Photocatalytic Degradation Study of Paroxetine with g-C3N4 Prepared Using Different Precursors in Lab- and Pilot-Scale Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Preliminary Study of PXT’s Photolytic Degradation and Its Degree of Mineralization (Lab-Scale Experiments)
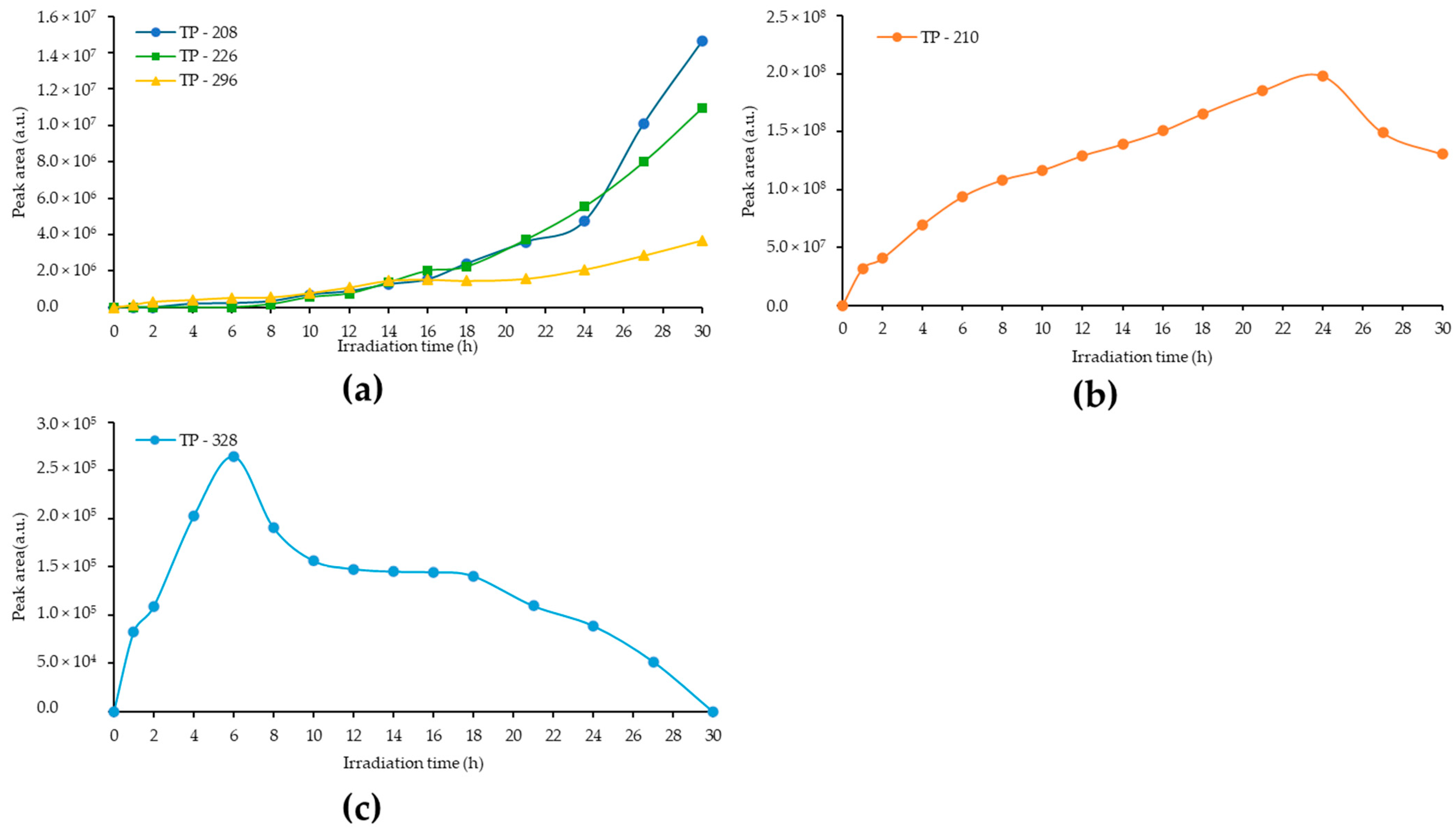
3.2. Identification of the Photolytically Produced TPs by High-Resolution LC–MS
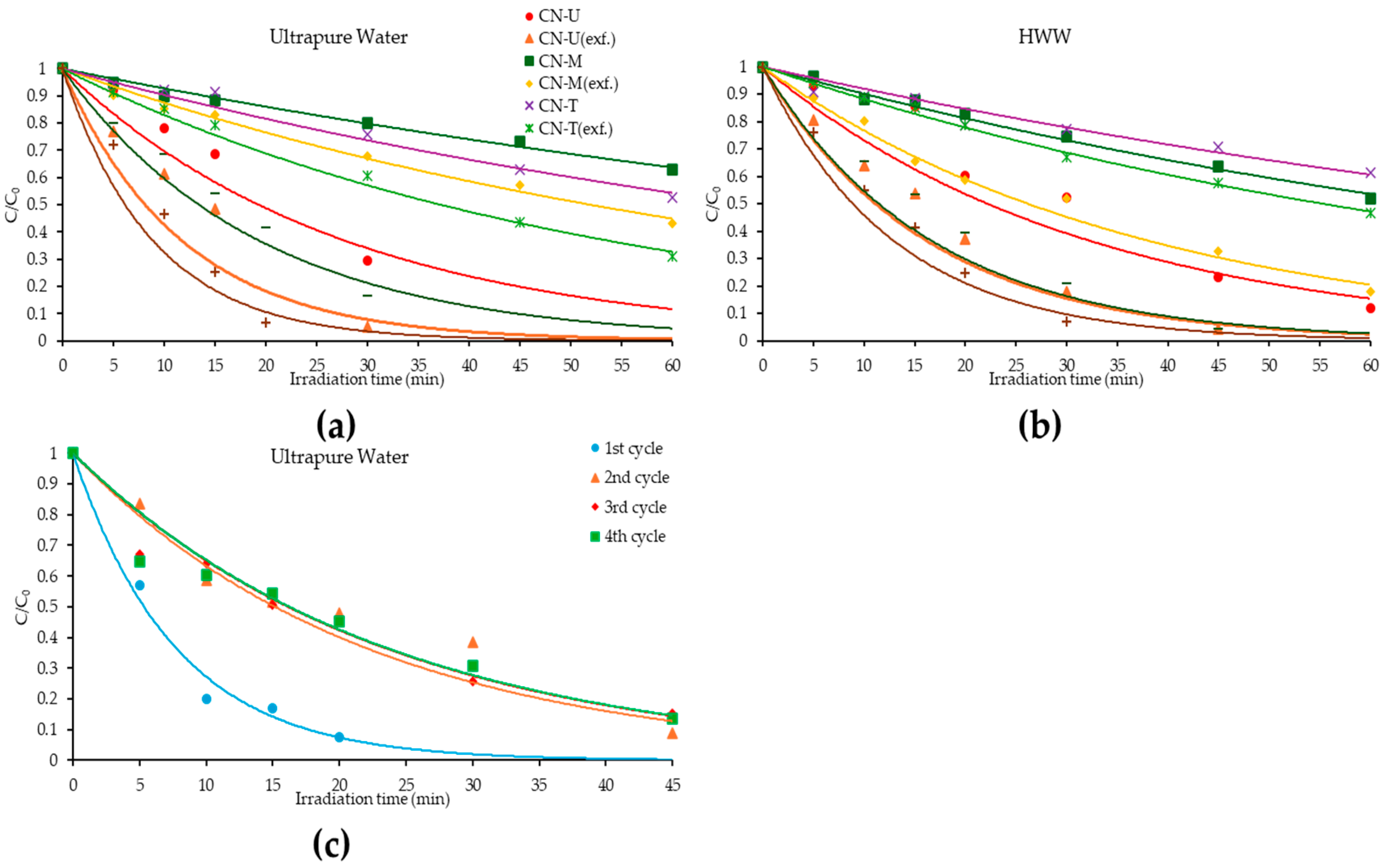
3.3. Photocatalytic Degradation of PXT with g-C3N4 in Lab-Scale Experiments
3.4. Reusability of CN-U(exf.) (Lab-Scale Experiments)
3.5. Scavenging Study of the Photogenerated Reactive Species with CN-U and CN-U(exf.)
3.6. Detection and Identification of the Photocatalytically Produced TPs with CN-U and CN-U(exf.)
3.7. In Silico Ecotoxicity Assessment (Lab-Scale Experiments)
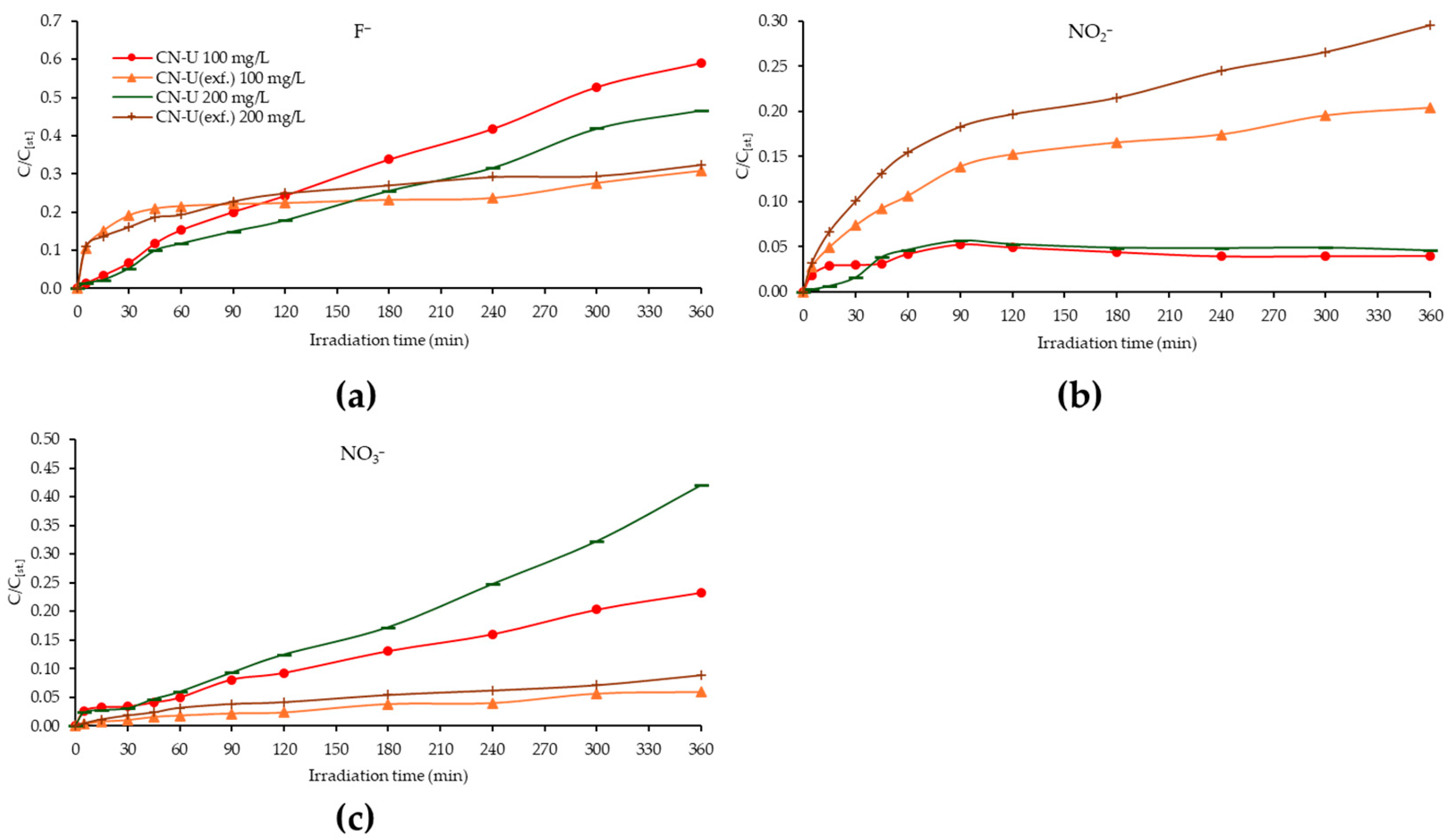
3.8. Photocatalytic Degradation of PXT with CN-U(exf.) in Lab Pilot-Scale Trial in Environmentally Relevant Concentrations
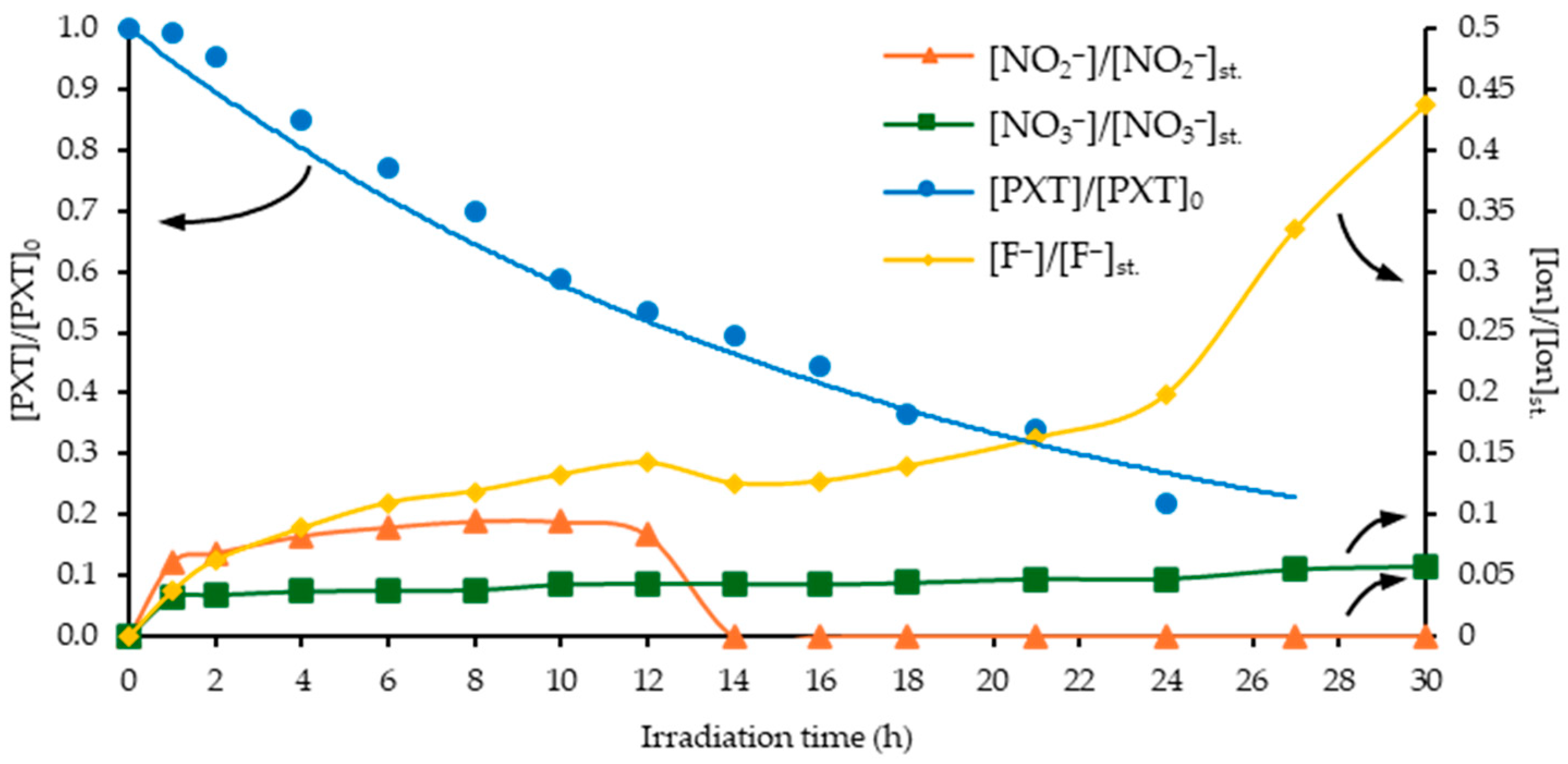
3.9. Photocatalytic Degradation of PXT with CN-U in Environmentally Relevant Concentrations (Solar Pilot-Scale Experiments)
3.10. In Vitro Toxicity Studies During the Photocatalytic Process (Pilot-Scale Experiments)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PXT | Paroxetine |
| WW | Wastewater |
| HWW | Hospital Wastewater |
| WWTPs | Wastewater Treatment Plants |
| CPCs | Concentrating Parabolic Collectors |
| PhACs | Pharmaceutical Active Compounds |
| PCPs | Personal Care Products |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| CN-U | g-C3N4 prepared by Urea |
| CN-U(exf.) | thermally exfoliated g-C3N4 prepared by Urea |
| CN-M | g-C3N4 prepared by Melamine |
| CN-M(exf.) | thermally exfoliated g-C3N4 prepared by Melamine |
| CN-T | g-C3N4 prepared by Thiourea |
| CN-T(exf.) | thermally exfoliated g-C3N4 prepared by Thiourea |
References
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present Applications of Titanium Dioxide for the Photocatalytic Removal of Pollutants from Water: A Review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef] [PubMed]
- Gurke, R.; Rößler, M.; Marx, C.; Diamond, S.; Schubert, S.; Oertel, R.; Fauler, J. Occurrence and Removal of Frequently Prescribed Pharmaceuticals and Corresponding Metabolites in Wastewater of a Sewage Treatment Plant. Sci. Total Environ. 2015, 532, 762–770. [Google Scholar] [CrossRef]
- Kosma, C.I.; Kapsi, M.G.; Konstas, P.S.G.; Trantopoulos, E.P.; Boti, V.I.; Konstantinou, I.K.; Albanis, T.A. Assessment of Multiclass Pharmaceutical Active Compounds (PhACs) in Hospital WWTP Influent and Effluent Samples by UHPLC-Orbitrap MS: Temporal Variation, Removals and Environmental Risk Assessment. Environ. Res. 2020, 191, 110152. [Google Scholar] [CrossRef]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. Occurrence, Fate and Removal of Pharmaceutically Active Compounds (PhACs) in Water and Wastewater Treatment Plants—A Review. J. Water Process Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- Wu, G.; Yao, R.; Zhang, Y.; Ying, M.; Wu, T.; Jiang, W.; Wang, D.; Zhang, X.X. Transformation Mechanisms of Antidepressants in Biological Wastewater Treatment: Removal Kinetic, Transformation Products and Pathways. Chem. Eng. J. 2024, 493, 152557. [Google Scholar] [CrossRef]
- Yuan, S.; Jiang, X.; Xia, X.; Zhang, H.; Zheng, S. Detection, Occurrence and Fate of 22 Psychiatric Pharmaceuticals in Psychiatric Hospital and Municipal Wastewater Treatment Plants in Beijing, China. Chemosphere 2013, 90, 2520–2525. [Google Scholar] [CrossRef]
- Gornik, T.; Carena, L.; Kosjek, T.; Vione, D. Phototransformation Study of the Antidepressant Paroxetine in Surface Waters. Sci. Total Environ. 2021, 774, 145380. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, J.H.; Jo, S.H. Inhibitory Effect of the Selective Serotonin Reuptake Inhibitor Paroxetine on Human Kv1.3 Channels. Eur. J. Pharmacol. 2021, 912, 174567. [Google Scholar] [CrossRef]
- Giatti, S.; Domizio, A.D.; Diviccaro, S.; Cioffi, L.; Marmorini, I.; Falvo, E.; Caruso, D.; Contini, A.; Melcangi, R.C. Identification of a Novel Off-Target of Paroxetine: Possible Role in Sexual Dysfunction Induced by This SSRI Antidepressant Drug. J. Mol. Struct. 2022, 1268, 133690. [Google Scholar] [CrossRef]
- Paíga, P.; Correia, M.; Fernandes, M.J.; Silva, A.; Carvalho, M.; Vieira, J.; Jorge, S.; Silva, J.G.; Freire, C.; Delerue-Matos, C. Assessment of 83 Pharmaceuticals in WWTP Influent and Effluent Samples by UHPLC-MS/MS: Hourly Variation. Sci. Total Environ. 2019, 648, 582–600. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Pereira, A.M.P.T.; Meisel, L.M.; Lino, C.M.; Pena, A. A One-Year Follow-up Analysis of Antidepressants in Portuguese Wastewaters: Occurrence and Fate, Seasonal Influence, and Risk Assessment. Sci. Total Environ. 2014, 490, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Schlüsener, M.P.; Hardenbicker, P.; Nilson, E.; Schulz, M.; Viergutz, C.; Ternes, T.A. Occurrence of Venlafaxine, Other Antidepressants and Selected Metabolites in the Rhine Catchment in the Face of Climate Change. Environ. Pollut. 2015, 196, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Smyth, S.A.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of Antidepressant Residues in Wastewater and Biosolids Following Different Treatment Processes by Municipal Wastewater Treatment Plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.; Almeida, C.M.R.; Fernandes, J.P.; Morais, D.; Lino, M.; Gomes, C.R.; Carvalho, M.F.; Mucha, A.P. Bioremediation of Bezafibrate and Paroxetine by Microorganisms from Estuarine Sediment and Activated Sludge of an Associated Wastewater Treatment Plant. Sci. Total Environ. 2019, 655, 796–806. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Lykos, C.; Kourkouta, T.; Konstantinou, I. Study on the Photocatalytic Degradation of Metronidazole Antibiotic in Aqueous Media with TiO2 under Lab and Pilot Scale. Sci. Total Environ. 2023, 870, 161877. [Google Scholar] [CrossRef]
- Pariente, M.I.; Segura, Y.; Álvarez-Torrellas, S.; Casas, J.A.; de Pedro, Z.M.; Diaz, E.; García, J.; López-Muñoz, M.J.; Marugán, J.; Mohedano, A.F.; et al. Critical Review of Technologies for the On-Site Treatment of Hospital Wastewater: From Conventional to Combined Advanced Processes. J. Environ. Manag. 2022, 320, 115769. [Google Scholar] [CrossRef]
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic Degradation of Pharmaceutical and Pesticide Compounds (PPCs) Using Doped TiO2 Nanomaterials: A Review. Water-Energy Nexus 2020, 3, 46–61. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and Mechanism of Semiconductor Photocatalysis on Degradation of Organic Pollutants in Water Treatment: A Review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Muhmood, T.; Ahmad, I.; Haider, Z.; Haider, S.K.; Shahzadi, N.; Aftab, A.; Ahmed, S.; Ahmad, F. Graphene-like Graphitic Carbon Nitride (g-C3N4) as a Semiconductor Photocatalyst: Properties, Classification, and Defects Engineering Approaches. Mater. Today Sustain. 2024, 25, 100633. [Google Scholar] [CrossRef]
- Patel, S.B.; Tripathi, A.; Vyas, A.P. Recent Development in the Structural Modification of Graphitic Carbon Nitride for Sustainable Photocatalysis: Advances, Challenges and Opportunities. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100589. [Google Scholar] [CrossRef]
- Qi, K.; Liu, S.; Zada, A. Graphitic Carbon Nitride, a Polymer Photocatalyst. J. Taiwan. Inst. Chem. Eng. 2020, 109, 111–123. [Google Scholar] [CrossRef]
- Bairamis, F.; Konstantinou, I. Photocatalytic Degradation Pathways of the Valsartan Drug by TiO2 and g-C3N4 Catalysts. Reactions 2022, 3, 160–171. [Google Scholar] [CrossRef]
- Cecconet, D.; Sturini, M.; Malavasi, L.; Capodaglio, A.G. Graphitic Carbon Nitride as a Sustainable Photocatalyst Material for Pollutants Removal. State-of-the Art, Preliminary Tests and Application Perspectives. Materials 2021, 14, 7368. [Google Scholar] [CrossRef]
- Wudil, Y.S.; Ahmad, U.F.; Gondal, M.A.; Al-Osta, M.A.; Almohammedi, A.; Sa’id, R.S.; Hrahsheh, F.; Haruna, K.; Mohamed, M.J.S. Tuning of Graphitic Carbon Nitride (g-C3N4) for Photocatalysis: A Critical Review. Arab. J. Chem. 2023, 16, 104542. [Google Scholar] [CrossRef]
- Farooq, N.; Khan, M.I.; ur Rehman, Z.; Wattoo, M.A.; Qureshi, A.M.; Shanableh, A.; ur Rehman, A. Barium-Doped Ceria-Fabricated Graphitic Carbon Nitride (BDC/g-C3N4) Nanomaterial as Improved Photocatalyst for Efficient Vitiation of Organic Dyes from Industrial Effluents. Inorg. Chem. Commun. 2024, 159, 111830. [Google Scholar] [CrossRef]
- Obregón, S. Exploring Nanoengineering Strategies for the Preparation of Graphitic Carbon Nitride Nanostructures. FlatChem 2023, 38, 100473. [Google Scholar] [CrossRef]
- Pei, J.; Li, H.; Zhuang, S.; Zhang, D.; Yu, D. Recent Advances in g-C3N4 Photocatalysts: A Review of Reaction Parameters, Structure Design and Exfoliation Methods. Catalysts 2023, 13, 1402. [Google Scholar] [CrossRef]
- Syed, J.A.S.; Zhang, X.Y.; Ding, W.J.; Li, A.D. An Overview of the Current Progress of Graphitic Carbon Nitride and Its Multifunctional Applications. J. Environ. Chem. Eng. 2022, 10, 108745. [Google Scholar] [CrossRef]
- Chen, L.; Maigbay, M.A.; Li, M.; Qiu, X. Synthesis and Modification Strategies of g-C3N4 Nanosheets for Photocatalytic Applications. Adv. Powder Mater. 2024, 3, 100150. [Google Scholar] [CrossRef]
- Molaei, M.J. Graphitic Carbon Nitride (g-C3N4) Synthesis and Heterostructures, Principles, Mechanisms, and Recent Advances: A Critical Review. Int. J. Hydrogen Energy 2023, 48, 32708–32728. [Google Scholar] [CrossRef]
- Rapti, I.; Kourkouta, T.; Malisova, E.M.; Albanis, T.; Konstantinou, I. Photocatalytic Degradation of Inherent Pharmaceutical Concentration Levels in Real Hospital WWTP Effluents Using g-C3N4 Catalyst on CPC Pilot Scale Reactor. Molecules 2023, 28, 1170. [Google Scholar] [CrossRef]
- Rapti, I.; Boti, V.; Albanis, T.; Konstantinou, I. Photocatalytic Degradation of Psychiatric Pharmaceuticals in Hospital WWTP Secondary Effluents Using g-C3N4 and g-C3N4/MoS2 Catalysts in Laboratory-Scale Pilot. Catalysts 2023, 13, 252. [Google Scholar] [CrossRef]
- Zheng, Q.; Aiello, A.; Choi, Y.S.; Tarr, K.; Shen, H.; Durkin, D.P.; Shuai, D. 3D Printed Photoreactor with Immobilized Graphitic Carbon Nitride: A Sustainable Platform for Solar Water Purification. J. Hazard. Mater. 2020, 399, 123097. [Google Scholar] [CrossRef]
- Lykos, C.; Sioulas, S.; Konstantinou, I. g-C3N4 as Photocatalyst for the Removal of Metronidazole Antibiotic from Aqueous Matrices under Lab and Pilot Scale Conditions. Catalysts 2023, 13, 254. [Google Scholar] [CrossRef]
- Fotiou, D.; Lykos, C.; Konstantinou, I. Photocatalytic Removal of the Antidepressant Fluoxetine from Aqueous Media Using TiO2 P25 and g-C3N4 Catalysts. J. Environ. Chem. Eng. 2024, 12, 111677. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Kosma, C.; Albanis, T.; Konstantinou, I. An Overview of Homogeneous and Heterogeneous Photocatalysis Applications for the Removal of Pharmaceutical Compounds from Real or Synthetic Hospital Wastewaters under Lab or Pilot Scale. Sci. Total Environ. 2021, 765, 144163. [Google Scholar] [CrossRef]
- Zheng, Q.; Shen, H.; Shuai, D. Emerging Investigators Series: Advances and Challenges of Graphitic Carbon Nitride as a Visible-Light-Responsive Photocatalyst for Sustainable Water Purification. Environ. Sci. 2017, 3, 982–1001. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Sannino, D. Main Parameters Influencing the Design of Photocatalytic Reactors for Wastewater Treatment: A Mini Review. J. Chem. Technol. Biotechnol. 2020, 95, 2608–2618. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar Photocatalysis: Materials, Reactors, Some Commercial, and Pre-Industrialized Applications. A Comprehensive Approach. Appl. Catal. B 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Kwon, J.-W.; Armbrust, K.L. Hydrolysis and photolysis of paroxetine, a selective serotonin reuptake inhibitor, in aqueous solutions. Environ. Toxicol. Chem. 2004, 23, 1394–1399. [Google Scholar] [CrossRef]
- Chand, H.; Kumar, A.; Goswami, S.; Krishnan, V. Comparison of Catalytic Activity of Graphitic Carbon Nitrides Derived from Different Precursors for Carbon Dioxide Conversion. Fuel 2024, 357, 129757. [Google Scholar] [CrossRef]
- Jiang, X.; Li, X.; Liu, Y.; Zhai, H.; Wang, G. The Oxidative Thermal Exfoliation-Dependent Microstructure and Photocatalytic Performance of g-C3N4 Nanosheets. Diam. Relat. Mater. 2024, 143, 110918. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Zhao, J.; Bao, Y.; Hou, L. an Exfoliation Method Matters: The Microstructure-Dependent Photoactivity of g-C3N4 Nanosheets for Water Purification. J. Hazard. Mater. 2022, 424, 127424. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Yue, D. Enhanced Adsorption of Humic/Fulvic Acids onto Urea-Derived Graphitic Carbon Nitride. J. Hazard. Mater. 2022, 424, 127643. [Google Scholar] [CrossRef]
- Yue, D.; Wang, J.; Cui, D.; Zhang, L.; Dong, X. Insights into Adsorption of Humic Substances on Graphitic Carbon Nitride. Environ. Sci. Technol. 2021, 55, 7910–7919. [Google Scholar] [CrossRef]
- Attri, P.; Garg, P.; Sharma, P.; Singh, R.; Chauhan, M.; Lim, D.K.; Kumar, S.; Chaudhary, G.R. Precursor-Dependent Fabrication of Exfoliated Graphitic Carbon Nitride (GCN) for Enhanced Photocatalytic and Antimicrobial Activity under Visible Light Irradiation. J. Clean. Prod. 2023, 422, 138538. [Google Scholar] [CrossRef]
- Tameu Djoko, S.Y.; Bashiri, H.; Njoyim, E.T.; Arabameri, M.; Djepang, S.; Tamo, A.K.; Laminsi, S.; Tasbihi, M.; Schwarze, M.; Schomäcker, R. Urea and Green Tea like Precursors for the Preparation of g-C3N4 Based Carbon Nanomaterials (CNMs) Composites as Photocatalysts for Photodegradation of Pollutants under UV Light Irradiation. J. Photochem. Photobiol. A Chem. 2020, 398, 112596. [Google Scholar] [CrossRef]
- Zhu, C.; Zhu, F.; Dionysiou, D.D.; Zhou, D.; Fang, G.; Gao, J. Contribution of Alcohol Radicals to Contaminant Degradation in Quenching Studies of Persulfate Activation Process. Water Res. 2018, 139, 66–73. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Sillanpää, M. A Review on Persulfates Activation by Functional Biochar for Organic Contaminants Removal: Synthesis, Characterizations, Radical Determination, and Mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Diniz, R.R.; Domingos, T.F.S.; Pinto, G.R.; Cabral, L.M.; de Pádula, M.; de Souza, A.M.T. Use of in Silico and in Vitro Methods as a Potential New Approach Methodologies (NAMs) for (Photo)Mutagenicity and Phototoxicity Risk Assessment of Agrochemicals. Sci. Total Environ. 2023, 904, 167320. [Google Scholar] [CrossRef]
- Toropova, A.P.; Toropov, A.A.; Roncaglioni, A.; Benfenati, E. The Enhancement Scheme for the Predictive Ability of QSAR: A Case of Mutagenicity. Toxicol. In Vitro 2023, 91, 105629. [Google Scholar] [CrossRef]
- Pandey, S.K.; Roy, K. Development of a Read-across-Derived Classification Model for the Predictions of Mutagenicity Data and Its Comparison with Traditional QSAR Models and Expert Systems. Toxicology 2023, 500, 153676. [Google Scholar] [CrossRef]
- Xu, J.Y.; Wang, K.; Men, S.H.; Yang, Y.; Zhou, Q.; Yan, Z.G. QSAR-QSIIR-Based Prediction of Bioconcentration Factor Using Machine Learning and Preliminary Application. Environ. Int. 2023, 177, 108003. [Google Scholar] [CrossRef]
- Gómez-Regalado, M.D.C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E.; Zafra-Gómez, A. Bioaccumulation/Bioconcentration of Pharmaceutical Active Compounds in Aquatic Organisms: Assessment and Factors Database. Sci. Total Environ. 2023, 861, 160638. [Google Scholar] [CrossRef]
- Khan, K.; Kumar, V.; Colombo, E.; Lombardo, A.; Benfenati, E.; Roy, K. Intelligent Consensus Predictions of Bioconcentration Factor of Pharmaceuticals Using 2D and Fragment-Based Descriptors. Environ. Int. 2022, 170, 107625. [Google Scholar] [CrossRef]
- Brienza, M.; Mahdi Ahmed, M.; Escande, A.; Plantard, G.; Scrano, L.; Chiron, S.; Bufo, S.A.; Goetz, V. Use of Solar Advanced Oxidation Processes for Wastewater Treatment: Follow-up on Degradation Products, Acute Toxicity, Genotoxicity and Estrogenicity. Chemosphere 2016, 148, 473–480. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Suspended TiO2-Assisted Photocatalytic Degradation of Emerging Contaminants in a Municipal WWTP Effluent Using a Solar Pilot Plant with CPCs. Chem. Eng. J. 2012, 198–199, 301–309. [Google Scholar] [CrossRef]
- Rapti, I.; Kosma, C.; Albanis, T.; Konstantinou, I. Solar Photocatalytic Degradation of Inherent Pharmaceutical Residues in Real Hospital WWTP Effluents Using Titanium Dioxide on a CPC Pilot Scale Reactor. Catal Today 2023, 423, 113884. [Google Scholar] [CrossRef]
- Germann, D.; Ma, G.; Han, F.; Tikhomirova, A. Paroxetine Hydrochloride. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 38, pp. 367–406. [Google Scholar] [CrossRef]


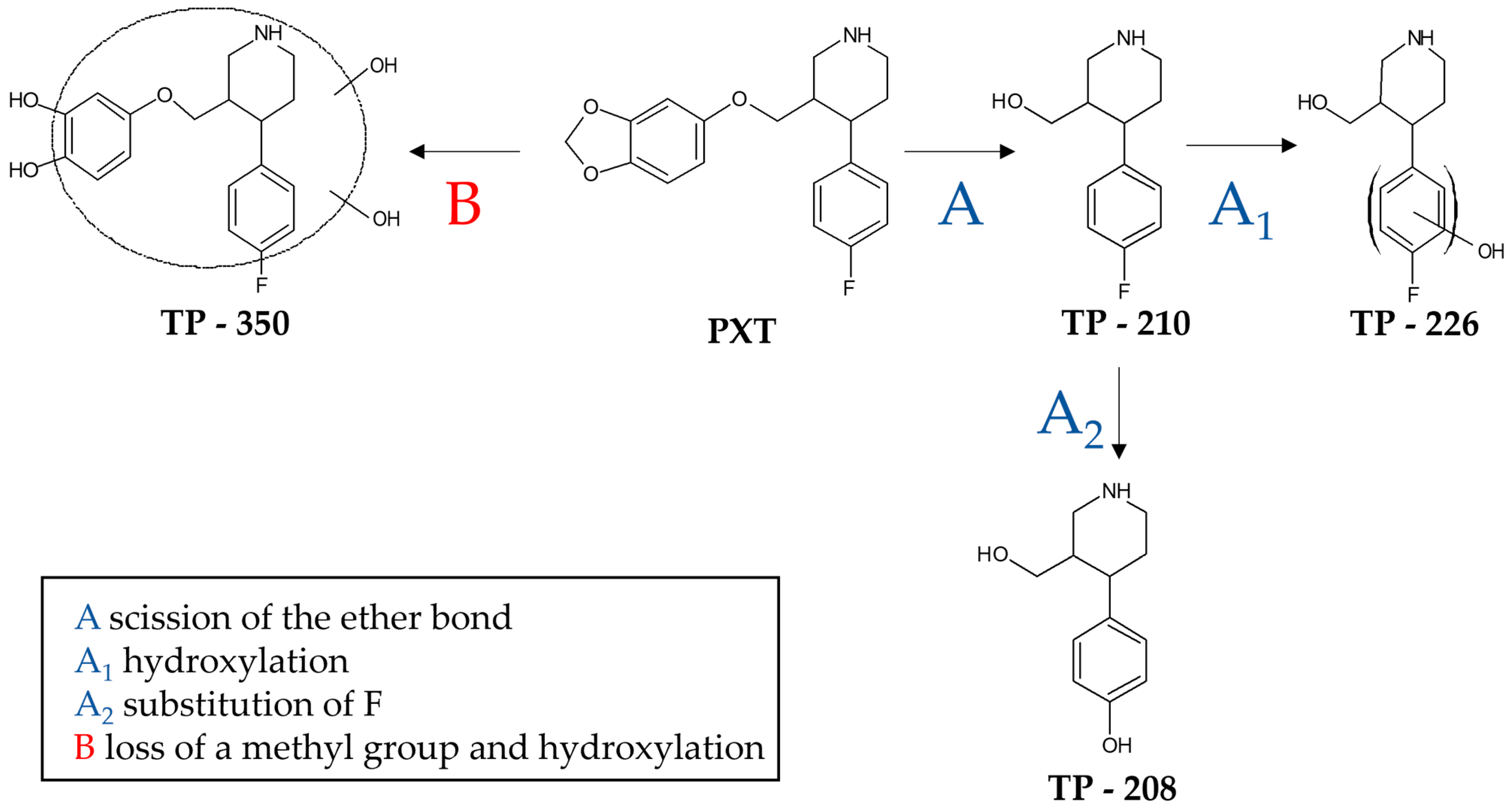
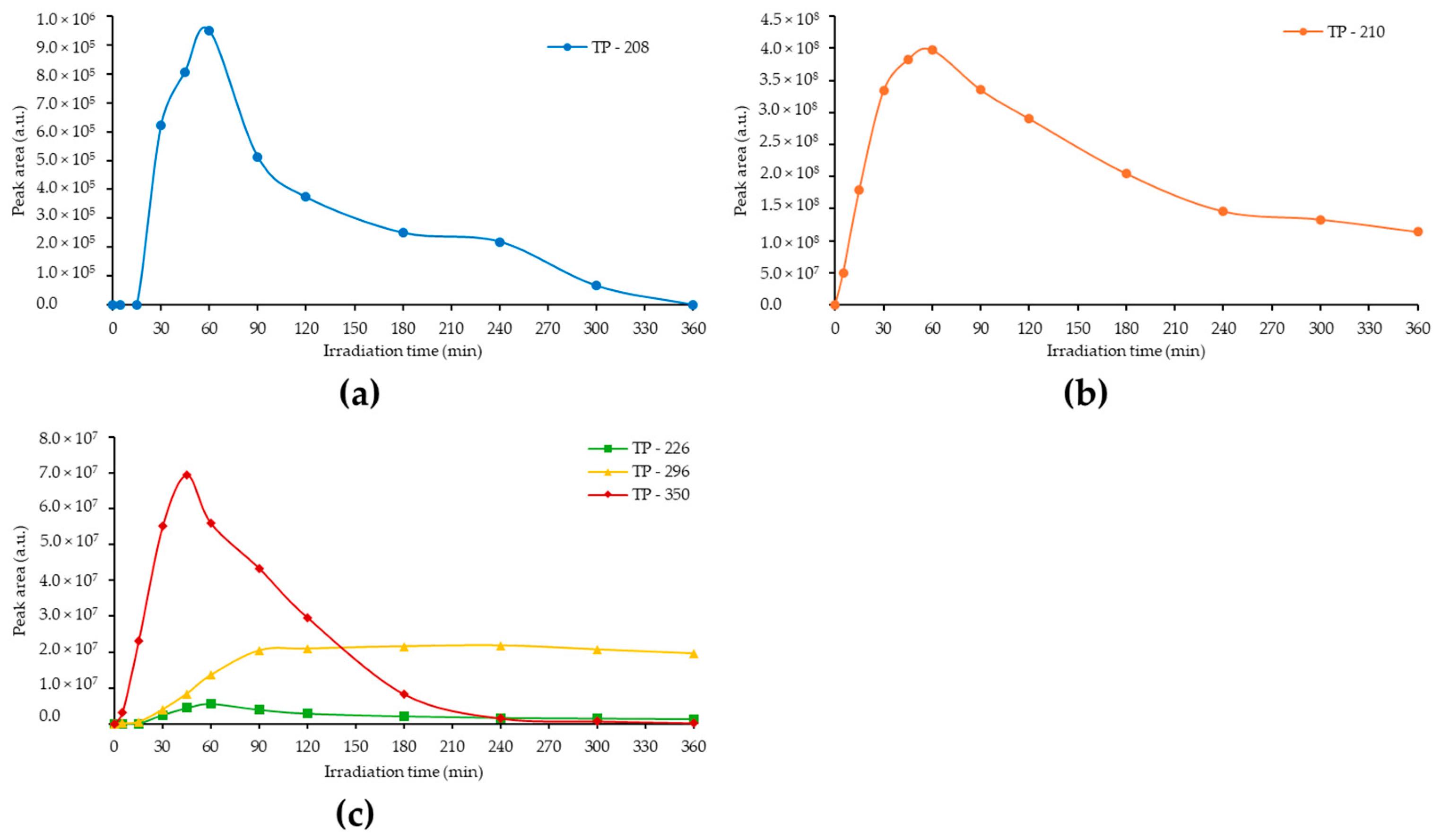
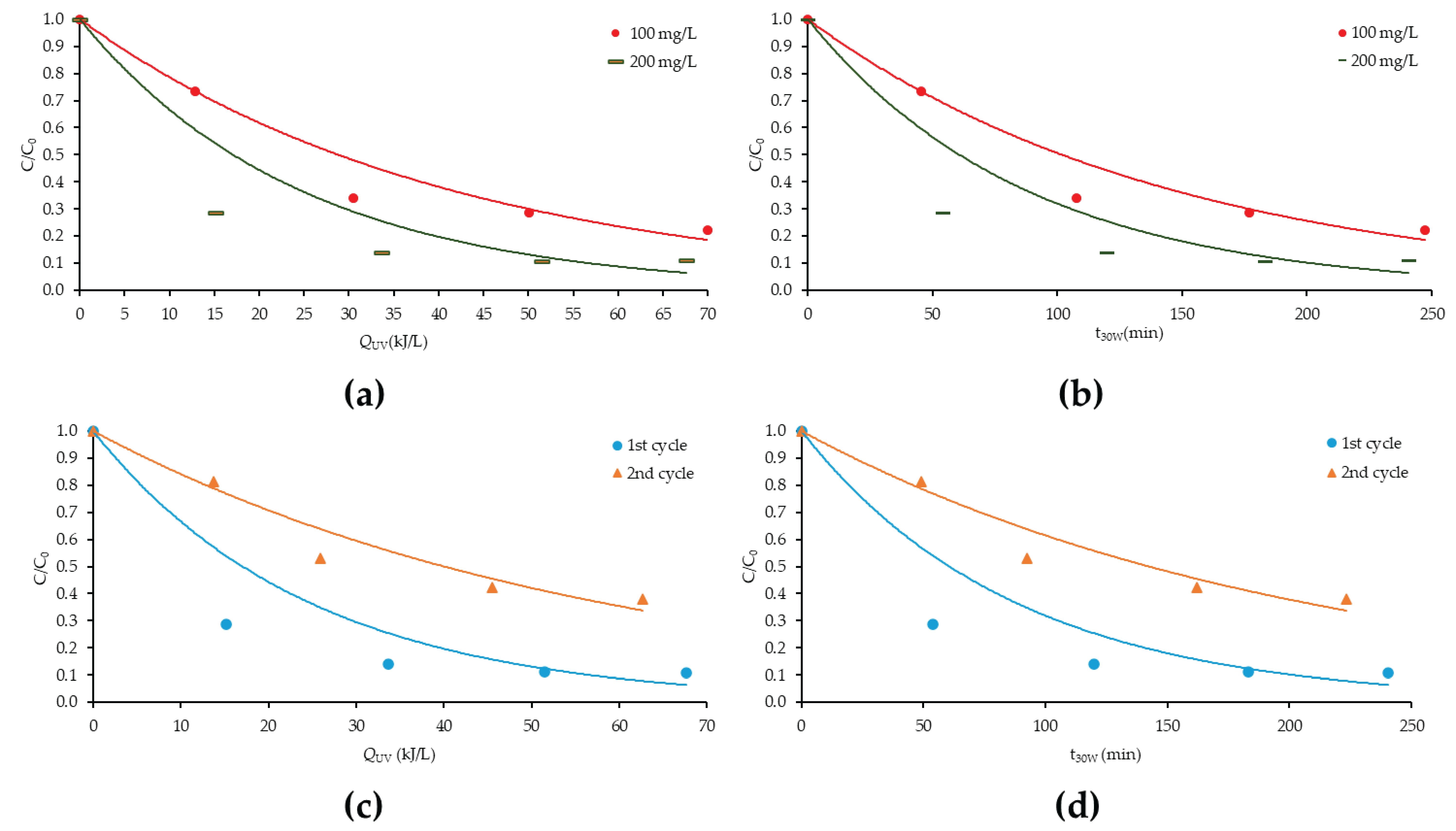
| Material | Concentration (mg/L) | Substrate | kapp (min−1) | t1/2 (min) | R2 | % Removal |
|---|---|---|---|---|---|---|
| CN-U | 100 | U.P. Water | 0.036 | 19.25 | 0.9487 | 100 |
| HWW | 0.031 | 22.36 | 0.9311 | 88.18 | ||
| 200 | U.P. Water | 0.052 | 13.33 | 0.9687 | 100 | |
| HWW | 0.061 | 11.36 | 0.9682 | 100 | ||
| CN-U(exf.) | 100 | U.P. Water | 0.085 | 8.15 | 0.9121 | 100 |
| HWW | 0.062 | 11.18 | 0.9701 | 100 | ||
| 200 | U.P. Water | 0.112 | 6.19 | 0.9497 | 100 | |
| HWW | 0.078 | 8.89 | 0.9754 | 100 | ||
| CN-M | 100 | U.P. Water | 0.008 | 86.64 | 0.9901 | 37.02 |
| HWW | 0.010 | 69.31 | 0.9916 | 48.10 | ||
| CN-M(exf.) | 100 | U.P. Water | 0.013 | 53.32 | 0.9913 | 56.75 |
| HWW | 0.026 | 26.66 | 0.9886 | 81.94 | ||
| CN-T | 100 | U.P. Water | 0.010 | 69.31 | 0.9841 | 47.21 |
| HWW | 0.008 | 86.64 | 0.9594 | 38.51 | ||
| CN-T(exf.) | 100 | U.P. Water | 0.019 | 36.48 | 0.9942 | 68.97 |
| HWW | 0.013 | 53.32 | 0.9969 | 53.26 |
| Material | Concentration (mg/L) | Catalytic Cycle | kapp (min−1) | R2 | Δkapp% |
|---|---|---|---|---|---|
| CN-U(exf.) | 200 | 1st | 0.13 | 0.9857 | - |
| 2nd | 0.046 | 0.9561 | 64.6 | ||
| 3rd | 0.043 | 0.9699 | 6.52 | ||
| 4th | 0.043 | 0.9535 | 0 |
| Material | Concentration (mg/L) | kapp | R2 | % Removal | Δkapp% |
|---|---|---|---|---|---|
| CN-U(exf.) | 100 | 0.034 min−1 | 0.9892 | 100 | - |
| CN-U | 100 | 0.024 L/kJ | 0.9586 | 77 | - |
| 200 (1st catalytic cycle) | 0.041 L/kJ | 0.9069 | 89 | - | |
| 200 (2nd catalytic cycle) | 0.017 L/kJ | 0.9510 | 62 | 58.5 |
| Irradiation Time (min) | % Bioluminescence Inhibition of Vibrio fischeri | ||
|---|---|---|---|
| 100 mg/L | 200 mg/L (1st Cycle) | 200 mg/L (2nd Cycle) | |
| 0 | 13.94 | 69.65 | 9.6 |
| 300 | Hormesis | 20.36 | Hormesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sioulas, S.; Lykos, C.; Konstantinou, I.; Albanis, T. Photocatalytic Degradation Study of Paroxetine with g-C3N4 Prepared Using Different Precursors in Lab- and Pilot-Scale Conditions. Water 2025, 17, 1609. https://doi.org/10.3390/w17111609
Sioulas S, Lykos C, Konstantinou I, Albanis T. Photocatalytic Degradation Study of Paroxetine with g-C3N4 Prepared Using Different Precursors in Lab- and Pilot-Scale Conditions. Water. 2025; 17(11):1609. https://doi.org/10.3390/w17111609
Chicago/Turabian StyleSioulas, Sotirios, Christos Lykos, Ioannis Konstantinou, and Triantafyllos Albanis. 2025. "Photocatalytic Degradation Study of Paroxetine with g-C3N4 Prepared Using Different Precursors in Lab- and Pilot-Scale Conditions" Water 17, no. 11: 1609. https://doi.org/10.3390/w17111609
APA StyleSioulas, S., Lykos, C., Konstantinou, I., & Albanis, T. (2025). Photocatalytic Degradation Study of Paroxetine with g-C3N4 Prepared Using Different Precursors in Lab- and Pilot-Scale Conditions. Water, 17(11), 1609. https://doi.org/10.3390/w17111609









