Calcium Determination by Complexometric Titration with Calcein Indicator Using Webcam for Endpoint Detection
Abstract
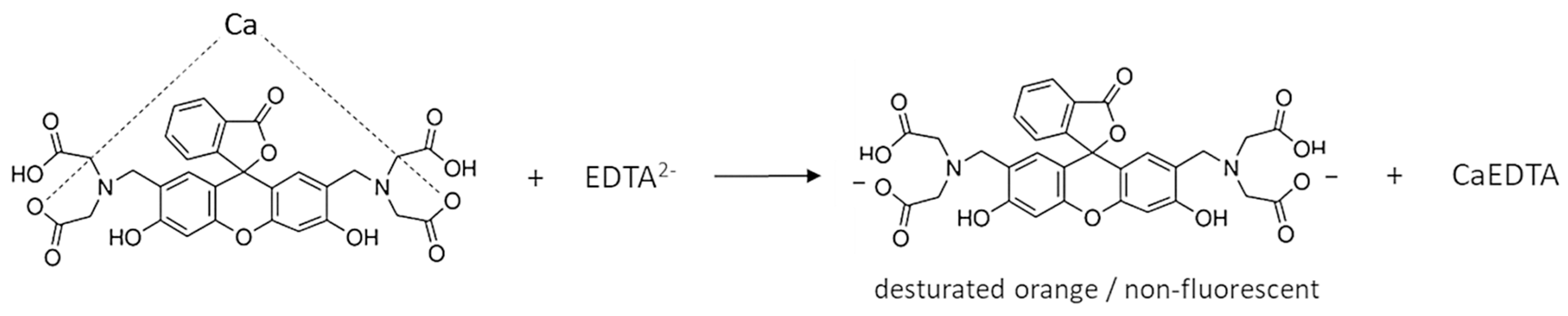
1. Introduction
2. Materials and Methods
2.1. Reagents, Instrumentation, and Lighting
2.2. Titration Procedure and Image Analysis
2.3. AAS Measurements
3. Results and Discussion
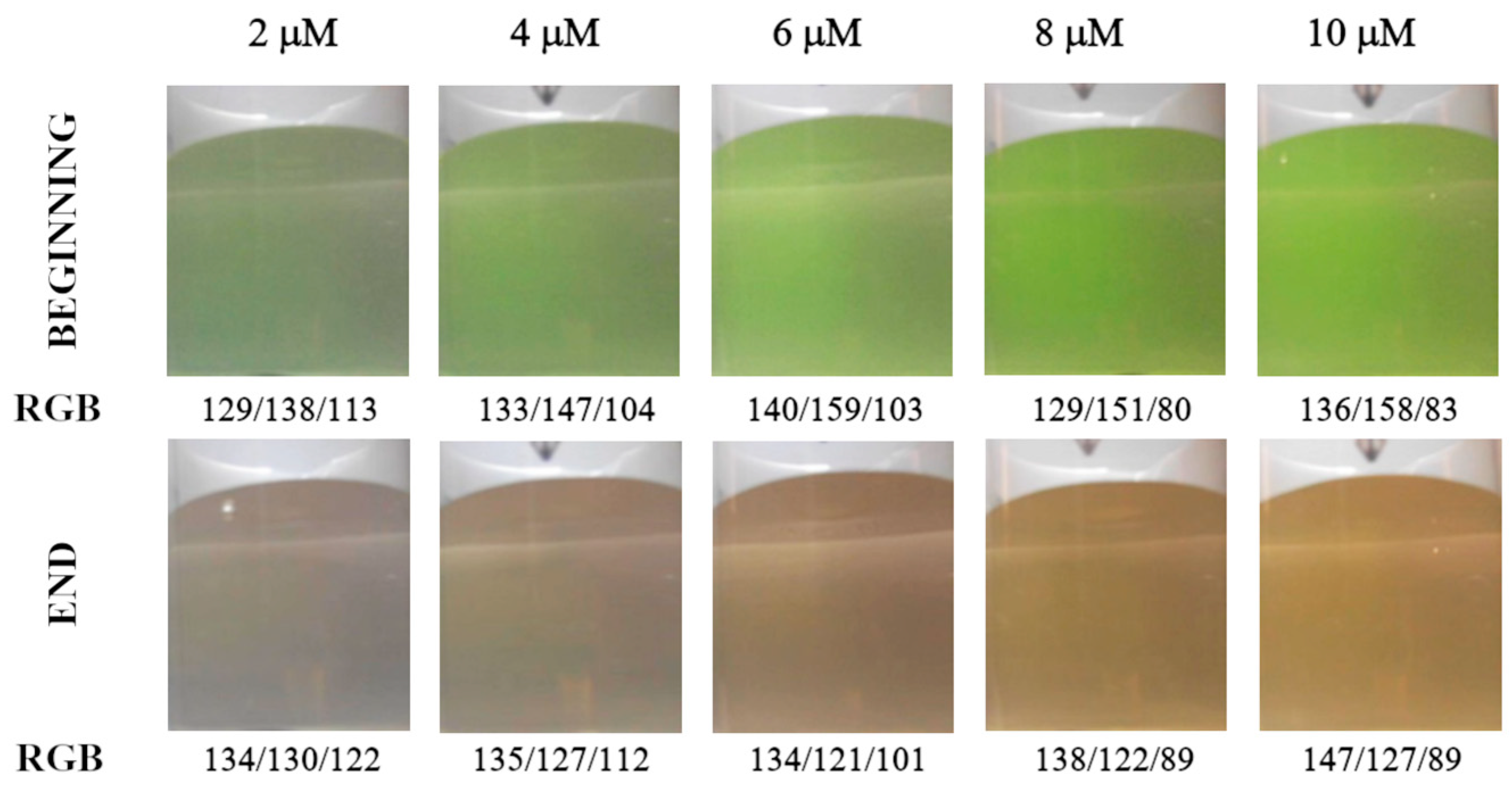
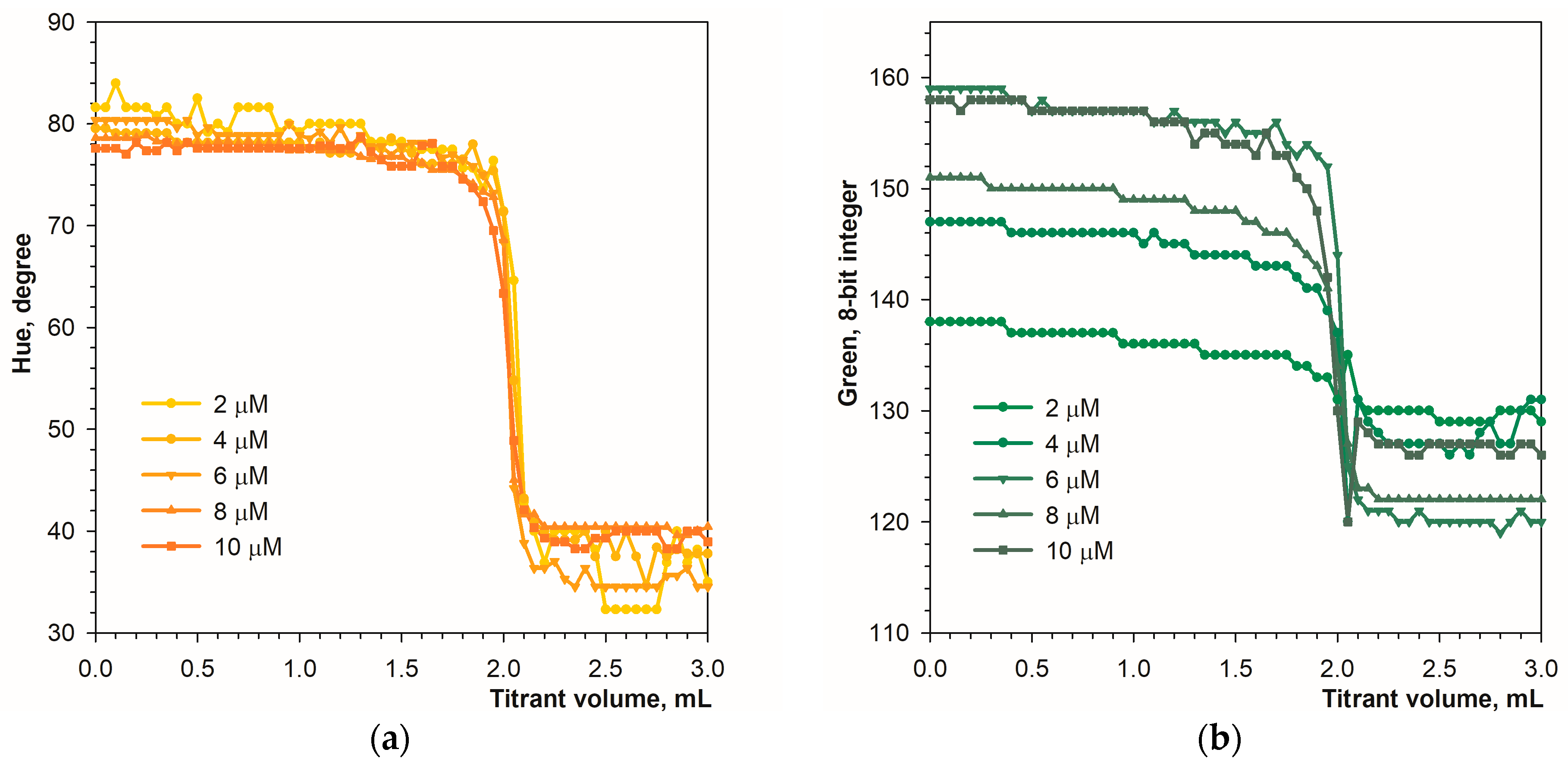
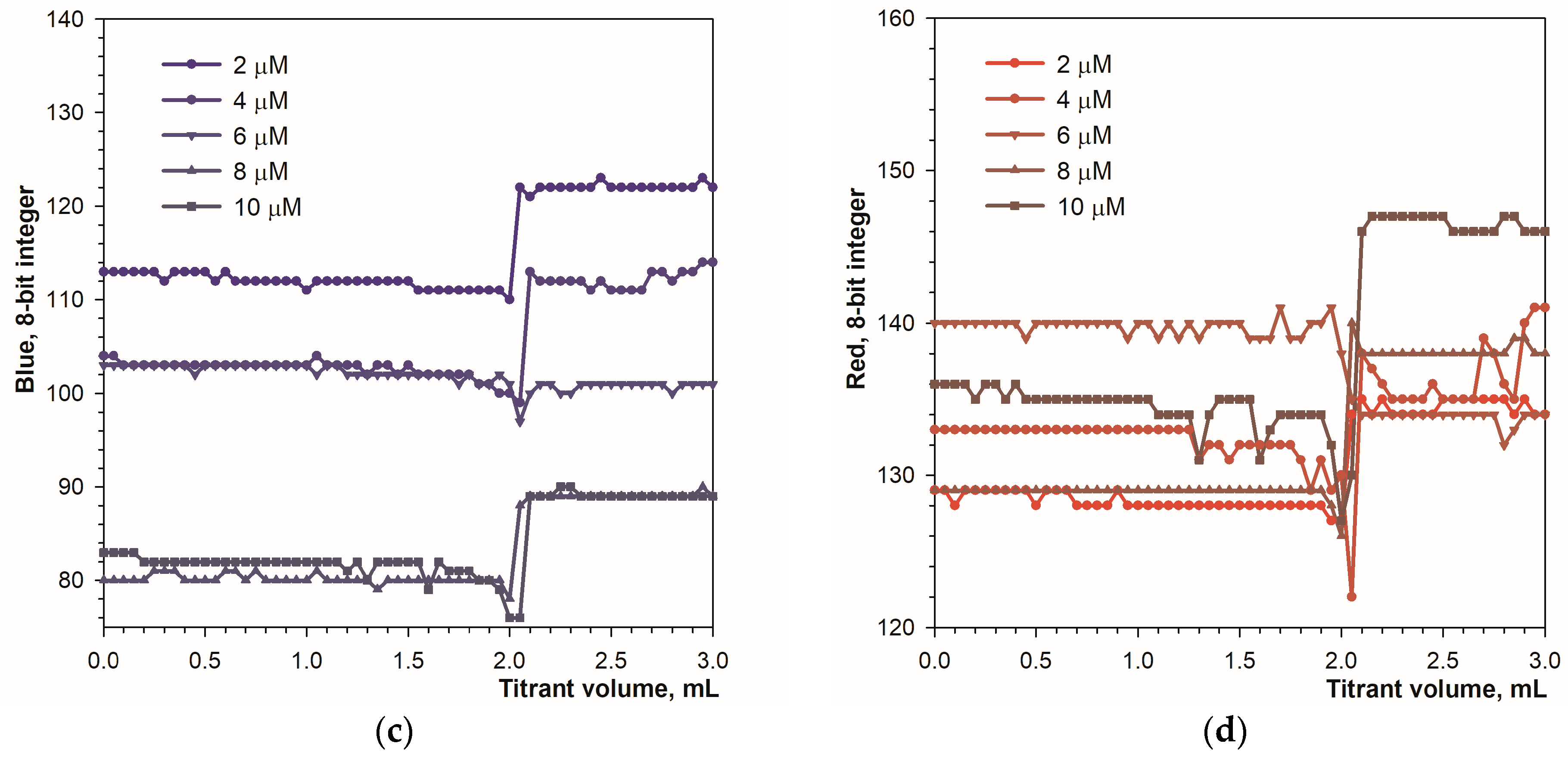
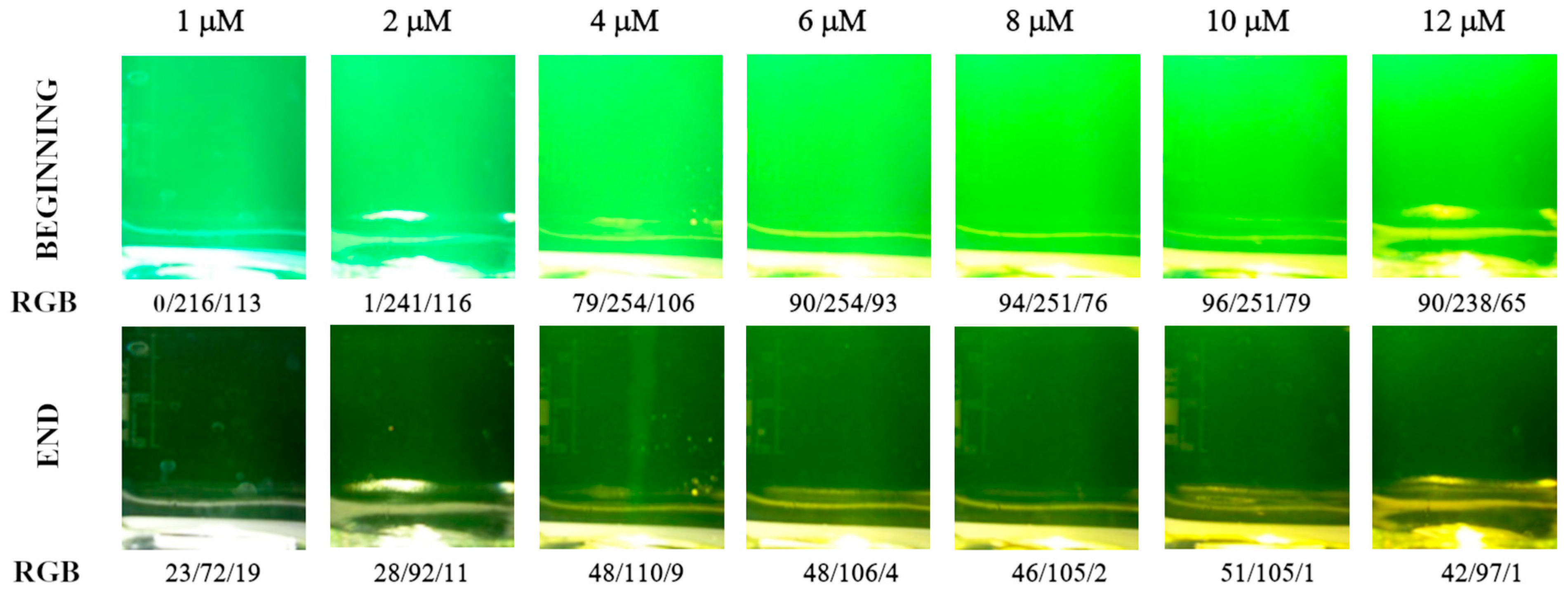
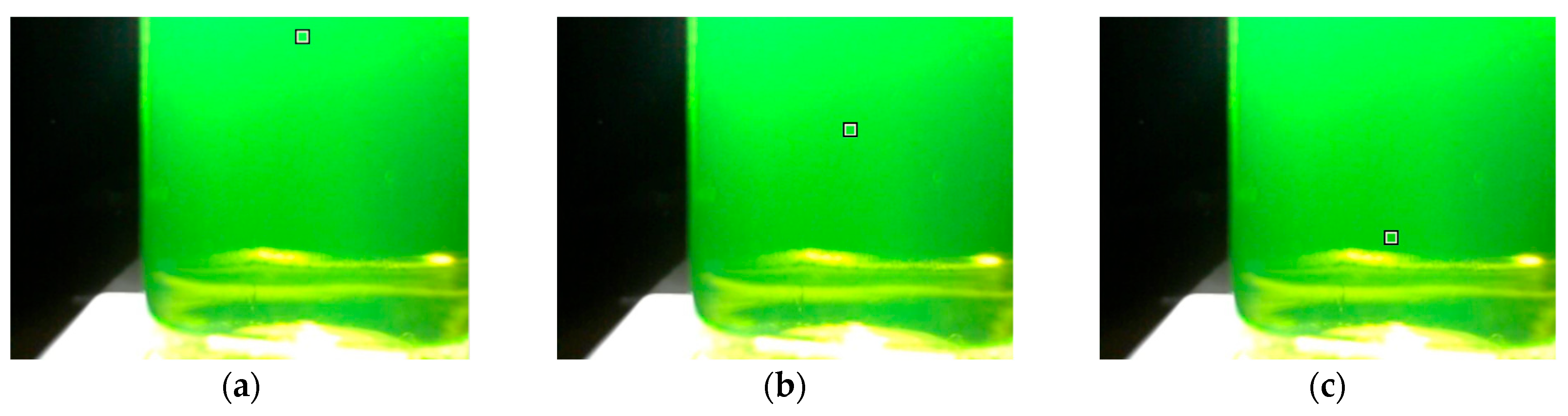
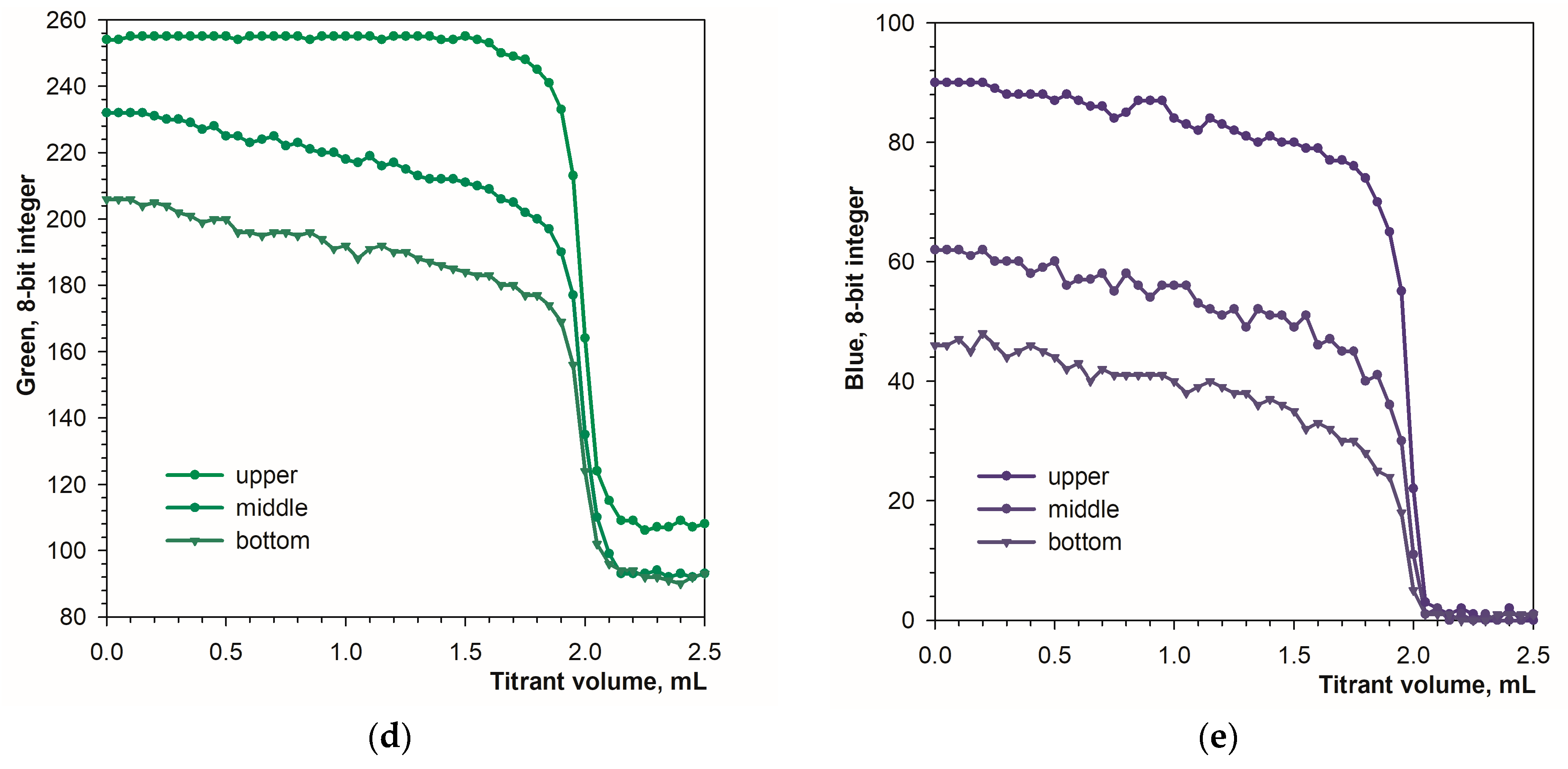
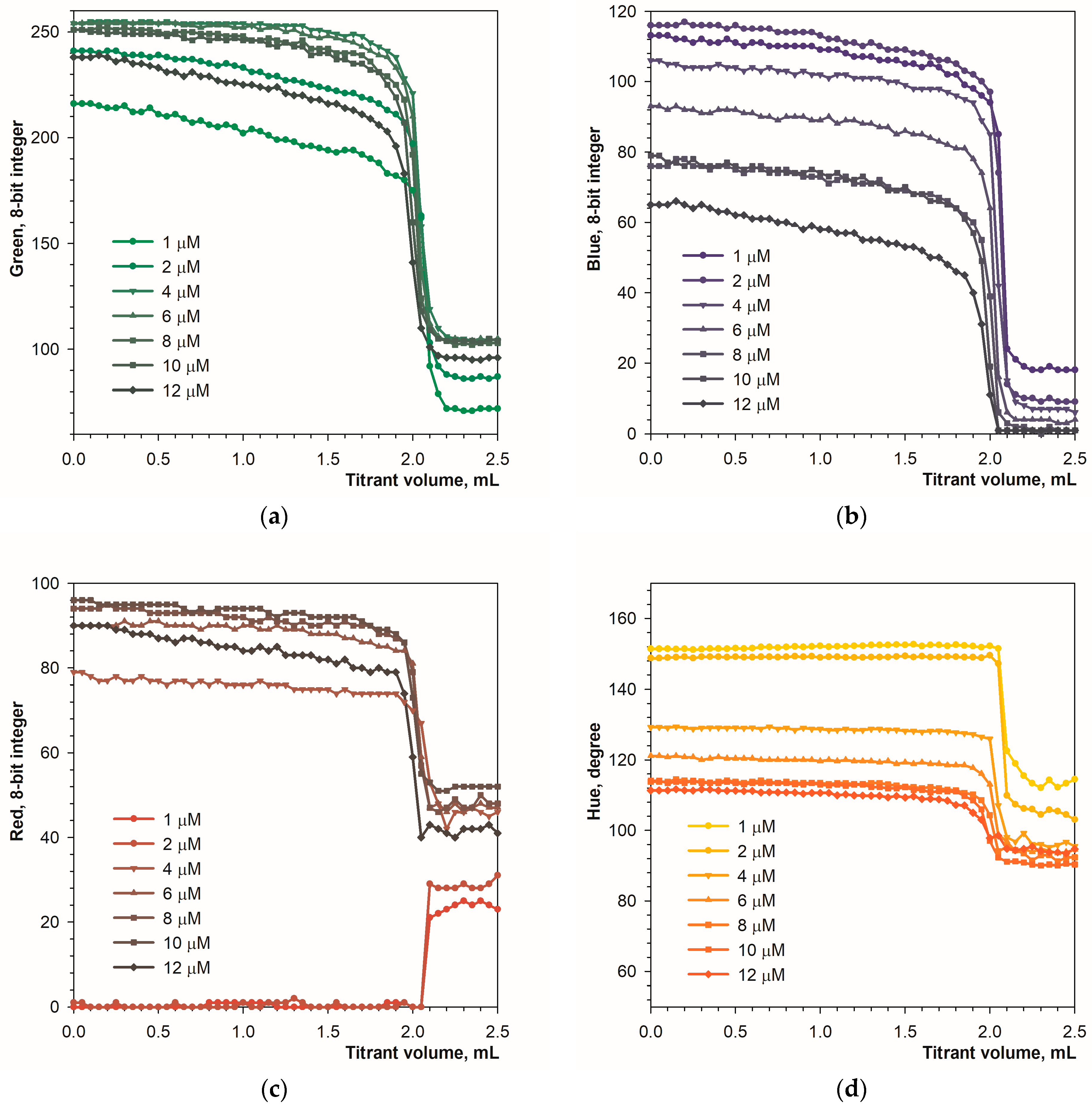
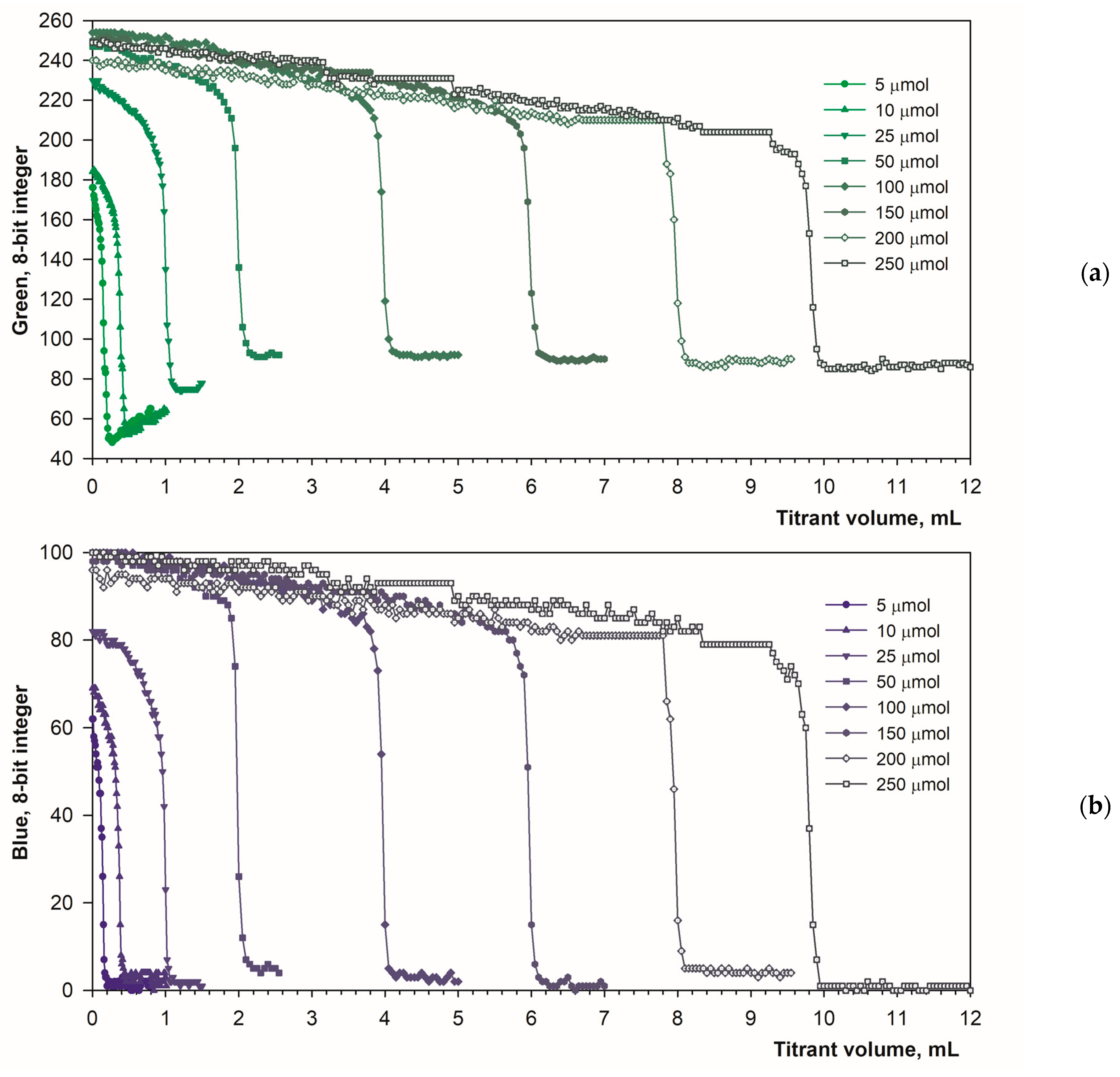
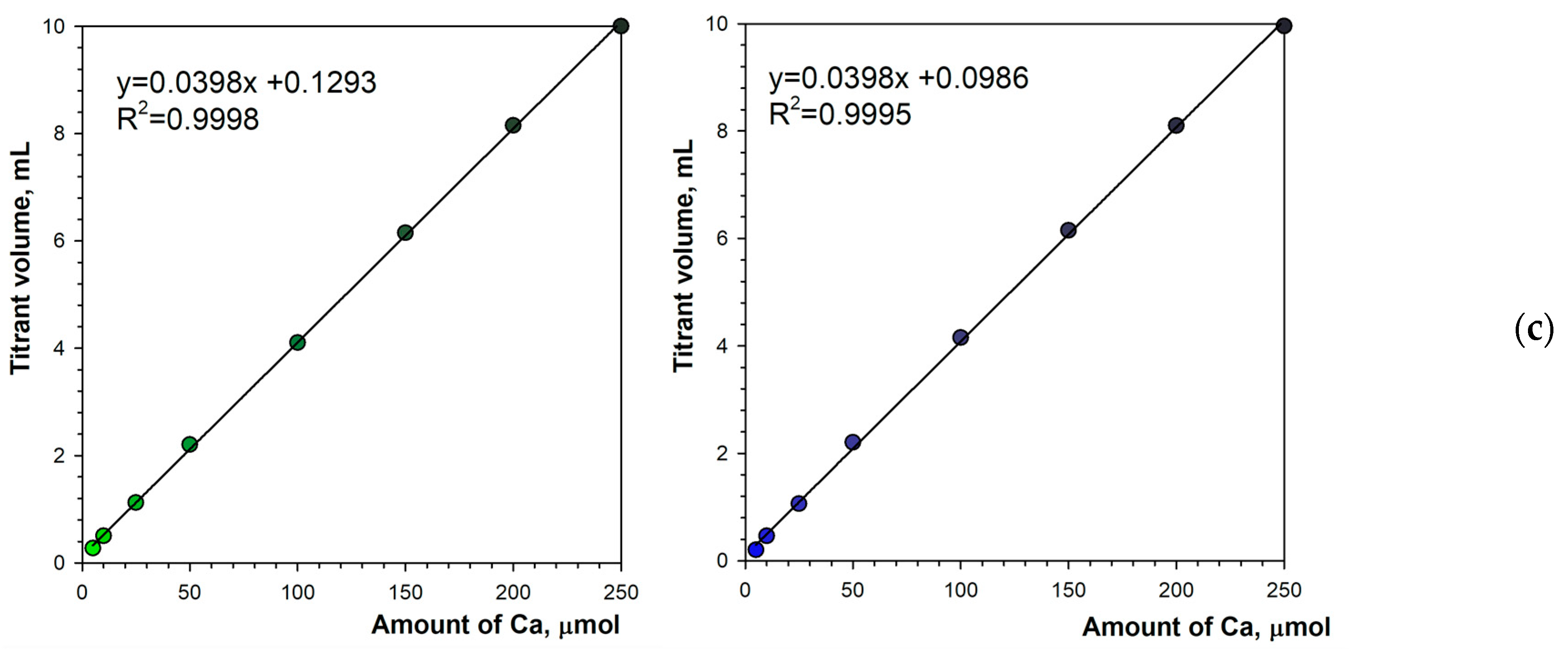
3.1. Detecting the Endpoint in Diffuse Reflection Mode
3.2. Detecting the Endpoint in Fluorescence Mode
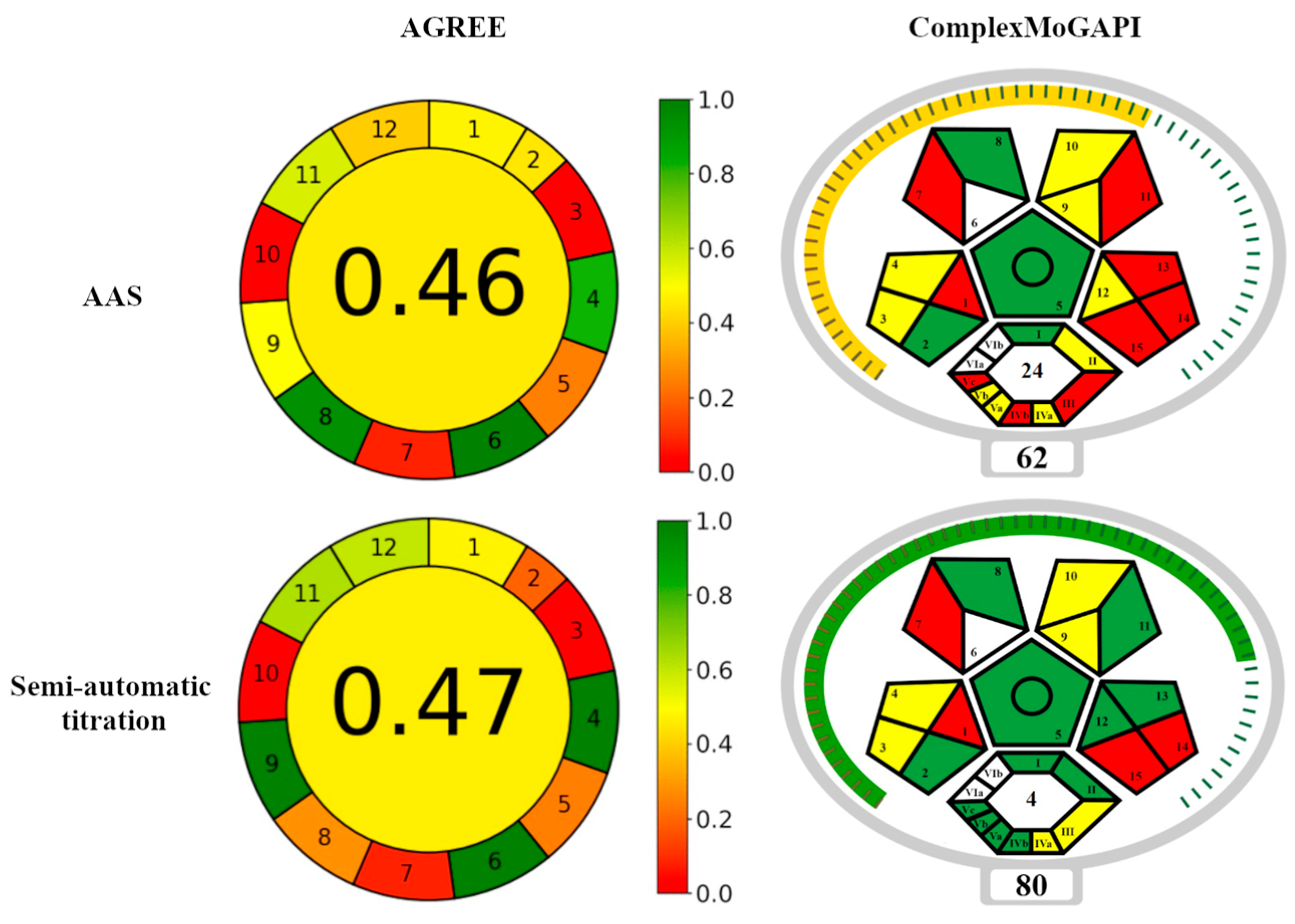
3.3. The Environmental Friendliness of the Titration Procedure
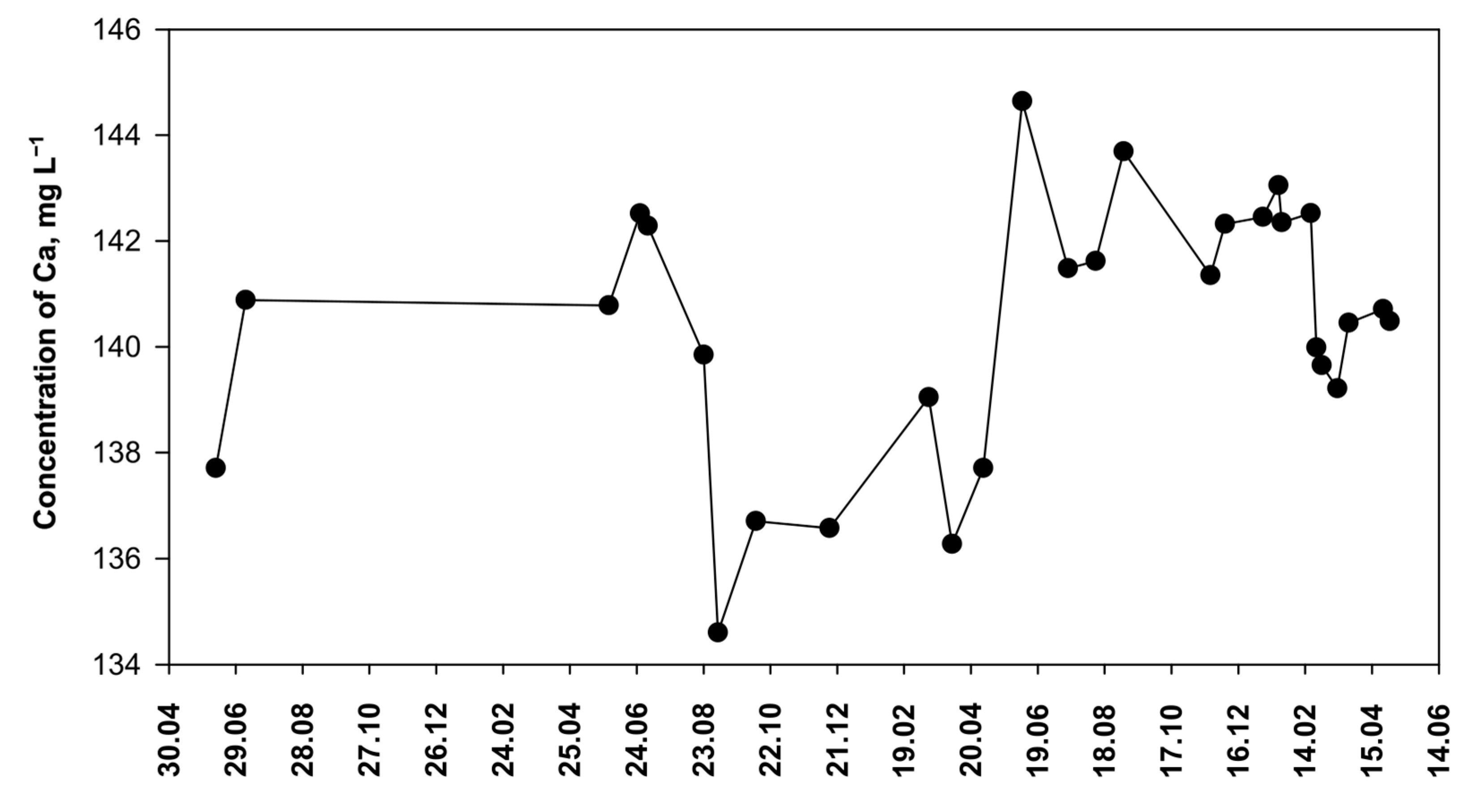
3.4. Determination of Calcium in Mineral Water Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hori, M.; Shozugawa, K.; Takizawa, T.; Watanabe, Y. Distribution of inorganic compositions of Japanese tap water: A nationwide survey in 2019–2024. Sci. Rep. 2024, 14, 14167. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, D.; Cichy, W.; Dobrzyńska, M.; Przysławski, J.; Drzymała-Czyż, S. Reasonableness of Enriching Cow’s Milk with Vitamins and Minerals. Foods 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Bykowska-Derda, A.; Spychala, M.; Czlapka-Matyasik, M.; Sojka, M.; Bykowski, J.; Ptak, M. The Relationship between Mortality from Cardiovascular Diseases and Total Drinking Water Hardness: Systematic Review with Meta-Analysis. Foods 2023, 12, 3255. [Google Scholar] [CrossRef]
- Kongpharm, K.; Nakklay, P.; Kongtong, C.; Tanapumchai, P.; Prapkree, L.; Rueangsri, N.; Singhato, A. Impacts of people at-risk of either cow milk allergies or lactose intolerance on their daily calcium intake and bone mineral density. Front. Nutr. 2024, 11, 1421275. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishy, S.M.; Shidfar, F. The Impact of Dairy Consumption on Anthropometric Indices. In Handbook of Public Health Nutrition; Preedy, V.R., Patel, V.B., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Ahmad, W.; Iqbal, J.; Nasir, M.J.; Ahmad, B.; Khan, M.T.; Khan, S.N.; Adnan, S. Impact of land use/land cover changes on water quality and human health in district Peshawar Pakistan. Sci. Rep. 2021, 11, 16526. [Google Scholar] [CrossRef]
- Mishra, S.; Chauhan, M.S.; Sundaramurthy, S. Assessing groundwater quality dynamics in Madhya Pradesh: Chemical contaminants and their temporal patterns. Environ. Res. 2024, 252, 118887. [Google Scholar] [CrossRef]
- Ocaña-Reyes, J.A.; Gutiérrez, M.; Paredes-Espinosa, R.; Riveros, C.A.; Cárdenas, G.P.; Bravo, N.; Quispe-Tomas, A.; Amaringo-Cordova, L.P.; Ocaña-Canales, J.C.; Zavala-Solórzano, J.W.; et al. Tillage Practices and Liming: Comparative Study of Soil Properties and Forage Corn Production. Agronomy 2024, 14, 558. [Google Scholar] [CrossRef]
- Wang, J.; Aalaei, K.; Skibsted, L.H.; Ahrné, L.M. Bioaccessibility of calcium in freeze-dried yogurt based snacks. LWT 2020, 129, 109527. [Google Scholar] [CrossRef]
- Wang, J.; Aalaei, K.; Skibsted, L.H.; Ahrné, L.M. Calcium bioaccessibility increased during gastrointestinal digestion of α-lactalbumin and β-lactoglobulin. Food Res. Int. 2023, 164, 112415. [Google Scholar] [CrossRef]
- Wang, J.; Munk, M.B.; Skibsted, L.H.; Ahrné, L.M. Impact of pectin and whey minerals solubilized by lime juice on calcium bioaccessibility in yogurt based snacks. Food Hydrocolloid 2022, 131, 107817. [Google Scholar] [CrossRef]
- Shao, J.; Wang, M.; Zhang, G.; Zhang, B.; Hao, Z. Preparation and characterization of sesame peptide-calcium chelate with different molecular weight. Int. J. Food Prop. 2022, 25, 2198–2210. [Google Scholar] [CrossRef]
- Qian, K.; Song, Y.; Lai, J.; Qian, X.; Zhang, Z.; Liang, Y.; Ruan, S. Characterization of historical mortar from ancient city walls of Xindeng in Fuyang, China. Constr. Build. Mater. 2022, 315, 125780. [Google Scholar] [CrossRef]
- Schäfer, J.; Hinrichs, J.; Kohlus, R.; Huppertz, T.; Atamer, Z. Pilot scale processing and characterisation of calcium-reduced micellar casein concentrate powders. Int. Dairy. J. 2021, 113, 104888. [Google Scholar] [CrossRef]
- Kozak, J.; Paluch, J.; Kozak, M.; Kochana, J.; Wieczorek, M.; Kościelniak, P. Novel Simultaneous Determination of Calcium and Magnesium Based on Flow Injection Gradient Titration. Anal. Lett. 2018, 51, 2157–2172. [Google Scholar] [CrossRef]
- Rosales-Conrado, N.; Peña-Martínez, J. Adding Sustainability in Analytical Chemistry Education through Monitoring Aquarium Water Quality. Sustain. Chem. 2023, 4, 282–303. [Google Scholar] [CrossRef]
- Zhai, W.; Lin, D.; Mo, R.; Zou, X.; Zhang, Y.; Zhang, L.; Ge, Y. Process Optimization, Structural Characterization, and Calcium Release Rate Evaluation of Mung Bean Peptides-Calcium Chelate. Foods 2023, 12, 1058. [Google Scholar] [CrossRef]
- Ouyang, Q.; Jiang, Y.; Liu, L.; Cai, C.; Bandara, N.; Li, P.; Wu, K.; Hong, H.; Chen, L. Preparation and characterization of Sipunculus nudus peptide-calcium chelate: Structural insights and osteogenic bioactivity assessment. J. Funct. Foods 2024, 122, 106497. [Google Scholar] [CrossRef]
- Bu, G.; Zhao, X.; Wang, M.; Ti, G.; Chen, F.; Duan, X.; Huang, Y.; Li, P. Identification of calcium chelating peptides from peanut protein hydrolysate and absorption activity of peptide–calcium complex. J. Sci. Food Agr. 2024, 104, 6676–6686. [Google Scholar] [CrossRef]
- Dai, H.; Cao, Y.; Fu, Y.; Tang, M.; Feng, X.; Ma, L.; Zhang, Y. Sustainable and one-pot fabrication of peptide chelated calcium from fish scale hydrolysates. Collagen Leather 2024, 6, 7. [Google Scholar] [CrossRef]
- Mueller, A.V.; Hemond, H.F. Statistical generation of training sets for measuring NO3−, NH4+ and major ions in natural waters using an ion selective electrode array. Environ. Sci.-Proc. Imp. 2016, 18, 590–599. [Google Scholar] [CrossRef]
- Tuan, V.; Khattak, A.; Zhu, H.; Gao, W.; Wang, M. Combination of Multivariate Standard Addition Technique and Deep Kernel Learning Model for Determining Multi-Ion in Hydroponic Nutrient Solution. Sensors 2020, 20, 5314. [Google Scholar] [CrossRef] [PubMed]
- Shyichuk, A.; Kowalska, M.; Shyychuk, I.; Lamkiewicz, J.; Ziółkowska, D. Determination of Calcium in Meat Products by Automatic Titration with 1,2-Diaminocyclohexane-N,N,N’,N’-tetraacetic Acid. Molecules 2023, 28, 6592. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.; Shah, H.S.; Sardhara, R.; Nahar, K.; Dave, R.H.; Morris, K.R. Protocol development, validation, and troubleshooting of in-situ fiber optic bathless dissolution system (FODS) for a pharmaceutical drug testing. J. Pharm. Biomed. Anal. 2021, 195, 113833. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Kumar, A.; Srivastava, M.; Srivastava, A.; Dwivedi, A.; Singh, R.K.; Srivastava, S.K. Recent advancements of smartphone-based sensing technology for diagnosis, food safety analysis, and environmental monitoring. Talanta 2024, 275, 126080. [Google Scholar] [CrossRef]
- Yadav, A.; Sagar, P.; Srivastava, M.; Srivastava, A.; Kumar, R.; Srivastava, S.K. A smartphone-enabled colorimetric sensor based on VS2 quantum dots for Rapid and on-site detection of ferric ions. Spectrochim. Acta A 2025, 329, 125609. [Google Scholar] [CrossRef]
- Lee, J.-K.; Lee, Y.-H.; Lee, D.-H. Proximal Absorbance Calibration Method Using an Embedded Blank Reference RGB Sensor for Determination of Ion Concentrations. Agriculture 2024, 14, 2171. [Google Scholar] [CrossRef]
- Qin, S.; Sun, X.; Zhao, X. Advances in smartphone-based biosensors for food testing. Curr. Opin. Food Sci. 2025, 61, 101236. [Google Scholar] [CrossRef]
- Danyliuk, N.; Tatarchuk, T.; Kannan, K.; Shyichuk, A. Optimization of TiO2-P25 photocatalyst dose and H2O2 concentration for advanced photo-oxidation using smartphone-based colorimetry. Water Sci. Technol. 2021, 84, 469–483. [Google Scholar] [CrossRef]
- Thanayutsiri, T.; Patrojanasophon, P.; Opanasopit, P.; Ngawhirunpat, T.; Chinsriwongkul, A.; Rojanarata, T. Green and facile short path smartphone-based assay for L-proline in dietary supplements using a mild chromogenic reaction combined with simple equipment. Sustain. Chem. Pharm. 2024, 42, 101787. [Google Scholar] [CrossRef]
- Keresteš, O.; Pohanka, M. A colour sensor integrated into a microcontroller platform as a reliable tool for measuring pH changes in biochemistry applications. Anal. Methods 2024, 16, 6487–6493. [Google Scholar] [CrossRef]
- Filgueiras, M.F.; de Oliveira Lima, B.; Borges, E.M. A high-throughput, cheap, and green method for determination of ethanol in cachaça and vodka using 96-well-plate images. Talanta 2022, 241, 123229. [Google Scholar] [CrossRef] [PubMed]
- Ruttanakorn, K.; Phadungcharoen, N.; Laiwattanapaisal, W.; Chinsriwongkul, A.; Rojanarata, T. Smartphone-based technique for the determination of a titration equivalence point from an RGB linear-segment curve with an example applica-tion to miniaturized titration of sodium chloride injections. Talanta 2021, 233, 122602. [Google Scholar] [CrossRef]
- Berasarte, I.; Bordagaray, A.; Garcia-Arrona, R.; Ostra, M.; Reis de Araujo, W.; Vidal, M. Microscale titration of acetic acid using digital colorimetry and paper-based analytical devices. Talanta 2024, 276, 126254. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, L.A.; Nunes, I.S.; Almeida Junior, P.L.; Lyra, W.S.; Andrade, R.A.N.; Araújo, M.C.U.; Almeida, L.F.; Lima, R.A.C. Accurate automatic titration procedure for low sharpness and dichroism in end point detection using digital movies as detection technique. Microchem. J. 2017, 133, 593–599. [Google Scholar] [CrossRef]
- Zhai, J.; Zhu, C.; Peng, X.; Xie, X. Ionophore-based Heterogeneous Calcium Optical Titration. Electroanalysis 2018, 30, 705–709. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Rathod, B.B. The Sound and Feel of Titrations: A Smartphone Aid for Color-Blind and Visually Im-paired Students. J. Chem. Educ. 2017, 94, 946–949. [Google Scholar] [CrossRef]
- Lima, R.A.C.; Almeida, L.F.; Lyra, W.S.; Siqueira, L.A.; Gaião, E.N.; Paiva Junior, S.S.L.; Lima, R.L.F.C. Digital movie-based on automatic titrations. Talanta 2016, 147, 226–232. [Google Scholar] [CrossRef]
- Zhai, J.; Xie, X.; Cherubini, T.; Bakker, E. Ionophore-Based Titrimetric Detection of Alkali Metal Ions in Serum. ACS Sens. 2017, 2, 606–612. [Google Scholar] [CrossRef]
- de Castro, C.M.; Olivi, P.; de Freitas Araújo, K.C.; Barbosa Segundo, I.D.; dos Santos, E.V.; Martínez-Huitle, C.A. Environmental application of a cost-effective smartphone-based method for COD analysis: Applicability in the electrochemical treatment of real wastewater. Sci. Total Environ. 2023, 855, 158816. [Google Scholar] [CrossRef]
- Kakiuchi, N.; Ochiai, J.; Takeuchi, M.; Tanaka, H. Inner product of RGB unit vectors for detecting color transition: Application to feedback-based flow ratiometric titration. Anal. Sci. 2022, 38, 623–626. [Google Scholar] [CrossRef]
- Boppana, N.P.D.; Snow, R.; Simone, P.S.; Emmert, G.L.; Brown, M.A. A low-cost automated titration system for colorimetric endpoint detection. Analyst 2023, 148, 2133–2140. [Google Scholar] [CrossRef]
- Lapanantnoppakhun, S.; Ganranoo, L.; Kradtap Hartwell, S.; Grudpan, K. Sequential Injection Ion Chromatography with Flow Injection Post Column Derivatization Capable of Using the Unstable Reagent Murexide to Determine Calcium and Magnesium in a Mixture. Chromatographia 2018, 81, 1269–1276. [Google Scholar] [CrossRef]
- Tian, C.; He, B.; Ye, F.; Wang, J.; Song, G. Using a decision-making approach to evaluate the effect of accelerators on calcium leaching of sprayed concrete. Eng. Fail. Anal. 2022, 138, 106349. [Google Scholar] [CrossRef]
- Vavrusova, M.; Liang, R.; Skibsted, L.H. Thermodynamics of Dissolution of Calcium Hydroxycarboxylates in Water. J. Agr. Food Chem. 2014, 62, 5675–5681. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, D.; Kaniewska, A.; Lamkiewicz, J.; Shyichuk, A. Determination of carrageenan by means of photometric titration with Methylene Blue and Toluidine Blue dyes. Carbohyd Polym. 2017, 165, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Agrawal, R.; Kotadiya, R. AQbD-guided stability indicating HPLC method for azelnidipine and chlorthalidone fixed-dose combination tablet: A green approach. J. Taibah Univ. Sci. 2024, 18, 2415156. [Google Scholar] [CrossRef]
- Mikawy, N.N.; Magdy, N.; Mohamed, M.H.; El-Kosasy, A.M. Green highly sensitive and selective spectroscopic detection of guaifenesin in multiple dosage forms and spiked human plasma. Sci. Rep. 2024, 14, 18694. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Syrgabek, Y.; Alimzhanova, M.; García-Encina, P.A.; Jiménez, J.J.; López-Serna, R. Greenness evaluation of sample preparation methods by GAPI for the determination of pesticides in grape: A review. Trends Environ. Anal. Chem. 2023, 39, e00206. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green. Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Mansour, F.R.; Omer, K.M.; Płotka-Wasylka, J. A total scoring system and software for complex modified GAPI (ComplexMoGAPI) application in the assessment of method greenness. Green. Anal. Chem. 2024, 10, 100126. [Google Scholar] [CrossRef]
- Siepak, M.; Lewandowska, A.; Sojka, M. Variability in the Chemical Composition of Spring Waters in the Postomia River Catchment (Northwest Poland). Water 2022, 15, 157. [Google Scholar] [CrossRef]
- Edet, A. Seasonal and spatio-temporal patterns, evolution and quality of groundwater in Cross River State, Nigeria: Implications for groundwater management. Sustain. Water Resour. Manag. 2019, 5, 667–687. [Google Scholar] [CrossRef]
- Mohammed, M.A.A.; Szabó, N.P.; Mikita, V.; Szűcs, P. Tracking the spatiotemporal evolution of groundwater chemistry in the Quaternary aquifer system of Debrecen area, Hungary: Integration of classical and unsupervised learning methods. Environ. Sci. Pollut. Res. 2025, 32, 6884–6903. [Google Scholar] [CrossRef]
- Sadeghi-Lari, A.; Bahrami, M.; Dastandaz, T. Temporal and spatial variations of groundwater quantity and quality for drinking and irrigation purposes in the arid and hot weather of Southern Iran. Phys. Chem. Earth 2024, 134, 103582. [Google Scholar] [CrossRef]
- Daneshvar Vousoughi, F.; Dinpashoh, Y.; Aalami, M.T.; Jhajharia, D. Trend analysis of groundwater using non-parametric methods (case study: Ardabil plain). Stoch. Environ. Res. Risk A 2013, 27, 547–559. [Google Scholar] [CrossRef]
- Reports of the Central Water and Sewage Testing Laboratory of the Municipal Water and Sewage Company in Bydgoszcz (Poland). Available online: https://bip.mwik.bydgoszcz.pl/w-zakresie-jakosci-wody-dostarczanej-odbiorcom/ (accessed on 14 May 2025).

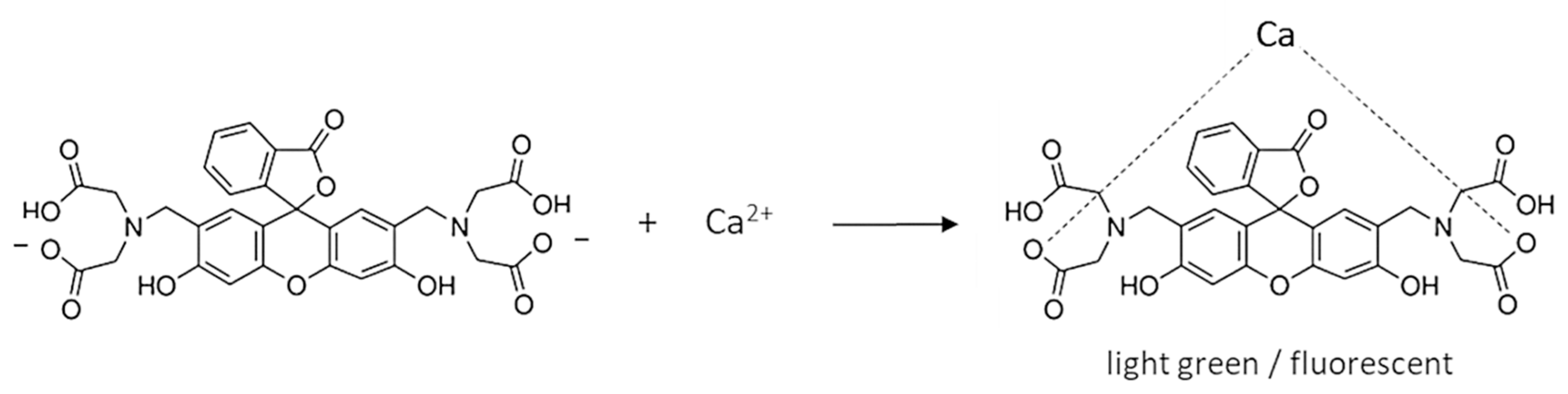

| Ca Amount, μmol | Signal | SD, μmol | RSD, % | Recovery, % |
|---|---|---|---|---|
| 50 | Green | 0.375 | 0.72 | 104.8 |
| 50 | Blue | 0.830 | 1.60 | 103.5 |
| 50 | Hue | 0.375 | 0.73 | 102.8 |
| 5 | Green | 0.305 | 6.07 | 100.5 |
| 5 | Blue | 0.250 | 5.00 | 100.0 |
| 5 | Hue | 0.100 | 2.08 | 96.0 |
| Ca Amount, μmol | Signal | SD, μmol | RSD, % | Recovery, % |
|---|---|---|---|---|
| 50 | Red | 1.63 | 3.14 | 103.7 |
| 50 | Green | 0.375 | 0.71 | 105.3 |
| 50 | Blue | 0.575 | 1.11 | 103.3 |
| 50 | Hue | 1.09 | 2.07 | 105.5 |
| 5 | Red | 0.295 | 5.92 | 100.0 |
| 5 | Green | 0.175 | 3.52 | 99.5 |
| 5 | Blue | 0.315 | 6.57 | 96.5 |
| 5 | Hue | 0.305 | 4.68 | 130.0 |
| Titration | AAS | |||
|---|---|---|---|---|
| Signal | Ca Amount, mg L−1 | Recovery, % | Ca Amount, mg L−1 | Recovery, % |
| Red | 10.52 | 105.2 | 9.72 | 97.2 |
| Green | 10.82 | 108.2 | ||
| Blue | 10.32 | 103.2 | ||
| Hue | 10.72 | 107.2 | ||
| Sample | Titration | AAS | ||||
|---|---|---|---|---|---|---|
| Signal | Mean Ca Concentration, mg L−1 | Intra-Day RSD, % | Inter-Day RSD, % | Mean Ca Concentration, mg L−1 | Intra-Day RSD, % | |
| A2 | Red | 2.89 | 7.44 | 9.35 | 2.70 | 1.98 |
| Green | 2.81 | 5.05 | 4.83 | |||
| Blue | 2.85 | 5.52 | 5.97 | |||
| Hue | 2.97 | 6.55 | 4.36 | |||
| C3 | Red | 302.2 | 3.05 | 1.15 | 301.1 | 0.95 |
| Green | 306.6 | 1.17 | 1.00 | |||
| Blue | 307.8 | 1.94 | 2.09 | |||
| Hue | 310.2 | 1.22 | 1.58 | |||
| Tap water | Red | 74.93 | 2.16 | 7.73 | 70.03 | 1.60 |
| Green | 75.45 | 1.04 | 0.96 | |||
| Blue | 73.95 | 1.90 | 2.62 | |||
| Hue | 76.25 | 1.37 | 1.00 | |||
| River water | Red | 79.53 | 1.99 | 4.38 | 74.38 | 0.64 |
| Green | 79.91 | 0.83 | 0.94 | |||
| Blue | 80.03 | 2.02 | 1.97 | |||
| Hue | 80.79 | 0.60 | 1.16 | |||
| Sample | Signal | Operator 1 | Operator 2 | ||
|---|---|---|---|---|---|
| Mean Ca Concentration, mg L−1 | RSD, % | Mean Ca Concentration, mg L−1 | RSD, % | ||
| C3 | Red | 302.2 | 3.05 | 308.6 | 2.22 |
| Green | 306.6 | 1.17 | 312.0 | 1.60 | |
| Blue | 307.8 | 1.94 | 312.0 | 2.31 | |
| Hue | 310.2 | 1.22 | 316.6 | 1.21 | |
| River water | Red | 79.53 | 1.99 | 87.37 | 17.22 |
| Green | 79.91 | 0.83 | 79.92 | 0.49 | |
| Blue | 80.03 | 2.02 | 78.96 | 1.87 | |
| Hue | 80.79 | 0.60 | 80.96 | 0.93 | |
| No | Principle | ASA | Semi-Automatic Titration |
|---|---|---|---|
| 1 | Direct Analytical Techniques Should Be Applied to Avoid Sample Treatment | Off-line analysis (2) | Off-line analysis (2) |
| 2 | Minimal Sample Size and Minimal Number of Samples Are Goals | 4 mL (1) | 25 mL (1) |
| 3 | In Situ Measurements Should Be Performed | Off-line (2) | Off-line (2) |
| 4 | Integration of Analytical Processes and Operations Saves Energy and Reduces the Use of Reagents | 4 steps (2) | 3 steps (2) |
| 5 | Automated and Miniaturized Methods Should Be Selected | Semi-automatic, not miniaturized (2) | Semi-automatic, not miniaturized (2) |
| 6 | Derivatization Should Be Avoided | No derivatization (2) | No derivatization (2) |
| 7 | Generation of a Large Volume of Analytical Waste Should Be Avoided and Proper Management of Analytical Waste Should Be Provided | 100 mL (2) | 100 mL (2) |
| 8 | Multianalyte or Multiparameter Methods Are Preferred versus Methods Using One Analyte at a Time | 1 analyte/run, 60 samples/h (2) | 1 analyte/run, 4 sample/h (2) |
| 9 | The Use of Energy Should Be Minimized | Flame atomic absorption spectrometry (2) | Titration (2) |
| 10 | Reagents Obtained from Renewable Source Should Be Preferred | None of the reagents are from bio-based sources (2) | None of the reagents are from bio-based sources (2) |
| 11 | Toxic Reagents Should Be Eliminated or Replaced | Toxic reagents: 0.60 g (2) | Toxic reagents: 0.36 g (2) |
| 12 | The Safety of the Operator Should Be Increased | Toxic to aquatic life; highly flammable; explosive (2) | Toxic to aquatic life, corrosive (2) |
| No | Criterion | Score | |
|---|---|---|---|
| AAS | Semi-Automatic Titration | ||
| Sample Preparation | |||
| 1 | Collection | Off-line | Off-line |
| 2 | Preservation | None | None |
| 3 | Transport | Required | Required |
| 4 | Storage | Under normal conditions | Under normal conditions |
| 5 | Type of method: direct or indirect | No sample preparation | No sample preparation |
| 6 | Scale of extraction | Not applicable | Not applicable |
| 7 | Solvents/reagents used | Non-green solvents/reagents used | Non-green solvents/reagents used |
| 8 | Additional treatments | None | None |
| Reagents and Solvents | |||
| 9 | Amount | 10–100 (10–100 g) | 10–100 mL (10–100 g) |
| 10 | Health hazard | Moderately toxic; could cause temporary incapacitation; NFPA = 2 or 3 | Moderately toxic; could cause temporary incapacitation; NFPA = 2 or 3 |
| 11 | Safety hazard | Highest NFPA flammability, instability score is 4 | Highest NFPA flammability, instability score of 0 or 1. No special hazards |
| Instrumentation | |||
| 12 | Energy | ≤1.5 kW h per sample | ≤0.1 kW h per sample |
| 13 | Occupational hazard | Emission of vapors into the atmosphere | Hermetic sealing of analytical process |
| 14 | Waste | >10 mL (>10 g) | >10 mL (>10 g) |
| 15 | Waste treatment | No treatment | No treatment |
| 16 | Quantification | Procedure for qualification and quantification | Procedure for qualification and quantification |
| Yield and Conditions | |||
| I | Yield | >89% | >89% |
| II | Temperature/time | Room temperature, >1 h, Heating, <1 h | Room temperature, <1 h |
| Relation to Green Economy | |||
| III | Number of rules met | 1 | 3 |
| Reagents and Solvents | |||
| IVa | Health hazard | Moderately toxic; could cause temporary incapacitation; NFPA = 2 or 3 | Moderately toxic; could cause temporary incapacitation; NFPA = 2 or 3 |
| IVb | Safety hazard | Highest NFPA flammability, instability score is 4 | Highest NFPA flammability, instability score of 0 or 1 No special hazards |
| Instrumentation | |||
| Va | Technical setup | Additional setups/semi-advanced instruments used | Common setup |
| Vb | Energy | ≤1.5 kW h per sample | ≤0.1 kW h per sample |
| Vc | Occupational hazard | Emission of vapors into the atmosphere | Hermetic sealing of analytical process |
| Workup and Purification | |||
| VIa | Workup and purification of the end product | Not applicable | Not applicable |
| VIb | Purity | Not applicable | Not applicable |
| E-Factor | 24 | 4 | |
| Brand | Carbonation | Ca Content, mg L−1 | |
|---|---|---|---|
| Determined | Declared | ||
| A1 | non-carbonated | 156 | 162 |
| A2 | non-carbonated | 2.81 | 3.27 |
| AK | non-carbonated | 50.7 | 40.3 |
| C1 | non-carbonated | 111 | 130 |
| K1 | non-carbonated | 56.3 | 68.1 |
| KB1 | non-carbonated | 45.3 | 41.0 |
| M1 | non-carbonated | 34.7 | 37.4 |
| NZ | non-carbonated | 101 | 96.2 |
| O1 | non-carbonated | 144 | 123 |
| OM | non-carbonated | 144 | 148 |
| P1 | non-carbonated | 51.3 | 48.1 |
| S1 | non-carbonated | 152 | 124 |
| SE | non-carbonated | 128 | 107 |
| Z1 | non-carbonated | 41.3 | 41.7 |
| A2 | low-carbonated | 224 | 198 |
| C2 | carbonated | 128 | 130.3 |
| C3 | low-carbonated | 306.6 | 325.8 |
| K2 | carbonated | 75.3 | 65 |
| KB2 | carbonated | 45.3 | 41 |
| M2 | carbonated | 42.7 | 37.4 |
| MU1 | low-carbonated | 212 | 208 |
| MU2 | low-carbonated | 131 | 200 |
| O2 | carbonated | 142.7 | 123 |
| P2 | carbonated | 54.7 | 48.1 |
| S2 | low-carbonated | 111 | 155 |
| Z2 | highly carbonated | 60.0 | 59.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shyichuk, A.; Ziółkowska, D.; Lamkiewicz, J.; Kowalska, M. Calcium Determination by Complexometric Titration with Calcein Indicator Using Webcam for Endpoint Detection. Water 2025, 17, 1757. https://doi.org/10.3390/w17121757
Shyichuk A, Ziółkowska D, Lamkiewicz J, Kowalska M. Calcium Determination by Complexometric Titration with Calcein Indicator Using Webcam for Endpoint Detection. Water. 2025; 17(12):1757. https://doi.org/10.3390/w17121757
Chicago/Turabian StyleShyichuk, Alexander, Dorota Ziółkowska, Jan Lamkiewicz, and Maria Kowalska. 2025. "Calcium Determination by Complexometric Titration with Calcein Indicator Using Webcam for Endpoint Detection" Water 17, no. 12: 1757. https://doi.org/10.3390/w17121757
APA StyleShyichuk, A., Ziółkowska, D., Lamkiewicz, J., & Kowalska, M. (2025). Calcium Determination by Complexometric Titration with Calcein Indicator Using Webcam for Endpoint Detection. Water, 17(12), 1757. https://doi.org/10.3390/w17121757





