Abstract
High turbidity in raw water poses a major challenge to drinking water quality and requires effective, sustainable treatment solutions. This work investigates the reduction in turbidity in raw water and the enhancement of overall drinking water quality through the coagulation–flocculation process. The performance of Pine cone extract as a bio-coagulant was evaluated using four different solvent-based extractions (PC-H2O, PC-HCl, PC-NaCl, and PC-NaOH). The effects of key operational parameters were analyzed, and jar tests were carried out to enhance the coagulation–flocculation process by identifying the optimal conditions. Experimental design was further refined using RSM based on a BBD, incorporating three factors: initial pH, coagulant dosage, and settling time, with turbidity removal efficiency as the response variable. Statistical analysis confirmed that initial pH, coagulant dosage, and settling time significantly influenced turbidity reduction at a confidence level of p-value < 0.05 for all four solvents. Among the extracts tested, PC-HCl demonstrated the highest turbidity removal efficiency. The optimal conditions achieving 78.57% turbidity reduction were a pH of 8.5, a coagulant dosage of 100 mL/L, and a settling time of 120 min. These findings highlight the significant potential of Pine cone extract as an effective, sustainable, and eco-friendly organic coagulant for raw water treatment.
1. Introduction
Drinking water is vital for human survival and having access to clean and safe water is a basic human right, regardless of nationality, religion, race, or socio-economic status. Polluted water and poor sanitation are major contributors to the spread of diseases such as cholera, diarrhea, dysentery, and polio, highlighting the significant impact of water quality on public health [1,2]. The treatment of surface or groundwater to produce potable water typically involves multiple steps to remove harmful substances. When surface water is used as the raw source, turbidity removal is often a critical part of the process. To ensure that clean water is accessible to as many people as possible, affordable, simple, and efficient treatment methods are required [3,4].
Various treatment technologies are employed to process raw water into potable water and to treat water and wastewater before it is released into natural water sources [5]. The coagulation–flocculation process is an integral stage in most water and wastewater treatment processes. These methods are widely used for their efficiency in eliminating suspended particles, organic matter, color, and turbidity from water [5,6,7]. The coagulation process uses inorganic coagulants to destabilize dispersed organic matter by forming microflocs, which then aggregate into larger flocs and settle out of the water by gravity [8,9]. However, the use of chemical coagulants presents several drawbacks, including high operational costs, reduced efficiency in cold water, the generation of large sludge volumes, significant impacts on the pH of treated water, and potential health risks, such as a link to Alzheimer’s disease [10,11]. In contrast, the use of natural, plant-based coagulants, such as fruit peels, seeds, and other agricultural by-products, provides a sustainable and eco-friendly alternative, decreasing reliance on chemical coagulants and lessening the environmental impact associated with chemically produced sludge disposal [8,12,13,14].
Recent research has increasingly focused on sustainable and environmentally eco-friendly natural coagulants as alternatives to chemical and synthetic coagulants for drinking water treatment [15,16,17]. Various bio-coagulants have been investigated, including aloe vera, as studied by Benalia et al. [18], as well as Moringa oleifera, Strychnos potatorum, Phaseolus vulgaris [19], chitosan [20,21], and Acorn leaves have also been explored for their coagulation properties [22]. However, to the best of our knowledge, no previous study has investigated the potential use of Pine cone-based coagulants for drinking water treatment, despite their richness in bioactive compounds such as polyphenols, polysaccharides, lignin, and proteins [6,23,24], which are known to play key roles in coagulation mechanisms like charge neutralization, adsorption, and bridging flocculation.
This study investigates the use of Pine cone extract, specifically from Pinus nigra, a species abundant in the Mediterranean region, as a novel and renewable bio-coagulant for drinking water treatment. To enhance the extraction of active compounds, four solvents were selected based on their extraction efficiency and suitability for simple extraction methods. Distilled water was used as a neutral and accessible medium to extract water-soluble compounds. Salt extraction using NaCl is known to effectively isolate proteins that can act as polyelectrolytes during the coagulation process [25]. Alkaline extraction with NaOH was applied to solubilize lignin and polysaccharides [26], while acidic extraction with HCl was employed to release polyphenols and bound carbohydrates [13]. These solvents offer the advantage of being low-cost, low-toxicity, and suitable for low-energy processes. The performance of the resulting extracts, namely PC-H₂O, PC-NaCl, PC-NaOH, and PC-HCl, was evaluated based on their ability to reduce turbidity in raw water collected from the Oued El Athmania drinking water treatment plant. A Response Surface Methodology (RSM) using the Box–Behnken Design (BBD) was applied to optimize key operational parameters, namely pH, coagulant dosage, and settling time, with turbidity removal as the main response variable. The performance of the four extracts was then compared based on their turbidity removal efficiency. This work aims to provide a scientific basis for the valorization of Pine cones as an accessible, cost-effective, and sustainable resource for water treatment.
2. Materials and Methods
2.1. Raw Water Collection and Analysis
The raw water for this study was sourced from the Oued El Athmania drinking water treatment plant in Algeria (coordinates: 36°49′34.89″ N, 6°31′11.81″ E). The physicochemical properties of the raw water, along with the regulatory quality limits set by Algerian water standards, are summarized in Table 1.

Table 1.
Raw water characterization.
2.2. Analytical Methods
Turbidity was assessed with a turbidimeter (Turbidimeter HI 98713-02-ISO 7027-Hanna instruments, Cluj-Napoca, Romania). Total hardness and alkalinity were determined using standard titrimetric methods. Measurements of pH, temperature, electrical conductivity, and salinity were carried out with a multi-parameter instrument (Jenway Model 3540, Camlab, Cambridge, UK). Total suspended solids (TSSs) in the effluent were measured by filtration using Whatman filter paper, followed by drying at 105 °C for 2 h. The solid content was determined by measuring the difference between the sludge initial weight and its dry weight after drying in an oven at 105 °C for 24 h. Aromatic organic matter was identified by UV–vis spectrophotometry (SHIMADZU UV 1201 model, Kyoto, Japan), with absorbance measured at 254 nm (UV254). All reagents were of high-purity grade.
2.3. Bio-Coagulant Preparation
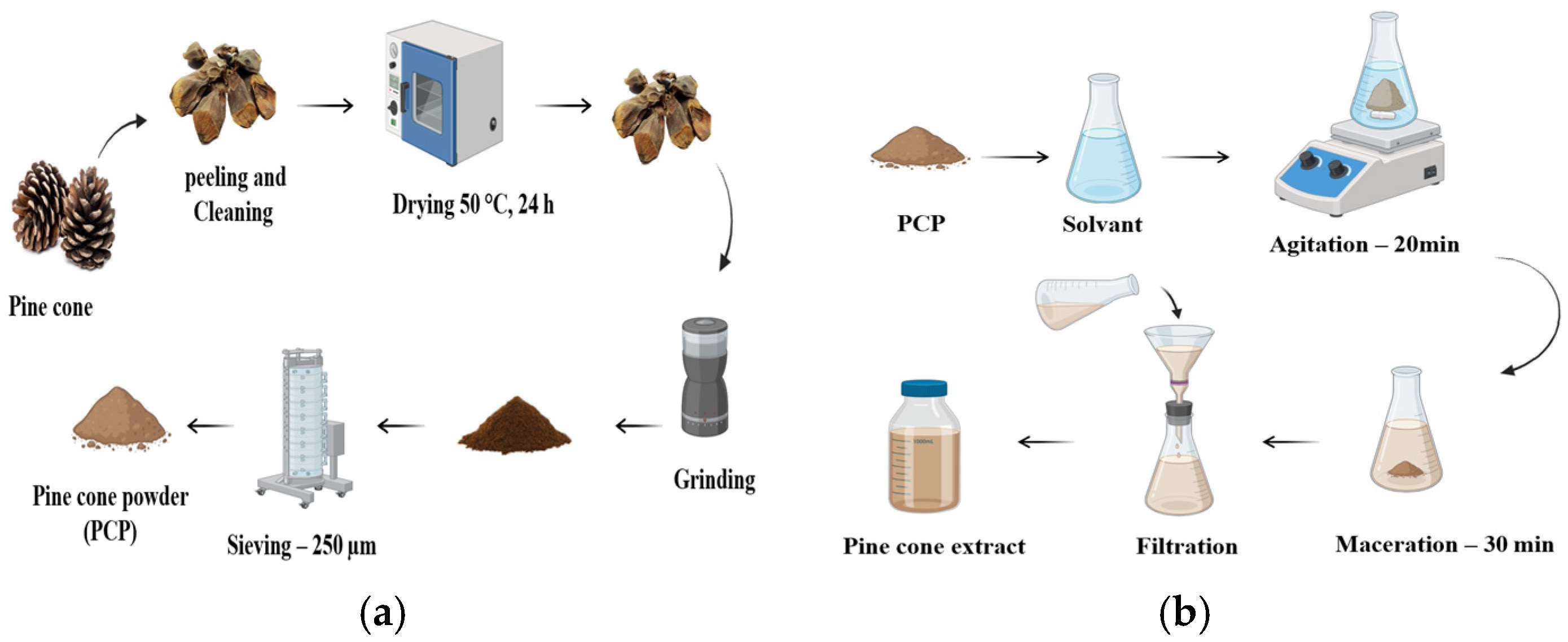
Pine cones were sourced from fields in Mila, Algeria. The preparation process for Pine cone powder (PCP) is outlined in Figure 1a. Initially, the cones were manually selected and peeled to remove unwanted materials. They were then dried in an oven at 50 °C ± 1 °C for 24 h to reduce moisture content. Once dried, the cones were finely ground using a domestic grinder and subsequently sieved through a 250 μm laboratory sieve to obtain a uniform powder.
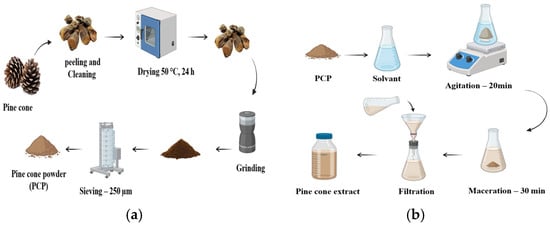
Figure 1.
Preparation of the bio-coagulant: (a) Steps for the preparation of Pine cone powder, (b) Steps for the extraction of Pine cone extracts using different solvents: H2O, NaCl (0.5 M), NaOH (0.05 M), and HCl (0.05 M).
The extraction was performed according to the steps shown in Figure 1b. A total of 5 g of prepared PCP was stirred with 1000 mL of each solvent: distilled water, NaCl (0.5 M), NaOH (0.05 M), and HCl (0.05 M), to obtain the corresponding aqueous extracts. The mixtures were stirred at 400 rpm for 20 min, followed by 30 min of maceration. The resulting suspensions were then filtered through standard filter paper with a porosity of less than 8 µm [23,24]. The resulting clear filtrates, stored at 4 °C, were labeled as PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl based on the solvent used.
For this investigation, sodium chloride (NaCl), sodium hydroxide (NaOH), and hydrochloric acid (HCl) were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
2.4. FTIR Analysis
Fourier Transform Infrared (FTIR) spectroscopy is a method used to analyze molecular structures by identifying functional groups and chemical bonds. It enables the identification of key functional groups that play a significant role in coagulation activities. The analysis of the absorption spectrum of Pine cone powder and extracts were obtained by using FTIR (Shimadzu, IRAffinity-1S, Kyoto, Japan).
2.5. Design of Experiment
A BBD was implemented using Minitab 21.0 software (Minitab, LLC, State College, PA, USA) as part of the RSM approach to optimize water treatment efficiency, focusing on turbidity removal and the reduction in physicochemical parameters that deviate from drinking water standards. Three key factors were selected to evaluate their impact on treatment performance: coagulant dose (X1), initial pH (X2), and settling time (X3) (see Table 2). The dependent variable, representing the response, was turbidity removal efficiency. These parameters were used to calculate removal efficiencies after each trial. Using RSM, a quadratic polynomial equation was developed to predict the response (treatment efficiency) based on the independent variables and their interactions (Equation (1)). The experimental design included 15 trials: 12 main trials and three replicates at the central point of the design.

Table 2.
The coded and actual levels of variables in BBD.
In this model, Yi represents the response (turbidity removal); K denotes the number of factors (K = 4); β0 is a constant term; while βi, βii, and βij correspond to the regression coefficients; and Xi represents the investigated parameters [6,28,29].
2.6. Experiments (Jar Test Assays)
Jar test experiments (Jar test Selecta-Flucomatica, Barcelona, Spain) were employed to evaluate the performance of the coagulation–flocculation process. Experiments were performed with a six-position stirrer and six beakers, each containing 500 mL of raw water samples. The pH was modified to the required level (Table 2) by adding 1 M hydrochloric acid or sodium hydroxide solutions. After adding the respective coagulant doses for the four extracts studied (PC-H2O, PC-HCl, PC-NaOH, PC-NaCl) as indicated in Table 2, the mixtures were agitated at 160 rpm for a duration of 3 min. The stirring speed was then reduced to 30 rpm for 20 min [30], followed by a settling period, the duration of which varied according to Table 2. The supernatant was collected from 2 cm below the surface [31] and analyzed to measure turbidity. The turbidity removal efficiency was then determined using Equation (2) [6].
where TRE is the turbidity removal efficiency (%), IT is the initial turbidity (NTU), and RT is the residual turbidity (NTU).
After determining the optimal conditions for each bio-coagulant (PC-H2O, PC-HCl, PC-NaOH, PC-NaCl), the treated water was analyzed for key parameters, including turbidity, pH, conductivity, salinity, total alkalinity (TAC), total hardness (TH), UV254, and total dissolved solids (TSSs).
2.7. Statistical Analysis
To assess the quality of the quadratic polynomial models, the R2 and adjusted R2, statistical tests (F and p-values), and normality plots were analyzed. A high F-value suggests that the regression model explains the response variability, while a p-value below 0.05 confirms the model’s statistical significance. R2 and adjusted R2 closer to 1 demonstrate better model fit [32,33,34]. The significance of regression coefficients was assessed using F and P-tests at a 95% confidence level [33,35,36].
3. Results and Discussion
3.1. Fourier Transform Infrared (FTIR) Spectroscopy
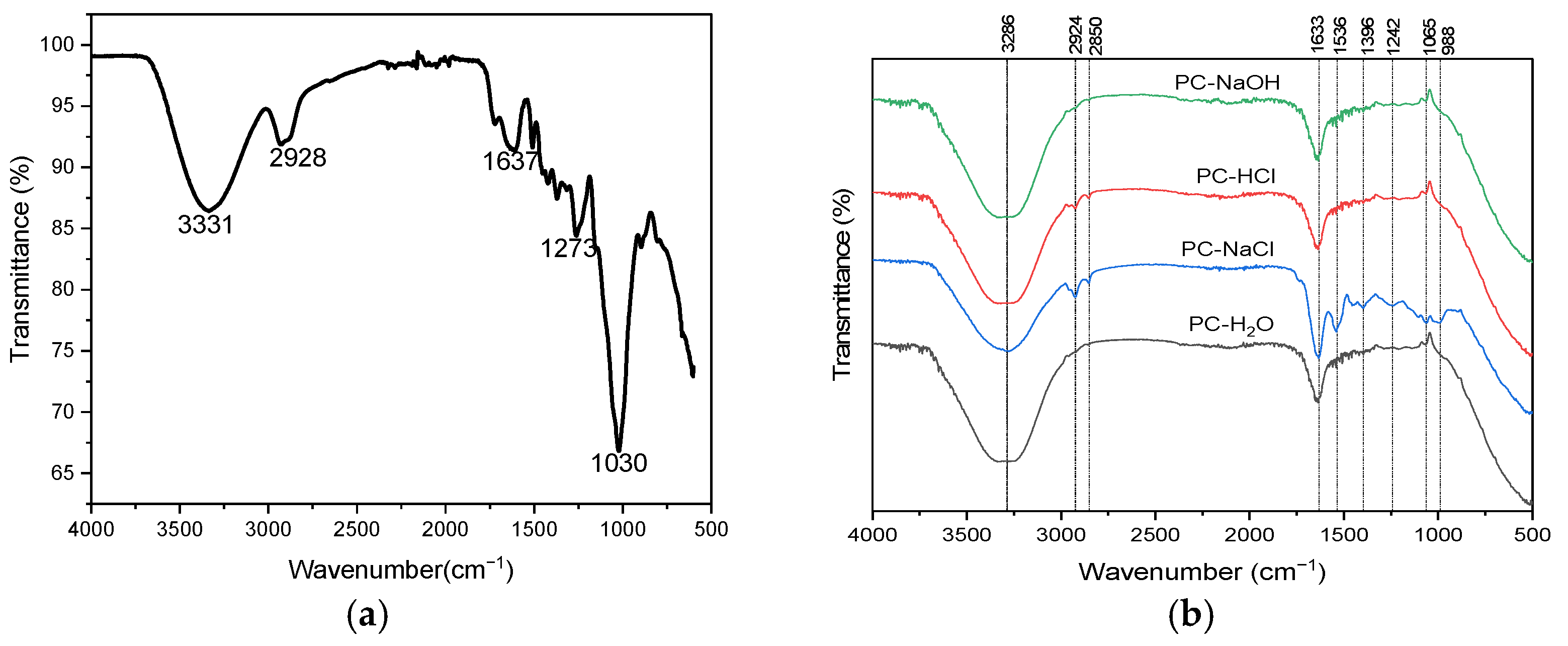
FTIR analysis was conducted to identify the functional groups in raw Pine cone powder and to evaluate their presence in the different extracts (PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl). This comparison aimed to confirm the extraction of key bioactive compounds such as proteins, polyphenols, and polysaccharides by detecting characteristic absorption bands of hydroxyl, carboxyl, and amide groups, which are linked to their coagulating activity.
Figure 2a shows the FTIR spectrum of the Pine cone powder. An intense absorption band is observed at 3331 cm⁻1, corresponding to the stretching vibrations of O-H functional groups (carboxylic acids, alcohols, or phenols) or associated with amine groups (NH) [37,38,39]. The CH group is detected at 2928 cm⁻1 [40]. The peak at 1637 cm⁻1 is most likely associated with carboxyl (C=O) functional groups [41]. The band at 1273 cm⁻1 indicates the presence of a COO stretching vibration, confirming the presence of functional groups such as carboxyl, alcohol, ether, or ester [23,41]. Additionally, the FTIR spectrum of the raw Pine cone confirms the probable presence of an ester (CO) functional group, as indicated by the pronounced peak at 1030 cm⁻1 [31].
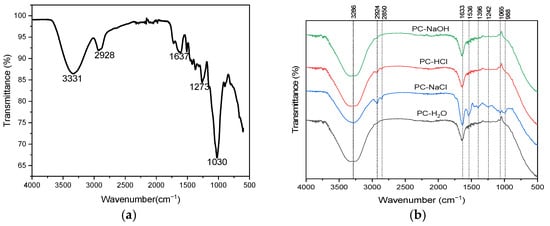
Figure 2.
FTIR spectra of (a) Pine cone powder and (b) Pine cone extracts: PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl.
Figure 2b presents the FTIR spectrum of the four Pine cone extracts (PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl). The spectra reveal similar absorption bands with varying intensities, indicating the presence of distinct molecules such as proteins, polyphenols, and polysaccharides extracted from the Pine cone. The figure presents a diverse range of broad infrared absorption bands positioned within the following spectral regions: 3039–3645 cm⁻1, 2792–3024 cm⁻1, and 906–1770 cm⁻1. A wide band centered at 3286 cm⁻1 corresponds to the stretching of O-H groups, commonly found in fatty acids, proteins, and polysaccharides within the different extracts [39,42]. The involvement of N-H stretching from amide groups is also observed in this region, primarily in the PC-NaCl extract, with lower intensity in PC-HCl, and its absence in the PC-NaOH and PC-H2O extracts. The peaks at 2924 cm⁻1 and 2850 cm⁻1 are associated with the symmetric and asymmetric stretching of C-H groups from CH₂, which are characteristic of fatty acids [43]. In the region between 1485 and 1770 cm⁻1, two intense bands are attributed to the stretching of the C=O bond, characteristic of carbonyl functions (primary amides). In this case, the spectra reveal a band at 1633 cm⁻1 associated with fatty acids, aromatic groups, phenol groups, and polysaccharides [31]. In the spectrum of the PC-NaCl extract, a band at 1536 cm⁻1 is observed, associated with the amide group of proteins and the aromatic groups of polyphenols. Three weak peaks are noted in the PC-NaCl extract: a peak at 1396 cm⁻1 attributed to the C-N group of aromatic primary amines, a peak at 1242 cm⁻1 corresponding to the stretching of the carboxylic acid COO bond, confirming the presence of ether, carboxyl, ester or alcohol functional groups, and finally, a weak peak at 1065 cm⁻1, which could be attributed to an ester CO group [31,39,44].
In summary, comparing the FTIR spectra of the raw Pine cone powder with those of the extracts clearly shows that the extraction process managed to recover the main bioactive functional groups. The presence of hydroxyl, carboxyl, and amide groups in the extracts confirms that important compounds like proteins, polysaccharides, and polyphenols originally found in the raw material were successfully extracted. These groups are essential because they provide active sites that help destabilize colloidal particles, which is a key step in removing pollutants during the coagulation–flocculation process [45].
3.2. Optimization and Modeling of the Turbidity of Drinking Water
Table 3 presents the results of the 15 analyses conducted according to the experimental plan defined by the Minitab software. The table shows the levels assigned to each studied variable, as well as the predicted and experimental values obtained for the turbidity reduction rate.

Table 3.
Results of the RSM methodology in terms of turbidity removal efficiency for the extracts: PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl.
According to the data presented in Table 3, it is observed that PC-H2O demonstrated remarkable turbidity removal efficiency, achieving 70.36%. These results were obtained under optimal conditions: a coagulant dose of 10 mL/L, a pH of 6.5, and a sedimentation time of 75 min. Using a concentration of 55 mL/L of PC-NaCl, with a pH of 6.5 and a sedimentation time of 120 min, a maximum turbidity reduction of 48.15% was observed. For PC-NaOH, the highest turbidity removal was 64.88%, with an optimal dose of 55 mL/L, an optimal pH of 7.5, and a sedimentation time of 120 min. Regarding PC-HCl, a maximum turbidity removal of 78.78% was achieved, with a dose of 100 mL/L, a pH of 7.5 and a sedimentation time of 120 min. These results indicate that PC-HCl proved to be the most effective in turbidity removal among the extracts tested.
The complete quadratic regression models for coagulation, using the four extracts PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl, are presented in coded form in Equations (3)–(6), respectively.
Turbidity removal (PC-H2O (%)) = 58.195 − 0.534 X1 − 4.298 X2 − 1.216X3 + 4.84X12
+ 2.089 X22 + 0.251X32 − 0.344X1X2 − 4.256 X1X3 + 0.334 X2X3.
+ 2.089 X22 + 0.251X32 − 0.344X1X2 − 4.256 X1X3 + 0.334 X2X3.
Turbidity removal (PC-NaCl (%)) = 37.35 + 0.753 X1 − 2.214 X2 + 6.145X3 − 6.256X12
+ 2.522 X22 + 1.852X32 + 2.007X1X2 + 1.337 X1X3 + 1.084 X2X3.
+ 2.522 X22 + 1.852X32 + 2.007X1X2 + 1.337 X1X3 + 1.084 X2X3.
Turbidity removal (PC-NaOH (%)) = 49.05 − 11.66 X1 − 2.72 X2 + 22.99X3 − 11.19X12
− 18.05 X22 − 1.24X32 + 0.75X1X2 − 1.84 X1X3 +2.68 X2X3.
− 18.05 X22 − 1.24X32 + 0.75X1X2 − 1.84 X1X3 +2.68 X2X3.
Turbidity removal (PC-HCl (%)) = 69.764 + 1.259 X1 + 0.305
X2 + 6.06X3 − 1.002X12 + 0.604 X22 + 0.292X32 + 1.121X1X2 + 2.617 X1X3 − 0.107 X2X3.
X2 + 6.06X3 − 1.002X12 + 0.604 X22 + 0.292X32 + 1.121X1X2 + 2.617 X1X3 − 0.107 X2X3.
The negative sign in Equations (2)–(5) denotes an antagonistic effect, whereas the positive sign represents a synergistic effect. It is clear from these equations that the individual operating variable, namely the coagulant dosage for the H2O and NaOH extracts, has a negative effect on turbidity removal, while for the NaCl and HCl extracts, the coagulant dosage has a net positive effect. Regarding the individual variable of pH, a negative effect on turbidity removal is observed for the H2O, NaOH, and NaCl extracts. In contrast, for the HCl extract, pH has a positive effect. As for the sedimentation time, a positive effect is noted for all extracts except for the H2O extract. Variability is observed for quadratic and interactive effects.
3.3. Analysis of Variance (ANOVA Test)
An ANOVA analysis was performed on the regression model in Equations (3)–(6) and the significance of each term is presented in Table 4.

Table 4.
Analysis of variance (ANOVA) of the fitted models developed for the turbidity removal of the extracts: PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl.
From Table 4, it can be observed that all regression models are statistically significant (p ≤ 0.05) for each of the four extracts analyzed, as well as for all the dependent variables investigated.
The models’ calculated F-values are greater than the critical tabulated F-values (3.4817), confirming their significance [46,47]. Furthermore, elevated mean square (MS) values across all models suggest their statistical significance and suitability for accurate prediction.
A well-fitted regression model is expected to have a computed F value that is at least four times greater than the tabulated F value [48,49]. The four regression models analyzed in this study all demonstrate this relationship.
The models describe the proportion of variation in the response as 98.50%, 97.25%, 94.75%, and 98.29% for PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl, respectively, these are the values of the determination coefficients (R2). The adjusted coefficient of determination values (adjusted R2) for turbidity removal were 95.79%, 92.31%, 85.29%, and 95.22% for PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl, respectively. The adjusted R2 represents the proportion of variation in the response explained by the model, considering the number of predictors in relation to the total observations. As stated by Sabeti et al. [50], a difference of less than 20.0% between R2 and adjusted R2 is considered ideal for assessing the model’s fit, and this is the case in our study.
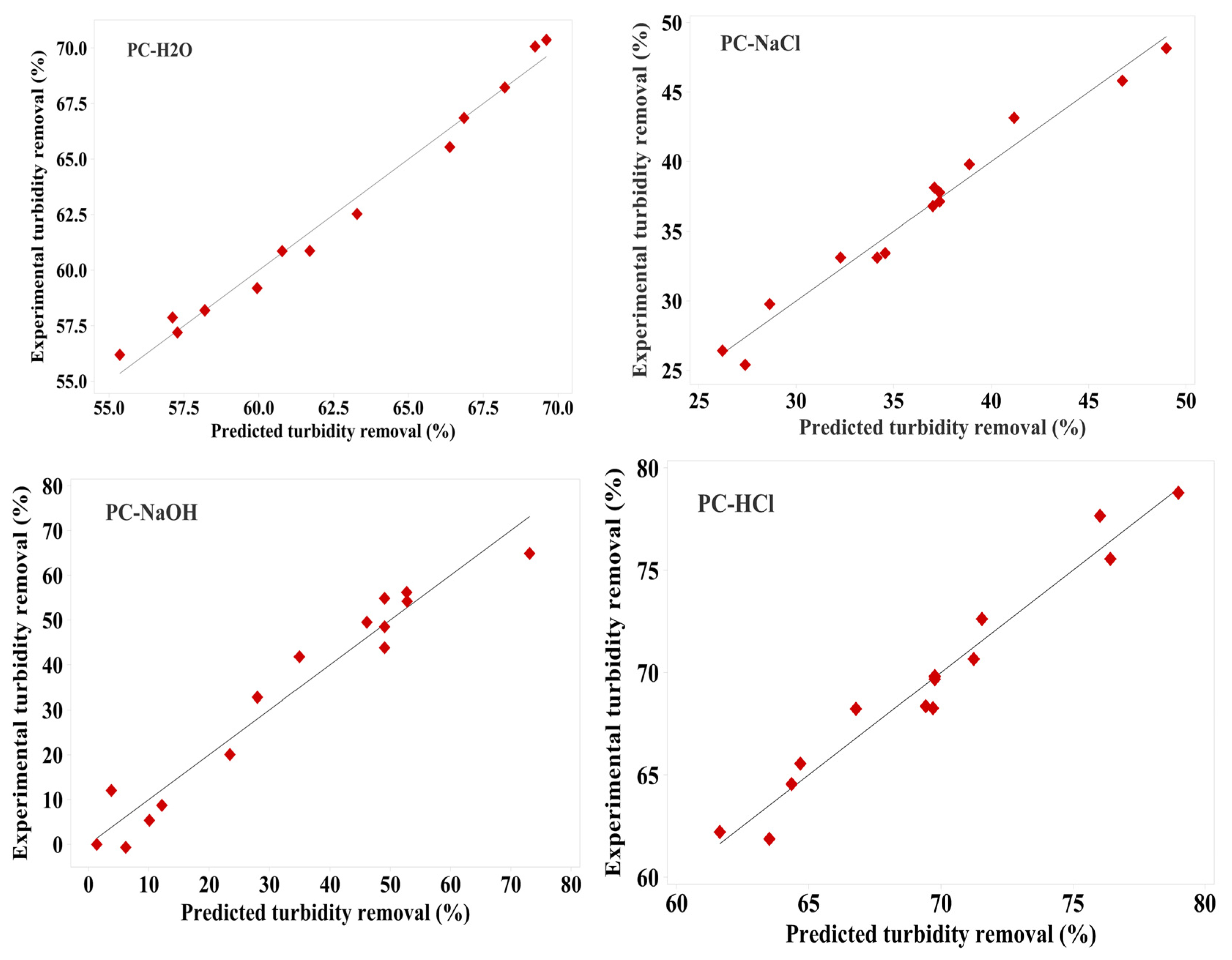
3.4. Evaluation Models
The models’ robustness further validates their predictive capability, as shown by the close clustering of points along the diagonal line, indicating a strong correlation between observed and predicted values [51,52,53]. This correlation is demonstrated in Figure 3. These figures illustrate the relationship between the actual and predicted values of turbidity removal from simulated drinking water using the four Pine cone extracts: PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl. The observed values represent the original turbidity removal measurement in solution, obtained experimentally, while the predicted values were generated using Equations (3)–(6).
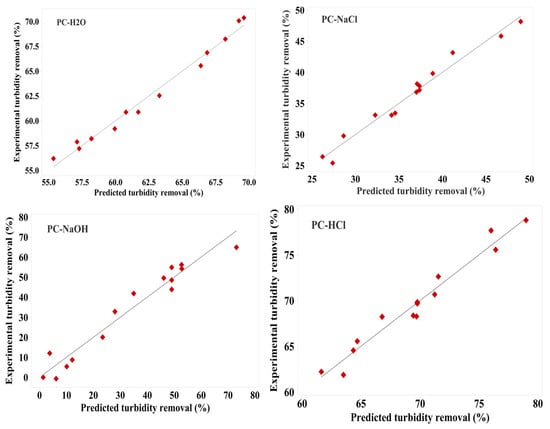
Figure 3.
The predicted and experimental responses for turbidity reduction for the four Pine cone extracts: PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl.
3.5. Effect of the Factors on Turbidity Reduction
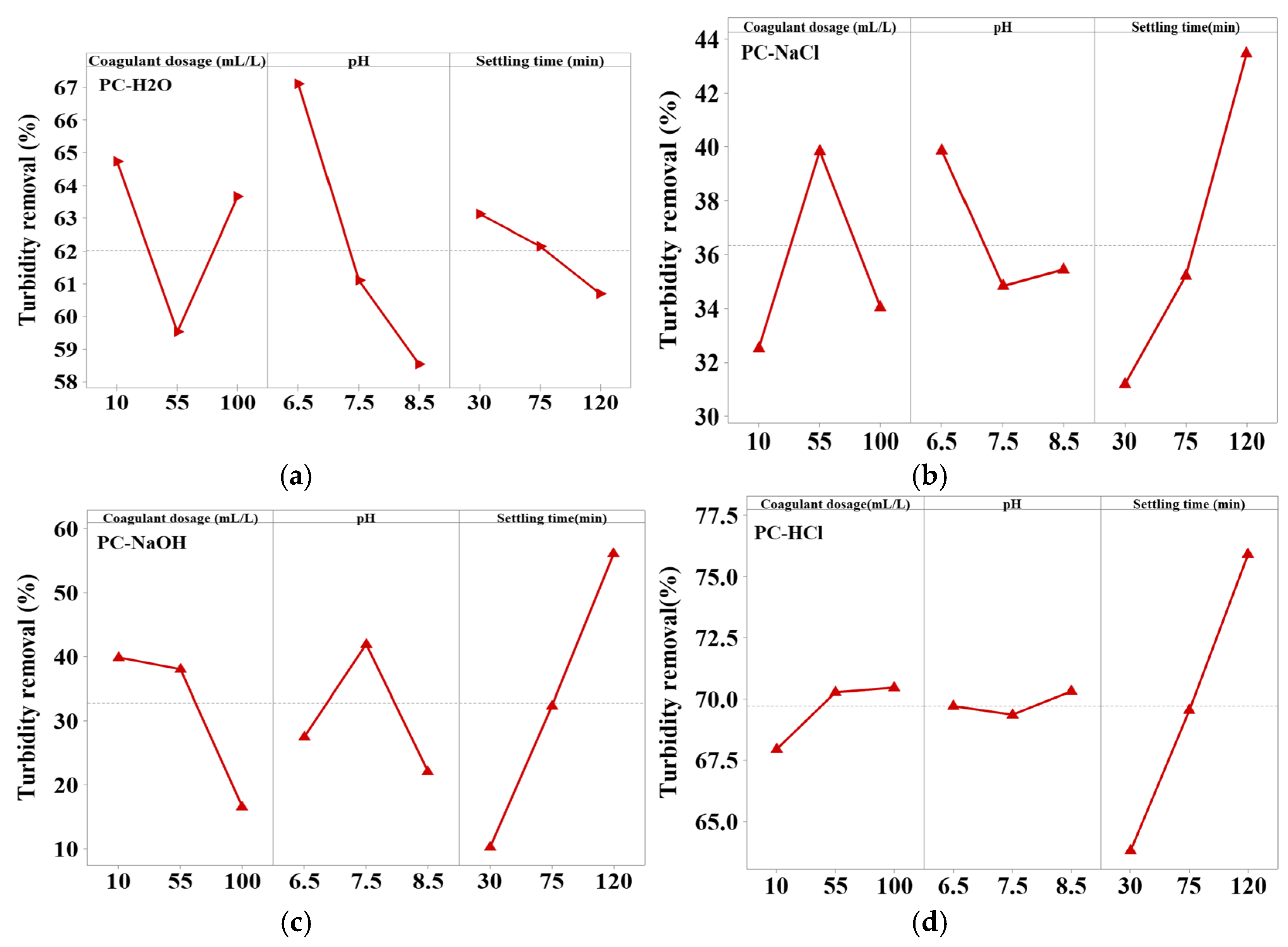
The most important parameters influencing the effectiveness of turbidity removal by the coagulation/flocculation process, and which have been studied, are the coagulant dosage, pH, and settling time. The main effect graphs of each parameter for turbidity reduction by different Pine cone extracts are presented in Figure 4.
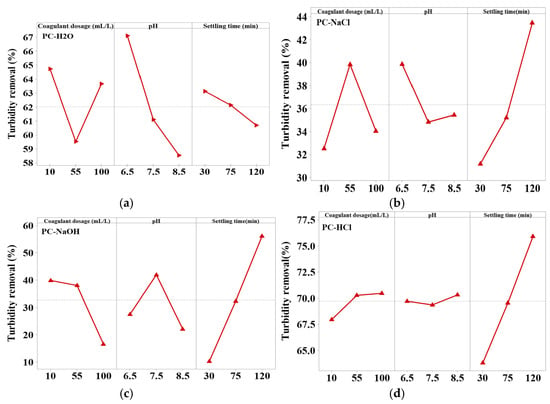
Figure 4.
Effects of factors on turbidity reduction for the four Pine cone extracts: (a) PC-H2O, (b) PC-NaCl, (c) PC-NaOH, and (d) PC-HCl.
As illustrated in Figure 4, both the optimal dosage and the turbidity removal efficiency vary considerably depending on the type of Pine cone extract used, highlighting clear differences in their bioactive compounds. The PC-H₂O and PC-NaOH extracts showed their best performance at a relatively low dose of 10 mL/L, achieving removal efficiencies of about 65% and 40%, respectively. In contrast, the PC-HCl extract reached its highest efficiency around 71% but at a much higher dose of 100 mL/L. Similarly, the PC-NaCl extract required 55 mL/L to reach an efficiency of about 40%. These variations are consistent with the findings of FTIR analysis, which confirmed the presence of functional groups associated with bioactive compounds responsible for coagulation. The higher turbidity removal at lower doses of PC-H2O is likely due to the presence of functional groups (C=O, C–O, –CH) identified by FTIR, which serve as active sites for adsorption. At low coagulant concentrations, these sites remain sufficiently available to interact with colloidal particles, favoring an adsorption–bridging mechanism that enables the formation of stable flocs without complete charge neutralization. This suggests that PC-H2O acts primarily through surface interactions and bridging, rather than relying on charge neutralization, which typically requires higher dosages [54,55]. The high turbidity removal observed with the PC-HCl extract at higher doses may be attributed to the presence of functional groups such as hydroxyl (–OH) and carboxyl (–COOH) in proteins, polyphenols and carbohydrates, whose protonation under acidic conditions enhances coagulation through charge neutralization, adsorption, and bridging mechanisms [56]. In the case of PC-NaCl, higher turbidity removal was observed at medium doses. FTIR spectra revealed amide and N–H bands, indicating the presence of proteins responsible for the coagulation activity. However, the lower efficiency at other doses may be due to a reduced concentration or activity of these proteins, which affects their ability to neutralize charges and form effective inter-particle bridges [56]. This observation, according to Naim et al., [57] supports the finding that an increased number of amino groups enhances aggregation and floc formation. However, at excessively high doses, repulsion between amino groups causes floc destabilization and decreases turbidity removal. As for PC-NaOH, the highest turbidity removal was observed at lower doses. FTIR analysis revealed strong O–H and C–O bands, indicating a high polysaccharide content. Under alkaline conditions, these biomolecules may experience structural changes that reduce their effectiveness at higher concentrations. Several studies, including that by Bahrodin et al., have shown that polysaccharide-based coagulants are more effective at lower doses, as higher concentrations can lead to active site saturation and conformational changes. In general, a higher polysaccharide content improves performance by reducing the coagulant dose required to achieve optimal turbidity removal [26]. Overall, the study revealed that the optimal coagulant dose varied for each extract of bio coagulant, and increasing the dosage did not necessarily result in enhanced coagulation activity. This implies that the coagulation process is strongly influenced by factors such as the concentration and functionality of active groups, as well as the nature of their interactions with suspended particles [56]. Moreover, using a dose that is either too high or too low compared to the optimal value can reduce efficiency, potentially increasing turbidity by destabilizing flocs or restabilizing suspended particles [58].
According to Figure 4a–d, the optimal pH for coagulation varies depending on the type of bio-coagulant extract used. The PC-H2O and PC-NaCl extracts exhibited their highest turbidity removal efficiencies at a slightly acidic pH of 6.5, reaching 67% and 40%, respectively. In contrast, the PC-HCl extract showed improved performance at a more alkaline pH of 8.5, achieving 71% removal. The PC-NaOH extract reached its maximum efficiency of 42% at a near-neutral pH of 7.5.
The higher turbidity removal observed under mildly acidic conditions for the PC-H2O and PC-NaCl extracts may be attributed to the protonation of hydroxyl and carboxyl groups present in the proteins and carbohydrates within the bio-coagulant extracts, which enhances their interaction with colloidal particles [56]. In contrast, the PC-HCl extract exhibits optimal performance at pH 8.5, likely due to the presence of acid-extracted polyphenols. Upon deprotonation, these compounds become more reactive, facilitating particle aggregation through bridging mechanisms, which leads to increased turbidity removal. Conversely, alkaline extraction solubilizes organic compounds rich in ionizable functional groups [59], as confirmed by FTIR analysis. However, these compounds can hinder floc formation at higher pH levels. Additionally, the increased negative surface charge of polysaccharides under strongly alkaline conditions may lead to electrostatic repulsion between the bio-coagulant and the negatively charged colloids, thereby reducing overall coagulation–flocculation efficiency [6].
These findings confirm that pH critically influences the surface charge, solubility, and functionality of the active biomolecules extracted from Pine cone. Similar to observations in studies on Opuntia ficus-indica [60] and Aloe vera [18], our results demonstrate that acidic to neutral conditions generally favor adsorption and bridging mechanisms. However, in some cases, alkaline pH can enhance removal efficiency depending on the specific composition of the extract, although it may also induce electrostatic repulsion that reduces coagulation efficiency.
The settling time also has a significant and measurable effect on coagulation efficiency. As shown in Figure 4, maximum turbidity removal was achieved for PC-NaCl, PC-HCl, and PC-NaOH extracts after 120 min (Figure 4b–d). This can be explained by the fact that, after 120 min of treatment, the maximum amount of active coagulant agents is available in the aqueous solution, enhancing coagulation activity. In contrast, for the PC-H2O extract, removal efficiency decreases significantly after 30 min of settling (Figure 4a). This is due to the reduced concentration of remaining suspended solid particles responsible for turbidity and the active coagulant agents in the extract. Therefore, more time is required for the active coagulant components to bind effectively to these suspended particles [58].
3.6. Response Surface
In the turbidity removal process, experimental factors such as coagulant dosage, settling time, and pH play a crucial role [61]. In Figure 5, Figure 6, Figure 7 and Figure 8, the 2D contour plots and 3D surface plots of the model illustrate the three experimental variables. They are plotted based on two factors while keeping the other factor at a fixed level. These plots can be used to explore the relationships between the variables.
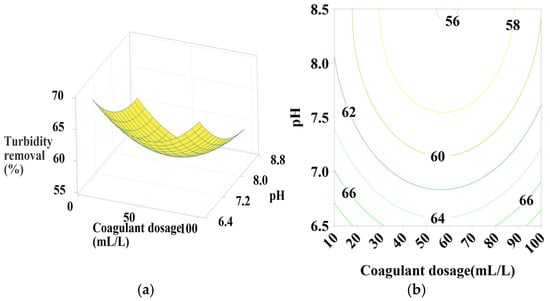
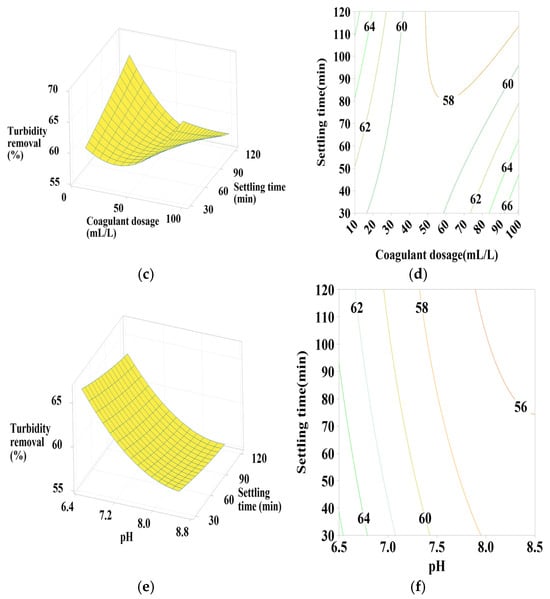
Figure 5.
Response surface and contour plots for turbidity removal using PC-H2O at (a,b) settling time of 75 min, (c,d) pH of 7.5, and (e,f) coagulant dosage of 55 mL/L.
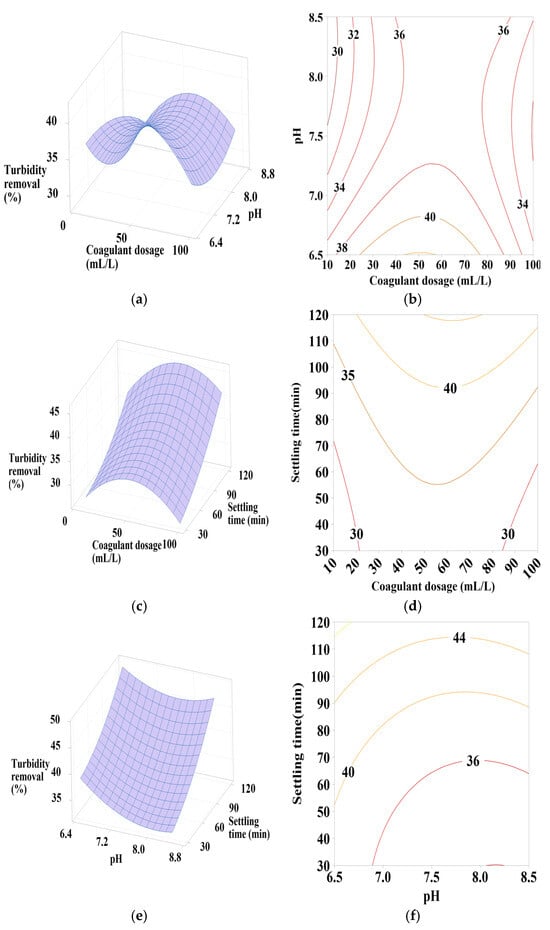
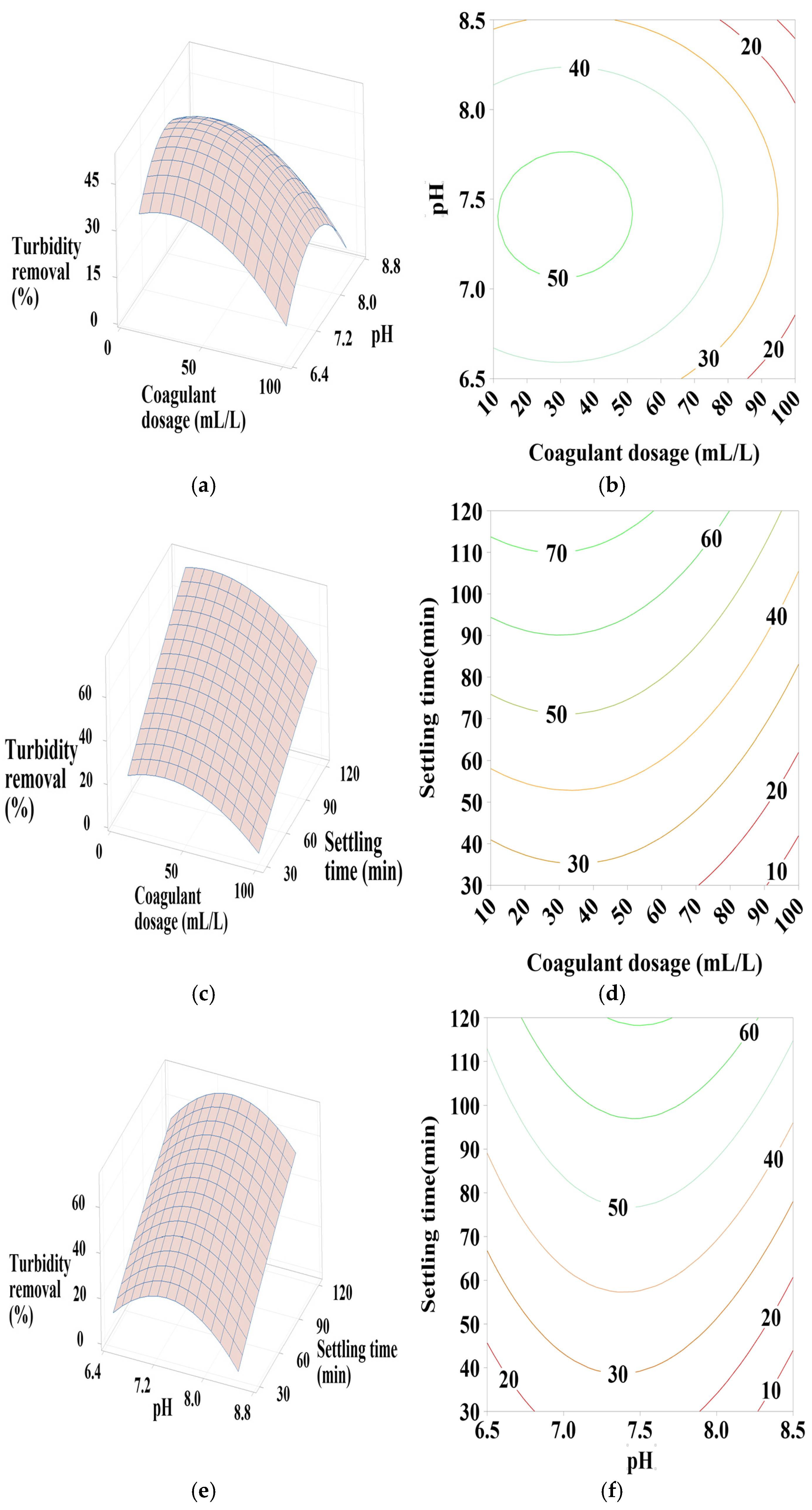
Figure 6.
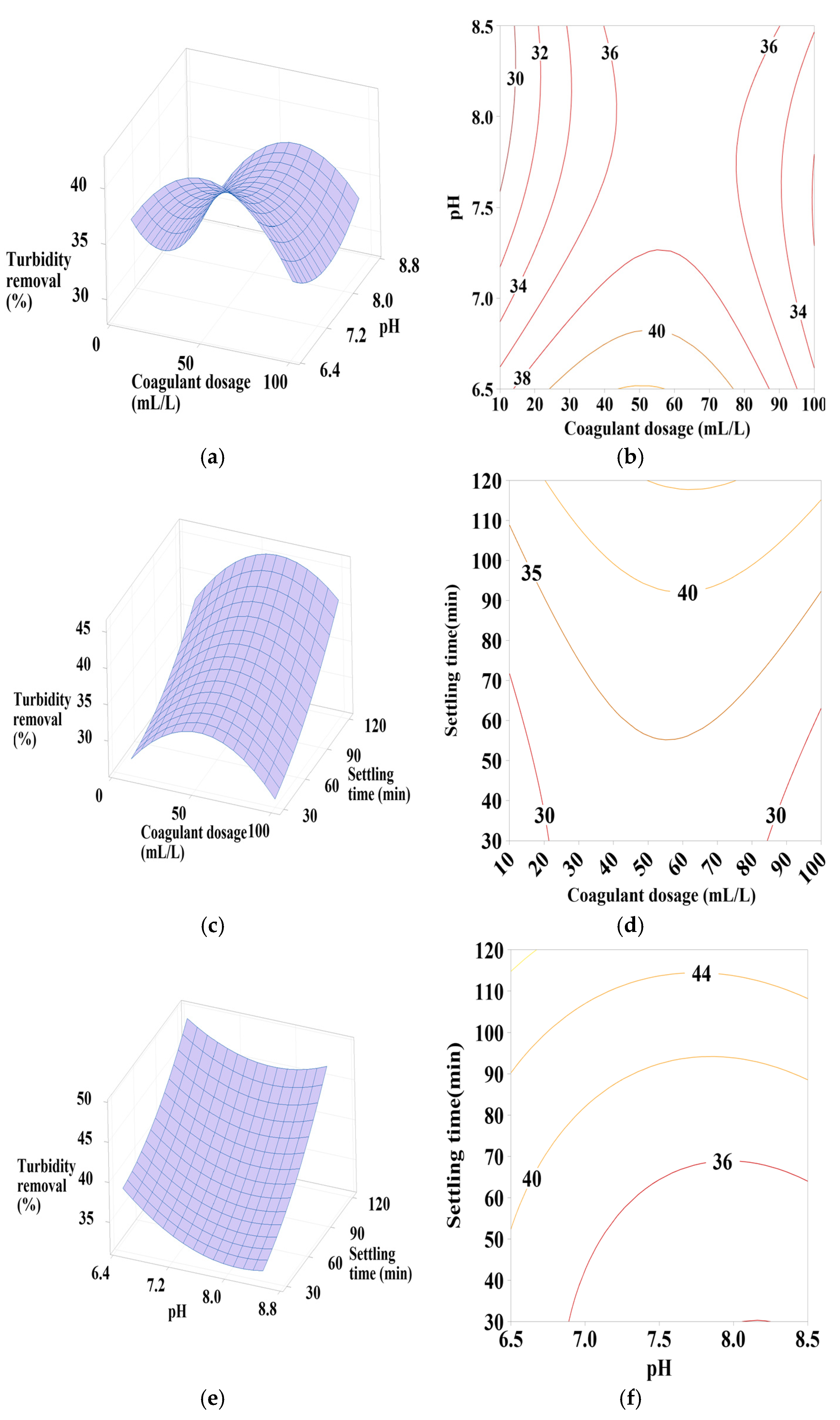
Response surface and contour plots for turbidity removal using PC-NaCl at (a,b) settling time of 75 min, (c,d) pH of 7.5, and (e,f) coagulant dosage of 55 mL/L.
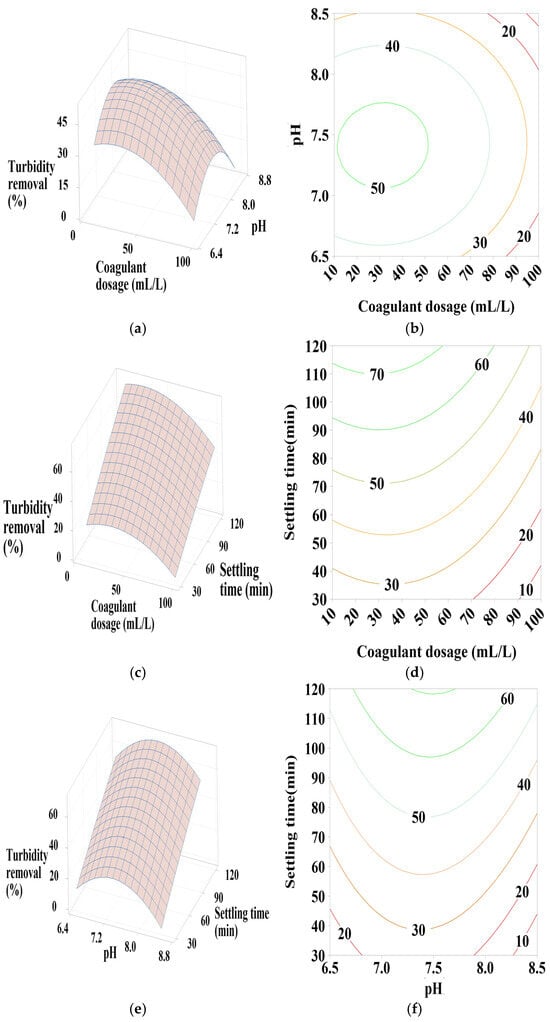
Figure 7.
Response surface and contour plots for turbidity removal using PC-NaOH at (a,b) settling time of 75 min, (c,d) pH of 7.5, and (e,f) coagulant dosage of 55 mL/L.
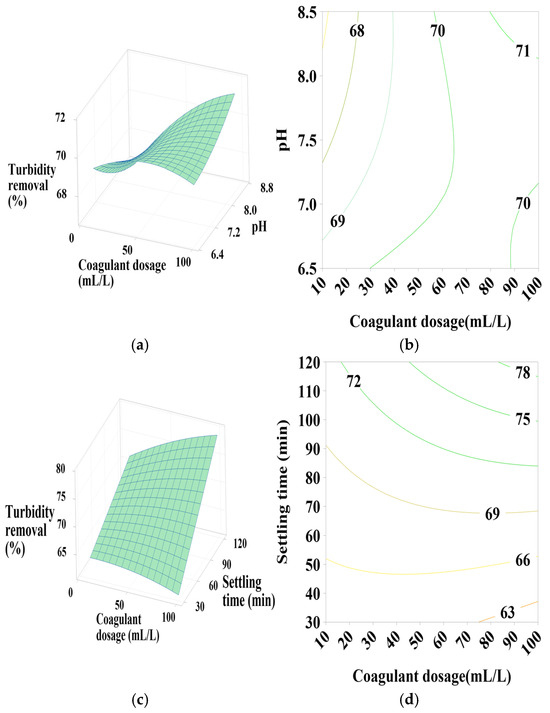
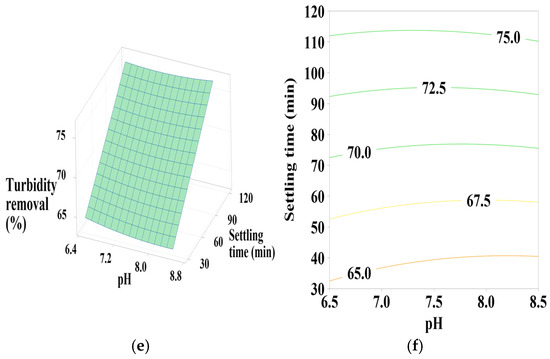
Figure 8.
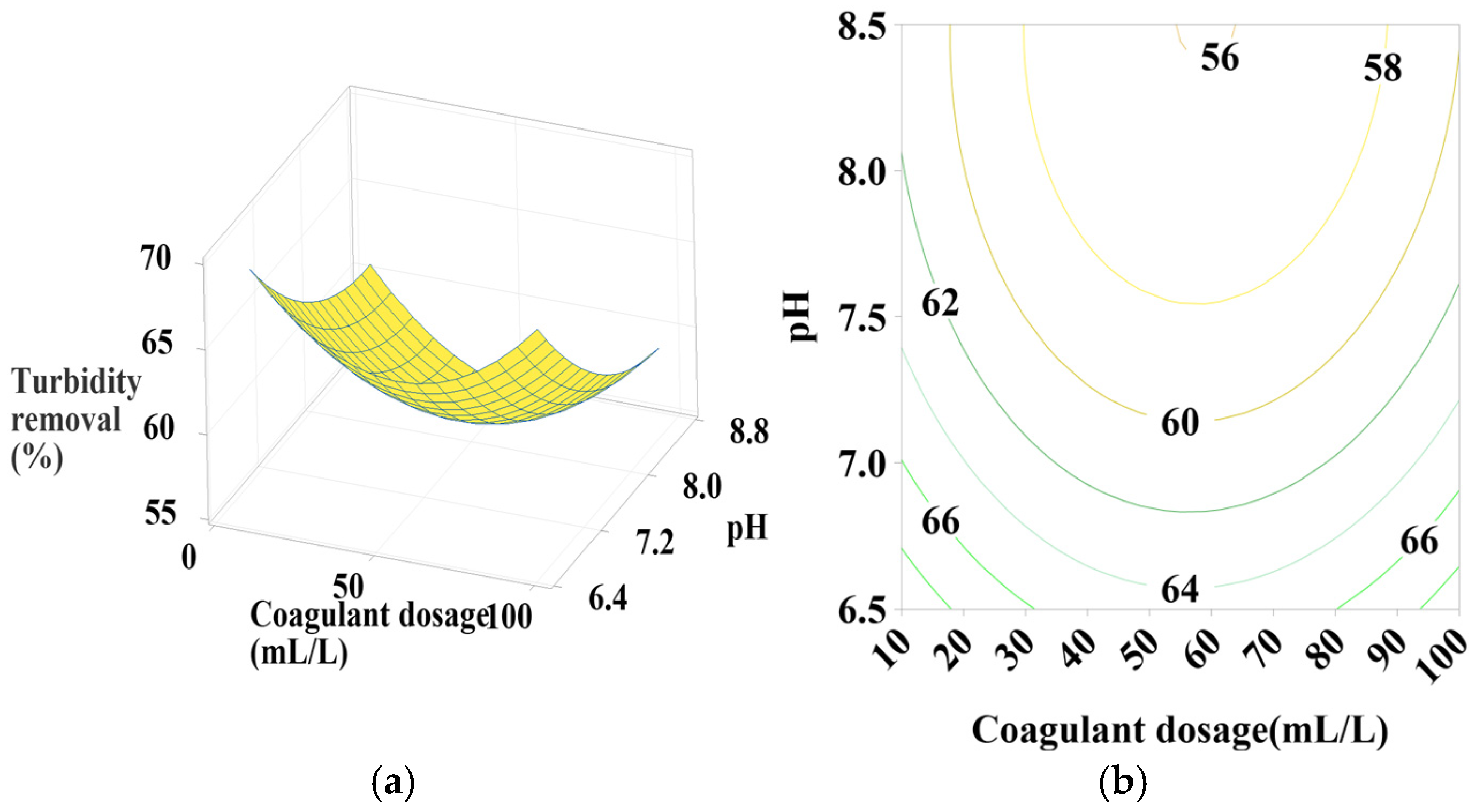
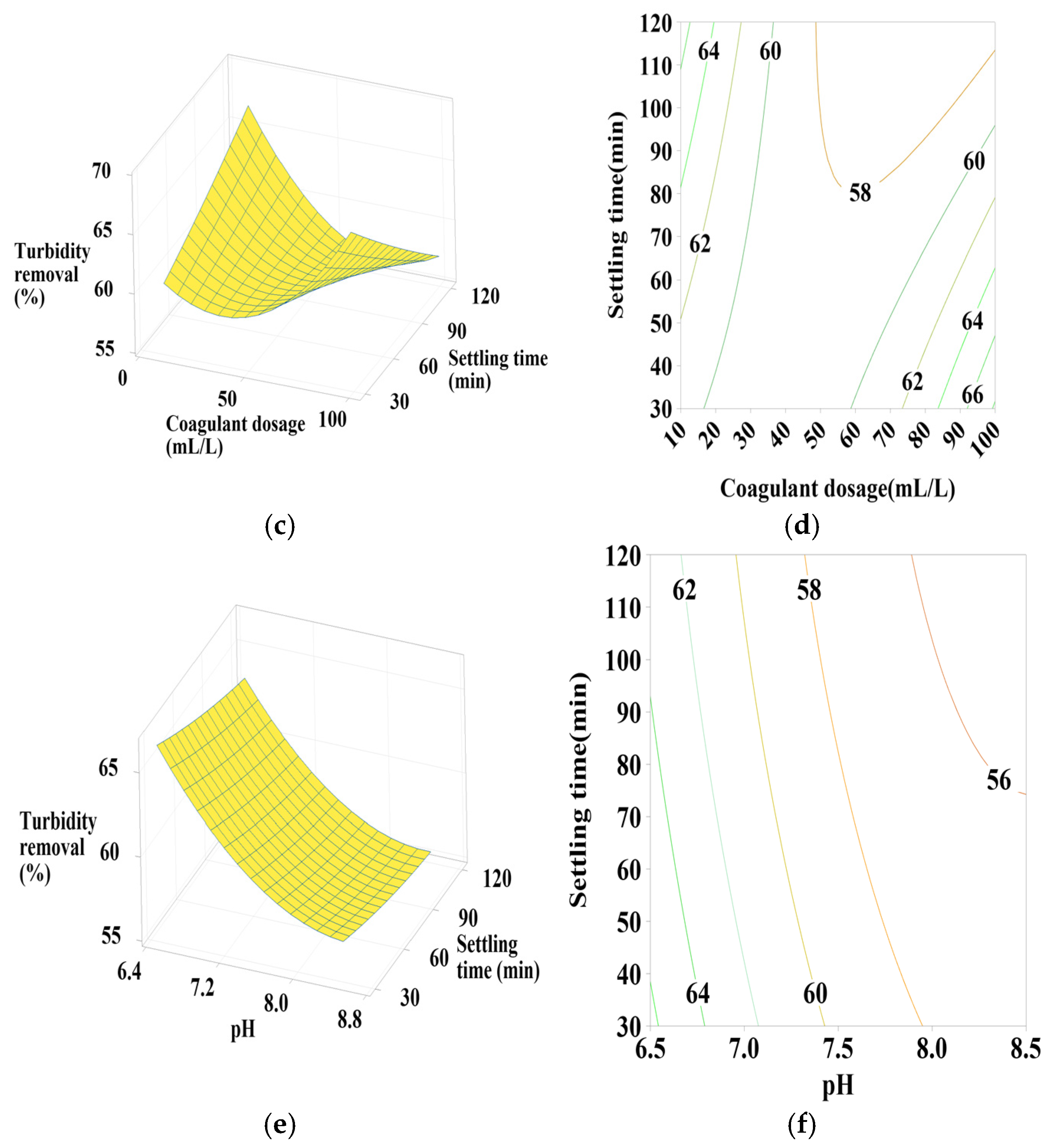
Response surface and contour plots for turbidity removal using PC-HCl at (a,b) settling time of 75 min, (c,d) pH of 7.5, and (e,f) coagulant dosage of 55 mL/L.
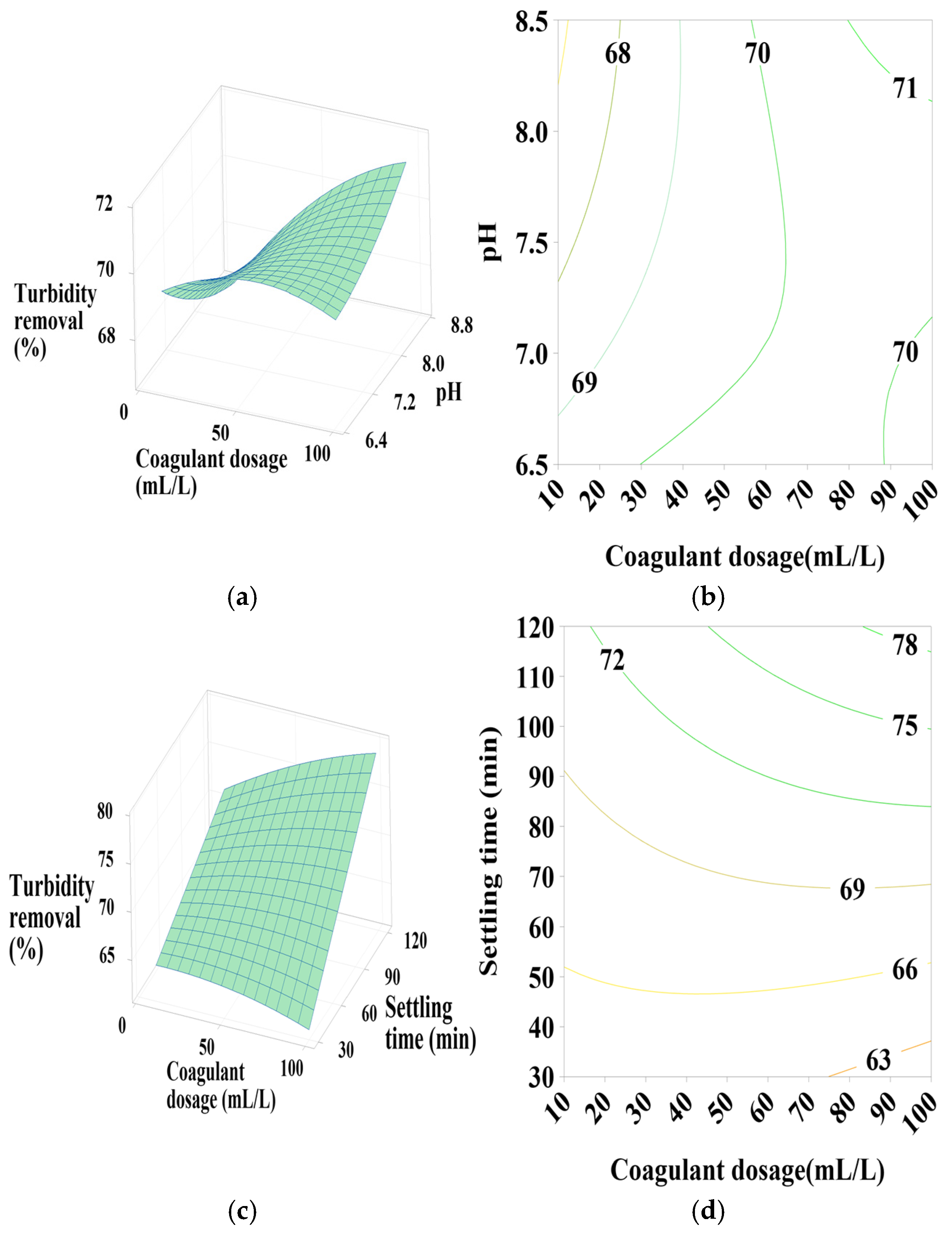
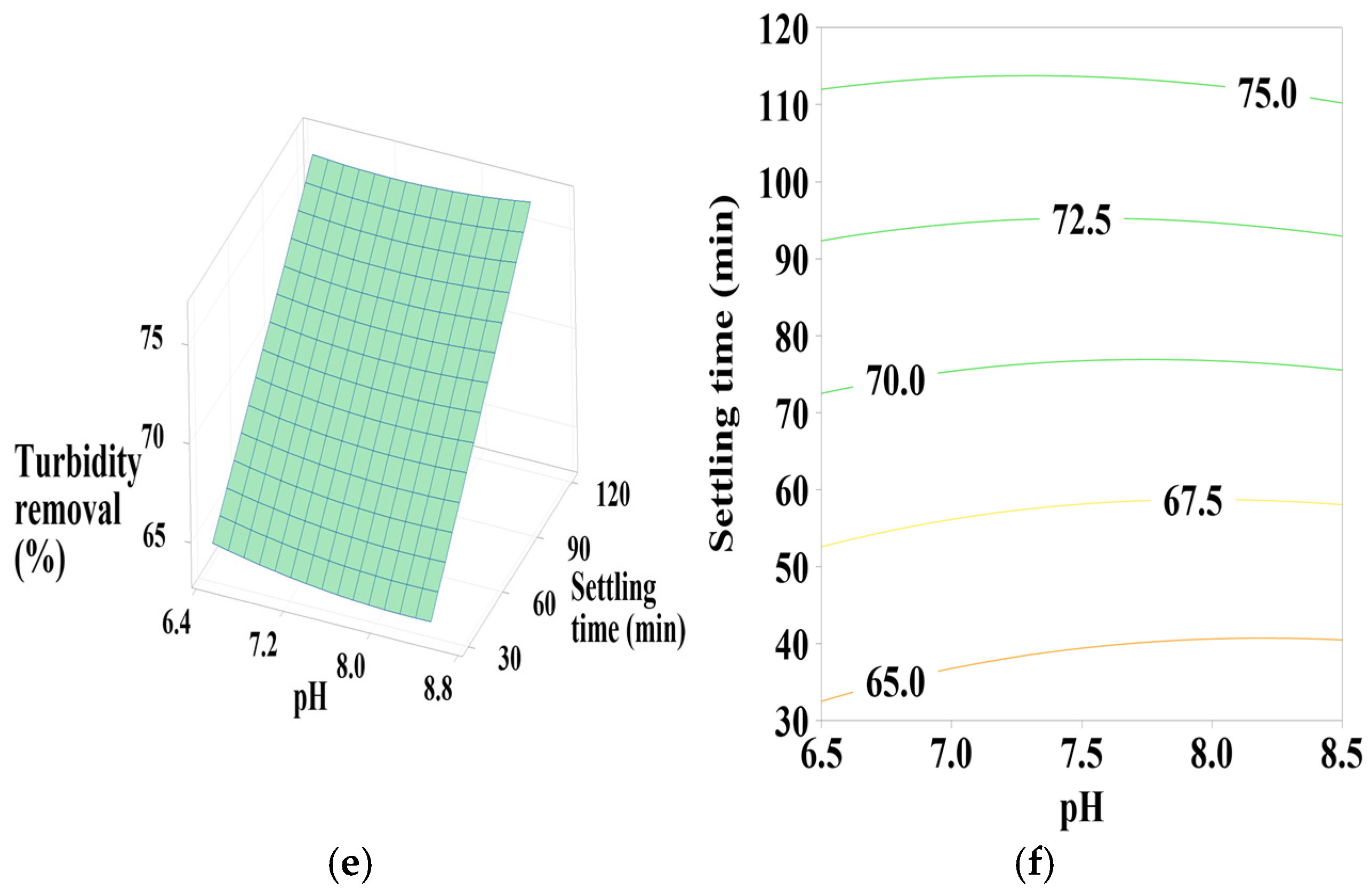
3.6.1. Effect of PC-H2O Dosage, pH, and Settling Time
According to Figure 5a,b, the contour plots (2D) and surface plots (3D) show that turbidity removal efficiency increases as the pH decreases from 8.5 to 6.5. The coagulant dosage takes a moderate value in the center, with a positive quadratic effect of both coagulant dosage and pH (Table 4). As indicated in Figure 5c,d, effective turbidity removal efficiency was observed within a settling time range of 30–120 min, with coagulant doses at minimum (10) and maximum (100) values, showing a positive linear effect of settling time, a positive quadratic effect of coagulant dosage, and a positive interactive effect between these two parameters (Table 4) in terms of p-values. Figure 5e,f shows that the best turbidity removal efficiency was achieved with a settling time between 30 and 90 min and a water pH of 6.5, with a positive linear effect of both pH and settling time (Table 4).
From these figures, a maximum turbidity reduction rate of approximately 67% was obtained under the following conditions: coagulant dosage between 10- and 20-mL L⁻1, pH between 6.5 and 6.75, and settling time between 30 and 90 min.
3.6.2. Effect of PC-NaCl Dosage, pH, and Settling Time
It is clear from Figure 6a,b that turbidity removal efficiency increases as the pH varies from 6.5 to 6.75, with the coagulant dosage maintaining an average value of 50 mL/L. The influence of these factors on turbidity removal is primarily characterized by their positive quadratic effect.
The increase in settling time also leads to an increase in turbidity reduction efficiency, as shown in Figure 6c,d. With a coagulant dosage of 50 mL/L, the positive quadratic effect of coagulant dosage is observed. Similarly, Figure 6e,f shows that maximum turbidity removal is achieved with maximum settling time and minimum pH values, indicating a positive linear and quadratic effect of pH.
From these figures, a maximum turbidity reduction rate of approximately 40% to 45% was obtained under the following conditions: coagulant dosage between 30 and 70 mL/L, pH between 6.5 and 6.75, and settling time of 120 min.
3.6.3. Effect of PC-NaOH Dosage, pH, and Settling Time
According to Figure 7a,b, increasing the added coagulant dosage and moving away from the neutral pH range significantly reduces turbidity removal efficiency. Therefore, maximum turbidity removal is observed at minimal coagulant doses with a neutral pH between 7 and 7.5, indicating a positive quadratic effect of both coagulant dosage and pH (Table 4).
As shown in Figure 7c,d, significant turbidity removal efficiency is observed with coagulant doses between 10 and 50 mL/L and a maximum settling time of 120 min, showing a positive quadratic effect of coagulant dosage and a positive linear effect of settling time. For the interactions between pH and settling time, Figure 7e,f shows that turbidity removal efficiency is maximal when pH is in the neutral range and with a maximum settling time of 120 min, indicating a positive linear effect of these parameters as well as a positive quadratic effect of pH.
3.6.4. Effect of PC-HCl Dosage, pH, and Settling Time
Figure 8a,b shows that turbidity removal efficiency is maximal when the coagulant dosage is between 70 and 100 mL/L and the pH is between 8 and 8.5, indicating a positive linear effect of coagulant dosage. According to Figure 8c,d, it is clear that maximization of turbidity removal occurs at the maximum intervals of the coagulant dosage and pH operating range, which is confirmed by the positive linear effect of these two parameters as well as a positive interaction between them. Regarding Figure 8e,f, maximum turbidity removal is observed across the entire pH range studied, with maximum settling time values, showing a positive linear effect of settling time.
3.7. Optimization of Experimental Conditions and Validation of Polynomial Models
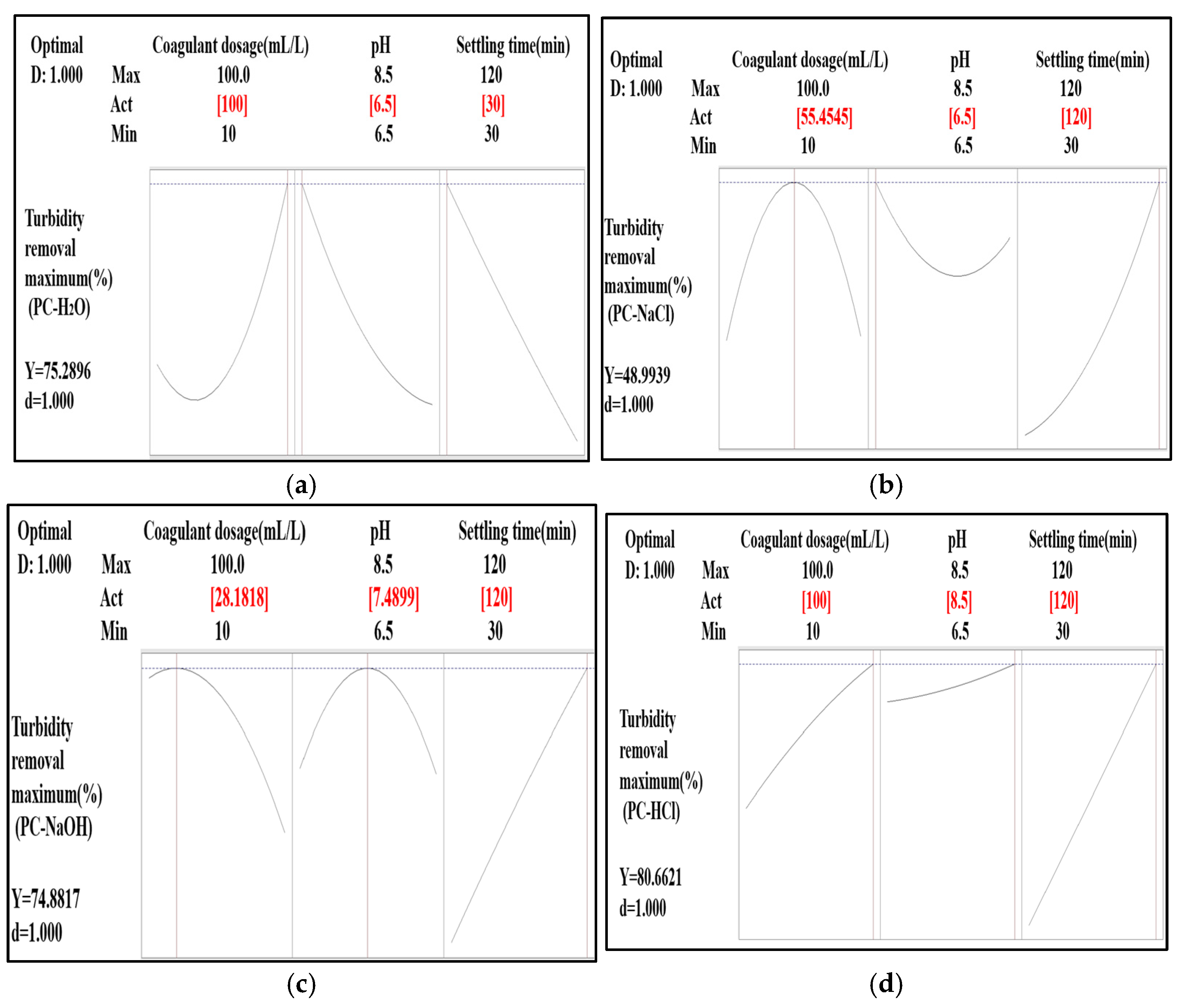
The determination of the optimal coagulant dosage, pH, and settling time for turbidity removal was carried out using the response surface optimization tool and the desirability function. For each extract used, the optimal point was identified (Figure 9).
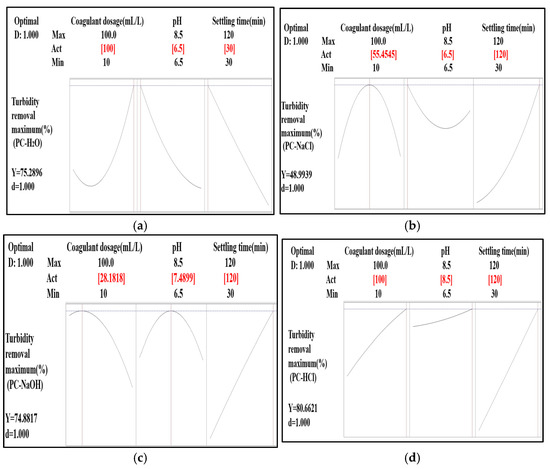
Figure 9.
Optimization of experimental conditions for highest turbidity removal efficiency: (a) PC-H2O, (b) PC-NaCl, (c) PC-NaOH, and (d) PC-HCl.
For PC-H2O, the best combination was obtained with a coagulant dosage of 100 mL/L at a pH of 6.50 and a settling time of 30 min. Under these conditions, the turbidity removal predicted by the fitted regression model was 75.28%, with a desirability of 1.000.
For PC-NaCl, the optimal combination was found with a coagulant dosage of 55.45 mL/L at a pH of 6.5 and a settling time of 120 min. Under these conditions, the predicted turbidity removal was 48.99%, with a desirability of 1.000.
For PC-NaOH, the best combination was found with a coagulant dosage of 28.18 mL/L at a pH of 7.48 and a settling time of 120 min. Under these conditions, the predicted turbidity removal was 74.88%, with a desirability of 1.000.
For PC-HCl, the optimal combination was obtained with a coagulant dosage of 100 mL/L at a pH of 8.5 and a settling time of 120 min. Under these conditions, the predicted turbidity removal was 80.66%, with a desirability of 1.000.
These results show that the optimal conditions vary depending on the pine cone extracts used as bio-coagulants. The PC-HCl extract demonstrated the best turbidity removal rate of 80.66%. This result is similar to that found by Benalia et al. [62], who reported that the HCl solvent was the best for reducing turbidity by 85.15% when treating drinking water with Aloe vera, compared to NaCl and NaOH.
The desirability of 1.000 for all optimal combinations indicates that the predictive models used are perfectly suited to estimate the efficiency of turbidity removal under the defined experimental conditions.
Once the optimal coagulation point was determined for the four extracts used, the regression models were validated through tests conducted under conditions corresponding to the optimal coagulant dose, pH, and settling time. The values predicted by the model were compared to those obtained experimentally, and the relative error was calculated. Table 5 presents the results obtained for turbidity removal for the four extracts studied.

Table 5.
Validation of polynomial models.
According to Table 5, it can be observed that the mathematical models developed are capable of satisfactorily predicting the turbidity removal from raw water. The relative error between the predicted values and the values obtained during experimentation for the PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl extracts were 2.6%, 0.61%, 5.3%, and 2.59%, respectively. It is noteworthy that all of these values are below 5%, which validates and confirms the reliability of the generated mathematical models.
3.8. Characterization of Treated Water Quality Under Optimal Conditions
Several water parameters were measured under the previously determined optimal conditions, including total hardness, total alkalinity, total suspended solids (SSs), UV254, dry residue, salinity, and conductivity. These results are summarized in Table 6.

Table 6.
The physico-chemical parameters of water treated with PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl.
According to Table 6, a variability in the effectiveness of Pine cone extracts used as bio-coagulants is observed. The PC-HCl extract demonstrates the best performance, reducing turbidity, total suspended solids (TSSs), and UV254 by 78.57%, 85.8%, and 66.80%, respectively. This efficiency is attributed to the presence of long protein chains, total sugars, and polyphenols in the extract. These molecules promote pollutant aggregation and floc formation, enabling their removal through sedimentation or filtration [45].
Regarding alkalinity, the results in Table 6 show a decrease during treatment with PC-H2O and PC-NaCl extracts (8 °F). This can be explained by the reaction between the released H+ ions and the alkaline ions (HCO3−, CO32−, OH−) present in the water. Treatment with PC-HCl shows an alkalinity of 0 °F, which is due to the complete neutralization of alkaline ions by the H+ ions released by hydrochloric acid (HCl). Conversely, treatment with PC-NaOH increases alkalinity to 19 °F, attributed to the rise in hydroxyl ion (OH−) concentration, which reduces the concentration of hydrogen ions (H+), thereby increasing the water’s alkalinity.
For the effect of Pine cone extracts on total hardness, an increase in hardness (34.4 °F and 31 °F) is observed after treatment with PC-NaOH and PC-HCl extracts, respectively. This increase is explained by the rise in hydroxyl ions (OH−) released during the pH adjustment to 7.48 for PC-NaOH and 8 for PC-HCl. In contrast, using PC-H2O and PC-NaCl extracts at an optimal pH of 6.5 results in a decrease in hardness to 8.6 °F and 8.8 °F. This reduction is linked to the reaction between H+ ions released by HCl used for pH adjustment and the HCO3−, OH−, CO32−, Ca2+, and Mg2+ ions present in the water.
The results regarding dry residue indicate a nearly identical reduction for treatments with PC-H2O, PC-NaCl, and PC-HCl extracts. This suggests that these treatments effectively removed a significant amount of solid matter (organic and/or mineral) from the water. Thus, a high dry residue content may indeed be attributed to the presence of mineral salts, heavy metals, or other undesirable substances, which are eliminated during treatment with these extracts.
3.9. Techno-Economic and Environmental Feasibility
Beyond evaluating the turbidity removal efficiency of Pine cone extract, this study also explores the economic and environmental potential of its large-scale use in water treatment. Pine cones, particularly from Pinus nigra, are abundant, renewable, and often considered forestry waste, making them an appealing, low-cost raw material.
The four extraction methods used in this work (PC-H2O, PC-NaCl, PC-NaOH, and PC-HCl) rely on relatively simple, low-energy processes and affordable solvents, which supports their potential for cost-effective application. From a techno-economic standpoint, this approach benefits from the absence of high-temperature processing, minimal chemical use (especially for PC-H2O and PC-NaCl), and the ease with which it could be scaled up for decentralized or small-scale treatment systems. Moreover, since the raw water used in the study came from an actual drinking water treatment plant, the process conditions and results reflect realistic, real-world scenarios.
From a life cycle perspective, the environmental impact of this bio-coagulant system is likely lower than that of traditional chemical coagulants. In particular, PC-H2O and PC-NaCl extracts use non-toxic, biodegradable solvents and natural biomass, suggesting a favorable environmental profile. While PC-HCl and PC-NaOH involve more reactive chemicals, their environmental impacts could potentially be reduced through optimized usage and strategies such as solvent recovery or neutralization.
Overall, the findings not only confirm the technical effectiveness of Pine cone extracts for turbidity removal (with PC-HCl achieving up to 78.57% reduction), but also highlight their promise as sustainable and economically viable alternatives to synthetic coagulants. These results encourage further investigation through comprehensive techno-economic and life cycle assessments in future studies.
3.10. Comparative Study of Natural Coagulants for Water Treatment Applications
To assess Pine cone efficacy in turbidity removal, its performance was benchmarked against other natural coagulants documented in the literature, with the comparative results detailed in Table 7.

Table 7.
Comparison of turbidity removal efficiency of Pine cone extract and other bio-coagulants from the literature.
4. Conclusions
This study highlights the suitability of Pine cone extract as a sustainable bio-coagulant for turbidity reduction in raw water. Based on the experimental design, the key factors influencing turbidity removal are coagulant dosage, settling time, and pH. The models showed excellent fit, as indicated by the high coefficients of determination: R2 = 0.985 and adj R2 = 0.9579 for PC-H2O, R2 = 0.9725 and adj R2 = 0.9231 for PC-NaCl, R2 = 0.9475 and adj R2 = 0.8529 for PC-NaOH and R2 = 0.9829 and adj R2 = 0.9522 for PC-HCl. Among the extracts studied, the HCl extract was the most effective for turbidity reduction. For the optimal turbidity removal efficiency of 78.57%, the ideal operating conditions are a coagulant dosage of 100 mL/L, pH 8.5, and a settling time of 120 min. These results highlight the importance of adjusting the pH, coagulant dosage, and settling time to achieve maximum efficiency. Furthermore, selecting the appropriate solvent for extracting the active molecules responsible for coagulation plays a critical role in raw water treatment. The findings from this research provide valuable insights for designing pilot or full-scale treatment plants, aiming to address raw water pollution. While Pine cone extract proved effective for turbidity removal, the results indicate that additional technologies, such as adsorption and filtration, are necessary to meet potable water standards and conform to environmental regulations. Moreover, future studies should evaluate the reusability of Pine cone extracts to determine their long-term applicability and operational sustainability.
Author Contributions
Conceptualization, O.B., A.B. and K.D.; methodology, O.B., A.B., K.D. and A.K.; formal analysis, O.B., A.B., A.B. and K.D.; investigation, A.B., A.B. and K.D.; data curation, O.B., A.B., K.D. and A.P.; writing—original draft preparation, O.B., A.B., K.D. and A.P.; writing review and editing, O.B., A.B., K.D., A.K. and A.P.; supervision, O.B., K.D., A.B. and A.P.; project administration, K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.
Acknowledgments
This work was supported by the National Polytechnic School of Constantine (Algeria).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, P.; Wu, J. Drinking Water Quality and Public Health. Expo. Health 2019, 11, 73–79. [Google Scholar] [CrossRef]
- Bora, A.J.; Dutta, R.K. Removal of Metals (Pb, Cd, Cu, Cr, Ni, and Co)from Drinking Water by Oxidation-Coagulation-Absorption at Optimized PH. J. Water Process Eng. 2019, 31, 100839. [Google Scholar] [CrossRef]
- Arnoldsson, E.; Bergman, M.; Matsinhe, N.; Persson, K.M. Assessment of Drinking Water Treatment Using Moringa oleifera Natural Coagulant. Vatten 2008, 64, 137–150. [Google Scholar]
- Sulaiman, M.; Zhigila, D.A.; Umar, D.M.; Babale, A.; Andrawus Zhigila, D.; Mohammed, K.; Mohammed Umar, D.; Aliyu, B.; Manan, F.A. Moringa oleifera Seed as Alternative Natural Coagulant for Potential Application in Water Treatment: A Review. J. Adv. Rev. Sci. Res. 2017, 30, 1–11. [Google Scholar]
- Bahrodin, M.B.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.D.; Syafiuddin, A. Recent Advances on Coagulation-Based Treatment of Wastewater: Transition from Chemical to Natural Coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Baatache, O.; Derbal, K.; Benalia, A.; Khalfaoui, A.; Pizzi, A. Optimization and Modeling of Bio-Coagulation Using Pine Cone as a Natural Coagulant: Jar Test and Pilot-Scale Applications. Water Air Soil Pollut. 2024, 235, 770. [Google Scholar] [CrossRef]
- El-taweel, R.M.; Mohamed, N.; Alrefaey, K.A.; Husien, S.; Abdel-Aziz, A.B.; Salim, A.I.; Mostafa, N.G.; Said, L.A.; Fahim, I.S.; Radwan, A.G. A Review of Coagulation Explaining Its Definition, Mechanism, Coagulant Types, and Optimization Models; RSM, and ANN. Curr. Res. Green Sustain. Chem. 2023, 6, 100358. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Gómez Castaño, J.A.; Cifuentes, G.R. Evaluation of Turbidity and Color Removal in Water Treatment: A Comparative Study between Opuntia Ficus-Indica Fruit Peel Mucilage and FeCl3. Polymers 2023, 15, 217. [Google Scholar] [CrossRef]
- Chatsungnoen, T.; Chisti, Y. Flocculation and Electroflocculation for Algal Biomass Recovery, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; ISBN 9780444641922. [Google Scholar]
- Ndive, J.N.; Obiora-Okafo, I.A.; Onukwuli, O.D. Optimization Studies on the Coagulation–Flocculation Process for PWW Treatment Using Cactus Oputia Extract: Comparative Studies for Performance Evaluation. J. Basic Appl. Res. Int. 2023, 29, 16–31. [Google Scholar] [CrossRef]
- Altaher, H.; Khalil, T.E.; Abubeah, R. An Agricultural Waste as a Novel Coagulant Aid to Treat High Turbid Water Containing Humic Acids. Glob. Nest J. 2016, 18, 279–290. [Google Scholar] [CrossRef]
- Elsergany, M. The Potential Use of Moringa Peregrina Seeds and Seed Extract as a Bio-Coagulant for Water Purification. Water 2023, 15, 2804. [Google Scholar] [CrossRef]
- Benalia, A.; Atime, L.; Baatache, O.; Khalfaoui, A.; Ghomrani, A.F.; Derbal, K.; Pizzi, A.; Panico, A.; Bouchareb, E.M.; Bouchareb, R.; et al. Removal of Lead in Water by Coagulation Flocculation Process Using Cactus-Based Natural Coagulant: Optimization and Modeling by Response Surface Methodology (RSM). Environ. Monit. Assess. 2024, 196, 244. [Google Scholar] [CrossRef] [PubMed]
- Diver, D.; Nhapi, I.; Ruziwa, W.R. The Potential and Constraints of Replacing Conventional Chemical Coagulants with Natural Plant Extracts in Water and Wastewater Treatment. Environ. Adv. 2023, 13, 100421. [Google Scholar] [CrossRef]
- Camacho, F.P.; Sousa, V.S.; Bergamasco, R.; Ribau Teixeira, M. The Use of Moringa oleifera as a Natural Coagulant in Surface Water Treatment. Chem. Eng. J. 2017, 313, 226–237. [Google Scholar] [CrossRef]
- Abderrezzaq, B.; Kerroum, D.; Amrouci, Z.; Baatache, O. Application of Plant-Based Coagulants and Their Mechanisms in Water Treatment: A Review. J. Renew. Mater. 2024, 12, 667–698. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of Natural Organic Matter in Drinking Water Treatment by Coagulation: A Comprehensive Review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Gisonni, C.; Crispino, G.; Pirozzi, F.; Pizzi, A. Use of Aloe Vera as an Organic Coagulant for Improving Drinking Water Quality. Water 2021, 13, 2024. [Google Scholar] [CrossRef]
- Muthuraman, G.; Sasikala, S. Removal of Turbidity from Drinking Water Using Natural Coagulants. J. Ind. Eng. Chem. 2014, 20, 1727–1731. [Google Scholar] [CrossRef]
- Fabris, R.; Chow, C.W.K.; Drikas, M. Evaluation of Chitosan as a Natural Coagulant for Drinking Water Treatment. Water Sci. Technol. 2010, 61, 2119–2128. [Google Scholar] [CrossRef]
- Jadhav, M.V.; Mahajan, Y.S. Investigation of the Performance of Chitosan as a Coagulant for Flocculation of Local Clay Suspensions of Different Turbidities. KSCE J. Civ. Eng. 2013, 17, 328–334. [Google Scholar] [CrossRef]
- Antov, M.G.; Šćiban, M.B.; Prodanović, J.M.; Kukić, D.V.; Vasić, V.M.; Đorđević, T.R.; Milošević, M.M. Common Oak (Quercus robur) Acorn as a Source of Natural Coagulants for Water Turbidity Removal. Ind. Crops Prod. 2018, 117, 340–346. [Google Scholar] [CrossRef]
- Baatache, O.; Derbal, K.; Benalia, A.; Aberkan, I.; Guizah, Q.E.; Khalfaoui, A.; Pizzi, A. Valorization of Pine Cones (Pinus nigras) for Industrial Wastewater Treatment and Crystal Violet Removal: A Sustainable Approach Based on Bio-Coagulants and a Bio-Adsorbent. Water 2024, 16, 260. [Google Scholar] [CrossRef]
- Baatache, O.; Derbal, K.; Benalia, A.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Pizzi, A. Use of Pine Cone as Bio-Coagulant for Heavy Metal Removal from Industrial Wastewater: Use of Box—Behnken Design. Ind. Crops Prod. 2024, 210, 118185. [Google Scholar] [CrossRef]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.C.; Bouzaza, A.; Amrane, A.; Tahraoui, H.; Mouni, L. Aleppo Pine Seeds (Pinus Halepensis Mill.) as a Promising Novel Green Coagulant for the Removal of Congo Red Dye: Optimization via Machine Learning Algorithm. J. Environ. Manag. 2023, 331, 117286. [Google Scholar] [CrossRef]
- Bahrodin, M.B.; Zaidi, N.S.; Kadier, A.; Hussein, N.; Syafiuddin, A.; Boopathy, R. A Novel Natural Active Coagulant Agent Extracted from the Sugarcane Bagasse for Wastewater Treatment. Appl. Sci. 2022, 12, 7972. [Google Scholar] [CrossRef]
- Boutteflika, A. Paramètres De Qualité De L’eau De Consommation Humaine. J. Off. De La Repub. Algériennes 2011, 18, 6–9. [Google Scholar]
- Reza, A.; Chen, L.; Mao, X. Response Surface Methodology for Process Optimization in Livestock Wastewater Treatment: A Review. Heliyon 2024, 10, e30326. [Google Scholar] [CrossRef]
- Mustapha, L.S.; Obayomi, K.S. Parametric Optimization of Aloe Vera Coagulant for Nitrate Removal from Textile Wastewater Using Response Surface Methodology (RSM). Results Chem. 2025, 14, 102111. [Google Scholar] [CrossRef]
- Benalia, A.; Baatache, O.; Derbal, K.; Khalfaoui, A. The Use of Central Composite Design ( CCD ) to Optimize and Model the Coagulation-Flocculation Process Using a Natural Coagulant: Application in Jar Test and Semi-Industrial Scale. J. Water Process Eng. 2024, 57, 104704. [Google Scholar] [CrossRef]
- Subramonian, W.; Wu, T.Y.; Chai, S.P. A Comprehensive Study on Coagulant Performance and Floc Characterization of Natural Cassia Obtusifolia Seed Gum in Treatment of Raw Pulp and Paper Mill Effluent. Ind. Crops Prod. 2014, 61, 317–324. [Google Scholar] [CrossRef]
- Chen, W.H.; Chiu, G.L.; Chyuan Ong, H.; Shiung Lam, S.; Lim, S.; Sik Ok, Y.; Kwon, E.E. Optimization and Analysis of Syngas Production from Methane and CO2 via Taguchi Approach, Response Surface Methodology (RSM) and Analysis of Variance (ANOVA). Fuel 2021, 296, 120642. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Amo-Duodu, G.; Tetteh, E.K.; Rathilal, S. Model Prediction of Coagulation by Magnetised Rice Starch for Wastewater Treatment Using Response Surface Methodology (RSM) with Artificial Neural Network (ANN). Sci. Afr. 2022, 17, e01282. [Google Scholar] [CrossRef]
- Polo-Castellano, C.; Mateos, R.M.; Visiedo, F.; Palma, M.; Barbero, G.F.; Ferreiro-González, M. Optimizing an Enzymatic Extraction Method for the Flavonoids in Moringa (Moringa oleifera Lam.) Leaves Based on Experimental Designs Methodologies. Antioxidants 2023, 12, 369. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Alidokht, L.; Khataee, A.R.; Karaca, S. Optimization of Comparative Removal of Two Structurally Different Basic Dyes Using Coal as a Low-Cost and Available Adsorbent. J. Taiwan Inst. Chem. Eng. 2014, 45, 1597–1607. [Google Scholar] [CrossRef]
- Obiora-Okafo, I.A.; Onukwuli, O.D.; Eli-Chukwu, N.C. Evaluation of Bio-Coagulants for Colour Removal from Dye Synthetic Wastewater: Characterization, Adsorption Kinetics, and Modelling Approach. Water SA 2020, 46, 300–312. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Panico, A.; Pirozzi, F. Use of Acorn Leaves as a Natural Coagulant in a Drinking Water Treatment Plant. Water 2018, 11, 57. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Utilization of Punica Granatum Peel as an Eco-Friendly Biosorbent for the Removal of Methylene Blue Dye from Aqueous Solution. J. Appl. Biotechnol. Bioeng. 2018, 5, 242–249. [Google Scholar] [CrossRef]
- Bouaouine, O.; Bourven, I.; Khalil, F.; Baudu, M. Identification of Functional Groups of Opuntia Ficus-Indica Involved in Coagulation Process after Its Active Part Extraction. Environ. Sci. Pollut. Res. 2018, 25, 11111–11119. [Google Scholar] [CrossRef]
- Choong Lek, B.L.; Peter, A.P.; Qi Chong, K.H.; Ragu, P.; Sethu, V.; Selvarajoo, A.; Arumugasamy, S.K. Treatment of Palm Oil Mill Effluent (POME) Using Chickpea (Cicer arietinum) as a Natural Coagulant and Flocculant: Evaluation, Process Optimization and Characterization of Chickpea Powder. J. Environ. Chem. Eng. 2018, 6, 6243–6255. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Huang, W.W.; Zhao, H.T.; Wang, J.; Xu, R.B.; Hu, X.L.; Shen, S.Y.; Qin, D. Characterization and Bioactivity of Polysaccharides Obtained from Pine Cones of Pinus Koraiensis by Graded Ethanol Precipitation. Molecules 2013, 18, 9933–9948. [Google Scholar] [CrossRef]
- Hamadi, Z.; Chaid, R.; Kebir, M.; Amirou, S.; Essawy, H.; Pizzi, A.; Séguin, P. Adsorption of Chromium Cr(VI) from Aqueous Solutions Using Algerian Pinus Halepensis Tannin Foam. Polymer 2020, 44, 425–435. [Google Scholar] [CrossRef]
- Fatombi, J.K.; Lartiges, B.; Aminou, T.; Barres, O.; Caillet, C. A Natural Coagulant Protein from Copra (Cocos nucifera): Isolation, Characterization, and Potential for Water Purification. Sep. Purif. Technol. 2013, 116, 35–40. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of Anionic Dye Congo Red from Aqueous Solution by Raw Pine and Acid-Treated Pine Cone Powder as Adsorbent: Equilibrium, Thermodynamic, Kinetics, Mechanism and Process Design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Y. Emerging Usage of Plant-Based Coagulants for Water and Wastewater Treatment. Process Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef]
- Beiramzadeh, Z.; Baqersad, M.; Aghababaei, M. Application of the Response Surface Methodology (RSM) in Heavy Metal Removal from Real Power Plant Wastewater Using Electrocoagulation. Eur. J. Environ. Civ. Eng. 2022, 26, 1–20. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Microwave-Assisted Extraction of Polyphenols from Eucalyptus Bark—A First Step for a Green Production of Tannin-Based Coagulants. Water 2023, 15, 317. [Google Scholar] [CrossRef]
- Muniz, G.L.; da Silva, T.C.F.; Borges, A.C. Assessment and Optimization of the Use of a Novel Natural Coagulant (Guazuma ulmifolia) for Dairy Wastewater Treatment. Sci. Total Environ. 2020, 744, 140864. [Google Scholar] [CrossRef]
- Ahmed, M.; Tounsadi, H. Box-Behnken Experimental Design for the Optimization of Methylene Blue Adsorption onto Aleppo Pine Cones. J. Mater. Environ. Sci. 2017, 8, 2184. [Google Scholar]
- Sabeti, Z.; Alimohammadi, M.; Yousefzadeh, S.; Aslani, H.; Ghani, M.; Nabizadeh, R. Application of Response Surface Methodology for Modeling and Optimization of Bacillus Subtilis Spores Inactivation by the UV/Persulfate Process. Water Sci. Technol. Water Supply 2017, 17, 342–351. [Google Scholar] [CrossRef]
- Azimi, S.C.; Shirini, F.; Pendashteh, A. Evaluation of COD and Turbidity Removal from Woodchips Wastewater Using Biologically Sequenced Batch Reactor. Process Saf. Environ. Prot. 2019, 128, 211–227. [Google Scholar] [CrossRef]
- Drury, C.; Paquet, V.; Kelly, H. Experimental Design and Analysis. In Evaluation of Human Work, 4th ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 37–60. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G.; Wang, Z. Box-Behnken Response Surface Design for the Optimization of Electrochemical Detection of Cadmium by Square Wave Anodic Stripping Voltammetry on Bismuth Film/Glassy Carbon Electrode. Sens. Actuators B Chem. 2016, 235, 67–73. [Google Scholar] [CrossRef]
- Mdee, A.L.O.J.; Sadiki, J.W.N.N. Performance Analysis of Plant—Based Coagulants in Water Purification: A Review. Discov. Water 2024, 4, 108. [Google Scholar] [CrossRef]
- Hameed, Y.T.; Idris, A.; Hussain, S.A.; Abdullah, N. A Tannin-Based Agent for Coagulation and Flocculation of Municipal Wastewater: Chemical Composition, Performance Assessment Compared to Polyaluminum Chloride, and Application in a Pilot Plant. J. Environ. Manag. 2016, 184, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.C.; Assadi, A.A.; Amrane, A.; Mouni, L. Comparison of Four Plant-Based Bio-Coagulants Performances against Alum and Ferric Chloride in the Turbidity Improvement of Bentonite Synthetic Water. Water 2022, 14, 3324. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Han, J.I. Use of Chitosan Acid Solutions to Improve Separation Efficiency for Harvesting of the Microalga Chlorella Vulgaris. Chem. Eng. J. 2013, 226, 238–242. [Google Scholar] [CrossRef]
- Hussain, S.; Sattar, A.; Ahmad, A. Pine Cone Extract as Natural Coagulant for Puri Fi Cation of Turbid Water. Heliyon 2019, 5, e01420. [Google Scholar] [CrossRef]
- Kleber, M.; Lehmann, J. Humic Substances Extracted by Alkali Are Invalid Proxies for the Dynamics and Functions of Organic Matter in Terrestrial and Aquatic Ecosystems. J. Environ. Qual. 2019, 48, 207–216. [Google Scholar] [CrossRef]
- Deshmukh, S.O.; Hedaoo, M.N. Wastewater Treatment Using Bio-Coagulant as Cactus Opuntia Ficus Indica—A Review. Int. J. Sci. Res. Dev. 2018, 6, 711–717. [Google Scholar] [CrossRef]
- Birhanu, Y.; Leta, S. Application of Response Surface Methodology to Optimize Removal Efficiency of Water Turbidity by Low-Cost Natural Coagulant (Odaracha Soil) from Saketa District, Ethiopia. Results Chem. 2021, 3, 100108. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Pizzi, A.; Medjahdi, G. The Use of Aloe Vera as Natural Coagulant in Algerian Drinking Water Treatment Plant. J. Renew. Mater. 2022, 10, 625–637. [Google Scholar] [CrossRef]
- Getahun, M.; Befekadu, A.; Alemayehu, E. Coagulation Process for the Removal of Color and Turbidity from Wet Coffee Processing Industry Wastewater Using Bio-Coagulant: Optimization through Central Composite Design. Heliyon 2024, 10, e27584. [Google Scholar] [CrossRef] [PubMed]
- Chik, C.E.N.C.E.; Kurniawan, S.B.; Shukri, Z.N.A.; Terkula, I.B.; Wahab, F.; Endut, A.; Lananan, F.; Hasan, H.A.; Abdullah, S.R.S.; Kasan, N.A. Chitosan Coagulant: Coagulation/Flocculation Studies on Turbidity Removal from Aquaculture Wastewater by Response Surface Methodology. Int. J. Environ. Sci. Technol. 2024, 21, 805–816. [Google Scholar] [CrossRef]
- Alnawajha, M.M.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Kurniawan, S.B. Effectiveness of Using Water-Extracted Leucaena Leucocephala Seeds as a Coagulant for Turbid Water Treatment: Effects of Dosage, PH, Mixing Speed, Mixing Time, and Settling Time; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2024; Volume 14, ISBN 1339902203233. [Google Scholar]
- Villabona-Ortiz, Á.; Tejada-Tovar, C.; Ortega-Toro, R. Development of a Natural Coagulant from Albizia Lebbeck Seeds for Application in Turbidity Removal from Water Intended for Human Consumption. S. Afr. J. Chem. Eng. 2025, 53, 331–341. [Google Scholar] [CrossRef]
- Shahzadi, F.; Haydar, S.; Tabraiz, S. Optimization of Coagulation to Remove Turbidity from Surface Water Using Novel Nature-Based Plant Coagulant and Response Surface Methodology. Sustainability. 2024, 16, 2941. [Google Scholar] [CrossRef]
- Maroneze, M.M.; Zepka, L.Q.; Vieira, J.G.; Queiroz, M.I.; Jacob-Lopes, E. A Tecnologia de Remoção de Fósforo: Gerenciamento Do Elemento Em Resíduos Industriais. Rev. Ambiente E Agua 2014, 9, 445–458. [Google Scholar] [CrossRef]
- Febrianti, N.; Permana, R.N.W.; Ariani, I.K. Utilization of Aloe Vera as a Biocoagulant for Turbidity, Total Dissolved Solid (TDS), and Iron (Fe) Removal in Well Water. IOP Conf. Ser. Earth Environ. Sci. 2024, 1312, 012007. [Google Scholar] [CrossRef]
- Kouniba, S.; Benbiyi, A.; Zourif, A.; EL Guendouzi, M. Optimization Use of Watermelon Rind in the Coagulation-Flocculation Process by Box Behnken Design for Copper, Zinc, and Turbidity Removal. Heliyon 2024, 10, e30823. [Google Scholar] [CrossRef]
- Alnawajha, M.M.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Jusoh, H.H.W.; Ismail, A.; Hasan, H.A. Water vs. Salt Extraction of Bio-Coagulant from Leucaena Leucocephala Pods for Removing Turbidity from Aquaculture Effluent. J. Indian Chem. Soc. 2025, 102, 101784. [Google Scholar] [CrossRef]
- Zourif, A.; Benbiyi, A.; Kouniba, S.; El Guendouzi, M. Avocado Seed as a Natural Coagulant for Removing Dyes and Turbidity from Wastewater: Behnken Box Design, Sustainable Reuse, and Economic Evaluation. Sustain. Chem. Pharm. 2024, 39, 101621. [Google Scholar] [CrossRef]
- Nouj, N.; Majbar, Z.; Abelouah, M.R.; Ben Hamou, A.; Chaoui, A.; Hafid, N.; Benafqir, M.; El Alem, N.; Jada, A.; Ouachtak, H.; et al. Eco-Friendly Wastewater Treatment Using a Crab Shell-Based Liquid Bio-Coagulant: Multi-Criteria Decision Analysis Related to Different Pollutants Separation. J. Environ. Chem. Eng. 2024, 12, 112318. [Google Scholar] [CrossRef]
- Kakoi, B.; Kaluli, J.W.; Ndiba, P.; Thiong’o, G. Optimization of Maerua Decumbent Bio-Coagulant in Paint Industry Wastewater Treatment with Response Surface Methodology. J. Clean. Prod. 2017, 164, 1124–1134. [Google Scholar] [CrossRef]
- Shahimi, N.S.W.; Zaidi, N.S.; Bahrodin, M.B.; Amran, A.H. Utilization of Fruit Wastes (Jackfruit and Mango Seeds and Banana Trunk) as Natural Coagulants in Treating Municipal Wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1144, 012049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).