Ecological Assessment of Rivers Under Anthropogenic Pressure: Testing Biological Indices Across Abiotic Types of Rivers
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection
2.2. Environmental Surveys
2.3. Biological Surveys
- EPT: the total number of families in the Ephemeroptera, Plecoptera and Trichoptera taxa.
- The Shannon–Wiener index (H′): H′ = −Σ(pi) (ln pi), where pi = ni/N, the proportion of individuals belonging to family is ni, and N is the total number of macroinvertebrate individuals.
- The total number of macroinvertebrate families.
- ASPT (Average Score per Taxon): the value of the BMWP (Biological Monitoring Working Party) divided by the number of BMWP families present in the taxa list. All Oligochaeta were considered as one taxon.
- 1 − GOLD: 1 − (relative abundance of Gastropoda + Oligochaeta + Diptera).
- Log10(Sel_EPTD + 1): log10 (sum of individuals of the families Heptageniidae, Ephemeridae, Leptophlebiidae, Brachycentridae, Georidae, Polycentropodidae, Limnephilidae, Odontoceridae, Dolichopodidae, Stratiomyidae, Dixidae, Empididae, Athericidae, Nemouridae + 1).
2.4. Statistical Analyses
3. Results
3.1. Environmental Parameters Across Abiotic Types of Rivers
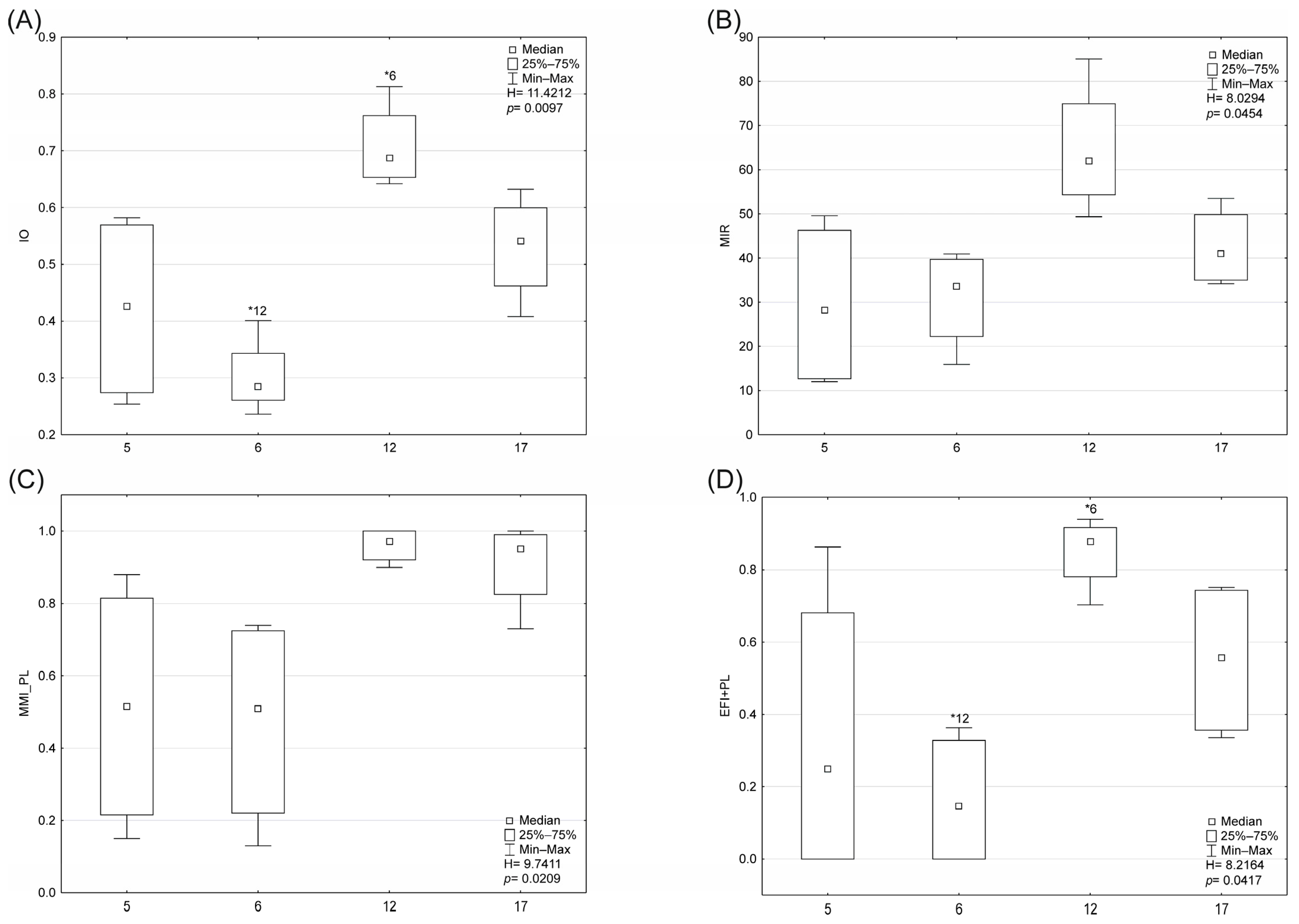
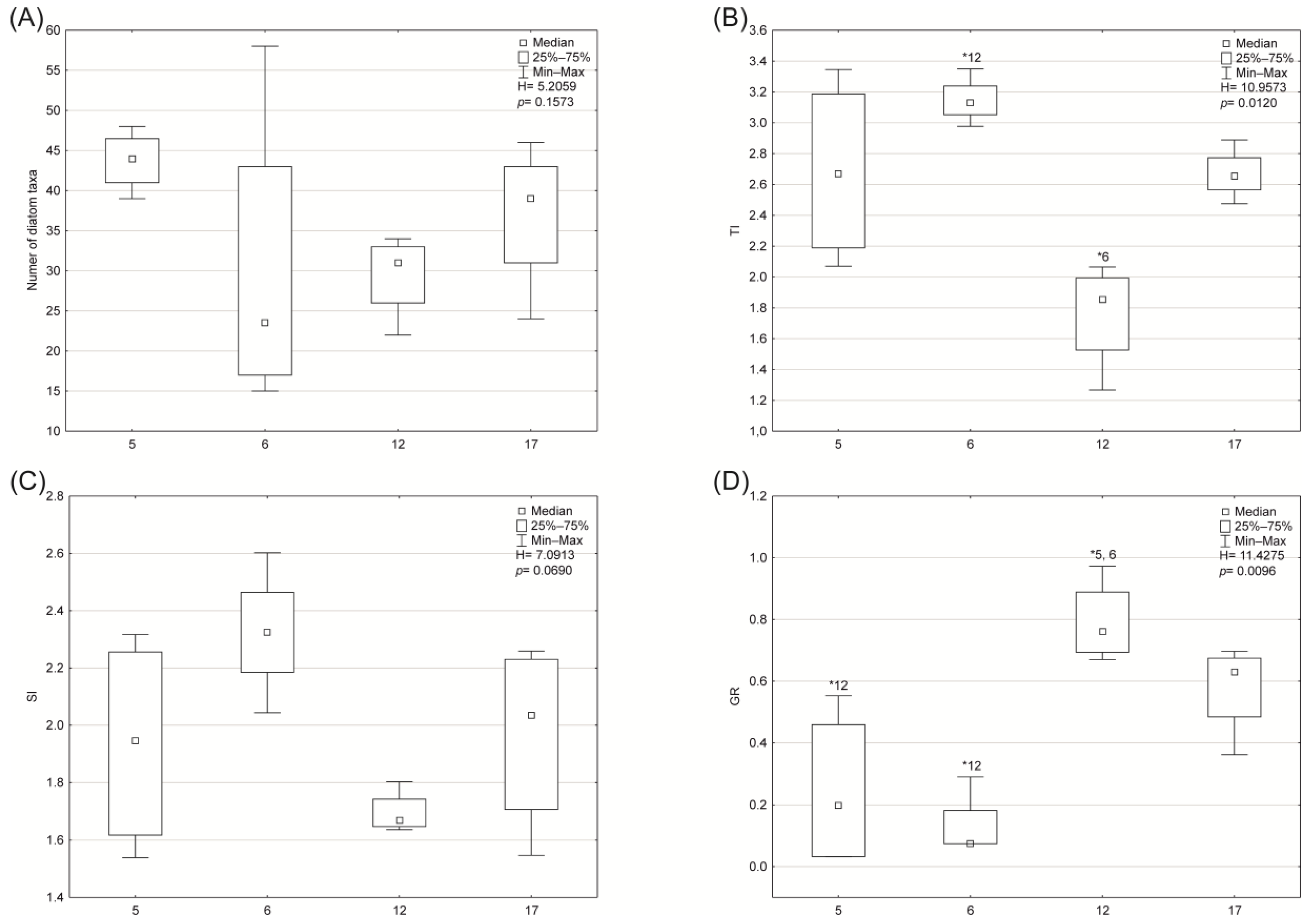
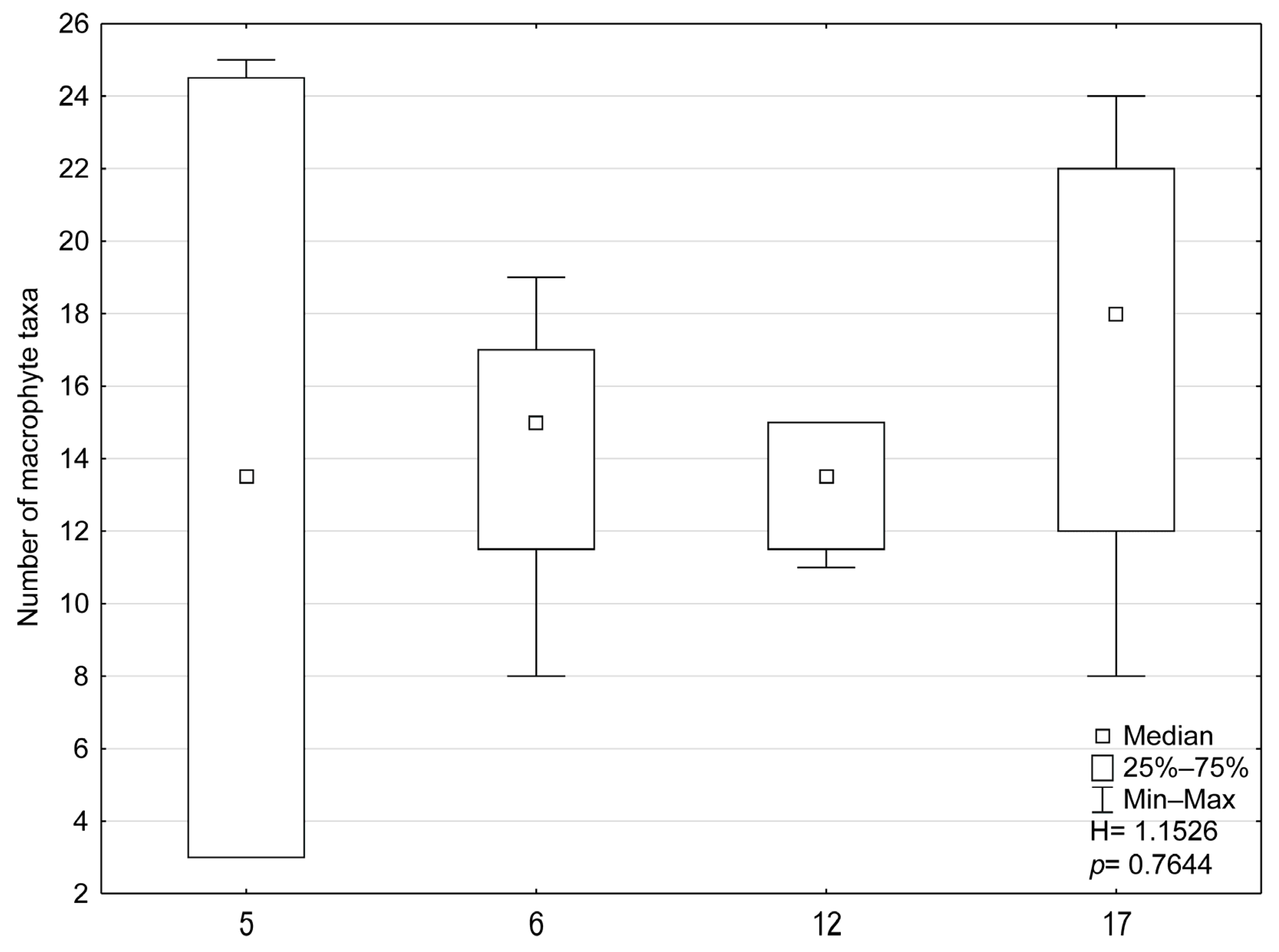
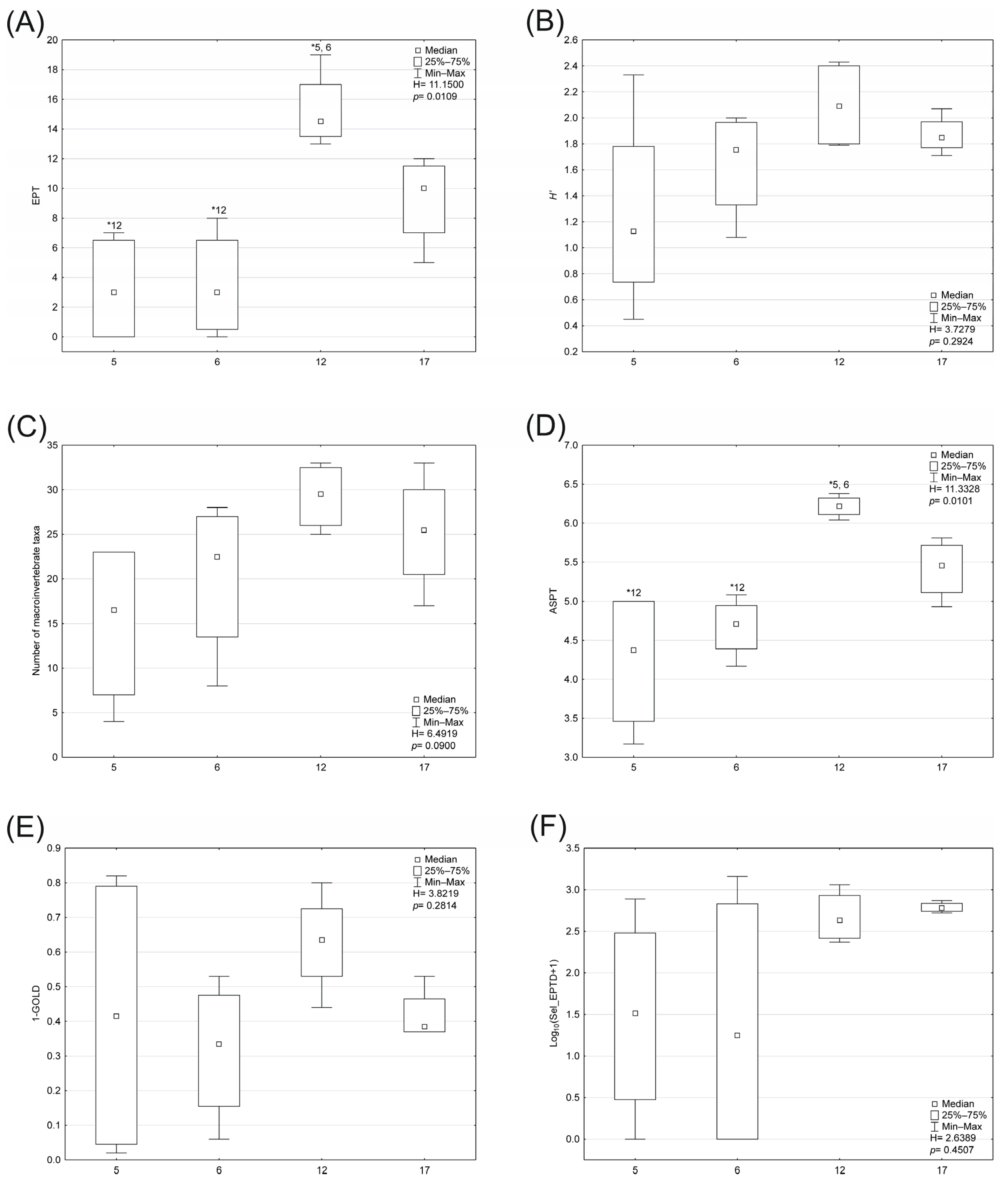
3.2. Biological Indices and Community Structure
3.3. Concordance Among Biological Indices and Environmental Gradients
- Diatoms: The RDA analysis based on diatom taxa and environmental variables showed that the first two axes explained 28.5% of the variance in biological data and 71.7% of the variance in species–environment relationships. According to forward selection, conductivity, TOC, and altitude were the most strongly associated variables (statistically significant) with the distribution of diatom taxa and biotic metrics (Figure 6). Three distinct patterns were identified: (1) the distribution of Pleurosigma salinarum, Halamphora coffeaeformis, Navicula flandriae, N. salinarum var. salinarum, Pleurosira laevis var. laevis, Haslea spicula, N. lanceolata, and Surirella brebissonii was positively associated with conductivity; (2) Cyclotella meneghiniana, Lemnicola hungarica, and the values of SI and TI indices were positively correlated with TOC; (3) Amphora inariensis, Reimeria uniseriata, Cocconeis neothumensis, C. placentula var. placentula, C. euglypta, and the GR and IO index values were positively associated with altitude and WRH. The relationship between diatom composition and environmental variables was statistically significant (Monte Carlo test for the first canonical axis: p = 0.006, F = 2.071; for all canonical axes: p = 0.002, F = 1.810).
- Macrophytes: The first two axes explained 79.0% of the variance in biological data and 93.9% of the variance in species–environment relationships in RDA analysis (Figure 7). Based on forward selection, conductivity, altitude, and WRH were the variables most significantly associated with macrophyte distribution. Lyngbya sp., Scapania undulata, Brachythecium rivulare, Plagiomnium affine, Platyhypnidium riparioides, Chiloscyphus polyanthos, Marchantia polymorpha, Berula erecta, Ranunculus aquatilis, and the MIR index were positively correlated with flow velocity, altitude, and WRH. In contrast, conductivity influenced the distribution of Phragmites australis, Enteromorpha sp., and Potamogeton pectinatus most. The relationship between macrophyte composition and environmental variables was statistically significant (Monte Carlo test for the first canonical axis: p = 0.002, F = 26.179; for all axes: p = 0.002, F = 7.956).
- Benthic macroinvertebrates: RDA showed that the first two axes explained 45.7% of the variance in biological data and 70.6% in species–environment relationships. Conductivity, alkalinity, and WRH were the most significant variables affecting macroinvertebrate distribution and biotic metrics (Figure 8). Three distribution patterns were established: (1) Hydrobiidae, Oligochaeta, Hydrophilidae, and Coenagrionidae were positively associated with higher conductivity, alkalinity, and temperature; (2) Gammaridae, Dixidae, Sericostomatidae, Glossosomatidae, Rhyacophilidae, and Odontoceridae were associated with altitude and coarse substrates (gravel and pebble); (3) the abundance of Ancylidae, Goeridae, Polycentropodidae, Lepidostomatidae, Limnephilidae, Chloroperlidae, Nemouridae, Leptophlebiidae, Baetidae, and Heptageniidae, along with high values of biotic metrics such as ASPT, 1-GOLD, H’, MMI_PL, and log10(Sel_EPTD + 1), was positively correlated with WRH. The relationship was statistically significant (Monte Carlo test for the first axis: p = 0.002, F = 2.814; for all axes: p = 0.002, F = 2.103).
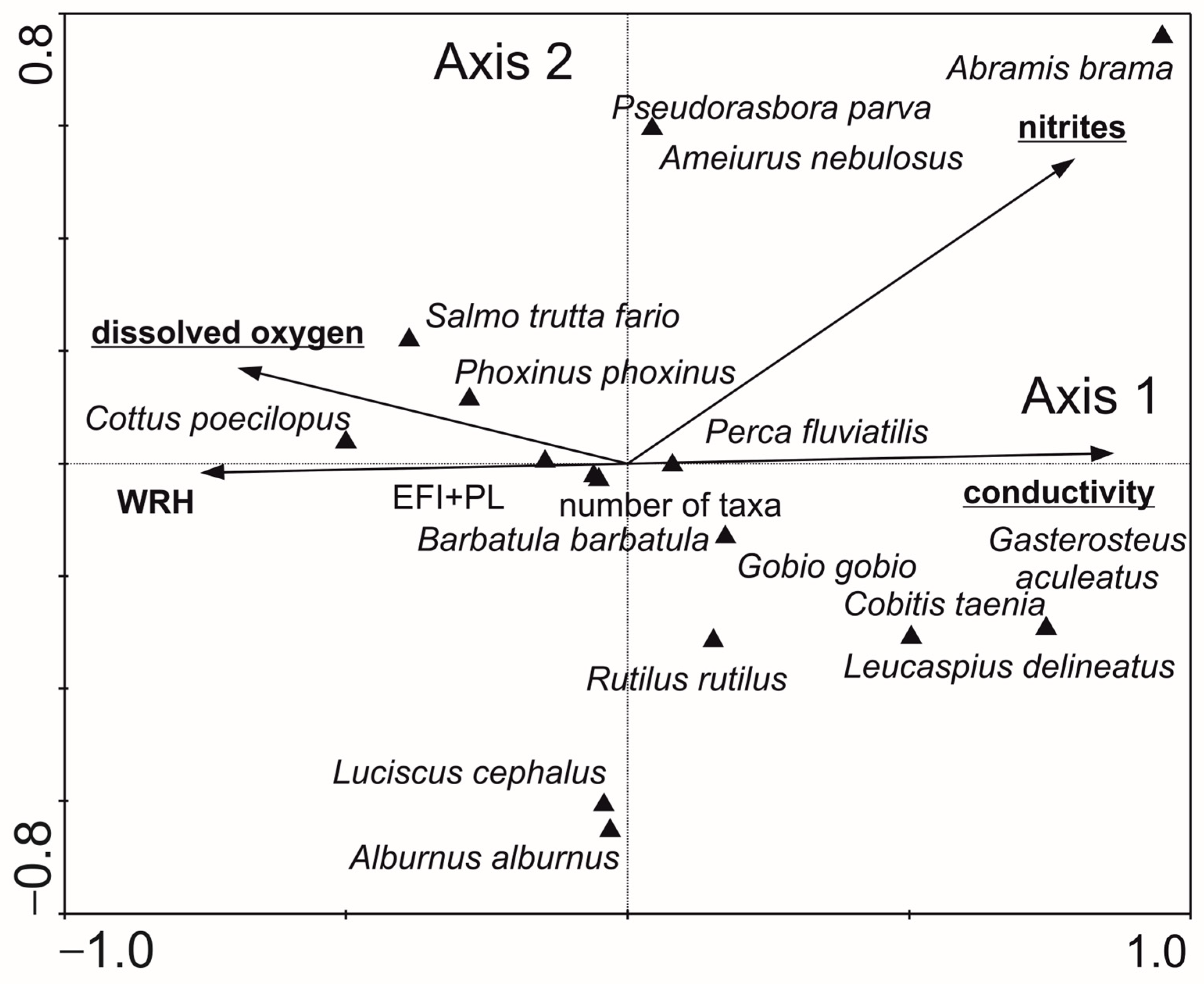
- Fish: The first two axes explained 32.1% of the variance in biological data and 72.9% in species–environment relationships in CCA analysis (Figure 9). Conductivity, dissolved oxygen, and nitrite concentration were the most significant variables. Gasterosteus aculeatus abundance was positively correlated with increasing conductivity. Salmo trutta fario, Phoxinus phoxinus, Cottus poecilopus, and Barbatula barbatula, as well as EFI + PL index values and total species number, were positively associated with a higher dissolved oxygen and WRH. The relationship between fish composition and environmental variables was statistically significant (Monte Carlo test for the first canonical axis: p = 0.006, F = 2.528; for all axes: p = 0.004, F = 2.165).
4. Discussion
4.1. Responses of Aquatic Organisms to Spatial and Anthropogenic Gradients
4.2. Diagnostic Performance of Biological Indices Across Types of Rivers
4.3. Typology-Specific Challenges in Ecological Assessment Under the WFD
4.4. Implications for Biomonitoring and Management Strategies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Fraction mm (%) | Type 5 | Type 6 | Type 12 | Type 17 | ||||
|---|---|---|---|---|---|---|---|---|
| Selected rivers | Bolina | Centuria | Mitręga | Mleczna | Dziechcinka | Vistula | Korzenica | Wiercica |
| <0.002 | 2.0–29.0 | 0.0–0.0 | 0.0–3.9 | 0.0–8.5 | 0.0–0.0 | 0.0–0.0 | 0.0–0.0 | 0.0–3.0 |
| 0.002–0.02 | 4.0–59.0 | 0.0–0.0 | 0.0–5.9 | 0.0–12.3 | 0.0–0.0 | 0.0–0.0 | 0.0–0.0 | 0.0–4.0 |
| 0.02–0.1 | 2.9–13.0 | 0.5–1.7 | 0.3–27.6 | 3.20–40.8 | 0.2–2.5 | 0.1–1.2 | 0.2–15.2 | 0.1–24.8 |
| 0.1–0.25 | 1.5–32.9 | 17.7–32.0 | 3.7–35.6 | 11.7–52.8 | 1.1–7.1 | 0.3–3.3 | 5.4–41.1 | 6.2–65.5 |
| 0.25–0.5 | 0.5–55.0 | 42.7–69.9 | 21.3–79.7 | 9.0–59.2 | 3.1–15.6 | 1.3–13.1 | 26.0–71.9 | 11.4–50.9 |
| 0.5–1 | 0.0–18.8 | 0.6–17.3 | 2.1–26.3 | 1.0–19.8 | 3.3–16.9 | 1.7–11.8 | 2.1–25.3 | 0.9–52.2 |
| 1–2 | 0.0–3.0 | 0.1–1.8 | 0.50–2.4 | 0.4–7.8 | 5.3–19.2 | 2.5–19.7 | 0.3–10.2 | 0.0–3.9 |
| 2–5 | 0.0–0.8 | 0.0–0.8 | 0.10–1.2 | 0.0–8.5 | 7.2–27.8 | 3.5–20.3 | 0.0–9.0 | 0.0–2.2 |
| 5–10 | 0.0–0.3 | 0.0–1.9 | 0.10–0.8 | 0.0–10.1 | 6.4–21.8 | 3.2–15.4 | 0.0–6.8 | 0.0–3.2 |
| 10–20 | 0.0–0.0 | 0.0–6.0 | 0.0–1.8 | 0.0–18.9 | 9.8–28.0 | 8.5–33.0 | 0.0–18.2 | 0.0–10.8 |
| >20 | 0.0–0.0 | 0.0–20.4 | 0.0–28.7 | 0.0–6.1 | 1.1–38.0 | 7.9–72.9 | 0.0–40.3 | 0.0–20.8 |
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Malmqvist, B.; Rundle, S. Threats to the running water ecosystems of the world. Environ. Conserv. 2002, 29, 134–153. [Google Scholar] [CrossRef]
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Pont, D.; Hugueny, B.; Rogers, C. Development of a fish-based index for the assessment of river health in Europe: The European Fish Index. Fish. Manag. Ecol. 2007, 14, 427–439. [Google Scholar] [CrossRef]
- Marzin, A.; Archaimbault, V.; Belliard, J.; Chauvin, C.; Delmas, F.; Pont, D. Ecological assessment of running waters: Do macrophytes, macroinvertebrates, diatoms and fish show similar responses to human pressures? Ecol. Indic. 2012, 23, 56–65. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; van de Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Birk, S.; Chapman, D.; Carvalho, L.; Spears, B.M.; Andersen, H.E.; Argillier, C.; Auer, S.; Baattrup-Pedersen, A.; Banin, L.; Beklioğlu, M.; et al. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 2020, 4, 1060–1068. [Google Scholar] [CrossRef]
- Sabater, S.; Elosegi, A.; Ludwig, R. Multiple Stressors in River Ecosystems: Status, Impacts and Prospects for the Future; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128117132. [Google Scholar]
- Lima, A.C.; Sayanda, D.; Wrona, F.J. A roadmap for multiple stressors assessment and management in freshwater ecosystems. Environ. Impact Assess. Rev. 2023, 102, 107191. [Google Scholar] [CrossRef]
- Johnson, R.K.; Hering, D. Response of taxonomic groups in streams to gradients in resource and habitat characteristics. J. Appl. Ecol. 2009, 46, 175–186. [Google Scholar] [CrossRef]
- Stubbington, R.; Sarremejane, R.; Laini, A.; Cid, N.; Csabai, Z.; England, J.; Munné, A.; Aspin, T.; Bonada, N.; Bruno, D.; et al. Disentangling responses to natural stressor and human impact gradients in river ecosystems across Europe. J. Appl. Ecol. 2022, 59, 537–548. [Google Scholar] [CrossRef]
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council—Establishing a Framework for Community Action in the Field of Water Policy; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Poikane, S.; Salas Herrero, F.; Kelly, M.G.; Borja, A.; Birk, S.; van de Bund, W. European aquatic ecological assessment methods: A critical review of their sensitivity to key pressures. Sci. Total Environ. 2020, 740, 140075. [Google Scholar] [CrossRef]
- Kolada, A.; Adamczyk, M.; Bielczyńska, A.; Bis, B.; Błachuta, J.; Błeńska, M.; Bociąg, K.; Brzeska-Roszczyk, P.; Ciecierska, H.; Dziemian, Ł.; et al. Handbook for the Biological Quality Elements (BQEs) Assessment and Ecological Status Classification of Surface Waters in Poland. Methodological Updating; Kolada, A., Ed.; Inspekcja Ochrony Środowiska: Warszawa, Poland, 2020; ISBN 978-83-950881-2-4. [Google Scholar]
- Bis, B. Assessing the Ecological Status Assessment of Freshwaters. In Freshwater Ecosystems in Europe—An Educational Approach.Natural History Museum of Crete; Voreadou, C., Ed.; Selena Press: Heraklion, Greece, 2008; pp. 56–59. [Google Scholar]
- Johnson, R.K.; Hering, D.; Furse, M.T.; Clarke, R.T. Detection of ecological change using multiple organism groups: Metrics and uncertainty. Hydrobiologia 2006, 566, 115–137. [Google Scholar] [CrossRef]
- Zgrundo, A.; Peszek, Ł.; Poradowska, A. Manual for Monitoring and Evaluation of River Surface Water Bodies Based on Phytobenthos; Główny Inspektorat Ochrony Środowiska: Gdańsk, Poland, 2018.
- Schneider, S. Macrophyte trophic indicator values from a European perspective. Limnologica 2007, 37, 281–289. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Zbierska, J.; Jusik, S.; Zgoła, T. Makrofitowa Metoda Oceny Rzek—Podręcznik Metodyczny Do Oceny i Klasyfikacji Stanu Ekologicznego Wód Płynących w Oparciu o Rośliny Wodne; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2010. [Google Scholar]
- Rosenberg, D.M.; Resh, V.H. Freshwater Biomonitoring and Benthic Macroinvertebrates; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Bis, B.; Mikulec, A. Guide to Assess the Ecological Status of Rivers Based on Benthic Macroinvertebrate; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2013. [Google Scholar]
- FAME CONSORTIUM Manual for the Application of the European Fish Index—EFI: A Fish-Based Method to Assess the Ecological Status of European Rivers in Support of the Water Framework Directive. Available online: https://pureportal.inbo.be/en/publications/manual-for-application-of-the-european-fish-index-efi-a-fish-base (accessed on 5 October 2019).
- Prus, P.; Wiśniewolski, W.; Adamczyk, M. Monitoring of Riverine Ichthyofauna. Methodological Guide; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2016. [Google Scholar]
- Szoszkiewicz, K.; Jusik, S.; Lewin, I.; Czerniawska-Kusza, I.; Kupiec, J.M.; Szostak, M. Macrophyte and macroinvertebrate patterns in unimpacted mountain rivers of two European ecoregions. Hydrobiologia 2018, 808, 327–342. [Google Scholar] [CrossRef]
- Hering, D.; Johnson, R.K.; Kramm, S.; Schmuts, S.; Szoszkiewicz, K.; Verdonschot, P.F.M. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: A comparative metric-based analysis of organism response to stress. Freshw. Biol. 2006, 51, 1757–1785. [Google Scholar] [CrossRef]
- Clarke, G.; Kernan, M.; Marchetto, A.; Sorvari, S.; Catalan, J. Using diatoms to assess geographical patterns of change in high-altitude European lakes from pre-industrial times to the present day. Aquat. Sci. 2005, 67, 224–236. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Szoszkiewicz, K.; Nijboer, R.; O’Hare, M.; Ferreira, T. Macrophyte communities in unimpacted European streams: Variability in assemblage patterns, abundance and diversity. Hydrobiologia 2006, 566, 179–196. [Google Scholar] [CrossRef]
- Jusik, S.; Szoszkiewicz, K.; Kupiec, J.M.; Lewin, I.; Samecka-Cymerman, A. Development of comprehensive river typology based on macrophytes in the mountain-lowland gradient of different Central European ecoregions. Hydrobiologia 2015, 745, 241–262. [Google Scholar] [CrossRef]
- Jupke, J.F.; Birk, S.; Álvarez-Cabria, M.; Aroviita, J.; Barquín, J.; Belmar, O.; Bonada, N.; Cañedo-Argüelles, M.; Chiriac, G.; Elexová, E.M.; et al. Evaluating the biological validity of European river typology systems with least disturbed benthic macroinvertebrate communities. Sci. Total Environ. 2022, 842, 156689. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Jusik, S.; Adynkiewicz-Piragas, M.; Gebler, D.; Achtenberg, K.; Radecki-Pawlik, A.; Okruszko, T.; Gielczewski, M.; Pietruczuk, K.; Przesmycki, M.; et al. Podręcznik Oceny Wód Płynących w Oparciu o Hydromorfologiczny Indeks Rzeczny; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2017. [Google Scholar]
- Regulation of the Minister of the Environment of 22 July 2009 on the Classification of the Ecological Status, Ecological Potential and Chemical Status of Surface Water Bodies [Dz. U. 2009, Item 1018]. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20091221018 (accessed on 5 October 2019). (In Polish)
- Regulation of the Minister of Infrastructure of 25 June 2021 on the Classification of Ecological Status, Ecological Potential and Chemical Status and the Method of Classifying the Status of Surface Water Bodies, as Well as Environmental Standards [Dz. U. 2021, Item 1475]. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001475 (accessed on 19 May 2025). (In Polish)
- Halabowski, D.; Bielańska-Grajner, I.; Lewin, I. Effect of underground salty mine water on the rotifer communities in the Bolina River (Upper Silesia, Southern Poland). Knowl. Manag. Aquat. Ecosyst. 2019, 420, 31. [Google Scholar] [CrossRef]
- Halabowski, D.; Lewin, I.; Buczyński, P.; Krodkiewska, M.; Płaska, W.; Sowa, A.; Buczyńska, E. Impact of the Discharge of Salinised Coal Mine Waters on the Structure of the Macroinvertebrate Communities in an Urban River (Central Europe). Water Air Soil Pollut. 2020, 231, 5. [Google Scholar] [CrossRef]
- Halabowski, D.; Lewin, I. Impact of anthropogenic transformations on the vegetation of selected abiotic types of rivers in two ecoregions (Southern Poland). Knowl. Manag. Aquat. Ecosyst. 2020, 421, 35. [Google Scholar] [CrossRef]
- Halabowski, D.; Lewin, I. Triggers for the Impoverishment of the Macroinvertebrate Communities in the Human-Impacted Rivers of Two Central European Ecoregions. Water Air Soil Pollut. 2021, 232, 55. [Google Scholar] [CrossRef]
- Halabowski, D.; Bielańska-Grajner, I.; Lewin, I.; Sowa, A. Diversity of Rotifers in Small Rivers Affected by Human Activity. Diversity 2022, 14, 127. [Google Scholar] [CrossRef]
- Myślińska, E. Organic and Laboratory Land Testing Methods; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 2001. [Google Scholar]
- Hermanowicz, W.; Dojlido, J.; Dożańska, W.; Koziorowski, B.; Zerbe, J. Physical and Chemical Studies of Water and Wastewater; Arkady: Warszawa, Poland, 1999. [Google Scholar]
- Szoszkiewicz, K.; Jusik, S.; Gebler, D.; Achtenberg, K.; Adynkiewicz-Piragas, M.; Artur Radecki-Pawlik, A.; Okruszko, T.; Pietruczuk, K.; Przesmycki, M.; Nawrocki, P. Hydromorphological Index for Rivers: A New Method for Hydromorphological Assessment and Classification for Flowing Waters in Poland. J. Ecol. Eng. 2020, 21, 261–271. [Google Scholar] [CrossRef]
- Picińska-Fałtynowicz, J.; Błachuta, J.; Kotowicz, J.; Mazurek, M.; Rawa, W. Select the Types of Water Bodies River and Lake to Assess the Ecological Status Based on Phytobenthos with the Recommendation of the Methodology of Collection and Analysis of Samples; Główny Inspektorat Ochrony Środowiska: Wrocław, Poland, 2006.
- Picińska-Fałtynowicz, J.; Błachuta, J. A Practical Guide: Sampling, Preparation and Processing of Diatom Phytobenthos Residing in Rivers and Lakes; Instytut Meteorologii i Gospodarki Wodnej: Wrocław, Poland, 2010. [Google Scholar]
- ISO 10870:2012; Water Quality—Guidelines for the Selection of Sampling Methods and Devices for Benthic Macroinvertebrates in Fresh Waters. Available online: https://www.iso.org/standard/46251.html (accessed on 19 May 2025).
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5), 2nd ed.; Microcomputer Power: New York, NY, USA, 2002. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Morin, S.; Gómez, N.; Tornés, E.; Licursi, M.; Rosebery, J. Benthic Diatom Monitoring and Assessment of Freshwater Environments: Standard Methods and Future Challenges. In Aquatic Biofilms: Ecology, Water Quality and Wastewater Treatment; Romaní, A.M., Guasch, H., Balaguer, M.D., Eds.; Caister Academic Press: Wymondham, UK, 2016; pp. 111–124. [Google Scholar]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardón, M.; Hopfensperger, K.N.; Lamers, L.P.M.; Gell, P. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 2015, 6, 206. [Google Scholar] [CrossRef]
- Bąk, M.; Halabowski, D.; Kryk, A.; Lewin, I.; Sowa, A. Mining salinisation of rivers: Its impact on diatom (Bacillariophyta) assemblages. Fottea 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Chen, K.; Sun, D.; Rajper, A.R.; Mulatibieke, M.; Hughes, R.M.; Pan, Y.; Tayibazaer, A.; Chen, Q.; Wang, B. Concordance in biological condition and biodiversity between diatom and macroinvertebrate assemblages in Chinese arid-zone streams. Hydrobiologia 2019, 829, 245–263. [Google Scholar] [CrossRef]
- Lewin, I.; Jusik, S.; Szoszkiewicz, K.; Czerniawska-Kusza, I.; Ławniczak, A.E. Application of the new multimetric MMI_PL index for biological water quality assessment in reference and human-impacted streams (Poland, the Slovak Republic). Limnologica 2014, 49, 42–51. [Google Scholar] [CrossRef]
- Sowa, A.; Krodkiewska, M.; Halabowski, D.; Lewin, I. Response of the mollusc communities to environmental factors along an anthropogenic salinity gradient. Sci. Nat. 2019, 106, 60. [Google Scholar] [CrossRef]
- Gorman, O.T.; Karr, J.R. Habitat Structure and Stream Fish Communities. Ecology 1978, 59, 507–515. [Google Scholar] [CrossRef]
- Przybylski, M.; Głowacki, Ł.; Grabowska, J.; Kaczkowski, Z.; Kruk, A.; Marszał, L.; Zięba, G.; Ziułkiewicz, M. Riverine Fish Fauna in Poland. In Polish River Basins and Lakes—Part II. The Handbook of Environmental Chemistry; Korzeniewska, E., Harnisz, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 195–238. [Google Scholar]
- Buffagni, A.; Erba, S.; Cazzola, M.; Kemp, J.L. The AQEM multimetric system for the southern Italian Apennines: Assessing the impact of water quality and habitat degradation on pool macroinvertebrates in Mediterranean rivers. Hydrobiologia 2004, 516, 313–329. [Google Scholar] [CrossRef]
- Lewin, I.; Czerniawska-Kusza, I.; Szoszkiewicz, K.; Ławniczak, A.E.; Jusik, S. Biological indices applied to benthic macroinvertebrates at reference conditions of mountain streams in two ecoregions (Poland, the Slovak Republic). Hydrobiologia 2013, 709, 183–200. [Google Scholar] [CrossRef]
- Vermaat, J.E.; De Bruyne, R.J. Factors limiting the distribution of submerged waterplants in the lowland River Vecht (The Netherlands). Freshw. Biol. 1993, 30, 147–157. [Google Scholar] [CrossRef]
- Šporka, F.; Pastuchová, Z.; Hamerlík, L.; Dobiašová, M.; Beracko, P. Assessment of running waters (Slovakia) using benthic macroinvertebrates—Derivation of ecological quality classes with respect to altitudinal gradients. Biologia 2009, 64, 1196–1205. [Google Scholar] [CrossRef]
- Hughes, S.J.; Santos, J.M.; Ferreira, M.T.; Caraça, R.; Mendes, A.M. Ecological assessment of an intermittent Mediterranean river using community structure and function: Evaluating the role of different organism groups. Freshw. Biol. 2009, 54, 2383–2400. [Google Scholar] [CrossRef]
- Haury, J.; Peltre, M.-C.; Trémolières, M.; Barbe, J.; Thiébaut, G.; Bernez, I.; Daniel, H.; Chatenet, P.; Haan-Archipof, G.; Muller, S.; et al. A new method to assess water trophy and organic pollution—The Macrophyte Biological Index for Rivers (IBMR): Its application to different types of river and pollution. Macrophytes Aquat. Ecosyst. Biol. Manag. 2006, 190, 153–158. [Google Scholar]
- Theodoropoulos, C.; Karaouzas, I.; Vourka, A.; Skoulikidis, N. ELF—A benthic macroinvertebrate multi-metric index for the assessment and classification of hydrological alteration in rivers. Ecol. Indic. 2020, 108, 105713. [Google Scholar] [CrossRef]
- Feio, M.J.; Hughes, R.M.; Callisto, M.; Nichols, S.J.; Odume, O.N.; Quintella, B.R.; Kuemmerlen, M.; Aguiar, F.C.; Almeida, S.F.P.; Alonso-EguíaLis, P.; et al. The Biological Assessment and Rehabilitation of the World’s Rivers: An Overview. Water 2021, 13, 371. [Google Scholar] [CrossRef]
- Assefa, W.W.; Eneyew, B.G.; Wondie, A. Development of a multi-metric index based on macroinvertebrates for wetland ecosystem health assessment in predominantly agricultural landscapes, Upper Blue Nile basin, northwestern Ethiopia. Front. Environ. Sci. 2023, 11, 1117190. [Google Scholar] [CrossRef]
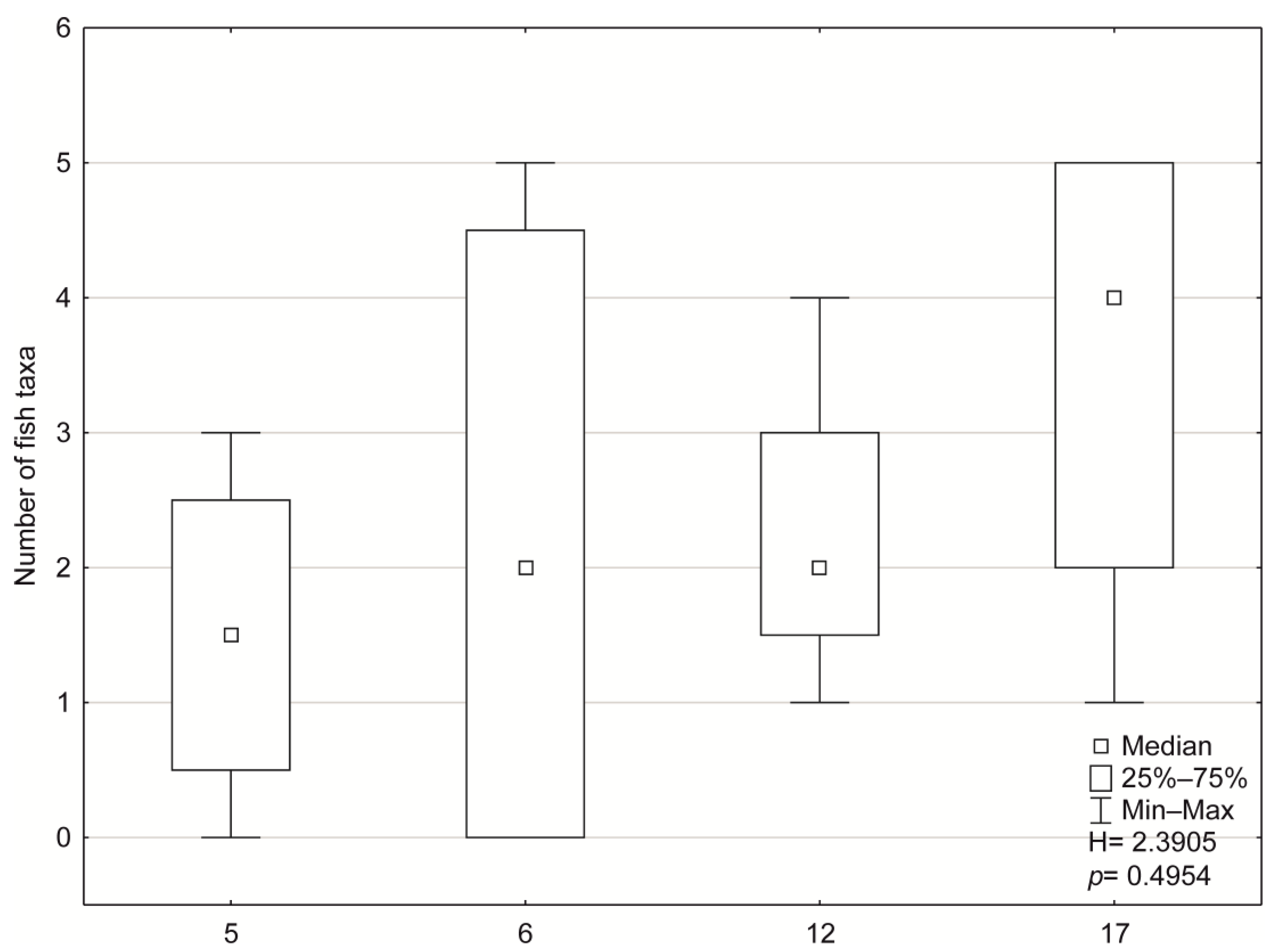
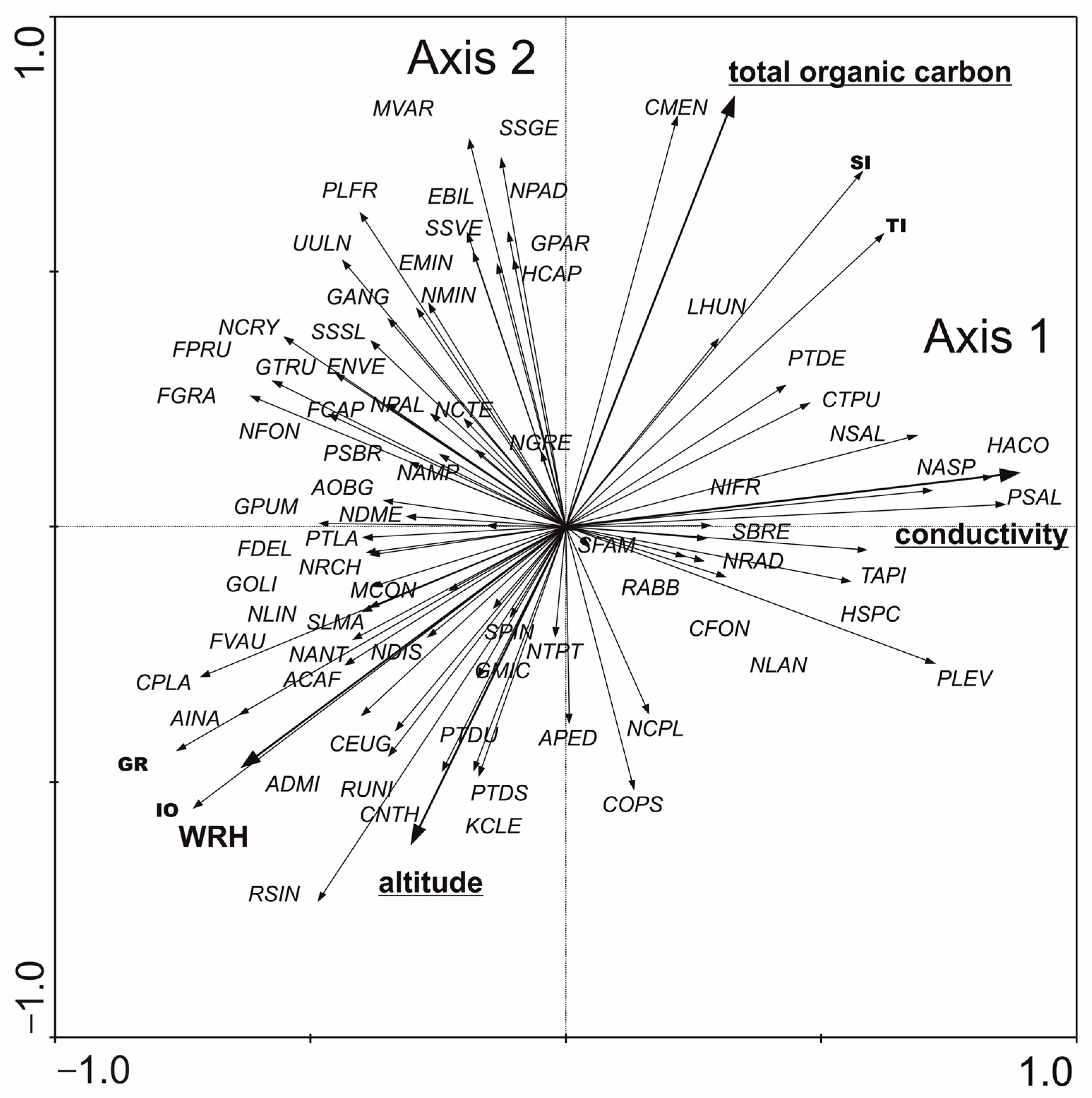
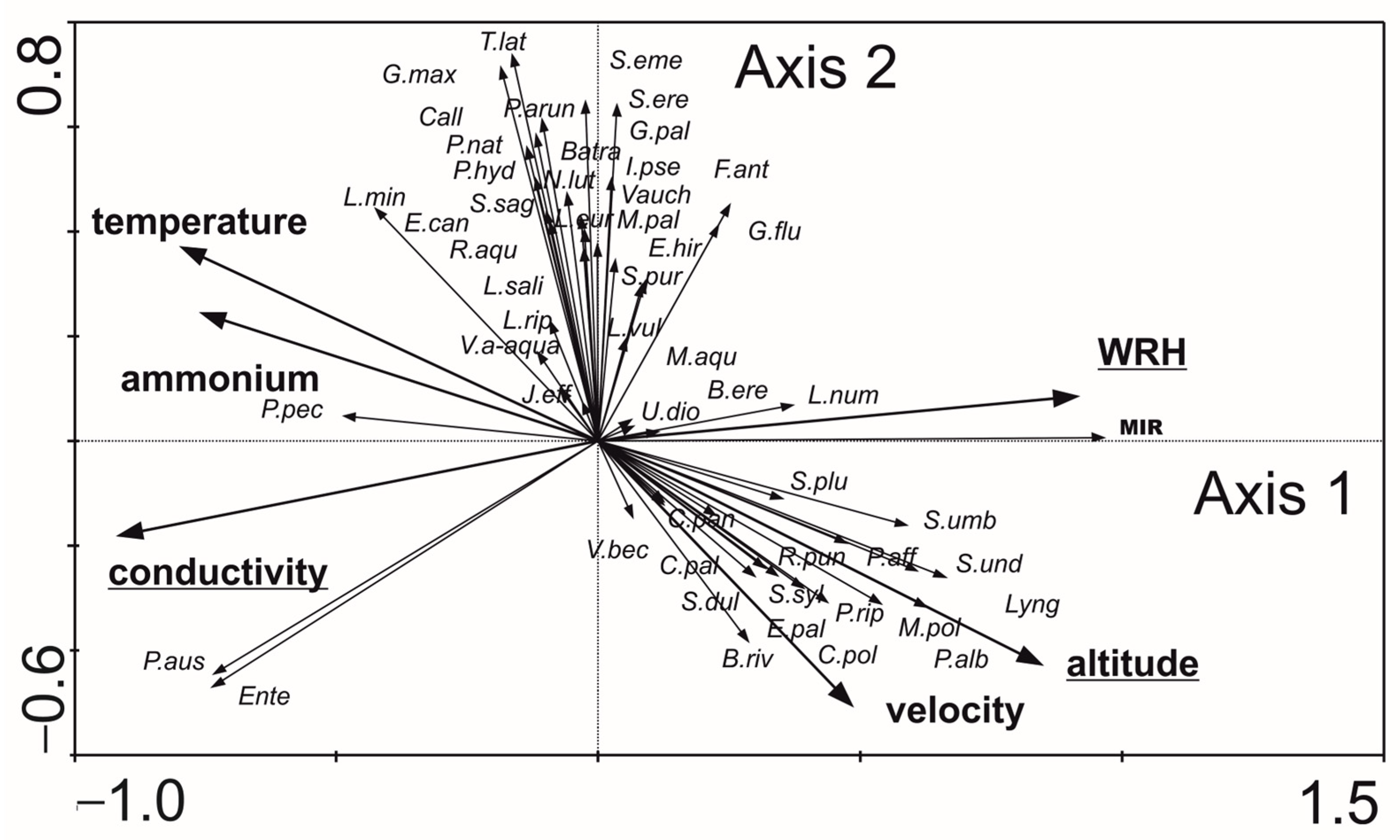
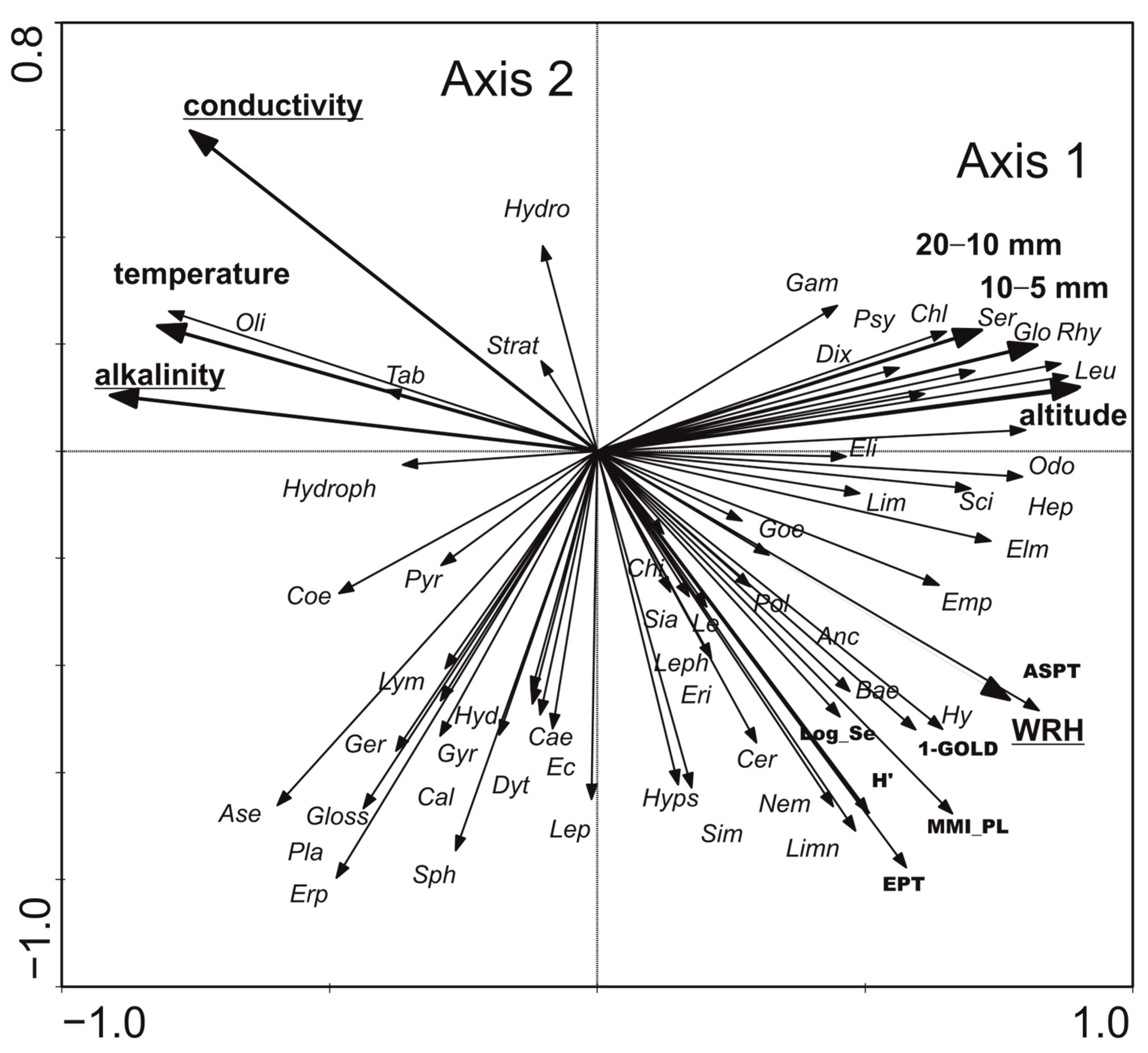
| Characteristic | Type 5 | Type 6 | Type 12 | Type 17 | ||||
|---|---|---|---|---|---|---|---|---|
| Selected rivers | Bolina | Centuria | Mitręga | Mleczna | Dziechcinka | Vistula | Korzenica | Wiercica |
| Geographical coordinates of the sampling sites | 50°14.742 N, 19°06.078 E; 50°13.793 N, 19°05.142 E | 50°21.920 N, 19°29.682 E; 50°24.879 N, 19°29.190 E | 50°26.070 N, 19°17.956 E; 50°24.797 N, 19°22.779 E | 50°07.018 N, 19°04.487 E; 52°09.754 N, 19°00.213 E | 49°38.789 N, 18°52.025 E; 49°38.021 N, 18°50.828 E | 49°38.728 N, 18°51.167 E; 49°37.190 N, 18°59.160 E | 50°01.850 N, 19°05.839 E; 50°03.509 N, 18°56.804 E | 50°52.471 N, 19°26.133 E; 50°41.117 N, 19°24.472 E |
| Main anthropogenic pressure in the upper course of the river | Salinisation from saline coal mine discharges, and river channel regulation | None | Dam reservoir, municipal sewage | Industrial and municipal sewage, and river regulation | None | None | Fish ponds and agricultural activity, municipal sewage | None |
| Main anthropogenic pressure in the lower course of the river | Salinisation from saline coal mine discharges, industrial and municipal wastewater, and river channel regulation | Organic pollution from agriculture and livestock grazing, as well as inputs from fish ponds | Dam reservoir, municipal sewage, river channel regulation | Salinisation from saline coal mine waters, industrial and municipal sewage, and river regulation | Riverbed regulation | Riverbed regulation, dam reservoir, municipal sewage | Fish ponds and agricultural activity | Agriculture, livestock grazing, and dam reservoirs |
| Parameter | Type 5 | Type 6 | Type 12 | Type 17 | p-Value |
|---|---|---|---|---|---|
| Altitude [m a.s.l.] | 257–343 c | 236–317 c | 415–748 a,b,d | 215–309 c | 0.0004 |
| Stream gradient [‰] | 3.00–10.89 d | 2.83–3.98 c | 45.59–177.50 b,d | 2.00–3.92 a,c | 0.0000 |
| Width of the river bed [m] | 3.600–7.480 | 2.636–8.100 | 3.247–12.450 | 1.190–13.100 | 0.1979 |
| Depth of the river bed [m] | 0.140–0.840 b | 0.206–1.375 a | 0.190–0.664 | 0.006–1.150 | 0.1116 |
| Flow velocity [m s−1] | 0.055–0.722 | 0.023–0.435 c | 0.107–0.897 b | 0.040–0.510 | 0.1979 |
| Temperature [°C] | 10.40–24.50 c | 16.50–20.70 c | 10.00–14.00 a,b | 10.70–20.20 | 0.0000 |
| pH | 6.20–8.70 | 6.60–8.90 | 5.90–9.00 | 6.60–8.50 | 0.3884 |
| Salinity [PSU] | 0.17–25.70 c | 0.25–10.58 c | 0.02–0.08 a,b,d | 0.17–0.29 | 0.0000 |
| Conductivity [μS cm−1] | 200–35,700 c | 330–14,690 c | 20–110 a,b,d | 220–371 c | 0.0000 |
| Total dissolved solids [mg dm−3] | 100–17,840 c | 160–7350 c | 10–50 a,b,d | 100–187 c | 0.0000 |
| Chlorides [mg dm−3] | 10–16,180 c | 18–4060 c | 4–10 a,b,d | 10–23 c | 0.0000 |
| Dissolved oxygen [mg dm−3] | 3.13–5.77 | 1.58–6.24 c | 3.87–5.36 b | 3.30–5.29 | 0.0054 |
| Biochemical Oxygen Demand (BOD) [mg dm−3] | 7–23 | <3–4 | <3–<3 | <3–<3 | 0.2112 |
| Sulfates [mg dm−3] | 40–780 c,d | 35–370 c,d | 11–20 a,b | 12–56 a,b | 0.0000 |
| Iron [mg dm−3] | 0.01–0.91 | 0.14–1.97 c | 0.00–0.60 b,d | 0.01–2.17 c | 0.0048 |
| Ammonium [mg dm−3] | 0.00–1.83 | 0.15–2.39 c | 0.00–0.30 b | 0.00–1.89 | 0.0089 |
| Nitrites [mg dm−3] | 0.000–2.687 c | 0.012–2.702 c | 0.000–0.020 a,b | 0.000–0.250 | 0.0001 |
| Nitrates [mg dm−3] | 0.00–57.59 | 0.00–78.41 | 1.33–8.42 | 0.89–29.68 | 0.4459 |
| Total nitrogen [mg dm−3] | 1.9–4.1 | 1.5–5.9 | 0.81–1.10 | 1.2–4.1 | 0.1116 |
| Phosphates [mg dm−3] | 0.00–0.36 b | 0.18–7.10 a,c | 0.00–0.35 b | 0.00–0.76 | 0.0040 |
| Total phosphorus [mg dm−3] | 0.100–0.240 | 0.065–0.460 | 0.064–0.190 | 0.140–0.180 | 0.5724 |
| Total Organic Carbon (TOC) [mg dm−3] | 2.5–3.5 | 7.8–11.0 c | <2.0–4.0 b | <2.0–8.1 | 0.1116 |
| Total hardness [mg CaCO3 dm−3] | 145–3700 c | 155–1339 c | 22–66 a,b,d | 95–315 c | 0.0005 |
| Alkalinity [mg CaCO3 dm−3] | 95–395 c | 120–305 c | 0.80–40 a,b,d | 75–300 c | 0.0001 |
| Calcium [mg dm−3] | 40–696 c | 50–632 c | 4–22 a,b,d | 28–80 c | 0.0004 |
| Magnesium [mg dm−3] | 0.12–476.64 c | 0.73–157.63 c | 0.12–8.25 a,b | 0.61–76.26 | 0.0001 |
| Organic matter [%] | 0.55–20.51 | 0.83–30.46 | 1.75–3.93 | 0.64–1.43 | 0.1352 |
| HIR | 0.37–0.87 | 0.34–0.56 | 0.42–0.93 | 0.49–0.88 | 0.5724 |
| WPH | 1.0–52.5 | 51.5–56.5 | 5.5–79.5 | 13.5–47.0 | 0.0460 |
| WRH | 33.5–73.0 | 31.0–69.0 | 65.0–88.5 | 49.5–87.5 | 0.1116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halabowski, D.; Lewin, I.; Bąk, M.; Płaska, W.; Rosińska, J.; Rechulicz, J.; Dukowska, M. Ecological Assessment of Rivers Under Anthropogenic Pressure: Testing Biological Indices Across Abiotic Types of Rivers. Water 2025, 17, 1817. https://doi.org/10.3390/w17121817
Halabowski D, Lewin I, Bąk M, Płaska W, Rosińska J, Rechulicz J, Dukowska M. Ecological Assessment of Rivers Under Anthropogenic Pressure: Testing Biological Indices Across Abiotic Types of Rivers. Water. 2025; 17(12):1817. https://doi.org/10.3390/w17121817
Chicago/Turabian StyleHalabowski, Dariusz, Iga Lewin, Małgorzata Bąk, Wojciech Płaska, Joanna Rosińska, Jacek Rechulicz, and Małgorzata Dukowska. 2025. "Ecological Assessment of Rivers Under Anthropogenic Pressure: Testing Biological Indices Across Abiotic Types of Rivers" Water 17, no. 12: 1817. https://doi.org/10.3390/w17121817
APA StyleHalabowski, D., Lewin, I., Bąk, M., Płaska, W., Rosińska, J., Rechulicz, J., & Dukowska, M. (2025). Ecological Assessment of Rivers Under Anthropogenic Pressure: Testing Biological Indices Across Abiotic Types of Rivers. Water, 17(12), 1817. https://doi.org/10.3390/w17121817








