Kinetics of the Process DAF-Culture Nannochloropsis oculata Remove Nutrients, Improve Water Quality, and Evaluate Rheological Parameters, Providing an Ecological Method for Treating Complex Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Physicochemical and Microbiological Characterization of Combined Wastewater
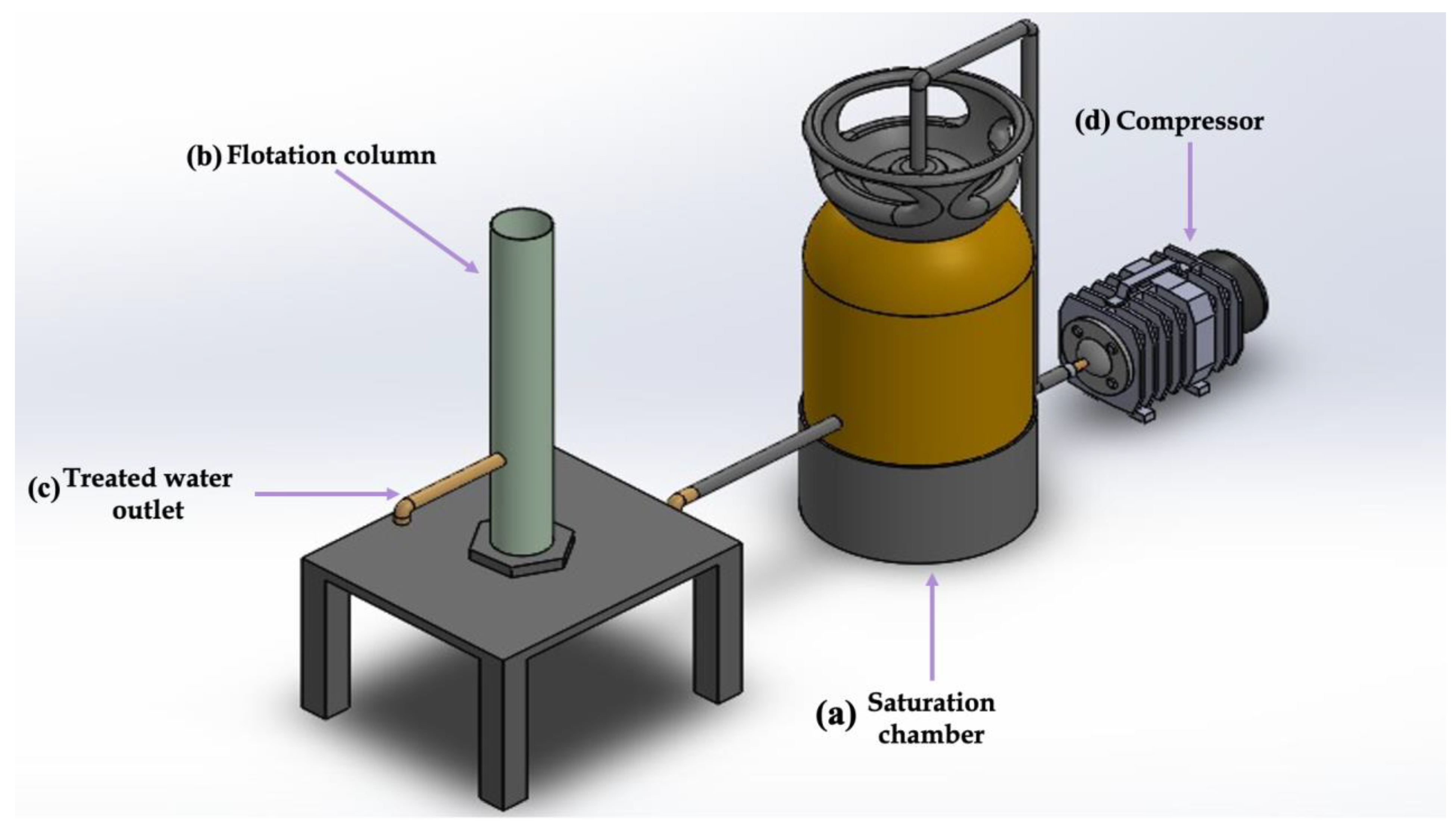
2.2. Implementation of the Dissolved Air Flotation System
2.3. Operating Conditions of the DAF System
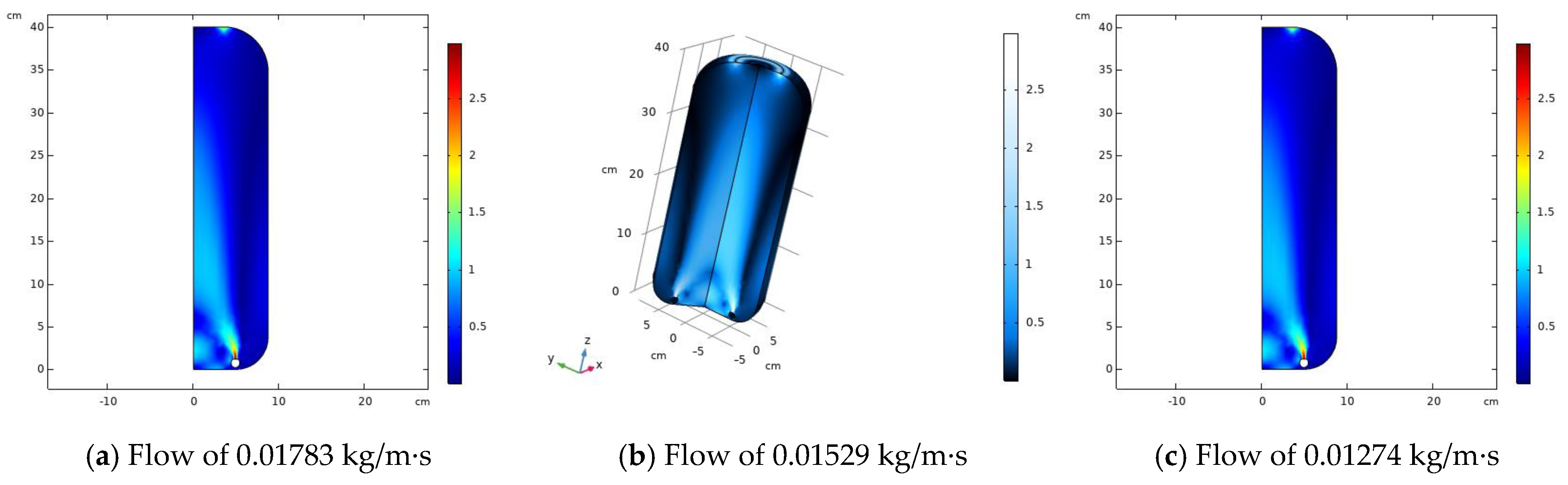
2.4. CFD Simulation of the Compressor Tank
2.5. Rheological Characterization of the Effluent
2.6. Obtaining and Propagating the Microalga Nannochloropsis Oculata
2.7. Growing Microalgae in Wastewater
2.8. Modeling of Microalgal Cell Growth by Mathematical Methods
2.9. Experimental Design
2.10. Statistical Techniques
3. Results and Discussion
3.1. Physicochemical and Microbiological Characterization of the Sample
3.2. CFD Simulation of Pressurizer Tank
3.3. Reological Analysis of the Sample
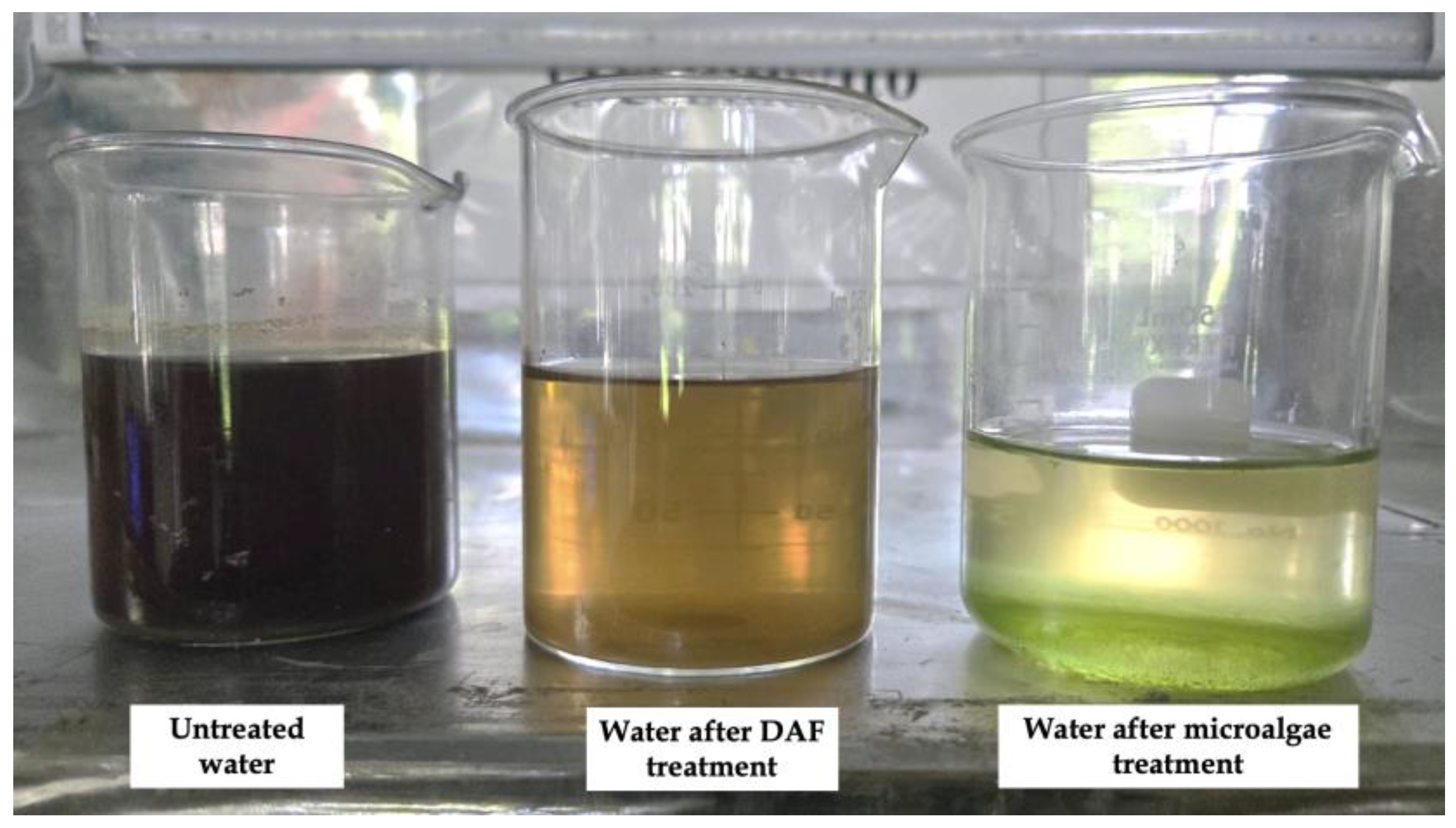
3.4. Primary Treatment of Wastewater with the DAF System
3.5. Secondary Treatment of Wastewater with Nannochloropsis Oculata
3.5.1. Removal of COD
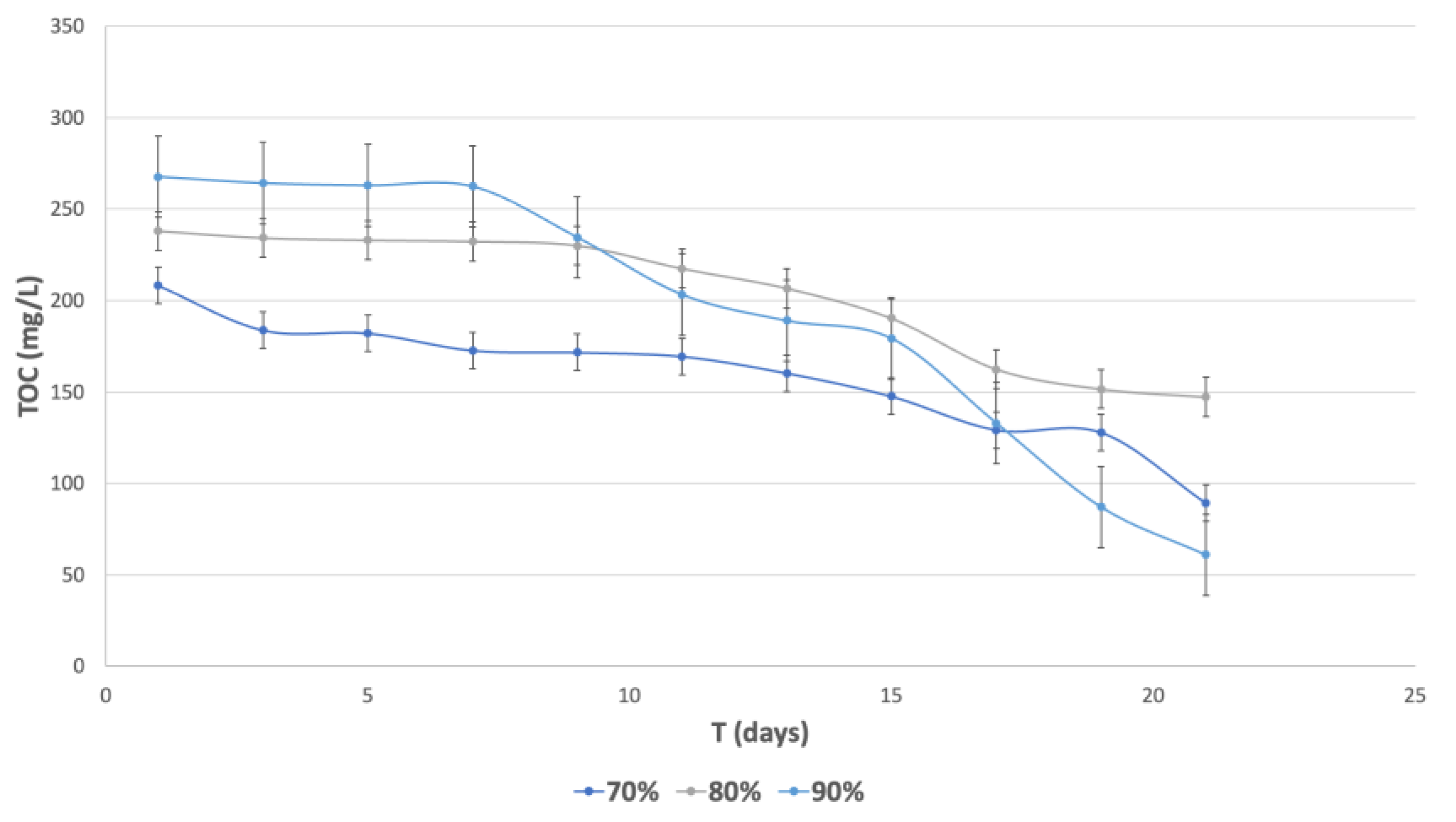
3.5.2. Removal of Total Organic Carbon
3.5.3. Removal of Nitrogen
3.5.4. Biomass Generation
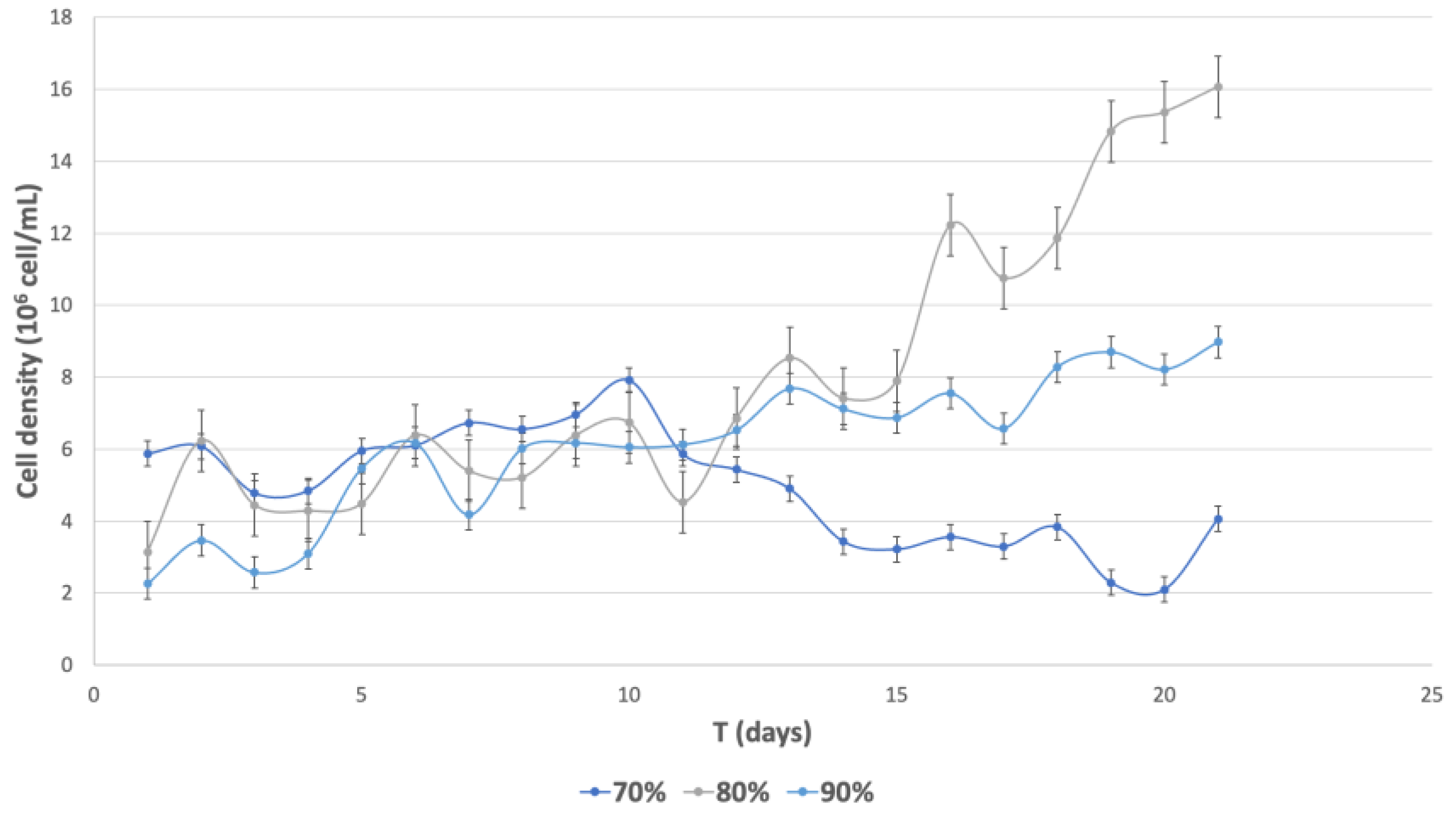
3.5.5. Cell Density Generation
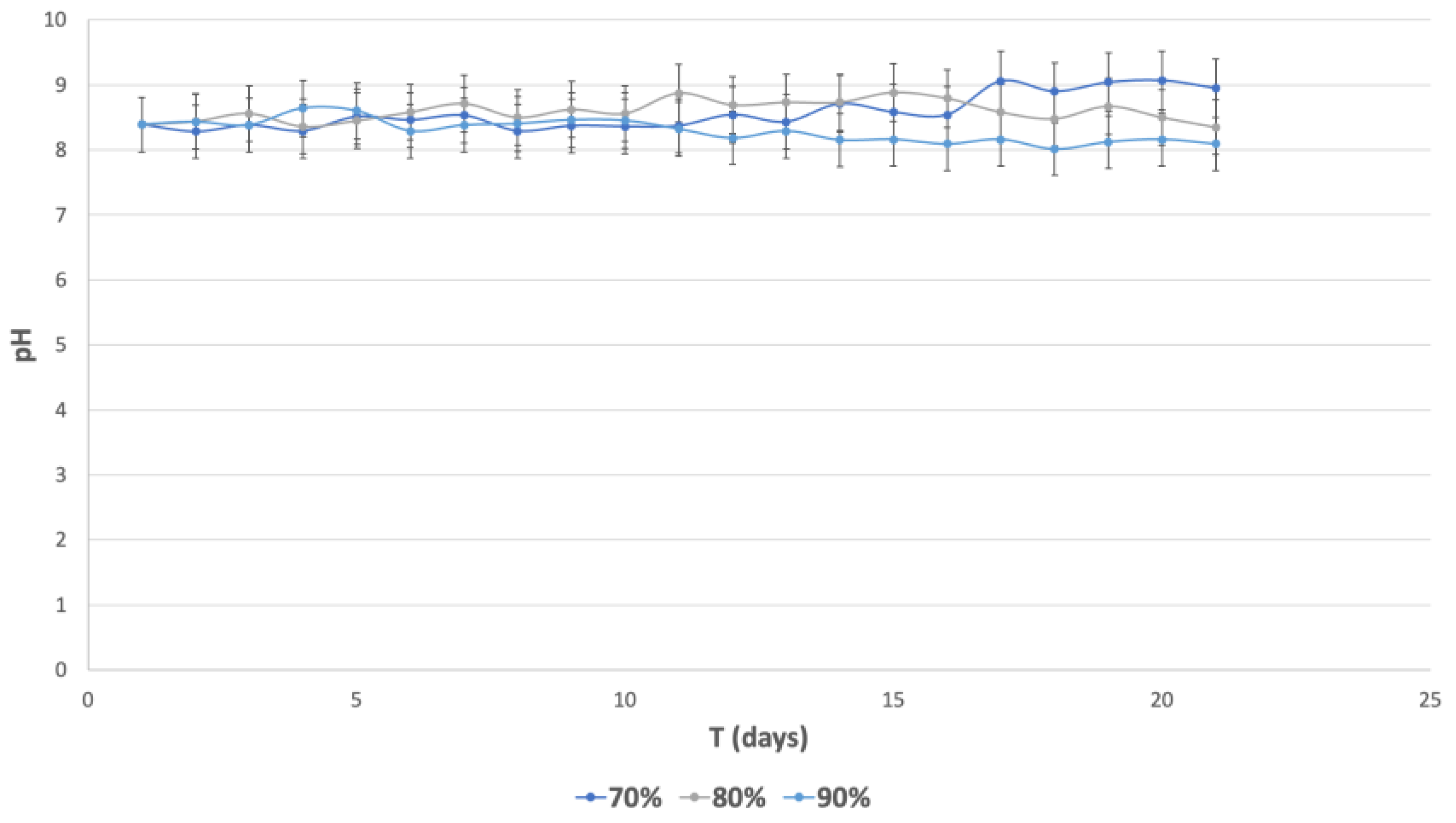
3.5.6. pH Monitoring
3.6. Kinetic Variables
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAF | Dissolved Air Flotation |
| COD | Chemical Oxygen Demand |
| TOC | Total Organic Carbon |
| TS | Total Solids |
| TVS | Total Volatile Solids |
| ULA | Ultra Low Adapter |
References
- Vázquez-Tapia, I.; Salazar-Martínez, T.; Acosta-Castro, M.; Meléndez-Castolo, K.A.; Mahlknetch, J.; Cervantes-Avilés, P.; Capparelli, M.V.; Mora, A. Occurrence of emerging organic contaminants and endocrine disruptors in different water compartments in Mexico—A review. Chemosphere 2022, 308, 136285. [Google Scholar] [CrossRef] [PubMed]
- Lesser, L.E.; Mora, A.; Moreau, C.; Mahlknetch, J.; Hernández-Antonio, A.; Ramírez, A.I.; Barrios-Piña, H. Survey of 218 organic contaminants in groundwater derived from the world’s largest untreated wastewater irrigation system: Mezquital Valley, Mexico. Chemosphere 2018, 198, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Calidad del agua en México. Available online: https://www.gob.mx/conagua/articulos/calidad-del-agua (accessed on 19 May 2025).
- CONAGUA. Estadísticas del agua en México 2021, 1st ed.; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2022; pp. 166–178. [Google Scholar]
- Brandt, M.J.; Johnson, K.M.; Elphinston, A.; Ratnayaka, D.D. Storage, clarification and chemical treatment (chapter 7). In Water Supply, 5th ed.; Twort, A.C., Ratnayaka, D.D., Brandt, M.J., Eds.; Elsevier Ltd.: Berkeley, CA, USA, 2000; pp. 323–366. [Google Scholar]
- Muñoz-Alegría, J.A.; Muñoz-España, E.; Flórez-Marulanda, J.F. Dissolved Air Flotation: A Review from the Perspective of System Parameters and Uses in Wastewater Treatment. TecnoLógicas 2021, 24, 281–303. [Google Scholar] [CrossRef]
- Fuad, N.; Omar, R.; Kamarudin, S.; Harun, R.; Idris, A.; Azlina, W. Mass harvesting of marine microalgae using different techniques. Food Bioprod. Process. 2018, 112, 169–184. [Google Scholar] [CrossRef]
- Aaltonen, A.; Le, T.M.K.; Saari, E.; Dahl, O.; Musuku, B.; Lang, A.; Hiidenheimo, S.; Dixon, R. Improving Nickel Recovery in Froth Flotation by Purifying Concentrators Process Water Using Dissolved Air Flotation. Minerals 2023, 13, 319. [Google Scholar] [CrossRef]
- Villar-Navarro, E.; Baena-Nogueras, R.M.; Paniw, M.; Perales, J.A.; Lara-Martín, P.A. Removal of pharmaceuticals in urban wastewater: High rate algae pond (HRAP) based technologies as an alternative to activated sludge based processes. Water Res. 2018, 139, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sanjayasari, D.; Rahmasari, F.; Khasanah, I.; Imlani, A. Density and Growth Rate of Nannochloropsis oculata with Different Photoperiods. MAIYAH 2023, 2, 25–32. [Google Scholar] [CrossRef]
- Osorio-Rivera, M.A.; Carrillo-Barahona, W.E.; Negrete-Costales, J.H.; Loor-Larvay, X.A.; Riera-Gualchichulca, E.J. La calidad de las aguas residuales domésticas. Pol. Con. 2020, 6, 228–245. [Google Scholar] [CrossRef]
- Zahran, E.; Elbahnaswy, S.; Ahmed, F.; Ibrahim, I.; Khaled, A.A.; Eldessouki, E.A. Nutritional and immunological evaluation of Nannochloropsis oculata as a potential Nile tilapia-aquafeed supplement. BMC Vet. Res. 2023, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Guiscafré Rodea, F.J.; García Trejo, J.F.; Vanthoor Koopmans, M. Cultivo de la microalga nannochloropsis limnetica en agua residual de invernadero. PCT 2018, 1, 10–19. Available online: https://revistas.uaq.mx/index.php/perspectivas/article/view/424 (accessed on 20 May 2025).
- Reyimu, Z.; Özçimen, R. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J. Clean. Prod. 2017, 150, 40–46. [Google Scholar] [CrossRef]
- Santos, B.; Araújo, J.; Carvalho, B.; Cotrim, C.; Bernardino, R.; Freitas, F.; Sobral, A.J.F.N.; Encarnação, T. Bioremediation of Synthetic Wastewater with Contaminants of Emerging Concern by Nannochloropsis sp. and Lipid Production: A Circular Approach. Bioengineering 2025, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Zezulka, Š.; Maršálek, B.; Maršálková, E.; Odehnalová, K.; Pavlíková, M.; Lamaczová, A. Suspended Particles in Water and Energetically Sustainable Solutions of Their Removal—A Review. Processes 2024, 12, 2627. [Google Scholar] [CrossRef]
- Sobolewska, E.; Borowski, S.; Nowicka-Krawczyk, P. Cultivation of Microalgae in Liquid Digestate to Remove Nutrients and Organic Contaminants. Bioenergy Res. 2024, 17, 1843–1855. [Google Scholar] [CrossRef]
- NMX-AA-008-SCFI-2016; Análisis de Aguas—Medición del pH en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2016.
- NMX-AA-007-SCFI-2013; Análisis de Agua—Medición de la Temperatura en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2013.
- NMX-AA-038-SCFI-2001; Análisis de Agua—Determinación de Turbiedad en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-034-SCFI-2015; Análisis de Agua—Medición de Sólidos y Sales Disueltas en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2015.
- NMX-AA-012-SCFI-2001; Análisis de Agua—Medición de Color Verdadero en Aguas Naturales, Residuales, Residuales Tratadas y Marinas—Mediante Coeficientes de Absorción Espectral—Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2021.
- NMX-AA-017-SCFI-2021; Análisis de Agua—Determinación de la Conductividad Electrolítica. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2000.
- NMX-AA-029-SCFI-2001; Diario Oficial de la Federación. Análisis de Aguas—Determinación de Fósforo Total en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-030-SCFI-2001; Análisis de Agua—Determinación de la Demanda Química de Oxígeno en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-026-SCFI-2010; Análisis de Agua—Medición de Nitrógeno Total Kjeldahl en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2010.
- NMX-AA-113-SCFI-2012; Análisis de Agua—Medición del Número de Huevos de Helminto en Aguas Residuales y Residuales Tratadas por Observación Microscópica. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2012.
- Ali, S.M.; Aziz, S.Q. Dissolved Air Flotation (DAF) Operational Parameters and Limitations for Wastewaters Treatment with Cost Study. Recycl. Sustain. Dev. 2023, 16, 91–97. [Google Scholar] [CrossRef]
- Fanaie, V.R.; Khiadani, M.; Ayres, T. Effects of internal geometry on hydrodynamics of dissolved air flotation (DAF) tank: An experimental study using particle image velocimetry (PIV). Colloids Surf. A 2019, 575, 382–390. [Google Scholar] [CrossRef]
- Gutiérrez-Casiano, N.; Hernández-Aguilar, E.; Alvarado-Lassman, A.; Méndez-Contreras, J.M. Removal of carbon and nitrogen in wastewater from a poultry processing plant in a photobioreactor cultivated with the microalga Chlorella vulgaris. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2022, 57, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Guzmán, S.M.; Hernández-Aguilar, E.; Alvarado-Lassman, A.; Méndez-Contreras, J.M. Kinetics of Obtaining Microalgal Biomass and Removal of Organic Contaminants in Photobiorreactors Operated with Microalgae—Study Case: Treatment of Wastewater from a Poultry Slaughterhouse. Water 2024, 16, 1558. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, J.; Jeon, J.T.; Lee, I.C.; Won, C.H.; Chung, J. Microbubble-inducing Characteristics Depending on Various Nozzle and Pressure in Dissolved Air Flotation Process. KSCE J. Civ. Eng. 2014, 19, 558–563. [Google Scholar] [CrossRef]
- Sirin, S.; Sillanpää, M. Cultivating and harvesting of marine alga Nannochloropsis oculata in local municipal wastewater for biodiesel. Bioresour. Tecnol. 2015, 191, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Macías, M.R.; Sánchez-Duarte, R.G.; Meza-Escalante, E.R.; Ulloa-Mercado, R.G.; Saldívar-Cabrales, J. Síntesis de lípidos de la microalga Nannochloropsis oculata para su uso potencial en la producción de biodiésel. Rev. Int. Contam. Ambient. 2017, 33, 85–91. [Google Scholar] [CrossRef]
- NOM-001-SEMARNAT-2021; Límites Permisibles de Contaminantes en las Descargas de Aguas Residuales en Cuerpos Receptores Propiedad de la Nación. Diario Oficial de la Federación: Mexico City, Mexico, 2021.
- Wei, Y.; Jiao, Y.; An, D.; Li, D.; Li, W.; Wei, Q. Review of Dissolved Oxygen Detection Technology: From Laboratory Analysis to Online Intelligent Detection. Sensors 2019, 19, 3995. [Google Scholar] [CrossRef] [PubMed]
- Bozorg-Haddad, O.; Delpasand, M.; Loáiciga, H. Water quality, hygiene and health. In Economical, Political and Social Issues in Water Resources; Elsevier: Tehrán, Iran, 2021. [Google Scholar]
- Palaniandy, P.; Abdul-Aziz, H.; Fared-Murshed, M. Dissolved Air Flotation (DAF) for Wastewater Treatment. In Waste Treatment in the Service and Utility Industries, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Luo, C.; Guo, L.; Zeng, S.; Long, T. Effects of turbulence fluctuation intensity in bioreactor of sewage treatment on physical and chemical properties of biofilms. Bioprocess Biosyst. Eng. 2021, 44, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Leyva-De La Cruz, B.; Guillen-Campo, Y.; Laurencio-Alfonso, H.L. Caracterización reológica de un lodo que se genera en el lavado de un árido. Rev. Ing. Mec. 2016, 19, 143–149. [Google Scholar]
- Farno, E.; Baudez, J.C.; Parthasarathy, R.; Eshtiaghi, N. Impact of temperature and duration of thermal treatment on different concentrations of anaerobic digested sludge: Kinetic similarity of organic matter solubilisation and sludge rheology. Chem. Eng. J. 2015, 273, 534–542. [Google Scholar] [CrossRef]
- Muhereid, M.A. Common Versus Herschel-Bulkley Drilling Fluid Models: Effect of Their Rheological Parameters on Dynamic Particle Settling Velocity. Am. Sci. Res. J. Eng. Technol. Sci. 2016, 16, 155–177. [Google Scholar]
- Mouzaoui, M.; Sauceau, M.; Devriendt, L.; Baudez, J.C.; Arlabosse, P. Rheological characterization of sludge in divided granular-like and pasty states using a granular rheometer. Powder Technol. 2020, 378, 521–527. [Google Scholar] [CrossRef]
- Button, C.; Poddar, N.; Lacassagne, T.; Pernice, M.; Gupta, V.; Aditya, L.; Kuzhiumparambil, U.; Ralph, P.J. Rheological characterization and modeling of freshwater and marine microalgae and cyanobacteria mixed cultures. Algal Res. 2025, 88, 103988. [Google Scholar] [CrossRef]
- Díaz-Díaz, M.A.; Rivas-Trasancos, L.; Fernández-Rangel, D.; Salazar-Alemán, D.; Miller-Palmer, S. Tratamiento de aguas residuales oleosas mediante flotación por aire disuelto. Tecnol. Química 2018, 38, 256–270. [Google Scholar]
- Pimiento, K.; Cárdenas-Gónzález, M.J. Evaluación del tratamiento preliminar y primario para las aguas residuales del procesamiento industrial de alimentos en La Grita (Venezuela). INGE 2021, 17, 1–14. [Google Scholar] [CrossRef]
- Monira, S.; Bhuiyan, M.A.; Haque, N.; Shah, K.; Roychand, R.; Hai, F.I.; Pramanik, B.K. Understanding the fate and control of road dust-associated microplastics in stormwater. Process. Saf. Environ. Prot. 2021, 152, 47–57. [Google Scholar] [CrossRef]
- Osorio-Trujillo, A.F.; Martínez-Cajigas, M.E. Validación de un método para el análisis de color real en agua. Rev. Fac. Cienc. 2018, 7, 143–155. [Google Scholar] [CrossRef]
- Lee, S.; Lee, N.; Oh, H.M.; Ahn, C.Y. Enhanced and balanced microalgal wastewater treatment (COD, N and P) by interval inoculation of activated sludge. J. Microbiol. Technol. 2019, 29, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, B.; Ngwa, F.F.; Deconinck, S.; Verstraete, W. Effluent Quality of a Conventional Activated Sludge and a Membrane Bioreactor System Treating Hospital Wastewater. Environ. Technol. 2010, 27, 395–402. [Google Scholar] [CrossRef] [PubMed]
- NMX-AA-187-SCFI-2021; Análisis de Agua—Medición de Carbono Orgánico Total en Aguas Naturales, Salinas, Residuales y Residuales Tratadas. Diario Oficial de la Federación: Mexico City, Mexico, 2021.
- Patel, A.K.; Choi, Y.Y.; Sim, S.J. Emerging prospects of mixotrophic microalgae: Way forward to sustainable bioprocess for environmental remediation and cost-effective biofuels. Bioresour. Technol. 2020, 300, 122741. [Google Scholar] [CrossRef] [PubMed]
- Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Suárez-Rumiche, J.A.; Castillo-Valdiviezo, A.; Souza-Najar, R.I. Obtención de azúcares reductores por hidrólisis ácida a partir de un consorcio de microalgas amazónicas cultivadas en agua residual. Rev. Cienc. Tecnol. 2021, 17, 45–51. [Google Scholar] [CrossRef]
- Rojo-Cebreros, A.H.; Morales-Plascencia, M.E.; Ibarra-Castro, L.; Martínez-Brown, J.M.; Medina-Jasso, M.A. Floculación de Nannochloropsis sp inducida por hidróxido de sodio: Eficiencia de floculación, efecto sobre la viabilidad microalgal y su uso como alimento para rotíferos. Lat. Am. J. Aquat. Res. 2016, 44, 662–670. [Google Scholar] [CrossRef]








| Parameter | Regulation | Equipment | Equipment Sensitivity |
|---|---|---|---|
| pH | NMX-AA-008-SCFI-2016 [18] | Hanna® potentiometer HI2002-01 model, Mexico City, Mexico. | 0.01 |
| Temperature | NMX-AA-007-SCFI-2013 [19] | Brannan® thermometer, Mexico City, Mexico. | 0.1 °C |
| Turbidity | NMX-AA-038-SCFI-2001 [20] | Hatch® turbidimeter 2100Q model, Mexico City, Mexico. | 0.1 NTU |
| TS and TVS | NMX-AA-034-SCFI-2015 [21] | Felisa® stove 4840 model and ThermoScientific® muffle furnace FE363 model, Mexico City, Mexico. | 1 °C |
| Dissolved oxygen and saturation | NMX-AA-012-SCFI-2001 [22] | Hatch® multiparameter HQ40D model, Mexico City, Mexico. | 0.1 mg/L, 0.1% |
| True color | NMX-AA-017-SCFI-2021 [23] | ThermoScientific® spectrophotometer UV-VIS Genesys 10S Model, Mexico City, Mexico. | 0.001 |
| Total and soluble COD | NMX-AA-030-SCFI-2012 [24] | ThermoScientific® spectrophotometer UV-VIS Genesys 10S Model, Mexico City, Mexico. | 0.001 |
| Total phosphorus | NMX-AA-029-SCFI-2001 [25] | ThermoScientific® spectrophotometer UV-VIS Genesys 10S Model, Mexico City, Mexico. | 0.001 |
| Total nitrogen | NMX-AA-026-SCFI-2016 [26] | Labconco® microdistiller 6030000 model, Mexico City, Mexico. | NA |
| Helminth eggs | NMX-AA-113-SCFI-2012 [27] | AmScope® microscope B250 model, Mexico City, Mexico. | NA |
| F:M Ratios | Wastewater Volume (mL) | Microalgae Volume (mL) |
|---|---|---|
| 70:30 | 350 | 150 |
| 80:20 | 400 | 100 |
| 90:10 | 450 | 50 |
| Parameter | Result | Maximum Permissible | Units | |
|---|---|---|---|---|
| pH | 8.19 | 6–9 | ||
| Temperature | 21 | 35 | °C | |
| Total COD | 155.91 | 150 | mg/L | |
| Soluble COD | 53.91 | mg/L | ||
| Phosphorus | 27.59 | 15 | mgP/L | |
| Total solids | 0.212 | % w/w | ||
| Total volatile solids | 43.353 | % w/w | ||
| Turbidity | 113.33 | NTU | ||
| Dissolved oxygen | 1.6 | 5 | mg/L | |
| Saturation | 22.5 | 80 | % | |
| True color | 436 nm | 10.53 | 7 | 1/m |
| 525 nm | 8.06 | 5 | 1/m | |
| 620 nm | 6.83 | 3 | 1/m | |
| Helminth eggs | Not found | 0 | HH/100 mL | |
| Temperature (°C) | Regression Equation | m (cP) | (Pa) | K (Pa·s) | n | R2 |
|---|---|---|---|---|---|---|
| 20 | 1.88 | 0.01566 | 0.00004964 | 1.687 | 0.9986 | |
| 25 | 1.76 | 0.008963 | 0.00005077 | 1.666 | 0.9989 | |
| 30 | 1.68 | 0 | 0.00004013 | 1.718 | 0.9983 | |
| 40 | 1.68 | 0 | 0.00004466 | 1.684 | 0.9981 |
| Temperature (°C) | Equation Regression | m (cP) | K (Pa·s) | n | R2 |
| 20 | 1.88 | 0.0001074 | 1.550 | 0.9986 | |
| 25 | 1.76 | 0.0000811 | 1.583 | 0.9980 | |
| 30 | 1.68 | 0.00003744 | 1.731 | 0.9982 | |
| 40 | 1.68 | 0.00003984 | 1.704 | 0.9980 |
| Raw Wastewater | Wastewater Before DAF | Wastewater Before Microalgae | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (Pa·s) | m (cP) | (Pa·s) | m (cP) | (Pa·s) | m (cP) | ||||
| 0.0000811 | 1.583 | 1.76 | 0.0005 | 1.2439 | 1.80 | 0.0007 | 1.1862 | 1.84 | 0.9981 |
| Parameter | Raw Wastewater | 40 PSI | 50 PSI | 60 PSI | Reference [45] |
|---|---|---|---|---|---|
| Total COD (mg/L) | 1654.67 | 1171.6 | 1489 | 1559.25 | 170 |
| Soluble COD (mg/L) | 625 | 513 | 453 | 377.84 | |
| Turbidity (NTU) | 110.44 | 106.08 | 108.64 | 99.92 | 21.9 |
| True Color 436 nm (m−1) | 119.43 | 103.2 | 92 | 81 | (465 nm) 70 |
| 525 nm (m−1) | 95.55 | 59.4 | 59.6 | 74 | |
| 620 nm (m−1) | 80.4 | 28.9 | 74.17 | 63.43 | |
| pH | 8.39 | 8.13 | 7.92 | 7.35 | 7.7 |
| a | b | c | μm | λ | G | R2 | |
|---|---|---|---|---|---|---|---|
| 70% | 0.6046 | 2.0116 | 0.1335 | 0.0807 | 7.5771 | 8.5859 | 99.58% |
| 80% | 1.5933 | 1.3524 | 0.0641 | 0.1022 | 5.4922 | 6.7787 | 99.11% |
| 90% | 0.3746 | 1.3076 | 6.2270 | 2.3323 | 0.0494 | 0.2970 | 99.12% |
| Std. Dev. | 0.02301 | 0.4143 | 0.0257 | 0.02367 | 2.1318 | 1.0127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Guzmán, S.M.; Alvarado-Lassman, A.; Hernández-Aguilar, E.; Sales-Pérez, R.E.; Méndez-Contreras, J.M. Kinetics of the Process DAF-Culture Nannochloropsis oculata Remove Nutrients, Improve Water Quality, and Evaluate Rheological Parameters, Providing an Ecological Method for Treating Complex Wastewater. Water 2025, 17, 2113. https://doi.org/10.3390/w17142113
Pérez-Guzmán SM, Alvarado-Lassman A, Hernández-Aguilar E, Sales-Pérez RE, Méndez-Contreras JM. Kinetics of the Process DAF-Culture Nannochloropsis oculata Remove Nutrients, Improve Water Quality, and Evaluate Rheological Parameters, Providing an Ecological Method for Treating Complex Wastewater. Water. 2025; 17(14):2113. https://doi.org/10.3390/w17142113
Chicago/Turabian StylePérez-Guzmán, Solmaría Mandi, Alejandro Alvarado-Lassman, Eduardo Hernández-Aguilar, Roger Emmanuel Sales-Pérez, and Juan Manuel Méndez-Contreras. 2025. "Kinetics of the Process DAF-Culture Nannochloropsis oculata Remove Nutrients, Improve Water Quality, and Evaluate Rheological Parameters, Providing an Ecological Method for Treating Complex Wastewater" Water 17, no. 14: 2113. https://doi.org/10.3390/w17142113
APA StylePérez-Guzmán, S. M., Alvarado-Lassman, A., Hernández-Aguilar, E., Sales-Pérez, R. E., & Méndez-Contreras, J. M. (2025). Kinetics of the Process DAF-Culture Nannochloropsis oculata Remove Nutrients, Improve Water Quality, and Evaluate Rheological Parameters, Providing an Ecological Method for Treating Complex Wastewater. Water, 17(14), 2113. https://doi.org/10.3390/w17142113









