Outstanding Adsorption of Reactive Red 2 and Reactive Blue 19 Dyes on MIL-101 (Cr): Novel Physicochemical Analysis of Underlying Mechanism Through Statistical Physics Modeling
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. RR2 and RB19 Adsorption Data Results
2.3. Statistical Physics Model Set-Up
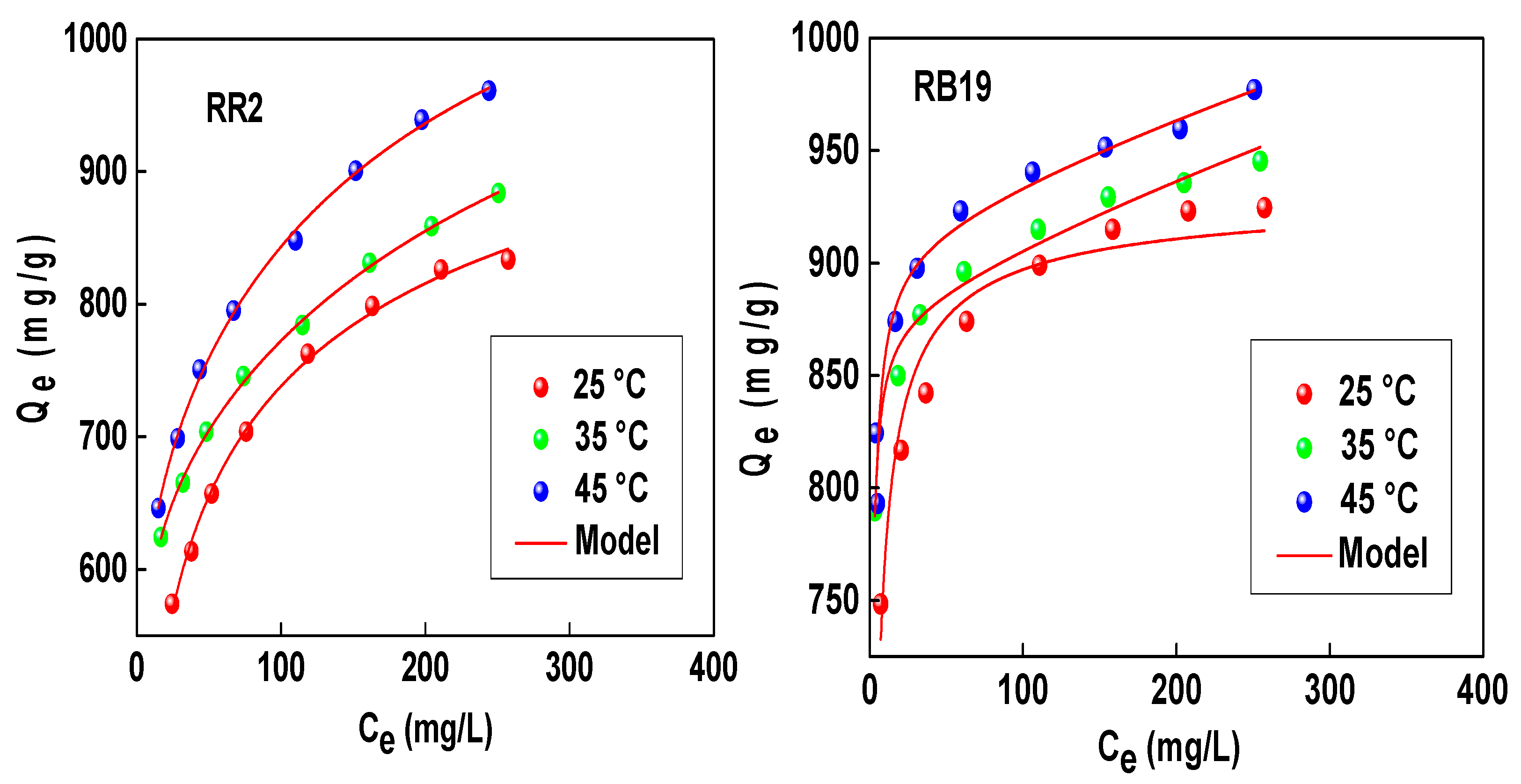
3. Discussion
3.1. Steric Aspect of Dye Adsorption Mechanisms
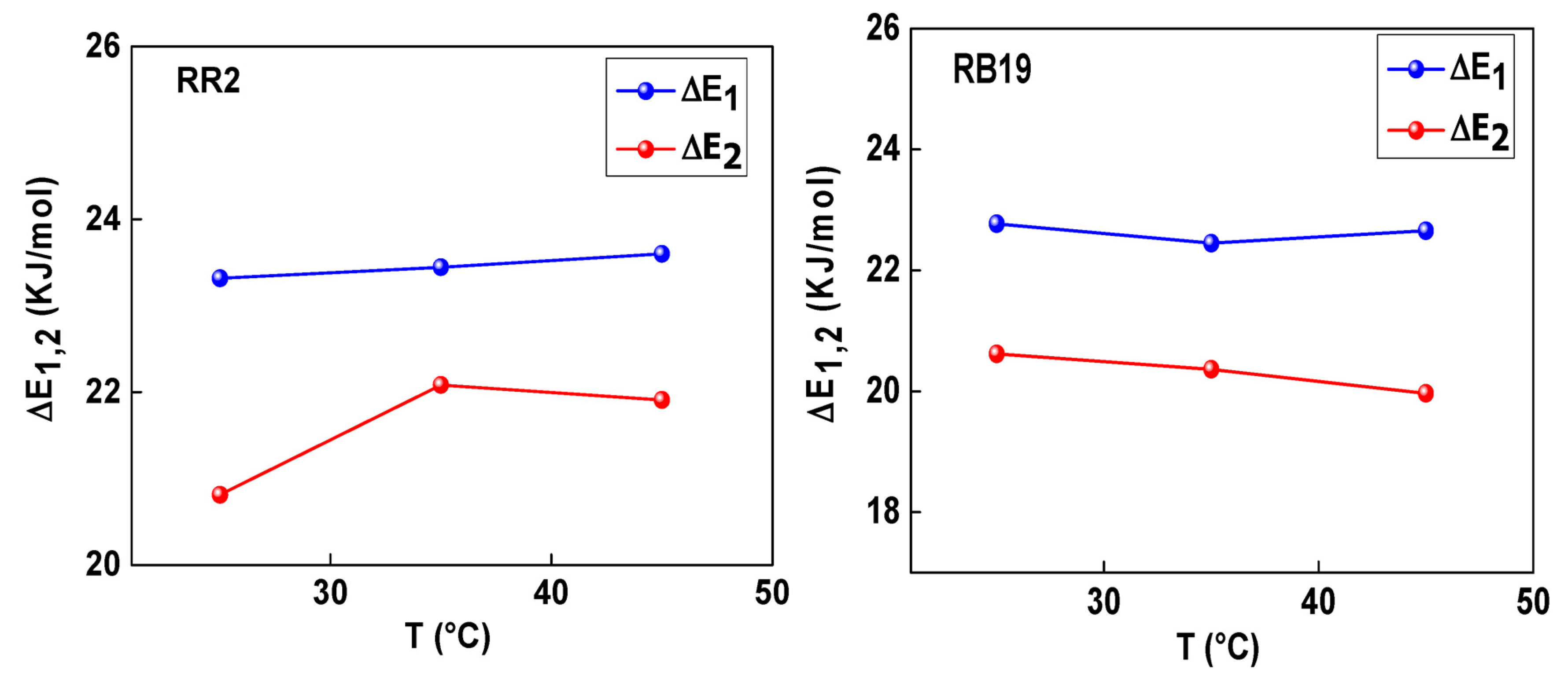
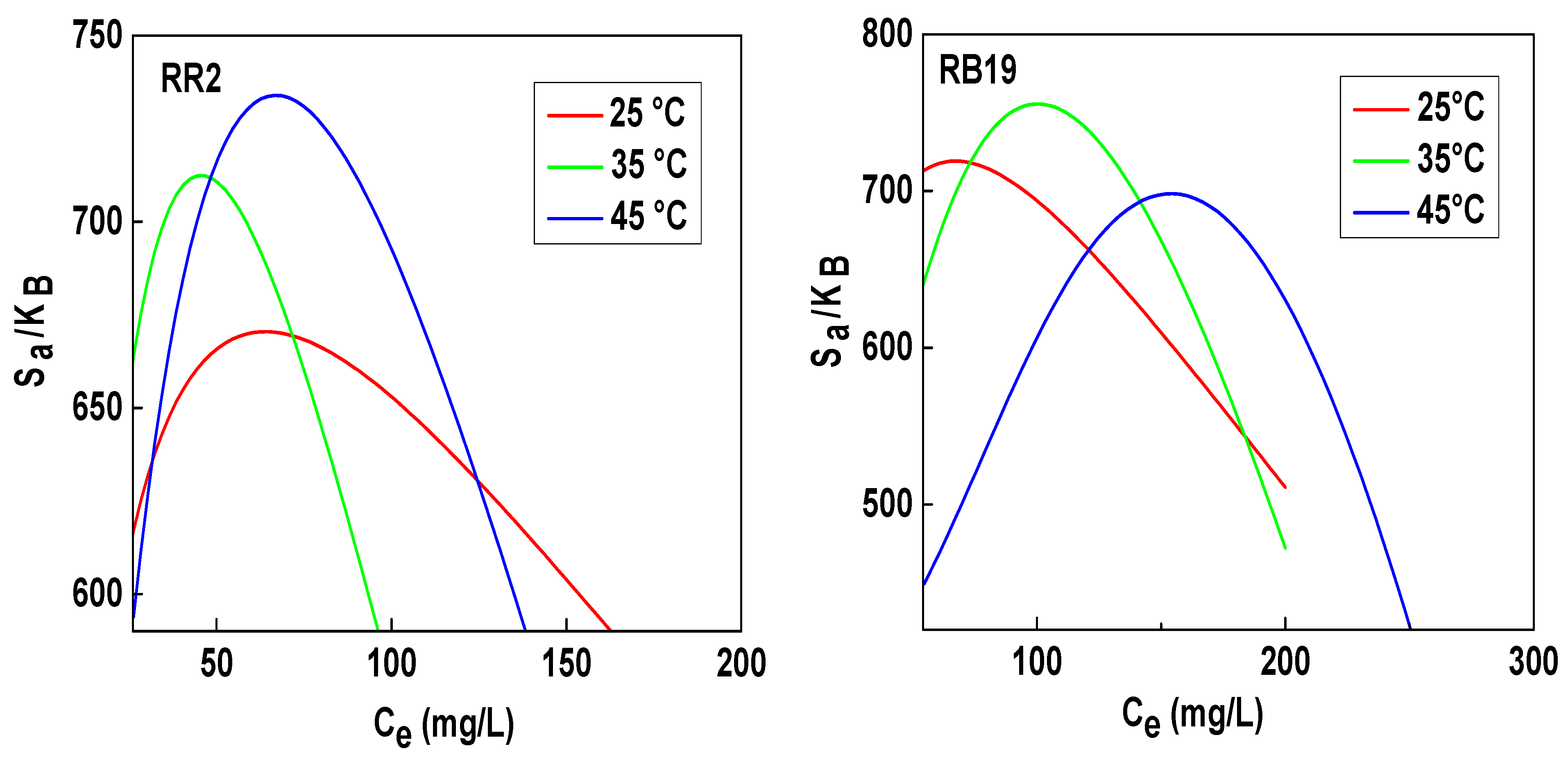
3.2. Energetic Aspects
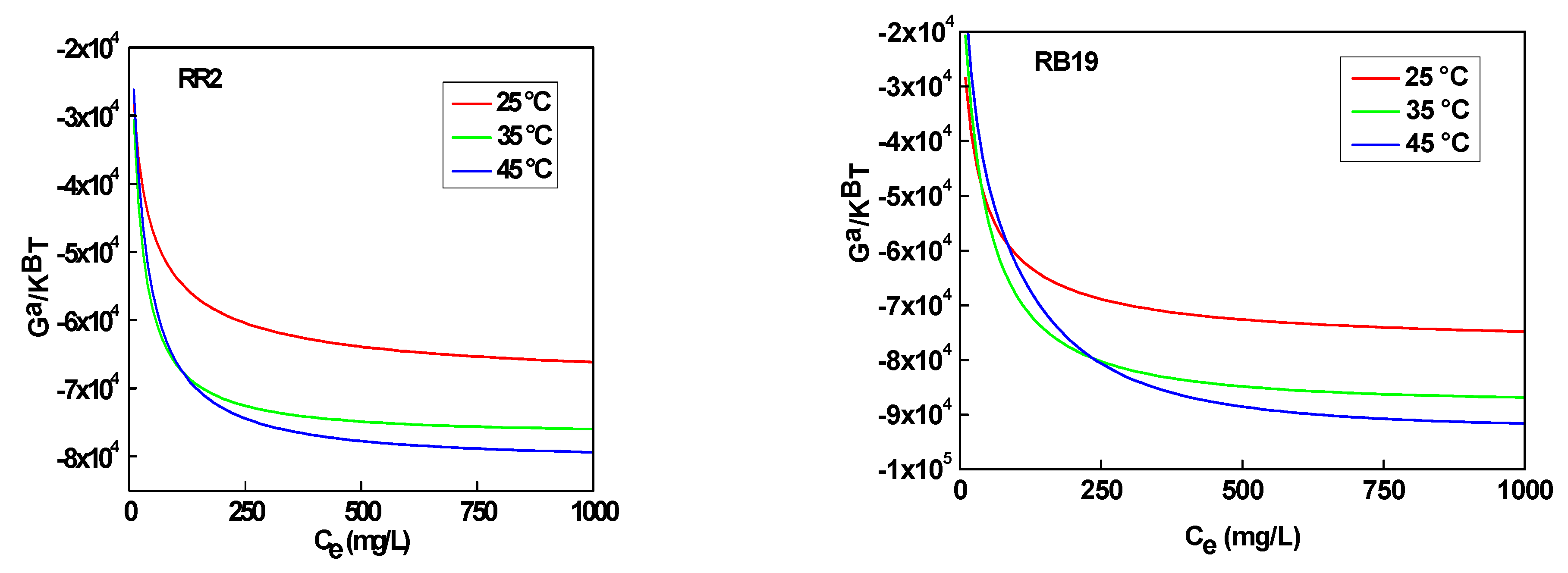
3.3. Analysis of Entropy and Free Enthalpy
3.3.1. Entropy
3.3.2. Free Enthalpy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crini, G. Non-Conventional Low-Cost Adsorbents for Dye Removal: A Review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gu, R.; Su, Y.; Li, Y.; Liu, G.; Cheng, S. Natural-Inspired Hierarchic Double-Janus Solar Evaporator for Stable Clean Water Production from High-Salinity Emulsions. J. Hazard. Mater. 2024, 474, 134739. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.I.; Usman, M.; Siddiq, M.; Rasool, N.; Bokhari, T.H.; Ibrahim, M.; Usman, A.R.; Khan, S.U.-D. Application of Micellar-Enhanced Ultrafiltration for the Removal of Reactive Blue 19 from Aqueous Media. J. Dispers. Sci. Technol. 2015, 36, 1208–1215. [Google Scholar] [CrossRef]
- Saeed, M.; Siddique, M.; Ibrahim, M.; Akram, N.; Usman, M.; Aleem, M.A.; Baig, A. Calotropis gigantea Leaves Assisted Biosynthesis of ZnO and Ag@ZnO Catalysts for Degradation of Rhodamine B Dye in Aqueous Medium. Environ. Prog. Sustain. Energy 2020, 39, e13408. [Google Scholar] [CrossRef]
- Maqsood, A.; Tazeen, K.; Hussain, S.; Mahmood, F.; Shahid, M. An Integrated Approach for Biodegradation of Toxic Azo Dyes and Wastewater Recycling by Azoreductase Gene–Harboring Bacillus Paramycoides Strain AM-3. Water Air Soil Pollut. 2023, 234, 727. [Google Scholar] [CrossRef]
- Alghamdi, H.M.; Mohammad, R.M.; Elwakeel, K.Z. Simultaneous Removal of Eriochrome Black T and Chromate Anions from Aqueous Solution Using Functionalized Hybrid Magnetic Polymers. J. Polym. Environ. 2024, 32, 6522–6543. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and Health Concerns of Persistent Coloring Pollutants of Textile Industry Wastewater and Treatment Approaches for Environmental Safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Das, R.S.; Warkhade, S.K.; Kumar, A.; Gaikwad, G.S.; Wankhade, A.V. Graphitic Carbon Nitride@ Silver Zirconate Nanocomposite (gC3N4@ Ag2ZrO3): A Type-II Heterojunction for an Effective Visible Light Photocatalysis and Bacterial Photo-Inactivation. J. Alloys Compd. 2020, 846, 155770. [Google Scholar] [CrossRef]
- Das, R.S.; Warkhade, S.K.; Kumar, A.; Wankhade, A.V. Graphene Oxide-Based Zirconium Oxide Nanocomposite for Enhanced Visible Light-Driven Photocatalytic Activity. Res. Chem. Intermed. 2019, 45, 1689–1705. [Google Scholar] [CrossRef]
- Jin, X.-C.; Liu, G.-Q.; Xu, Z.-H.; Tao, W.-Y. Decolorization of a Dye Industry Effluent by Aspergillus Fumigatus XC6. Appl. Microbiol. Biotechnol. 2007, 74, 239–243. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Water Purification by Using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Mon, P.P.; Cho, P.P.; Chandana, L.; Srikanth, V.; Madras, G.; Ch, S. Biowaste-Derived Ni/NiO Decorated-2D Biochar for Adsorption of Methyl Orange. J. Environ. Manag. 2023, 344, 118418. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chang, Q.; Zhang, T.; Song, G.; Sun, Y.; Ding, G. A Review on Selective Dye Adsorption by Different Mechanisms. J. Environ. Chem. Eng. 2022, 10, 108639. [Google Scholar] [CrossRef]
- Abdelaziz, M.A.; Owda, M.E.; Abouzeid, R.E.; Alaysuy, O.; Mohamed, E.I. Kinetics, Isotherms, and Mechanism of Removing Cationic and Anionic Dyes from Aqueous Solutions Using Chitosan/Magnetite/Silver Nanoparticles. Int. J. Biol. Macromol. 2023, 225, 1462–1475. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Javanbakht, V. Fabrication of Zn-Based Magnetic Zeolitic Imidazolate Framework Bionanocomposite Using Basil Seed Mucilage for Removal of Azo Cationic and Anionic Dyes from Aqueous Solution. Int. J. Biol. Macromol. 2021, 167, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, N.; Khani, H.; Gupta, V.K.; Amereh, E.; Agarwal, S. Adsorption Process of Methyl Orange Dye onto Mesoporous Carbon Material–Kinetic and Thermodynamic Studies. J. Colloid Interface Sci. 2011, 362, 457–462. [Google Scholar] [CrossRef]
- Ahmadi, A.; Hajilou, M.; Zavari, S.; Yaghmaei, S. A Comparative Review on Adsorption and Photocatalytic Degradation of Classified Dyes with Metal/Non-Metal-Based Modification of Graphitic Carbon Nitride Nanocomposites: Synthesis, Mechanism, and Affecting Parameters. J. Clean. Prod. 2023, 382, 134967. [Google Scholar] [CrossRef]
- Hamad, N.; Galhoum, A.A.; Wageh, S. Enhanced Chromate Sorption Using Zinc-Manganese Oxide Mesoporous Structures Decorated Carbon Nanocomposite Prepared from Battery Wastes. J. Environ. Chem. Eng. 2024, 12, 113055. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of Heavy Metals on Functionalized-Mesoporous Silica: A Review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A Review on Heavy Metal Ions Adsorption from Water by Graphene Oxide and Its Composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Asghar, A. A Review of the Applications of Organo-Functionalized Magnetic Graphene Oxide Nanocomposites for Heavy Metal Adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Ibrahim, M.; Usman, M.; Adrees, M.; Mehmood, M.A.; Abbas, F.; Rasool, N.; Rashid, U. Removal of Reactive Blue 21 from Aqueous Solution by Sorption and Solubilization in Micellar Media. J. Dispers. Sci. Technol. 2016, 37, 144–154. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Kurup, L.; Mittal, J. Adsorption of a Hazardous Dye, Erythrosine, over Hen Feathers. J. Colloid Interface Sci. 2006, 304, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Bello, O.S.; Auta, M.; Ayodele, O.B. Ackee Apple (Blighia sapida) Seeds: A Novel Adsorbent for the Removal of Congo Red Dye from Aqueous Solutions. Chem. Ecol. 2013, 29, 58–71. [Google Scholar] [CrossRef]
- Bello, O.S.; Ahmad, M.A. Coconut (Cocos nucifera) Shell Based Activated Carbon for the Removal of Malachite Green Dye from Aqueous Solutions. Sep. Sci. Technol. 2012, 47, 903–912. [Google Scholar] [CrossRef]
- Erto, A.; Giraldo, L.; Lancia, A.; Moreno-Piraján, J.C. A Comparison between a Low-Cost Sorbent and an Activated Carbon for the Adsorption of Heavy Metals from Water. Water Air Soil Pollut. 2013, 224, 1531. [Google Scholar] [CrossRef]
- Di Natale, F.; Erto, A.; Lancia, A. Desorption of Arsenic from Exhaust Activated Carbons Used for Water Purification. J. Hazard. Mater. 2013, 260, 451–458. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Surip, S.N.; Alothman, Z.A. Hybrid Multifunctional Biocomposite of Chitosan Grafted Benzaldehyde/Montmorillonite/Algae for Effective Removal of Brilliant Green and Reactive Blue 19 Dyes: Optimization and Adsorption Mechanism. J. Clean. Prod. 2023, 393, 136334. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Y.; Yu, Z.; Su, Y. Efficient Adsorption Removal of Anionic Dyes by Waste PET-Derived MIL-101 (Cr). Sep. Purif. Technol. 2025, 354, 128985. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, W.; Xing, S.; Guo, H.; Wang, S.; Sun, M.; Bi, J. Insights into the Synergistic, Neutral, and Antagonistic Adsorption Effects in Cobalt-Containing Wastewater Treatment. Chem. Eng. Sci. 2024, 298, 120318. [Google Scholar] [CrossRef]
- Bououden, W.; Benguerba, Y.; Darwish, A.S.; Attoui, A.; Lemaoui, T.; Balsamo, M.; Erto, A.; Alnashef, I.M. Surface Adsorption of Crizotinib on Carbon and Boron Nitride Nanotubes as Anti-Cancer Drug Carriers: COSMO-RS and DFT Molecular Insights. J. Mol. Liq. 2021, 338, 116666. [Google Scholar] [CrossRef]
- Sellaoui, L.; Nour, S.; Dhaouadi, F.; Bonilla-Petriciolet, A. Physicochemical Study of the Adsorption of Chlorophenols on Activated Carbon Using an Advanced Double Layer Model. J. Mol. Liq. 2024, 414, 126098. [Google Scholar] [CrossRef]
- Ghorbali, R.; Sellaoui, L.; Ghalla, H.; Bonilla-Petriciolet, A.; Trejo-Valencia, R.; Sánchez-Barroso, A.; Deng, S.; Lamine, A.B. In-Depth Study of Adsorption Mechanisms and Interactions in the Removal of Pharmaceutical Contaminants via Activated Carbon: A Physicochemical Analysis. Environ. Sci. Pollut. Res. 2024, 31, 39208–39216. [Google Scholar] [CrossRef]
- Sellaoui, L.; Dhaouadi, F.; Reynel-Avila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Trejo-Valencia, R.; Taamalli, S.; Louis, F.; El Bakali, A.; Chen, Z. Physicochemical Assessment of Anionic Dye Adsorption on Bone Char Using a Multilayer Statistical Physics Model. Environ. Sci. Pollut. Res. 2021, 28, 67248–67255. [Google Scholar] [CrossRef] [PubMed]
- Ghorbali, R.; Sellaoui, L.; Dhaouadi, F.; Ghalla, H.; Bonilla-Petriciolet, A.; Badawi, M.; Lamine, A.B. Adsorption of Phenolic Compounds on Polymeric Ionic Liquids: Adsorption Isotherms Interpretation and Thermodynamic Study. J. Mol. Liq. 2024, 414, 126155. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Reynel-Ávila, H.E.; Landín-Sandoval, V.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Lima, E.C.; Bonilla-Petriciolet, A.; Lamine, A.B. Adsorption Mechanism of Zn2+, Ni2+, Cd2+, and Cu2+ Ions by Carbon-Based Adsorbents: Interpretation of the Adsorption Isotherms via Physical Modelling. Environ. Sci. Pollut. Res. Int. 2021, 28, 30943–30954. [Google Scholar] [CrossRef]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Ben Lamine, A.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.; et al. Adsorption of Methylene Blue from Aqueous Solution on Activated Carbons and Composite Prepared from an Agricultural Waste Biomass: A Comparative Study by Experimental and Advanced Modeling Analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Aumeier, B.M.; Augustin, A.; Thönes, M.; Sablotny, J.; Wintgens, T.; Wessling, M. Linking the Effect of Temperature on Adsorption from Aqueous Solution with Solute Dissociation. J. Hazard. Mater. 2022, 429, 128291. [Google Scholar] [CrossRef]
- Yanan, C.; Ali, J.; Sellaoui, L.; Dhaoudi, F.; Franco, D.S.; Georgin, J.; Erto, A.; Vieillard, J.; Badawi, M. Elucidating the Adsorption Mechanism of Herbicide Diuron onto Activated Carbons via Steric, Energetic and Thermodynamic Investigations. J. Water Process Eng. 2023, 53, 103910. [Google Scholar] [CrossRef]
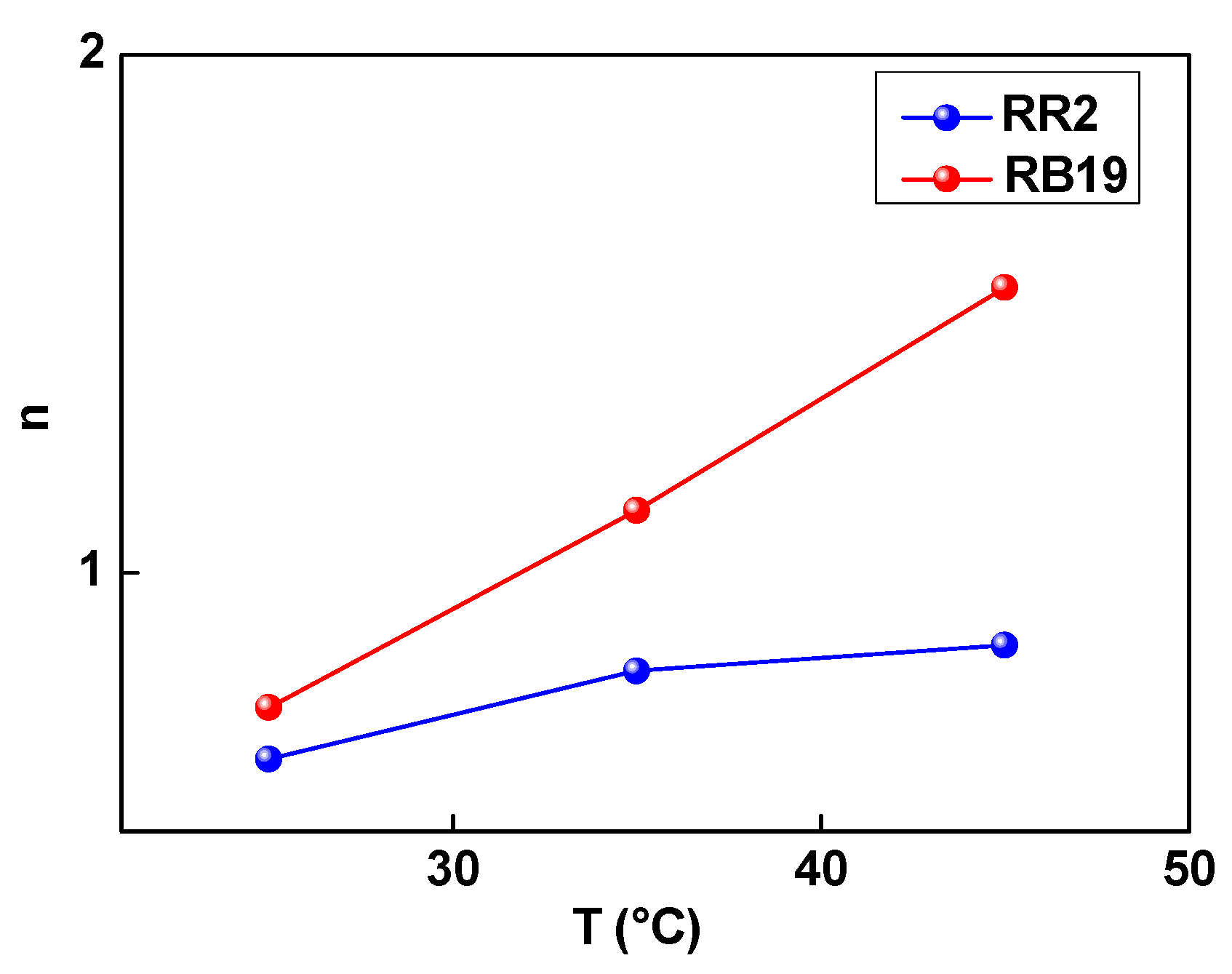

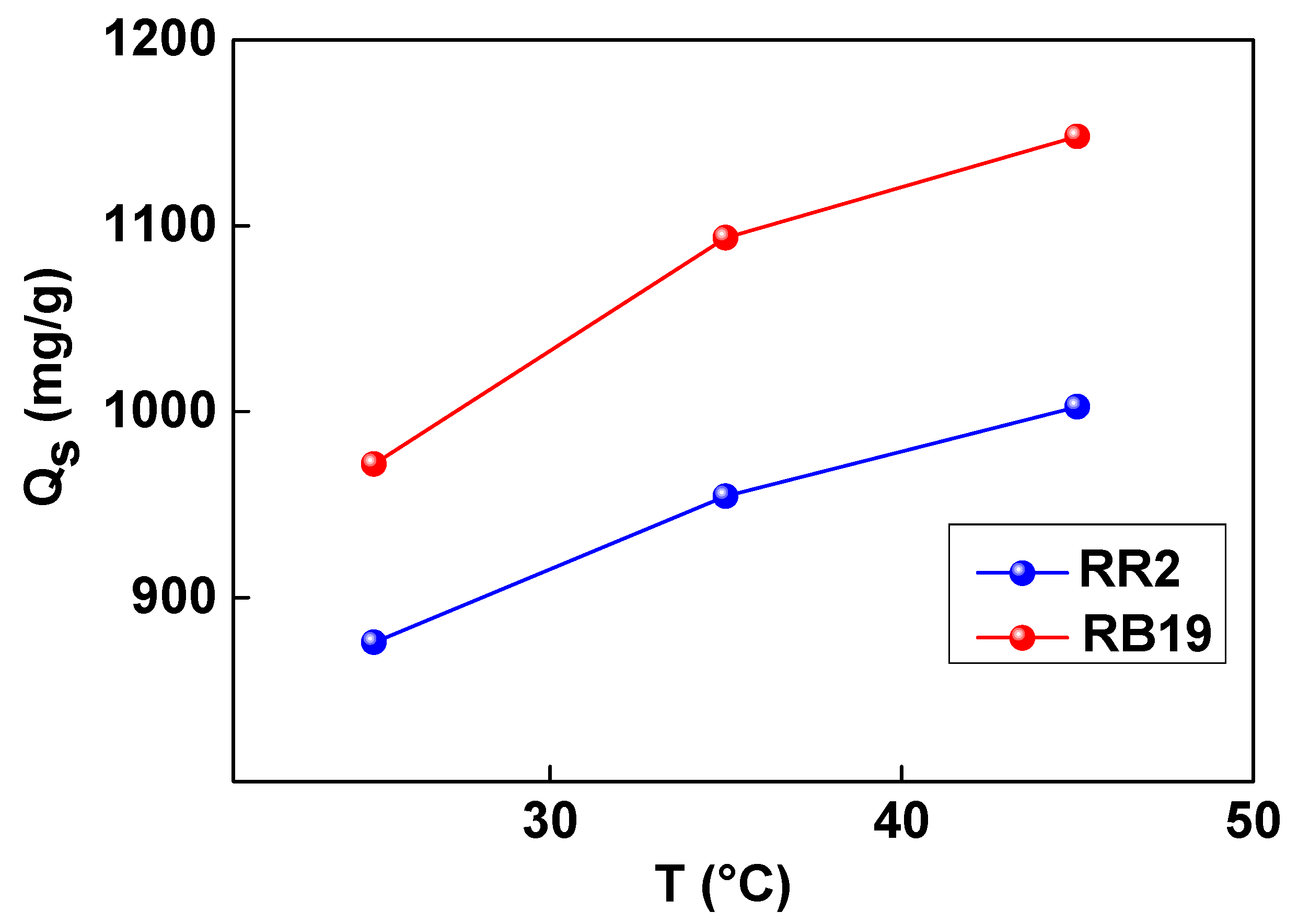
| T, °C | R2 | n | Nm (mg/g) | C1 (mg/L) | C2 (mg/L) | MAC (mg/g) |
|---|---|---|---|---|---|---|
| RR2 | ||||||
| 25 | 0.995 | 0.64 | 684.25 | 8.18 | 22.48 | 875.84 |
| 35 | 0.997 | 0.81 | 589.19 | 10.56 | 17.98 | 954.48 |
| 45 | 0.997 | 0.86 | 582.98 | 13.28 | 25.18 | 1002.72 |
| RB19 | ||||||
| 25 | 0.946 | 0.74 | 656.66 | 10.19 | 24.29 | 971.85 |
| 35 | 0.977 | 1.12 | 488.26 | 15.58 | 35.17 | 1093.70 |
| 45 | 0.956 | 1.55 | 370.36 | 18.98 | 52.52 | 1148.116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellaoui, L.; Sghaier, N.; Erto, A. Outstanding Adsorption of Reactive Red 2 and Reactive Blue 19 Dyes on MIL-101 (Cr): Novel Physicochemical Analysis of Underlying Mechanism Through Statistical Physics Modeling. Water 2025, 17, 1665. https://doi.org/10.3390/w17111665
Sellaoui L, Sghaier N, Erto A. Outstanding Adsorption of Reactive Red 2 and Reactive Blue 19 Dyes on MIL-101 (Cr): Novel Physicochemical Analysis of Underlying Mechanism Through Statistical Physics Modeling. Water. 2025; 17(11):1665. https://doi.org/10.3390/w17111665
Chicago/Turabian StyleSellaoui, Lotfi, Nour Sghaier, and Alessandro Erto. 2025. "Outstanding Adsorption of Reactive Red 2 and Reactive Blue 19 Dyes on MIL-101 (Cr): Novel Physicochemical Analysis of Underlying Mechanism Through Statistical Physics Modeling" Water 17, no. 11: 1665. https://doi.org/10.3390/w17111665
APA StyleSellaoui, L., Sghaier, N., & Erto, A. (2025). Outstanding Adsorption of Reactive Red 2 and Reactive Blue 19 Dyes on MIL-101 (Cr): Novel Physicochemical Analysis of Underlying Mechanism Through Statistical Physics Modeling. Water, 17(11), 1665. https://doi.org/10.3390/w17111665








