Research Progress on the Influence Factors of the Quorum Sensing System Regulating the Growth of Wastewater Treatment Biofilm
Abstract
1. Introduction
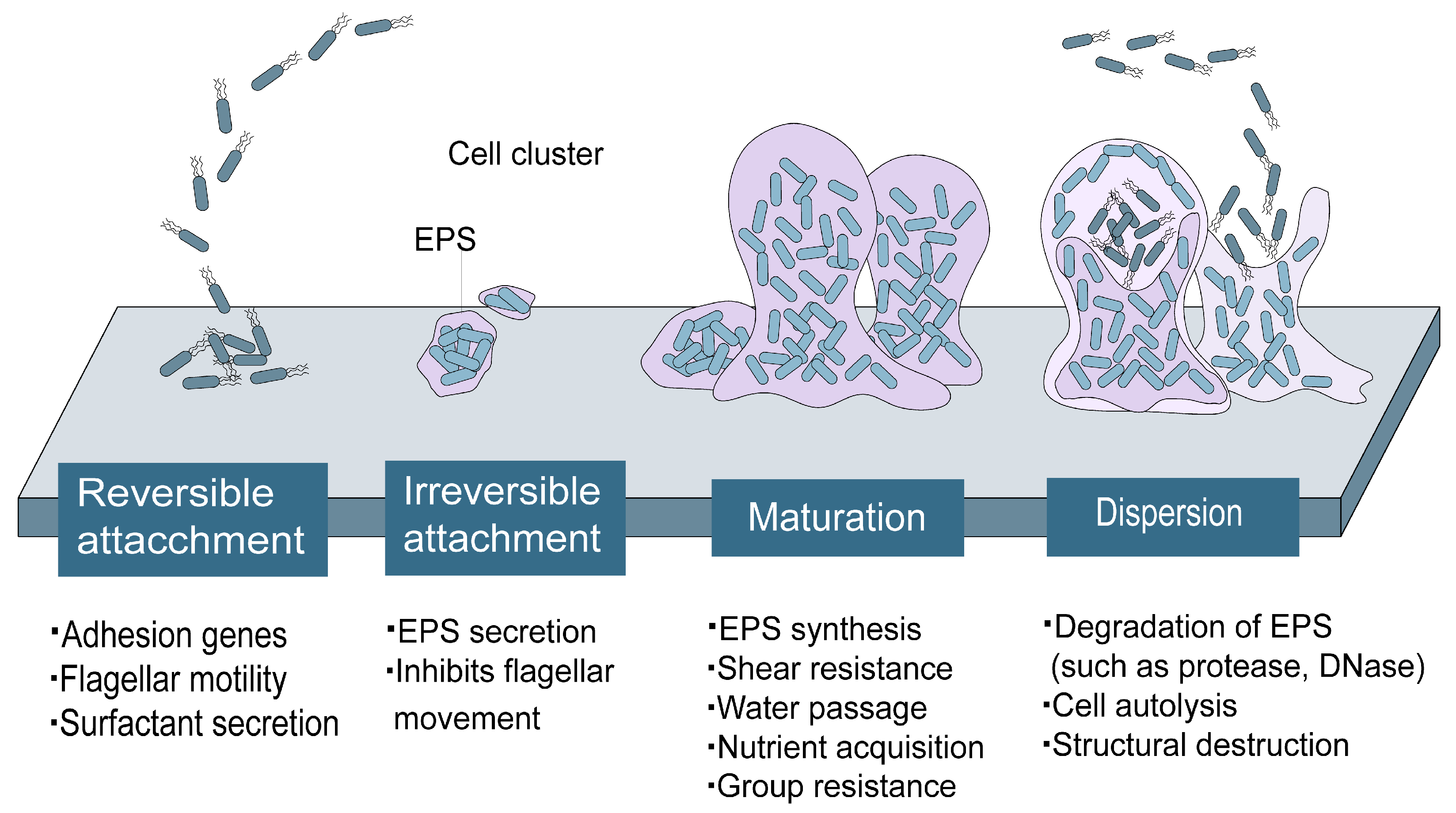
2. The Effect of the QS System on Biofilm Formation and Regulation
2.1. Effect of QS on the Initial Attachment of Biofilm
2.2. The Effect of the QS System on the Irreversible Adhesion Stage
2.3. The Effect of QS System on the Mature Stage of Biofilm
2.4. The Effect of QS System on the Shedding Stage
3. The Influence of Conventional Factors on the Regulation of Biofilm by the QS System
3.1. Substrate
3.2. Operation Parameters
3.3. Temperature
3.4. pH
3.5. Other Conventional Parameters
4. The Influence of Emerging Factors on the Regulation of Biofilm by the QS System
4.1. Metal Nanoparticles
4.2. Antibiotics and Antibiotic Resistance Genes
4.3. Microplastics and Nanoplastics
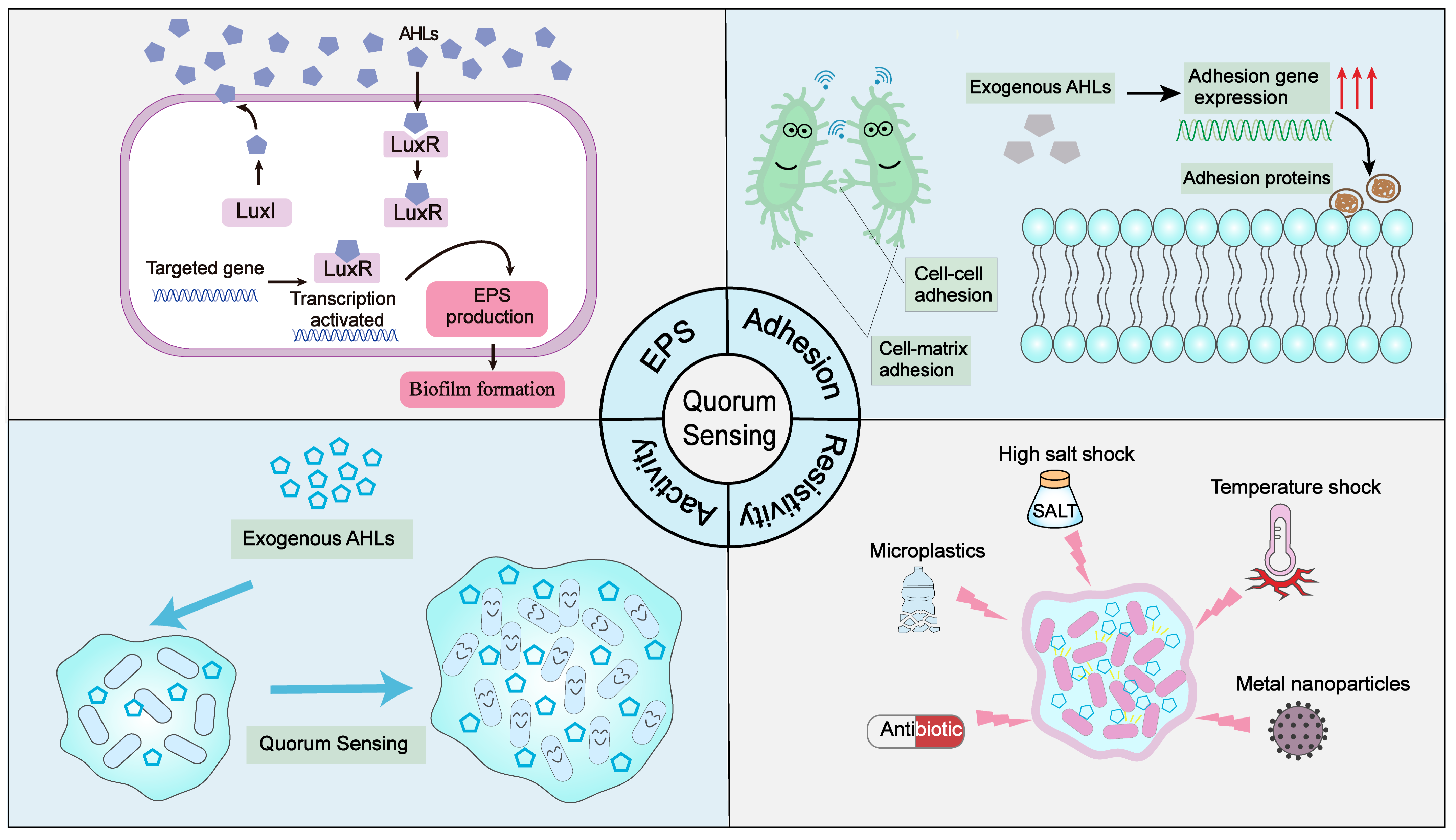
5. Strengthening Effect of QS on the Biofilm System
5.1. Enhancement of EPS Production by QS
5.2. The Enhancement of Biofilm Adhesion Ability by QS
5.3. Enhancement of Biofilm Activity by QS
5.4. The Enhancement of Biofilm Shock Resistance by QS
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coenye, T.; Ahonen, M.; Anderson, S.; Cámara, M.; Chundi, P.; Fields, M.; Foidl, I.; Gnimpieba, E.Z.; Griffin, K.; Hinks, J.; et al. Global challenges and microbial biofilms: Identification of priority questions in biofilm research, innovation and policy. Biofilm 2024, 8, 100210. [Google Scholar] [CrossRef] [PubMed]
- Savadiya, B.; Pandey, G.; Misra, S.K. Remediation of pharmacophoric laboratory waste by using biodegradable carbon nanoparticles of bacterial biofilm origin. Environ. Res. 2024, 252, 118969. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Lu, Z.D.; Sun, W.J.; Zhang, X.H. Influence of pore structure on biologically activated carbon performance and biofilm microbial characteristics. Front. Environ. Sci. Eng. 2021, 15, 131. [Google Scholar] [CrossRef]
- Verma, S.; Kuila, A.; Jacob, S. Role of Biofilms in Waste Water Treatment. Appl. Biochem. Biotechnol. 2023, 195, 5618–5642. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yi, K.; Zeng, G.; Shi, Y.; Gu, Y.; Shi, L.; Yu, H. The role of quorum sensing in granular sludge: Impact and future application: A review. Chemosphere 2019, 236, 124310. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, D.; Wang, X.; Lin, S. Effects of the quorum sensing related luxS gene and lsr operon on Klebsiella michiganensis resisting copper stress. Environ. Res. 2024, 256, 119244. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, L.; Lv, Z.; Xin, F.; Dong, W.; Liu, G.; Li, Y.; Jia, H. N-acyl-homoserine lactones in extracellular polymeric substances from sludge for enhanced chloramphenicol-degrading anode biofilm formation in microbial fuel cells. Environ. Res. 2022, 207, 112649. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.-m.; Wang, J.; Ren, H.; Ding, L. Acceleration of start-up of moving bed biofilm reactor at low temperature by adding specialized quorum sensing bacteria. Bioresour. Technol. 2022, 358, 127249. [Google Scholar] [CrossRef]
- Wang, H.; Liao, L.; Chen, S.; Zhang, L.-H. A quorum quenching bacterial isolate contains multiple substrate-inducible genes conferring degradation of diffusible signal factor. Appl. Environ. Microbiol. 2020, 86, e02930-19. [Google Scholar] [CrossRef]
- Yingsong, W.; Zeran, B.; Yan, W. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl. Microbiol. Biotechnol. 2022, 106, 6365–6381. [Google Scholar]
- Wang, J.; Liu, Q.; Dong, D.; Hu, H.; Wu, B.; Ren, H. AHLs-mediated quorum sensing threshold and its response towards initial adhesion of wastewater biofilms. Water Res. 2021, 194, 116925. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Usman, T.M.; Varjani, S. Exploring the role of microbial biofilm for industrial effluents treatment. Bioengineered 2022, 13, 6420–6440. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xi, J.; Yang, C.; Cong, W. Quorum sensing regulation methods and their effects on biofilm in biological waste treatment systems: A review. Front. Environ. Sci. Eng. 2021, 16, 87. [Google Scholar] [CrossRef]
- Sahreen, S.; Mukhtar, H.; Imre, K.; Morar, A.; Herman, V.; Sharif, S. Exploring the Function of Quorum Sensing Regulated Biofilms in Biological Wastewater Treatment: A Review. Int. J. Mol. Sci. 2022, 23, 9751. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Qian, F.; Luo, L.; Chen, X.; Zhang, K.; Fang, F.; Xue, Z.; Li, C.; Cao, J.; Luo, J. Facilitating biofilm formation of Pseudomonas aeruginosa via exogenous N-Acy-L-homoserine lactones stimulation: Regulation on the bacterial motility, adhesive ability and metabolic activity. Bioresour. Technol. 2021, 341, 125727. [Google Scholar] [CrossRef]
- Shi, N.; Gao, Y.; Yin, D.; Song, Y.; Kang, J.; Li, X.; Zhang, Z.; Feng, X.; Duan, J. The effect of the sub-minimal inhibitory concentration and the concentrations within resistant mutation window of ciprofloxacin on MIC, swimming motility and biofilm formation of Pseudomonas aeruginosa. Microb. Pathog. 2019, 137, 103765. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Ma, S.; Hu, H.; Wu, B.; Zhang, X.-X.; Ren, H. Distribution characteristics of N-acyl homoserine lactones during the moving bed biofilm reactor biofilm development process: Effect of carbon/nitrogen ratio and exogenous quorum sensing signals. Bioresour. Technol. 2019, 289, 121591. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Li, X.; Ma, S.; Hu, H.; Wu, B.; Zhang, X.-x.; Ren, H. In-situ monitoring AHL-mediated quorum-sensing regulation of the initial phase of wastewater biofilm formation. Environ. Int. 2020, 135, 105326. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K.K.; Tan, H.S. The role of quorum sensing, biofilm formation, and iron acquisition as key virulence mechanisms in Acinetobacter baumannii and the corresponding anti-virulence strategies. Microbiol. Res. 2022, 260, 127032. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Wang, F.; Sun, D.; Liu, X.; Xin, B. Formation, Development, and Cross-Species Interactions in Biofilms. Front. Microbiol. 2022, 12, 757327. [Google Scholar] [CrossRef]
- Baraquet, C.; Harwood, C.S. FleQ DNA Binding Consensus Sequence Revealed by Studies of FleQ-Dependent Regulation of Biofilm Gene Expression in Pseudomonas aeruginosa. J. Bacteriol. 2016, 198, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Upmanyu, K.; Haq, Q.M.R.; Singh, R. Factors mediating Acinetobacter baumannii biofilm formation: Opportunities for developing therapeutics. Curr. Res. Microb. Sci. 2022, 3, 100131. [Google Scholar] [CrossRef]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef]
- Liu, C.; Sun, D.; Zhu, J.; Liu, J.; Liu, W. The Regulation of Bacterial Biofilm Formation by cAMP-CRP: A Mini-Review. Front. Microbiol. 2020, 11, 802. [Google Scholar] [CrossRef]
- Zorraquino, V.; García, B.; Latasa, C.; Echeverz, M.; Toledo-Arana, A.; Valle, J.; Lasa, I.; Solano, C. Coordinated Cyclic-Di-GMP Repression of Salmonella Motility through YcgR and Cellulose. J. Bacteriol. 2013, 195, 417–428. [Google Scholar] [CrossRef]
- Petrova, O.E.; Cherny, K.E.; Sauer, K. The Pseudomonas aeruginosa Diguanylate Cyclase GcbA, a Homolog of P. fluorescens GcbA, Promotes Initial Attachment to Surfaces, but Not Biofilm Formation, via Regulation of Motility. J. Bacteriol. 2014, 196, 2827–2841. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Zhou, J.-W.; Zhang, P.-P.; Luo, H.-Z.; Tang, S.; Li, J.-J.; Deng, S.-M.; Jia, A.-Q. Quorum sensing inhibition and tobramycin acceleration in Chromobacterium violaceum by two natural cinnamic acid derivatives. Appl. Microbiol. Biotechnol. 2020, 104, 5025–5037. [Google Scholar] [CrossRef]
- Erkihun, M.; Asmare, Z.; Endalamew, K.; Getie, B.; Kiros, T.; Berhan, A. Medical Scope of Biofilm and Quorum Sensing during Biofilm Formation: Systematic Review. Bacteria 2024, 3, 118–135. [Google Scholar] [CrossRef]
- Hadla, M.; Halabi, M.A. Chapter Three—Effect of Quorum Sensing. In Comprehensive Analytical Chemistry; Chormey, D.S., Bakırdere, S., Turan, N.B., Engin, G.Ö., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 81, pp. 95–116. [Google Scholar]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance—Development, composition and regulation—Therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, X.; Zheng, J.; Zou, Y.; Qiu, S.; Dai, Y. AHL-mediated quorum sensing to regulate bacterial substance and energy metabolism: A review. Microbiol. Res. 2022, 262, 127102. [Google Scholar] [CrossRef]
- Cayetano, R.D.A.; Kim, G.-B.; Park, J.-H.; Lee, M.-J.; Kim, S.-H. Anaerobic digestion of waste activated sludge using dynamic membrane at varying substrate concentration reveals new insight towards methanogenic pathway and biofilm formation. Chem. Eng. J. 2021, 423, 130249. [Google Scholar] [CrossRef]
- Sun, Y.; Guan, Y.; Zeng, D.; He, K.; Wu, G. Metagenomics-based interpretation of AHLs-mediated quorum sensing in Anammox biofilm reactors for low-strength wastewater treatment. Chem. Eng. J. 2018, 344, 42–52. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Zhao, B.-H.; Zhang, Y.-C.; Wang, X.-J.; Chen, G.-H. Long-term effects of N-acyl-homoserine lactone-based quorum sensing on the characteristics of ANAMMOX granules in high-loaded reactors. Chemosphere 2019, 218, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.-c.; Wang, X.-j.; Li, J.; Zhou, R.-x.; Wei, J.; Liang, D.-b.; Zhang, K. Effects of substrate shock on release of AHL signals in ANAMMOX granules and properties of granules. Environ. Sci. Water Res. Technol. 2019, 5, 756–768. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Deng, D.; Yang, Z.; Wang, C.; Li, Y.; Zhu, L.; Pan, Y. Nitrite enhanced denitrifying phosphorus removal in alternating aerobic/anaerobic biofilm system by promoting quorum sensing activity. Chem. Eng. J. 2025, 505, 159284. [Google Scholar] [CrossRef]
- Yan, H.; Li, J.; Meng, J.; Li, J.; Kumar Jha, A.; Zhang, Y.; Fan, Y.; Wang, X. Effects of reflux ratio on the anaerobic sludge and microbial social behaviors in an expanded granular sludge bed reactor: From the perspective of acyl-homoserine lactones-mediated quorum sensing. Bioresour. Technol. 2021, 337, 125360. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Z.; Wang, Z.; Ma, K.; Xu, X.; Alvarezc, P.J.J.; Zhu, L. Understanding of aerobic sludge granulation enhanced by sludge retention time in the aspect of quorum sensing. Bioresour. Technol. 2019, 272, 226–234. [Google Scholar] [CrossRef]
- Liu, L.; Ji, M.; Wang, F.; Tian, Z.; Yan, Z.; Wang, S. N-acyl- -homoserine lactones release and microbial community changes in response to operation temperature in an anammox biofilm reactor. Chemosphere 2021, 262, 127602. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Zhao, Z. Deciphering the internal mechanism of nitrogen removal from sludge and biofilm under low temperature from the perspective of microbial function metabolism. Environ. Res. 2025, 267, 120688. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.C.; Feng, H.J.; Huang, W.K.; Li, N.; Zhou, Y.Y.; Wang, M.Z.; Zhang, X.Q.; Shen, D.S. The effect of quorum sensing on anaerobic granular sludge in different pH conditions. Biochem. Eng. J. 2015, 103, 270–276. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.Z.; Zhao, B.H.; Zhang, K.; Liang, D.B.; Wei, J.; Wang, X.J.; Li, J.; Chen, G.H. Effects of pH on AHL signal release and properties of ANAMMOX granules with different biomass densities. Environ. Sci.-Water Res. Technol. 2019, 5, 1723–1735. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, S.; Xie, Y.; Wang, M.; Cai, T.; Li, J.; Guo, D.; Zhao, L.; Xu, Y.; Liang, S.; et al. Inactivation of Pseudomonas aeruginosa Biofilms by 405-Nanometer-Light-Emitting Diode Illumination. Appl. Environ. Microbiol. 2020, 86, e00092-20. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.; Lens, P.N.L.; Zhang, Z.; Shi, W.; Cui, F.; Tay, J.H. Effect of light intensity on the characteristics of algal-bacterial granular sludge and the role of N-acyl-homoserine lactone in the granulation. Sci. Total Environ. 2019, 659, 372–383. [Google Scholar] [CrossRef]
- Gao, P.; Zhao, A.; Zhang, X.; Tang, P.; Li, D.; Liu, T.; Li, J.; Zhu, Y.; Wang, Z. Potential role of N-acyl homoserine lactone-mediated quorum sensing in the adaptation of anammox granular sludge system to salinity stress. Bioresour. Technol. 2025, 416, 131758. [Google Scholar] [CrossRef]
- Xue, J.; Ma, H.; Dong, X.; Shi, K.; Zhou, X.; Qiao, Y.; Gao, Y.; Liu, Y.; Feng, Y.; Jiang, Q. Insights into the response of electroactive biofilm with petroleum hydrocarbons degradation ability to quorum sensing signals. J. Hazard. Mater. 2024, 471, 134407. [Google Scholar] [CrossRef]
- Gómez-Gómez, B.; Arregui, L.; Serrano, S.; Santos, A.; Pérez-Corona, T.; Madrid, Y. Unravelling mechanisms of bacterial quorum sensing disruption by metal-based nanoparticles. Sci. Total Environ. 2019, 696, 133869. [Google Scholar] [CrossRef]
- Choi, S.; Johnston, M.V.; Wang, G.-S.; Huang, C.P. Looking for engineered nanoparticles (ENPs) in wastewater treatment systems: Qualification and quantification aspects. Sci. Total Environ. 2017, 590–591, 809–817. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Ni, S.-Q.; Zhuang, X.; Lee, T. Nano zero-valent iron improves anammox activity by promoting the activity of quorum sensing system. Water Res. 2021, 202, 117491. [Google Scholar] [CrossRef] [PubMed]
- de Celis, M.; Belda, I.; Marquina, D.; Santos, A. Phenotypic and transcriptional study of the antimicrobial activity of silver and zinc oxide nanoparticles on a wastewater biofilm-forming Pseudomonas aeruginosa strain. Sci. Total Environ. 2022, 826, 153915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Liu, L.; Zhou, L.; Zhao, Z. Impacts of antibiotics on biofilm bacterial community and disinfection performance on simulated drinking water supply pipe wall. Environ. Pollut. 2021, 288, 117736. [Google Scholar] [CrossRef]
- Bruchmann, J.; Kirchen, S.; Schwartz, T. Sub-inhibitory concentrations of antibiotics and wastewater influencing biofilm formation and gene expression of multi-resistant Pseudomonas aeruginosa wastewater isolates. Environ. Sci. Pollut. Res. 2013, 20, 3539–3549. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, Y.; Xia, T.; Feng, Y.; Liu, X.; Graham, N.J.D.; Choo, K.-H.; Takizawa, S.; Ng, H.Y.; Hou, L.-a. Overlooked interference of antibiotics on quorum sensing inhibitors for membrane biofouling mitigation by affecting AHLs and PQS pathway. Sep. Purif. Technol. 2025, 363, 132116. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Chen, P.; Maddela, N.R.; Li, S. Insights into the membrane fouling aggravation under polyethylene terephthalate microplastics contamination: From a biochemical point of view. J. Clean. Prod. 2023, 424, 138905. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, J.; Li, R.; Chen, X.; Cao, M. Dissecting the negative influence mechanisms of microplastics on plant and microbial mediated nitrogen removal in constructed wetlands. Chem. Eng. J. 2024, 491, 151910. [Google Scholar] [CrossRef]
- Zhang, B.; Qi, B.; Shi, W.; Huang, S.; Xu, W.; Yan, P.; Zhang, B.; Lens, P.N.L.; Peng, Y. Impaired denitrification of aerobic granules in response to micro/nanoplastic stress: Insights from interspecies interactions and electron transfer processes. Water Res. 2025, 279, 123472. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.J.; Liu, Z.; Li, H.T.; Zhang, A.N. Dynamic characteristics of AHLs-secreting strain Aeromonas sp. A-L2 and its bioaugmentation during quinoline biodegradation. J. Appl. Microbiol. 2020, 128, 1060–1073. [Google Scholar] [CrossRef]
- Fang, Y.; Deng, C.; Chen, J.; Lü, J.; Chen, S.; Zhou, S. Accelerating the start-up of the cathodic biofilm by adding acyl-homoserine lactone signaling molecules. Bioresour. Technol. 2018, 266, 548–554. [Google Scholar] [CrossRef]
- Shi, K.; Cheng, W.; Cheng, D.; Xue, J.; Qiao, Y.; Gao, Y.; Jiang, Q.; Wang, J. Stability improvement and the mechanism of a microbial electrolysis cell biocathode for treating wastewater containing sulfate by quorum sensing. Chem. Eng. J. 2023, 455, 140597. [Google Scholar] [CrossRef]
- Hu, H.; He, J.; Liu, J.; Yu, H.; Zhang, J. Biofilm activity and sludge characteristics affected by exogenous N-acyl homoserine lactones in biofilm reactors. Bioresour. Technol. 2016, 211, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, Y.; Zhang, J.; Zhang, Y.; Li, J. Biofilm activity, ammonia removal and cell growth of the heterotrophic nitrifier, Acinetobacter sp., facilitated by exogenous N-acyl-homoserine lactones. RSC Adv. 2018, 8, 30783–30793. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Feng, C.; Li, W.; Ren, Z.; Wang, P.; Liu, X.; Gao, W.; Sun, L.; Zhang, G. Accelerated performance recovery of anaerobic granular sludge after temperature shock: Rapid construction of protective barriers (EPS) to optimize microbial community composition base on quorum sensing. J. Clean. Prod. 2023, 392, 136243. [Google Scholar] [CrossRef]
- Zhou, S.; An, W.; Zhao, K.; Lin, L.; Yang, S.; Zhang, Y.; Xu, M. Protection of electroactive biofilms against hypersaline shock by quorum sensing. Water Res. 2023, 233, 119823. [Google Scholar] [CrossRef]
- Jiang, K.; Yang, X.; Gao, Q.; Ni, J.; Feng, J.; Wu, D.; Zou, X.; Hu, L.; Liu, X.; Song, Z.; et al. Exogenous signaling molecules N-acyl-homoserine lactones promotes the reconstruction of sludge particles after impact with highly concentrated urea-formaldehyde resin microplastics. J. Environ. Manag. 2024, 371, 123179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, S.; Liu, L.; Qiu, C.; Xiao, S.; Ouyang, Q.; Ji, M. Research Progress on the Influence Factors of the Quorum Sensing System Regulating the Growth of Wastewater Treatment Biofilm. Water 2025, 17, 1944. https://doi.org/10.3390/w17131944
Wang R, Wang S, Liu L, Qiu C, Xiao S, Ouyang Q, Ji M. Research Progress on the Influence Factors of the Quorum Sensing System Regulating the Growth of Wastewater Treatment Biofilm. Water. 2025; 17(13):1944. https://doi.org/10.3390/w17131944
Chicago/Turabian StyleWang, Rao, Shaopo Wang, Lingjie Liu, Chunsheng Qiu, Shumin Xiao, Qinghua Ouyang, and Min Ji. 2025. "Research Progress on the Influence Factors of the Quorum Sensing System Regulating the Growth of Wastewater Treatment Biofilm" Water 17, no. 13: 1944. https://doi.org/10.3390/w17131944
APA StyleWang, R., Wang, S., Liu, L., Qiu, C., Xiao, S., Ouyang, Q., & Ji, M. (2025). Research Progress on the Influence Factors of the Quorum Sensing System Regulating the Growth of Wastewater Treatment Biofilm. Water, 17(13), 1944. https://doi.org/10.3390/w17131944






