Characterization of Water Quality and the Relationship Between WQI and Benthic Macroinvertebrate Communities as Ecological Indicators in the Ghris Watershed, Southeast Morocco
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Setting
2.2. Data Collection and Processing
2.2.1. Surface Water Sampling
2.2.2. Water Quality Index WQI
2.2.3. Systematic Identification of Taxa
2.3. IBMWP (Iberian Biological Monitoring Working Group) Calculation
2.4. Statistical Analyses
3. Results
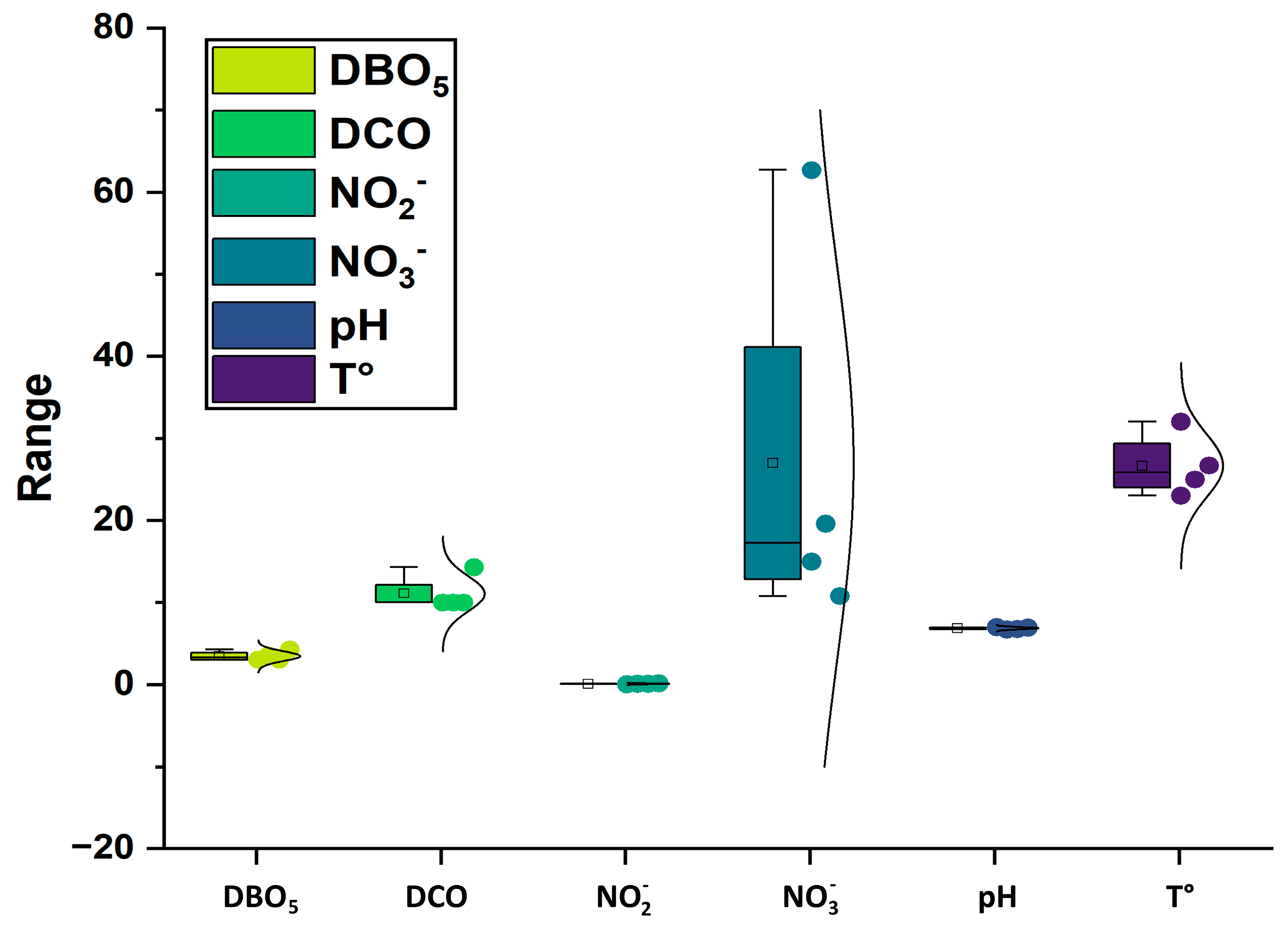
3.1. Physico-Chemical Analysis of Water
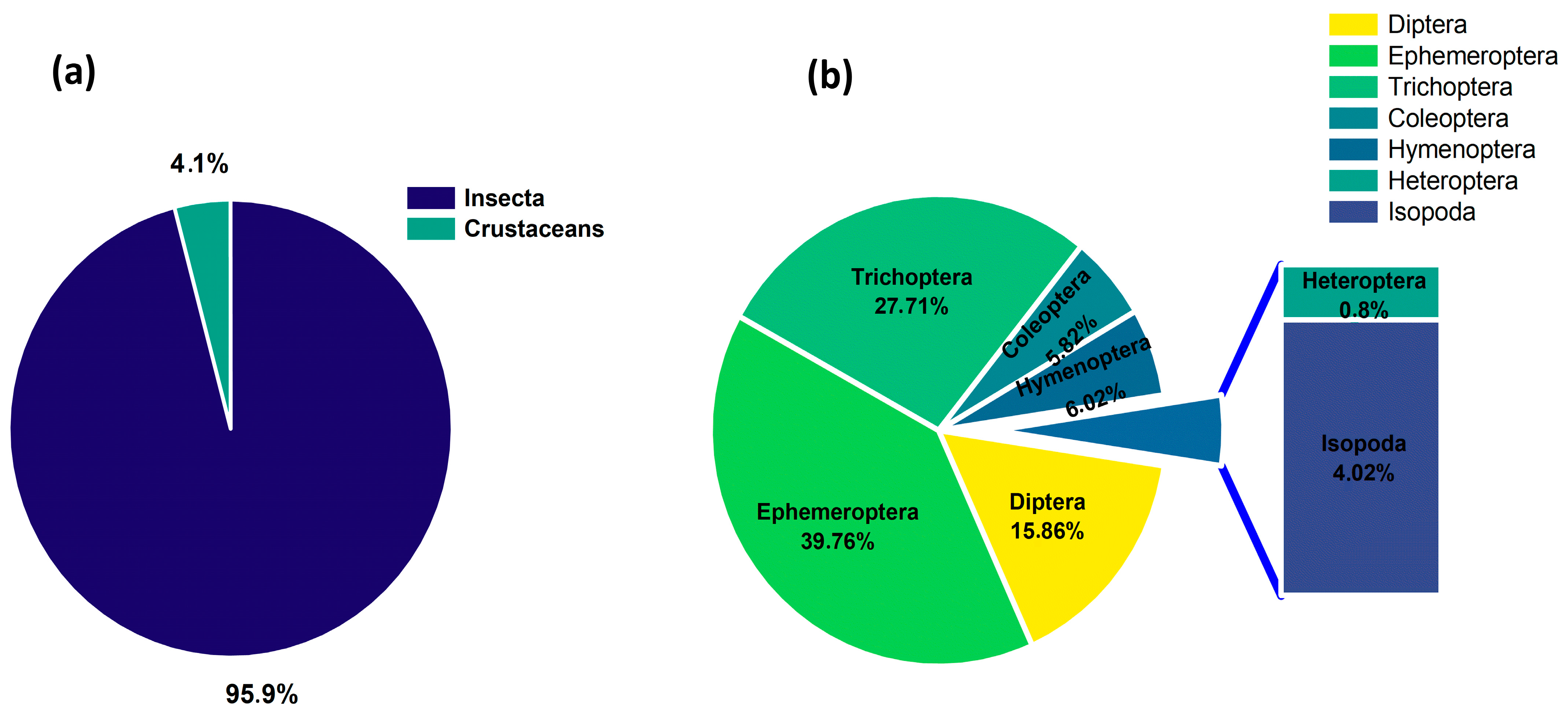
3.2. Benthic Macroinvertebrate Community Structure
3.3. Biological Monitoring of Water Quality and Water Quality Index
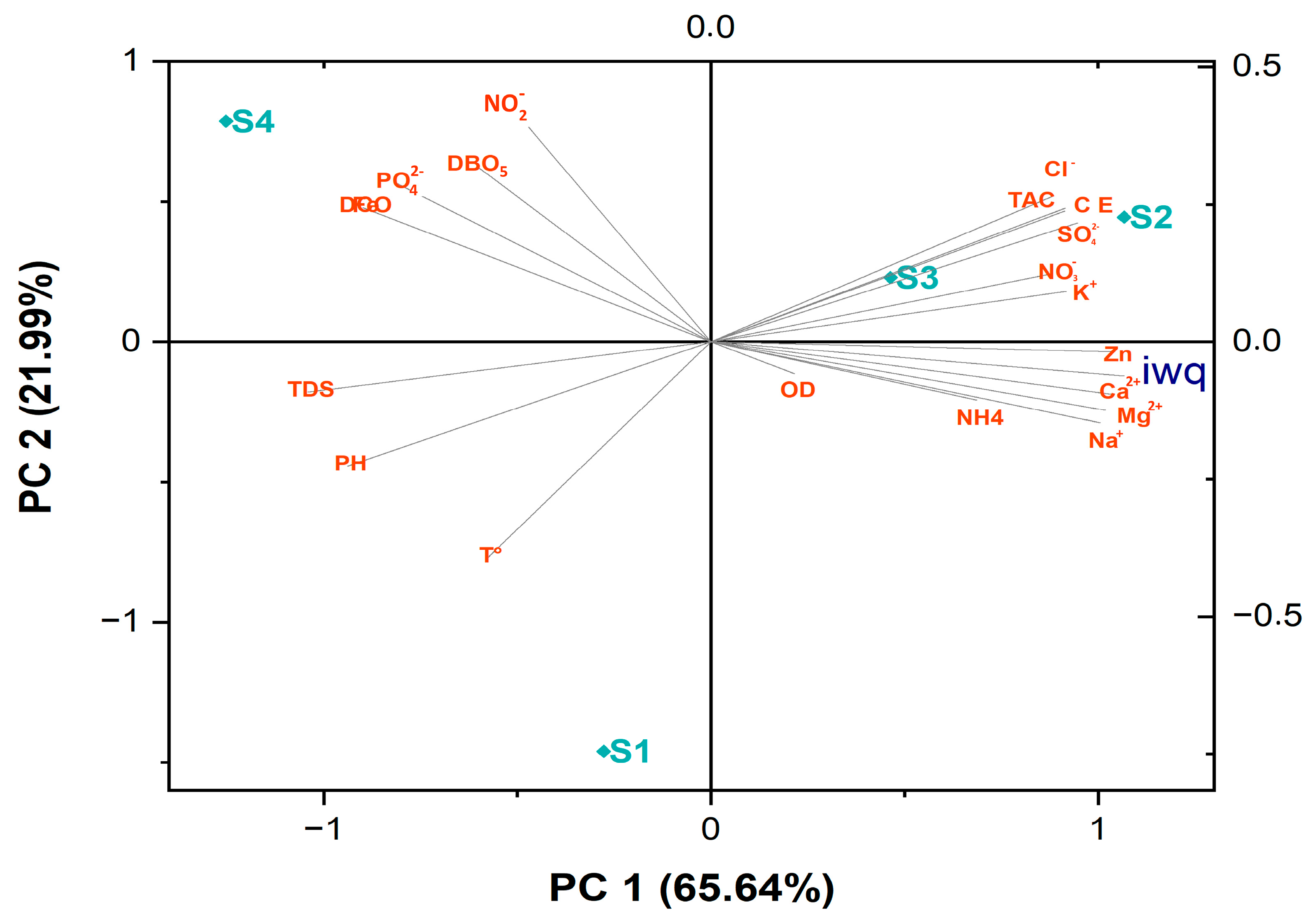
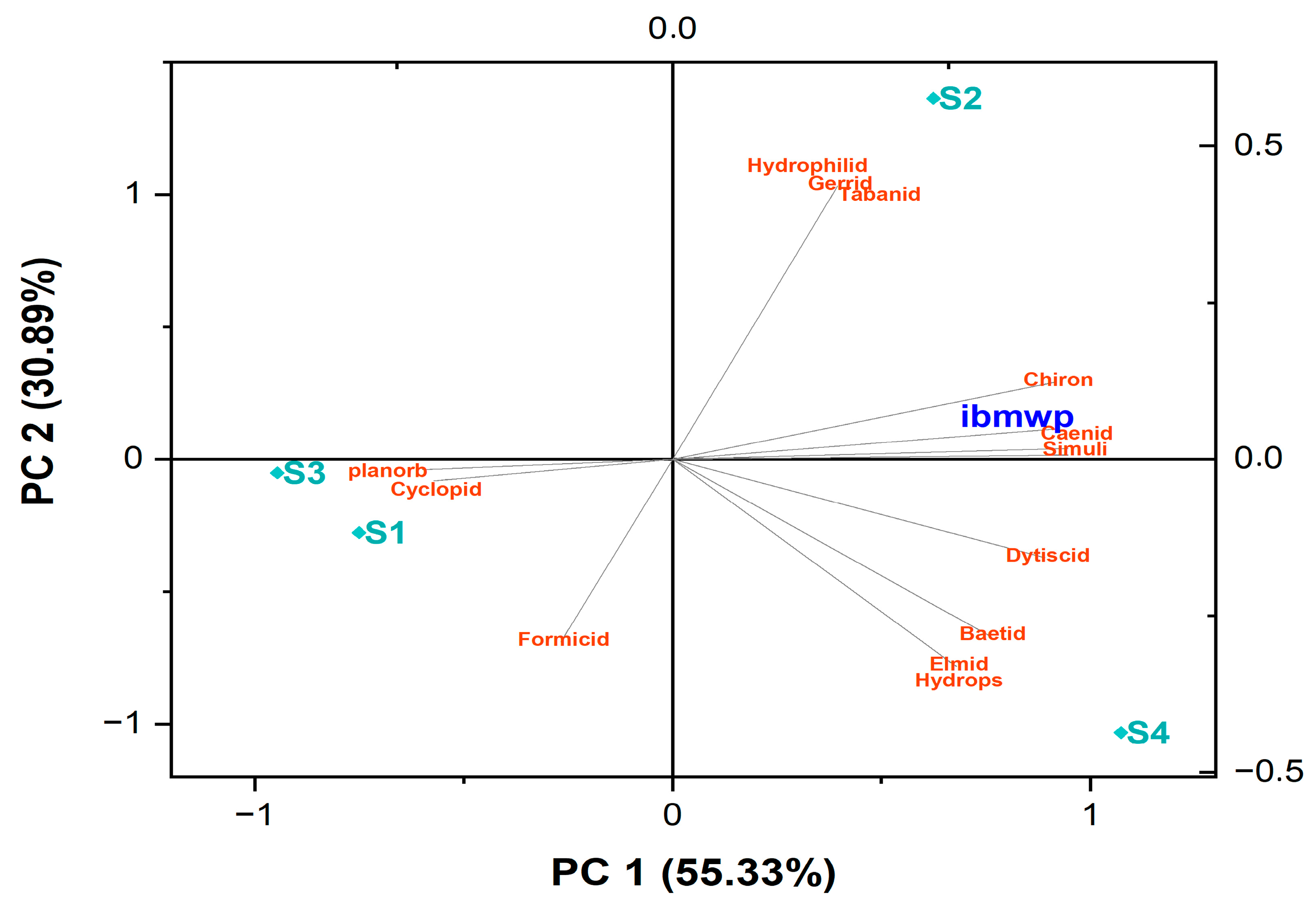
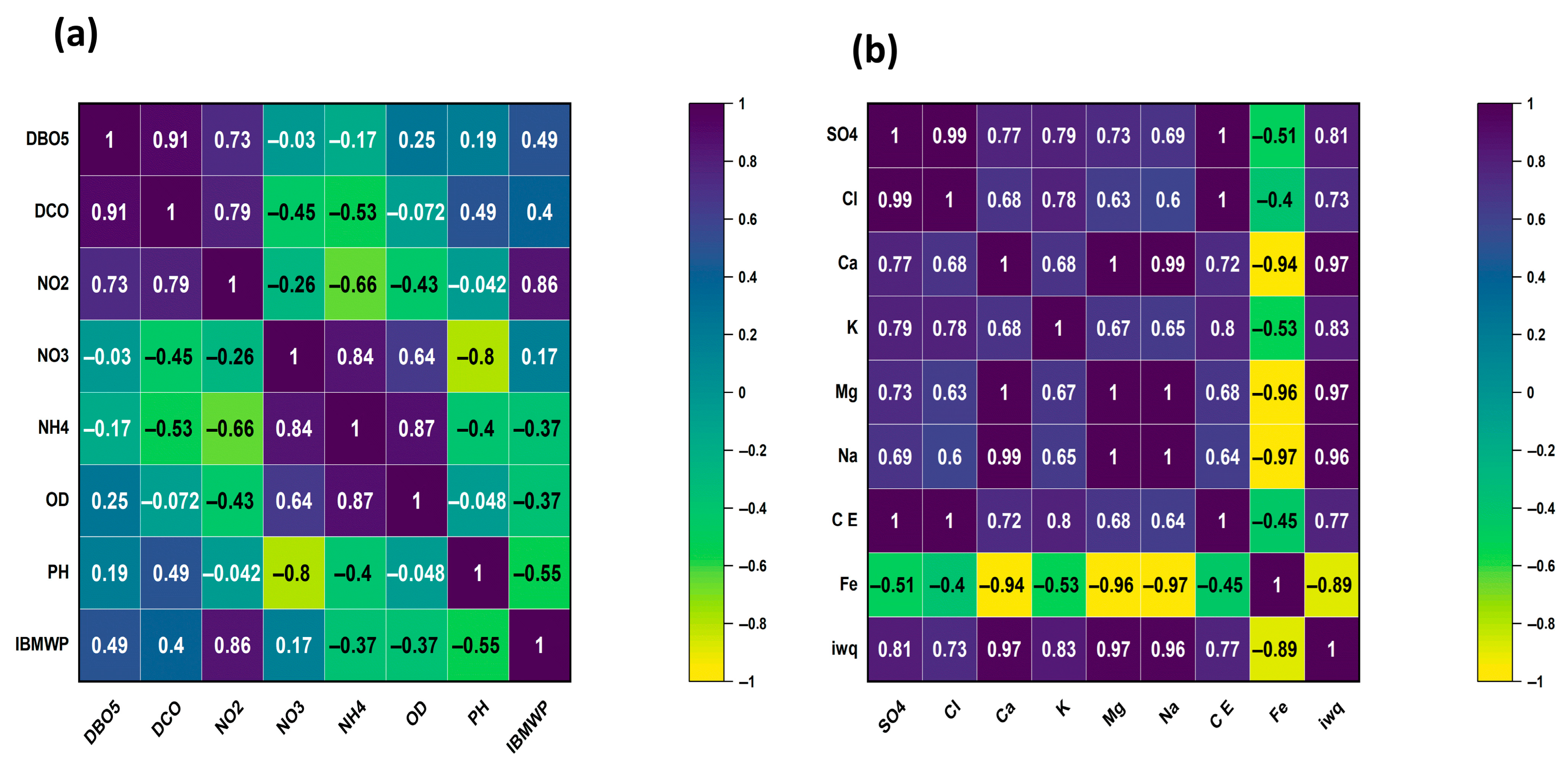
3.4. Statistical Analyses
4. Discussion
4.1. Spatial Variation in Surface Water Quality
4.2. Relationship Between WQI and IBMWP
4.3. Relationship Between Substrate and Benthic Macroinvertebrate Distribution
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gleick, P.H. (Ed.) An Introduction to Global Fresh Water Issues. In Water in Crisis; Oxford University Press: New York, NY, USA, 1993; pp. 3–12. [Google Scholar]
- Botkin, D.B.; Keller, E.A. Environmental Science: Earth as a Living Planet; Wiley: New York, NY, USA, 1995; ISBN 978-0-471-54548-4. [Google Scholar]
- Noutcha, M.; Damiete, O.; Johnny, M.; Ngozi, O.; Ezera, C.; Okiwelu, S. Quantity and Quality of Water Condensate from Air Conditioners and Its Potential Uses at the University of Port Harcourt, Nigeria. Adv. Appl. Sci. Res. 2016, 7, 45–48. [Google Scholar]
- Ganiyu, S.A.; Mabunmi, A.A.; Olurin, O.T.; Adeyemi, A.A.; Jegede, O.A.; Okeh, A. Assessment of Microbial and Heavy Metal Contamination in Shallow Hand-Dug Wells Bordering Ona River, Southwest Nigeria. Environ. Monit. Assess. 2021, 193, 126. [Google Scholar] [CrossRef] [PubMed]
- Kawo, N.S.; Karuppannan, S. Groundwater Quality Assessment Using Water Quality Index and GIS Technique in Modjo River Basin, Central Ethiopia. J. Afr. Earth Sci. 2018, 147, 300–311. [Google Scholar] [CrossRef]
- Rivett, M.O.; Miller, A.V.M.; MacAllister, D.J.; Fallas, A.; Wanangwa, G.J.; Mleta, P.; Phiri, P.; Mannix, N.; Monjerezi, M.; Kalin, R.M. A Conceptual Model Based Framework for Pragmatic Groundwater-Quality Monitoring Network Design in the Developing World: Application to the Chikwawa District, Malawi. Groundw. Sustain. Dev. 2018, 6, 213–226. [Google Scholar] [CrossRef]
- Ouhamdouch, S.; Bahir, M.; Souhel, A.; Paula, C. Vulnerability and Impact of Climate Change Processes on Water Resource in Semi-Arid Areas: In Essaouira Basin (Morocco). In Energy, Transportation and Global Warming; Grammelis, P., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 719–736. ISBN 978-3-319-30127-3. [Google Scholar]
- Dahan, S.; Johan, G.; Anouar, K.; Bekhechi, M.; Jalil, M.; Abdulnour, R. Gestion de La Rarete de l’Eau En Milieu Urbain Au Maroc; Banque mondiale: Washington, DC, USA, 2017. [Google Scholar]
- Julien, F. Maîtrise de l’eau et développement durable en Afrique de l’ouest: De la nécessité d’une coopération régionale autour des systèmes hydrologiques transfrontaliers. VertigO Rev. Electronique Sci. L’environnement 2006, 7–2. [Google Scholar] [CrossRef]
- Brierley, G.J. The Socio-Ecological River: Socio-Economic, Cultural and Environmental Relations to River Systems. In Finding the Voice of the River: Beyond Restoration and Management; Brierley, G.J., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 29–60. ISBN 978-3-030-27068-1. [Google Scholar]
- Fretwell, J.D. National Water Summary on Wetland Resources; U.S. Government Printing Office: Washington, DC, USA, 1996; ISBN 978-0-607-85696-5. [Google Scholar]
- Lougheed, V.L.; Crosbie, B.; Chow-Fraser, P. Predictions on the Effect of Common Carp (Cyprinus carpio) Exclusion on Water Quality, Zooplankton, and Submergent Macrophytes in a Great Lakes Wetland. Can. J. Fish. Aquat. Sci. 1998, 55, 1189–1197. [Google Scholar] [CrossRef]
- Riens, J.R.; Schwarz, M.S.; Mustafa, F.; Hoback, W.W. Aquatic Macroinvertebrate Communities and Water Quality at Buffered and Non-Buffered Wetland Sites on Federal Waterfowl Production Areas in the Rainwater Basin, Nebraska. Wetlands 2013, 33, 1025–1036. [Google Scholar] [CrossRef]
- Dunson, W.A.; Travis, J. The Role of Abiotic Factors in Community Organization. Am. Nat. 1991, 138, 1067–1091. [Google Scholar] [CrossRef]
- Kingsford, R.T.; Biggs, H.C.; Pollard, S.R. Strategic Adaptive Management in Freshwater Protected Areas and Their Rivers. Biol. Conserv. 2011, 144, 1194–1203. [Google Scholar] [CrossRef]
- Tahraoui, H.; Belhadj, A.E.; Moula, N.; Bouranene, S.; Amrane, A. Optimisation and Prediction of the Coagulant Dose for the Elimination of Organic Micropollutants Based on Turbidity. Kem. Ind. 2021, 70, 675–691. [Google Scholar] [CrossRef]
- Tahraoui, H.; Belhadj, A.-E.; Triki, Z.; Boudellal, N.R.; Seder, S.; Amrane, A.; Zhang, J.; Moula, N.; Tifoura, A.; Ferhat, R.; et al. Mixed Coagulant-Flocculant Optimization for Pharmaceutical Effluent Pretreatment Using Response Surface Methodology and Gaussian Process Regression. Process Saf. Environ. Prot. 2023, 169, 909–927. [Google Scholar] [CrossRef]
- Smith, V.H. Effects of Eutrophication on Maximum Algal Biomass in Lake and River Ecosystems. Inland. Waters 2016, 6, 147–154. [Google Scholar] [CrossRef]
- Adimalla, N.; Li, P.; Venkatayogi, S. Hydrogeochemical Evaluation of Groundwater Quality for Drinking and Irrigation Purposes and Integrated Interpretation with Water Quality Index Studies. Environ. Process. 2018, 5, 363–383. [Google Scholar] [CrossRef]
- Maghrebi, M.; Noori, R.; Bhattarai, R.; Mundher Yaseen, Z.; Tang, Q.; Al-Ansari, N.; Danandeh Mehr, A.; Karbassi, A.; Omidvar, J.; Farnoush, H.; et al. Iran’s Agriculture in the Anthropocene. Earth’s Future 2020, 8, e2020EF001547. [Google Scholar] [CrossRef]
- Bouriqi, A.; Ouazzani, N.; Benaissa, H.; Benaddi, R.; Deliège, J.-F. Typology and Classification of Water Quality in an Intermittent River in a Semi-Arid Mediterranean Climate. Environ. Monit. Assess. 2024, 196, 381. [Google Scholar] [CrossRef]
- Ramírez Ulate, L.A. Evaluación de La Calidad Ecológica de Los Ríos En Puerto Rico: Principales Amenazas y Herramientas de Evaluación; Evaluación de La Calidad Ecológica de Los Ríos En Puerto Rico: Principales Amenazas y Herramientas de Evaluación; Ecological Evaluation of Streams in Puerto Rico: Major Threats and Evaluation Tools. Hidrobiologica 2016, 26, 433–441. [Google Scholar]
- Gutiérrez-Fonseca, P.E.; Lorion, C.M. Application of the BMWP-Costa Rica biotic index in aquatic biomonitoring: Sensitivity to collection method and sampling intensity. Rev. Biol. Trop. 2014, 62, 275–289. [Google Scholar] [CrossRef]
- Ruiz-Picos, R.A.; Sedeño-Díaz, J.E.; López-López, E.; Ruiz-Picos, R.A.; Sedeño-Díaz, J.E.; López-López, E. Calibrating and Validating the Biomonitoring Working Party (BMWP) Index for the Bioassessment of Water Quality in Neotropical Streams. In Water Quality; IntechOpen: London, UK, 2017; ISBN 978-953-51-2882-3. [Google Scholar]
- Amina, W.; Ensaf, E.-C.; Salhddine, D.; Zakaria, O. Hydrogeological and Hydrochemical Study of Mellaha Village, Oued Guir, Er-Rachidia, Morcco. J. Water Sci. Environ. Technol. 2019, 4, 411–427. [Google Scholar]
- Rachid, L. Evaluation of the groundwater quality in the Ghallil quaternary basin (Todgha downstream, South—Eastern Morocco). Environ. Water Sci. Public Health Territ. Intell. J. 2020, 4, 289–299. [Google Scholar] [CrossRef]
- El Hafyani, M.; Essahlaoui, A.; El Baghdadi, M.; Teodoro, A.C.; Mohajane, M.; El Hmaidi, A.; El Ouali, A. Modeling and Mapping of Soil Salinity in Tafilalet Plain (Morocco). Arab. J. Geosci. 2019, 12, 35. [Google Scholar] [CrossRef]
- Mariouh, A.; Bouaouinate, A. La conception d’une vision de développement territorial pour le Tafilalet: L’apport de la réflexion prospective. Espace Géographique Société Marocaine 2022, 1, 169–189. [Google Scholar] [CrossRef]
- Mainguet, M.; Dumay, F.; Kabiri, L.; Rémini, B. Prodromes of Desertification in the Oasis of Tafilalet (Morocco) and Specific Local Solutions. In Coping with Global Environmental Change, Disasters and Security: Threats, Challenges, Vulnerabilities and Risks; Brauch, H.G., Oswald Spring, Ú., Mesjasz, C., Grin, J., Kameri-Mbote, P., Chourou, B., Dunay, P., Birkmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 901–919. ISBN 978-3-642-17776-7. [Google Scholar]
- Baki, S.; Hilali, M.; Kacimi, I.; Mahboub, A. Caractérisation Hydrogéologique et Cartographie Des Ressources En Eau Dans Le Bassin Versant de l’oued Rhéris (Sud-Est Du Maroc). Bull. l’Institut Sci. Rabat 2016, 38, 29–43. [Google Scholar]
- Ait Lahssaine, I.; Kabiri, L.; Messaoudi, B.; Saadi, Y.; Essahlaoui, A.; Essafraoui, B.; Ouali, L.; Kadiri, A. Integrated Assessment of Groundwater Pollution Vulnerability in Goulmima: A Comparative Analysis of DRASTIC and GOD Methods. Euro-Mediterr. J. Environ. Integr. 2024, 9, 2109–2122. [Google Scholar] [CrossRef]
- Alaoui, K.S.; Daoud, M.; Bouaouinate, A. Les Ksour Du Tafilalet Au Cøeur Du Développement Du Pays Présaharien. Espace Géographique Société Marocaine 2022, 1, 151–164. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012; ISBN 978-0-87553-013-0. [Google Scholar]
- Jehan, S.; Ullah, I.; Khan, S.; Muhammad, S.; Khattak, S.A.; Khan, T. Evaluation of the Swat River, Northern Pakistan, Water Quality Using Multivariate Statistical Techniques and Water Quality Index (WQI) Model. Environ. Sci. Pollut. Res. 2020, 27, 38545–38558. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Fact Sheet. 15 November 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 22 December 2024).
- Horton, R.K. An Index Number System for Rating Water Quality. J. Water Pollut. Control Fed. 1965, 37, 300–306. [Google Scholar]
- Azzirgue, E.M.; Cherif, E.K.; Tchakoucht, T.A.; Azhari, H.E.; Salmoun, F. Testing Groundwater Quality in Jouamaa Hakama Region (North of Morocco) Using Water Quality Indices (WQIs) and Fuzzy Logic Method: An Exploratory Study. Water 2022, 14, 3028. [Google Scholar] [CrossRef]
- Rupias, O.J.B.; Pereira, S.Y.; de Abreu, A.E.S. Hydrogeochemistry and Groundwater Quality Assessment Using the Water Quality Index and Heavy-Metal Pollution Index in the Alluvial Plain of Atibaia River- Campinas/SP, Brazil. Groundw. Sustain. Dev. 2021, 15, 100661. [Google Scholar] [CrossRef]
- Masoud, M.; El Osta, M.; Alqarawy, A.; Elsayed, S.; Gad, M. Evaluation of Groundwater Quality for Agricultural under Different Conditions Using Water Quality Indices, Partial Least Squares Regression Models, and GIS Approaches. Appl. Water Sci. 2022, 12, 244. [Google Scholar] [CrossRef]
- Kulisz, M.; Kujawska, J.; Przysucha, B.; Cel, W. Forecasting Water Quality Index in Groundwater Using Artificial Neural Network. Energies 2021, 14, 5875. [Google Scholar] [CrossRef]
- Kachroud, M.; Trolard, F.; Kefi, M.; Jebari, S.; Bourrié, G. Water Quality Indices: Challenges and Application Limits in the Literature. Water 2019, 11, 361. [Google Scholar] [CrossRef]
- Hecq, J.H. Cycle Annuel Du Zooplancton A Boulogne-Sur-Mer (Pas-de-Calais) 1970-1971. II. L’holoplancton. Bull. Société R. Sci. Liège 1976, 9, 443–457. [Google Scholar]
- Marghade, D.; Malpe, D.B.; Subba Rao, N. Applications of Geochemical and Multivariate Statistical Approaches for the Evaluation of Groundwater Quality and Human Health Risks in a Semi-Arid Region of Eastern Maharashtra, India. Environ. Geochem. Health 2021, 43, 683–703. [Google Scholar] [CrossRef]
- Sengupta, P. Potential Health Impacts of Hard Water. Int. J. Prev. Med. 2013, 4, 866–875. [Google Scholar] [PubMed]
- Listyani, T.; Prabowo, I.A. Groundwater in Bener Area, Its Quality and Contribution for Agriculture. IOP Conf. Ser. Earth Environ. Sci. 2022, 985, 012028. [Google Scholar]
- Dimri, D.; Daverey, A.; Kumar, A.; Sharma, A. Monitoring Water Quality of River Ganga Using Multivariate Techniques and WQI (Water Quality Index) in Western Himalayan Region of Uttarakhand, India. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100375. [Google Scholar] [CrossRef]
- Abessolo, J.-R.Z.; Khebiza, M.Y.; Messouli, M. Réponse des macroinvertébrés benthiques (éphéméroptères, plécoptères, trichoptères) aux pressions anthropiques dans un contexte de changement climatique sur le bassin versant de l’Ourika (Haut-Atlas du Maroc). Hydroécol. Appl. 2021, 21, 115–155. [Google Scholar] [CrossRef]
- Cid, N.; Bonada, N.; Carlson, S.M.; Grantham, T.E.; Gasith, A.; Resh, V.H. High Variability Is a Defining Component of Mediterranean-Climate Rivers and Their Biota. Water 2017, 9, 52. [Google Scholar] [CrossRef]
- Guellaf, A.; Kettani, K. Assessing the Ecological Status Using Physico-Chemical, Bacteriological Parameters and Biotic Indices of the Oued Martil River Basin in Northwestern Morocco. Biologia 2021, 76, 585–598. [Google Scholar] [CrossRef]
- Saal, I.; Bouchelouche, D.; Hamache, C.; Arab, A. Evaluation of the Surface Water Quality in the Kebir-Rhumel Catchment Area (Northeast Algeria) Using Biotic Indices and Physico-Chemical Analyses. Environ. Sci. Pollut. Res. 2021, 28, 46565–46579. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Mahadevan, H.; Arun, V.; Krishnakumar, A.P.; Krishnan, K.A. A Multivariate Statistical Approach in Assessing the Quality of Potable and Irrigation Water Environs of the Netravati River Basin (India). Groundw. Sustain. Dev. 2020, 11, 100462. [Google Scholar] [CrossRef]
- Hou, W.; Sun, S.; Wang, M.; Li, X.; Zhang, N.; Xin, X.; Sun, L.; Li, W.; Jia, R. Assessing Water Quality of Five Typical Reservoirs in Lower Reaches of Yellow River, China: Using a Water Quality Index Method. Ecol. Indic. 2016, 61, 309–316. [Google Scholar] [CrossRef]
- Young, R.G.; Quarterman, A.J.; Eyles, R.F.; Smith, R.A.; Bowden, W.B. Water Quality and Thermal Regime of the Motueka River: Influences of Land Cover, Geology and Position in the Catchment. N. Z. J. Mar. Freshw. Res. 2005, 39, 803–825. [Google Scholar] [CrossRef]
- Prathumratana, L.; Sthiannopkao, S.; Kim, K.W. The Relationship of Climatic and Hydrological Parameters to Surface Water Quality in the Lower Mekong River. Environ. Int. 2008, 34, 860–866. [Google Scholar] [CrossRef]
- Bellos, D.; Sawidis, T. Chemical Pollution Monitoring of the River Pinios (Thessalia—Greece). J. Environ. Manag. 2005, 76, 282–292. [Google Scholar] [CrossRef]
- Rajwa-Kuligiewicz, A.; Bialik, R.J.; Rowiński, P.M. Dissolved Oxygen and Water Temperature Dynamics in Lowland Rivers over Various Timescales. J. Hydrol. Hydromech. 2015, 63, 353–363. [Google Scholar] [CrossRef]
- Sidabutar, N.V.; Namara, I.; Hartono, D.M.; Soesilo, T.E.B. The Effect of Anthropogenic Activities to the Decrease of Water Quality. IOP Conf. Ser. Earth Environ. Sci. 2017, 67, 012034. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Reichl, F.X. Reactive Water-Soluble Forms of Nitrogen and Phosphorus and Their Impacts on Environment and Human Health. In Soil Components and Human Health; Nieder, R., Benbi, D.K., Reichl, F.X., Eds.; Springe: Dordrecht, The Netherlands, 2018; pp. 223–255. ISBN 978-94-024-1222-2. [Google Scholar]
- Omar, A.A.-K.; Hani, M.A.; Ahmad, M.A.J.; Ala’a, H.A.-M. Monitoring and Assessing of Spring Water Quality in Southwestern Basin of Jordan. Open J. Mod. Hydrol. 2017, 7, 331–349. [Google Scholar]
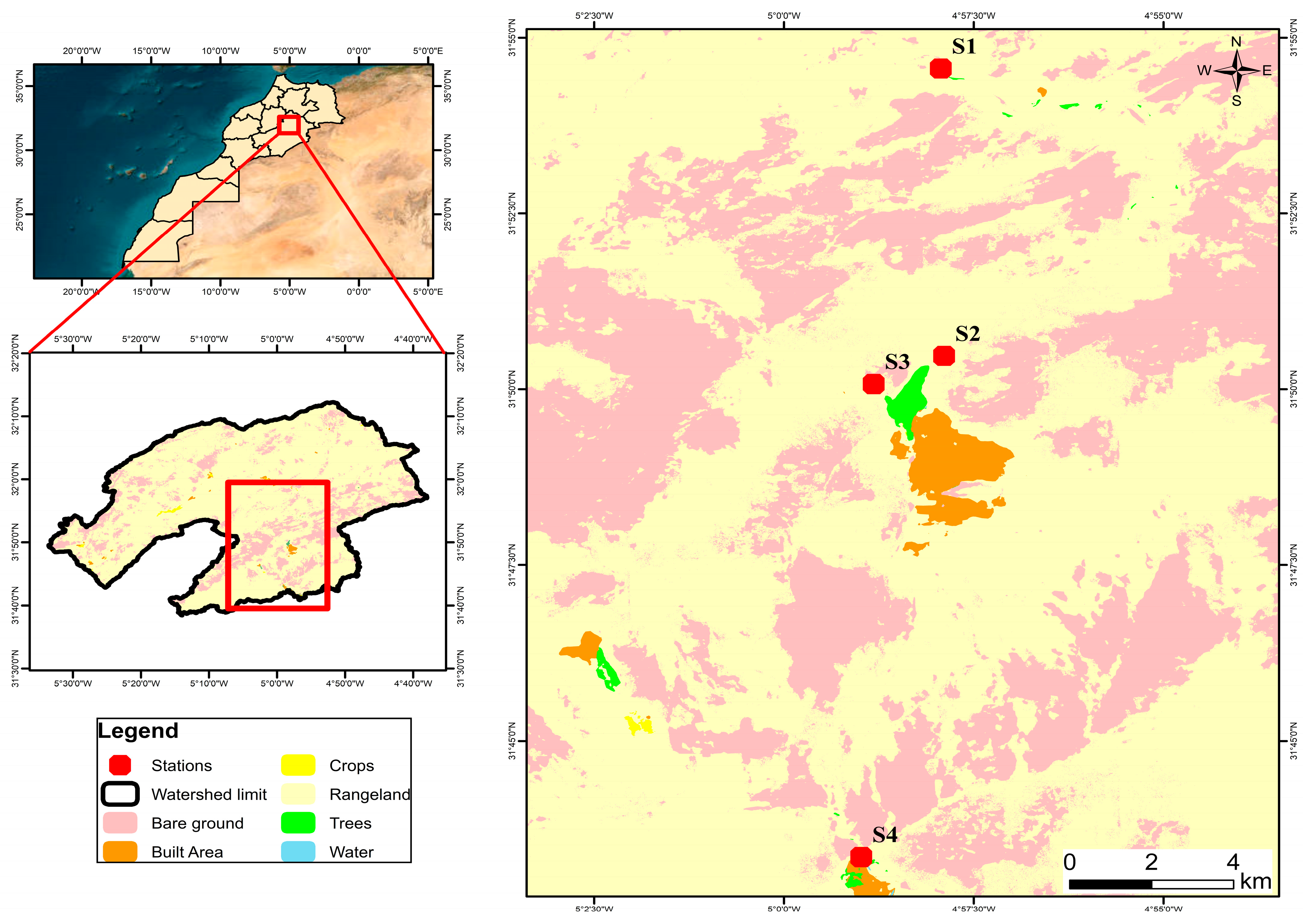






| Site Code | X (m) | Y (m) | Location and Type of Station | Measured Temperature (°C) | Köppen Classification | Altitude (m) |
|---|---|---|---|---|---|---|
| S1 | 31.543368 | −4.575596 | Tihereint Nigrane, Source | 30 | BWh | 1140 |
| S2 | 31.502852 | −4.575302 | Ogoug, Source | 25 | BWh | 988 |
| S3 | 31.50452 | −4.584902 | Mouy, Source | 27 | BWh | 1422 |
| S4 | 31.432090 | −4.585830 | Tifounasin, a stream | 26.7 | BWh | 1085 |
| Water Quality Parameters | Unit | WHO 2017 | Weight (Wi) | Wr |
|---|---|---|---|---|
| EC | µS/cm | 1000 | 4 | 0.072 |
| pH | - | 6.5–8.5 | 4 | 0.072 |
| TH | mg/L | 500 | 3 | 0.053 |
| Cl− | mg/L | 250 | 3 | 0.043 |
| PO4 | mg/L | 3–5 | 3 | 0.043 |
| NO2− | mg/L | 3 | 3 | 0.053 |
| NO3− | mg/L | 50 | 5 | 0.089 |
| NH4+ | mg/L | 35 | 3 | 0.053 |
| Ca2+ | mg/L | 75 | 2 | 0.035 |
| Mg2+ | mg/L | 50 | 2 | 0.035 |
| DO | mg/L | 5 | 5 | 0.089 |
| SO42− | mg/L | 250 | 4 | 0.072 |
| TDS | mg/L | 1000 | 5 | 0.089 |
| Fe2+ | mg/L | 0.3 | 3 | 0.100 |
| Na+ | mg/L | 200 | 2 | 0.067 |
| Total | 51 | 1 |
| Ranking | WQI Value | Explanation |
|---|---|---|
| <50 | Excellent water | Good for human health |
| 50–100 | Good water | Suitable for human consumption |
| 100–200 | Poor water | Water in poor condition |
| 200–300 | Very poor water | Needs special attention before use |
| >300 | Unsuitable for drinking | Requires too much attention |
| Variable | Unit | Minimum | Maximum | Mean | Standard Deviation | WHO 2017 |
|---|---|---|---|---|---|---|
| T | °C | 25 | 30 | 27.17 | 1.02 | 25–35 |
| pH | - | 6.8 | 7.08 | 6.86 | 0.11 | 6.5–8.5 |
| EC | µS/cm | 2288 | 3721 | 2677.7 | 628.45 | 1000 |
| DO | mg/L | 7.07 | 7.29 | 7.19 | 0.12 | 2 |
| TDS | ppm | 42.1 | 1030 | 618.75 | 121.95 | 1000 |
| SO42− | mg/L | 162.5 | 385.2 | 295.80 | 57.66 | 250 |
| DBO5 | mg/L | 3 | 4.29 | 3.46 | 0.59 | 3 |
| COD | mg/L | 10 | 14.3 | 11.83 | 2.24 | - |
| NO2− | mg/L | 0.02 | 0.13 | 0.08 | 0.06 | 3 |
| NO3− | mg/L | 10.76 | 62.69 | 27.75 | 26.16 | 50 |
| NH4+ | mg/L | 0.02 | 0.19 | 0.09 | 0.08 | 35 |
| Cl− | mg/L | 412.4 | 728.3 | 645.85 | 102.94 | 50 |
| TAC | mg/L | 28.53 | 47.28 | 38.41 | 7.60 | 200 |
| PO42− | mg/L | 0.02 | 0.09 | 0.05 | 0.02 | 3–5 |
| Ca2+ | mg/L | 17.08 | 252.81 | 171.64 | 106.77 | 75 |
| K+ | mg/L | 0.54 | 42.54 | 15.32 | 16.38 | 12 |
| Mg2+ | mg/L | 8.71 | 86.33 | 80.30 | 42.53 | 50 |
| Na+ | mg/L | 28 | 209.46 | 166.75 | 100.32 | 200 |
| Fe | mg/L | 0.01 | 0.02 | 0.01 | 0.01 | 0.3 |
| Zn | mg/L | 0.05 | 0.07 | 0.06 | 0.01 | 3 |
| Family | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| Simuliidae | 6 | 24 | ||
| Chironomidae | 12 | 10 | ||
| Tabanidae | 2 | |||
| Caenidae | 2 | 24 | ||
| Baetidae | 2 | 15 | 17 | 90 |
| Hydropsychidae | 3 | 129 | ||
| Gerridae | 66 | 2 | ||
| Elmidae | 1 | |||
| Dytiscidae | 10 | 6 | ||
| Planorbidae | 4 | 2 | ||
| Cyclopidae | 2 |
| Class | Order | Family | Tolerance Value IBMWP |
|---|---|---|---|
| Insecta | Diptères | Simuliidae | 6 |
| Chironomidae | 4 | ||
| Tabanidae | 5 | ||
| Ephemeroptera | Caenidae | 4 | |
| Baetidae | 3 | ||
| Trichoptera | Hydropsychidae | 6 | |
| Heteroptera | Gerridae | 5 | |
| Coleoptera | Elmidae | 5 | |
| Dytiscidae | 5 | ||
| Hymenoptera | Formicidae | 3 | |
| Gastropoda | Pulmonata | Planorbidae | 4 |
| Crustaceans | Isopode | Cyclopidae | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Mansour, A.; Ait Boughrous, S.; Mansouri, I.; Abdaoui, A.; Squalli, W.; Nouayti, A.; Abdellaoui, M.; Beyouda, E.M.; Piscart, C.; Ait Boughrous, A. Characterization of Water Quality and the Relationship Between WQI and Benthic Macroinvertebrate Communities as Ecological Indicators in the Ghris Watershed, Southeast Morocco. Water 2025, 17, 2055. https://doi.org/10.3390/w17142055
El Mansour A, Ait Boughrous S, Mansouri I, Abdaoui A, Squalli W, Nouayti A, Abdellaoui M, Beyouda EM, Piscart C, Ait Boughrous A. Characterization of Water Quality and the Relationship Between WQI and Benthic Macroinvertebrate Communities as Ecological Indicators in the Ghris Watershed, Southeast Morocco. Water. 2025; 17(14):2055. https://doi.org/10.3390/w17142055
Chicago/Turabian StyleEl Mansour, Ali, Saida Ait Boughrous, Ismail Mansouri, Abdellali Abdaoui, Wafae Squalli, Asmae Nouayti, Mohamed Abdellaoui, El Mahdi Beyouda, Christophe Piscart, and Ali Ait Boughrous. 2025. "Characterization of Water Quality and the Relationship Between WQI and Benthic Macroinvertebrate Communities as Ecological Indicators in the Ghris Watershed, Southeast Morocco" Water 17, no. 14: 2055. https://doi.org/10.3390/w17142055
APA StyleEl Mansour, A., Ait Boughrous, S., Mansouri, I., Abdaoui, A., Squalli, W., Nouayti, A., Abdellaoui, M., Beyouda, E. M., Piscart, C., & Ait Boughrous, A. (2025). Characterization of Water Quality and the Relationship Between WQI and Benthic Macroinvertebrate Communities as Ecological Indicators in the Ghris Watershed, Southeast Morocco. Water, 17(14), 2055. https://doi.org/10.3390/w17142055








