The Impact of Rainwater Quality Harvested from Asbestos Cement Roofs on Leaf Temperature in Solanum lycopersicum as a Plant Water Stress Indicator
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Growth Conditions
2.2. Preparation of Simulated Contaminated Rainwater
2.3. Irrigation Regimen
2.4. Leaf Temperature Measurement
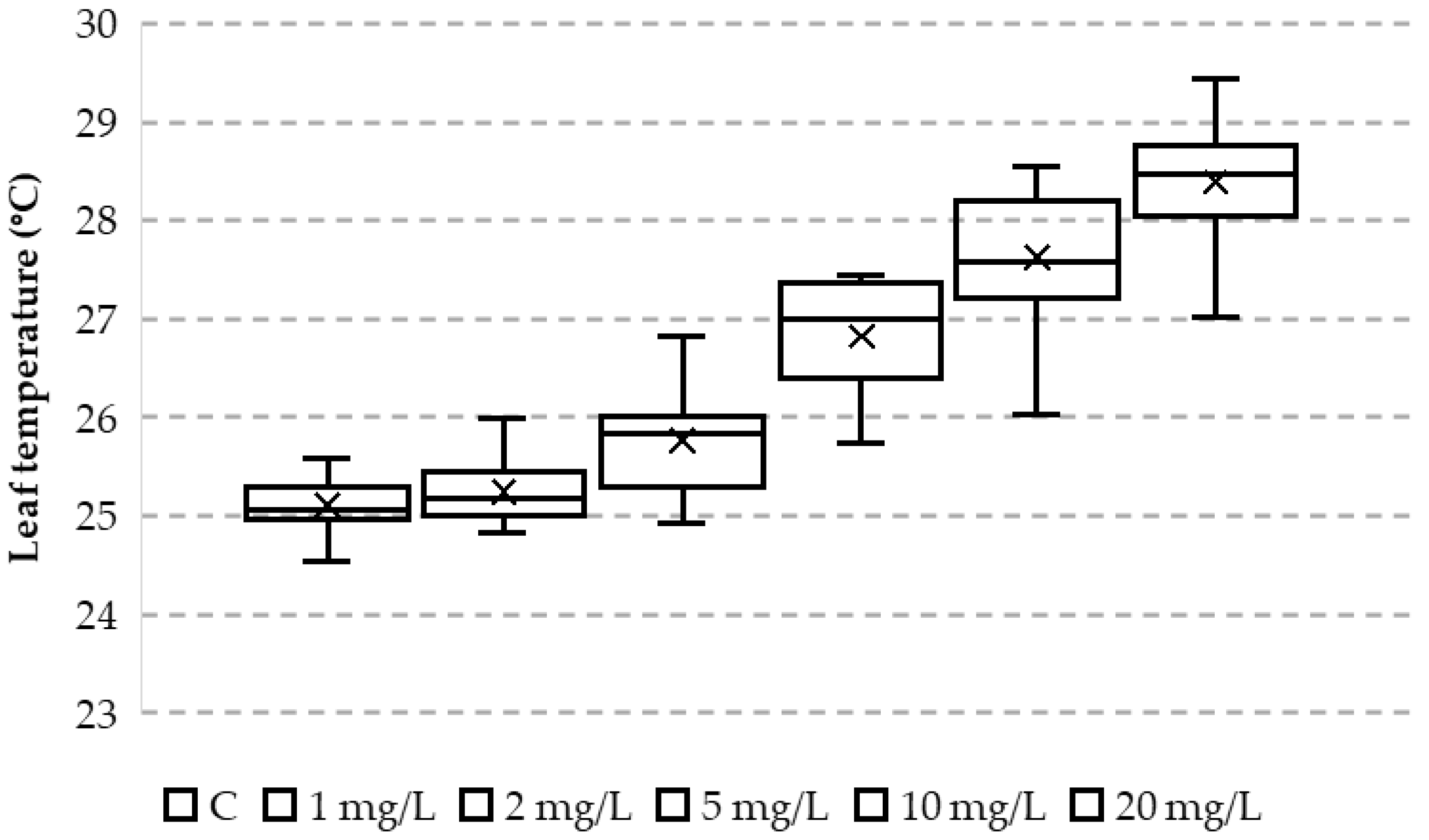
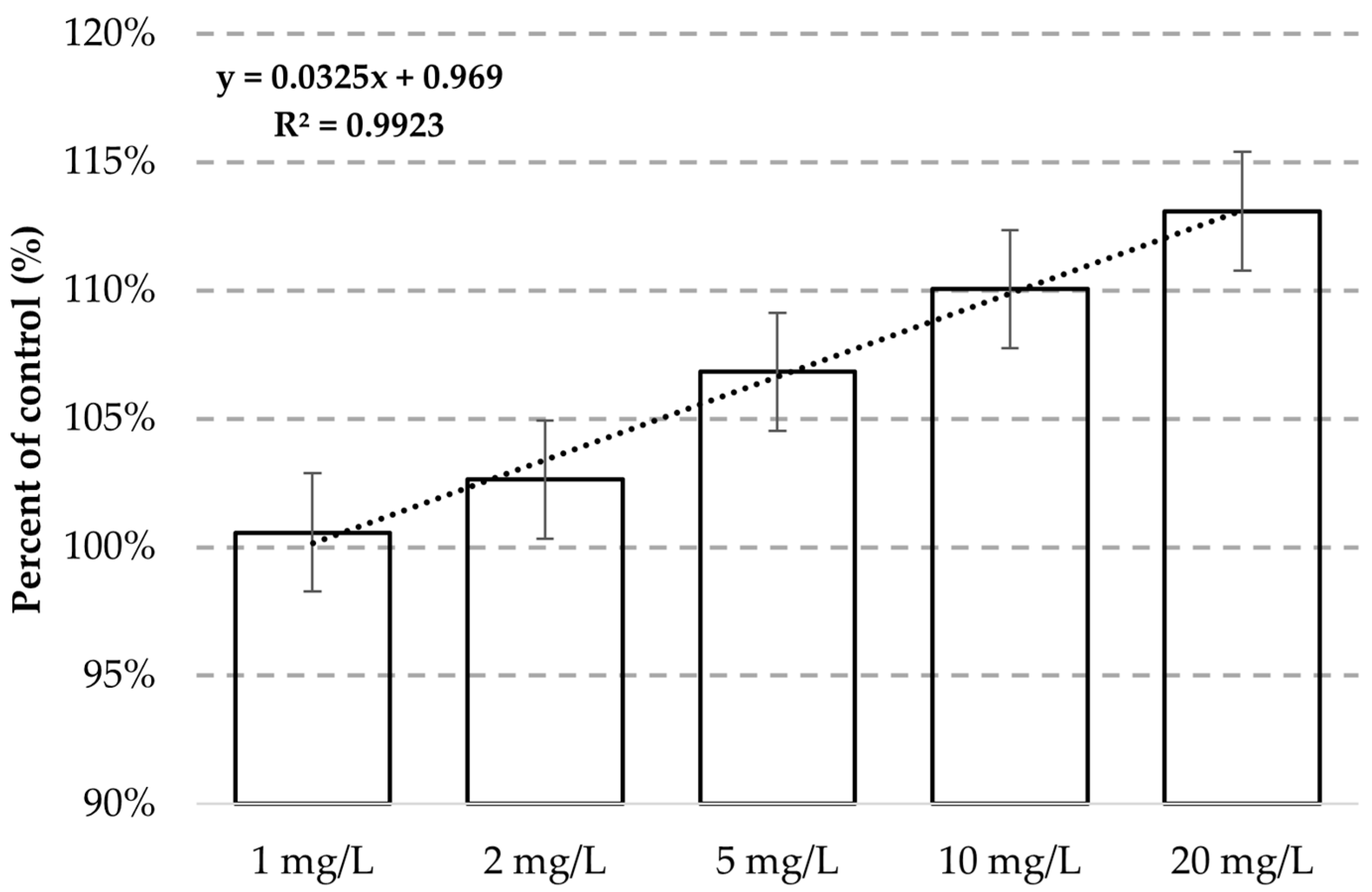
3. Results
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | asbestos cement |
| ACM | asbestos-containing material |
| ROS | reactive oxygen species |
| RRWH | rooftop rainwater harvesting |
| RWH | rainwater harvesting |
References
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef]
- Molnár, R.; Molnár, J.; Beke, D.; Papp, B.; Kuti, R. Shelf life of bottled water—Field conditions in Hungary. J. Food Nutr. Res. 2020, 59, 361–366. [Google Scholar]
- Oishy, M.N.; Shemonty, N.A.; Fatema, S.I.; Mahbub, S.; Mim, E.L.; Hasan Raisa, M.B.; Anik, A.H. Unravelling the effects of climate change on the soil-plant-atmosphere interactions: A critical review. Soil Environ. Health 2025, 3, 100130. [Google Scholar] [CrossRef]
- Tabari, H. Climate change impact on flood and extreme precipitation increases with water availability. Sci. Rep. 2020, 10, 13768. [Google Scholar] [CrossRef]
- Nabayi, A.; The, C.B.S.; Tan, A.K.Z.; Tan, N.P.; Beke, D. Combined benefits of fermented washed rice water and NPK mineral fertilizer on plant growth and soil fertility over three field planting cycles. Heliyon 2023, 9, e20213. [Google Scholar] [CrossRef]
- Gorelick, S.M.; Zheng, C. Global change and the groundwater management challenge. Water Resour. Res. 2015, 51, 3031–3051. [Google Scholar] [CrossRef]
- Mancosu, N.; Snyder, R.; Kyriakakis, G.; Spano, D. Water Scarcity and Future Challenges for Food Production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Bernal, D.; Restrepo, I.; Grueso-Casquete, S. Key criteria for considering decentralization in municipal wastewater management. Heliyon 2021, 7, e06375. [Google Scholar] [CrossRef]
- Gikas, P.; Tchobanoglous, G. The role of satellite and decentralized strategies in water resources management. J. Environ. Manag. 2009, 90, 144–152. [Google Scholar] [CrossRef]
- Sari, S.P. Suhendri. Potential of Rainwater System for Domestic Building in Jakarta. IOP Conf. Ser. Earth Environ. Sci. 2018, 152, 012002. [Google Scholar] [CrossRef]
- Oyilieze Akanwa, A. Rural Harvested Rainwater: Effect of Roof Types and its Design on Water Quality and Health: A Case for CBP Approach in Anambra State. Rev. Environ. Earth Sci. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Traboulsi, H.; Traboulsi, M. Rooftop level rainwater harvesting system. Appl. Water Sci. 2017, 7, 769–775. [Google Scholar] [CrossRef]
- Amos, C.; Rahman, A.; Gathenya, J.; Friedler, E.; Karim, F.; Renzaho, A. Roof-Harvested Rainwater Use in Household Agriculture: Contributions to the Sustainable Development Goals. Water 2020, 12, 332. [Google Scholar] [CrossRef]
- Razali, M.H.; Puteh, A.Q.; Sulaiman, A.H.; Yatim, M.H. Smart Rainwater Harvesting System for Sustainable Agricultural Irrigation and Drainage System. In Irrigation and Drainag—Recent Advances; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Qammar, S.W.; Khan, F.A.; Rehan, R. Evaluating the Potential of Roof Water Harvesting System for Drinking Water Supplies During Emergencies Under the Impacts of Climate Change: ‘A Case Study of Swat District, Pakistan’. Standards 2025, 5, 11. [Google Scholar] [CrossRef]
- Meili, N.; Manoli, G.; Burlando, P.; Carmeliet, J.; Chow, W.T.L.; Coutts, A.M.; Roth, M.; Velasco, E.; Vivoni, E.R.; Fatichi, S. Tree effects on urban microclimate: Diurnal, seasonal, and climatic temperature differences explained by separating radiation, evapotranspiration, and roughness effects. Urban For. Urban Green. 2021, 58, 126970. [Google Scholar] [CrossRef]
- Kumar, P.; Debele, S.E.; Khalili, S.; Halios, C.H.; Sahani, J.; Aghamohammadi, N.; de Fatima Andrade, M.; Athanassiadou, M.; Bhui, K.; Calvillo, N.; et al. Urban heat mitigation by green and blue infrastructure: Drivers, effectiveness, and future needs. Innovation 2024, 5, 100588. [Google Scholar] [CrossRef]
- Gunawardena, K.R.; Wells, M.J.; Kershaw, T. Utilising green and bluespace to mitigate urban heat island intensity. Sci. Total Environ. 2017, 584, 1040–1055. [Google Scholar] [CrossRef]
- Saba, M.; Coronado-Hernández, O.E.; Gil, L.K.T. Energy Efficiency in Subtropical Homes: Replacing Asbestos–Cement Roofs with Sustainable Alternatives. Buildings 2024, 14, 4082. [Google Scholar] [CrossRef]
- Gil, L.K.; Martínez, D.V.; Franco, K.B.; Pastrana, A.A.; Saba, M. Mapping roof coverings of asbestos-cement, the first step to control the technical condition/threat and establish priorities for replacement in developing countries. Heliyon 2024, 10, e37522. [Google Scholar] [CrossRef]
- Lee, E.-S.; Kim, Y.-K. Asbestos Exposure Level and the Carcinogenic Risk Due to Corrugated Asbestos-Cement Slate Roofs in Korea. Int. J. Environ. Res. Public Health 2021, 18, 6925. [Google Scholar] [CrossRef]
- Frank, A.L.; van Zandwijk, N. Asbestos history and use. Lung Cancer 2024, 193, 107828. [Google Scholar] [CrossRef]
- Ervik, T.; Eriksen Hammer, S.; Graff, P. Mobilization of asbestos fibers by weathering of a corrugated asbestos cement roof. J. Occup. Environ. Hyg. 2021, 18, 110–117. [Google Scholar] [CrossRef]
- Kottek, M.; Yuen, M.L. Public health risks from asbestos cement roofing. Am. J. Ind. Med. 2022, 65, 157–161. [Google Scholar] [CrossRef]
- Curado, A.; Nunes, L.J.R.; Carvalho, A.; Abrantes, J.; Lima, E.; Tomé, M. The Use of Asbestos and Its Consequences: An Assessment of Environmental Impacts and Public Health Risks. Fibers 2024, 12, 102. [Google Scholar] [CrossRef]
- Frank, A.L.; Joshi, T.K. The Global Spread of Asbestos. Ann. Glob. Health 2014, 80, 257. [Google Scholar] [CrossRef]
- Campopiano, A.; Ramires, D.; Zakrzewska, A.M.; Ferri, R.; D’annibale, A.; Pizzutelli, G. Risk Assessment of the Decay of Asbestos Cement Roofs. Ann. Occup. Hyg. 2009, 53, 627–638. [Google Scholar] [CrossRef]
- Ojo, O.M. Effects of roofing materials on harvested rain water quality. J. Appl. Sci. Environ. Manag. 2019, 23, 735. [Google Scholar] [CrossRef]
- Lai, Y.H.; Ahmad, Y.; Yusoff, I.; Bong, C.W.; Kong, S.Y. Effects of roof pitch gradient and material to harvested rainwater quality. IOP Conf. Ser. Mater. Sci. Eng. 2018, 401, 012011. [Google Scholar] [CrossRef]
- Galgani, F.; Bouchoucha, M.; Brach Papa, C.; Andral, B.; Connes, C.; Gonzalez, J.L.; Tomasino, C.; Paoli, C.; Baldi, Y.; Chiffoleau, J.F. Long term changes of associated metal contamination of marine sediments affected by coastal mining of asbestos. Mar. Pollut. Bull. 2025, 217, 118093. [Google Scholar] [CrossRef]
- Saleem, K.; Asghar, M.A.; Saleem, M.H.; Raza, A.; Kocsy, G.; Iqbal, N.; Ali, B.; Albeshr, M.F.; Bhat, E.A. Chrysotile-Asbestos-Induced Damage in Panicum virgatum and Phleum pretense Species and Its Alleviation by Organic-Soil Amendment. Sustainability 2022, 14, 10824. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Ahmad, I. Effects of Chrysotile Asbestos Contaminated Soil on Crop Plants. Soil Sediment Contam. Int. J. 2011, 20, 767–776. [Google Scholar] [CrossRef]
- Gomes, M.; Ralph, T.J.; Humphries, M.S.; Graves, B.P.; Kobayashi, T.; Gore, D.B. Waterborne contaminants in high intensity agriculture and plant production: A review of on-site and downstream impacts. Sci. Total Environ. 2025, 958, 178084. [Google Scholar] [CrossRef]
- Hamilton, K.; Reyneke, B.; Waso, M.; Clements, T.; Ndlovu, T.; Khan, W.; DiGiovanni, K.; Rakestraw, E.; Montalto, F.; Haas, C.N.; et al. A global review of the microbiological quality and potential health risks associated with roof-harvested rainwater tanks. NPJ Clean Water 2019, 2, 7. [Google Scholar] [CrossRef]
- Vidal, P.; Leiva, A.M.; Gómez, G.; Salgado, M.; Vidal, G. Water Quality of Rainwater Harvesting Systems and Acceptance of Their Reuse in Young Users: An Exploratory Approach. Resources 2024, 13, 159. [Google Scholar] [CrossRef]
- Alim, M.A.; Rahman, A.; Tao, Z.; Samali, B.; Khan, M.M.; Shirin, S. Suitability of roof harvested rainwater for potential potable water production: A scoping review. J. Clean. Prod. 2020, 248, 119226. [Google Scholar] [CrossRef]
- García-Ávila, F.; Guanoquiza-Suárez, M.; Guzmán-Galarza, J.; Cabello-Torres, R.; Valdiviezo-Gonzales, L. Rainwater harvesting and storage systems for domestic supply: An overview of research for water scarcity management in rural areas. Results Eng. 2023, 18, 101153. [Google Scholar] [CrossRef]
- Sperdouli, I. Heavy Metal Toxicity Effects on Plants. Toxics 2022, 10, 715. [Google Scholar] [CrossRef]
- Yang, Z.; Chu, C. Towards Understanding Plant Response to Heavy Metal Stress. In Abiotic Stress in Plants—Mechanisms and Adaptations; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef][Green Version]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methylglyoxal and in Heavy Metal Chelation. J. Bot. 2012, 2012, 1–37. [Google Scholar] [CrossRef]
- Zdeb, M.; Zamorska, J.; Papciak, D.; Słyś, D. The Quality of Rainwater Collected from Roofs and the Possibility of Its Economic Use. Resources 2020, 9, 12. [Google Scholar] [CrossRef]
- Sanjeeva, A.; Puttaswamaiah, S.G. Influence of Atmospheric Deposition and Roof Materials on Harvested Rainwater Quality. J. Environ. Eng. 2018, 144, 04018121. [Google Scholar] [CrossRef]
- Son, K.-H.; Sim, H.-S.; Lee, J.-K.; Lee, J. Precise Sensing of Leaf Temperatures for Smart Farm Applications. Horticulturae 2023, 9, 518. [Google Scholar] [CrossRef]
- Blaya-Ros, P.J.; Blanco, V.; Domingo, R.; Soto-Valles, F.; Torres-Sánchez, R. Feasibility of Low-Cost Thermal Imaging for Monitoring Water Stress in Young and Mature Sweet Cherry Trees. Appl. Sci. 2020, 10, 5461. [Google Scholar] [CrossRef]
- Udompetaikul, V.; Upadhyaya, S.K.; Slaughter, D.C.; Lampinen, B.D. Development of a sensor suite to determine plant water potential. In Advances in Agricultural Engineering, Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting, Pittsburgh, PA, USA, 20–23 June 2010; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2010. [Google Scholar] [CrossRef]
- Udompetaikul, V.; Upadhyaya, S.K.; Slaughter, D.C.; Lampinen, B.D.; Shackel, K.A. Plant Water Stress Detection Using Leaf Temperature and Microclimatic Information. In Advances in Agricultural Engineering, Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting, Louisville, KY, USA, 7–10 August 2011; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2011. [Google Scholar] [CrossRef]
- Ranilović, B.; Cukrov, A.; Boras, I.; Švaić, S.; Zovko, M. Infrared Thermography as a Prediction Tool for the Irrigation Requirement in Agriculture. Trans. FAMENA 2021, 45, 23–34. [Google Scholar] [CrossRef]
- Pineda, M.; Barón, M.; Pérez-Bueno, M.-L. Thermal Imaging for Plant Stress Detection and Phenotyping. Remote Sens 2020, 13, 68. [Google Scholar] [CrossRef]
- Turan, M.; Ekinci, M.; Argin, S.; Brinza, M.; Yildirim, E. Drought stress amelioration in tomato (Solanum lycopersicum L.) seedlings by biostimulant as regenerative agent. Front. Plant Sci. 2023, 14, 1211210. [Google Scholar] [CrossRef]
- Mustafa, M.; Mwangi, R.W.; Szalai, Z.; Kappel, N.; Csambalik, L. Sustainable responses to open field tomato (Solanum lycopersicum L.) stress impacts. J. Agric. Food Res. 2025, 21, 101825. [Google Scholar] [CrossRef]
- Krishna, R.; Ansari, W.A.; Soumia, P.S.; Yadav, A.; Jaiswal, D.K.; Kumar, S.; Singh, A.K.; Singh, M.; Verma, J.P. Biotechnological Interventions in Tomato (Solanum lycopersicum) for Drought Stress Tolerance: Achievements and Future Prospects. BioTech 2022, 11, 48. [Google Scholar] [CrossRef]
- Jiang, Z.; van Zanten, M.; Sasidharan, R. Mechanisms of plant acclimation to multiple abiotic stresses. Commun. Biol. 2025, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Ramegowda, V.; Da Costa, M.V.J.; Harihar, S.; Karaba, N.N.; Sreeman, S.M. Abiotic and biotic stress interactions in plants: A cross-tolerance perspective. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–302. [Google Scholar] [CrossRef]
- Mundim, F.M.; Pringle, E.G. Whole-Plant Metabolic Allocation Under Water Stress. Front. Plant Sci. 2018, 9, 852. [Google Scholar] [CrossRef]
- Paes de Melo, B.; Carpinetti, P.D.; Fraga, O.T.; Rodrigues-Silva, P.L.; Fioresi, V.S.; de Camargos, L.F.; Ferreira, M.F. Abiotic Stresses in Plants and Their Markers: A Practice View of Plant Stress Responses and Programmed Cell Death Mechanisms. Plants 2022, 11, 1100. [Google Scholar] [CrossRef]
- Mertens, S.; Verbraeken, L.; Sprenger, H.; De Meyer, S.; Demuynck, K.; Cannoot, B.; Merchie, J.; De Block, J.; Vogel, J.T.; Bruce, W.; et al. Monitoring of drought stress and transpiration rate using proximal thermal and hyperspectral imaging in an indoor automated plant phenotyping platform. Plant Methods 2023, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, L.; Arteaga, M.; Mirza, H.; Coleman, M.; Mitchell, D.; Huang, X.; Ortiz, H.; Santiago, L.S. Plant physiological indicators for optimizing conservation outcomes. Conserv. Physiol. 2023, 11, coad073. [Google Scholar] [CrossRef] [PubMed]
- Ezemonye, M.N.; Isueken, C.O.; Emeribe, C.N. Physicochemical and bacteriological characteristics of rainwater harvested from rooftops in Esan-West Local Government Area of Edo State, Nigeria. J. Appl. Sci. Environ. Manag. 2016, 20, 748. [Google Scholar] [CrossRef]
- Selala, M.; Thenga, H.; Jewitt, G.; Chaplot, V. Comparison of the chemical quality of rainwater harvested from roof and surface run-off systems. Water SA 2018, 44, 223–231. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Yuan, X.; Yuan, M.; Huang, L.; Wang, S.; Liu, C.E.; Duan, C. Effects of Heavy Metals on Stomata in Plants: A Review. Int. J. Mol. Sci. 2023, 24, 9302. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Matarredona, L.; Zafrilla, B.; Camacho, M.; Bonete, M.; Esclapez, J. Understanding the tolerance of halophilic archaea to stress landscapes. Environ. Microbiol. Rep. 2024, 16, e70039. [Google Scholar] [CrossRef]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.P. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014, 202, 35–49. [Google Scholar] [CrossRef]
- Steward, K.F.; Refai, M.; Dyer, W.E.; Copié, V.; Lachowiec, J.; Bothner, B. Acute stress reduces population-level metabolic and proteomic variation. BMC Bioinform. 2023, 24, 87. [Google Scholar] [CrossRef]
- Baker, B.P.; Van Wie, I.; Braun, E.; Jimenez, A.G. Thermal stability vs. variability: Insights in oxidative stress from a eurytolerant fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 249, 110767. [Google Scholar] [CrossRef]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S. Multilevel Regulation of Abiotic Stress Responses in Plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef]
- Vaverková, M.D. Landfill Impacts on the Environment—Review. Geosciences 2019, 9, 431. [Google Scholar] [CrossRef]
- Ali, A.; Hussain, T.; Zahid, A. Smart Irrigation Technologies and Prospects for Enhancing Water Use Efficiency for Sustainable Agriculture. AgriEngineering 2025, 7, 106. [Google Scholar] [CrossRef]
- Adhikari, U.; Nejadhashemi, A.P.; Woznicki, S.A. Climate change and eastern Africa: A review of impact on major crops. Food Energy Secur. 2015, 4, 110–132. [Google Scholar] [CrossRef]
- Et-taibi, B.; Abid, M.R.; Boufounas, E.-M.; Morchid, A.; Bourhnane, S.; Abu Hamed, T.; Benhaddou, D. Enhancing water management in smart agriculture: A cloud and IoT-Based smart irrigation system. Results Eng. 2024, 22, 102283. [Google Scholar] [CrossRef]
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant. Signal. Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef]
- Wallis, S.L.; Emmett, E.A.; Hardy, R.; Casper, B.B.; Blanchon, D.J.; Testa, J.R.; Menges, C.W.; Gonneau, C.; Jerolmack, D.J.; Seiphoori, A. Challenging Global Waste Management—Bioremediation to Detoxify Asbestos. Front. Environ. Sci. 2020, 8, 20. [Google Scholar] [CrossRef]
- Aust, A.E.; Cook, P.M.; Dodson, R.F. Morphological and Chemical Mechanisms of Elongated Mineral Particle Toxicities. J. Toxicol. Environ. Health Part B 2011, 14, 40–75. [Google Scholar] [CrossRef]
- Barceló, J.; Poschenrieder, C. Plant water relations as affected by heavy metal stress: A review. J. Plant. Nutr. 1990, 13, 1–37. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Gao, Z.-F.; Qiu, C.-W.; Shi, S.-H.; Chen, Z.-H.; Ali, M.A.; Wang, F.; Wu, F. Integrated physiological and omics analyses reveal the mechanism of beneficial fungal Trichoderma sp. alleviating cadmium toxicity in tobacco (Nicotiana tabacum L.). Ecotoxicol. Environ. Saf. 2023, 267, 115631. [Google Scholar] [CrossRef]
- Bridges, J. Human health and environmental risk assessment: The need for a more harmonised and integrated approach. Chemosphere 2003, 52, 1347–1351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macher, G.Z. The Impact of Rainwater Quality Harvested from Asbestos Cement Roofs on Leaf Temperature in Solanum lycopersicum as a Plant Water Stress Indicator. Water 2025, 17, 2070. https://doi.org/10.3390/w17142070
Macher GZ. The Impact of Rainwater Quality Harvested from Asbestos Cement Roofs on Leaf Temperature in Solanum lycopersicum as a Plant Water Stress Indicator. Water. 2025; 17(14):2070. https://doi.org/10.3390/w17142070
Chicago/Turabian StyleMacher, Gergely Zoltán. 2025. "The Impact of Rainwater Quality Harvested from Asbestos Cement Roofs on Leaf Temperature in Solanum lycopersicum as a Plant Water Stress Indicator" Water 17, no. 14: 2070. https://doi.org/10.3390/w17142070
APA StyleMacher, G. Z. (2025). The Impact of Rainwater Quality Harvested from Asbestos Cement Roofs on Leaf Temperature in Solanum lycopersicum as a Plant Water Stress Indicator. Water, 17(14), 2070. https://doi.org/10.3390/w17142070





