Optimization of Ozonation in Drinking Water Production at Lake Butoniga
Abstract
1. Introduction
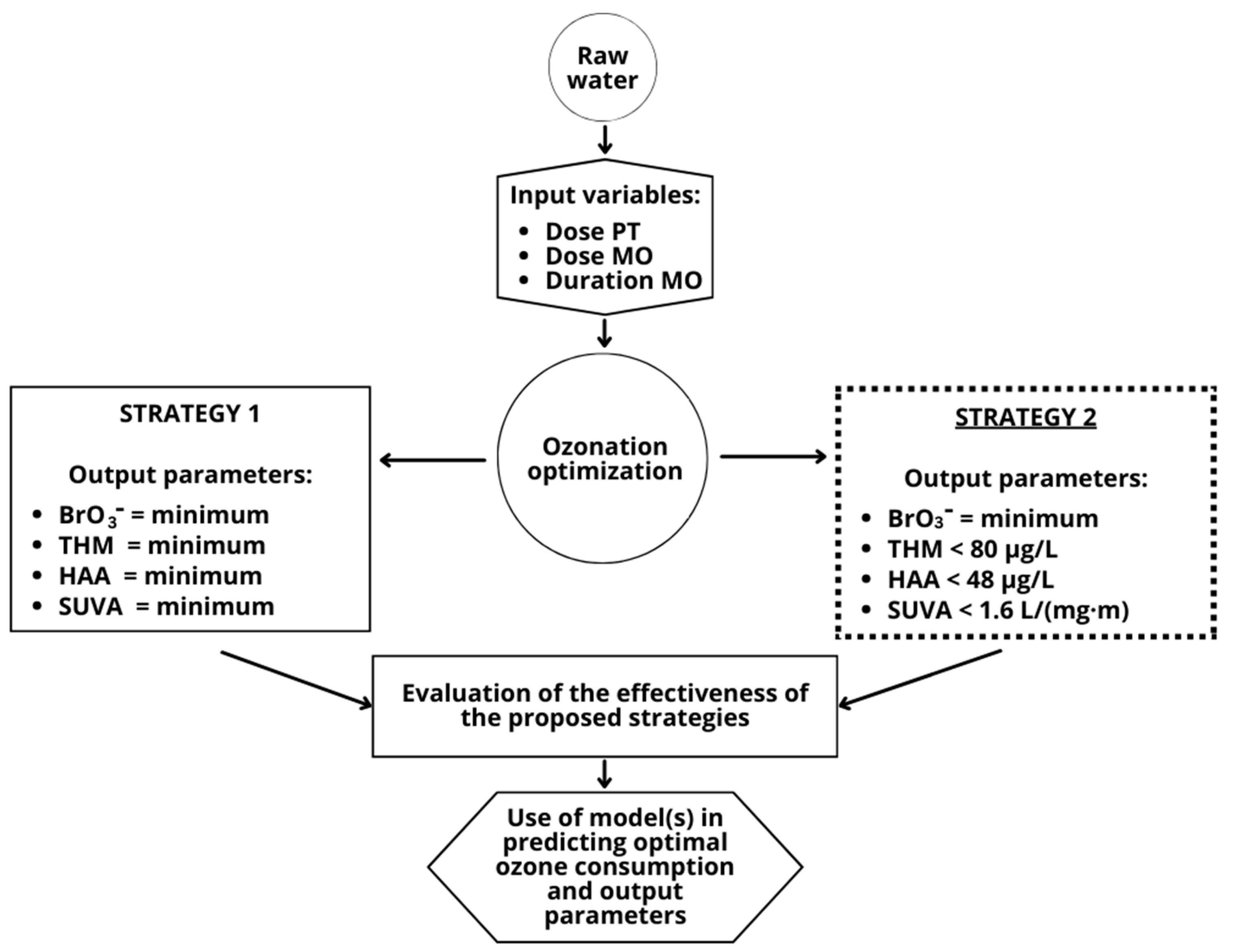
2. Materials and Methods
2.1. Water Samples and Design of Experiment
2.2. Ozonation Experiment
2.3. Chemical Analysis
2.4. Data Analysis and Modelling
- Trihalomethanes formation potential (FP THM);
- Haloacetic acids formation potential (FP HAA);
- Specific UV absorbance (SUVA);
- Bromate concentration.
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament. Directive (EU) 2020/2184. Qual. Water Intend. Hum. Consum. 2020. Available online: https://eur-lex.europa.eu/legal-content/HR/TXT/?uri=uriserv%3AOJ.L_.2020.435.01.0001.01.HRV&toc=OJ%3AL%3A2020%3A435%3AFULL (accessed on 13 April 2023).
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 6 June 2024).
- Tzvi, Y.; Paz, Y. Highly efficient method for oxidation of dissolved hydrogen sulfide in water, utilizing a combination of UVC light and dissolved oxygen. J. Photochem. Photobiol. A Chem. 2019, 372, 63–70. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res.-Rev. Mutat. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Pham, M. Two-Stage Filtration to Control Manganese and DBPS at the Lantern Hill Water Treatment Plant. Environ. Water Resour. Eng. Masters Proj. 2010, 51, 11–12. Available online: https://scholarworks.umass.edu/cee_ewre (accessed on 28 December 2023).
- O’Donnell, C.; Tiwari, B.K.; Cullen, P.J.; Rice, R.G. Ozone in Food Processing; Blackwell Publishing: West Sussex, UK, 2012. [Google Scholar]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Mezcua, M.; Hernando, M.D.; Letón, P.; García-Calvo, E.; Agüera, A.; Fernán-dez-Alba, A.R. Removal of pharmaceuticals and kinetics of mineralization by O3/H2O2 in a biotreated municipal wastewater. Water Res. 2008, 42, 3719–3728. [Google Scholar] [CrossRef]
- IVB. Akumulacija Butoniga. 2023. Available online: https://www.ivb.hr/o-vodovodu/distribucija-i-izvori/akumulacija-butoniga/ (accessed on 6 June 2024).
- Britto, F.B.; Vasco, A.N.D.; Aguiar Netto, A.D.O.; Garcia, C.A.B.; Moraes, G.F.O.; Silva, M.G.D. Surface water quality assessment of the main tributaries in the lower São Francisco River, Sergipe. Rev. Bras. Recur. Hidricos 2018, 23, e28. Available online: https://www.scielo.br/j/rbrh/a/BBJ9HdwX9wrtFdYcP3RLkFx/?lang=en (accessed on 4 June 2024). [CrossRef]
- Paun, I.; Cruceru, L.V.; Chiriac, F.L.; Niculescu, M.; Vasile, G.G.; Marin, N.M. Water Quality Indices-Methods for Evaluating the Quality of Drinking Water. In The Environment and the Industry, Proceedings of the INCD ECOIND—International Symposium—SIMI 2016, Bucharest, Romania, 13–14 October 2016; 2016; pp. 395–402. Available online: https://www.researchgate.net/publication/309304038_WATER_QUALITY_INDICES_-_METHODS_FOR_EVALUATING_THE_QUALITY_OF_DRINKING_WATER (accessed on 4 June 2024).
- Montgomery, D.C.; Wiley, J. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- HRN EN 1484:2002; Water Analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC) (EN 1484:1997) (Reference Method). HZN: Zagreb, Croatia, 2002.
- HRN EN ISO 10304-1:2009/Ispr.1:2012; Water Quality—Determination of Dissolved Anions by Liquid Chromatography of Ions—Part 1: Determination of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate—Technical Corrigendum 1 (ISO 10304-1:2007/Cor 1:2010; EN ISO 10304-1:2009/AC:2012) (Reference Method). HZN: Zagreb, Croatia, 2012.
- HRN EN ISO 15061:2001; Water Quality—Determination of Dissolved Bromate—Method by Liquid Chromatography of Ions (ISO 15061:2001; EN ISO 15061:2001) (Reference Method). HZN: Zagreb, Croatia, 2001.
- USEPA. Disinfectants and Disinfection Byproducts Rules (Stage 1 and Stage 2). 2020. Available online: http://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P100C8XW.txt (accessed on 10 June 2024).
- Chen, B.; Westerhoff, P. Predicting disinfection by-product formation potential in water. Water Res. 2010, 44, 3755–3762. [Google Scholar] [CrossRef]
- Ikehata, K. Recent Research on Ozonation By-products in Water and Wastewater Treatment: Formation, Control, Mitigation, and Other Relevant Topics. In Energy, Environment, and Sustainability; Springer: Singapore, 2019; pp. 117–144. [Google Scholar]
- Von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide, or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Morrison, C.M.; Hogard, S.; Pearce, R.; Mohan, A.; Pisarenko, A.N.; Dickenson, E.R.V.; von Gunten, U.; Wert, E.C. Critical Review on Bromate Formation during Ozonation and Control Options for Its Minimization. Environ. Sci. Technol. 2023, 57, 18393–18409. [Google Scholar] [CrossRef]
- Jeirani, Z.; Sadeghi, A.; Soltan, J.; Roshani, B.; Rindall, B. Effectiveness of advanced oxidation processes for the removal of manganese and organic compounds in membrane concentrate. Sep. Purif. Technol. 2015, 149, 110–115. [Google Scholar] [CrossRef]
- Sadrnourmohamadi, M.; Gorczyca, B. Effects of ozone as a stand-alone and coagulation-aid treatment on the reduction of trihalomethanes precursors from high DOC and hardness water. Water Res. 2015, 73, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Agbaba, J.; Molnar, J.; Tubić, A.; Watson, M.; Maletić, S.; Dalmacija, B. Effects of water matrix and ozonation on natural organic matter fractionation and corresponding disinfection by-products formation. Water Sci. Technol. Water Supply 2015, 15, 75–83. [Google Scholar] [CrossRef]
- Mazloum, S.; Jasim, S.; Biswas, N.; Rakness, K.; Hunter, G. Improvement and optimization of the A.H. Weeks water treatment plant processes, Windsor, ON, Canada. Ozone Sci. Eng. 2004, 26, 125–140. [Google Scholar] [CrossRef]
- Lind, P.; Sauvignet, P.; Flodin, J.; Bourghardt, C.; Bengtsson, S. Pilot scale removal of organic micropollutants by combining ozonation with powdered activated carbon. Water Pract. Technol. 2024, 19, 2255–2266. [Google Scholar] [CrossRef]
- May, R.J.; Dandy, G.C.; Maier, H.R.; Nixon, J.B. Application of partial mutual information variable selection to ANN forecasting of water quality in water distribution systems. Environ. Model. Softw. 2008, 23, 1289–1299. [Google Scholar] [CrossRef]
- Tang, W.; Liu, Y.; Li, Q.; Chen, L.; Li, Q.; Li, P.; Xia, S. The Impact of Extreme Weather Events on Bacterial Communities and Opportunistic Pathogens in a Drinking Water Treatment Plant. Water 2022, 14, 54. [Google Scholar] [CrossRef]
- Wilske, C.; Herzsprung, P.; Lechtenfeld, O.J.; Kamjunke, N.; Einax, J.W.; von Tümpling, W. New insights into the seasonal variation of DOM quality of a humic-rich drinking-water reservoir—coupling 2D-fluorescence and FTICR MS measurements. Water 2021, 13, 1703. [Google Scholar] [CrossRef]
- Von Gunten, U. Oxidation Processes in Water Treatment: Are We on Track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef]
- Gregov, M.; Jurinjak Tušek, A.; Valinger, D.; Benković, M.; Jurina, T.; Surać, L.; Kurajica, L.; Matošić, M.; Gajdoš Kljusurić, J.; Ujević Bošnjak, M.; et al. Linear and Non-Linear Modelling of Bromate Formation during Ozonation of Surface Water in Drinking Water Production. Water 2023, 15, 1516. [Google Scholar] [CrossRef]
- Von Sonntag, C.; von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; IWA Publishing: London, UK, 2015. [Google Scholar]
- Beltrán, F.J.; Rey, A.; Gimeno, O. The Role of Catalytic Ozonation Processes on the Elimination of DBPs and Their Precursors in Drinking Water Treatment. Catalysts 2021, 11, 521. [Google Scholar] [CrossRef]
- Aliashrafi, A.; Zhang, Y.; Groenewegen, H.; Peleato, N.M. A review of data-driven modelling in drinking water treatment. Rev. Environ. Sci. Biotechnol. 2021, 20, 985–1009. [Google Scholar] [CrossRef]
| Date of Sampling | DOC | UV254 | Br− | SUVA | FP 1 THM | FP 1 HAA |
|---|---|---|---|---|---|---|
| mg/L | cm−1 | mg/L | L/(mg·m) | µg/L | µg/L | |
| 6.7 m above the lake bottom | ||||||
| April 2021 | 2.2 ± 0.1 a | 0.057 ± 0.002 b | 0.017 ± 0.001 a | 2.6 ± 0.2 c | 106.7 ± 3.1 c,d | 101.5 ± 2.5 d |
| May 2021 | 2.1 ± 0.1 a | 0.055 ± 0.001 b,# | 0.018 ± 0.001 a | 2.7 ± 0.1 c | 103.2 ± 0.8 c,# | 96.9 ± 1.7 c |
| June 2021 | 2.3 ± 0.1 a | 0.052 ± 0.002 b | 0.019 ± 0.001 b | 2.3 ± 0.1 b,c | 98.8 ± 3.2 c | 93.9 ± 3.6 c |
| July 2021 | 2.3 ± 0.1 a | 0.052 ± 0.001 a | 0.019 ± 0.001 a,b | 2.3 ± 0.1 b,c | 99.4 ± 1.2 c | 94.7 ± 1.8 c |
| September 2021 | 2.4 ± 0.1 a,b | 0.047 ± 0.001 a,b,# | 0.021 ± 0.001 b | 2.0 ± 0.0 b,# | 91.2 ± 1.6 c,# | 86.8 ± 2.3 c,# |
| November 2021 | 2.5 ± 0.1 b | 0.033 ± 0.002 a | 0.025 ± 0.001 b | 1.3 ± 0.0 a | 68.3 ± 2.8 a | 63.9 ± 3.1 a |
| April 2022 | 2.2 ± 0.1 a,# | 0.037 ± 0.002 a | 0.083 ± 0.110 c | 1.7 ± 0.0 a,b,# | 75.4 ± 0.9 b | 66.7 ± 6.8 a |
| May 2022 | 2.5 ± 0.1 b | 0.036 ± 0.001 a | 0.023 ± 0.001 b | 1.5 ± 0.1 a | 73.3 ± 1.7 a,b | 68.8 ± 1.5 a |
| June 2022 | 3.1 ± 0.1 c,# | 0.038 ± 0.002 a | 0.022 ± 0.001 b | 1.2 ± 0.1 a,# | 75.9 ± 2.6 b | 74.4 ± 2.5 b |
| July 2022 | 2.4 ± 0.1 a,b | 0.039 ± 0.001 a,# | 0.026 ± 0.001 b | 1.7 ± 0.1 a,b | 78.9 ± 0.8 b,# | 74.0 ± 0.7 b,# |
| August 2022 | 2.8 ± 0.2 b,c | 0.039 ± 0.001 a,# | 0.027 ± 0.002 b | 1.4 ± 0.1 a | 78.4 ± 1.8 a,# | 75.4 ± 0.9 b,# |
| November 2022 | 3.1 ± 0.1 c,# | 0.065 ± 0.001 c | 0.028 ± 0.002 b | 2.1 ± 0.0 c,# | 119.7 ± 1.6 d | 120.8 ± 2.3 d |
| 4 m above the lake bottom | ||||||
| April 2021 | 2.2 ± 0.2 a | 0.057 ± 0.001 a,b | 0.018 ± 0.002 a | 2.6 ± 0.3 c | 106.5 ± 1.0 c | 101.2 ± 1.0 c |
| May 2021 | 2.1 ± 0.1 a | 0.057 ± 0.001 a,b,# | 0.018 ± 0.001 a | 2.7 ± 0.1 c | 107.1 ± 1.6 c,# | 101.1 ± 2.5 c |
| June 2021 | 2.3 ± 0.1 a | 0.054 ± 0.002 a,b | 0.019 ± 0.001 a | 2.4 ± 0.0 b,c | 102.2 ± 3.2 c | 97.6 ± 3.5 c |
| July 2021 | 2.5 ± 0.2 a | 0.053 ± 0.001 b | 0.019 ± 0.001 a | 2.1 ± 0.1 b | 100.6 ± 1.6 c | 97.3 ± 2.7 c |
| September 2021 | 2.5 ± 0.1 a | 0.057 ± 0.001 b,# | 0.023 ± 0.001 b | 2.3 ± 0.1 b,# | 106.5 ± 0.9 c,# | 103.1 ± 0.5 d,# |
| November 2021 | 2.4 ± 0.1 a | 0.031 ± 0.001 a | 0.025 ± 0.001 b | 1.3 ± 0.0 a | 64.7 ± 1.7 a | 59.9 ± 2.2 a |
| April 2022 | 2.5 ± 0.0 a,# | 0.037 ± 0.001 a | 0.020 ± 0.001 a,b | 1.5 ± 0.0 a,# | 74.7 ± 1.7 a,b | 70.7 ± 1.8 b |
| May 2022 | 2.4 ± 0.1 a | 0.036 ± 0.001 a | 0.023 ± 0.001 b | 1.5 ± 0.1 a | 73.2 ± 1.7 a | 68.4 ± 1.4 a,b |
| June 2022 | 2.4 ± 0.2 a,# | 0.039 ± 0.001 a | 0.021 ± 0.002 b | 1.6 ± 0.1 a,# | 77.9 ± 1.7 a,b | 73.4 ± 2.4 b |
| July 2022 | 2.5 ± 0.2 a,b | 0.043 ± 0.001 a,b,# | 0.025 ± 0.001 b | 1.8 ± 0.2 a,b | 85.2 ± 1.1 b,# | 80.9 ± 0.8 b,# |
| August 2022 | 2.7 ± 0.1 b | 0.042 ± 0.001 a,# | 0.027 ± 0.001 b | 1.6 ± 0.1 a | 83.4 ± 1.7 b,# | 80.0 ± 1.3 b,# |
| November 2022 | 3.4 ± 0.1 c,# | 0.066 ± 0.001 c | 0.027 ± 0.001 b | 2.0 ± 0.1 b,# | 121.3 ± 1.6 d | 124.1 ± 1.4 e |
| Date | Input Variables | Predicted Output Parameters | |||||
|---|---|---|---|---|---|---|---|
| Dose PT | Dose MO | Duration MO | BrO3− | SUVA | FP 1 THM | FP 1 HAA | |
| mg/L | mg/L | min | µg/L | L/(mg·m) | µg/L | µg/L | |
| 6.7 m above the lake bottom | |||||||
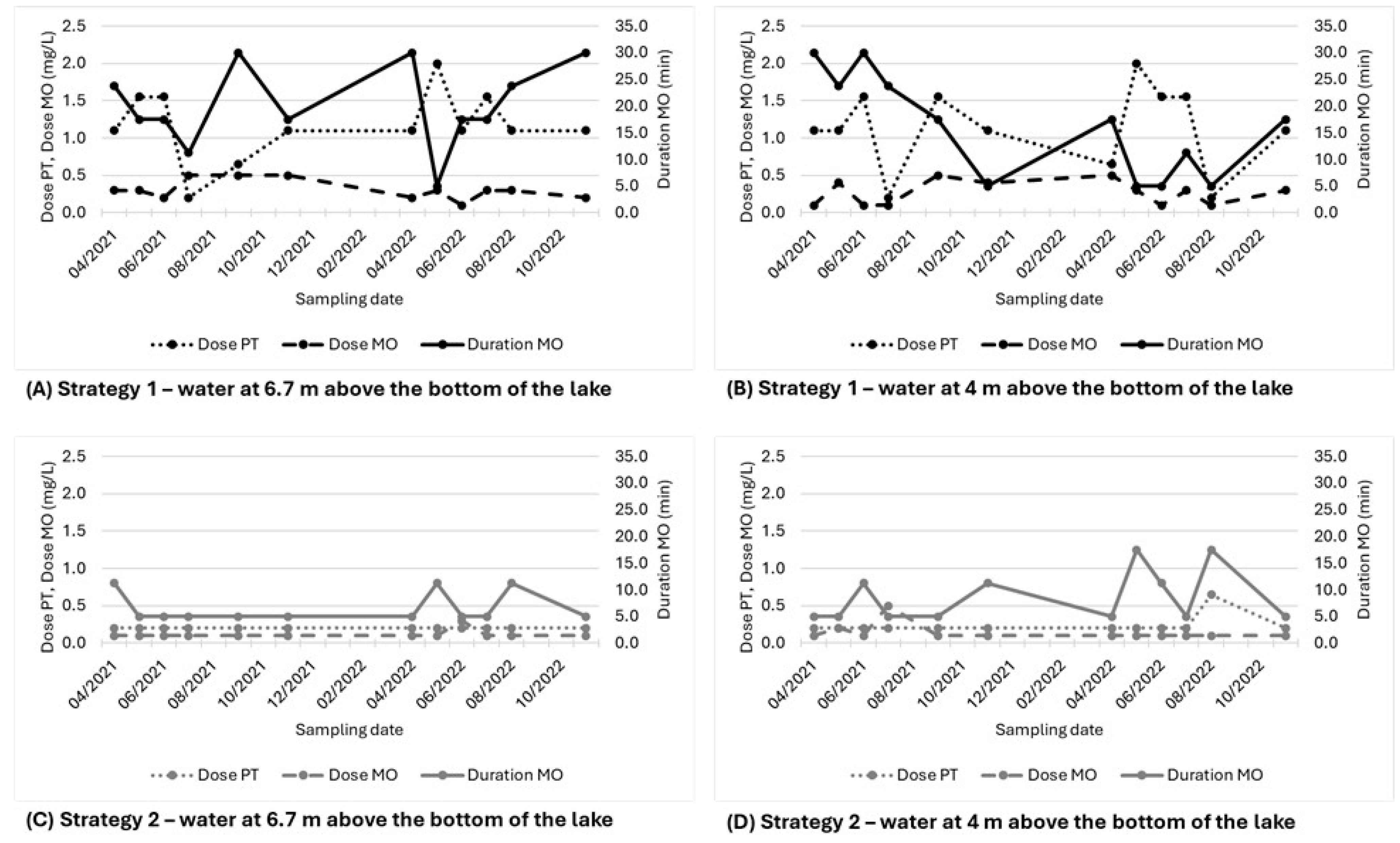
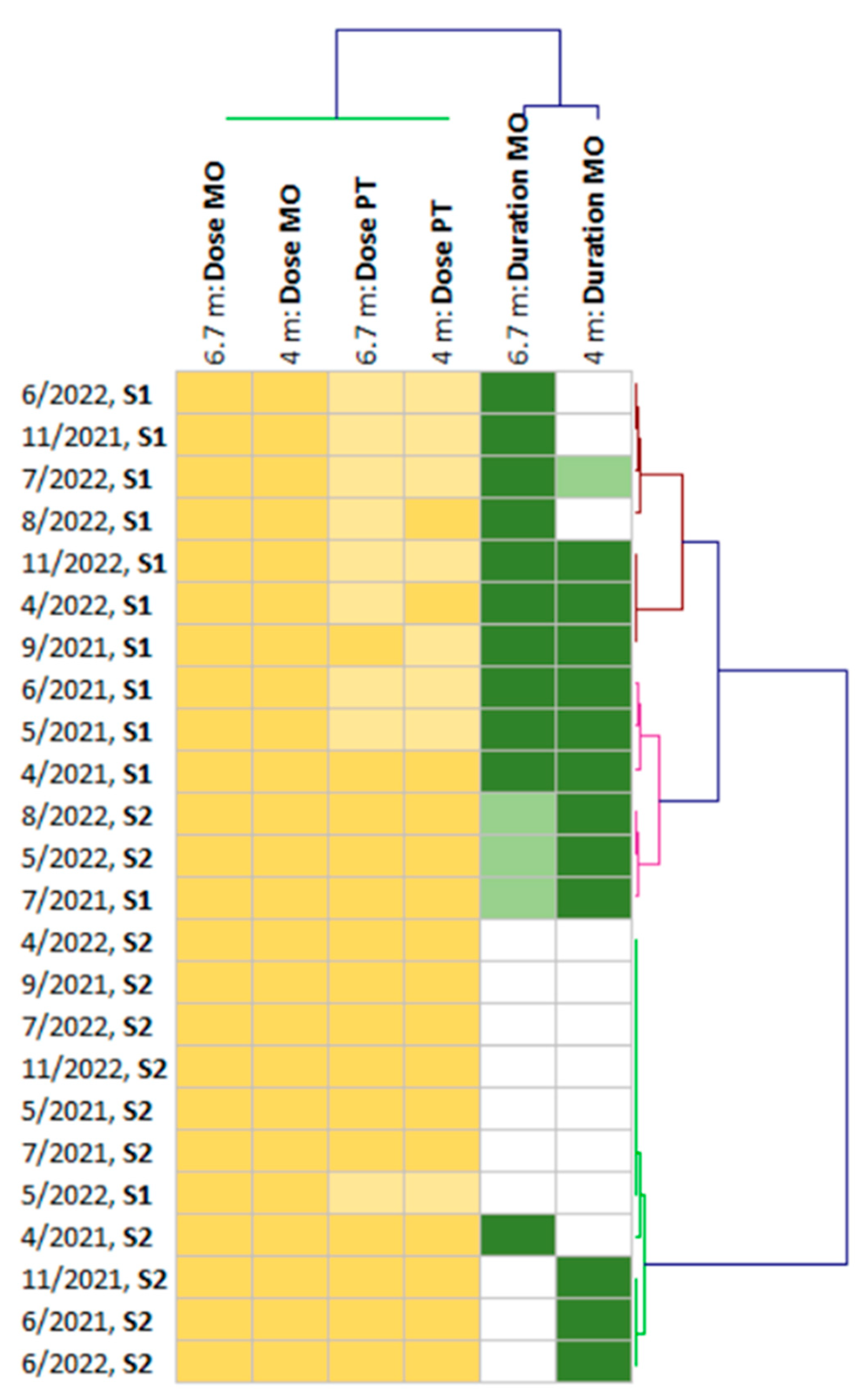
| April 2021 | 1.1 | 0.3 | 23.8 | 7.0 | 0.6 | 30 | 25 |
| May 2021 | 1.6 | 0.3 | 17.5 | 7.1 | 0.7 | 31 | 27 |
| June 2021 | 1.6 | 0.2 | 17.5 | 8.0 | 0.5 | 29 | 25 |
| July 2021 | 0.2 | 0.5 | 11.3 | 7.2 | 0.6 | 32 | 28 |
| September 2021 | 0.7 | 0.5 | 30.0 | 5.1 | 0.5 | 30 | 26 |
| November 2021 | 1.1 | 0.5 | 17.5 | 8.0 | 0.4 | 22 | 18 |
| April 2022 | 1.1 | 0.2 | 30.0 | 9.9 | 0.5 | 26 | 22 |
| May 2022 | 2.0 | 0.3 | 5.0 | 9.3 | 0.4 | 22 | 19 |
| June 2022 | 1.1 | 0.1 | 17.5 | 6.7 | 0.4 | 25 | 22 |
| July 2022 | 1.6 | 0.3 | 17.5 | 11.0 | 0.3 | 20 | 17 |
| August 2022 | 1.1 | 0.3 | 23.8 | 15.0 | 0.4 | 25 | 21 |
| November 2022 | 1.1 | 0.2 | 30.0 | 9.6 | 0.6 | 36 | 32 |
| 4 m above the lake bottom | |||||||
| April 2021 | 1.1 | 0.1 | 30.0 | 2.4 | 0.6 | 26 | 22 |
| May 2021 | 1.1 | 0.4 | 23.8 | 7.0 | 0.6 | 31 | 26 |
| June 2021 | 1.6 | 0.1 | 30.0 | 5.5 | 0.5 | 29 | 25 |
| July 2021 | 0.2 | 0.1 | 23.8 | 6.2 | 0.5 | 27 | 23 |
| September 2021 | 1.6 | 0.5 | 17.5 | 1.9 | 0.6 | 34 | 30 |
| November 2021 | 1.1 | 0.4 | 5.0 | 5.3 | 0.5 | 23 | 19 |
| April 2022 | 0.7 | 0.5 | 17.5 | 7.8 | 0.5 | 26 | 22 |
| May 2022 | 2.0 | 0.3 | 5.0 | 10.3 | 0.3 | 20 | 17 |
| June 2022 | 1.6 | 0.1 | 5.0 | 4.1 | 0.5 | 29 | 26 |
| July 2022 | 1.6 | 0.3 | 11.3 | 5.2 | 0.4 | 22 | 19 |
| August 2022 | 0.2 | 0.1 | 5.0 | 3.2 | 0.5 | 26 | 22 |
| November 2022 | 1.1 | 0.3 | 17.5 | 7.3 | 0.6 | 38 | 34 |
| Date | Model | Input Variables | Predicted Output Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| Dose PT | Dose MO | Duration MO | BrO3− | SUVA | FP 1 THM | FP 1 HAA | ||
| mg/L | mg/L | min | µg/L | L/(mg·m) | µg/L | µg/L | ||
| April 2021 | Individual | 0.2 | 0.1 | 11.3 | 0.0 | 1.1 | 53 | 47 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 1.2 | 56 | 50 | |
| May 2021 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 1.0 | 50 | 44 |
| June 2021 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 0.7 | 41 | 37 |
| July 2021 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 0.8 | 45 | 41 |
| September 2021 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 0.9 | 42 | 40 |
| November 2021 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 0.6 | 35 | 31 |
| April 2022 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 0.7 | 37 | 32 |
| May 2022 | Individual | 0.2 | 0.1 | 11.3 | 0.0 | 0.6 | 33 | 29 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 0.5 | 31 | 28 | |
| June 2022 | Individual | 0.2 | 0.3 | 5.0 | 1.3 | 0.6 | 37 | 34 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 0.5 | 34 | 31 | |
| July 2022 | Individual | 0.2 | 0.1 | 5.0 | 0.2 | 0.5 | 32 | 28 |
| August 2022 | Individual | 0.2 | 0.1 | 11.3 | 0.0 | 0.6 | 32 | 28 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 0.6 | 33 | 28 | |
| November 2022 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 0.8 | 48 | 44 |
| Date | Model | Input Variables | Predicted Output Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| Dose PT | Dose MO | Duration MO | BrO3− | SUVA | FP 1 THM | FP 1 HAA | ||
| mg/L | mg/L | min | µg/L | L/(mg·m) | µg/L | µg/L | ||
| April 2021 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 1.2 | 51 | 45 |
| May 2021 | Individual | 0.2 | 0.2 | 5.0 | 0.0 | 1.0 | 46 | 40 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 1.0 | 48 | 42 | |
| June 2021 | Individual | 0.2 | 0.1 | 11.3 | 0.2 | 0.7 | 39 | 35 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 0.7 | 39 | 35 | |
| July 2021 | Individual | 0.2 | 0.5 | 5.0 | 0.0 | 0.8 | 45 | 41 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 0.8 | 45 | 41 | |
| September 2021 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 0.9 | 49 | 44 |
| November 2021 | Individual | 0.2 | 0.1 | 11.3 | 0.0 | 0.6 | 32 | 27 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 0.6 | 32 | 27 | |
| April 2022 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 0.7 | 34 | 30 |
| May 2022 | Individual | 0.2 | 0.1 | 17.5 | 1.4 | 0.6 | 32 | 28 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 0.6 | 30 | 26 | |
| June 2022 | Individual | 0.2 | 0.1 | 11.3 | 0.0 | 0.7 | 34 | 30 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 0.7 | 34 | 30 | |
| July 2022 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 0.6 | 35 | 31 |
| August 2022 | Individual | 0.7 | 0.1 | 17.5 | 1.1 | 0.7 | 37 | 33 |
| General | 0.2 | 0.1 | 5.0 | 0.0 | 0.6 | 31 | 26 | |
| November 2022 | Individual | 0.2 | 0.1 | 5.0 | 0.0 | 0.9 | 52 | 47 |
| Date | Parameters in the Plant | Analysis from the Plant | Model Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose PT | Dose MO | Duration MO | BrO3− | SUVA | FP 1 THM | FP 1 HAA | BrO3− | SUVA | FP 1 THM | FP 1 HAA | |
| April 2021 | 0.7 | 0.5 | 17.5 | 2.0 | 1.0 | 43 | 38 | 6.7 | 0.6 | 32 | 28 |
| May 2021 | 0.8 | 0.6 | 14.6 | 0.0 | 1.2 | 45 | 38 | 6.4 | 0.6 | 33 | 29 |
| June 2021 | 0.8 | 0.6 | 14.6 | 0.0 | 1.1 | 42 | 35 | 6.0 | 0.6 | 34 | 30 |
| July 2021 | 0.8 | 0.4 | 17.5 | 1.5 | 0.7 | 28 | 23 | 6.9 | 0.6 | 31 | 27 |
| September 2021 | 0.9 | 0.1 | 17.5 | 1.8 | 1.1 | 43 | 36 | 4.0 | 0.6 | 33 | 29 |
| November 2021 | 1.0 | 0.6 | 17.5 | 1.3 | 1.1 | 42 | 36 | 7.3 | 0.6 | 33 | 29 |
| April 2022 | 1.2 | 0.4 | 14.6 | 0.0 | 0.7 | 30 | 26 | 7.8 | 0.6 | 30 | 26 |
| May 2022 | 1.3 | 0.6 | 14.6 | 2.2 | 0.8 | 34 | 29 | 7.8 | 0.6 | 33 | 28 |
| July 2022 | 1.3 | 0.3 | 8.7 | 1.6 | 0.7 | 32 | 27 | 6.4 | 0.6 | 31 | 27 |
| August 2022 | 1.4 | 0.5 | 8.7 | 1.6 | 0.6 | 37 | 33 | 7.4 | 0.6 | 31 | 27 |
| November 2022 | 1.6 | 0.5 | 17.5 | 0.0 | 0.9 | 46 | 41 | 10.0 | 0.5 | 29 | 25 |
| Date | Dose PT | Dose MO | Duration MO | BrO3− | SUVA | FP 1 THM | FP 1 HAA |
|---|---|---|---|---|---|---|---|
| April 2021 | 0.3 | 0.1 | 17.5 | 1.1 | 0.7 | 37 | 33 |
| May 2021 | 0.3 | 0.1 | 14.6 | 0.9 | 0.7 | 37 | 33 |
| June 2021 | 0.3 | 0.1 | 14.6 | 1.2 | 0.7 | 37 | 32 |
| July 2021 | 0.3 | 0.1 | 17.5 | 0.9 | 0.7 | 37 | 33 |
| September 2021 | 0.4 | 0.0 | 17.5 | 0.0 | 0.7 | 39 | 34 |
| November 2021 | 0.4 | 0.1 | 17.5 | 2.1 | 0.7 | 36 | 31 |
| April 2022 | 0.5 | 0.1 | 14.6 | 1.0 | 0.7 | 37 | 33 |
| May 2022 | 0.6 | 0.1 | 14.6 | 2.0 | 0.7 | 36 | 31 |
| July 2022 | 0.6 | 0.1 | 8.7 | 0.0 | 0.7 | 38 | 34 |
| August 2022 | 0.6 | 0.1 | 8.7 | 0.4 | 0.7 | 37 | 33 |
| November 2022 | 0.7 | 0.1 | 17.5 | 2.6 | 0.7 | 35 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregov, M.; Gajdoš Kljusurić, J.; Valinger, D.; Benković, M.; Jurina, T.; Jurinjak Tušek, A.; Crnek, V.; Matošić, M.; Ujević Bošnjak, M.; Ćurko, J. Optimization of Ozonation in Drinking Water Production at Lake Butoniga. Water 2025, 17, 97. https://doi.org/10.3390/w17010097
Gregov M, Gajdoš Kljusurić J, Valinger D, Benković M, Jurina T, Jurinjak Tušek A, Crnek V, Matošić M, Ujević Bošnjak M, Ćurko J. Optimization of Ozonation in Drinking Water Production at Lake Butoniga. Water. 2025; 17(1):97. https://doi.org/10.3390/w17010097
Chicago/Turabian StyleGregov, Marija, Jasenka Gajdoš Kljusurić, Davor Valinger, Maja Benković, Tamara Jurina, Ana Jurinjak Tušek, Vlado Crnek, Marin Matošić, Magdalena Ujević Bošnjak, and Josip Ćurko. 2025. "Optimization of Ozonation in Drinking Water Production at Lake Butoniga" Water 17, no. 1: 97. https://doi.org/10.3390/w17010097
APA StyleGregov, M., Gajdoš Kljusurić, J., Valinger, D., Benković, M., Jurina, T., Jurinjak Tušek, A., Crnek, V., Matošić, M., Ujević Bošnjak, M., & Ćurko, J. (2025). Optimization of Ozonation in Drinking Water Production at Lake Butoniga. Water, 17(1), 97. https://doi.org/10.3390/w17010097












