ. Sci. 2022, 7, 288–293. [Google Scholar] [CrossRef]
- Gayathri, P.V.; Joseph, S.; Mohan, M.; Pillai, D. Advanced Oxidation Processes for the Degradation of Microplastics from the Environment: A Review. Water Environ. J. 2023, 37, 686–701. [Google Scholar] [CrossRef]
- Nabi, I.; Bacha, A.-U.-R.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete Photocatalytic Mineralization of Microplastic on TiO2 Nanoparticle Film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and Effects of Plastic Additives on Marine Environments and Organisms: A Review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Rist, S.; Hartmann, N.B. Aquatic Ecotoxicity of Microplastics and Nanoplastics: Lessons Learned from Engineered Nanomaterials. In Freshwater Microplastics: Emerging Environmental Contaminants? Springer: Cham, Switzerland, 2018; pp. 25–49. [Google Scholar]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Gottschalk, F.; Nowack, B. The Release of Engineered Nanomaterials to the Environment. J. Environ. Monit. 2011, 13, 1145. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the Environment: Behavior, Fate, Bioavailability, and Effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the Aquatic Environment. Critical Review. In Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; pp. 325–340. [Google Scholar]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater Treatment Plants as a Pathway for Microplastics: Development of a New Approach to Sample Wastewater-Based Microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Parimaladevi, R.; Umadevi, M.; Rekha, T.N.; Benial, A.M.F. TiO2-Based Nanomaterials for Wastewater Treatment. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–24. [Google Scholar]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnology 2018, 16, 84. [Google Scholar] [CrossRef]
- Beegam, A.; Prasad, P.; Jose, J.; Oliveira, M.; Costa, F.G.; Soares, A.M.V.M.; Gonçalves, P.P.; Trindade, T.; Kalarikkal, N.; Thomas, S.; et al. Environmental Fate of Zinc Oxide Nanoparticles: Risks and Benefits. In Toxicology—New Aspects to This Scientific Conundrum; InTech: London, UK, 2016. [Google Scholar]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the Aquatic Environment: Adsorption, Dispersion, Toxicity and Transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef]
- Chen, C.; Shen, L.; Wang, B.; Lu, X.; Raza, S.; Xu, J.; Li, B.; Lin, H.; Chen, B. Environmental Applications of Metal–Organic Framework-Based Three-Dimensional Macrostructures: A Review. Chem. Soc. Rev. 2025, 54, 2208–2245. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Chen, Q.; Kalogerakis, N.; Ji, R.; Ma, Y. Interactions between Microplastics and Organic Pollutants: Effects on Toxicity, Bioaccumulation, Degradation, and Transport. Sci. Total Environ. 2020, 748, 142427. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Gong, G.; Lee, H.-J.; Seong, J.; Hong, S.W.; Lee, C. Transformation of Microplastics by Oxidative Water and Wastewater Treatment Processes: A Critical Review. J. Hazard. Mater. 2023, 443, 130313. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, X.; Ding, W.; Zhang, Z.; Nghiem, L.D.; Sun, J.; Wang, Q. Do Microplastics Affect Biological Wastewater Treatment Performance? Implications from Bacterial Activity Experiments. ACS Sustain. Chem. Eng. 2019, 7, 20097–20101. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hidayaturrahman, H.; Peera, S.G.; Lee, T.G. Elimination of Microplastics at Different Stages in Wastewater Treatment Plants. Water 2022, 14, 2404. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to Microplastic Pollution—Removal of Microplastics from Wastewater Effluent with Advanced Wastewater Treatment Technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Kasmuri, N.; Rosli, M.S.; Zaini, N.; Nayono, S.E. Reduction of Microplastic in Wastewater Via Electrocoagulation Process. IOP Conf. Ser. Earth Environ. Sci. 2024, 1303, 012020. [Google Scholar] [CrossRef]
- Saggioro, E.M.; Oliveira, A.S.; Moreira, J.C. Heterogeneous Photocatalysis Remediation of Wastewater Polluted by Indigoid Dyes. In Textile Wastewater Treatment; InTech: London, UK, 2016. [Google Scholar]
- Heydari, G.; Hollman, J.; Achari, G.; Langford, C.H. Comparative Study of Four TiO2-Based Photocatalysts to Degrade 2,4-D in a Semi-Passive System. Water 2019, 11, 621. [Google Scholar] [CrossRef]
- Rex, M.C.; Mukherjee, A. Prospects of TiO2-Based Photocatalytic Degradation of Microplastic Leachates Related Disposable Facemask, a Major COVID-19 Waste. Front. Nanotechnol. 2022, 4, 1072227. [Google Scholar] [CrossRef]
- Nair, N.; Gandhi, V.; Shukla, A.; Ghotekar, S.; Nguyen, V.-H.; Varma, K. Mechanisms in the Photocatalytic Breakdown of Persistent Pharmaceutical and Pesticide Molecules over TiO2-Based Photocatalysts: A Review. J. Phys. Condens. Matter 2024, 36, 413003. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Yu, X.; Ke, X.; Zhao, J. A Novel Route for Microplastic Mineralization: Visible-Light-Driven Heterogeneous Photocatalysis and Photothermal Fenton-Like Reaction. Environ. Sci. Nano 2024, 11, 113–122. [Google Scholar] [CrossRef]
- Skaf, D.W. Photocatalytic Oxidation of Dimethyl Methylphosphonate in Aqueous Suspensions of TiO2. J. Chem. Eng. Process Technol. 2015, 6, 3. [Google Scholar] [CrossRef]
- Fadli, M.H.; Ibadurrohman, M.; Slamet, S. Microplastic Pollutant Degradation in Water Using Modified TiO2 Photocatalyst Under UV-Irradiation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012055. [Google Scholar] [CrossRef]
- Maulana, D.A.; Ibadurrohman, M. Slamet Synthesis of Nano-Composite Ag/TiO2 for Polyethylene Microplastic Degradation Applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012054. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; Rivera De la Rosa, J.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. Microplastic Pollution Reduction by a Carbon and Nitrogen-Doped TiO2: Effect of PH and Temperature in the Photocatalytic Degradation Process. J. Hazard. Mater. 2020, 395, 122632. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Ruiz, A.A.; Bailón-Ruiz, S.J. Titanium Nanostructures: Advancing Photocatalysis in Complex Systems. Photochem. 2024, 4, 222–232. [Google Scholar] [CrossRef]
- Ochanda, F.O.; Rajukada, S.; Barnett, M.R. Controlled Synthesis of TiO2 Hierarchical Nanofibre Structures via Electrospinning and Solvothermal Processes: Photocatalytic Activity for Degradation of Methylene Blue. Nanomater. Nanotechnol. 2012, 2, 9. [Google Scholar] [CrossRef]
- Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts. Catalysts 2019, 9, 819. [Google Scholar] [CrossRef]
- Wang, X.; Hwa, T.S.; Shamsuddin, M.R.; Wid, N. Photocatalytic Degradation of Microplastic Polyamide 66 by Ag/TiO2 Composite: Preparation of Catalysts and Photocatalytic Degradation Mechanism. In Environmental Governance, Ecological Remediation and Sustainable Development; Springer: Cham, Switzerland, 2024; pp. 37–45. [Google Scholar]
- Ullattil, S.G.; Pumera, M. Light-Powered Self-Adaptive Mesostructured Microrobots for Simultaneous Microplastics Trapping and Fragmentation via in Situ Surface Morphing. Small 2023, 19, 2301467. [Google Scholar] [CrossRef]
- Uogintė, I.; Pleskytė, S.; Skapas, M.; Stanionytė, S.; Lujanienė, G. Degradation and Optimization of Microplastic in Aqueous Solutions with Graphene Oxide-Based Nanomaterials. Int. J. Environ. Sci. Technol. 2023, 20, 9693–9706. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Modi, G.; Cretu, V.; Postica, V.; Lupan, O.; Reimer, T.; Paulowicz, I.; Hrkac, V.; Benecke, W.; Kienle, L.; et al. Direct Growth of Freestanding ZnO Tetrapod Networks for Multifunctional Applications in Photocatalysis, UV Photodetection, and Gas Sensing. ACS Appl. Mater. Interfaces 2015, 7, 14303–14316. [Google Scholar] [CrossRef]
- Baruah, S.; Mahmood, M.A.; Myint, M.T.Z.; Bora, T.; Dutta, J. Enhanced Visible Light Photocatalysis through Fast Crystallization of Zinc Oxide Nanorods. Beilstein J. Nanotechnol. 2010, 1, 14–20. [Google Scholar] [CrossRef]
- Xie, M.; Wang, L.; Xu, L.; Shen, Y. Preparation and Application of Ag-In/ZnO Nanorods. AATCC J. Res. 2021, 8, 34–39. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, C.; Huang, H.; Li, W.; Du, D.; Han, D.; Qiu, T.; Chu, P.K. Aluminum Plasmonic Photocatalysis. Sci. Rep. 2015, 5, 15288. [Google Scholar] [CrossRef]
- Li, X.; Niu, F.; Su, J.; Guo, L. Photoelectrochemical Performance Dependence on Geometric Surface Area of Branched ZnO Nanowires. ChemElectroChem 2018, 5, 3717–3722. [Google Scholar] [CrossRef]
- Ivanova, D.; Simeonova, S.; Kaneva, N. Enhanced Photocatalytic Properties of MnOx Co-Catalytic Modified ZnO Nanostructured Films for Organic Dye Degradation. J. Chem. Technol. Metall. 2024, 59, 343–350. [Google Scholar] [CrossRef]
- Gunasekaran, D.; Chandrasekaran, N.; Jenkins, D.; Mukherjee, A. Plain Polystyrene Microplastics Reduce the Toxic Effects of ZnO Particles on Marine Microalgae Dunaliella Salina. J. Environ. Chem. Eng. 2020, 8, 104250. [Google Scholar] [CrossRef]
- Saoud, K.; Alsoubaihi, R.; Bensalah, N.; Bora, T.; Bertino, M.; Dutta, J. Synthesis of Supported Silver Nano-Spheres on Zinc Oxide Nanorods for Visible Light Photocatalytic Applications. Mater. Res. Bull. 2015, 63, 134–140. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kuk, Y.-S.; Kwon, O.H.; Park, Y.W.; Park, M. Synthesis and Characterization of ZnO-TiO2/Carbon Fiber Composite with Enhanced Photocatalytic Properties. Nanomaterials 2020, 10, 1960. [Google Scholar] [CrossRef] [PubMed]
- Bi Irié Williams, I.; Kouadio Fodjo, E.; Bi Boussou Narcisse, P.; Alla Martin, A.; Koffi Koffi Kra Sylvestre, K.; Albert, T.; Gu, Z. Effect of Synthesized Carbon Quantum Dots on the Photocatalytic Properties of ZnO. Bull. Chem. Soc. Ethiop. 2022, 37, 463–476. [Google Scholar] [CrossRef]
- Tripta; Vikas; Nehra, P.; Rana, P.S. Photocatalytic Treatment of MB Dye Using ZnO Nanoparticles. ECS Trans. 2022, 107, 16213–16221. [Google Scholar] [CrossRef]
- Smitiukh, O.M.; Marchuk, O.V.; Yanchuk, O.M.; Khmaruk, Y.O. Nanoparticles of ZnO/ZnS: Electrochemical Synthesis, Analysis and Prospective Applications. J. Nano-Electron. Phys. 2024, 16, 01024. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.-C.; Balram, D. Ultrasensitive Electrochemical Detection and Plasmon-Enhanced Photocatalytic Degradation of Rhodamine B Based on Dual-Functional, 3D, Hierarchical Ag/ZnO Nanoflowers. Sensors 2022, 22, 5049. [Google Scholar] [CrossRef]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible Light Photocatalytic Degradation of Microplastic Residues with Zinc Oxide Nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.F.; Allabadi, O.; Aljarrah, M.T. Photocatalytic Activity of Graphene Oxide/Zinc Oxide Nanocomposites with Embedded Metal Nanoparticles for the Degradation of Organic Dyes. ACS Omega 2020, 5, 28046–28055. [Google Scholar] [CrossRef] [PubMed]
- Ullattil, S.G.; Periyat, P.; Naufal, B.; Lazar, M.A. Self-Doped ZnO Microrods—High Temperature Stable Oxygen Deficient Platforms for Solar Photocatalysis. Ind. Eng. Chem. Res. 2016, 55, 6413–6421. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Z.; Jiang, J.; Li, R.; Xiong, J. Preparation of Heterojunction C3N4/WO3 Photocatalyst for Degradation of Microplastics in Water. Chemosphere 2023, 337, 139206. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yao, H.; Li, Y.; Zhu, Y. Microplastic Addition Alters the Microbial Community Structure and Stimulates Soil Carbon Dioxide Emissions in Vegetable-Growing Soil. Environ. Toxicol. Chem. 2021, 40, 352–365. [Google Scholar] [CrossRef]
- Vas, N.V.; Sundari, S.K.; Jeyachandran, S. Degradation of Microplastics by Zinc Oxide Nanoparticles Synthesized Using Piper Longum Leaf Extract. Cureus 2024, 16, e69876. [Google Scholar] [CrossRef]
- Razali, N.; Wan Abdullah, W.R.; Mohd Zikir, N. Effect of Thermo-Photocatalytic Process Using Zinc Oxide on Degradation of Macro/Micro-Plastic in Aqueous Environment. J. Sustain. Sci. Manag. 2020, 15, 1–14. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, G.; Yan, Z.; Liu, J.; Wu, D.; Wang, Y. Microplastic Degradation by Hydroxy-Rich Bismuth Oxychloride. J. Hazard. Mater. 2021, 405, 124247. [Google Scholar] [CrossRef]
- Saputri, D.; Hamdhani, H.; Suryana, I. Microplastic in Water and Sediment from the Middle Segment of Karang Mumus River, Samarinda City, East Kalimantan. Nusant. Trop. Fish. Sci. J. 2023, 2, 128–134. [Google Scholar] [CrossRef]
- Wu, D.; Lim, B.X.H.; Seah, I.; Xie, S.; Jaeger, J.E.; Symons, R.K.; Heffernan, A.L.; Curren, E.E.M.; Leong, S.C.Y.; Riau, A.K.; et al. Impact of Microplastics on the Ocular Surface. Int. J. Mol. Sci. 2023, 24, 3928. [Google Scholar] [CrossRef]
- Sultanov, F.; Daulbayev, C.; Azat, S.; Kuterbekov, K.; Bekmyrza, K.; Bakbolat, B.; Bigaj, M.; Mansurov, Z. Influence of Metal Oxide Particles on Bandgap of 1D Photocatalysts Based on SrTiO3/PAN Fibers. Nanomaterials 2020, 10, 1734. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of Orthodox Seeds during Ageing: Influencing Factors, Physiological Alterations and the Role of Reactive Oxygen Species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef]
- Zhang, S.; Zhuo, Y.; Ezugwu, C.I.; Wang, C.; Li, C.; Liu, S. Synergetic Molecular Oxygen Activation and Catalytic Oxidation of Formaldehyde over Defective MIL-88B(Fe) Nanorods at Room Temperature. Environ. Sci. Technol. 2021, 55, 8341–8350. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, T.; Zhang, Q.; Wang, T.; Hou, X. Rapid Microwave Synthesis of Fe3O4-PVP@ZIF-67 as Highly Effective Peroxymonosulfate Catalyst for Degradation of Bisphenol F and Its Mechanism Analysis. Chem. Eng. J. 2021, 404, 126453. [Google Scholar] [CrossRef]
- Li, Y.; Fang, Y.; Cao, Z.; Li, N.; Chen, D.; Xu, Q.; Lu, J. Construction of G-C3N4/PDI@MOF Heterojunctions for the Highly Efficient Visible Light-Driven Degradation of Pharmaceutical and Phenolic Micropollutants. Appl. Catal. B 2019, 250, 150–162. [Google Scholar] [CrossRef]
- Tung, M.H.T.; Hien, T.T.T.; Le Chi, N.T.P.; Quoc, N.T.; Binh, N.T.T.; Mai, N.V.N.; Hiep, P.P.M.; Cam, N.T.D. Synthesis of WO3/AgI Photocatalysts Applying for Degradation of Antibiotics in Water. Vietnam J. Catal. Adsorpt. 2022, 11, 76–81. [Google Scholar] [CrossRef]
- Wang, L.; Kaeppler, A.; Fischer, D.; Simmchen, J. Photocatalytic TiO2 Micromotors for Removal of Microplastics and Suspended Matter. ACS Appl. Mater. Interfaces 2019, 11, 32937–32944. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, R.; Wu, Y.; Gao, W.; He, Z.; Ren, B. Light-Driven Au-WO3@C Janus Micromotors for Rapid Photodegradation of Dye Pollutants. ACS Appl. Mater. Interfaces 2017, 9, 4674–4683. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, R.; Zhang, Q.; Ren, B. Dye-Enhanced Self-Electrophoretic Propulsion of Light-Driven TiO2–Au Janus Micromotors. Nanomicro Lett. 2017, 9, 30. [Google Scholar] [CrossRef]
- Kong, L.; Mayorga-Martinez, C.C.; Guan, J.; Pumera, M. Fuel-Free Light-Powered TiO2/Pt Janus Micromotors for Enhanced Nitroaromatic Explosives Degradation. ACS Appl. Mater. Interfaces 2018, 10, 22427–22434. [Google Scholar] [CrossRef]
- Mou, F.; Kong, L.; Chen, C.; Chen, Z.; Xu, L.; Guan, J. Light-Controlled Propulsion, Aggregation and Separation of Water-Fuelled TiO2/Pt Janus Submicromotors and Their “on-the-Fly” Photocatalytic Activities. Nanoscale 2016, 8, 4976–4983. [Google Scholar] [CrossRef]
- Li, J.; He, X.; Jiang, H.; Xing, Y.; Fu, B.; Hu, C. Enhanced and Robust Directional Propulsion of Light-Activated Janus Micromotors by Magnetic Spinning and the Magnus Effect. ACS Appl. Mater. Interfaces 2022, 14, 36027–36037. [Google Scholar] [CrossRef] [PubMed]
- Pourrahimi, A.M.; Villa, K.; Ying, Y.; Sofer, Z.; Pumera, M. ZnO/ZnO2/Pt Janus Micromotors Propulsion Mode Changes with Size and Interface Structure: Enhanced Nitroaromatic Explosives Degradation under Visible Light. ACS Appl. Mater. Interfaces 2018, 10, 42688–42697. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Silk, R.; Smith, M.; Dong, Y.; Chen, W.-T.; Waterhouse, G.I.N. Hierarchical TiO2 Nanoflower Photocatalysts with Remarkable Activity for Aqueous Methylene Blue Photo-Oxidation. ACS Omega 2020, 5, 18919–18934. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.T.; Ma, X. Machine Learning Prediction of Adsorption Behavior of Xenobiotics on Microplastics under Different Environmental Conditions. ACS EST Water 2024, 4, 991–999. [Google Scholar] [CrossRef]
- Meephon, S.; Puttamat, S.; Pavarajarn, V. Dependence of Catalyst Surface in Photocatalytic Degradation of Phenyl Urea Herbicides. Eng. J. 2018, 22, 57–66. [Google Scholar] [CrossRef]
- Kovačić, M.; Tomić, A.; Tonković, S.; Pulitika, A.; Papac Zjačić, J.; Katančić, Z.; Genorio, B.; Kušić, H.; Lončarić Božić, A. Pristine and UV-Weathered PET Microplastics as Water Contaminants: Appraising the Potential of the Fenton Process for Effective Remediation. Processes 2024, 12, 844. [Google Scholar] [CrossRef]
- Li, S.; Chen, Z.; Wu, D.; Qin, Y.; Kong, Y. Graphene Quantum Dots Enhanced Photocatalytic Activity of Sb2WO6 Under Ultraviolet-and Visible-Light Irradiation. Bull. Korean Chem. Soc. 2020, 41, 552–557. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.; Deganello, F.; Bianco Prevot, A.; Magnacca, G. Revisiting the Catalytic Activity of a Doped SrFeO3 for Water Pollutants Removal: Effect of Light and Temperature. Appl. Catal. B 2017, 207, 174–181. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, Y.; Chu, Z.; Long, J.; An, X.; Zhang, H.; Lin, H.; Zhang, Z.; Wang, X. Synergy of Metal and Nonmetal Dopants for Visible-Light Photocatalysis: A Case-Study of Sn and N Co-Doped TiO2. Phys. Chem. Chem. Phys. 2016, 18, 9636–9644. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, Z.; Xu, C.; Shen, X.; Ma, L.; Liu, X. Hydrothermal Syntheses of Silver Phosphate Nanostructures and Their Photocatalytic Performance for Organic Pollutant Degradation. Cryst. Res. Technol. 2014, 49, 975–981. [Google Scholar] [CrossRef]
- Dhaundiyal, A.; Mittal, A. Unveiling the Microplastics: Sources, Distribution, Toxicological Impacts, Extraction Methods, Degradational Strategies, Paving the Path to a Sustainable Future. Water Air Soil. Pollut. 2024, 235, 691. [Google Scholar] [CrossRef]
- Jancik-Prochazkova, A.; Jašek, V.; Figalla, S.; Pumera, M. Photocatalytic Microplastics “On-The-fly” Degradation via Motile Quantum Materials-Based Microrobots. Adv. Opt. Mater. 2023, 11, 2300782. [Google Scholar] [CrossRef]
- Vital-Grappin, A.D.; Ariza-Tarazona, M.C.; Luna-Hernández, V.M.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; Siligardi, C.; Cedillo-González, E.I. The Role of the Reactive Species Involved in the Photocatalytic Degradation of HDPE Microplastics Using C,N-TiO2 Powders. Polymers 2021, 13, 999. [Google Scholar] [CrossRef]
- Zhou, H.; Mayorga-Martinez, C.C.; Pumera, M. Microplastic Removal and Degradation by Mussel-Inspired Adhesive Magnetic/Enzymatic Microrobots. Small Methods 2021, 5, 2100230. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, R.F.; Wypych, A.; Pérez-García, P.; Danso, D.; Chow, J.; Streit, W.R. An Ultra-Sensitive Comamonas Thiooxidans Biosensor for the Rapid Detection of Enzymatic Polyethylene Terephthalate (PET) Degradation. Appl. Environ. Microbiol. 2023, 89, e01603-22. [Google Scholar] [CrossRef]
- Fa, W.; Guo, L.; Wang, J.; Guo, R.; Zheng, Z.; Yang, F. Solid-phase Photocatalytic Degradation of Polystyrene with TiO2/Fe(St)3 as Catalyst. J. Appl. Polym. Sci. 2013, 128, 2618–2622. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Li, W.; Zhao, W.; Zhu, H.; Li, Z.-J.; Wang, W. State of the Art in the Photochemical Degradation of (Micro)Plastics: From Fundamental Principles to Catalysts and Applications. J. Mater. Chem. A Mater. 2023, 11, 2503–2527. [Google Scholar] [CrossRef]
- Xie, A.; Jin, M.; Zhu, J.; Zhou, Q.; Fu, L.; Wu, W. Photocatalytic Technologies for Transformation and Degradation of Microplastics in the Environment: Current Achievements and Future Prospects. Catalysts 2023, 13, 846. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhang, G.H.; Sun, L.; Ma, J. Performance of Bi20TiO32 for Photocatalytic Degradation of Organic Pollutants in Water. Adv. Mat. Res. 2011, 233–235, 724–727. [Google Scholar] [CrossRef]
- Chen, X.; Li, N.; Xu, S.; Wang, H.; Cai, Y. Study on the Visible-Light Photocatalytic Performance and Degradation Mechanism of Diclofenac Sodium under the System of Hetero-Structural CuBi2O4/Ag3PO4 with H2O2. Materials 2018, 11, 511. [Google Scholar] [CrossRef]
- Kongsong, P.; Sikong, L.; Niyomwas, S.; Rachpech, V. Photocatalytic Degradation of Glyphosate in Water by N-Doped SnO2/TiO2 Thin-Film-Coated Glass Fibers. Photochem. Photobiol. 2014, 90, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.M.; Aazam, E.S. New Visible-Light Pt/PbS Nanoparticle Photocatalysts for the Photocatalytic Oxidation of Thiophene. Clean 2015, 43, 421–426. [Google Scholar] [CrossRef]
- Atitar, M.F.; Dillert, R.; Bahnemann, D.W. Surface Interactions between Imazapyr and the TiO2 Surface: An In Situ ATR-FTIR Study. J. Phys. Chem. C 2017, 121, 4293–4303. [Google Scholar] [CrossRef]
- Jancik-Prochazkova, A.; Pumera, M. Light-Powered Swarming Phoretic Antimony Chalcogenide-Based Microrobots with “on-the-Fly” Photodegradation Abilities. Nanoscale 2023, 15, 5726–5734. [Google Scholar] [CrossRef]
- Song, R.; Yao, L.; Sun, C.; Yu, D.; Lin, H.; Li, G.; Lian, Z.; Zhuang, S.; Zhang, D. Electrospun Membranes Anchored with G-C3N4/MoS2 for Highly Efficient Photocatalytic Degradation of Aflatoxin B1 under Visible Light. Toxins 2023, 15, 133. [Google Scholar] [CrossRef]
- Priyanka; Srivastava, V.C. Photocatalytic Oxidation of Dye Bearing Wastewater by Iron Doped Zinc Oxide. Ind. Eng. Chem. Res. 2013, 52, 17790–17799. [Google Scholar] [CrossRef]
- Kapinus, E.I.; Viktorova, T.I. Kinetics of the Photocatalytic Degradation of Methylene Blue on Titanium Dioxide. Theor. Exp. Chem. 2010, 46, 163–167. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Kumar, E.; Durai, S.C.V. Structural, Biological and Photocatalytic Properties of Zirconium Oxide Nanoparticles Synthesized by Microwave Assisted Solution Method. Asian J. Chem. 2019, 31, 1729–1732. [Google Scholar] [CrossRef]
- Khan, W.Z.; Najeeb, I.; Tuiyebayeva, M.; Makhteyeva, Z. Refinery Wastewater Degradation with Titanium Dioxide, Zinc Oxide, and Hydrogen Peroxide in a Photocatalytic Reactor. In Proceedings of the SPE African Health, Safety, Security, and Environment and Social Responsibility Conference and Exhibition, Maputo, Mozambique, 15 September 2014. [Google Scholar]
- Chen, R.; Ding, S.; Wang, B.; Ren, X. Preparation of ZnFe2O4@TiO2 Novel Core-Shell Photocatalyst by Ultrasonic Method and Its Photocatalytic Degradation Activity. Coatings 2022, 12, 1407. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Baeissa, E.S.; Mkhalid, I.A.; Al-Rayyani, M.A. Optimization of Preparation Conditions of ZnO–SiO2 Xerogel by Sol–Gel Technique for Photodegradation of Methylene Blue Dye. Appl. Nanosci. 2013, 3, 57–63. [Google Scholar] [CrossRef][Green Version]
- Liu, F.; Leung, Y.H.; Djurišić, A.B.; Ng, A.M.C.; Chan, W.K.; Ng, K.L.; Wong, K.S.; Liao, C.; Shih, K.; Surya, C. Effect of Plasma Treatment on Native Defects and Photocatalytic Activities of Zinc Oxide Tetrapods. J. Phys. Chem. C 2014, 118, 22760–22767. [Google Scholar] [CrossRef]
- Wei, W.; Huang, Q.-S.; Sun, J.; Wang, J.-Y.; Wu, S.-L.; Ni, B.-J. Polyvinyl Chloride Microplastics Affect Methane Production from the Anaerobic Digestion of Waste Activated Sludge through Leaching Toxic Bisphenol-A. Environ. Sci. Technol. 2019, 53, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- Sutkar, P.R.; Gadewar, R.D.; Dhulap, V.P. Recent Trends in Degradation of Microplastics in the Environment: A State-of-the-Art Review. J. Hazard. Mater. Adv. 2023, 11, 100343. [Google Scholar] [CrossRef]
- Idris, F.; Febrianto, T.; Hidayati, J.R.; Rajib; Nugraha, A.H. Microplastic Abundance in Sea Cucumber at Seagrass Ecosystem of Bintan Island and Surrounding Area, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 967, 012009. [Google Scholar] [CrossRef]
- Molinari, R.; Limonti, C.; Lavorato, C.; Siciliano, A.; Argurio, P. Upgrade of a Slurry Photocatalytic Membrane Reactor Based on a Vertical Filter and an External Membrane and Testing in the Photodegradation of a Model Pollutant in Water. Chem. Eng. J. 2023, 451, 138577. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Lee, J.; Echavarría-Isaza, A.; Torres-Palma, R.A. Possibilities and Limitations of the Sono-Fenton Process Using Mid-High-Frequency Ultrasound for the Degradation of Organic Pollutants. Molecules 2023, 28, 1113. [Google Scholar] [CrossRef]
- Pishahang, M.; Larring, Y.; McCann, M.; Bredesen, R. Ca0.9Mn0.5Ti0.5O3−δ: A Suitable Oxygen Carrier Material for Fixed-Bed Chemical Looping Combustion under Syngas Conditions. Ind. Eng. Chem. Res. 2014, 53, 10549–10556. [Google Scholar] [CrossRef]
- Bustos-Terrones, Y.A.; Estrada-Vázquez, R.; Ramírez-Pereda, B.; Bustos-Terrones, V.; Rangel-Peraza, J.G. Kinetics of a Fixed Bed Reactor with Immobilized Microorganisms for the Removal of Organic Matter and Phosphorous. Water Environ. Res. 2020, 92, 1956–1965. [Google Scholar] [CrossRef]
- Cloteaux, A.; Gérardin, F.; Thomas, D.; Midoux, N.; André, J.-C. Fixed Bed Photocatalytic Reactor for Formaldehyde Degradation: Experimental and Modeling Study. Chem. Eng. J. 2014, 249, 121–129. [Google Scholar] [CrossRef]
- Surana, M.; Pattanayak, D.S.; Yadav, V.; Singh, V.K.; Pal, D. An Insight Decipher on Photocatalytic Degradation of Microplastics: Mechanism, Limitations, and Future Outlook. Environ. Res. 2024, 247, 118268. [Google Scholar] [CrossRef] [PubMed]
- Piazza, V.; Uheida, A.; Gambardella, C.; Garaventa, F.; Faimali, M.; Dutta, J. Ecosafety Screening of Photo-Fenton Process for the Degradation of Microplastics in Water. Front. Mar. Sci. 2022, 8, 791431. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New Strategy for Microplastic Degradation: Green Photocatalysis Using a Protein-Based Porous N-TiO2 Semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Z.; Zheng, Y.; Cui, C.; Li, C.; Li, Z. Controlled Growth of Metal–Organic Framework on Upconversion Nanocrystals for NIR-Enhanced Photocatalysis. ACS Appl. Mater. Interfaces 2017, 9, 2899–2905. [Google Scholar] [CrossRef]
- Sun, M.-H.; Huang, S.-Z.; Chen, L.-H.; Li, Y.; Yang, X.-Y.; Yuan, Z.-Y.; Su, B.-L. Applications of Hierarchically Structured Porous Materials from Energy Storage and Conversion, Catalysis, Photocatalysis, Adsorption, Separation, and Sensing to Biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Millward, F.; Zysman-Colman, E. Mechanophotocatalysis: A Generalizable Approach to Solvent-minimized Photocatalytic Reactions for Organic Synthesis. Angew. Chem. 2024, 63, e202316169. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic Reactors with Suspended and Immobilized TiO2: Comparative Efficiency Evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Giannakis, T.; Zervou, S.-K.; Triantis, T.M.; Christophoridis, C.; Bizani, E.; Starinskiy, S.V.; Koralli, P.; Mousdis, G.; Hiskia, A.; Kandyla, M. Enhancing the Photocatalytic Activity of Immobilized TiO2 Using Laser-Micropatterned Surfaces. Appl. Sci. 2024, 14, 3033. [Google Scholar] [CrossRef]
- Cao, T.; Xia, T.; Zhou, L.; Li, G.; Chen, X.; Tian, H.; Zhao, J.; Wang, J.; Zhang, W.; Li, S.; et al. Distribution and Concentration of Surface Oxygen Vacancy of TiO2 and Its Photocatalytic Activity. J. Phys. D Appl. Phys. 2020, 53, 424001. [Google Scholar] [CrossRef]
- Katuri, J.; Ma, X.; Stanton, M.M.; Sánchez, S. Designing Micro- and Nanoswimmers for Specific Applications. Acc. Chem. Res. 2017, 50, 2–11. [Google Scholar] [CrossRef]
- He, T.; Liu, S.; Yang, Y.; Chen, X. Application of Micro/Nanomotors in Environmental Remediation: A Review. Micromachines 2024, 15, 1443. [Google Scholar] [CrossRef]
- Wagner, M.; Lambert, S. (Eds.) Freshwater Microplastics; Springer International Publishing: Cham, Switzerland, 2018; Volume 58, ISBN 978-3-319-61614-8. [Google Scholar]
- Kinyua, E.M.; Nyakairu, G.W.A.; Tebandeke, E.; Odume, O.N. Visible Light Photocatalytic Degradation of HDPE Microplastics Using Vanadium-Doped Titania. Cent. Asian J. Water Res. 2024, 10, 126–141. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Huo, P. Plastic Degradation and Conversion by Photocatalysis; American Chemical Society: Washington, DC, USA, 2024; pp. 1–22. [Google Scholar]
- Ye, J.; Chen, Y.; Gao, C.; Wang, C.; Hu, A.; Dong, G.; Chen, Z.; Zhou, S.; Xiong, Y. Sustainable Conversion of Microplastics to Methane with Ultrahigh Selectivity by a Biotic–Abiotic Hybrid Photocatalytic System. Angew. Chem. 2022, 134, e202213244. [Google Scholar] [CrossRef]
- Wang, Q.; Hong, L.; Wu, K.; Li, M.; Zhang, J.; Li, X.; Jin, J.; Liu, B. Research Progress in Microbial Degradation of Microplastics. J. Phys. Conf. Ser. 2024, 2706, 012043. [Google Scholar] [CrossRef]
- Nam, J.-W.; Pham, V.N.; Ha, J.M.; Shin, M.; Lee, H.; Youn, Y.-S. Photocatalysis of Bifunctional Cr- and Fe-Doped CeO2 Nanoparticles toward Selective Oxidation of 5-Hydroxymethylfurfural and Decomposition of High-Density Polyethylene. Res. Sq. 2022. [Google Scholar] [CrossRef]
- He, Y.; Rehman, A.U.; Xu, M.; Not, C.A.; Ng, A.M.C.; Djurišić, A.B. Photocatalytic Degradation of Different Types of Microplastics by TiOx/ZnO Tetrapod Photocatalysts. Heliyon 2023, 9, e22562. [Google Scholar] [CrossRef]
- Farrugia, C.; Di Mauro, A.; Lia, F.; Zammit, E.; Rizzo, A.; Privitera, V.; Impellizzeri, G.; Buccheri, M.A.; Rappazzo, G.; Grech, M.; et al. Suitability of Different Titanium Dioxide Nanotube Morphologies for Photocatalytic Water Treatment. Nanomaterials 2021, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Zandieh, M.; Liu, J. Removal and Degradation of Microplastics Using the Magnetic and Nanozyme Activities of Bare Iron Oxide Nanoaggregates. Angew. Chem. 2022, 134, e202212013. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Fehn, S.; Steegmüller, T.; Rauwolf, S.; Löwe, H.; Pflüger-Grau, K.; Berensmeier, S. Immobilization of PETase Enzymes on Magnetic Iron Oxide Nanoparticles for the Decomposition of Microplastic PET. Nanoscale Adv. 2021, 3, 4395–4399. [Google Scholar] [CrossRef]
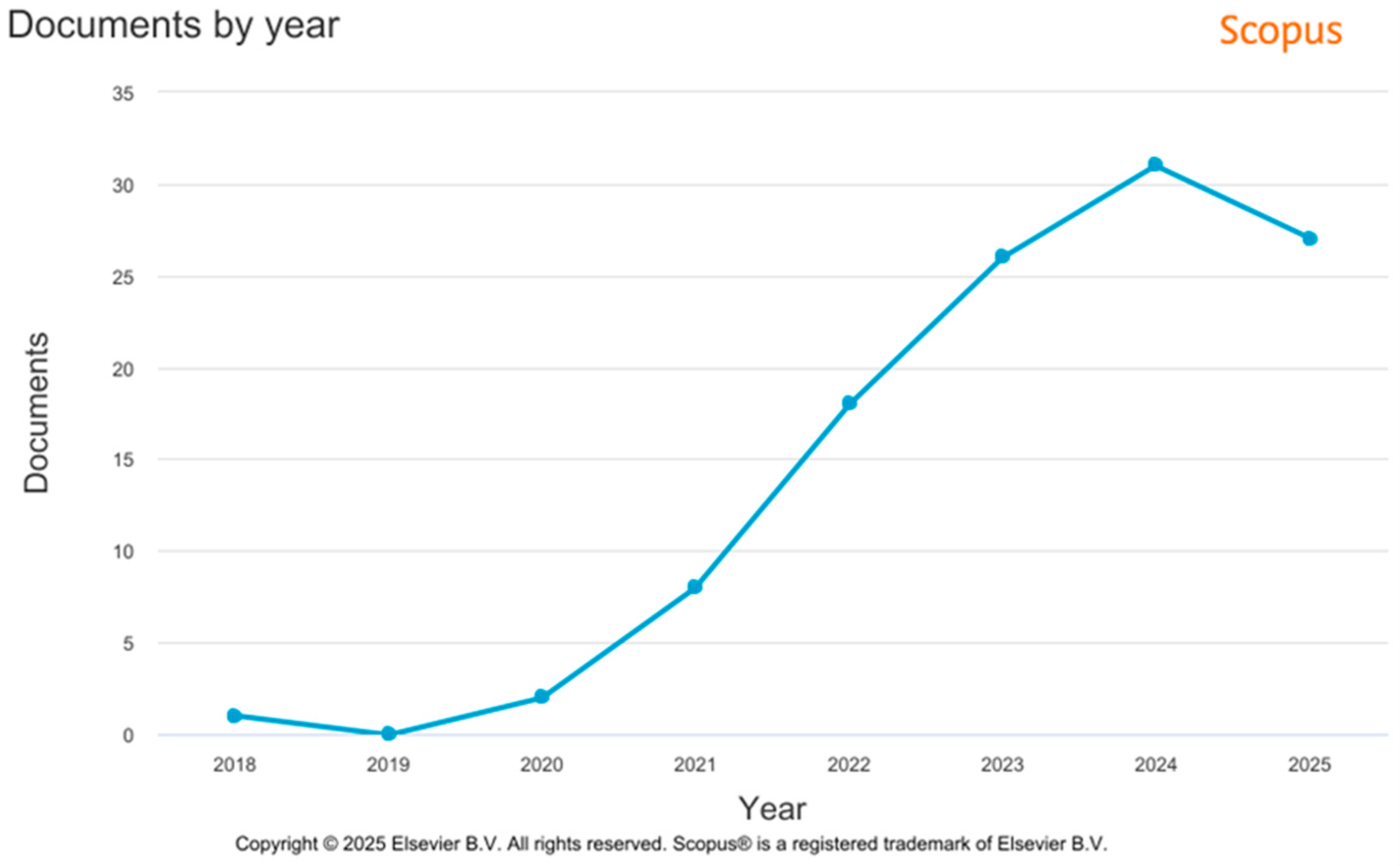
| Material | Bandgap Energy (eV) | Light Absorption | Physicochemical Properties | Synthesis/Modification Method | Photocatalytic Efficiency and Limitations |
|---|---|---|---|---|---|
| TiO2 | ≈3.0–3.2 (rutile and anatase) | Primarily UV | High stability, electron-hole recombination issues | Nanostructuring (nanotubes, nanowires), doping, composites (e.g., with activated carbon) | Efficient under ultraviolet light, with minimal visible light exposure and a high rate of electron-to-proton recombination. The degradation rates reported for polyethylene and polystyrene microplastics under optimal laboratory conditions range from 70 to 99%. |
| ZnO | ≈3.2 | Primarily UV | High surface area, adaptable morphology | Nanostructuring (nanorods, nanoflowers), doping (e.g., Ag, In), composites with reduced GO | Similarly to TiO2, it is primarily active under ultraviolet light due to its large energy gap (~3.3 eV). However, it performs poorly under natural sunlight and is susceptible to the recombination of electrons and protons. The activity of this material is dependent on its morphology. |
| GO | 2.4–4.3 eV | UV and Visible | High surface area, excellent electron mobility | Used as a composite with semiconductors like TiO2 to enhance charge separation | Improves the efficiency of charge transfer and increases the surface area in composite materials. The cost and complexity of synthesis are primarily linked to the production of pure GO and GO-based nanocomposites through chemical exfoliation or the Hummers method. |
| WO3 | ≈2.7 | Visible | Moderately stable, good ROS production | Doping, composite formation | Limited efficiency under sunlight without modifications. |
| CdS | ≈2.4 | Visible | High ROS production, toxicity concerns | Used in composites to stabilize and enhance degradation | Effective, but limited by toxicity and environmental safety concerns. |
| MOFs | Varied depending on metal nodes, linkers, structure, defects, and modifications | UV and Visible | Porous structure, tunable, high adsorption | Hybrid with metals, organic ligands | High charge separation, scalable; complex synthesis. |
| Material | Reactor Type | Reaction Condition | Degradation Efficiency | Reference |
|---|---|---|---|---|
| TiO2 nanofilms | Batch reactor | UV radiation | After 12 h, the decomposition of 98.4% was observed, and after 36 h, the decomposition was complete for both the polyethylene and polystyrene microspheres. | [18] |
| TiO2 nanorods | Batch reactor | pH of 3 and a temperature of 0 °C, irradiation with a 50 W LED lamp | The mass of microplastic particles decreased by 60% | [46,49] |
| N-modified TiO2 | Batch reactor | pH = 3, temperature 0 after 50 h reaction under visible light irradiation | The highest mean weight loss was 71.77% for HDPE | [46] |
| GO-TiO2 | Batch reactor | 72 W UV lamp with a wavelength of about 350 nm | 50.46% after 480 min of degradation | [52] |
| TiO2 nanoparticle films | Batch reactor | Made with Triton X-100; under UV light irradiation | The complete mineralization of 400 nm PS particles within 12 h, achieving a mineralization rate of 98.40%. | [18] |
| Ag/TiO2 composite | Batch reactor | UVA irradiation, mass ratio of photocatalyst to PA66 1:1 and 3:1 | significant degradation of PA66 | [50] |
| Factor | Effect on Photocatalytic Degradation | Key Considerations |
|---|---|---|
| Light wavelength |
|
|
| Light intensity |
|
|
| pH |
|
|
| Catalyst dosage |
|
|
| Type of MPs |
|
|
| Size of MPs |
|
|
| Temperature |
|
|
| ROS |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeszhan, Y.; Bexeitova, K.; Yermekbayev, S.; Toktarbay, Z.; Lee, J.; Berndtsson, R.; Azat, S. Photocatalytic Degradation of Microplastics in Aquatic Environments: Materials, Mechanisms, Practical Challenges, and Future Perspectives. Water 2025, 17, 2139. https://doi.org/10.3390/w17142139
Yeszhan Y, Bexeitova K, Yermekbayev S, Toktarbay Z, Lee J, Berndtsson R, Azat S. Photocatalytic Degradation of Microplastics in Aquatic Environments: Materials, Mechanisms, Practical Challenges, and Future Perspectives. Water. 2025; 17(14):2139. https://doi.org/10.3390/w17142139
Chicago/Turabian StyleYeszhan, Yelriza, Kalampyr Bexeitova, Samgat Yermekbayev, Zhexenbek Toktarbay, Jechan Lee, Ronny Berndtsson, and Seitkhan Azat. 2025. "Photocatalytic Degradation of Microplastics in Aquatic Environments: Materials, Mechanisms, Practical Challenges, and Future Perspectives" Water 17, no. 14: 2139. https://doi.org/10.3390/w17142139
APA StyleYeszhan, Y., Bexeitova, K., Yermekbayev, S., Toktarbay, Z., Lee, J., Berndtsson, R., & Azat, S. (2025). Photocatalytic Degradation of Microplastics in Aquatic Environments: Materials, Mechanisms, Practical Challenges, and Future Perspectives. Water, 17(14), 2139. https://doi.org/10.3390/w17142139








