Damage Mechanisms of Stabilized/Solidified Sediments in Dry–Wet Cycles: Insights from Microporous Structure Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparations
2.2.1. Sample Molding
2.2.2. Sample Curing and DWs
2.2.3. Freeze-Drying of the Sample
2.3. Tests
2.3.1. SEM
2.3.2. MIP
2.3.3. NAP
3. Results
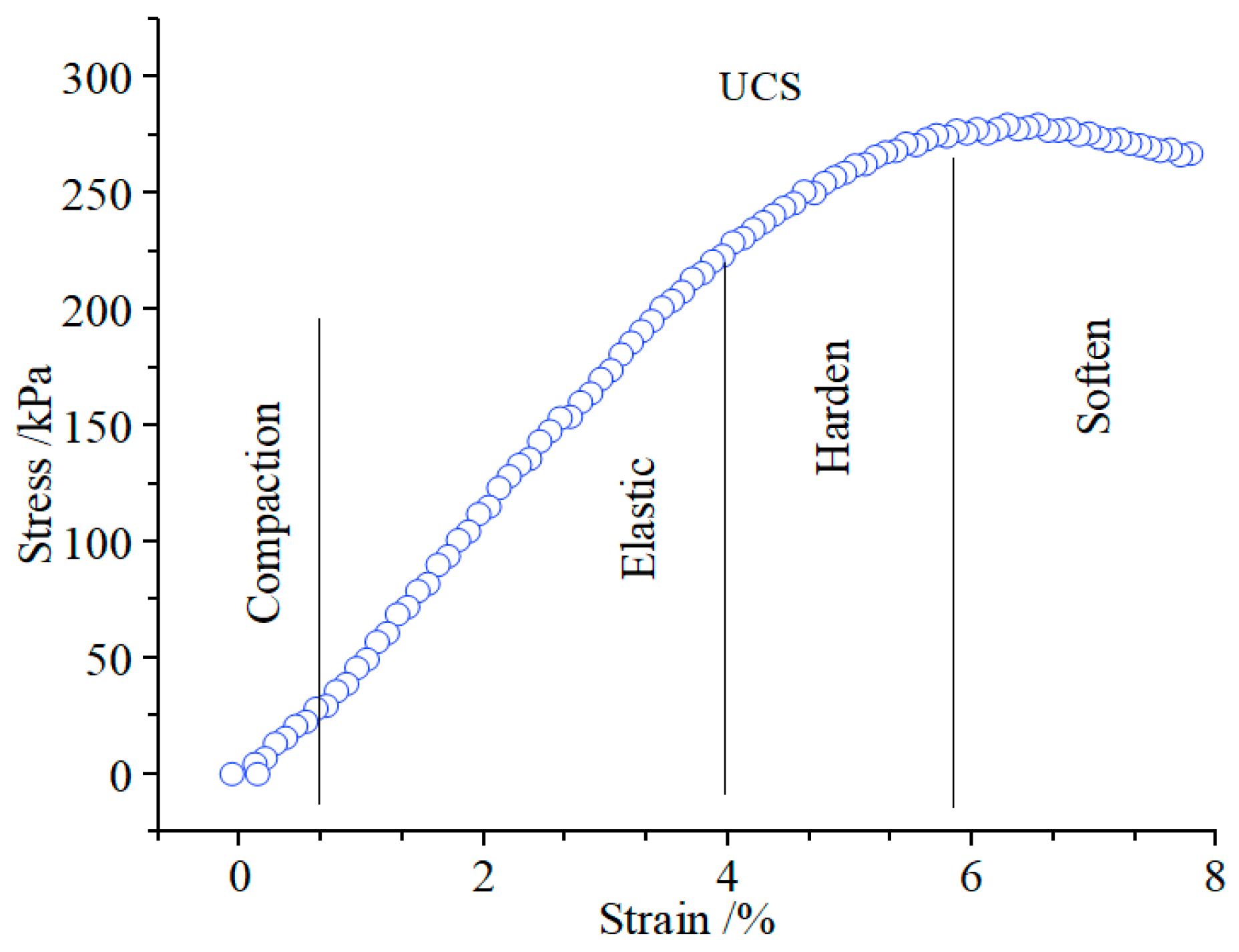
3.1. Macro Performance Analysis Using the UCS Test
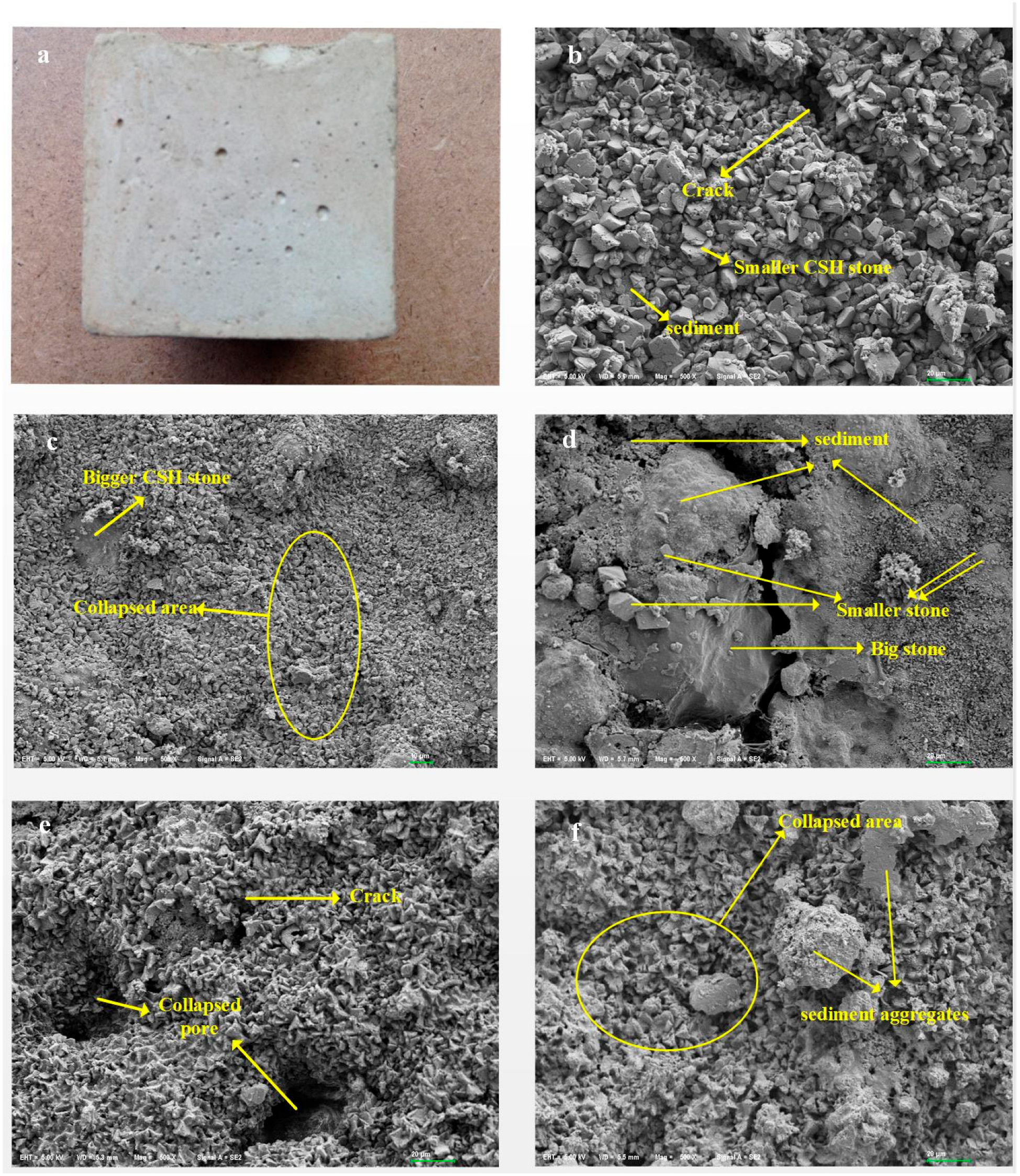
3.2. Qualitative Pore Structure Analysis Using SEM
3.3. Quantitative Pore Structure Analysis Using MIP
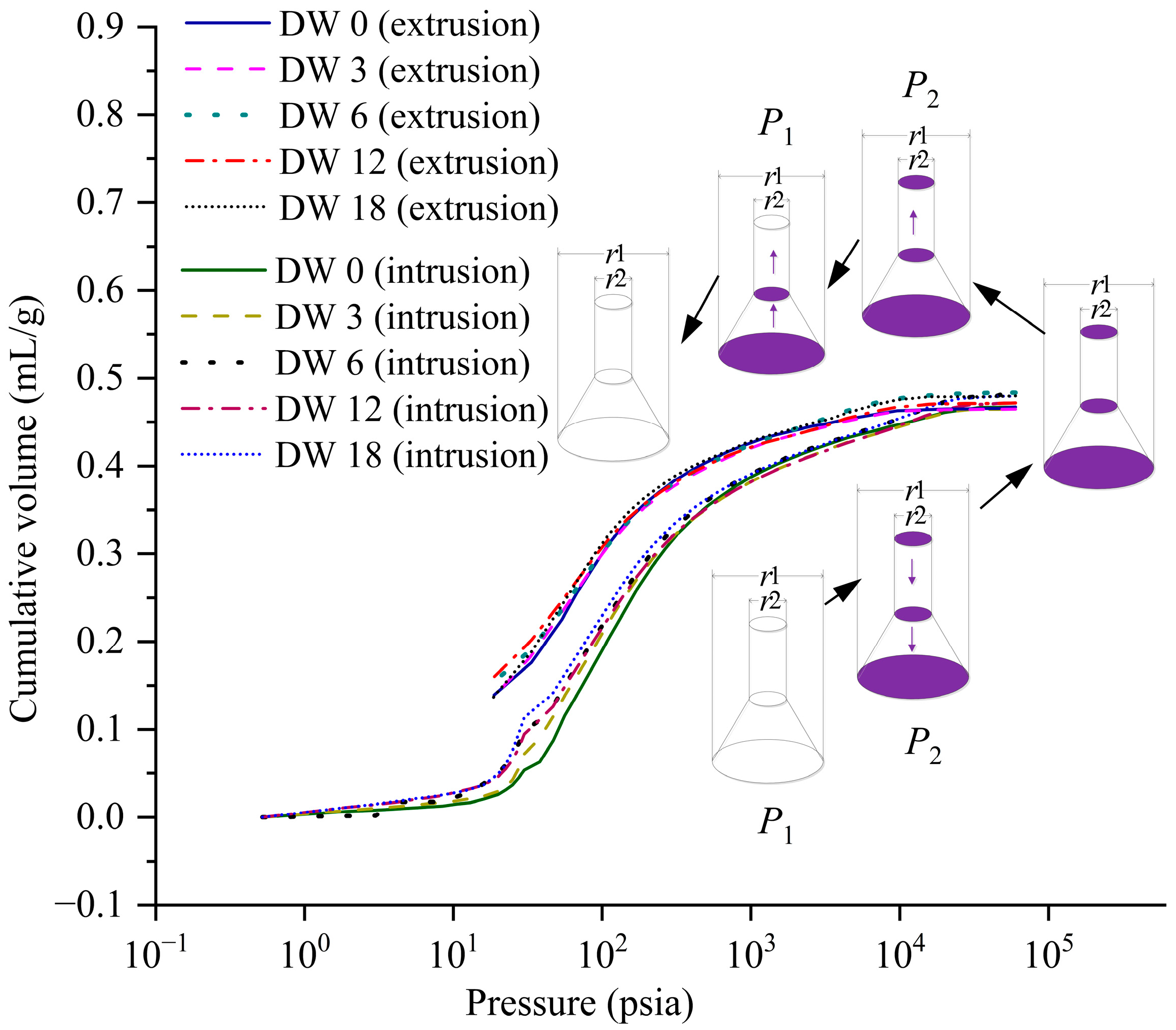
3.3.1. Intrusion–Extrusion Characteristics of S/S Sediments
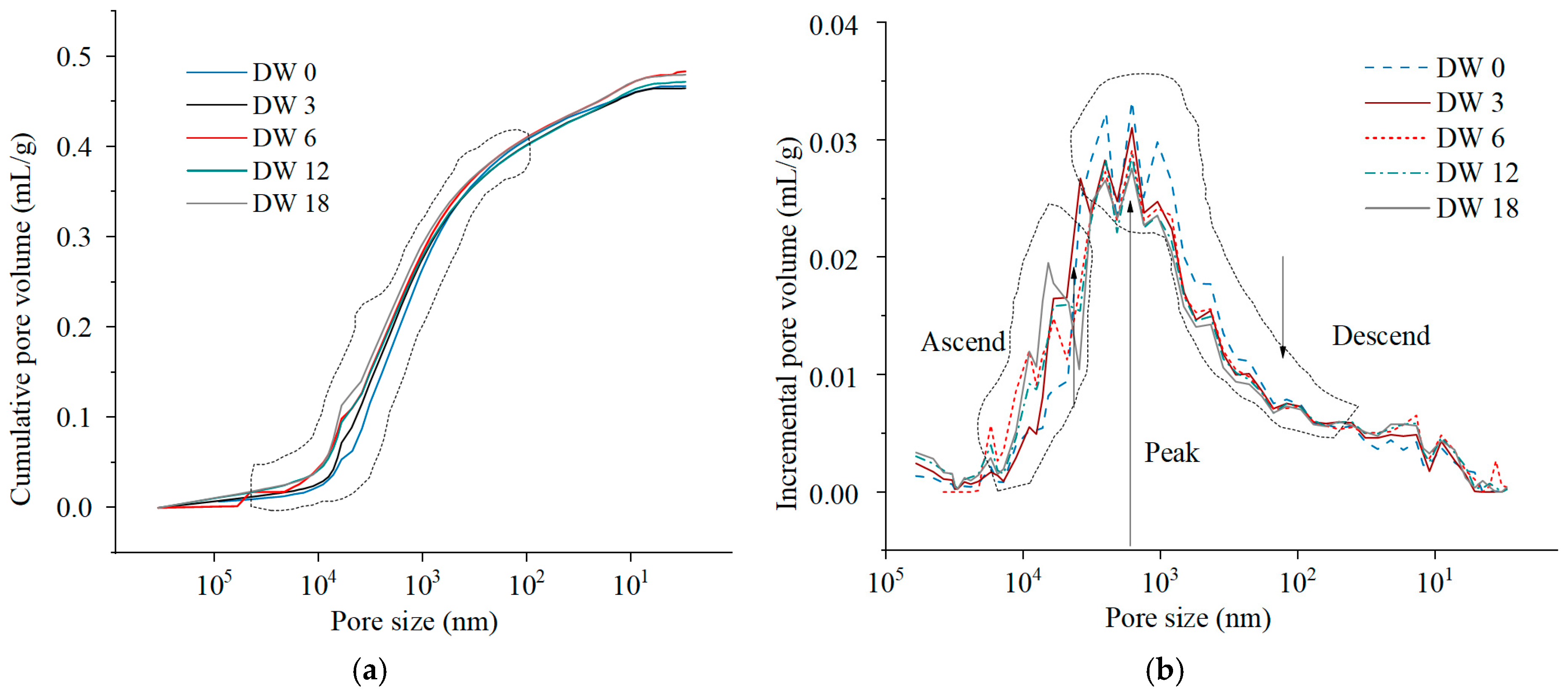
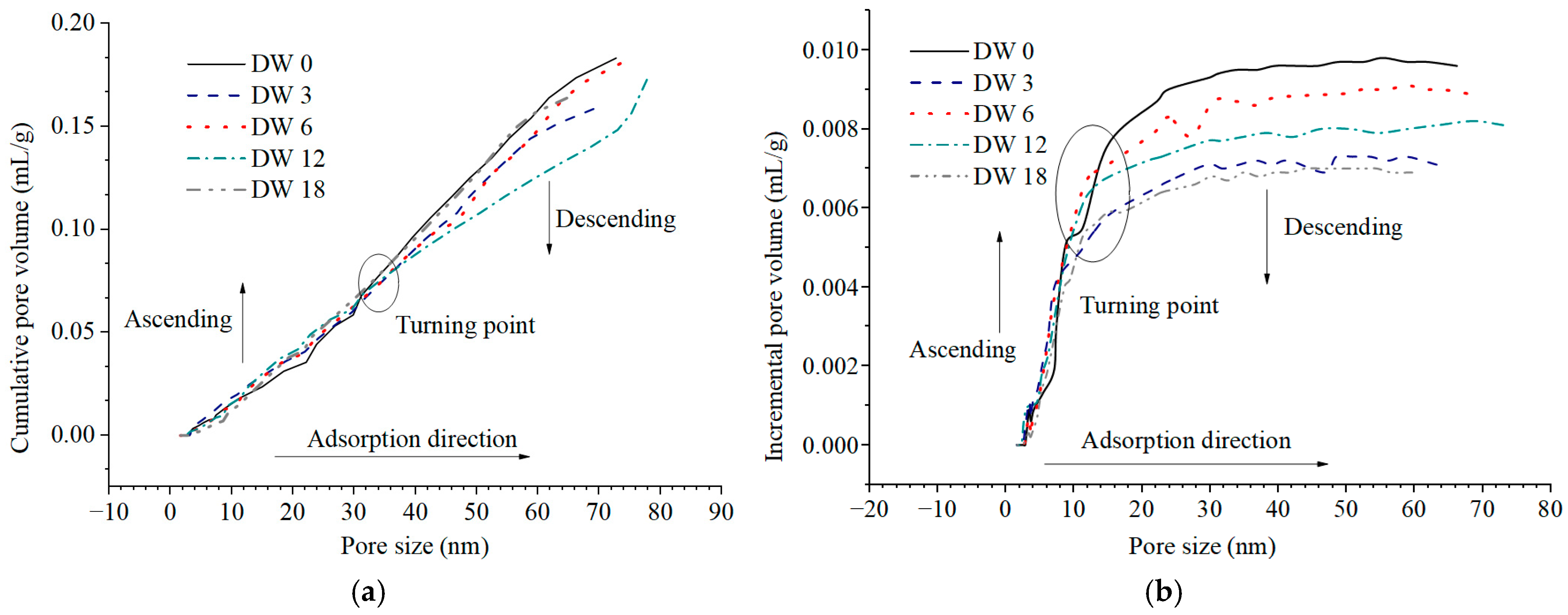
3.3.2. Pore Volume Evolution Characteristics During DWs Using MIP
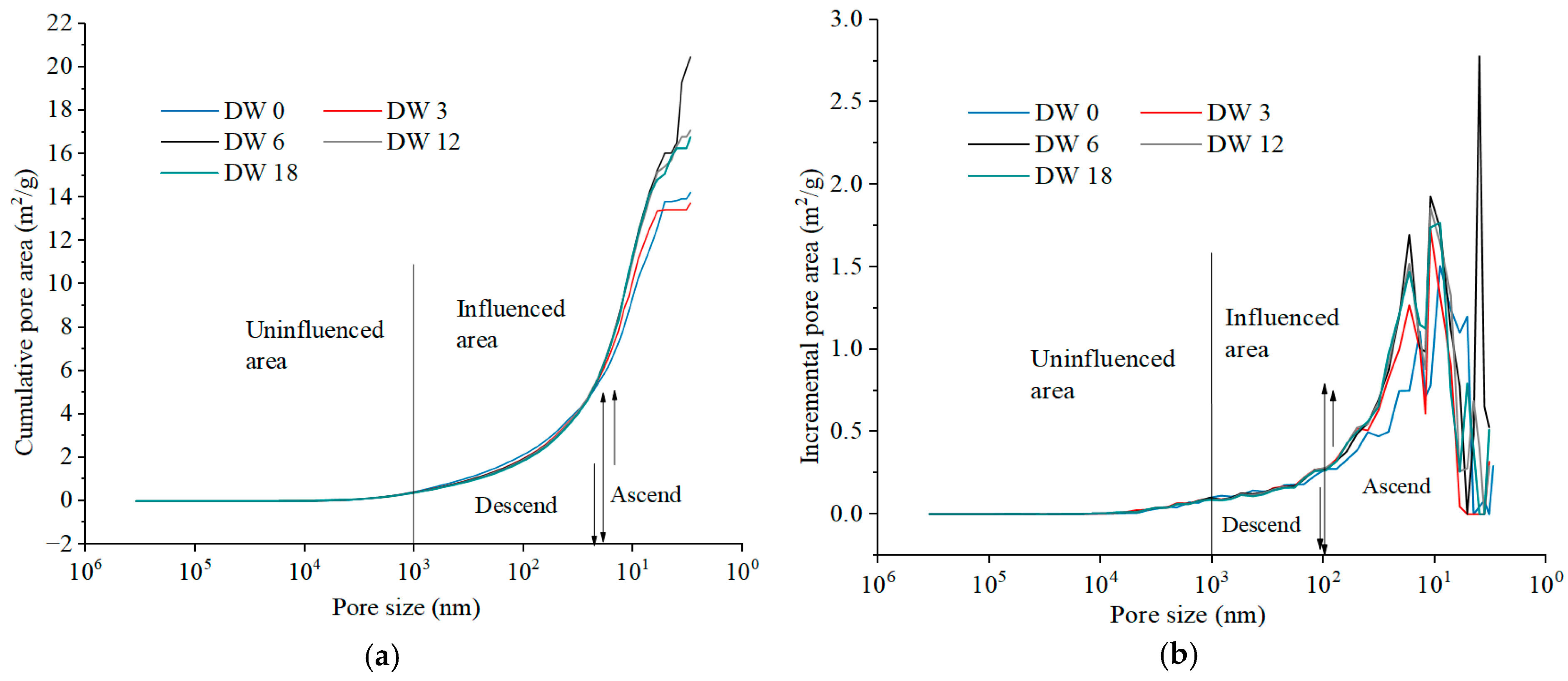
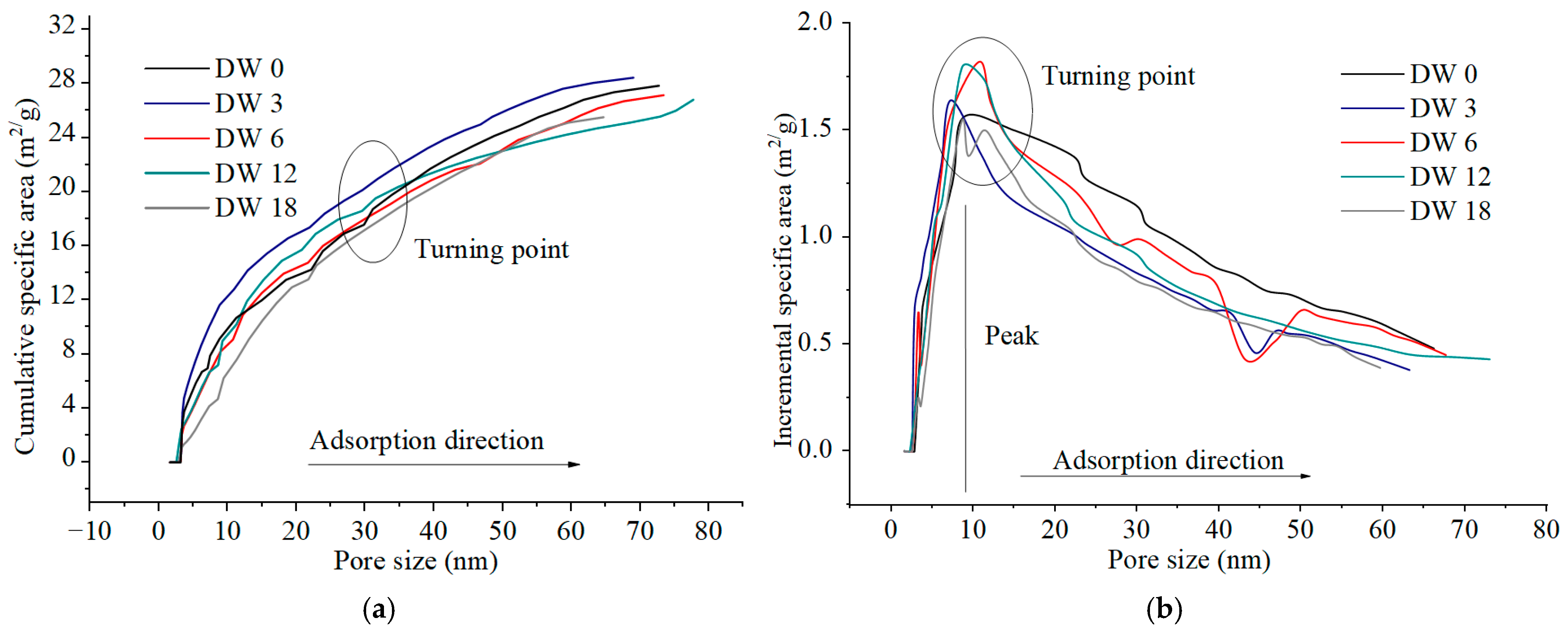
3.3.3. Pore Surface Area Evolution Characteristics During DWs Using MIP
3.4. Quantitative Micropore Structure Analysis Using NAP
3.4.1. Adsorption/Desorption Characteristic of S/S Sediments
3.4.2. Pore Volume Evolution Characteristics During DWs Using NAP
3.4.3. Pore Area Evolution Characteristics in DWs Using NAP
4. Damage Mechanisms of S/S Sediments Under DWs
4.1. Generation and Transmission of Expansion and Shrinkage Forces
4.2. Identification of Micropores
4.3. Damage Mechanisms of S/S Sediment During DWs
5. Conclusions
- (1)
- The strength of S/S sediments declined as the number of DWs increased, as did the rates of decline.
- (2)
- The primary micropores were those within the sediment particles, CSH component, and sediment aggregates and among the CSH skeletons. Due to the expansion/shrinkage forces that originated from the sediment particles in DWs, pores in the sediment aggregates and CHS skeletons gradually collapsed and were reconstructed due to DWs, leading to structural damage in the S/S sediment and a decrease in UCS.
- (3)
- Pores in the sediment aggregates in the range of 102 nm < d < 104 nm accounted for a larger proportion, those among the CSH skeleton (d > 104) and those in the CSH components and sediment particles accounted for a minor proportion (d < 102 nm), and pores in the range of d < 102 contributed 90% of the pore area. Pores in the CSH skeleton were expanded by DWs, while pores in sediment aggregates were gradually compressed. Under the joint control of the CSH skeleton and sediment aggregates, the UCS of the S/S sediments gradually decreased due to DWs, though the decrease rate also slowed.
- (4)
- Due to the expansion and shrinkage elastic deformation in sediment particles with pores in the range of d < 10 nm, the CSH component joints with pores in the range of 10 nm < d < 102 nm were gradually destroyed, leading to plastic deformation and damage of the sediment aggregates and CSH skeletons.
- (5)
- With the damage and reconstruction of sediment aggregates and CSH skeletons, the S/S sediments were adjusted. As a result, the expansion and shrinkage forces acting on the sediment aggregates and CSH skeletons gradually became weaker, leading to a slower decrease in UCS.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| S/S | Stabilized/solidified |
| UCS | Unconfined compression strength |
| DWs | Dry–wet cycles |
| SEM | Scanning electron microscope |
| MIP | Mercury intrusion porosimetry |
| NAP | Nitrogen adsorption porosimetry |
References
- Liu, H.; Kong, T.; Qiu, L.; Xu, R.; Li, F.; Kolton, M.; Lin, H.; Zhang, L.; Lin, L.; Chen, J.; et al. Solar-driven, self-sustainable electrolysis for treating eutrophic river water: Intensified nutrient removal and reshaped microbial communities. Sci. Total Environ. 2021, 764, 144293. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Meng, H.; Liu, Z.; Sun, H.; Zhang, X.; Jin, Q.; Wang, L. Development, Performance, and Mechanism of Fluidized Solidified Soil Treated with Multi-Source Industrial Solid Waste Cementitious Materials. Buildings 2025, 15, 864. [Google Scholar] [CrossRef]
- Wang, L.; Tsang, D.C.W.; Poon, C.S. Green remediation and recycling of contaminated sediment by waste-incorporated stabili-zation/solidification. Chemosphere 2015, 122, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhao, X.; Wang, H. Synthesis and Property Characterization of Low-Activity Waste-Derived Quaternary Cementitious Materials. Buildings 2025, 15, 1426. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Luan, L.; Sha, F.; Liu, X. Technology and Equipment of Deep-sea Mining: State of the Art and Perspectives. Earth Energy Sci. 2025, 1, 65–84. [Google Scholar] [CrossRef]
- Zhang, W.-L.; Zhao, L.-Y.; McCabe, B.A.; Chen, Y.-H.; Morrison, L. Dredged marine sediments stabilized/solidified with cement and GGBS: Factors affecting mechanical behaviour and leachability. Sci. Total Environ. 2020, 733, 138551. [Google Scholar] [CrossRef] [PubMed]
- Banoune, B.; Melbouci, B.; Rosquoët, F.; Langlet, T. Treatment of river sediments by hydraulic binders for valorization in road construction. Bull. Eng. Geol. Environ. 2016, 75, 1505. [Google Scholar] [CrossRef]
- Tang, I.Y.; Yan, D.Y.S.; Lo, I.M.C.; Liu, T. Pulverized fuel ash solidification/stabilization of waste: Comparison between beneficial reuse of contaminated marine mud and sediment. J. Environ. Eng. Landsc. Manag. 2015, 23, 202–210. [Google Scholar] [CrossRef]
- De Gisi, S.; Labianca, C.; Todaro, F.; Notarnicola, M. Stabilization/Solidification of Contaminated Marine Sediment. In Low Carbon Stabilization and Solidification of Hazardous Wastes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 113–127. [Google Scholar]
- Dubois, V.; Abriak, N.E.; Zentar, R.; Ballivy, G. The use of marine sediments as a pavement base material. Waste Manag. 2009, 29, 774–782. [Google Scholar] [CrossRef]
- Zhang, Y.; Labianca, C.; Chen, L.; De Gisi, S.; Notarnicola, M.; Guo, B.; Sun, J.; Ding, S.; Wang, L. Sustainable ex-situ remediation of contaminated sediment: A review. Environ. Pollut. 2021, 287, 117333. [Google Scholar] [CrossRef]
- Dalmacija, M.B.; Prica, M.D.J.; Dalmacija, B.D.; Roncevic, S.D.; Rajic, L.M. Correlation between the results of sequential extraction and effectiveness of immobilization treatment of lead-and cadmium-contaminated sediment. Sci. World J. 2010, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Seo, J.; Phan, H.Q.H.; Ahn, J.; Hwang, I. MgO-based binder for treating contaminated sediments: Characteristics of metal stabilization and mineral carbonation. CLEAN–Soil Air Water 2014, 42, 355–363. [Google Scholar] [CrossRef]
- Cho, H.; Shim, J.H.; Park, J.Y. Performance evaluation of solidification/stabilization of dredged sediment using alkali-activated slag. Desalin. Water Treat. 2016, 57, 10159–10168. [Google Scholar] [CrossRef]
- Silitonga, E. Stabilization/Solidification of Polluted Marine Dredged Sediment of Port en Bessin, France, Using Hydraulic Binders and Silica Fume. In Proceedings of the 1st Nommensen International Conference on Technology and Engineering, Medan, Indonesia, 11–12 July 2017. [Google Scholar]
- Todaro, F.; Vitone, C.; Notarnicola, M. Stabilization and Recycling of Contaminated Marine Sediments. In Proceedings of the 7th International Symposium on Deformation Characteristics of Geomaterials, Strathclyde’s Technology & Innovation Centre, Glasgow, UK, 26–28 June 2019. [Google Scholar]
- Li, J.-S.; Zhou, Y.-F.; Wang, Q.-M.; Xue, Q.; Poon, C.S. Development of a novel binder using lime and incinerated sewage sludge ash to stabilize and solidify contaminated marine sediments with high water content as a fill material. J. Mater. Civ. Eng. 2019, 31, 04019245. [Google Scholar] [CrossRef]
- Cheng, S.; Shui, Z.; Gao, X.; Lu, J.; Sun, T.; Yu, R. Degradation progress of Portland cement mortar under the coupled effects of multiple corrosive ions and drying-wetting cycles. Cem. Concr. Compos. 2020, 111, 103629. [Google Scholar] [CrossRef]
- Zha, F.; Huang, K.; Kang, B.; Sun, X.; Su, J.; Li, Y.; Lu, Z. Deterioration characteristic and constitutive model of red-bed argillaceous siltstone subjected to drying-wetting cycles. Lithosphere 2022, 2022, 8786210. [Google Scholar] [CrossRef]
- Pu, H.; Yi, Q.; Jivkov, A.P.; Bian, Z.; Chen, W.; Wu, J. Effect of dry-wet cycles on dynamic properties and microstructures of sandstone: Experiments and modelling. Int. J. Min. Sci. Technol. 2024, 34, 655–679. [Google Scholar] [CrossRef]
- Wang, H.-X.; Wu, Z.-Z.; Tan, Y.-Z.; Cui, X.-Z.; Zuo, Q.-J.; Wang, L.-H.; Lu, L.-Q. Characteristics of the micro pore structures of stabilized/solidified sediments during freeze-thaw cycles. Constr. Build. Mat. 2019, 211, 1–22. [Google Scholar]
- Li, J.-S.; Zhou, Y.; Chen, X.; Wang, Q.; Xue, Q.; Tsang, D.C.; Poon, C.S. Engineering and microstructure properties of contaminated marine sediments solidified by high content of incinerated sewage sludge ash. J. Rock Mech. Geotech. Eng. 2021, 13, 643–652. [Google Scholar] [CrossRef]
- ISO 14688-1:2017; Geotechnical Investigation and Testing—Identification and Classification of Soil—Part 1: Identification and Description. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 5667-12:2017; Part 12: Guidance on Sampling of Bottom Sediments from Rivers, Lakes and Estuarine Areas. International Organization for Standardization: Geneva, Switzerland, 2017.
- ASTM D4823-95; Standard Guide for Core Sampling Submerged, Unconsolidated Sediments. ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 19901-8:2023; Oil and Gas Industries Including Lower Carbon Energy—Offshore Structures. International Organization for Standardization: Geneva, Switzerland, 2023.
- DB 42/T 1255-2017; Technical Specification for Inland Freshwater Lake Sedimentary Deposits in Still Water. Hubei Provincial Market Supervision Administration: Wuhan, China, 2017.
- Kamei, T.; Ahmed, A.; Shibi, T. The use of recycled bassanite and coal ash to enhance the strength of very soft clay in dry and wet environmental conditions. Constr. Build. Mater. 2013, 38, 224–235. [Google Scholar] [CrossRef]
- ASTM D559-03; Standard Test Methods for Wetting and Drying Compacted Soil-Cement Mixtures. ASTM International: West Conshohocken, PA, USA, 2003.
- ASTM D4219-02 (2003); Standard Test Method for Unconfined Compressive Strength Index of Chemical-Grouted Soils. ASTM International: West Conshohocken, PA, USA, 2003.
- Sasanian, S.; Newson, T.A. Use of mercury intrusion porosimetry for microstructural investigation of reconstituted clays at high water contents. Eng. Geol. 2013, 158, 15–22. [Google Scholar] [CrossRef]
- Abbaslou, R.M.; Vosoughi, V.; Dalai, A.K. Comparison of nitrogen adsorption and transmission electron microscopy analyses for structural characterization of carbon nanotubes. Appl. Surf. Sci. 2017, 419, 817–825. [Google Scholar] [CrossRef]
- Ojha, S.P.; Misra, S.; Tinni, A.; Sondergeld, C.; Rai, C. Pore connectivity and pore size distribution estimates for Wolfcamp and Eagle Ford shale samples from oil, gas and condensate windows using adsorption-desorption measurements. J. Pet. Sci. Eng. 2017, 158, 454–468. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Wang, H.; Wu, S.; Jing, X.; Liu, K.; Ren, L. Strength and Stiffness Degradation Mechanisms of Stabilized/Solidified Sediments by Freeze–Thaw Cycles. Front. Earth Sci. 2022, 10, 905482. [Google Scholar] [CrossRef]




| Property | Value |
|---|---|
| Initial water content, Wi (%) | 178 |
| Plastic limit, WP (%) | 73 |
| Liquid limit, WL (%) | 34 |
| Plasticity index | 39 |
| Total organic content (%) | 4.0 |
| pH | 6.3 |
| Sand particle fraction (d > 0.75 mm) (%) | 18.7 |
| Silt particle fraction (0.75 mm > d > 0.005 mm) (%) | 38.1 |
| Clay particle fraction (0.005 mm > d) (%) | 43.2 |
| Composition | SiO2 | Fe2O3 | Al2O3 | TiO2 | CaO | MgO | SO3 | Na2O | K2O | Loi 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Percentage | 23.35 | 4.03 | 7.92 | 0.33 | 55.76 | 2.09 | 2.82 | 0.31 | 0.22 | 3.16 |
| DWs | Total | d > 104 nm | 104 nm > d > 103 nm | 103 nm > d > 102 nm | d < 102 nm |
|---|---|---|---|---|---|
| 0 | 0.467 | 0.024 | 0.242 | 0.142 | 0.059 |
| 3 | 0.465 | 0.027 | 0.250 | 0.125 | 0.063 |
| 6 | 0.484 | 0.045 | 0.239 | 0.127 | 0.073 |
| 12 | 0.472 | 0.042 | 0.237 | 0.122 | 0.070 |
| 18 | 0.480 | 0.044 | 0.250 | 0.117 | 0.070 |
| DWs | Total | d > 104 nm | 104 nm > d > 103 nm | 103 nm > d > 102 nm | d < 102 nm |
|---|---|---|---|---|---|
| 0 | 14.22239 | 0.004 | 0.432 | 1.722 | 12.065 |
| 3 | 13.74129 | 0.004 | 0.409 | 1.569 | 11.758 |
| 6 | 20.47247 | 0.010 | 0.391 | 1.566 | 18.506 |
| 12 | 17.08244 | 0.007 | 0.385 | 1.534 | 15.157 |
| 18 | 16.77475 | 0.008 | 0.388 | 1.476 | 14.903 |
| DWs | Total | Micropores (r < 2 nm) | Mesopores (2 nm < r < 50 nm) | Macropores (r > 50 nm) | |
|---|---|---|---|---|---|
| 0 | Volume (mL/g) | 0.1832 | 0 | 0.128 | 0.0552 |
| Proportion /% | 100 | 0 | 69.869 | 30.131 | |
| 3 | Volume (mL/g) | 0.1585 | 0 | 0.1223 | 0.0362 |
| Proportion /% | 100 | 0 | 77.16088 | 22.83912 | |
| 6 | Volume (mL/g) | 0.1806 | 0 | 0.1177 | 0.0629 |
| Proportion /% | 100 | 0 | 65.17165 | 34.82835 | |
| 12 | Volume (mL/g) | 0.1483 | 0 | 0.1081 | 0.0402 |
| Proportion /% | 100 | 0 | 72.89278 | 27.10722 | |
| 18 | Volume (mL/g) | 0.1638 | 0 | 0.129 | 0.0348 |
| Proportion /% | 100 | 0 | 78.75458 | 21.24542 |
| DWs | Total | Micropores (r < 2 nm) | Mesopores (2 nm < r < 50 nm) | Macropores (r > 50 nm) | |
|---|---|---|---|---|---|
| 0 | Volume (m2/g) | 27.84 | 0 | 24.37 | 3.47 |
| Proportion /% | 100 | 0 | 87.53592 | 12.46408 | |
| 3 | Volume (m2/g) | 28.43 | 0 | 26.09 | 2.34 |
| Proportion /% | 100 | 0 | 91.76926 | 8.23074 | |
| 6 | Volume (m2/g) | 27.14 | 0 | 23.17 | 3.97 |
| Proportion /% | 100 | 0 | 85.37214 | 14.62786 | |
| 12 | Volume (m2/g) | 25.56 | 0 | 23.1 | 2.46 |
| Proportion /% | 100 | 0 | 90.37559 | 9.62441 | |
| 18 | Volume (m2/g) | 25.51 | 0 | 23.16 | 2.35 |
| Proportion /% | 100 | 0 | 90.78793 | 9.21207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Luo, Y.; Wang, H.; Liu, W.; Zhao, T.; Wu, S.; Ruan, J.; Wu, Y. Damage Mechanisms of Stabilized/Solidified Sediments in Dry–Wet Cycles: Insights from Microporous Structure Evolution. Water 2025, 17, 1667. https://doi.org/10.3390/w17111667
Li K, Luo Y, Wang H, Liu W, Zhao T, Wu S, Ruan J, Wu Y. Damage Mechanisms of Stabilized/Solidified Sediments in Dry–Wet Cycles: Insights from Microporous Structure Evolution. Water. 2025; 17(11):1667. https://doi.org/10.3390/w17111667
Chicago/Turabian StyleLi, Keyao, Yun Luo, Hongxing Wang, Wei Liu, Tiantian Zhao, Shangwei Wu, Junhao Ruan, and Yanping Wu. 2025. "Damage Mechanisms of Stabilized/Solidified Sediments in Dry–Wet Cycles: Insights from Microporous Structure Evolution" Water 17, no. 11: 1667. https://doi.org/10.3390/w17111667
APA StyleLi, K., Luo, Y., Wang, H., Liu, W., Zhao, T., Wu, S., Ruan, J., & Wu, Y. (2025). Damage Mechanisms of Stabilized/Solidified Sediments in Dry–Wet Cycles: Insights from Microporous Structure Evolution. Water, 17(11), 1667. https://doi.org/10.3390/w17111667







