Hydrochemical Characteristics and Association of Hot Springs on Small-Scale Faults in Southern Yunnan–Tibet Geothermal Zone
Abstract
1. Introduction
2. Materials and Methods
2.1. Geological Setting
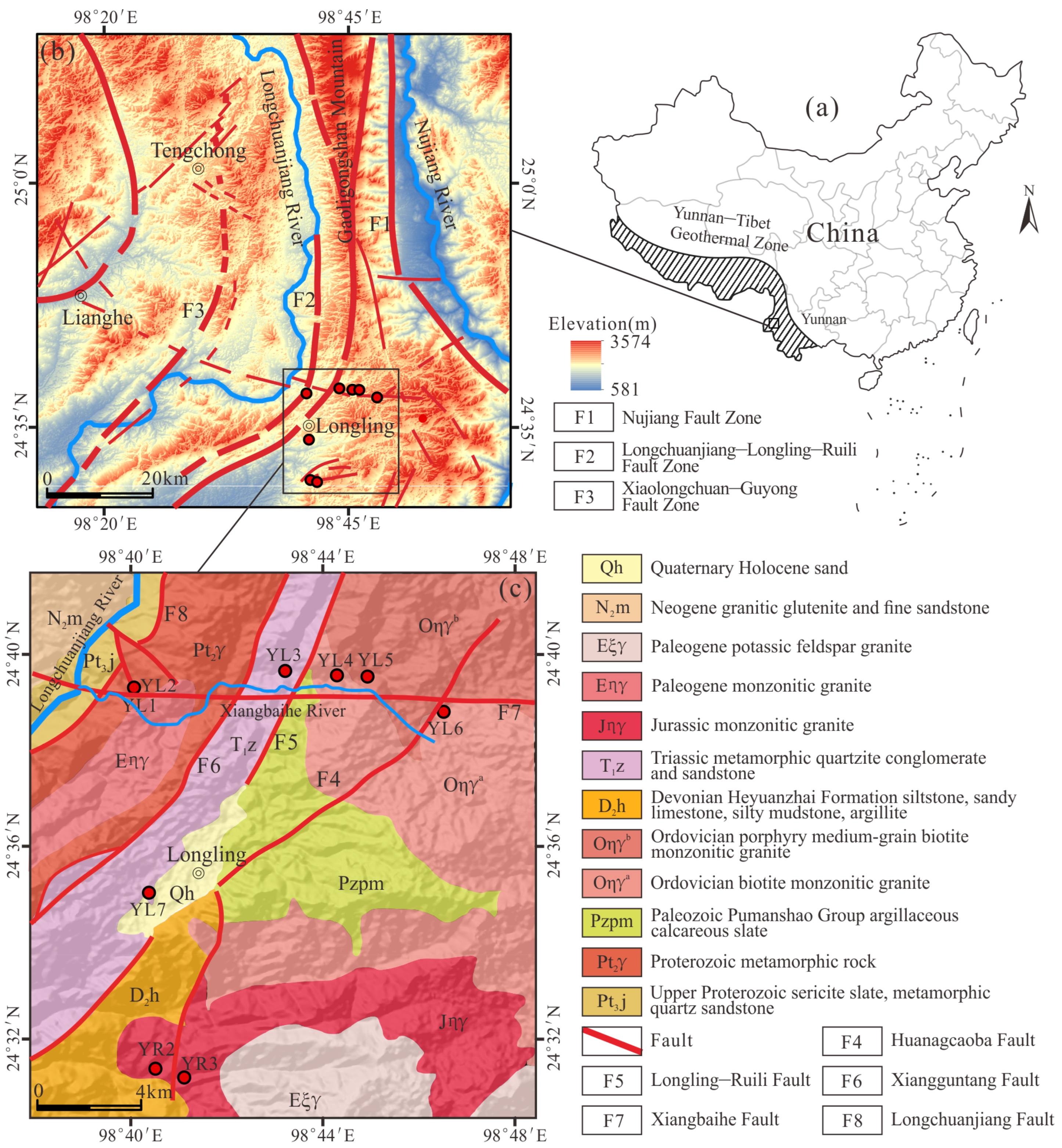
2.2. Sampling and Analytical Methods
3. Results
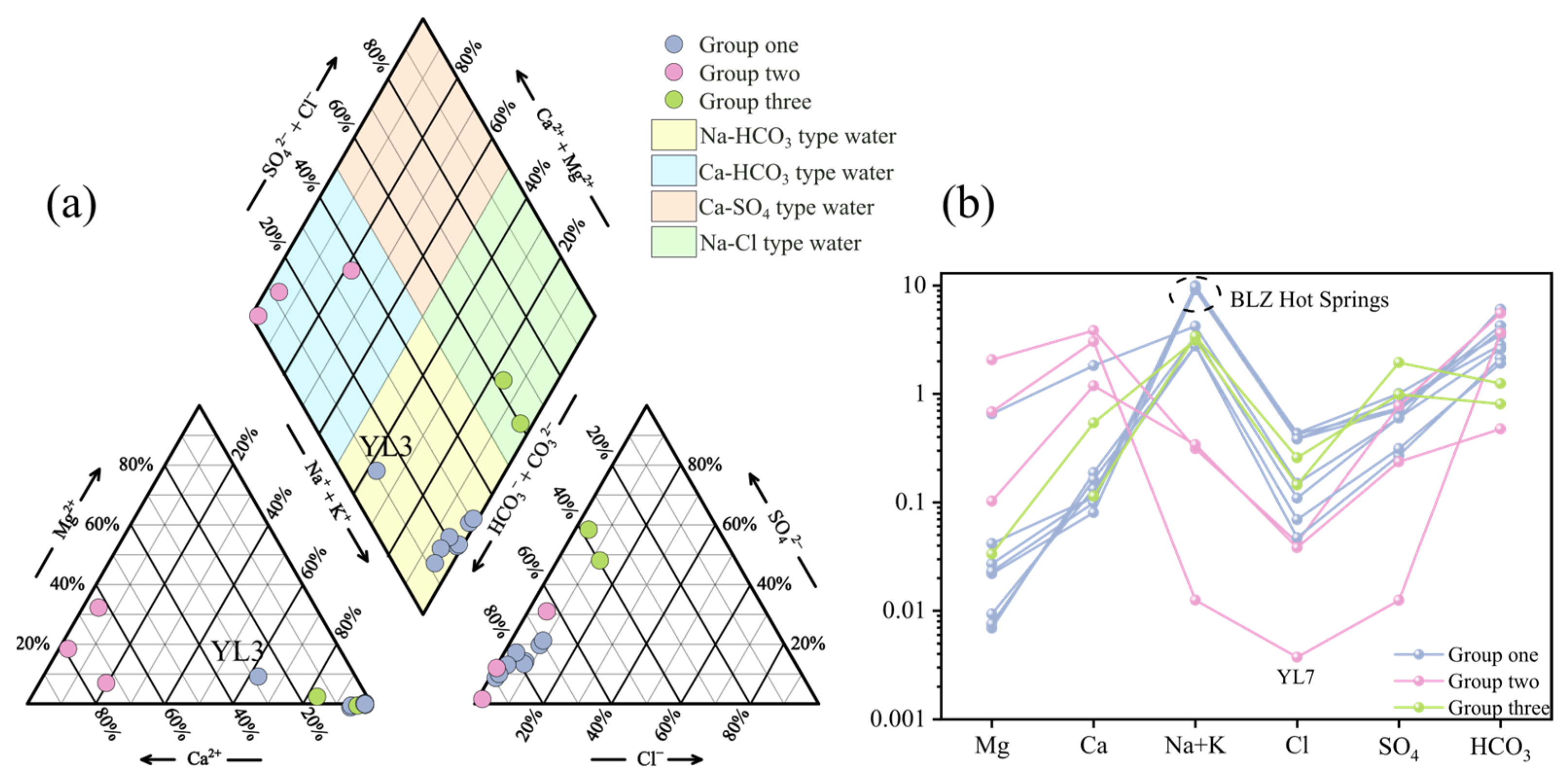
3.1. Physical and Chemical Properties of Hot Springs
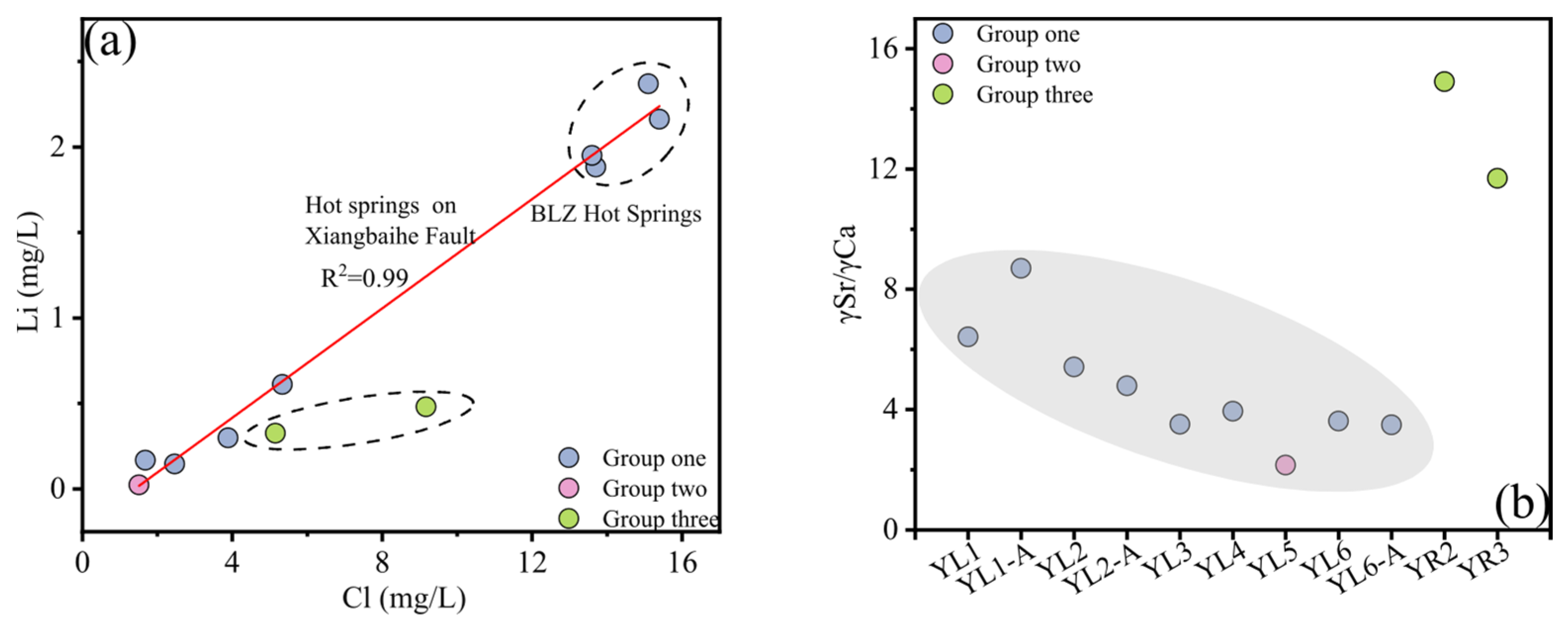
3.2. Trace Element Characteristics of Spring Water
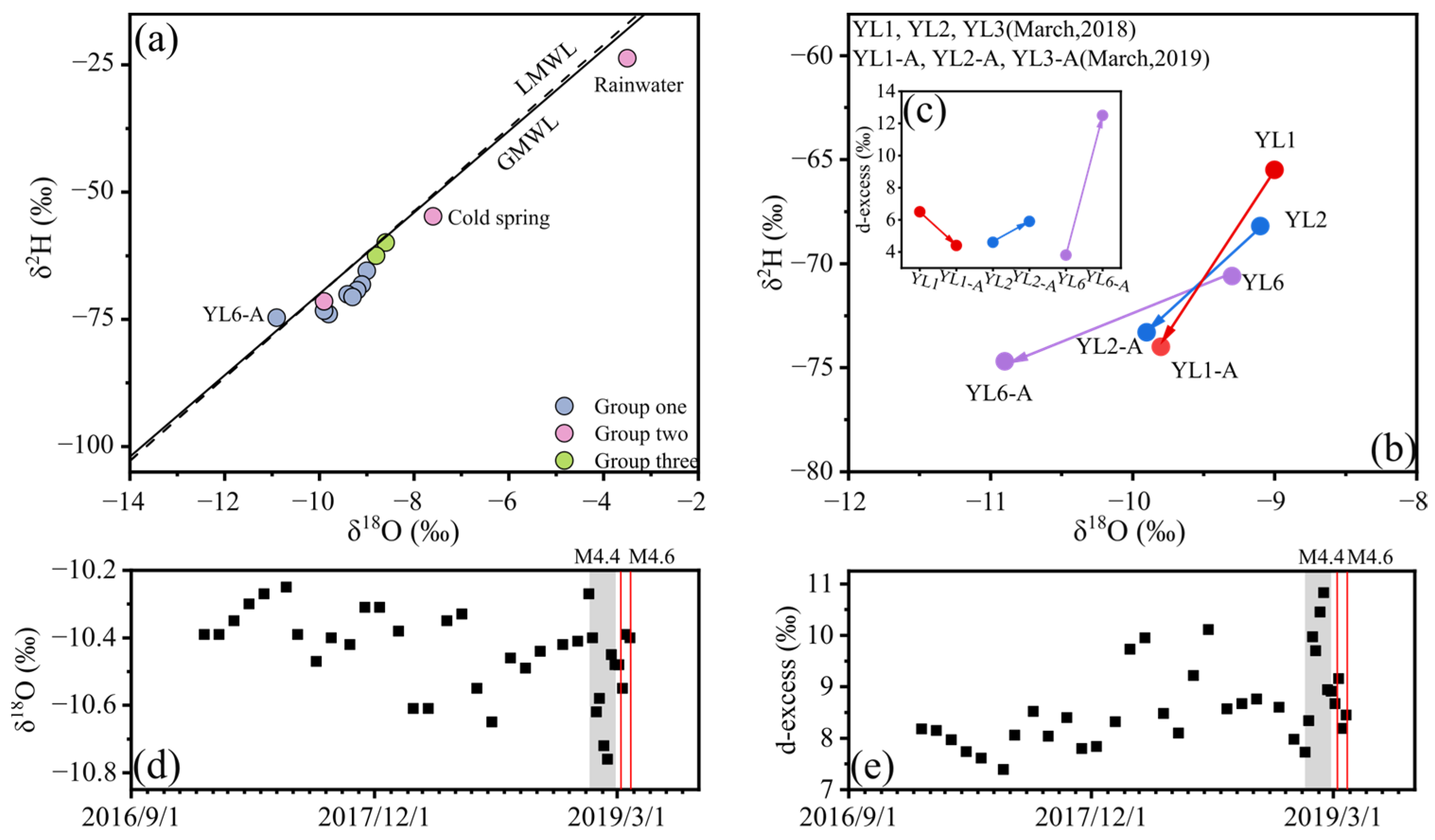
3.3. Stable Hydrogen and Oxygen Isotopes
4. Discussion
4.1. Origin of Hot Springs
4.2. Water–Rock Interactions
4.2.1. Water–Rock Interaction Indicators
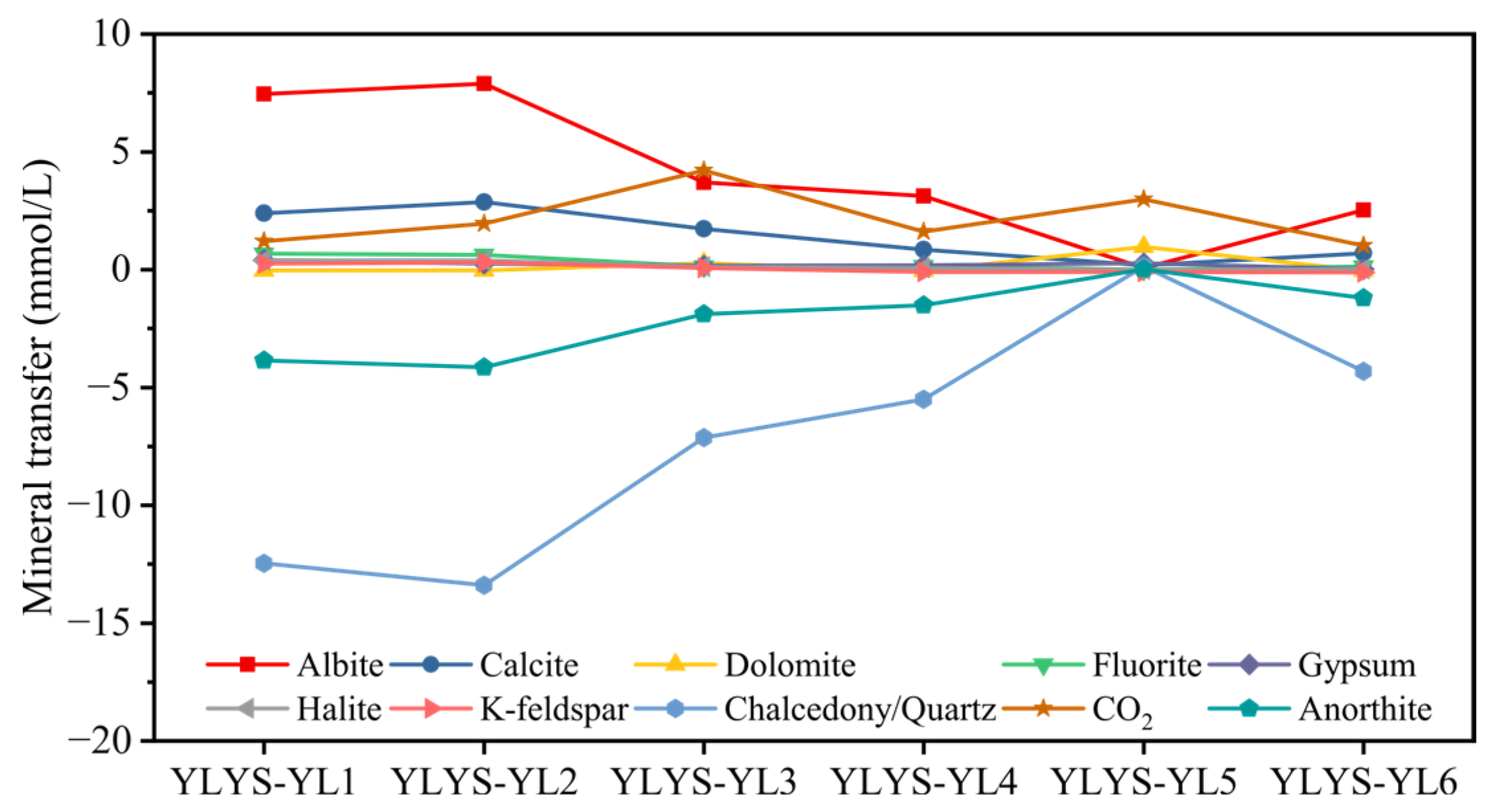
4.2.2. Inverse Hydrochemical Modeling and Mineral Equilibria
4.3. Reservoir Temperature
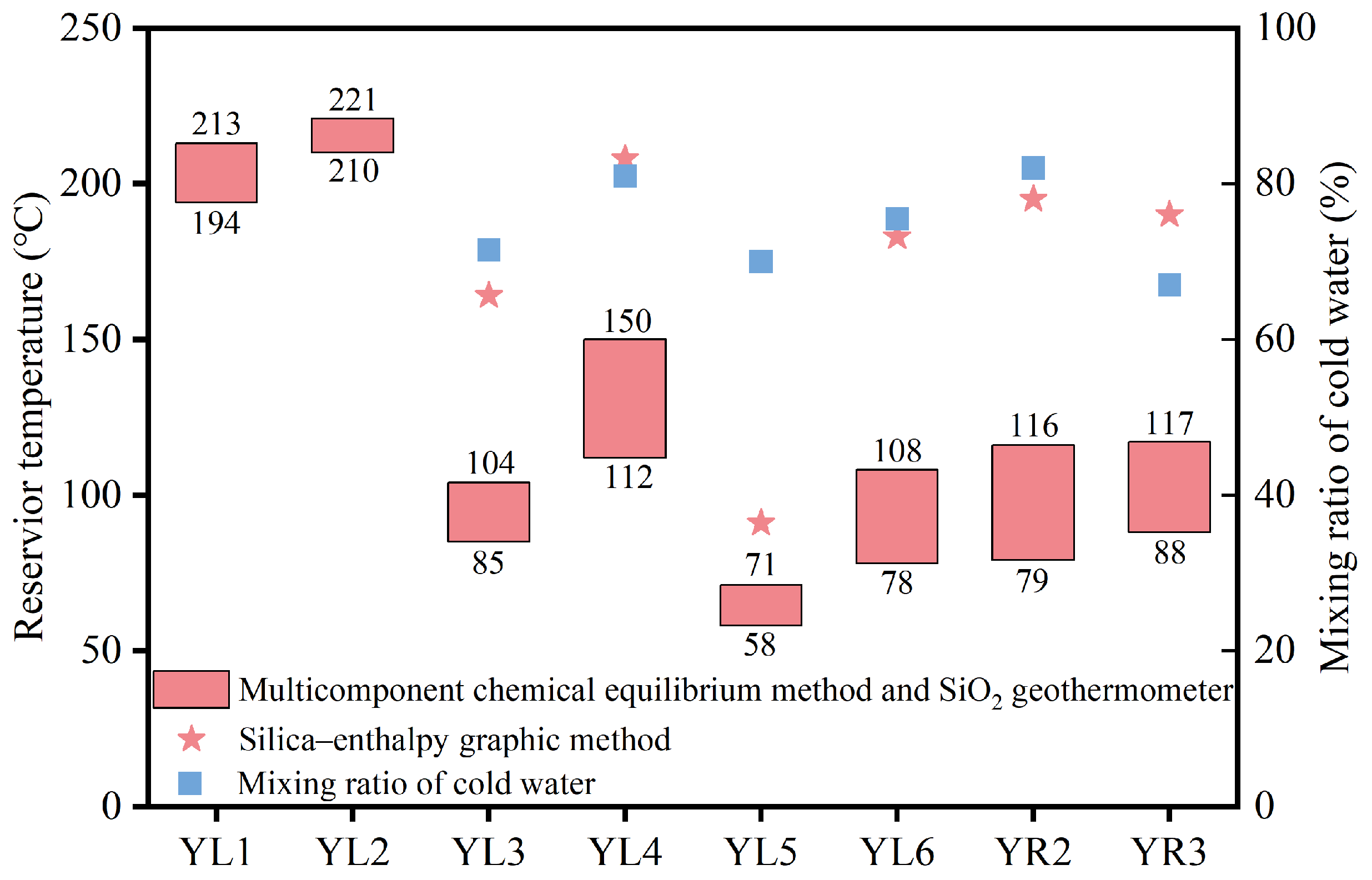
4.3.1. Multicomponent Chemical Equilibrium Method
4.3.2. SiO2 Geothermometer
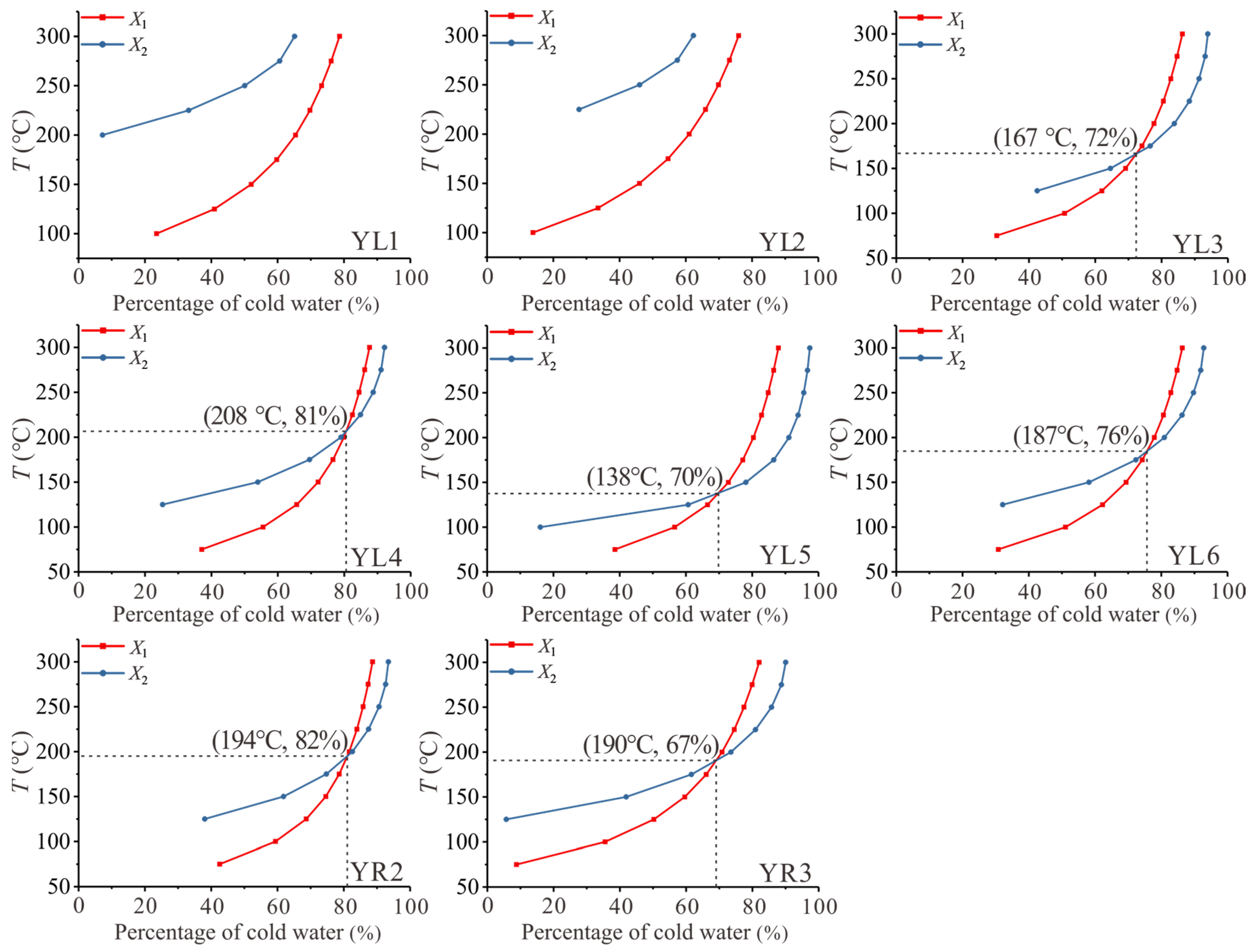
4.3.3. Silica–Enthalpy Mixing Equation Method
4.4. Genesis Mode of the Hot Springs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Zhou, X. Structural control effects on hot springs’ hydrochemistry in the northern Red River Fault Zone: Implications for geothermal systems in fault zones. J. Hydrol. 2023, 623, 129836. [Google Scholar] [CrossRef]
- Jaworowski, C.; Lynne, B.Y.; Heasler, H.; Foley, D.; Smith, I.J.; Smith, G.J. Detecting natural fractures with ground penetrating radar and airborne night-thermal infrared imagery around Old Faithful Geyser, Yellowstone National Park, USA. Geothermics 2020, 85, 101775. [Google Scholar] [CrossRef]
- Furukawa, T.; Ueda, A. Tamagawa hyper-acidic hot spring and phreatic eruptions at Mt. Akita-Yakeyama: Part 1. The isotopic and chemical characteristics of the hot spring water. J. Volcanol. Geotherm. Res. 2021, 412, 107179. [Google Scholar] [CrossRef]
- Guillou-Frottier, L.; Guillou-Frottier, L.; Duwiquet, H.; Duwiquet, H.; Duwiquet, H.; Launay, G.; Taillefer, A.; Roche, V.; Link, G. On the morphology and amplitude of 2D and 3D thermal anomalies induced by buoyancy-driven flow within and around fault zones. Solid Earth 2020, 11, 1571–1595. [Google Scholar] [CrossRef]
- Qi, J.; Li, X.; Xu, M.; Yi, L.; Zhang, Q.; Qin, L.; Li, K. Origin of saline springs in Yanjing, Tibet: Hydrochemical and isotopic characteristics. Appl. Geochem. 2018, 96, 164–176. [Google Scholar] [CrossRef]
- Benmarce, K.; Hadji, R.; Zahri, F.; Khanchoul, K.; Chouabi, A.; Zighmi, K.; Hamed, Y. Hydrochemical and geothermometry characterization for a geothermal system in semiarid dry climate: The case study of Hamma spring (Northeast Algeria). J. Afr. Earth Sci. 2021, 182, 104285. [Google Scholar] [CrossRef]
- White, D.E. Geothermal Energy Reservoirs. AAPG Bull. 1968, 52, 568. [Google Scholar] [CrossRef]
- Panza, E.; Sessa, E.; Agosta, F.; Giorgioni, M. Discrete Fracture Network modelling of a hydrocarbon-bearing, oblique-slip fault zone: Inferences on fault-controlled fluid storage and migration properties of carbonate fault damage zones. Mar. Pet. Geol. 2018, 89, 263–279. [Google Scholar] [CrossRef]
- Cappa, F.; Guglielmi, Y.; Nussbaum, C.; De Barros, L.; Birkholzer, J. Fluid migration in low-permeability faults driven by decoupling of fault slip and opening. Nat. Geosci. 2022, 15, 747–751. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, L.; Wang, Y.; Liu, D.; Liu, Y.; Li, J. Continuous monitoring of hydrogen and oxygen stable isotopes in a hot spring: Significance for distant earthquakes. Appl. Geochem. 2020, 112, 104488. [Google Scholar] [CrossRef]
- Grand, R.V. Controls, Characterization and Small Scale Chemical Variations of a Karst System: A Geochemical Assessment of Tuscarora Creek Watershed, West Virginia. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2005. [Google Scholar]
- Meng, B.; Zhou, S.; Li, J.; Sun, Z. Mantle-Derived Helium Distribution and Tectonic Implications in the Sichuan-Yunnan Block, China. ACS Omega 2021, 6, 30674–30685. [Google Scholar] [CrossRef]
- Yang, B. Study on hydrochemical and isotopic characteristics of from Ruili Hot field. Coal Geol. China 1999, 11, 47–50. (In Chinese) [Google Scholar]
- Song, Y. The Characteristics and Origin of Longling Hot Spring in Yunnan. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2021. [Google Scholar]
- Liu, Y. A Study of Hydrochemistry and Geyser of Thermal Groundwater in the Banglazhang Geothermal Field in Longling, Yunnan. Master’s Thesis, China University of Geosciences, Beijing, China, 2009. [Google Scholar]
- Zhou, C.; Wu, Z. The characteristics of geothermal field along the Dali-Ruili railway in western Yunnan Province and their implications for geo-engineering. Geol. Bull. China 2012, 31, 326–336. [Google Scholar]
- Bai, D.; Meju, M.A. Deep structure of the Longling-Ruili fault underneath Ruili basin near the eastern Himalayan syntaxis: Insights from magnetotelluric imaging. Tectonophysics 2003, 364, 135–146. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, M.; Li, J.; Zhang, X.; Guo, W.; Wang, Y. Fluid geochemical constraints on the heat source and reservoir temperature of the Banglazhang hydrothermal system, Yunnan-Tibet Geothermal Province, China. J. Geochem. Explor. 2017, 172, 109–119. [Google Scholar] [CrossRef]
- Stefánsson, A.; Keller, N.S.; Robin, J.; Kaasalainen, H.; Björnsdóttir, S.; Pétursdóttir, S.; Jóhannesson, H.; Hreggvidsson, Ó.G. Quantifying mixing, boiling, degassing, oxidation and reactivity of thermal waters at Vonarskard, Iceland. J. Volcanol. Geotherm. Res. 2016, 309, 53–62. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Liang, L.; Zhu, Z.; Zhang, A. Constraining the properties of the heat sources of high-temperature hydrogeothermal systems: Evidence from the lithium concentrations of geothermal waters. J. Hydrol. 2024, 640, 131696. [Google Scholar] [CrossRef]
- National Geological Archives. Available online: https://geocloud.cgs.gov.cn/geological/database?type=dzsjk&code=%E5%9F%BA%E7%A1%80%E5%9C%B0%E8%B4%A8 (accessed on 10 April 2023).
- Guo, T. Research on the Characteristics and Formation of the Rehai Geothermal Field in Tengchong. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2012. [Google Scholar]
- Li, B.; Wang, G.; Liu, F.; Shi, Z.; Kong, Q.; Zhang, S.; Yan, X.; Liao, F.; Guo, L.; Liu, C. Hydrochemical characteristics and evolution of geothermal waters in western Yunnan, China based on self-organizing map and hydrogeochemical simulation. Appl. Geochem. 2025, 181, 106291. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 1997; pp. 102–147. [Google Scholar]
- Wang, J.; Xiong, L.; Pang, Z. Medium and Low Temperature Convection Type Geothermal System; Science Press: Beijing, China, 1993; pp. 23–56. [Google Scholar]
- Schoeller, H. Geochemistry of groundwaters. In Groundwater Studies: An International Guide for Research and Practice; The Unesco Press: Paris, France, 1977; pp. 89–100. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Inverse_modeling. In User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Reaction-Path, One-Dimensional Transport, and Inverse Geothermics Geochemical Calculations; U.S. Geological Survey: Denver, CO, USA, 1999; pp. 98–105. [Google Scholar]
- Rafighdoust, Y.; Eckstein, Y.; Harami, R.M.; Gharaie, M.H.M.; Mahboubi, A. Using inverse modeling and hierarchical cluster analysis for hydrochemical characterization of springs and Talkhab River in Tang-Bijar oilfield, Iran. Arab. J. Geosci. 2016, 9, 241. [Google Scholar] [CrossRef]
- Pang, Z.; Reed, M. Theoretical chemical thermometry on geothermal waters: Problems and methods. Geochim. Cosmochim. Acta 1998, 62, 1083–1091. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Deng, C.; Li, X.; Zhao, X.; Feng, T.; Wang, Y.; Wang, Y. Identifying the genesis of hydrothermal activities in the Xiangcheng fault belt, southwestern China: Evidence from hydrochemistry and stable isotopes. Ore Geol. Rev. 2024, 173, 106221. [Google Scholar] [CrossRef]
- Zhou, X.; Zhuo, L.; Wu, Y.; Tao, G.; Ma, J.; Jiang, Z.; Sui, L.; Wang, Y.; Wang, C.; Cui, J. Origin of some hot springs as conceptual geothermal models. J. Hydrol. 2023, 624, 129927. [Google Scholar] [CrossRef]
- Fournier, R.O. Chemical geothermometers and mixing models for geothermal systems. Geothermics 1977, 5, 41–50. [Google Scholar] [CrossRef]
- Truesdell, A.H.; Fournier, R.O. Geochemical indicators of subsurface temperature-Part 2, Estimation of temperature and fraction of hot water mixed with cold water. J. Res. U.S. Geol. Surv. 1974, 2, 263–270. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Wang, Y. Distribution and origin of warmsprings in Shandong Peninsula. J. Ocean Univ. Qingdao 1997, 27, 389–396. [Google Scholar]
- Truesdell, A.H.; Nathenson, M.; Rye, R.O. The effects of subsurface boiling and dilution on the isotopic compositions of Yellowstone thermal waters. J. Geophys. Res. 1977, 82, 3694–3704. [Google Scholar] [CrossRef]
- Nicholson, K. Geothermal Fluids: Chemistry and Exploration Techniques; Springer: Berlin/Heidelberg, Germany, 1993; pp. 134–150. [Google Scholar]
- Li, J.; Sagoe, G.; Wang, X.; Yang, Z. Assessing the suitability of lithium-related geothermometers for estimating the temperature of felsic rock reservoirs. Geothermics 2021, 89, 101950. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Zhang, X.; Wu, H.; Sun, G.; Huang, Y. Stable hydrogen and oxygen isotopes characteristics of atmospheric precipitation from Tengchong, Yunnan. Resour. Environ. Yangtza Basin 2013, 22, 1458–1465. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Skelton, A.; Liljedahl-Claesson, L.; Wästeby, N.; Andrén, M.; Stockmann, G.; Sturkell, E.; Mörth, C.-M.; Stefansson, A.; Tollefsen, E.; Siegmund, H.; et al. Hydrochemical Changes Before and After Earthquakes Based on Long-Term Measurements of Multiple Parameters at Two Sites in Northern Icelan—A Review. J. Geophys. Res. Solid Earth 2019, 124, 2702–2720. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, H.; Yu, F.; Liu, D. Oxygen and hydrogen isotopic compositions of meteoric waters in the eastern part of Xizang. Geochemistry 1984, 3, 93–101. [Google Scholar] [CrossRef]
- Boschetti, T.; Venturelli, G.; Toscani, L.; Barbieri, M.; Mucchino, C. The Bagni di Lucca thermal waters (Tuscany, Italy): An example of Ca-SO4 waters with high Na/Cl and low Ca/SO4 ratios. J. Hydrol. 2005, 307, 270–293. [Google Scholar] [CrossRef]
- Sasaki, K.; Morita, J.; Iwaki, C.; Ueda, A. Geochemical evaluation of geothermal resources in Toyama Prefecture, Japan, based on the chemical and isotopic characteristics of hot spring waters. Geothermics 2021, 93, 102071. [Google Scholar] [CrossRef]
- Kenoyer, G.J.; Bowser, C.J. Groundwater chemical evolution in a sandy silicate aquifer in northern Wisconsin. I. Patterns and rates of change. Water Resour. Res. 1992, 28, 579–589. [Google Scholar] [CrossRef]
- Rajmohan, N.; Elango, L. Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ. Geol. 2004, 46, 47–61. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative Evaluation of Groundwater Resources. In Methods and Techniques of Groundwater Investigations and Development; UNESCO: Paris, France, 1965; pp. 21–46. [Google Scholar]
- Chidambaram, S.; Anandhan, P.; Prasanna, M.V.; Srinivasamoorthy, K.; Vasanthavigar, M. Major ion chemistry and identification of hydrogeochemical processes controlling groundwater in and around Neyveli Lignite Mines, Tamil Nadu, South India. Arab. J. Geosci. 2013, 6, 3451–3467. [Google Scholar] [CrossRef]
- Hou, Z.; Duan, L.; Lu, Y.; Zheng, Y.; Zhu, D.; Yang, Z.; Yang, Z.; Wang, B.; Pei, Y.; Zhao, Z.; et al. Lithospheric Architecture of the Lhasa Terrane and Its Control on Ore Deposits in the Himalayan-Tibetan Orogen. Econ. Geol. 2015, 110, 1541–1575. [Google Scholar] [CrossRef]
- Gupta, H.K.; Roy, S. Geothermal Energy: An Alternative Resource for the 21st Century; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 73–77. [Google Scholar]
- Ferguson, G.; Grasby, S.E.; Hindle, S.R. What do aqueous geothermometers really tell us? Geofluids 2009, 9, 39–48. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, F.; He, J.; Wang, X.; Fang, B. Introduction to Groundwater Science, 2nd ed.; Geological Publishing House: Beijing, China, 2014; pp. 187–196. [Google Scholar]



| Sample ID | YL1 | YL1-A | YL2 | YL2-A | YL3 | YL4 | YL5 | YL6 | YL6-A | YL7 | YR2 | YR3 | YLYS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 80.0 | 77.7 | 88.2 | 88.2 | 56.7 | 52.6 | 51.7 | 56.4 | 47.1 | 14.6 | 49.3 | 69.7 | 18.4 |
| pH (in field) | 8.6 | 9.4 | 8.3 | 9.1 | 7.0 | 7.1 | 7.6 | 8.3 | 9.0 | 7.5 | 8.0 | 8.4 | 9.8 |
| Vent elevation (m) | 1280 | 1280 | 1290 | 1290 | 1610 | 1590 | 1630 | 1760 | 1760 | 1540 | 1170 | 1260 | 1240 |
| TDS (mg/L) | 520 | 501 | 553 | 531 | 369 | 201 | 321 | 162 | 161 | 183 | 269 | 228 | 104 |
| Hydrochemical type | Na-HCO3· CO3 | Na-CO3· HCO3 | Na-HCO3· CO3 | Na-CO3· HCO3 | Na·Ca-HCO3 | Na-HCO3 | Ca·Mg-HCO3 | Na-HCO3 | Na-HCO3 | Ca-HCO3 | Na-SO4·HCO3 | Na-SO4·CO3· HCO3 | Na-CO3· HCO3 |
| H2SiO3 (mg/L) | 320.4 | 367.7 | 345.4 | 384.4 | 64.1 | 80.2 | 30.6 | 73.8 | 88.3 | 10.1 | 68.3 | 98.6 | 23.7 |
| SiO2 (mg/L) | 246.4 | 282.9 | 265.7 | 295.7 | 49.3 | 61.7 | 23.6 | 56.8 | 67.9 | 7.7 | 52.5 | 75.9 | 18.2 |
| Na (mg/L) | 205.0 | 203.0 | 216.0 | 214.0 | 91.9 | 77.1 | 6.1 | 63.0 | 63.7 | 0.2 | 71.0 | 76.8 | 4.3 |
| K (mg/L) | 15.7 | 11.3 | 21.5 | 16.1 | 8.7 | 2.2 | 1.9 | 1.4 | 1.0 | 0.1 | 2.2 | 2.2 | 6.0 |
| Ca (mg/L) | 2.3 | 1.6 | 2.2 | 2.0 | 36.5 | 3.8 | 76.9 | 2. 7 | 3.3 | 60.7 | 10.8 | 2.3 | 23.8 |
| Mg (mg/L) | 0.33 | 0.26 | 0.50 | 0.28 | 7.88 | 0.08 | 24.80 | 0.11 | 0.09 | 8.22 | 0.40 | 0.00 | 1.23 |
| HCO3 (mg/L) | 212.0 | 172.0 | 260.0 | 232.0 | 369.0 | 158.0 | 339.0 | 130.0 | 117.0 | 223.0 | 76.0 | 49.3 | 29.0 |
| CO3 (mg/L) | 104.0 | 123.0 | 110.0 | 115.0 | 0.0 | 0.0 | 0.0 | 9.8 | 10.3 | 0.0 | 3.9 | 27.9 | 26.3 |
| SO4 (mg/L) | 48.3 | 41.9 | 35.5 | 34.2 | 28.8 | 29.0 | 37.4 | 13.0 | 15.0 | 0.6 | 93.7 | 47.6 | 11.4 |
| Cl (mg/L) | 15.4 | 13.7 | 15.1 | 13.6 | 5.3 | 3.9 | 1.5 | 1.7 | 2.5 | 0.1 | 5.2 | 9.2 | 1.4 |
| Li (μg/L) | 2162.0 | 1883.0 | 2370.0 | 1950.0 | 611.0 | 299.0 | 22.7 | 168.0 | 146.0 | 1.7 | 325.0 | 480.0 | 4.4 |
| Sr (μg/L) | 32.5 | 30.7 | 26.6 | 20.9 | 281.0 | 32.6 | 363.0 | 21.2 | 24.9 | 19.8 | 353.0 | 58.7 | 45.5 |
| γNa/γCl | 20.55 | 22.87 | 22.08 | 24.29 | 26.56 | 30.59 | 6.26 | 57.88 | 39.97 | * | 21.28 | 12.93 | * |
| γ(Ca + Mg − SO4 − HCO3) /γ(Na − Cl) | 0.49 | 0.41 | 0.51 | 0.47 | 1.02 | 0.91 | 1.57 | 0.83 | 0.76 | * | 0.88 | 0.54 | * |
| Recharge elevation (m) | 2744 | 3102 | 2838 | 3094 | 3117 | 3153 | 3286 | 3269 | 3093 | 2951 | 2465 | 2628 | * |
| Circulation depth (m) | 3197–3536 | 3483–3680 | 1247–1586 | 1730–2409 | 763–996 | 1121–1657 | 1139–1801 | 1300–1819 |
| Ca | Mg | Na + K | HCO3 | Cl | SO4 | |
|---|---|---|---|---|---|---|
| Ca | 1 | |||||
| Mg | 0.986 ** | 1 | ||||
| Na + K | −0.522 | −0.475 | 1 | |||
| HCO3 | 0.661 * | 0.651 * | 0.151 | 1 | ||
| Cl | −0.461 | −0.407 | 0.947 ** | 0.074 | 1 | |
| SO4 | 0.000 | −0.055 | 0.052 | −0.280 | 0.217 | 1 |
| Sample ID | YL1 | YL1-A | YL2 | YL2-A | YL3 | YL4 |
|---|---|---|---|---|---|---|
| CAI-I | −20 | −23 | −22 | −24 | −27 | −30 |
| CAI-II | −1 | −1 | −1 | −1 | −1 | −1 |
| Sample ID | YL5 | YL6 | YL6-A | YR2 | YR3 | |
| CAI-I | −6 | −58 | −39 | −21 | −12 | |
| CAI-II | 0 | −1 | −1 | −1 | −1 |
| Reactant | Molecular Formula | Mineral Transfer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| YLYS–YL1 | YLYS–YL2 | YLYS–YL3 | YLYS–YL4 | YLYS–YL5 | YLYS–YL6 | YLYS–YR2 | YLYS–YR3 | ||
| Albite | NaAlSi3O8 | 7.447 | 7.887 | 3.699 | 3.123 | 0.074 | 2.523 | 2.794 | 2.623 |
| Calcite | CaCO3 | 2.393 | 2.867 | 1.731 | 0.842 | 0.186 | 0.691 | 0.176 | 0.190 |
| Dolomite | CaMg(CO3)2 | −0.037 | −0.030 | 0.274 | −0.047 | 0.970 | −0.046 | −0.034 | −0.051 |
| Fluorite | CaF2 | 0.671 | 0.631 | 0.139 | 0.159 | 0.005 | 0.137 | 0.148 | 0.333 |
| Gypsum | CaSO4·2H2O | 0.403 | 0.255 | 0.178 | 0.180 | 0.267 | 0.013 | 0.853 | 0.373 |
| Halite | NaCl | 0.396 | 0.388 | 0.112 | 0.071 | 0.004 | 0.009 | 0.107 | 0.220 |
| K-feldspar | KAlSi3O8 | 0.248 | 0.307 | 0.070 | −0.096 | −0.106 | −0.117 | −0.098 | −0.097 |
| Chalcedony/Quartz | SiO2 | −12.470 | −13.400 | −7.139 | −5.496 | 0.133 | −4.319 | −4.954 | −4.314 |
| CO2(g) | CO2 | 1.212 | 1.954 | 4.209 | 1.616 | 2.987 | 1.012 | 0.596 | 0.414 |
| Anorthite | CaAl2Si2O8 | −3.848 | −4.142 | −1.884 | −1.513 | 0.016 | −1.203 | −1.348 | −1.263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, L.; Zhou, X.; Zou, C.; Wu, Y.; Tao, G.; Cheng, R.; Wang, Y.; Ma, J. Hydrochemical Characteristics and Association of Hot Springs on Small-Scale Faults in Southern Yunnan–Tibet Geothermal Zone. Water 2025, 17, 1481. https://doi.org/10.3390/w17101481
Zhuo L, Zhou X, Zou C, Wu Y, Tao G, Cheng R, Wang Y, Ma J. Hydrochemical Characteristics and Association of Hot Springs on Small-Scale Faults in Southern Yunnan–Tibet Geothermal Zone. Water. 2025; 17(10):1481. https://doi.org/10.3390/w17101481
Chicago/Turabian StyleZhuo, Linyang, Xun Zhou, Changpei Zou, Yanqiu Wu, Guangbin Tao, Ruirui Cheng, Yixuan Wang, and Jingru Ma. 2025. "Hydrochemical Characteristics and Association of Hot Springs on Small-Scale Faults in Southern Yunnan–Tibet Geothermal Zone" Water 17, no. 10: 1481. https://doi.org/10.3390/w17101481
APA StyleZhuo, L., Zhou, X., Zou, C., Wu, Y., Tao, G., Cheng, R., Wang, Y., & Ma, J. (2025). Hydrochemical Characteristics and Association of Hot Springs on Small-Scale Faults in Southern Yunnan–Tibet Geothermal Zone. Water, 17(10), 1481. https://doi.org/10.3390/w17101481






