Wetland Creation and Reforestation of Legacy Surface Mines in the Central Appalachian Region (USA): A Potential Climate-Adaptation Approach for Pond-Breeding Amphibians?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Treatment Types
2.3. Wetland Attributes
2.4. Amphibian Surveys
2.5. Data Analysis
2.5.1. Wetland Attributes
2.5.2. Multi-Species Occupancy Models
2.5.3. N-Mixture (Abundance) Models
3. Results
3.1. Wetland Attributes
3.2. Dipnet Surveys
3.3. Occupancy and Species Richness
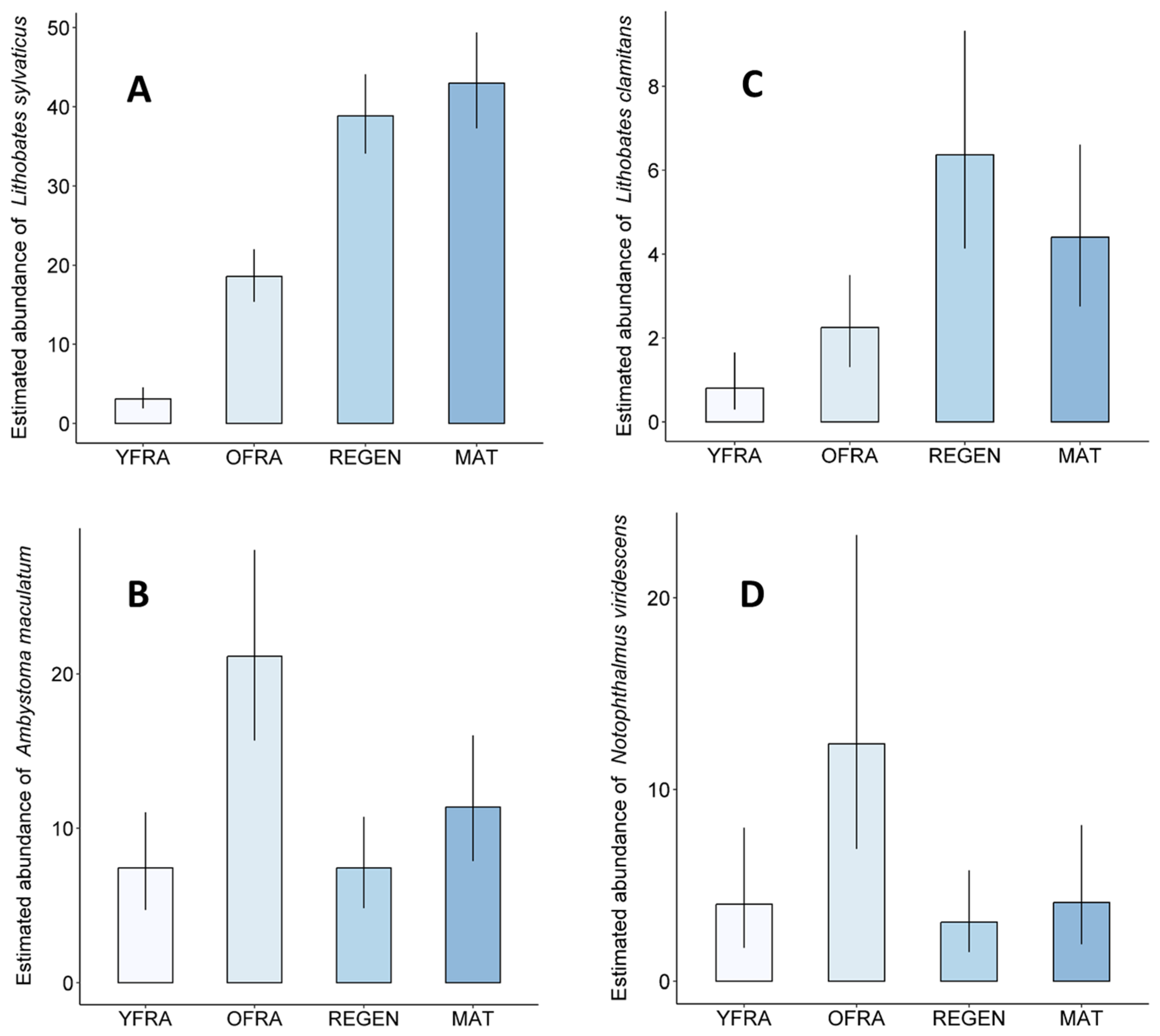
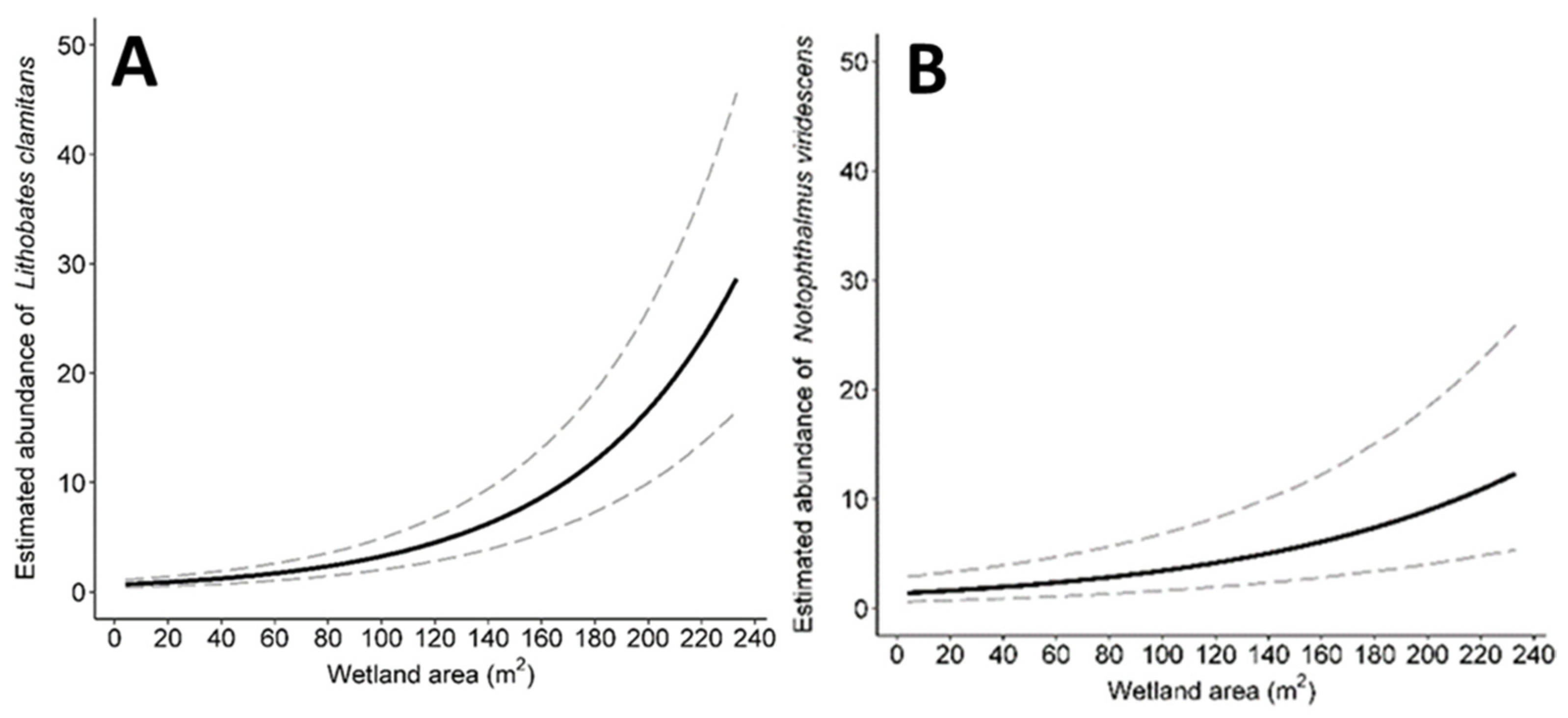
3.4. Abundance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeDee, O.E.; Handler, S.D.; Hoving, C.L.; Swanston, C.W.; Zuckerberg, B. Preparing wildlife for climate change: How far have we come? J. Wildl. Manag. 2021, 85, 7–16. [Google Scholar] [CrossRef]
- Mawdsley, J.R.; O’Malley, R.; Ojima, D.S. A review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conserv. Biol. 2009, 23, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Handler, S.D.; Ledee, O.E.; Hoving, C.L.; Zuckerberg, B.; Swanston, C.W. A menu of climate change adaptation actions for terrestrial wildlife management. Wildl. Soc. Bull. 2002, 46, e1331. [Google Scholar] [CrossRef]
- Fischlin, A.; Midgley, G.F.; Price, J.; Leemans, R.; Gopal, B.; Turley, C.; Rounsevell, M.; Dube, P.; Tarazona, J.; Velichko, A. Ecosystems, their properties, goods and services. In Climate Change 2007: Impacts, Adaptation and Vulnerability; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 211–272. [Google Scholar]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, N.S.; Bickford, D.; Diesmos, A.C.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Sekercioglu, C.H.; Bradshaw, C.J. Measuring the meltdown: Drivers of global amphibian extinction and decline. PLoS ONE 2008, 3, e1636. [Google Scholar] [CrossRef]
- Calhoun, A.J.; Mushet, D.M.; Bell, K.P.; Boix, D.; Fitzsimons, J.A.; Isselin-Nondedeu, F. Temporary wetlands: Challenges and solutions to conserving a ‘disappearing’ ecosystem. Biol. Conserv. 2017, 211, 3–11. [Google Scholar] [CrossRef]
- Walls, S.C.; Barichivich, W.J.; Brown, M.E. Drought, deluge and declines: The impact of precipitation extremes on amphibians in a changing climate. Biology 2013, 2, 399–418. [Google Scholar] [CrossRef]
- Shoo, L.P.; Olson, D.H.; McMenamin, S.K.; Murray, K.A.; Van Sluys, M.; Donnelly, M.A.; Stratford, D.; Terhivuo, J.; Merino-Viteri, A.; Herbert, S.M. Engineering a future for amphibians under climate change. J. Appl. Ecol. 2011, 48, 487–492. [Google Scholar] [CrossRef]
- Miller, D.A.W.; Campbell Grant, E.H.; Muths, E.; Amburgey, S.M.; Adams, M.J.; Joseph, M.B.; Waddle, J.H.; Johnson, P.T.J.; Ryan, M.E.; Schmidt, B.R.; et al. Quantifying climate sensitivity and climate driven change in North American amphibian communities. Nat. Commun. 2018, 9, 3926. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Bodie, J.R. Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv. Biol. 2003, 17, 1219–1228. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Principles for management of aquatic-breeding amphibians. J. Wildl. Manag. 2000, 64, 615–631. [Google Scholar] [CrossRef]
- Lambert, M.; Drayer, A.N.; Leuenberger, W.; Price, S.J.; Barton, C. Evaluation of created wetlands as amphibian habitat on a reforested surface mine. Ecol. Eng. 2021, 171, 106386. [Google Scholar] [CrossRef]
- Byers, E.A.; Vanderhorst, V.P.; Streets, B.P. Classification and conservation assessment of upland red spruce communities in West Virginia. In West Virginia Natural Heritage Program; WVDNR: Elkins, WV, USA, 2010. [Google Scholar]
- Beane, N.R.; Rentch, J.S. Using known occurrences to model suitable habitat for a rare forest type in West Virginia under select climate change scenarios. Ecol. Restor. 2015, 33, 178–189. [Google Scholar] [CrossRef]
- Burkett, V.; Kusler, J. Climate change: Potential impacts and interactions in wetlands of the United States 1. J. Am. Water Resour. Assoc. 2000, 36, 313–320. [Google Scholar] [CrossRef]
- Rhodes, B. Evaluating Restoration Outcomes: Red Spruce Reforestation in the West Virginia Highlands. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2022. [Google Scholar]
- Burger, J.; Zipper, C.; Angel, P.; Hall, N.; Skousen, J.; Barton, C.; Eggerud, S. Establishing Native Trees on Legacy Surface Mines, Forest Reclamation Advisory No. 11; US Department of Interior Office of Surface Mining: Washington, DC, USA, 2013.
- Branduzzi, A.M.; Barton, C.D.; Lovell, A. First-year survival of native wetland plants in created vernal pools on an Appalachian surface mine. Ecol. Restor. 2020, 38, 70–73. [Google Scholar] [CrossRef]
- Branduzzi, A.M.; Barton, C.D.; Baskin, C.C.; Davis, A.G. Evaluating the use of woody debris to enhance native plant establishment from seeds on legacy coal mines in West Virginia (USA). Nat. Plants 2022, 23, 273–287. [Google Scholar] [CrossRef]
- Groninger, J.; Skousen, J.; Angel, P.; Barton, C.; Burger, J.; Zipper, C. Chapter 8: Mine reclamation practices to enhance forest development through natural succession. In The Forestry Reclamation Approach: Guide to Successful Reforestation of Mined Lands; Adams, M., Ed.; Gen. Tech. Rep. NRS-169; Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2007; pp. 8-1–8-7. [Google Scholar]
- Greenberg, A.E.; Clesceri, L.S.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Denton, R.D.; Richter, S.C. Amphibian communities in natural and constructed ridge top wetlands with implications for wetland construction. J. Wildl. Manag. 2013, 77, 886–896. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 13 April 2024).
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Zipkin, E.F.; DeWan, A.; Royle, A.J. Impacts of forest fragmentation on species richness: A hierarchical approach to community modelling. J. Appl. Ecol. 2009, 46, 815–822. [Google Scholar] [CrossRef]
- Pacifici, K.; Zipkin, E.F.; Collazo, J.A.; Irizarry, J.I.; DeWan, A. Guidelines for a priori grouping of species in hierarchical community models. Ecol. Evol. 2014, 4, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Link, W.A.; Cam, E.; Nichols, J.D.; Cooch, E.G. Of BUGS and birds: Markov chain Monte Carlo for hierarchical modeling in wildlife research. J. Wildl. Manag. 2002, 66, 277–291. [Google Scholar] [CrossRef]
- Gelman, A.; Rubin, D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992, 7, 457–472. [Google Scholar] [CrossRef]
- Sturtz, S.; Ligges, U.; Gelman, A. R2WinBUGS: A Package for Running WinBUGS from R. J. Stat. Soft. 2005, 12, 1–16. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Thomas, A.; Best, N.; Lunn, D. WinBUGS Version 1.4; MRC Biostatistics Unit: Cambridge, UK, 2003. [Google Scholar]
- Royle, J.A. N-mixture models for estimating population size from spatially replicated counts. Biometrics 2004, 60, 108–115. [Google Scholar] [CrossRef]
- Hughes, J. Freshwater Ecology and Conservation: Approaches and Techniques; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Elias, P.E.; Burger, J.A.; Adams, M.B. Acid deposition effects on forest composition and growth on the Monongahela National Forest, West Virginia. For. Ecol. Manag. 2009, 258, 2175–2182. [Google Scholar] [CrossRef]
- Agouridis, C.T.; Angel, P.N.; Taylor, T.J.; Barton, C.D.; Warner, R.C.; Yu, X.; Wood, C. Water quality characteristics of discharge from reforested loose-dumped mine spoil in eastern Kentucky. J. Environ. Qual. 2012, 41, 454–468. [Google Scholar] [CrossRef]
- Ahn, C.; Jones, S. Assessing organic matter and organic carbon contents in soils of created mitigation wetlands in Virginia. Environ. Eng. Res. 2013, 18, 151–156. [Google Scholar] [CrossRef]
- Freda, J. The influence of acidic pond water on amphibians: A review. Water Air Soil Poll. 1986, 30, 439–450. [Google Scholar] [CrossRef]
- Freda, J. The effects of aluminum and other metals on amphibians. Environ. Pollut. 1991, 71, 305–328. [Google Scholar] [CrossRef]
- US EPA (U.S. Environmental Protection Agency) Final Aquatic Life Ambient Water Quality Criteria for Aluminum. 2018. Available online: https://www.epa.gov/sites/default/files/2018-12/documents/aluminum-final-national-recommended-awqc.pdf (accessed on 13 April 2024).
- Lindsay, W.L.; Walthall, P.M. The solubility of aluminum in soils. In The Environmental Chemistry of Aluminum; Sposito, G., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 333–361. [Google Scholar]
- Lindberg, T.T.; Bernhardt, E.S.; Bier, R.; Helton, A.M.; Merola, R.B.; Wengosh, A.; DiGiulio, R.T. Cumulative impacts of mountaintop mining on an Appalachian watershed. Proc. Natl. Acad. Sci. USA 2011, 108, 20929–20934. [Google Scholar] [CrossRef]
- Hutton, J.M.; Price, S.J.; Bonner, S.J.; Richter, S.C.; Barton, C.D. Occupancy and abundance of stream salamanders along a specific conductance gradient. Freshw. Sci. 2020, 39, 433–446. [Google Scholar] [CrossRef]
- Buckardt, E.M.; Rega-Brodsky, C.C.; George, A.D. Post-mined wetlands provide breeding habitat for amphibians. Wetlands 2023, 43, 75. [Google Scholar] [CrossRef]
- US EPA (U.S. Environmental Protection Agency). A Field-Based Aquatic Life Benchmark for Conductivity in Central Appalachian Streams; EPA/600/R-10/023F; National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Stiles, R.M.; Swan, J.W.; Klemish, J.L.; Lannoo, M.J. Amphibian habitat creation on postindustrial landscapes: A case study in a reclaimed coal strip-mine area. Can. J. Zool. 2017, 95, 67–73. [Google Scholar] [CrossRef]
- Laan, R.; Verboom, B. Effects of pool size and isolation on amphibian communities. Biol. Conserv. 1990, 54, 251–262. [Google Scholar] [CrossRef]
- Resetarits, W.J., Jr.; Wilbur, H.M. Choice of oviposition site by Hyla chrysoscelis: Role of predators and competitors. Ecology 1989, 70, 220–228. [Google Scholar] [CrossRef]
- Kross, C.S.; Richter, S.C. Species interactions in constructed wetlands result in population sinks for wood frogs (Lithobates sylvaticus) while benefiting eastern newts (Notophthalmus viridescens). Wetlands 2016, 36, 385–393. [Google Scholar] [CrossRef]
- Marsh, D.M. Fluctuations in amphibian populations: A meta-analysis. Biol. Conserv. 2001, 101, 327–335. [Google Scholar] [CrossRef]
- Drayer, A.N.; Guzy, J.C.; Caro, R.; Price, S.J. Created wetlands managed for hydroperiod provide habitat for amphibians in Western Kentucky, USA. Wetl. Ecol. Manag. 2020, 28, 543–558. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Peterman, W.E.; Anderson, T.L.; Drake, D.L.; Ousterhout, B.H. Intermediate pond sizes contain the highest density, richness, and diversity of pond-breeding amphibians. PLoS ONE 2015, 10, e0123055. [Google Scholar] [CrossRef]
- Babbitt, K.J. The relative importance of wetland size and hydroperiod for amphibians in southern New Hampshire, USA. Wetl. Ecol. Manag. 2005, 13, 269–279. [Google Scholar] [CrossRef]
- Millikin, A.R.; Woodley, S.K.; Davis, D.R.; Anderson, J.T. Habitat characteristics in created vernal pools impact spotted salamander water-borne corticosterone levels. Wetlands 2019, 39, 803–814. [Google Scholar] [CrossRef]
- Burrow, A.; Maerz, J. How plants affect amphibian populations. Biol. Rev. 2022, 97, 1749–1767. [Google Scholar] [CrossRef]
- Sawatzky, M.E.; Martin, A.E.; Fahrig, L. Landscape context is more important than wetland buffers for farmland amphibians. Agric. Ecosyst. Environ. 2019, 269, 97–106. [Google Scholar] [CrossRef]


| Variable | p-Value | F-Stat | YFRA | OFRA | REGEN | MAT |
|---|---|---|---|---|---|---|
| Wetland Area (m2) | 0.729 | 0.436 | 57.99 a | 71.63 a | 81.66 a | 49.66 a |
| Canopy Cover (%) | 0.004 | 5.67 | 0 b | 10.45 ab | 53.68 a | 54.75 a |
| Conductivity (μS cm−1) | 0.316 | 1.23 | 44.4 a | 52.09 a | 23.24 a | 33.39 a |
| pH (H+) | 0.001 | 6.71 | 6.74 a | 6.78 a | 5.77 b | 6.15 ab |
| NO3-N (mg L−1) | 0.407 | 1 | 0.002 a | 0 a | 0 a | 0 a |
| Turbidity (FTU) | 0.328 | 1.2 | 8.57 a | 4.02 a | 6.35 a | 2.88 a |
| TOC (mg L−1) | 0.022 | 3.76 | 3.75 ab | 4.31 a | 2.73 b | 3.19 ab |
| Ca (mg L−1) | 0.47 | 0.87 | 4.77 a | 6.78 a | 1.78 a | 5.14 a |
| Mg (mg L−1) | 0.131 | 2.04 | 3.04 a | 2.78 a | 1.03 a | 0.49 a |
| Fe (mg L−1) | 0.397 | 1.02 | 1.74 a | 3.9 a | 7.81 a | 1.74 a |
| NO2-N (mg L−1) | 0.179 | 1.76 | 0.29 a | 0.2 a | 0.42 a | 0.39 a |
| Al (mg L−1) | 0.054 | 2.87 | 0.15 a | 0.1 a | 0.68 a | 0.3 a |
| Na (mg L−1) | 0.505 | 0.8 | 0.75 a | 0.56 a | 0.55 a | 0.83 a |
| Mn (mg L−1) | 0.261 | 1.410 | 0.31 a | 1.02 a | 0.99 a | 0.13 a |
| K (mg L−1) | 0.117 | 2.15 | 1.39 a | 1.39 a | 0.76 a | 0.67 a |
| Cl (mg L−1) | 0.172 | 1.790 | 0.67 a | 0.7 a | 0.71 a | 1.04 a |
| NH4-N (mg L−1) | 0.569 | 0.685 | 0.02 a | 0.01 a | 0.1 a | 0.01 a |
| SO4 (mg L−1) | 0.188 | 1.71 | 4.77 a | 2.23 a | 2.74 a | 2.42 a |
| Species | YFRA | OFRA | REGEN | MAT | Wetland Area | Date |
|---|---|---|---|---|---|---|
| Anaxyrus americanus | 0.90 (−0.76, 2.73) | 0.24 (−2.12, 1.90) | −0.24 (−2.30, 1.35) | −3.38 (−6.48, −0.25) | −0.01 (−1.68, 0.87) | 0.15 (−0.14, 0.44) |
| Ambystoma maculatum | −0.42 (−2.34, 1.21) | 1.05 (−0.53, 2.71) | −0.17 (−1.89, 1.33) | 1.68 (−1.15, 4.83) | 0.63 (−0.18, 1.78) | 0.19 (0.01, 0.58) |
| Hemidactylium scutatum | 0.15 (−2.05, 2.32) | 0.61 (−1.62, 2.50) | 0.25 (−1.46, 2.23) | 1.62 (−2.29, 5.93) | 0.52 (−0.51, 1.76) | 0.22 (−0.40, 0.67) |
| Hyla versicolor | 0.93 (−0.62, 2.66) | 1.09 (−0.34, 2.64) | −0.26 (−2.09, 1.18) | −1.42 (−4.29, 1.55) | 0.51 (−0.24, 1.40) | 0.12 (−0.12, 0.53) |
| Lithobates clamitans | −0.09 (−1.79, 1.40) | 0.41 (−1.28, 1.87) | 0.24 (−1.14, 1.80) | −0.13 (−2.8, 2.69) | 0.97 (0.20, 2.34) | 0.08 (−0.35, 0.42) |
| Lithobates palustris | 0.02 (−2.32, 2.15) | 0.81 (−1.18, 2.58) | −0.11 (−2.22, 1.74) | −1.05 (−6.28, 5.72) | 0.53 (−0.80, 2.01) | 0.18 (−0.33, 0.78) |
| Lithobates sylvaticus | 0.08 (−1.46, 1.53) | 0.30 (−1.37, 1.69) | 0.46 (−0.88, 2.10) | −0.66 (−3.28, 1.99) | 0.34 (−0.37, 1.00) | 0.07 (−0.42, 0.44) |
| Notophthalmus viridescens | −0.14 (−1.89, 1.40) | 1.29 (−0.20, 2.82) | 0.20 (−1.23, 1.79) | 0.63 (−2.08, 3.65) | 0.73 (−0.02, 1.94) | 0.2 (−0.13, 0.59) |
| Pseudacris crucifer | 0.49 (−1.15, 2.30) | 0.60 (−1.07, 2.08) | −0.22 (−2.06, 1.24) | −1.90 (−4.80, 0.97) | 0.63 (−0.07, 1.53) | 0.15 (−0.28, 0.59) |
| Species | ΨYFRA | ΨOFRA | ΨREGEN | ΨMAT |
|---|---|---|---|---|
| Anaxyrus americanus | 0.12 (0.02, 0.36) | 0.08 (0.01, 0.25) | 0.05 (0.003, 0.18) | 0.06 (0.003, 0.25) |
| Ambystoma maculatum | 0.79 (0.49, 0.96) | 0.93 (0.79, 0.99) | 0.82 (0.55, 0.97) | 0.79 (0.39, 0.98) |
| Hemidactylium scutatum | 0.79 (0.29, 0.99) | 0.85 (0.36, 0.99) | 0.83 (0.41, 0.99) | 0.74 (0.18, 0.99) |
| Hyla versicolor | 0.44 (0.15, 0.79) | 0.48 (0.18, 0.82) | 0.22 (0.04, 0.55) | 0.24 (0.03, 0.70) |
| Lithobates clamitans | 0.50 (0.19, 0.82) | 0.61 (0.29, 0.89) | 0.58 (0.28, 0.86) | 0.47 (0.10, 0.89) |
| Lithobates palustris | 0.38 (0.01, 0.99) | 0.46 (0.02, 0.99) | 0.37 (0.01, 0.99) | 0.36 (0.004, 0.99) |
| Lithobates sylvaticus | 0.42 (0.15, 0.72) | 0.47 (0.19, 0.76) | 0.51 (0.23, 0.81) | 0.37 (0.06, 0.80) |
| Notophthalmus viridescens | 0.65 (0.32, 0.92) | 0.87 (0.66, 0.98) | 0.72 (0.43, 0.94) | 0.62 (0.19, 0.95) |
| Pseudacris crucifer | 0.26 (0.05, 0.63) | 0.28 (0.07, 0.58) | 0.16 (0.02, 0.41) | 0.18 (0.02, 0.57) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherman, L.; Barton, C.D.; Guzy, J.C.; Davenport, R.N.; Cox, J.J.; Larkin, J.L.; Fearer, T.; Newman, J.C.; Price, S.J. Wetland Creation and Reforestation of Legacy Surface Mines in the Central Appalachian Region (USA): A Potential Climate-Adaptation Approach for Pond-Breeding Amphibians? Water 2024, 16, 1202. https://doi.org/10.3390/w16091202
Sherman L, Barton CD, Guzy JC, Davenport RN, Cox JJ, Larkin JL, Fearer T, Newman JC, Price SJ. Wetland Creation and Reforestation of Legacy Surface Mines in the Central Appalachian Region (USA): A Potential Climate-Adaptation Approach for Pond-Breeding Amphibians? Water. 2024; 16(9):1202. https://doi.org/10.3390/w16091202
Chicago/Turabian StyleSherman, Lauren, Christopher D. Barton, Jacquelyn C. Guzy, Rebecca N. Davenport, John J. Cox, Jeffery L. Larkin, Todd Fearer, Jillian C. Newman, and Steven J. Price. 2024. "Wetland Creation and Reforestation of Legacy Surface Mines in the Central Appalachian Region (USA): A Potential Climate-Adaptation Approach for Pond-Breeding Amphibians?" Water 16, no. 9: 1202. https://doi.org/10.3390/w16091202
APA StyleSherman, L., Barton, C. D., Guzy, J. C., Davenport, R. N., Cox, J. J., Larkin, J. L., Fearer, T., Newman, J. C., & Price, S. J. (2024). Wetland Creation and Reforestation of Legacy Surface Mines in the Central Appalachian Region (USA): A Potential Climate-Adaptation Approach for Pond-Breeding Amphibians? Water, 16(9), 1202. https://doi.org/10.3390/w16091202







