Influence of Distribution Spacing on Intraspecific Competition in the Brown Seaweed Sargassum thunbergii Along the Luhua Coast, China
Abstract
1. Introduction
2. Methods
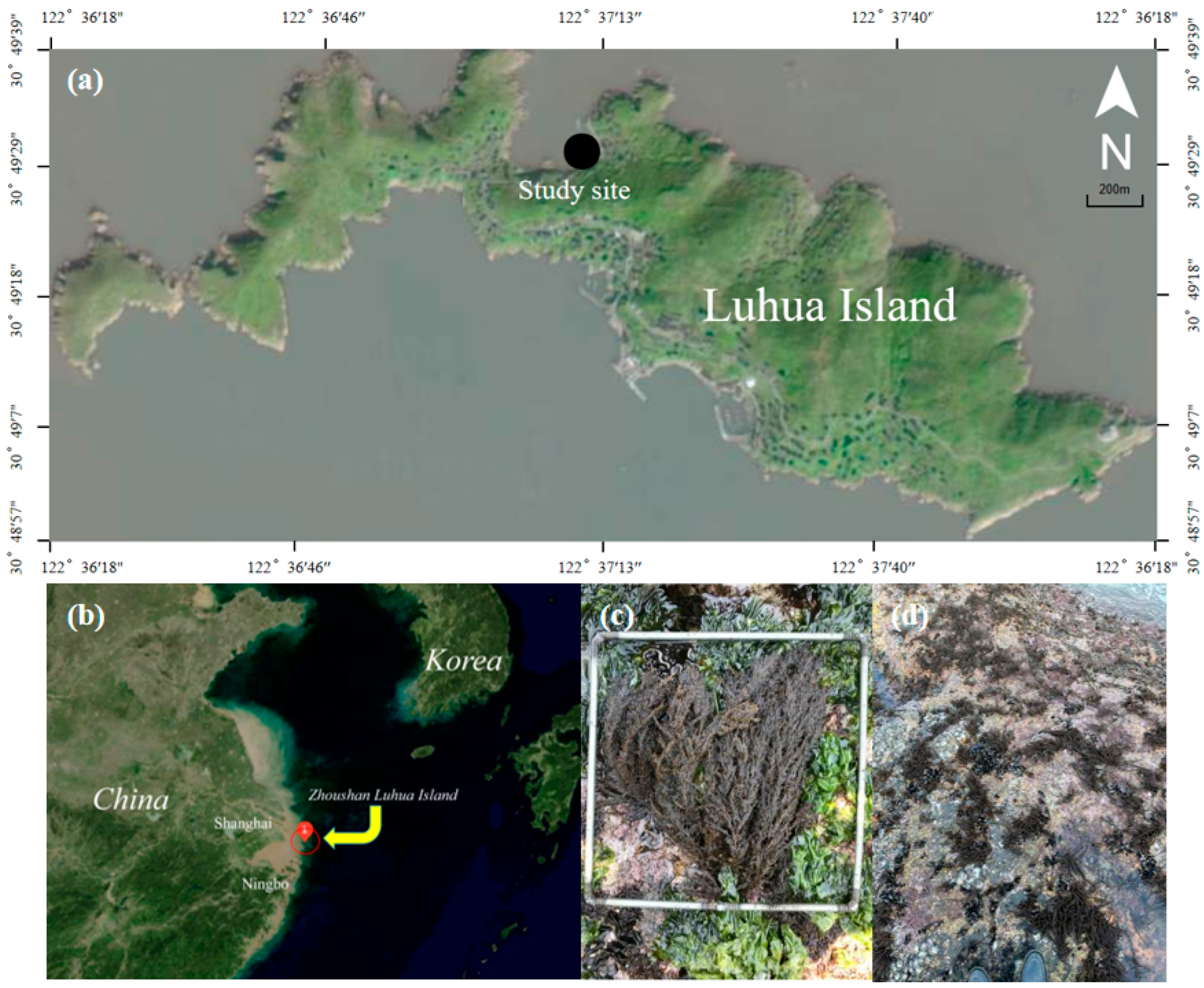
2.1. Study Site
2.2. Study Period
2.3. Determination of Competition Index
3. Results
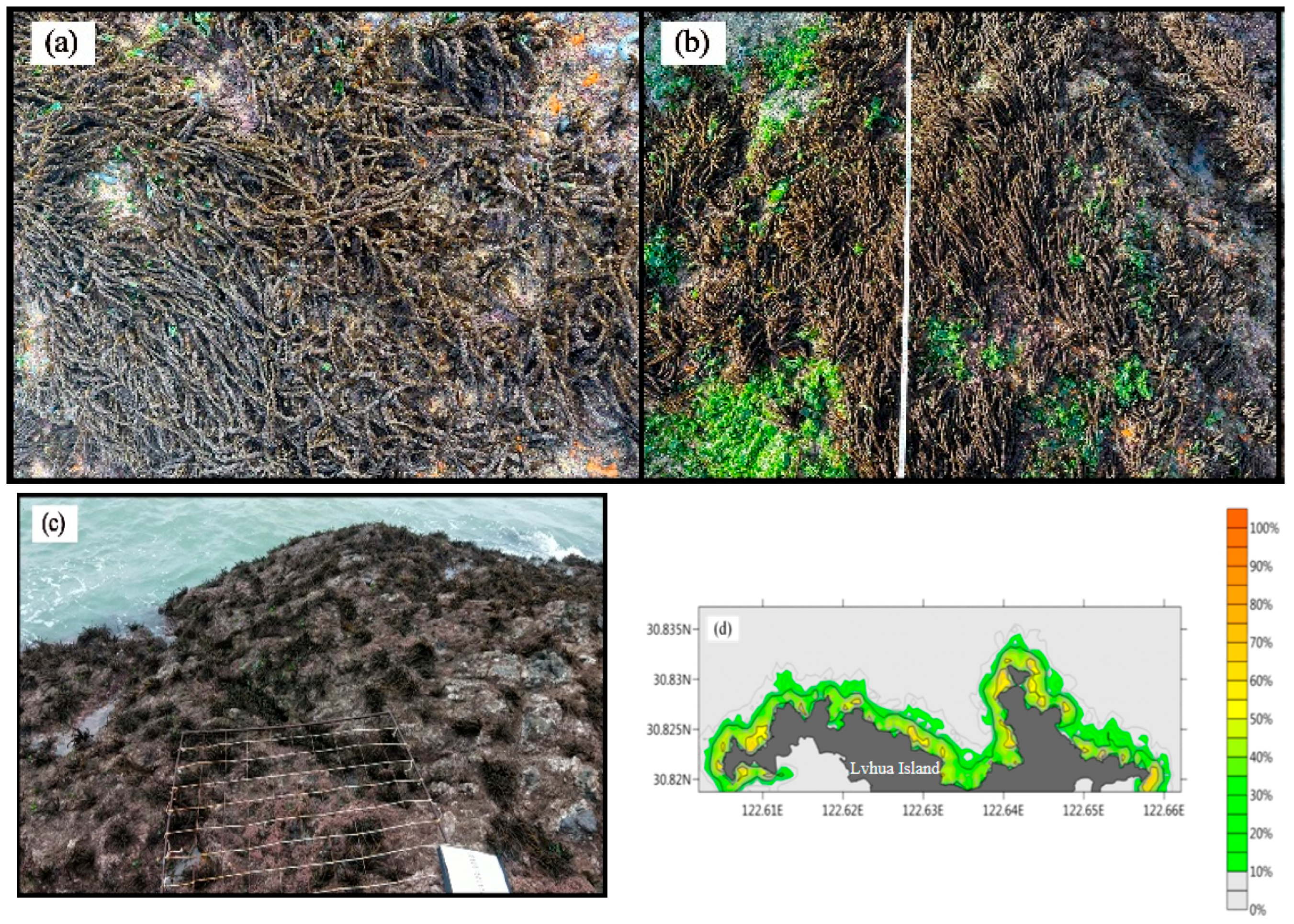
3.1. Distribution
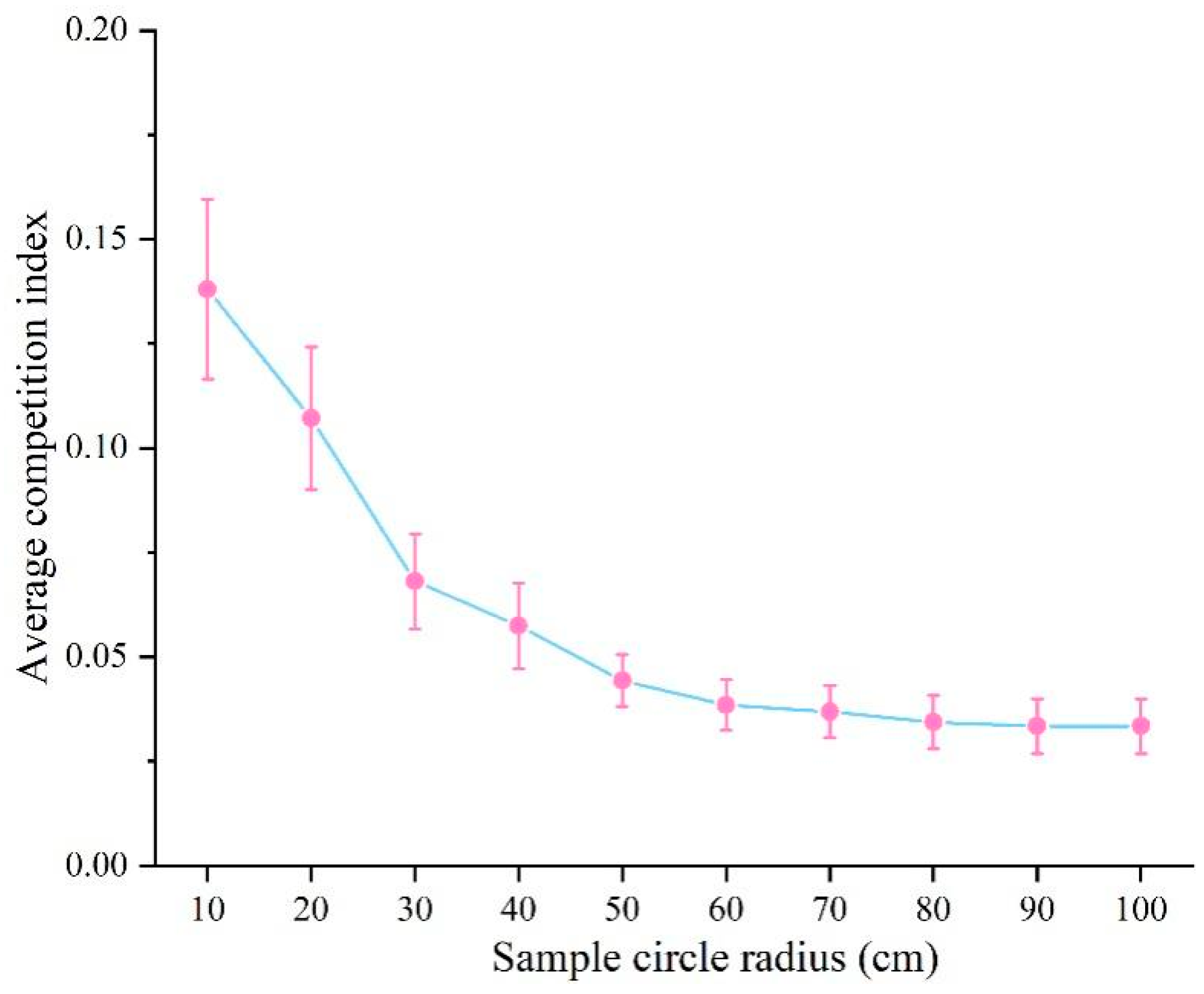
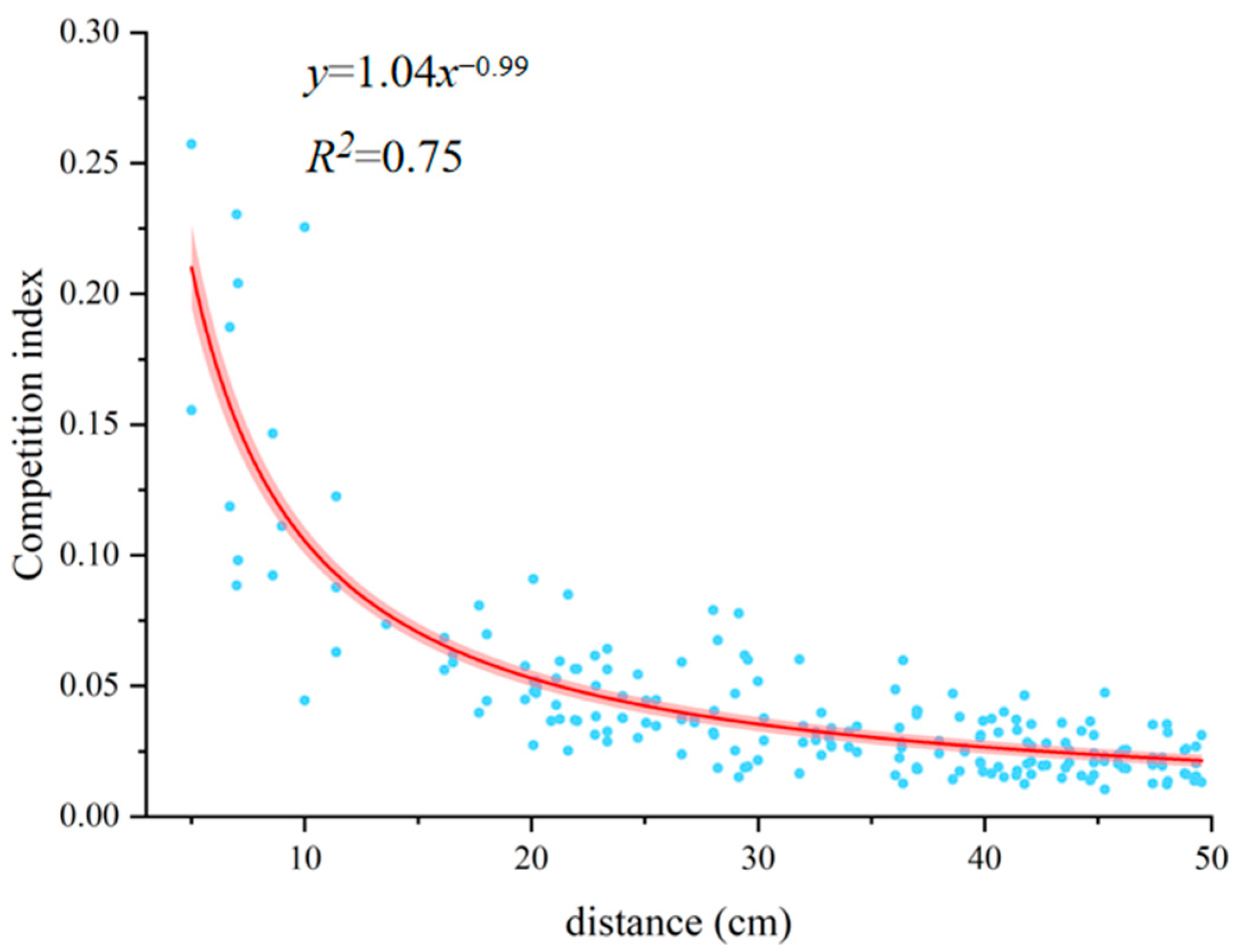
3.2. Optimal Competitive Distance
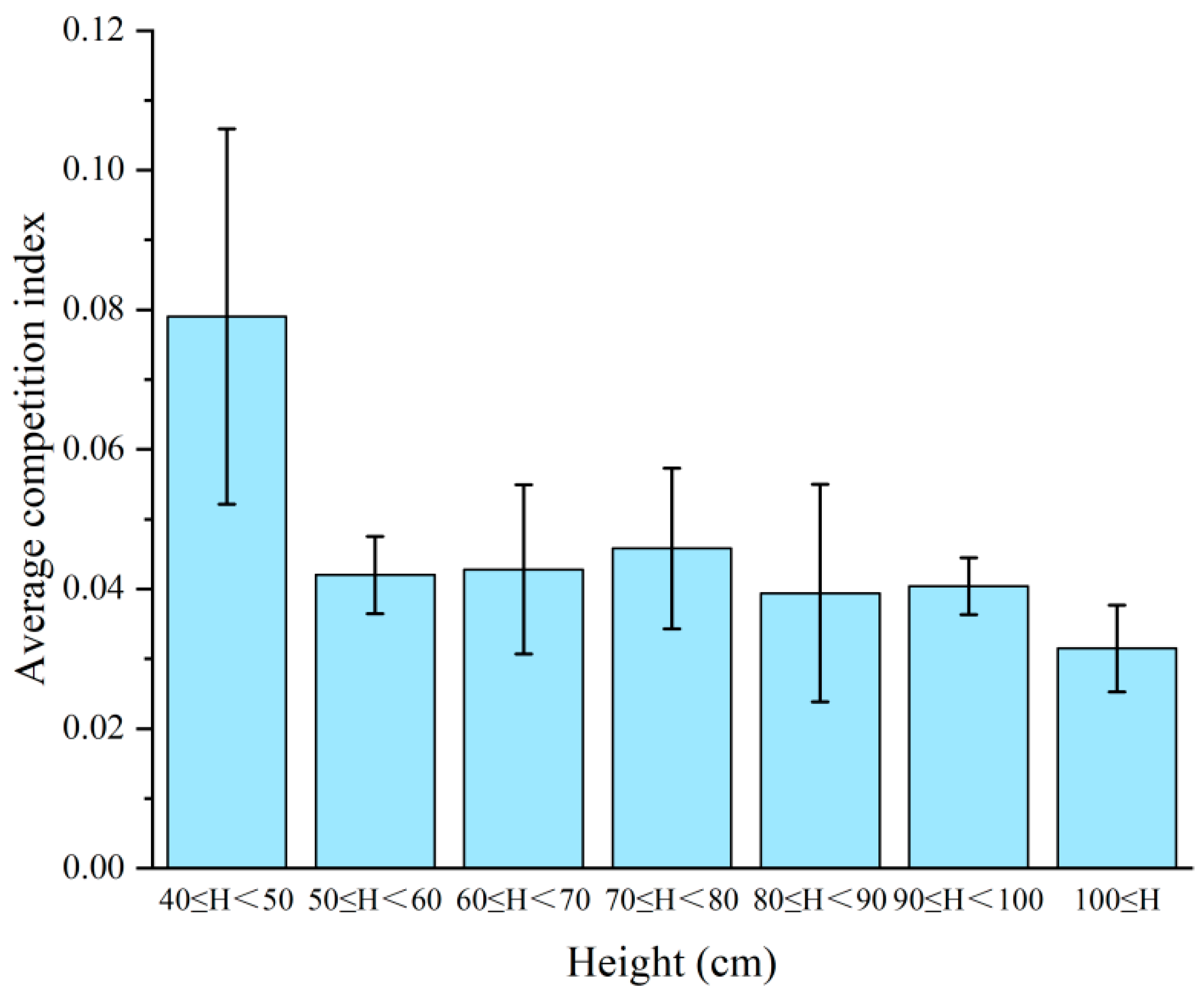
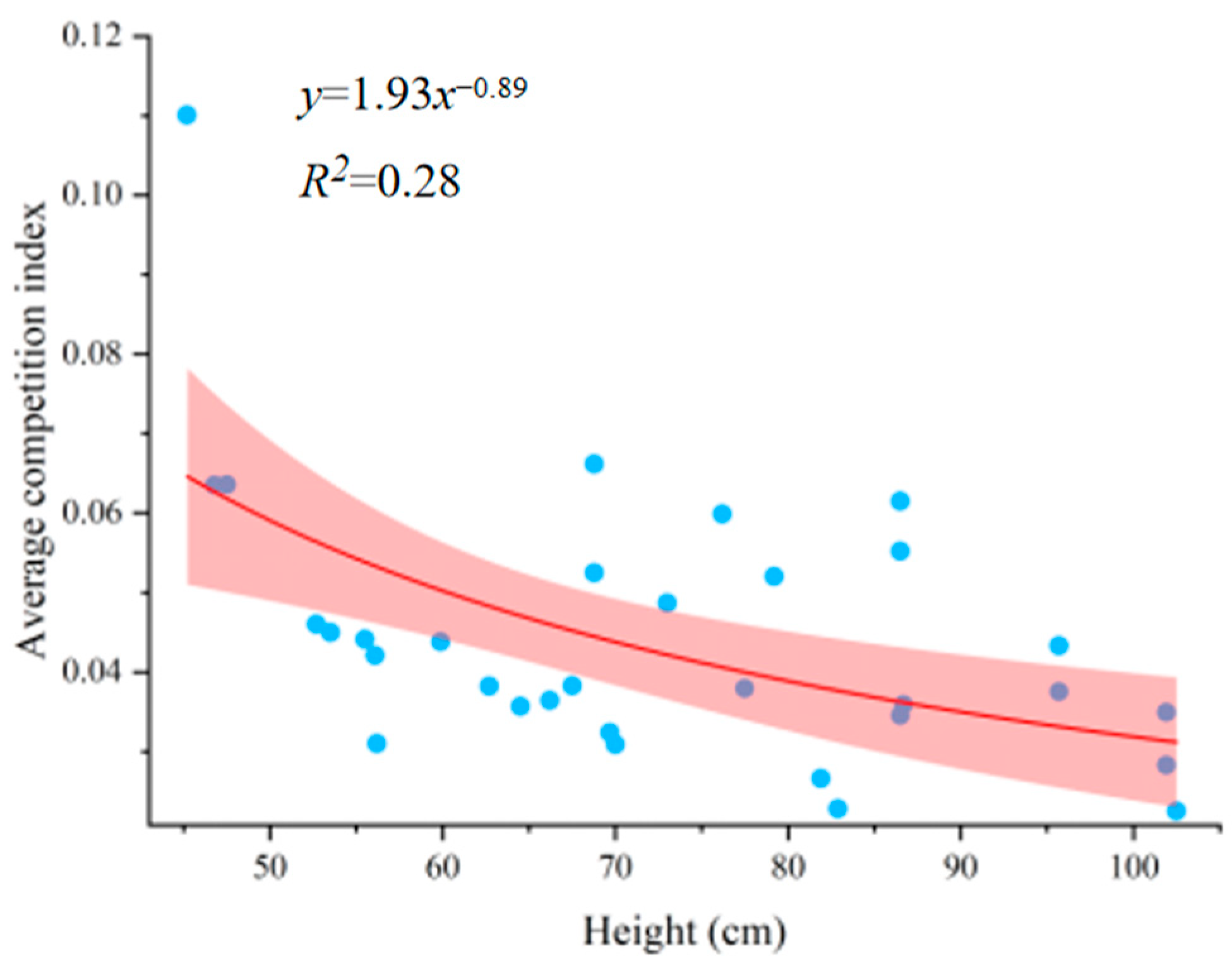
3.3. Optimal Competitive Height
3.4. Relationship Between the Competitive Index and Height of Sargassum thunbergii
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, S.Z. Ecological investigation of wild Sargassum thunbergii populations in Dalian, Qingdao and Wenzhou. Shanxi For. Sci. Technol. 2024, 53, 37–39. [Google Scholar]
- Liu, J.; Zhan, D.; Zhang, R.; Zeng, L.; Wang, G.; Hu, Z.-M. AFLP analysis revealed a north to south genetic break in the brown alga Sargassum thunbergii along the coast of China. J. Appl. Phycol. 2023, 30, 2697–2705. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Qin, S.; Liu, Z.Y.; Tang, J.; Xiao, S.; Zhong, Z. Response of carbon sequestration capacity of Sargassum thunbergii to water flow speed. Haiyang Xuebao 2022, 44, 113–122. [Google Scholar]
- Chen, H.F.; Zou, Q. Eulerian–Lagrangian flow-vegetation interaction model using immersed boundary method and OpenFOAM. Adv. Water Resour. 2019, 126, 176–192. [Google Scholar] [CrossRef]
- Chen, H.F.; Liu, X.F.; Zou, Q. Wave-driven flow induced by suspended and submerged canopies. Adv. Water Resour. 2019, 123, 160–172. [Google Scholar] [CrossRef]
- Rousset, O.; Lepart, J. Positive and negative interactions at different life stages of a colonizing species (Quercus humilis). J. Ecol. 2000, 88, 401–412. [Google Scholar] [CrossRef]
- Xiang, X.Y.; Wu, G.L.; Duan, R.Y.; Yan, Y.M.; Zhang, X.P. Intraspecific and interspecific competition of Pinus dabeshanesis. Acta Ecol. Sin. 2015, 35, 389–395. [Google Scholar]
- Rehling, F.; Sandner, T.M.; Matthies, D. Biomass partitioning in response to intraspecific competition depends on nutrients and species characteristics: A study of 43 plant species. J. Ecol. 2021, 109, 2219–2233. [Google Scholar] [CrossRef]
- Lin, F.X.; Xu, Q.L.; Qu, X.Y.; Huang, Y.; Chen, Y.; Lin, K.; Cao, G. Effects of Light Environment Differences on Understory Vegetation Diversity and Root Interspecific Competition of Young Cunning-hamia lanceolata Plantation. J. Southwest For. Univ. 2024, 44, 35–43. [Google Scholar]
- Wu, Z.L.; Cui, X.S.; Tang, F.H.; Xiong, M.S. Research on genecology of benthic macroalgae. Fish. Inf. Strategy 2018, 33, 36–44. [Google Scholar]
- Liu, Q.; Wang, X.B.; Li, L.X. Physiological response of intertidal marine macroalgae Ulvapertusa and Sargassum thunbergii to heavy metal copper stress. Mar. Sci. 2018, 42, 35–42. [Google Scholar]
- Zeng, C.K. Seaweed in the Yellow Sea and Bohai Seas of China; Science Press: Beijing, China, 2009; p. 395. [Google Scholar]
- Koh, C.H.; Kim, Y.; Kang, S.G. Size distribution, growth and production of Sargassum thunbergii in an intertidal zone of Padori, west coast of Korea. Hydrobiologia 1993, 260, 207–214. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Cui, X.; Wang, Z.H.; Wang, K.; Cheng, X.-P.; Liu, S.-R.; Shen, H. Community structure of epiphytic macroalgae on mussel culture rafts in Gouqi Island. J. Fish. China 2021, 45, 726–739. [Google Scholar]
- Chen, X.X.; Huang, Z.H.; Zhou, C.S.; Wu, Y.; Lu, R.M.; Zhang, P. Analysis of heavy metal and harmful element content characteristics of large seaweeds in Dongtou sea area. J. Zhejiang Agric. Sci. 2020, 61, 125–128. [Google Scholar]
- Yuan, Y.M.; Liu, F.L.; Liang, Z.R.; Zhang, P.; Liu, Y.; Zheng, Y.; Zhang, H. Study on the Physiological and Biochemical Influence of Sargassum thunbergii Under Dehydration. Prog. Fish. Sci. 2023, 44, 149–160. [Google Scholar]
- Yang, Y.F.; Luo, H.T.; Wang, Q.; He, Z.; Long, A. Large-scale Cultivation of Seaweed is Effective Approach to Increase Marine Carbon Sequestration and Solve Coastal Environmental Problems. Bull. Chin. Acad. Sci. 2021, 36, 259–269. [Google Scholar]
- Wu, H.Y.; Liu, H.J.; Zhang, D.M.; Li, M.Z.H. Research present situation of Sargassum thunbergii. Territ. Nat. Resour. Study 2010, 1, 95–96. [Google Scholar]
- Zhou, W.; Zhang, H.J.; Li, Z.D.; Zhao, Z.-G. Effects of dietary protein levels on growth in juvenile sea cucumber Apostichopus japonicus. J. Dalian Ocean Univ. 2010, 25, 359–364. [Google Scholar]
- Fraver, S.; D’Amato, A.; Bradford, J.; Jonsson, B.G.; Jönsson, M.; Esseen, P.-A. Tree growth and competition in an old - growth Picea abies forest of boreal Sweden: Influence of tree spatial patterning. J. Veg. Sci. 2013, 24, 374–385. [Google Scholar]
- Gossard, D.J.; Steller, D.L. Population persistence of the perennial kelp Eisenia arborea varies across local spatial scales. Algae 2022, 37, 63–74. [Google Scholar] [CrossRef]
- Chu, Y.Y.; Liu, Y.; Ren, L.Y.; Gong, Q.L. Ecological investigation of wild Sargassum thunbergii populations in Dalian, Qingdao and Wenzhou. Mar. Environ. Sci. 2019, 38, 840–847. [Google Scholar]
- Bi, Y.X.; Wang, W.D. Effects of wind wave on distribution pattern of Sargassum horneri around Gouqi Island. Chin. J. Ecol. 2016, 35, 1595–1600. [Google Scholar]
- Ou, J.M.; Wang, S.H.; Zhao, X.; Chen, J.; Sun, J.; Zou, Q.; Wang, K.; Zhang, S.; Wang, K. Response of photosynthetic activity to temperature rise and light quality of four intertidal macroalgae from Luhua Island. Zhejiang China J. Trop. Oceanogr. 2025, 44, 72–84. [Google Scholar]
- Sun, J.N.; Wang, Y.Q.; Zhang, S.Y.; Wang, K. Structure of macrobenthic community in the seaweed beds of Luhua Island waters and its response to environmental factors. J. Trop. Oceanogr. 2025, 1–16. [Google Scholar]
- Xue, J.; Li, T.; Su, J.Q.; Zhu, C.; Zhang, B.; Chen, S. Survey of economic marine macroalgae on intertidal areas of ten islands in Guangdong Province. South China Fish. Sci. 2024, 20, 98–106. [Google Scholar]
- Li, X.; Zhao, X.; Yuan, H.; Guo, Y.; Li, J.; Zhang, S.; Chen, J.; Wang, Z.; Wang, K. Diversity and carbon sequestration of seaweed in the Ma’an Archipelago. China Divers. 2022, 15, 12. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.J.; Zhang, S.L.; Wang, Z.B.; Dai, W.; Bi, X.D. The competition and allelopathy of Microcystis aeruginosa with Cyanoidetes longissima and Scenedesmus quadricauda. J. Tianjin Agric. Univ. 2023, 30, 32–38. [Google Scholar]
- Heggi, F. A Simulation Models for Managing Jack Pine Stands, Growth Models for Tree and Stand Simulation. For. Res. 1974, 30, 74–90. [Google Scholar]
- Xu, Y. Intraspecifie Competition of Quercus Wutaishanica and Janzen-Connell Effectin Loess Plateau; Northwest University: Xi’an, China, 2020. [Google Scholar]
- Yu, D.Y.; Fan, Z.F.; Ma, C.L.; Li, M.M. Intra-and Inter-specific Competition of Torreya yunnanensis Community. J. Northwest For. Univ. 2022, 37, 47–52. [Google Scholar]
- Zhao, Q.L.; Zhang, S.Z.; Wang, J.H.; YUN, H.L.; FENG, X.Q. Study on the effects of underground and aboveground competition of Catalpa ovata seedlings. Acta Bot. Boreal.-Occident. Sin. 2012, 32, 802–806. [Google Scholar]
- Liu, D.H.; Chen, Z.T.; Shu, X.Z.; Zhou, X.F.; Wang, G.F.; Zhong, X.H.; Huang, J.Z. Interaspecific and Intraspecific Competitions of Ormosia boluoensis with Extremely Small Population. For. Sci. Technol. 2024, 49, 14–18. [Google Scholar]
- Si, C.; Zhang, L.M.; Yu, F.H. Effects of physical space and nutrients on the growth and intraspecific competition of a floating fern. Aquat. Ecol. 2019, 53, 295–302. [Google Scholar] [CrossRef]
- Li, Z.H.; Hu, S.Y.; Li, W.; Li, Q. Intra-specifi c and inter-specific competitions among major trees species in Pinus massoniana and Schima superba mixed forest in northeastern Guangdong Province. J. Cent. South. Univ. For. Technol. 2013, 33, 91–95. [Google Scholar]
- White, L.F.; Shurin, J.B. Density dependent effects of an exotic marine macroalga on native community diversity. J. Exp. Mar. Biol. Ecol. 2011, 405, 111–119. [Google Scholar] [CrossRef]
- Chen, C.J.; Han, L.S.; Yao, M.K.; Shi, L.; Zhang, M.; Shi, Y.; Liu, C.; Bai, X.; Liu, X.; Liu, X.; et al. Comparative studies on antioxidant, angiotensin-converting enzyme inhibitory and anticoagulant activities of the methanol extracts from two brown algae (Sargassum horneri and Sargassum thunbergii). Russ. J. Mar. Biol. 2021, 47, 380–387. [Google Scholar]
- Zhu, L.H.; Zou, Q.P. Three-layer analytical solution for wave attenuation by suspended and nonsuspended vegetation canopy. In Proceedings of the 35th International Conference on Coastal Engineering, Antalya, Turkey, 17–20 November 2016. [Google Scholar]
- Schiel, D.R.; Foster, M.S. The population biology of large brown seaweeds: Ecological consequences of multiphase life histories in dynamic coastal environments. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 343–372. [Google Scholar] [CrossRef]
- Liu, F.L.; Li, J.-J.; Liang, Z.-R.; Zhang, Q.-S.; Zhao, F.-J.; Jueterbock, A.; Critchley, A.T.; Morrell, S.L.; Assis, J.; Tang, Y.-Z.; et al. A concise review of the brown seaweed Sargassum thunbergii-a knowledge base to inform large-scale cultivation efforts. J. Appl. Phycol. 2021, 33, 3469–3482. [Google Scholar] [CrossRef]
- Jin, Z.X.; Zhang, W.B. The quantitative relation of intraspecific and interspecific competition in endangered plant Heptacodium miconioides. Bull. Bot. Res. 2004, 24, 53–58. [Google Scholar]
- Liu, W.S.; Li, X.; Chen, F.Y.; Zhu, M.-T.; Mu, L.-Q. Intraspecific and Interspecific Competition of Quercus mongolica. Forest. Bull. Bot. Res. 2020, 40, 552–558. [Google Scholar]
- Xie, D.; Zou, Q.P.; Mignone, A.; MacRae, J.D. Coastal flooding from wave overtopping and sea level rise adaptation in the northeastern USA. Coast. Eng. 2019, 150, 39–58. [Google Scholar] [CrossRef]
- Zhu, L.H.; Huguenard, K.; Zou, Q.P.; Fredriksson, D.W.; Xie, D. Aquaculture farms as nature-based coastal protection: Random wave attenuation by suspended and submerged canopies. Coast. Eng. 2020, 160, 103737. [Google Scholar] [CrossRef]
- Yin, X.A.; Li, H. Study on interspecifie competition of algal species under combination of flow velocity and nutrient status. Yangtze River 2023, 54, 62–68. [Google Scholar]
- McConnico, L.A.; Foster, M.S. Population biology of the intertidal kelp, Alaria marginata Postels and Ruprecht: A non-fugitive annual. J. Exp. Mar. Biol. Ecol. 2005, 324, 61–75. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zou, Q.P.; Huguenard, K.; Fredriksson, D.W. Mechanisms for the asymmetric motion of submerged aquatic vegetation in waves: A consistent-mass cable model. J. Geophys. Res. Ocean. 2020, 125, e2019JC015517. [Google Scholar] [CrossRef]
- Cong, X.; Kuang, C.P.; Huang, G.W. Experimental study on the morphodynamic evolution of sandbar-lagoon system with emergent vegetation. Coast. Eng. 2023, 184, 104340. [Google Scholar] [CrossRef]

| Height (cm) | Objective Seaweed | Competitive Seaweed | ||||
|---|---|---|---|---|---|---|
| Percentage | Average Height | Wet Weight | Number | Percentage | Average Height | |
| 40 ≤ H < 50 | 9.38% | 46.50 ± 1.18 | 93.68 ± 26.13 | 19 | 4.40% | 74.17 |
| 50 ≤ H < 60 | 18.75% | 55.65 ± 2.53 | 109.01 ± 29.80 | 40 | 9.26% | 74.69 |
| 60 ≤ H < 70 | 21.88% | 66.89 ± 2.56 | 116.72 ± 51.27 | 81 | 18.75% | 74.61 |
| 70 ≤ H < 80 | 15.63% | 75.18 ± 3.68 | 161.23 ± 73.93 | 71 | 16.44% | 71.4 |
| 80 ≤ H < 90 | 18.75% | 85.17 ± 2.17 | 157.63 ± 77.45 | 104 | 24.07% | 72.47 |
| 90 ≤ H < 100 | 6.25% | 95.7 | / | 39 | 9.03% | 75.39 |
| 100 ≤ H | 9.38% | 102.1 ± 0.35 | 203.34 ± 6.74 | 78 | 18.06% | 71.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, F.; Zong, K.; Ni, J.; Lan, S.; Lu, J.; Daniel, T.; Feng, D.; Yang, X.; Zhang, G.; Mei, L.; et al. Influence of Distribution Spacing on Intraspecific Competition in the Brown Seaweed Sargassum thunbergii Along the Luhua Coast, China. Water 2025, 17, 1735. https://doi.org/10.3390/w17121735
Gui F, Zong K, Ni J, Lan S, Lu J, Daniel T, Feng D, Yang X, Zhang G, Mei L, et al. Influence of Distribution Spacing on Intraspecific Competition in the Brown Seaweed Sargassum thunbergii Along the Luhua Coast, China. Water. 2025; 17(12):1735. https://doi.org/10.3390/w17121735
Chicago/Turabian StyleGui, Fukun, Kai Zong, Jinhuai Ni, Sunzhaocong Lan, Jianpeng Lu, Tumusenge Daniel, Dejun Feng, Xu Yang, Guangyang Zhang, Lili Mei, and et al. 2025. "Influence of Distribution Spacing on Intraspecific Competition in the Brown Seaweed Sargassum thunbergii Along the Luhua Coast, China" Water 17, no. 12: 1735. https://doi.org/10.3390/w17121735
APA StyleGui, F., Zong, K., Ni, J., Lan, S., Lu, J., Daniel, T., Feng, D., Yang, X., Zhang, G., Mei, L., Li, J., Lin, X., Li, X., Chen, H., & Zou, Q. (2025). Influence of Distribution Spacing on Intraspecific Competition in the Brown Seaweed Sargassum thunbergii Along the Luhua Coast, China. Water, 17(12), 1735. https://doi.org/10.3390/w17121735









