Contrasting Microplastic Characteristics in Macroinvertebrates from Two Independent but Adjacent Rivers in Kruger National Park, South Africa
Abstract
1. Introduction
2. Materials and Methods
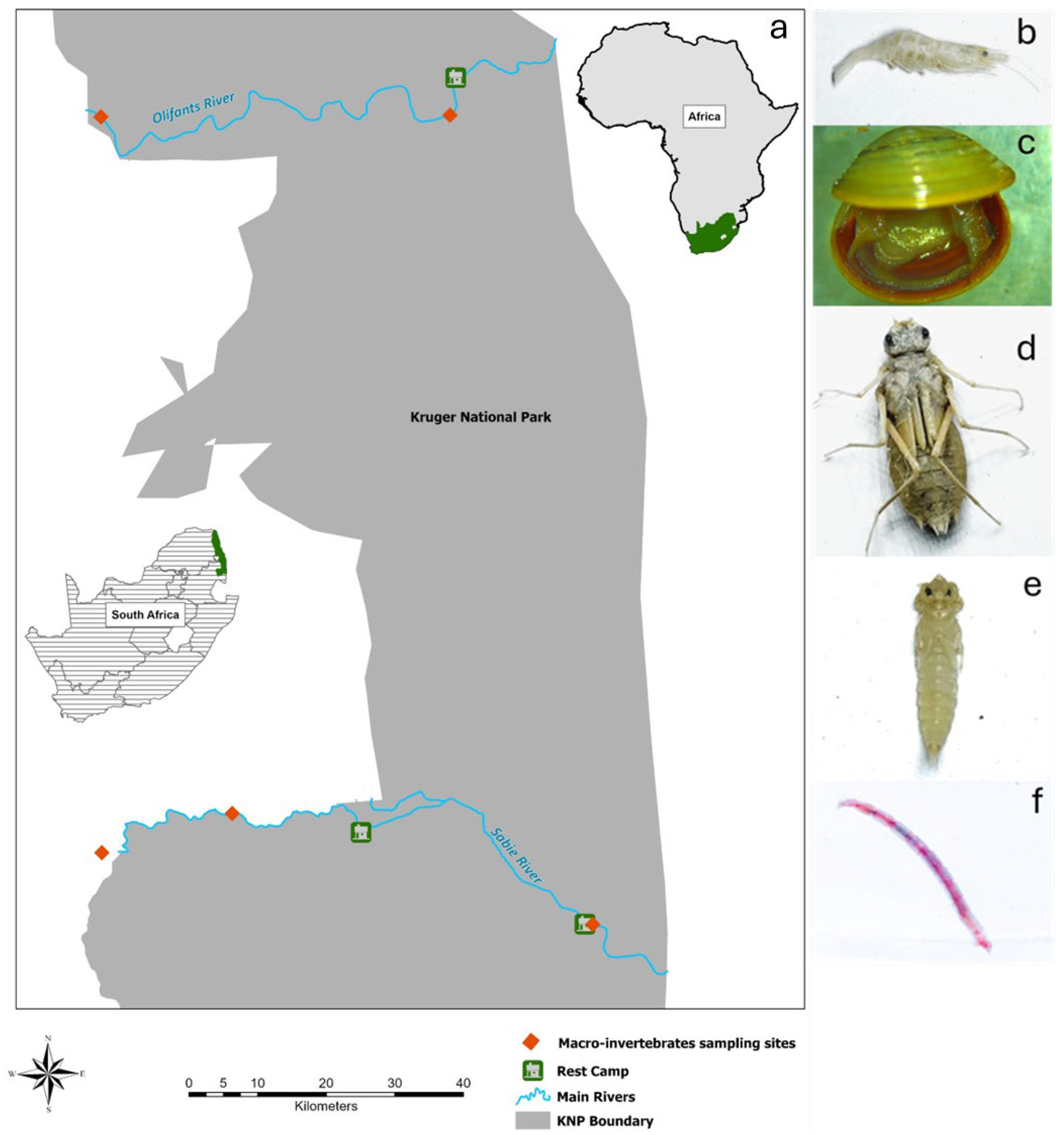
2.1. Study Area
2.2. Macroinvertebrate Collection
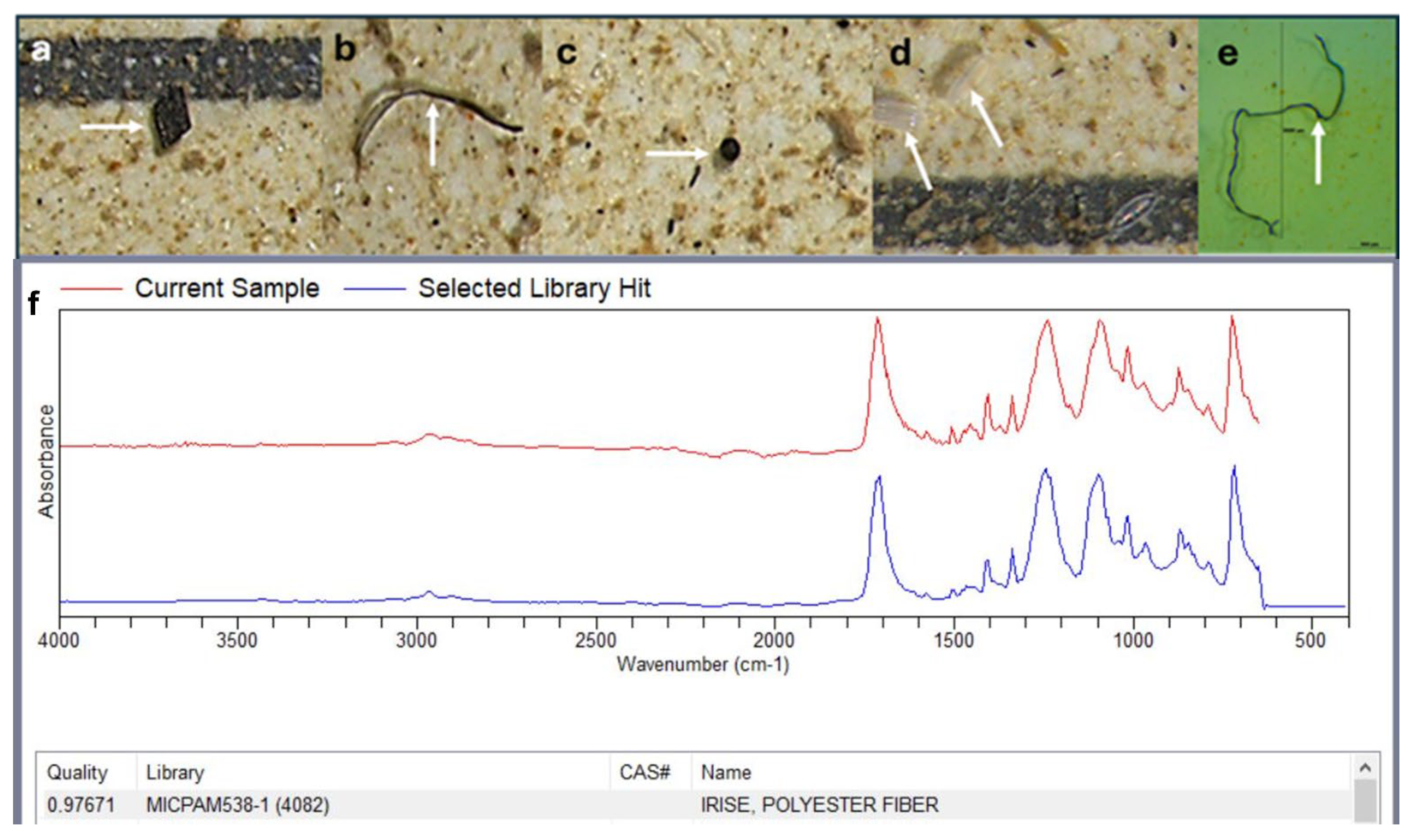
2.3. Isolation and Identification of Mps in Benthic Macroinvertebrates
3. Quality Control
Statistical Analysis
4. Results
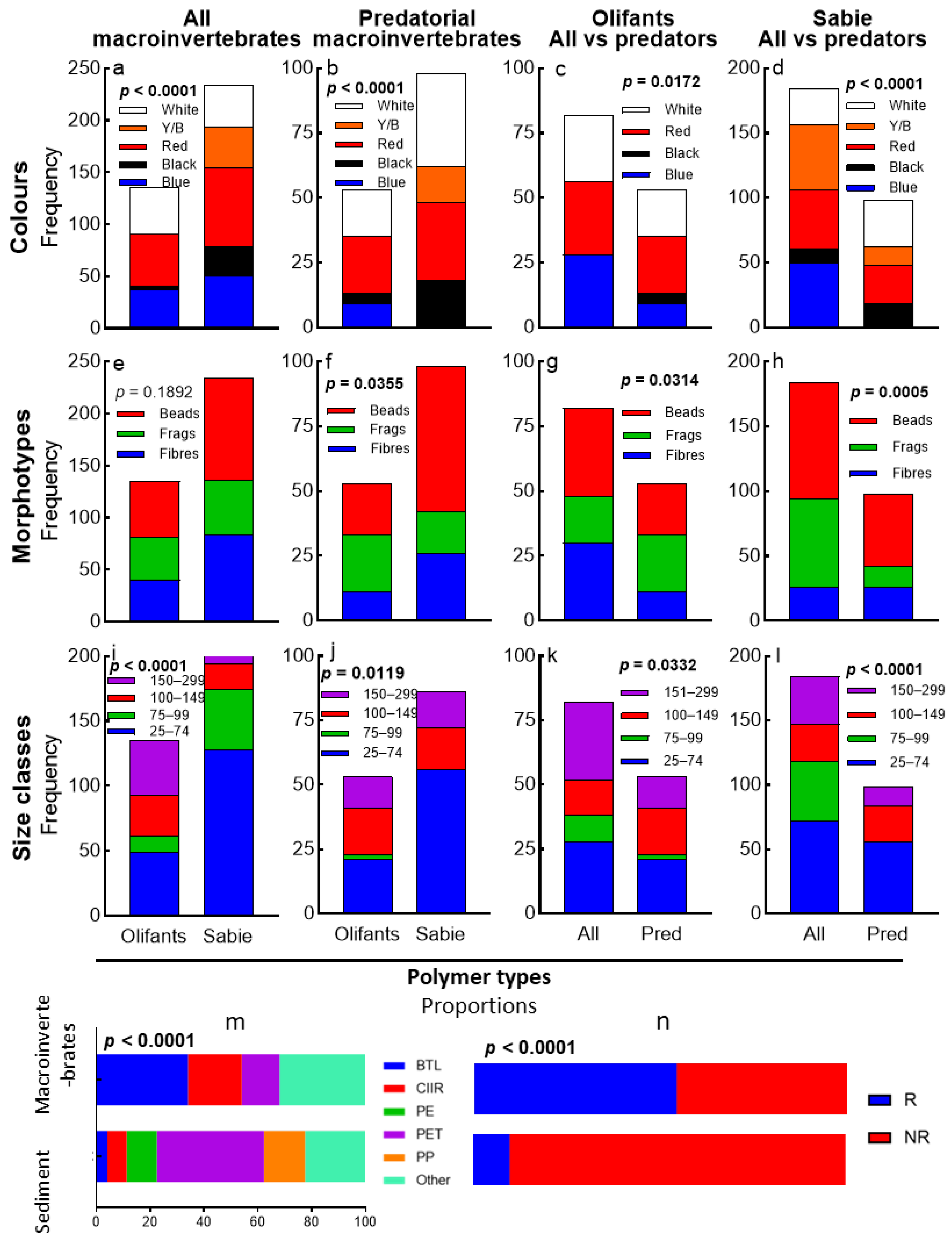
4.1. Overall MP Content
4.2. Microplastic Colour, Morphotype, and Size Class Proportions Between Rivers and Guilds
4.3. MP Polymer Types
5. Discussion
5.1. Microplastic Colour, Morphotype, and Size Class Proportions Between Rivers and Their Guilds
5.2. Overall MP Contents
5.3. Evidence of Trophic Transfer
5.4. MP Polymer Types
6. Conclusions and Recommendations
7. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aragaw, T.A. Microplastic pollution in African countries’ water systems: A review on findings, applied methods, characteristics, impacts, and managements. SN Appl. Sci. 2021, 3, 629. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, L.; Dai, Y.; Sun, H.; Liu, C. Behavior of Microplastics in Inland Waters: Aggregation, Settlement, and Transport. Bull. Environ. Contam. Toxicol. 2021, 107, 700–709. [Google Scholar] [CrossRef]
- Saad, D. Why Microplastics Are Exceptional Contaminants? 2023. Available online: https://www.intechopen.com/ (accessed on 4 May 2024).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made [Producción, uso y destino de todos los plásticos jamás fabricados]. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Wagner, M.; Lambert, S. Freshwater Microplastics The Handbook of Environmental Chemistry 58 Series; Barceló, D., Kostianoy, A.G., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Covernton, G.A.; Cox, K. Commentary on: Abundance and distribution of microplastics within surface sediments of a key shellfish growing region of Canada. PLoS ONE 2019, 14, e0225945. [Google Scholar] [CrossRef]
- Hernandez, E.; Nowack, B.; Mitrano, D.M. Polyester Textiles as a Source of Microplastics from Households: A Mechanistic Study to Understand Microfiber Release during Washing. Environ. Sci. Technol. 2017, 51, 7036–7046. [Google Scholar] [CrossRef]
- Verster, C.; Bouwman, H. Land-based sources and pathways of marine plastics in a South African context. S. Afr. J. Sci. 2020, 116, 1–9. [Google Scholar] [CrossRef]
- Maleka, T.; Greenfield, R.; Muniyasamy, S.; Modley, L.A. An overview on the characterization of microplastics (MPs) in waste water treatment plants (WWTPs). Sustain. Water Resour. Manag. 2024, 10, 182. [Google Scholar] [CrossRef]
- Tibbetts, J.; Krause, S.; Lynch, I.; Smith, G.H.S. Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water 2018, 10, 1597. [Google Scholar] [CrossRef]
- Dahms, H.T.J.; van Rensburg, G.J.; Greenfield, R. The microplastic profile of an urban African stream. Sci. Total Environ. 2020, 731, 138893. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, S.; Miao, B.; Hou, M.; Zhao, Y. A review of the ecotoxicological status of microplastic pollution in African freshwater systems. Sci. Total Environ. 2024, 946, 174092. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Costa, M.F.; Duarte, A.C. Biotechnology advances for dealing with environmental pollution by micro(nano)plastics: Lessons on theory and practices. Curr. Opin. Environ. Sci. Health 2018, 1, 30–35. [Google Scholar] [CrossRef]
- Chang, M. Reducing microplastics from facial exfoliating cleansers in wastewater through treatment versus consumer product decisions. Mar. Pollut. Bull. 2015, 101, 330–333. [Google Scholar] [CrossRef]
- Clapp, J.; Swanston, L. Doing away with plastic shopping bags: International patterns of norm emergence and policy implementation. Environ. Politics 2009, 18, 315–332. [Google Scholar] [CrossRef]
- Xanthos, D.; Walker, T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Mar. Pollut. Bull. 2017, 118, 17–26. [Google Scholar] [CrossRef]
- Royer, S.; Greco, F.; Kogler, M.; Deheyn, D.D. Not so biodegradable: Polylactic acid and cellulose/plastic blend textiles lack fast biodegradation in marine waters. PLoS ONE 2023, 18, e0284681. [Google Scholar] [CrossRef]
- Leblanc, R. How Long Garbage Decomposes. 2015. Available online: https://www.richlandcenterwi.gov/sites/default/files/fileattachments/parks_amp_recreation/page/2534/howlonggarbagedecomposes.pdf (accessed on 5 May 2024).
- Saad, D.; Alamin, H. The first evidence of microplastic presence in the River Nile in Khartoum, Sudan: Using Nile tilapia fish as a bio-indicator. Heliyon 2024, 10, e23393. [Google Scholar] [CrossRef]
- Ma, Y.; You, X. Microplastics in freshwater ecosystems: A significant force of disrupting health and altering trophic transfer patterns by reduced assimilation efficiency of aquatic organisms. Aquaculture 2025, 594, 741463. [Google Scholar] [CrossRef]
- Emon, F.J.; Hasan, J.; Shahriar, S.I.M.; Islam, N.; Islam, M.S.; Shahjahan, M. Increased ingestion and toxicity of polyamide microplastics in Nile tilapia with increase of salinity. Ecotoxicol. Environ. Saf. 2024, 282, 116730. [Google Scholar] [CrossRef]
- Devriese, L.I.; De Witte, B.; Vethaak, A.D.; Hostens, K.; Leslie, H.A. Bioaccumulation of PCBs from microplastics in Norway lobster (Nephrops norvegicus): An experimental study. Chemosphere 2017, 186, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.; Matias, M.G.; Castillo-Escrivà, A.; Messyasz, B.; Leoni, B. Microalgae colonization of different microplastic polymers in experimental mesocosms across an environmental gradient. Glob. Change Biol. 2022, 28, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Taurozzi, D.; Cesarini, G.; Scalici, M. Diatom and macroinvertebrate communities dynamic: A co-occurrence pattern analysis on plastic substrates. Sci. Total Environ. 2024, 912, 169071. [Google Scholar] [CrossRef] [PubMed]
- Taurozzi, D.; Cesarini, G.; Scalici, M. New ecological frontiers in the plastisphere: Diatoms and macroinvertebrates turnover assessment by a traits-based approach. Sci. Total Environ. 2023, 887, 164186. [Google Scholar] [CrossRef]
- Sun, L.; Cheng, Z.; Wang, M.; Wei, C.; Liu, H.; Yang, Y. A multi-levels analysis to evaluate the toxicity of microplastics on aquatic insects: A case study with damselfly larvae (Ischnura elegans). Ecotoxicol. Environ. Saf. 2025, 289, 117447. [Google Scholar] [CrossRef]
- Sbarberi, R.; Magni, S.; Ponti, B.; Tediosi, E.; Neri, M.C.; Binelli, A. Multigenerational effects of virgin and sampled plastics on the benthic macroinvertebrate Chironomus riparius. Aquat. Toxicol. 2025, 279, 107205. [Google Scholar] [CrossRef]
- Ghosh, T. Microplastics bioaccumulation in fish: Its potential toxic effects on hematology, immune response, neurotoxicity, oxidative stress, growth, and reproductive dysfunction. Toxicol. Rep. 2025, 14, 101854. [Google Scholar] [CrossRef]
- Akindele, E.O.; Ehlers, S.M.; Koop, J.H.E. Freshwater insects of different feeding guilds ingest microplastics in two Gulf of Guinea tributaries in Nigeria. Environ. Sci. Pollut. Res. 2020, 27, 33373–33379. [Google Scholar] [CrossRef]
- Parra, S.; Varandas, S.; Santos, D.; Félix, L.; Fernandes, L.; Cabecinha, E.; Gago, J.; Monteiro, S.M. Multi-biomarker responses of Asian clam Corbicula fluminea (Bivalvia, Corbiculidea) to cadmium and microplastics pollutants. Water 2021, 13, 394. [Google Scholar] [CrossRef]
- Dickens, C.W.S.; Graham, P.M.; Dickens, C.W.S.; Graham, P.M. The South African Scoring System (SASS) Version 5 Rapid Bioassessment Method for Rivers The South African Scoring System (SASS) Version 5 Rapid Bioassessment. Afr. J. Aquat. Sci. 2010, 27, 1–10. [Google Scholar] [CrossRef]
- Mangadze, T.; Bere, T.; Mwedzi, T. Choice of biota in stream assessment and monitoring programs in tropical streams: A comparison of diatoms, macroinvertebrates and fish. Ecol. Indic. 2016, 63, 128–143. [Google Scholar] [CrossRef]
- Porter, A.; Godbold, J.A.; Lewis, C.N.; Savage, G.; Solan, M.; Galloway, T.S. Microplastic burden in marine benthic invertebrates depends on species traits and feeding ecology within biogeographical provinces. Nat. Commun. 2023, 14, 8023. [Google Scholar] [CrossRef] [PubMed]
- Benhadji, N.; Kurniawan, S.B.; Imron, M.F. Review of mayflies (Insecta Ephemeroptera) as a bioindicator of heavy metals and microplastics in freshwater. Sci. Total Environ. 2025, 958, 178057. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Song, S.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef]
- Mbedzi, R.; Cuthbert, R.N.; Wasserman, R.J.; Murungweni, F.M.; Dalu, T. Spatiotemporal variation in microplastic contamination along a subtropical reservoir shoreline. Environ. Sci. Pollut. Res. 2020, 27, 23880–23887. [Google Scholar] [CrossRef]
- Riddell, E.S.; Govender, D.; Botha, J.; Sithole, H.; Petersen, R.M.; Shikwambana, P. Pollution impacts on the aquatic ecosystems of the Kruger National Park, South Africa. Sci. Afr. 2019, 6, e00195. [Google Scholar] [CrossRef]
- Shikwambana, P.; Foxcroft, L.C.; Taylor, J.C.; Bouwman, H. Microplastic Concentrations in Sediments and Waters Do Not Decrease in Two Rivers Flowing Through the Kruger National Park, South Africa. Water Air Soil Pollut. 2024, 235, 675. [Google Scholar] [CrossRef]
- Pollard, S.; Laporte, A. Overview of the Olifants Catchment. No. March. 2014. Available online: http://award.org.za/wp/wp-content/uploads/2021/04/AWARD-TECH-REPORT-53-Overview-of-the-Olifants-Catchment-March-2014-v1.pdf (accessed on 5 May 2024).
- Moolman, J.; King, J.; Thirion, C. Channel Slopes in the Olifants, Crocodile and Sabie River Catchments. 2002; pp. 1–42. Available online: https://award.org.za/wp/wp-content/uploads/2020/07/AWARD-TECH-REPORT-40-Overview-of-the-Olifants-catchment-2014-v1.pdf (accessed on 4 May 2024).
- Pollard, E.S. Testing Strategic Adaptive Management during Crisis: Management of the Perennial Rivers of the Kruger National Park during Drought. In Proceedings of the 14th International Water Association (IWA) Specialist Conference on Watershed and River Basin Management, Skukuza, South Africa, 9–11 October 2017. [Google Scholar]
- Biggs, H.C.; Clifford-Holmes, J.K.; Freitag, S.; Venter, F.J.; Venter, J. Cross-scale governance and ecosystem service delivery: A case narrative from the Olifants River in north-eastern South Africa. Ecosyst. Serv. 2017, 28, 173–184. [Google Scholar] [CrossRef]
- Statistics South Africa. Midyear Population Estimate 2019; Statistics South Africa: Cape Town, South Africa, 2019; p. 24. [Google Scholar]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Gerber, A.; Gabriel, M.J.M. Aquatic Invertebrates of South African Rivers Field Guide; Department of Water Affairs and Forestry: Pretoria, South Africa, 2002. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past Palaeontological Statistics, Ver. 1.79. 2001; pp. 1–88. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 20 January 2023).
- Nel, H.A.; Dalu, T.; Wasserman, R.J. Sinks and sources: Assessing microplastic abundance in river sediment and deposit feeders in an Austral temperate urban river system. Sci. Total Environ. 2018, 612, 950–956. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, M.; Pastorino, P.; Lesa, D.; Renzi, M.; Anselmi, S.; Prearo, M.; Pizzul, E. Microplastics accumulation in functional feeding guilds and functional habit groups of freshwater macrobenthic invertebrates: Novel insights in a riverine ecosystem. Sci. Total Environ. 2022, 804, 150207. [Google Scholar] [CrossRef]
- Dahms, H.T.J.; Tweddle, G.P.; Greenfield, R. Gastric Microplastics in Clarias gariepinus of the Upper Vaal River, South Africa. Front. Environ. Sci. 2022, 10, 931073. [Google Scholar] [CrossRef]
- Weeks, D.C.; O’Keeffe, J.H.; Fourie, A.; Davies, B.R. A Pre-Impoundment Study of the Sabie-Sand River System, Mpumalanga With Special Reference To Predicted Impacts on the Kruger National Park. Water Res. Comm. 1996, 294, 96. [Google Scholar]
- Monira, S.; Bhuiyan, M.; Haque, N.; Shah, K.; Roychand, R.; Hai, F.I.; Premanik, B.K. Understanding the fate and control of road dust-associated microplastics in stormwater. Process Saf. Environ. Prot. 2021, 152, 47–57. [Google Scholar] [CrossRef]
- Peller, J.R.; Tabor, G.; Davis, C.; Iceman, C.; Nwachukwu, O.; Doudrick, K.; Wilson, A.; Suprenant, A.; Dabertin, D.; McCool, J.-P. Distribution and Fate of Polyethylene Microplastics Released by a Portable Toilet Manufacturer into a Freshwater Wetland and Lake. Water 2024, 16, 11. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef]
- Dahms, H.T.J.; Greenfield, R.; Dalu, T.; Themba, N.N.; Dondofema, F.; Cuthbert, R.N. Nowhere to go! Microplastic abundances in freshwater fishes living near wastewater plants. Environ. Toxicol. Pharmacol. 2023, 101, 104210. [Google Scholar] [CrossRef]
- Szałkiewicz, E.; Kałuża, T.; Grygoruk, M. Detailed analysis of habitat suitability curves for macroinvertebrates and functional feeding groups. Sci. Rep. 2022, 12, 10757. [Google Scholar] [CrossRef]
- Goss, H.; Jaskiel, J.; Rotjan, R. Thalassia testudinum as a potential vector for incorporating microplastics into benthic marine food webs. Mar. Pollut. Bull. 2018, 135, 1085–1089. [Google Scholar] [CrossRef]
- Guo, F.; Bunn, S.E.; Brett, M.T.; Fry, B.; Hager, H.; Ouyang, X.; Kainz, M.J. Feeding strategies for the acquisition of high-quality food sources in stream macroinvertebrates: Collecting, integrating, and mixed feeding. Limnol. Oceanogr. 2018, 63, 1964–1978. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, C.C.; Hoellein, T.J. Bivalve impacts in freshwater and marine ecosystems. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 183–208. [Google Scholar] [CrossRef]
- Antczak-Orlewska, O.; Płóciennik, M.; Sobczyk, R.; Okupny, D.; Stachowicz-Rybka, R.; Rzodkiewicz, M.; Siciński, J.; Mroczkowska, A.; Krąpiec, M.; Słowiński, M.; et al. Chironomidae Morphological Types and Functional Feeding Groups as a Habitat Complexity Vestige. Front. Ecol. Evol. 2021, 8, 583831. [Google Scholar] [CrossRef]
- Makgoale, M.M.; Addo-Bediako, A.; Ayisi, K.K. Distribution Pattern of Macroinvertebrate Functional Feeding Groups in the Steelpoort River, South Africa. Appl. Ecol. Environ. Res. 2022, 20, 189–206. [Google Scholar] [CrossRef]
- Scherer, C.; Brennholt, N.; Reifferscheid, G.; Wagner, M. Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci. Rep. 2017, 7, 17006. [Google Scholar] [CrossRef]
- Uwadiae, R.E. Macroinvertebrates functional feeding groups as indices of biological assessment in a tropical aquatic ecosystem: Implications for ecosystem functions. N. Y. Sci. J. 2010, 3, 6–15. [Google Scholar]
- Nhiwatiwa, T.; Brendonck, L.; Dalu, T. Understanding factors structuring zooplankton and macroinvertebrate assemblages in ephemeral pans. Limnologica 2017, 64, 11–19. [Google Scholar] [CrossRef]
- Addo-Bediako, A. Spatial distribution patterns of benthic macroinvertebrate functional feeding groups in two rivers of the olifants river system, South Africa. J. Freshw. Ecol. 2021, 36, 97–109. [Google Scholar] [CrossRef]
- Lourenço, P.M.; Serra-Gonçalves, C.; Ferreira, J.L.; Catry, T.; Granadeiro, J.P. Plastic and other microfibers in sediments, macroinvertebrates and shorebirds from three intertidal wetlands of southern Europe and west Africa. Environ. Pollut. 2017, 231, 123–133. [Google Scholar] [CrossRef]
- Paduvilan, J.K.; Velayudhan, P.; Amanulla, A.; Maria, H.J.; Saiter-fourcin, A.; Thomas, S. Assessment of Graphene Oxide and Nanoclay Based Hybrid Filler in Chlorobutyl-Natural Rubber Blend for Advanced Gas Barrier Applications. Nanomaterials 2021, 11, 1098. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and road wear particles (TRWP)—A review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total Environ. 2020, 733, 137823. [Google Scholar] [CrossRef] [PubMed]
- Munari, C.; Infantini, V.; Scoponi, M.; Rastelli, E.; Corinaldesi, C.; Mistri, M. Microplastics in the sediments of Terra Nova Bay (Ross Sea, Antarctica). Mar. Pollut. Bull. 2017, 122, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 2017, 127, 249–257. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Shen, Y.; Zhan, L.; Xu, Z. An ignored potential microplastic contamination of a typical waste glass recycling base. J. Hazard. Mater. 2022, 422, 126854. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Niu, S.; Wu, J. The role of algae in regulating the fate of microplastics: A review for processes, mechanisms, and influencing factors. Sci. Total Environ. 2024, 949, 175227. [Google Scholar] [CrossRef]
- Valentine, K.; Hughes, C.; Boxall, A. Plastic Litter Emits the Foraging Infochemical Dimethyl Sulfide after Submersion in Freshwater Rivers. Environ. Toxicol. Chem. 2024, 47, 1485–1496. [Google Scholar] [CrossRef]
- Nenadović, T.; Šarčević, T.; Čižmek, H.; Godrijan, J.; Marić Pfannkuchen, D.; Pfannkuchen, M.; Ljubešić, Z. Development of periphytic diatoms on different artificial substrates in the Eastern Adriatic Sea. Acta Bot. Croat. 2015, 74, 377–392. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Ranking of potential hazards from microplastics polymers in the marine environment. J. Hazard. Mater. 2022, 429, 128399. [Google Scholar] [CrossRef]
- Cowger, W.; Markley, L.A.T.; Moore, S.; Gray, A.B.; Upadhyay, K.; Koelmans, A.A. How many microplastics do you need to (sub)sample? Ecotoxicol. Environ. Saf. 2024, 275, 116243. [Google Scholar] [CrossRef]
| Sample | Macroinvertebrate | Guild | River | Number | Site ID | Lat | Long |
|---|---|---|---|---|---|---|---|
| O10GOM | Gomphidae | Predator | Olifants | 7 | O6 | 31.42029° | −24.3162° |
| O5LAB | Libellulidae | Predator | Olifants | 11 | O2 | 31.37197° | −23.9215° |
| O6GOM | Gomphidae | Predator | Olifants | 23 | O6 | 31.42029° | −24.0527° |
| O7COR | Corbiculidae | Filter | Olifants | 26 | O2 | 31.22318° | −24.0471° |
| O8COR | Corbiculidae | Filter | Olifants | 24 | O6 | 31.42029° | −24.3162° |
| O9LAB | Libellulidae | Predator | Olifants | 17 | O6 | 31.42029° | −24.3162° |
| S11COR | Corbiculidae | Filter | Sabie | 17 | S2 | 31.29239° | −24.9883° |
| S11ATY | Atyidae | Filter | Sabie | 25 | S8 | 31.92460° | −25.1219° |
| S2CHI | Chironomidae | Grazer | Sabie | 156 | S1 | 31.21729° | −25.0194° |
| S3GOM | Gomphidae | Predator | Sabie | 56 | S2 | 31.29239° | −24.9883° |
| S4LAB | Libellulidae | Predator | Sabie | 14 | S2 | 31.29239° | −24.9883° |
| Feeding Guild | MP Size Classes | ||||
|---|---|---|---|---|---|
| 25–75 µm | 76–100 µm | 101–150 µm | 151–299 µm | Total | |
| Filter feeder (n = 92) | 3.1 | 2.2 | 0.72 | 2.8 | 8.8 |
| Fibre | ND | ND | 0.16 | 2.8 | 3.0 |
| Fragment | 0.71 | 1.3 | 0.56 | ND | 2.6 |
| Bead | 2.4 | 0.95 | ND | ND | 3.3 |
| Grazer (n = 156) | 0.16 | ND | ND | ND | 0.16 |
| Fibre | 0.03 | ND | ND | ND | 0.03 |
| Fragment | 0.11 | ND | ND | ND | 0.1 |
| Bead | 0.01 | ND | ND | ND | 0.01 |
| Predator (n = 128) | 4.2 | 0.08 | 2.4 | 1.8 | 8.5 |
| Fibre | 0.14 | ND | 0.14 | 1.2 | 1.5 |
| Fragment | 0.5 | ND | 2.2 | 0.6 | 3.3 |
| Bead | 3.5 | 0.08 | ND | ND | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shikwambana, P.; Foxcroft, L.C.; Bouwman, H.; Botha, J.; Taylor, J.C. Contrasting Microplastic Characteristics in Macroinvertebrates from Two Independent but Adjacent Rivers in Kruger National Park, South Africa. Water 2025, 17, 1579. https://doi.org/10.3390/w17111579
Shikwambana P, Foxcroft LC, Bouwman H, Botha J, Taylor JC. Contrasting Microplastic Characteristics in Macroinvertebrates from Two Independent but Adjacent Rivers in Kruger National Park, South Africa. Water. 2025; 17(11):1579. https://doi.org/10.3390/w17111579
Chicago/Turabian StyleShikwambana, Purvance, Llewellyn C. Foxcroft, Hindrik Bouwman, Judith Botha, and Jonathan C. Taylor. 2025. "Contrasting Microplastic Characteristics in Macroinvertebrates from Two Independent but Adjacent Rivers in Kruger National Park, South Africa" Water 17, no. 11: 1579. https://doi.org/10.3390/w17111579
APA StyleShikwambana, P., Foxcroft, L. C., Bouwman, H., Botha, J., & Taylor, J. C. (2025). Contrasting Microplastic Characteristics in Macroinvertebrates from Two Independent but Adjacent Rivers in Kruger National Park, South Africa. Water, 17(11), 1579. https://doi.org/10.3390/w17111579







