Abstract
The presence of tetracycline hydrochloride (TC) in the environment poses significant risks to human health and ecological stability, necessitating the development of effective and rapid removal strategies. In this research, we investigate the efficacy of degrading tetracycline hydrochloride using cobalt-doped-biochar (Co-BC)-activated peroxymonosulfate (PMS) and the underlying mechanisms of this process. The research objectives and conclusions were as follows: (1) Co-BC materials were synthesized from balsa wood powder through a process of impregnation followed by high-temperature calcination. Characterization techniques such as SEM, XRD, FTIR, and XPS were used to confirm the material’s structure and composition. (2) In a TC solution of 20 mg L−1, the use of 100.0 mg L−1 of Co-BC and 1.0 mM PMS led to a TC degradation efficiency of 96.2% within 30 min. (3) The Co-BC+PMS system exhibited wide pH adaptability (4.34–9.02) and strong resistance to environmental matrix interference (Cl−, , and ). (4) Free-radical quenching experiments indicated that sulfate radicals () were the primary reactive species in TC degradation. The 11 intermediates of TC were analyzed using LC-MS, and two possible degradation pathways were deduced. In summary, this study offers significant, valuable insights into and technical support for the green, efficient, and environmentally friendly removal of antibiotics from sewage.
1. Introduction
Antibiotics, categorized as emerging pollutants, often accumulate as residuals in aquatic environments, posing potential risks to human health and aquatic ecosystems [1,2,3]. Tetracycline hydrochloride (TC), a broad-spectrum antibiotic characterized by its biotoxicity and resistance to degradation, is frequently detected in environmental water bodies [4,5]. Prolonged exposure to TC can induce antibiotic resistance in pathogenic bacteria, further threatening human health [6]. However, conventional wastewater treatment methods, including biodegradation, membrane separation, and adsorption, do not facilitate efficient TC removal [7,8]. Consequently, there is an urgent need to develop efficient and eco-friendly technologies to eliminate residual TC from water.
In recent decades, advanced oxidation processes (AOPs) have become increasingly prevalent in the degradation of various recalcitrant and toxic pollutants. This trend is attributed to their rapid degradation kinetics, environmental friendliness, ease of induction, lack of harmful byproducts, and cost-effectiveness [9,10]. Sulfate radicals () have drawn significant attention in water and wastewater treatment for their strong oxidizing power and high reactivity towards recalcitrant organic pollutants [11]. exhibits a high redox potential (2.5–3.1 V) [12], a relatively long half-life (3–4 × 10−5 s), and a broad pH reaction window [13]. Typically, permonosulfate (PMS, ) and peroxydisulfate (PDS, ) can be activated to generate through the cleavage of the peroxygen bond [14,15]. The literature suggests that PMS is more readily activated than PDS and demonstrates superior performance in the degradation of organic pollutants; this performance can be attributed to its asymmetric structure and longer O-O bonds [16]. However, the practical application of inactivated PMS is limited by its slow autoxidation kinetics [17]. Consequently, the development of catalysts with high catalytic activity and environmental adaptability for PMS activation is of critical importance [18,19].
Biochar (BC), an environmentally friendly material characterized by its abundant raw materials, straightforward preparation process, and low cost, is widely used for the catalytic activation of PMS [20,21]. However, pure biochar has few active sites, seriously affecting its effectiveness in activating PMS for pollutant removal. Consequently, biochar must be modified and optimized to improve its activation capacity [22]. Doping biochar with transition metals (Co, Fe, Ni, etc.) is an effective way of enhancing its catalytic performance [23]. Among these metals, Co and its composites show superior performance to other metals because of reversible Co2+/Co3+ redox reactions when activating PMS to produce reactive oxygen species [24]. The incorporation of Co onto a biochar substrate with a high specific surface area and stability not only mitigates the agglomeration of Co metal particles but also enhances the rate of PMS activation by the catalytic materials, thereby improving the efficiency of pollutant degradation [25,26]. Therefore, the introduction of metal ions into biochar is a feasible and eco-friendly method of improving the performance of biochar-activated PMS.
In this study, cobalt-doped biochar composites (Co-BCs) were synthesized through an impregnation method, followed by high-temperature calcination under an inert atmosphere, and used to activate PMS for TC degradation. The synthesized Co-BC was characterized to analyze its surface morphology, crystal structure, and elemental composition. The effects of the catalyst dosage, PMS concentration, initial solution pH, inorganic anions, and different aqueous matrices on TC removal in the Co-BC+PMS system were investigated. Finally, free-radical quenching experiments were performed to determine the primary active species involved in TC degradation, and the TC degradation intermediates and degradation pathways were detected and analyzed using LC-MS.
2. Results and Discussion
2.1. Characterization of the Catalyst
2.1.1. SEM Characterization
The morphology and structural characteristics of the catalyst were examined through scanning electron microscopy (SEM) analysis characterization, and the results are shown in Figure 1. Figure 1a shows that the BC has an abundant open structure with a smooth surface, and this structure is favorable for making contact with PMS during the catalytic process. The Co-BC obtained after Co doping still retained an intact open structure, which did not have much of an effect on the surface morphology of the BC (Figure 1b). Analyzing the surface elements of Co-BC using EDS revealed that C, N, O, and Co were uniformly distributed on the surface of the material (Figure 1c,d), indicating that Co was successfully doped into the biochar. Figure 1e indicates that the content of Co in the Co-BC composite is 5.95 wt %. This characterization underscores the efficacy of biochar as a support for cobalt, facilitating the dispersion of Co particles on its surface, thereby mitigating particle agglomeration and enhancing the catalytic performance of Co-BC [27].
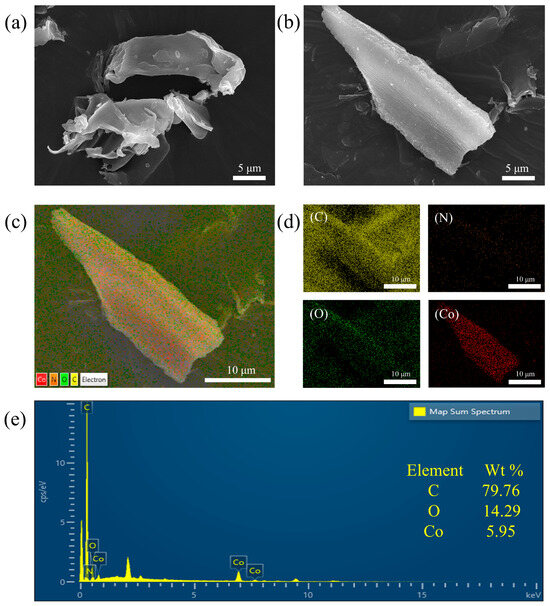
Figure 1.
SEM images of (a) BC and (b) Co-BC; (c,d) EDS energy spectra and elemental distribution maps of Co-BC; and (e) elemental ratio diagram of Co-BC.
2.1.2. XRD Characterization
The crystalline phase and chemical structure of the material were examined using X-ray diffraction (XRD). As illustrated in Figure 2, the broad diffraction peak at 22.6° in the XRD pattern of BC represents the (002) crystalline plane of graphitic carbon [28,29]. The diffraction peaks of graphitic carbon can also be observed in the XRD pattern of Co-BC, indicating that the process of Co doping also helped enhance the graphitization of the biochar. Furthermore, the two characteristic peaks appearing at 44.2° and 51.5° correspond to the (111) and (200) crystallographic planes of the Co standard card (PDF#15-0806) [30]. This analysis further confirms the successful incorporation of elemental cobalt into the biochar matrix.
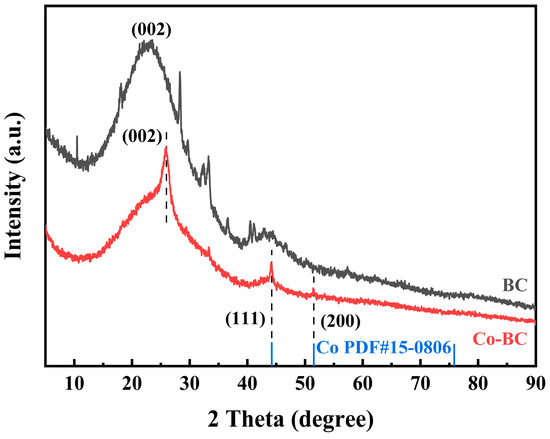
Figure 2.
XRD patterns of BC and Co-BC.
2.1.3. FTIR Characterization
The FTIR spectra of BC and Co-BC are shown in Figure 3. The absorption peaks of BC and Co-BC at 1045 cm−1 and 1381 cm−1 are mainly related to the stretching vibration of C-O [31] and the deformation vibration of C-O-H [32], while the absorption band at 1633 cm−1 was caused by the vibration of C=O [33,34]. In addition, the peak at 2918 cm−1 corresponds to the stretching vibrations of C-H, while the peak at 3444 cm−1 was mainly caused by the stretching vibration of O-H present in the biochar [35,36].
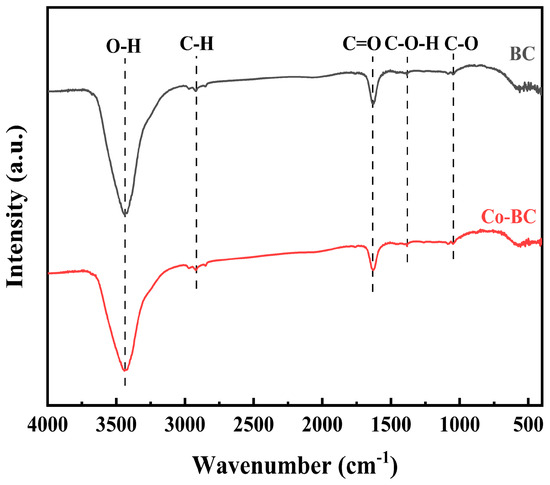
Figure 3.
FTIR patterns of BC and Co-BC.
2.1.4. XPS Characterization
The chemical composition and valence states of the Co-BC composites were analyzed using XPS tests. As shown in Figure 4, the XPS spectrum of Co 2p (Figure 4a) indicates the presence of Co0 (780.2 eV), Co3+ (781.8 eV), and Co2+ (783.1 eV) [37,38] as well as a satellite peak located at 787.0 eV [39]. The weaker signal for Co 2p is due to the smaller Co doping. As shown in Figure 4b, the C 1s peak (284.8 eV) was used to correct all the binding energies obtained from the XPS spectra, and the C 1s peak spectrogram of Co-BC was categorized into four peaks at 284.8, 285.4, 288.5, and 291.1 eV, which correspond to C-C, C-N, C-O, and O-C=O, respectively [38,40]. Figure 4c shows three peaks of the N 1s spectrum corresponding to pyridine N (397.9 eV), pyrrole N (400.9 eV), and graphite N (404.0 eV) [30,38]. Previous studies have shown that the presence of N facilitates the activation of PMS, which may add additional active sites or accelerate electron transfer, wherein graphite N is able to enhance the activity towards PMS by accelerating the electron transfer between neighboring carbon atoms, thus improving the catalytic performance of the materials [41,42]. Additionally, the fine spectrum of O 1s (Figure 4d), with peaks at 532.1 and 533.4 eV, can be attributed to O-H and C-O [43,44]. The above results indicate the successful synthesis of cobalt-doped biochar Co-BC composites.
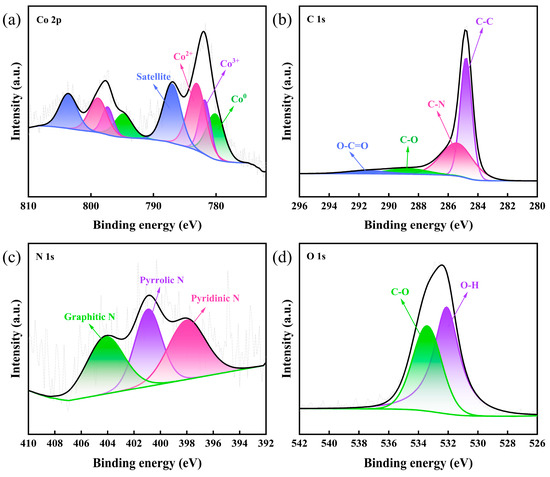
Figure 4.
XPS spectra of Co-BC: (a) Co 2p, (b) C 1s, (c) N 1s, and (d) O 1s.
2.2. Catalytic Performance
The catalytic performance of Co-BC composites was evaluated by analyzing the TC degradation efficiency of different systems, and the results are illustrated in Figure 5a. The Co-BC adsorption experiments showed that the catalyst alone had a poor adsorption effect on TC, adsorbing only about 10% of the TC, while the degradation rate of the PMS system alone was 44.5% in 50 min, attributed to the fact that PMS has a strong oxidizing property by itself, but its oxidation rate was slow and the degradation efficiency of TC was not high. Additionally, the degradation rate of the BC+PMS system was comparable to that of PMS alone, indicating that the activation effect of BC on PMS was weak. In addition, the Co-BC+PMS system exhibited a significant TC degradation effect. Its TC degradation rate reached 97% after the addition of PMS for 30 min, and the degradation rate constant reached 0.1118 min−1 (Figure 5b). The enhanced activation performance of PMS induced by Co-BC is likely due to the doping of Co, and the homogeneous distribution of Co on the biochar provided effective catalytic active sites for the activation of PMS.
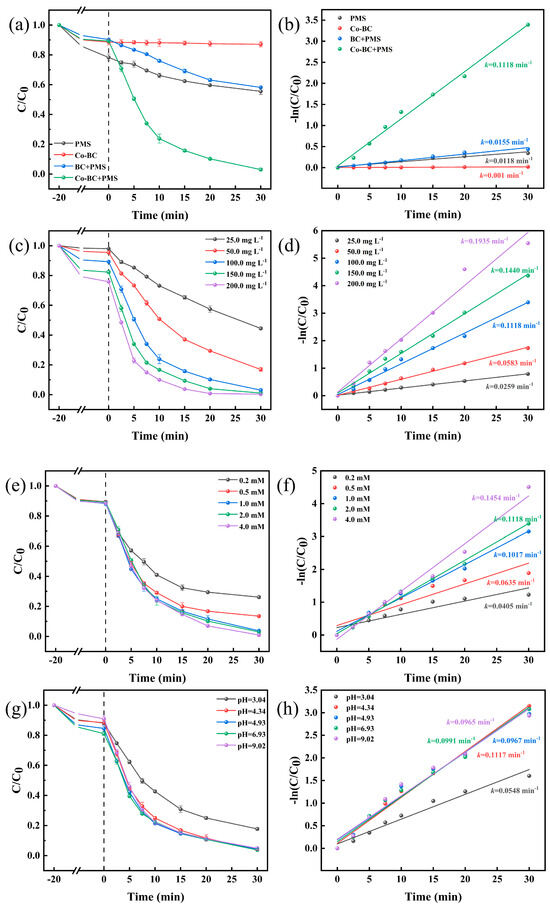
Figure 5.
(a) Degradation performance and (b) rate constants of different catalytic systems. The effects of (c) catalyst dosage and (d) rate constants, (e) PMS dosage and (f) rate constants, and (g) initial pH and (h) rate constants on TC removal ([TC] = 20.0 mg L−1, [temperature] = 25 °C, [catalyst] = 100.0 mg L−1, [PMS] = 2.0 mM, and [initial pH] = 4.34).
The influence of catalyst dosage on TC degradation was investigated (Figure 5c). The effect of an increasing catalyst dosage on the degradation process is shown in Figure 5. With the gradual increase in catalyst dosage from 25.0 mg L−1 to 200.0 mg L−1, the adsorption effect of the material increased from 2% to 24.1%. Concurrently, the TC removal rate improved from 55.5% to 99.7%, and its degradation rate constant increased from 0.0259 min−1 to 0.1935 min−1 (Figure 5d). When the catalyst dosage was increased from 25.0 mg L−1 to 100.0 mg L−1, the degradation performance was significantly improved, a result attributed to the introduction of more active centers in the reaction system, an action that facilitated the activation of PMS and thus improved the TC degradation efficiency. However, further increases in catalyst dosing served to decrease the additional increase in the overall removal rate of the system. This result is consistent with a previous report, which showed that more catalyst does not necessarily equate to better performance [45] and is contrary to the concept of green chemistry. Therefore, a catalyst dosage of 100.0 mg L−1 was selected for the subsequent experiments.
The influence of the PMS concentration is illustrated in Figure 5e. The findings indicate that an increase in the PMS concentration from 0.2 mM to 1.0 mM significantly improved the catalytic performance. Additionally, the TC degradation rate increased from 73.8% to 96.2%, and the degradation rate constant increased from 0.0405 min−1 to 0.1017 min−1 (Figure 5f). These results are due to the rapid consumption of PMS by TC, resulting from the insufficient production of active species when the amount of PMS was low (0.2 mM). When 1.0 mM PMS was added, almost complete degradation of TC was achieved, while a further increase in the PMS dosage decreased the rate of increase. This observation suggests that while elevated PMS concentrations generally facilitate TC removal, the incremental benefit is attenuated beyond a certain concentration. This attenuating effect may be attributed to the quenching of free radicals by excess PMS [15]. From both economic and environmental perspectives, a PMS concentration of 1.0 mM was selected for all of the subsequent experiments.
The initial pH of a solution is a critical determinant of the catalytic efficacy of the Co-BC+PMS system. As illustrated in Figure 5g, the ultimate degradation rate of TC was typically over 95% when the pH ranged from 4.34 to 9.02. This indicates that the system is effective in a wide pH range. However, when the initial pH was lowered to 3.04, the final TC degradation rate decreased to 82.3%, and its degradation rate constant decreased to 0.0548 min−1 (Figure 5h). This diminished performance at a strongly acidic pH can be attributed to the formation of CoOH+ (Equation (1)), in addition to the acidic conditions at pH 3.04, at which point H+ binds to free radicals and quenches some of them (Equations (2) and (3)) [46], further contributing to the reduction in the degradation rate.
Co2+ + H2O → CoOH+ + H+
•OH + H+ + e− → H2O
Environmental matrices, including inorganic substances, are commonly found in natural water bodies. They can affect the performance of catalysts through various pathways, such as by adjusting pH, quenching free radicals, or influencing the decomposition of PMS [47]. As shown in Figure 6, the effects of different anions on the TC degradation rate at 1 mM, 10 mM, and 100 mM concentrations were investigated. Based on previous studies, it is generally believed that the presence of Cl− has an inhibitory effect on the degradation of organic pollutants [48,49]. As shown in Figure 6a, it can be seen that 1 mM, 10 mM, and 100 mM of Cl− reduced the TC degradation rate to 89.6%, 85.4%, and 77.1%, respectively. This phenomenon may be attributed to the fact that Cl− quenches and •OH radicals, producing less reactive chloride anions (Cl•, , and ) (Equations (4)–(6)) [50]. Figure 6b shows that had a small effect on the system, and the TC degradation rate was reduced to 86.5% when the concentration of 1 mM was increased to 100 mM. Figure 6c shows that had no effect on TC degradation. The effects of different ions on the Co-BC+PMS system are different. Overall, the Co-BC+PMS system has some resistance to ionic interference.
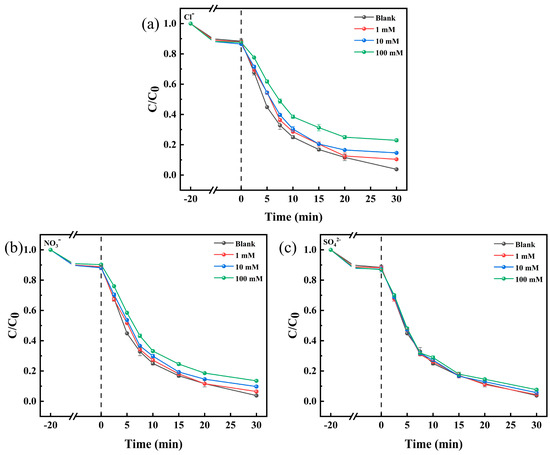
Figure 6.
Effects of the anions (a) Cl−, (b) , and (c) on TC removal ([TC] = 20.0 mg L−1, [temperature] = 25 °C, [catalyst] = 100.0 mg L−1, [PMS] = 1.0 mM, and [initial pH] = 4.34).
Furthermore, we examined the degradation of TC in various aqueous matrices. As illustrated in Figure 7, compared with deionized water, tap water and river water slightly decreased the TC degradation rate to 87.6% and 86.1%, respectively, which might have been a result of the different types and contents of ions and natural organic matter in the different types of water with different levels of purity, but the overall difference was not significant. In conclusion, the Co-BC+PMS system demonstrates resilience to variations in water quality, indicating its potential for widespread application across diverse aquatic environments.
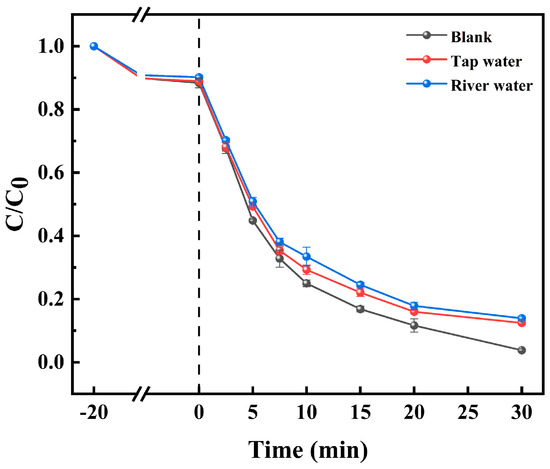
Figure 7.
Effects of different waterbodies on TC removal ([TC] = 20.0 mg L−1, [temperature] = 25 °C, [catalyst] = 100.0 mg L−1, [PMS] = 1.0 mM, and [initial pH] = 4.34).
The reusability of the synthesized Co-BC catalyst was assessed through cyclic degradation experiments. A series of four consecutive tetracycline (TC) degradation trials were conducted under identical reaction conditions, as illustrated in Figure 8a. The findings indicated a decline in the TC degradation rate from 96.5% to 67.2% over the course of the four cycles. This reduction can be attributed to the oxidation of the active sites on the surface of Co-BC, as well as the accumulation of TC degradation intermediates, which may have impeded the efficiency of TC degradation [51]. The stability of the catalyst was further examined through X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR) analyses of Co-BC before and after the reaction, as depicted in Figure 8b,c. The results revealed no significant alterations in the XRD peaks or the functional groups of the catalyst, indicating that the Co-BC catalyst maintained its stability throughout the experiments.
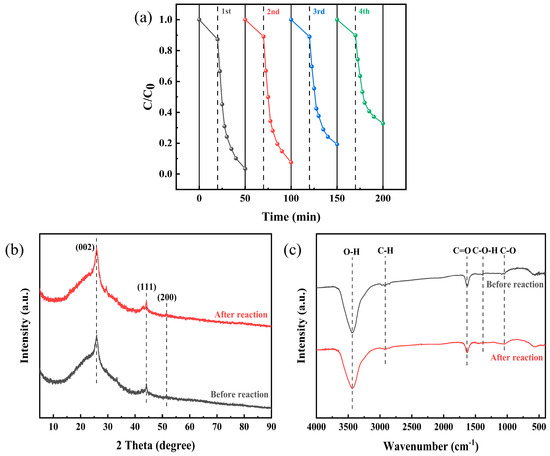
Figure 8.
(a) Cycling experiments, before and after the reaction; (b) XPS spectrum; and (c) FTIR spectrum ([TC] = 20.0 mg L−1, [temperature] = 25 °C, [catalyst] = 100.0 mg L−1, and [PMS] = 1.0 mM, [initial pH] = 4.34).
2.3. Free Radical Identification
In order to compare the contributions of various free radicals to TC degradation, we employed methanol (MeOH) and tert-butanol (TBA) as free-radical quenchers. MeOH and TBA are commonly used to quench and •OH, respectively [52]. As shown in Figure 9, the degradation rate of TC without adding any quencher was up to 96.2% at 30 min after casting PMS. The final degradation rate of TC was 87.4% after 500 mM of TBA was added to the system, and this rate decreased to 61.9% after 500 mM of MeOH was added to the system. This finding suggests that both •OH and were involved in the degradation of TC by the Co-BC+PMS system, with being the major reactive oxygen contributor. Our findings align with the research conducted by Luo et al. [14,15], who examined the degradation of hygromycin hydrochloride and ciprofloxacin by a cobalt-based MOF catalyst (ZIF-67) activated by PMS. The results revealed that ZIF-67 has significant activation potential for PMS, with being the main active species.
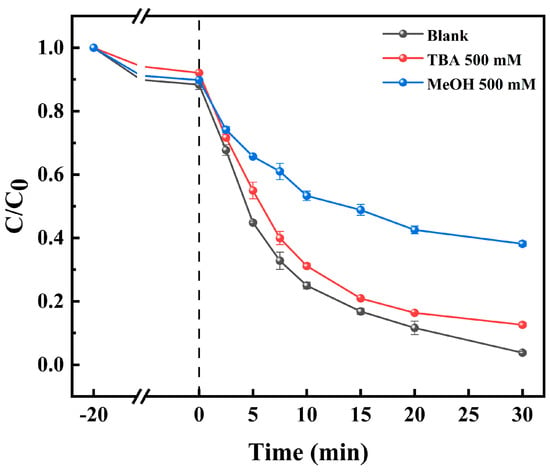
Figure 9.
Effects of different radical scavengers on TC removal ([TC] = 20.0 mg L−1, [temperature] = 25 °C, [catalyst] = 100.0 mg L−1, [PMS] = 1.0 mM, and [initial pH] = 4.34).
3. Materials and Methods
3.1. Materials
Balsa wood powder (100 mesh) was sourced from China Changsha Jirui Chemical Glass Instrument and Equipment Co. (Changsha, China). Tetracycline hydrochloride (TC, C22H24N2O8·HCl), potassium persulfate (PMS, KHSO5·0.5KHSO4·0.5K2SO4), and cobalt nitrate hexahydrate (Co(NO3)2·6H2O) were obtained from McLean Biochemistry Co. Ltd. (Shanghai, China). Methanol (MeOH, CH3OH), concentrated sulfuric acid (H2SO4), sodium hydroxide (NaOH), sodium nitrate (NaNO3), anhydrous sodium sulfate (Na2SO4), sodium chloride (NaCl), and tert-butanol (TBA, C4H10O) were supplied by Sinopharm Chemical Reagent Co. (Shanghai, China). All chemicals were of analytical grade and used without further purification. Deionized water (18.24 MΩ·cm) was utilized for all experiments. River water samples were collected from the Jinjiang River in Changsha, Hunan Province, China, and tap water samples were taken from the municipal water supply in Changsha, Hunan Province, China.
3.2. Synthesis of Catalysts
The 100-mesh balsa wood powder was cleaned with deionized water to remove surface impurities and then dried at 80 °C. Subsequently, 2 g of the treated powder was dispersed in 200 mL of deionized water. Then, 1.75 g of Co(NO3)2·6H2O was added to the solution, which was then stirred for 12 h. The resultant material was then separated, washed with deionized water, and dried overnight at 60 °C. The material was subsequently heated to 800 °C in a tube furnace under a nitrogen atmosphere at a rate of 5 °C min−1 and held at this temperature for 2 h to yield Co-BC. For comparison, pristine biochar (BC) was synthesized by directly calcining balsa wood powder using a similar method.
3.3. Experimental Procedure
A TC stock solution was prepared using deionized water. All catalytic experiments were conducted in a 200 mL beaker containing 100 mL of TC solution (20 mg L−1) at 25 °C with constant stirring. Typically, a measured amount of catalyst was added to the TC solution and stirred for 20 min to establish an adsorption equilibrium. Subsequently, a predetermined amount of PMS was introduced to initiate the degradation process. The initial pH of the TC solution was adjusted using 0.1 mol L−1 H2SO4 or NaOH solutions. At designated time intervals, samples were collected, and the absorbance at 355 nm was measured using a UV–visible spectrophotometer. The TC concentration in the degradation system was determined using a pre-established standard curve. The degradation efficiency of TC was calculated using Equation (7):
where R represents the degradation efficiency of TC, C0 is the initial concentration of TC, and C is the final concentration (mg L−1).
R = (1 − C/C0) × 100%
3.4. Material Characterization
The samples’ morphologies were examined using scanning electron microscopy (SEM, Sigma 300, ZEISS, Oberkochen, Germany), and elemental distribution was analyzed via EDS. X-ray diffractograms were acquired with an X-ray diffractometer (XRD, MiniFlex600, Rigaku, Tokyo, Japan), operating at a scan rate of 5° min−1 over a 5°~90° range with 40 mA and 40 kV settings. Fourier-transform infrared spectra were recorded using an infrared spectrometer (FT-IR, NICOLET iS20, Thermo Scientific, Waltham, MA, USA) across a 4000–400 cm−1 range with 32 scans. X-ray photoelectron spectroscopy (XPS, PHI VersaProbe 4, ULVAC-PHI, Kanagawa, Japan) was employed to analyze the chemical states of the samples’ main elements.
4. Conclusions
In this study, cobalt-doped biochar catalysts were successfully prepared via a simple impregnation method and high-temperature calcination using balsa wood powder as biomass. These catalysts were employed to activate PMS for TC degradation. The successful preparation of the catalyst was proved through various characterization techniques, such as SEM, XRD, FTIR, and XPS. Using 100.0 mg L−1 of Co-BC and 1.0 mM of PMS, an initial concentration of 20 mg L−1 of TC was effectively removed in 30 min, with a degradation rate constant of 0.1017 min−1. In addition, the Co-BC+PMS system exhibited a high TC degradation efficiency over a wide pH range (4.34–9.02). Different ions had different effects on the Co-BC+PMS system. Overall, the Co-BC+PMS system has some resistance to ionic interference. Only when 100 mM Cl− was added to the system did the TC degradation rate decrease to 77.1%. At the same time, the effects of water from different water bodies on the TC degradation rate were weak. The free-radical quenching experiments revealed that was the main reactive oxygen species in the TC degradation process. Therefore, the Co-BC+PMS system shows great potential for practical application in wastewater treatment and enables the efficient removal of antibiotics from polluted water sources.
Author Contributions
Conceptualization, B.S., L.Z., M.Z., R.S., X.H. and Y.L.; data curation, B.S., X.Z., J.C., H.L., Y.M., T.X., Y.Z. and Y.C.; formal analysis, R.S., L.Z., M.Z., J.C., H.L., Y.M., T.X., Y.Z. and Y.C.; methodology, L.Z., M.Z., J.C., H.L. and Y.M.; validation, L.Z., M.Z., J.C., H.L., Y.M., T.X. and Y.Z.; investigation, L.Z., M.Z., X.Z., J.C., H.L., Y.M. and T.X.; supervision, J.C., H.L., Y.M. and T.X.; writing—original draft, B.S., Y.Z., L.Z., X.Z. and Y.M.; writing—review and editing, R.S., X.H., Y.L., T.X. and Y.C.; funding acquisition, R.S. and Y.L.; project administration, R.S., X.H. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hunan Provincial Natural Science Foundation of China (2023JJ31010, 2024JJ7094, 2025JJ70604), the Key Scientific Research Project of the Hunan Provincial Department of Education (23A0225), the Hunan Province Environmental Protection Research Project (HBKYXM-2023038), the Scientific Research Foundation for Talented Scholars of CSUFT (2020YJ010), and the Graduate science and technology innovation project of CSUFT (2023CX02106).
Data Availability Statement
The data are contained within this article.
Acknowledgments
The authors thank all the participants who devoted their free time to participate in this study.
Conflicts of Interest
Author Xiaojie Zhuang was employed by the company PowerChina Zhongnan Engineering Corporation Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Roy, N.; Alex, S.A.; Chandrasekaran, N.; Mukherjee, A.; Kannabiran, K. A comprehensive update on antibiotics as an emerging water pollutant and their removal using nano-structured photocatalysts. J. Environ. Chem. Eng. 2021, 9, 104796. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Pham, T.H.; Viet, N.M.; Thang, P.Q.; Rajagopal, R.; Sathya, R.; Jung, S.H.; Kim, T. Improved photodegradation of antibiotics pollutants in wastewaters by advanced oxidation process based on Ni-doped TiO2. Chemosphere 2022, 302, 134837. [Google Scholar] [CrossRef]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef]
- Liu, S.; Kang, Y. Underwater bubbling plasma assisted with persulfate activation for the synergistic degradation of tetracycline hydrochloride. Environ. Res. 2024, 240, 117539. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Su, R. Preparation of NH2-MIL-101(Fe) metal organic framework and its performance in adsorbing and removing tetracycline. Int. J. Mol. Sci. 2024, 25, 9855. [Google Scholar] [CrossRef]
- Hou, C.; Niu, M.; Hao, J.; Liu, Q.; Wang, X.; Zhang, M.; Wang, L. Construction of an S-scheme g-C3N4/TiOF2 Heterostructures with abundant O vacancies: Enhanced photocatalytic activity and mechanism insight. J. Alloys Compd. 2023, 938, 168560. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Luo, X.; Huang, L.; Gong, X.; Tian, J. Sulfur anchored on N-doped porous carbon as metal-free peroxymonosulfate activator for tetracycline hydrochloride degradation: Nonradical pathway mechanism, performance and biotoxicity. Chem. Eng. J. 2023, 457, 141149. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R.; Yao, H.; Zhang, A.; Xiang, S.; Huang, L. Degradation of trimethoprim by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Environ. Sci. Pollut. Res. 2021, 28, 62572–62582. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole degradation by UV and UV/H2O2 advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef]
- Su, R.; Li, Z.; Cheng, F.; Dai, X.; Wang, H.; Luo, Y.; Huang, L. Advances in the degradation of emerging contaminants by persulfate oxidation technology. Water Air Soil Pollut. 2023, 234, 754. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.M. Oxidation of bisoprolol in heated persulfate/H2O systems: Kinetics and products. Chem. Eng. J. 2012, 183, 162–171. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R. Cobalt-based mof material activates persulfate to degrade residual ciprofloxacin. Water 2024, 16, 2299. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Z.; Ye, M.; Zhou, Y.; Su, R.; Huang, S.; Chen, Y.; Dai, X. Synergistic enhancement of oxytetracycline hydrochloride removal by UV/ZIF-67 (Co)-activated peroxymonosulfate. Water 2024, 16, 2586. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of sulfate radical-based advanced oxidation processes for water and wastewater treatment: A review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen reduction reaction in the field of water environment for application of nanomaterials. Nanomaterials 2020, 10, 1719. [Google Scholar] [CrossRef]
- Su, R.; Yao, H.; Wang, H.; Chen, Y.; Huang, S.; Luo, Y.; Ma, X. Metal-organic frameworks for removing emerging organic pollutants: A review. J. Water Process Eng. 2025, 70, 107096. [Google Scholar] [CrossRef]
- Su, R.; Wang, Z.; Liu, Z.; Chen, Y.; Wang, H.; Dai, X.; Ge, X.; Luo, Y. Single atoms in environmental catalysis: Breakthroughs in synthesis and application. J. Water Process Eng. 2024, 68, 106319. [Google Scholar] [CrossRef]
- Song, G.; Qin, F.; Yu, J.; Tang, L.; Pang, Y.; Zhang, C.; Wang, J.; Deng, L. Tailoring biochar for persulfate-based environmental catalysis: Impact of biomass feedstocks. J. Hazard. Mater. 2022, 424, 127663. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Sun, Y.; Yu, I.K.; Tsang, D.C.; Hou, D.; Gupta, J.; Bhaskar, T.; Pandey, A. Critical review on biochar-supported catalysts for pollutant degradation and sustainable biorefinery. Adv. Sustain. Syst. 2020, 4, 1900149. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, L.; Dai, H.; Li, N.; Song, Y.; Yan, B.; Chen, G.; Hou, L. Activation of peroxymonosulfate by food waste digestate derived biochar for sulfamethoxazole degradation: Performance and mechanism. Sep. Purif. Technol. 2023, 327, 124935. [Google Scholar] [CrossRef]
- Shi, Q.; Deng, S.; Zheng, Y.; Du, Y.; Li, L.; Yang, S.; Zhang, G.; Du, L.; Wang, G.; Cheng, M. The application of transition metal-modified biochar in sulfate radical based advanced oxidation processes. Environ. Res. 2022, 212, 113340. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; Oliveira, M.C.; Florêncio, M.H. Visible light photocatalytic degradation of amitriptyline using cobalt doped titanate nanowires: Kinetics and characterization of transformation products. J. Environ. Chem. Eng. 2020, 8, 103585. [Google Scholar] [CrossRef]
- Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; Oliveira, M.C.; Florêncio, M.H. Photocatalytic degradation of amitriptyline, trazodone and venlafaxine using modified cobalt-titanate nanowires under UV–Vis radiation: Transformation products and in silico toxicity. Chem. Eng. J. 2019, 373, 1338–1347. [Google Scholar] [CrossRef]
- Yi, Y.; Fu, Y.; Wang, Y.; Cai, Y.; Liu, Y.; Xu, Z.; Diao, Z. Persulfate oxidation of norfloxacin by cobalt doped water hyacinth biochar composite: The key role of cobalt and singlet oxygen. J. Water Process Eng. 2024, 59, 104967. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Lan, M.-Y.; Wang, F.; Wang, C.-C.; Wang, P.; Ge, C.; Liu, W. Immobilized N-C/Co derived from ZIF-67 as PS-AOP catalyst for effective tetracycline matrix elimination: From batch to continuous process. Chem. Eng. J. 2022, 450, 138082. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K. MOFs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.; Xiong, W.; Zhou, Y.; Wu, Y.; Jia, M.; Sun, S.; Zhou, C.; Zhang, Y.; Zhong, R. Peroxymonosulfate activation of magnetic Co nanoparticles relative to an N-doped porous carbon under confinement: Boosting stability and performance. Sep. Purif. Technol. 2020, 250, 117237. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Meng, X.; Christodoulatos, C.; Boddu, V.M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Liu, G.; Ma, X.; Gong, J.; Ma, B.; Yan, Q.; Chen, Q.; Ma, D.; Zhang, G.; Gao, M. High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: Economical synthesis, catalytic performance and mechanism. Appl. Catal. B Environ. 2021, 280, 119386. [Google Scholar] [CrossRef]
- Yan, J.; Gong, L.; Chai, S.; Guo, C.; Zhang, W.; Wan, H. ZIF-67 loaded lotus leaf-derived biochar for efficient peroxymonosulfate activation for sustained levofloxacin degradation. Chem. Eng. J. 2023, 458, 141456. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, Y. Co3O4/C-PC composite derived from pomelo peel-loaded ZIF-67 for activating peroxymonosulfate (PMS) to degrade ciprofloxacin. J. Water Process Eng. 2022, 49, 103043. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, L.; Fang, J.; Liu, X.; Qi, J.; Li, H. Tetracycline degradation by activated persulfate with enhancement of ZIF-67 loaded wood-microreactor. J. Environ. Chem. Eng. 2024, 12, 111901. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Xie, T.; Liu, Y.; Shen, Q.; Yang, J.; Cao, L.; Yang, J. Highly efficient Hg2+ removal via a competitive strategy using a Co-based metal organic framework ZIF-67. J. Environ. Sci. 2022, 119, 33–43. [Google Scholar] [CrossRef]
- Wang, A.; Ni, J.; Wang, W.; Liu, D.; Zhu, Q.; Xue, B.; Chang, C.-C.; Ma, J.; Zhao, Y. MOF Derived Co−Fe nitrogen doped graphite carbon@crosslinked magnetic chitosan Micro−nanoreactor for environmental applications: Synergy enhancement effect of adsorption−PMS activation. Appl. Catal. B Environ. 2022, 319, 121926. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yang, Y.; Feng, Y.; Wu, D.; Mao, S. Activation of persulfate with metal–organic framework-derived nitrogen-doped porous Co@C nanoboxes for highly efficient p-Chloroaniline removal. Chem. Eng. J. 2019, 358, 408–418. [Google Scholar] [CrossRef]
- Ban, J.; Xu, G.; Zhang, L.; Xu, G.; Yang, L.; Sun, Z.; Jia, D. Efficient Co–N/PC@CNT bifunctional electrocatalytic materials for oxygen reduction and oxygen evolution reactions based on metal–organic frameworks. Nanoscale 2018, 10, 9077–9086. [Google Scholar] [CrossRef]
- Ling, Z.; Gu, Y.; He, B.; Chen, Z.; Hu, H.; Liu, H.; Ding, W. Biochar-Supported FeCo-MOF derivative catalyzes PDS-Mediated degradation of tetracycline. Sep. Purif. Technol. 2024, 349, 127841. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Liu, B.; Jiang, W.; Zhou, T.; Ma, Y.; Che, G.; Liu, C. Superhydrophilic N,S,O-doped Co/CoO/Co9S8@carbon derived from metal-organic framework for activating peroxymonosulfate to degrade sulfamethoxazole: Performance, mechanism insight and large-scale application. Chem. Eng. J. 2022, 446, 137361. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, X.; Chen, Q.; Zhou, J. Removal of 2,4-dichlorophenoxyacetic acid by the boron-nitrogen co-doped carbon nanotubes: Insights into peroxymonosulfate adsorption and activation. Sep. Purif. Technol. 2021, 259, 118196. [Google Scholar] [CrossRef]
- Liu, D.; Li, M.; Li, X.; Ren, F.; Sun, P.; Zhou, L. Core-shell Zn/Co MOFs derived Co3O4/CNTs as an efficient magnetic heterogeneous catalyst for persulfate activation and oxytetracycline degradation. Chem. Eng. J. 2020, 387, 124008. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Wang, L.; Wei, Y.; Zhao, Z.; Du, K.; Chen, D.; Li, X.; Zhou, C.; Liu, G. ZIF-67-derived Co@N-PC anchored on tracheid skeleton from sawdust with micro/nano composite structures for boosted methylene blue degradation. Sep. Purif. Technol. 2021, 278, 119489. [Google Scholar] [CrossRef]
- Abdul-wahid, I.K.; Ammar, S.H.; Elaibi, A.I.; Jabbar, Z.H. Enhanced synergistic photocatalytic degradation of oxytetracycline antibiotic using novel Ag2MoO4/Co-zeolitic imidazolate framework (ZIF-67) Z-type heterojunction. Inorg. Chem. Commun. 2023, 156, 111277. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Huang, Y.-F.; Huang, C.-I.; Chen, C.-Y. Efficient decolorization of azo dye Reactive Black B involving aromatic fragment degradation in buffered Co2+/PMS oxidative processes with a ppb level dosage of Co2+-catalyst. J. Hazard. Mater. 2009, 170, 1110–1118. [Google Scholar] [CrossRef]
- Jawad, A.; Lang, J.; Liao, Z.; Khan, A.; Ifthikar, J.; Lv, Z.; Long, S.; Chen, Z.; Chen, Z. Activation of persulfate by CuOx@Co-LDH: A novel heterogeneous system for contaminant degradation with broad pH window and controlled leaching. Chem. Eng. J. 2018, 335, 548–559. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, K.; Liu, T.; Cui, M.; Ding, Y.; Chen, Y.; Chen, X.; Li, W.-W.; Li, C.-X. Enhanced degradation of sulfamethoxazole by non-radical-dominated peroxymonosulfate activation with Co/Zn co-doped carbonaceous catalyst: Synergy between Co and Zn. Sci. Total Environ. 2022, 850, 158055. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, S.; Sun, J.; Meng, X.; Luo, J.; Zhou, D.; Crittenden, J. Impact of chloride ions on UV/H2O2 and UV/persulfate advanced oxidation processes. Environ. Sci. Technol. 2018, 52, 7380–7389. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, L.; Jiang, M.; Lu, J.; Ferronato, C.; Chovelon, J.-M. The role of nitrite in sulfate radical-based degradation of phenolic compounds: An unexpected nitration process relevant to groundwater remediation by in-situ chemical oxidation (ISCO). Water Res. 2017, 123, 249–257. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, L. Magnetic porous carbon materials derived from metal-organic framework in-situ growth on natural cellulose of wood for sulfadiazine degradation: Role of delignification and mechanisms. Int. J. Biol. Macromol. 2023, 248, 125902. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Yan, J.; Fan, G.; Liu, Y.; Chai, B.; Wang, C.; Song, G. Built-in electric field mediated peroxymonosulfate activation over biochar supported-Co3O4 catalyst for tetracycline hydrochloride degradation. Chem. Eng. J. 2022, 444, 136589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).