Effect of Environmental Variables on Zooplankton in Various Habitats of the Nile River
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Physico-Chemical Parameters in Water
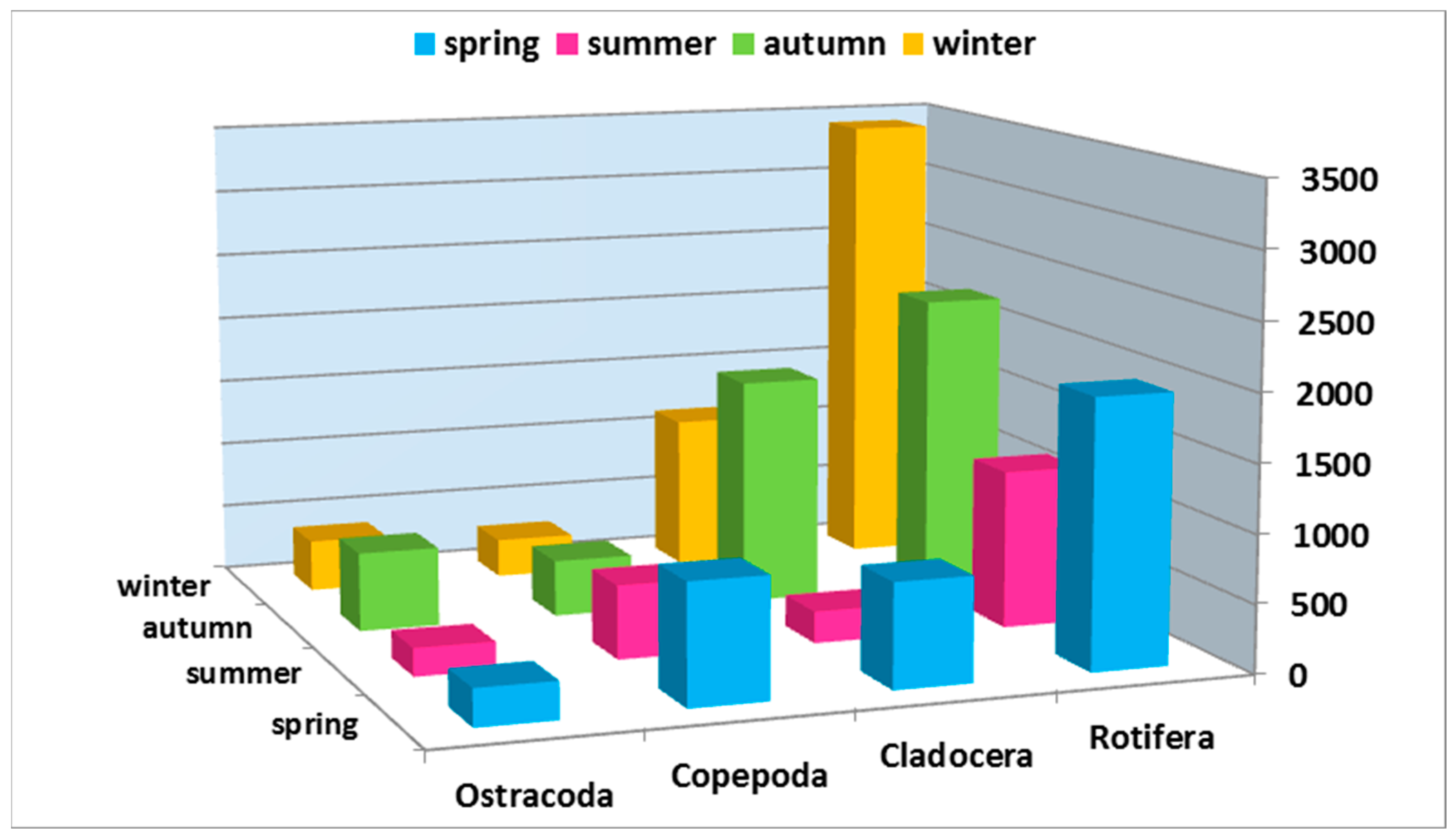
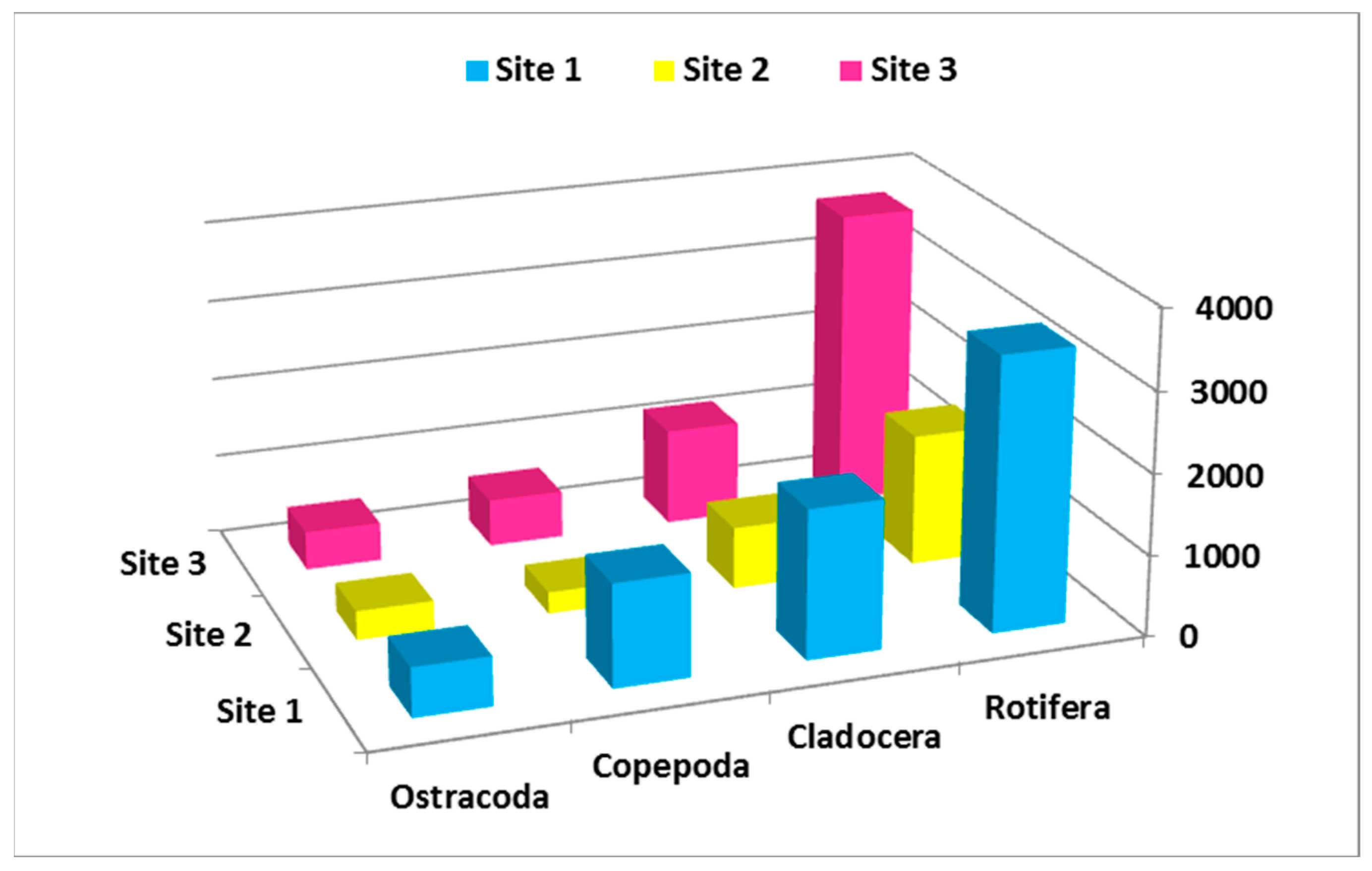
3.2. Abundance and Seasonal Differences of Zooplankton
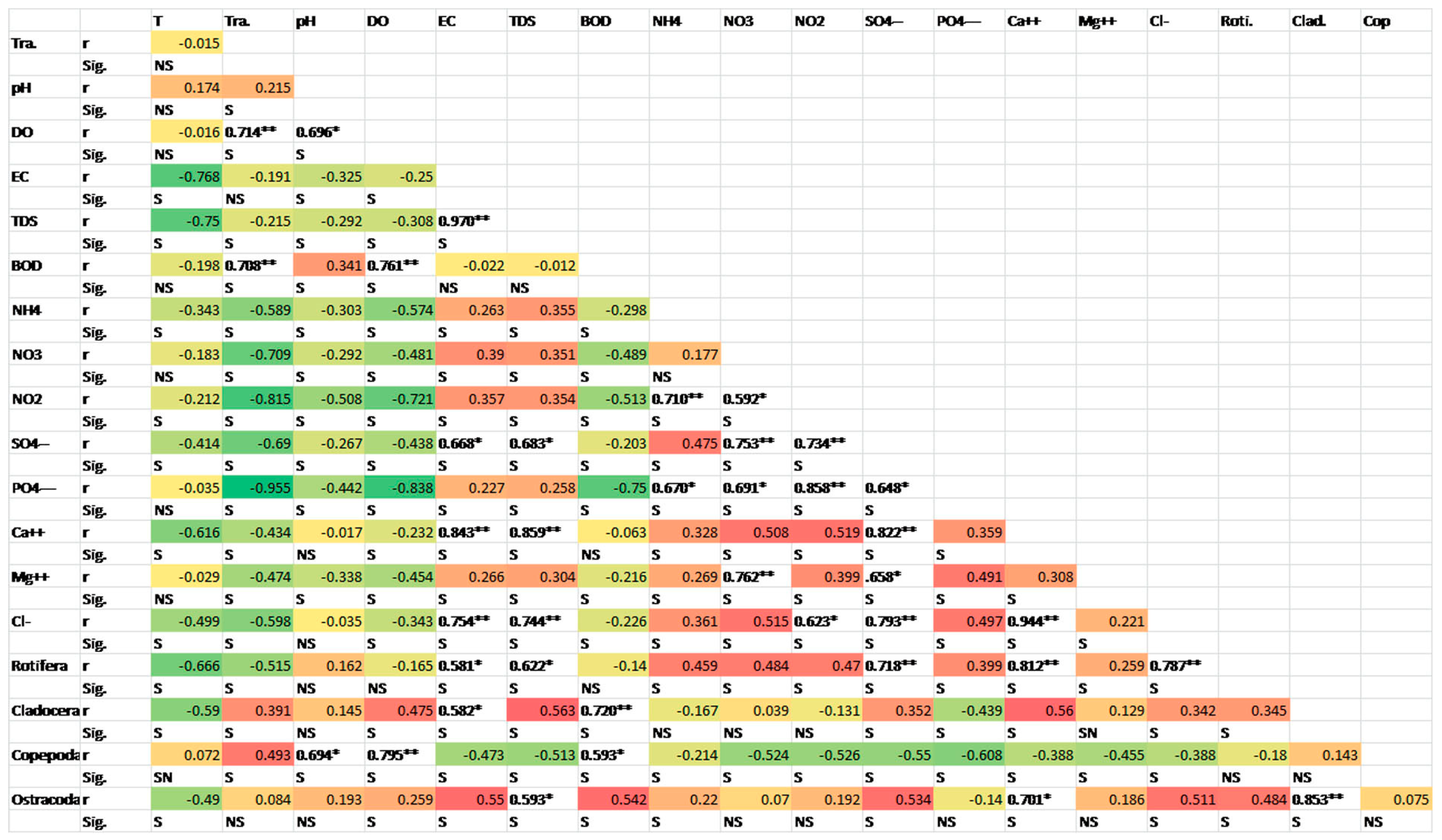
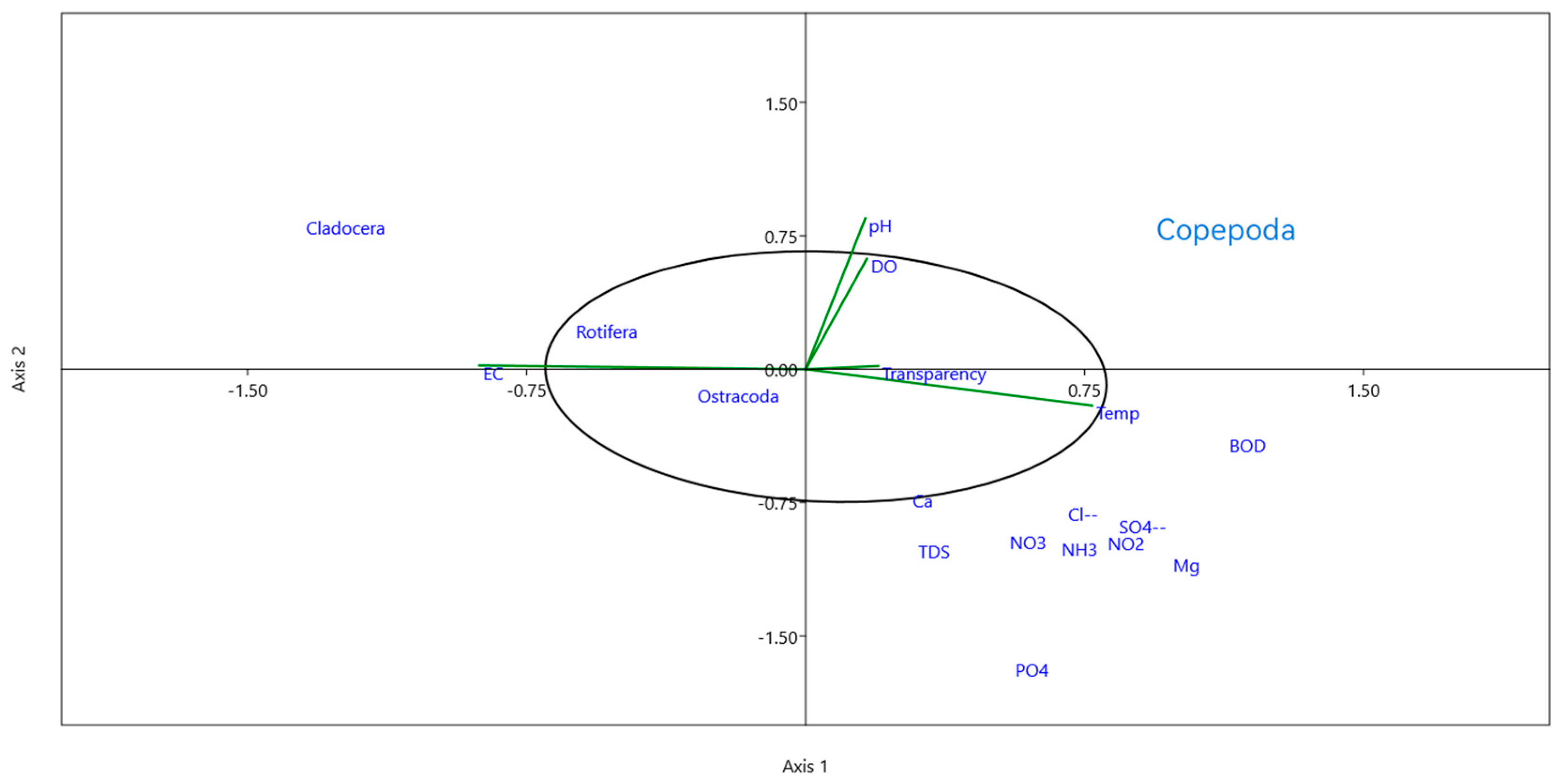
3.3. Correlation between Different Water Parameters and Zooplankton Groups
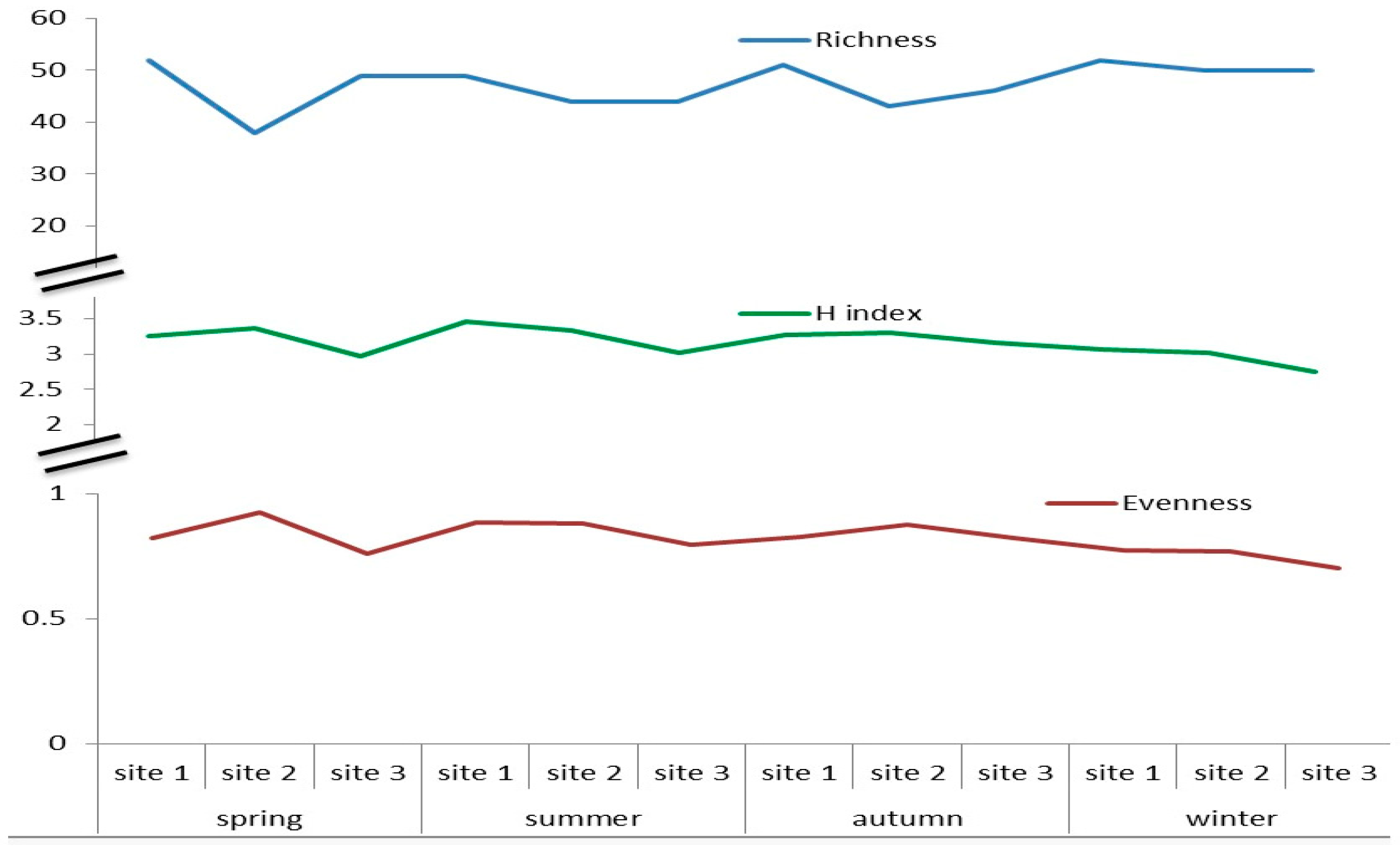
3.4. Shannon–Weaver Diversity Indexes, Richness and Evenness
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ali, E.M.; Shabaan-Dessouki, S.A.; Soliman, A.I.; El Shenawy, A.S. Characterization of chemical water quality in the Nile River. Egypt. Int. J. Pure Appl. Biosci. 2014, 2, 35–53. [Google Scholar]
- Dhote, S.; Dixit, S. Water quality improvement through macrophytes—A review. Environ. Monit. Assess. 2009, 152, 149–153. [Google Scholar] [CrossRef]
- El-Sherbini, A.; El-Moatassem, M. River Nile water quality monitoring. In Proceedings of the National Seminar on Physical Response of the River Nile to Interventions, Cairo, Egypt, 12–13 November 1990. [Google Scholar]
- Ezzat, M.N.; Shehab, H.; Hassan, A.A.; El Sharkawy, M.; El Diasty, A.; El Assiouty, I.; El-Gohary, F.; Tczap, A. Survey of Nile System pollution Sources. Report No 64, for United States Agency for International Development/Egypt. 2002. Available online: https://pdf.usaid.gov/pdf_docs/Pnacr168.pdf (accessed on 3 September 2002).
- Hassan, M.M. Ecological Studies on Zooplankton and Macrobenthos of Lake Edku, Egypt. Ph.D. Thesis, Ain Shams University, Faculty of Science, Zoology Department, Cairo, Egypt, 2008. [Google Scholar]
- Rocha, O.; Matsumura-Tundisi, T.; Espindola, E.L.G.; Roche, K.F.; Rietzler, A.C. Ecological theory applied to reservoir zooplankton. In Theoretical Reservoir Ecology and Its Applications; Tundisi, J.G., Straskraba, M., Eds.; International Institute of Ecology, Brazilian Academy of Sciences: Rio de Janeiro, Brazil; Backhuys Publishers: Leiden, Holland, 1999; pp. 29–51. [Google Scholar]
- Arthington, A.H.; Naiman, R.J.; McClain, M.E.; Nilsson, C. Preserving the biodiversity and ecological services of rivers: New challenges and research opportunities. Freshw. Biol. 2015, 5, 1–16. [Google Scholar] [CrossRef]
- Sameoto, D.; Wiebe, P.; Runge, J.; Postel, L.; Dunn, J.; Miller, C.; Coombs, S. Collecting zooplankton. In ICES Zooplankton Methodology Manual; Harris, R.P., Wiebe, P.H., Lenz, J., Skjoldal, H.R., Huntley, M., Eds.; Academic Press: London, UK, 2000; pp. 55–78. [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewaters Analysis, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Edmonson, W.T. Freshwater Biology; John Wiley and Sons: New York, NY, USA, 1959. [Google Scholar]
- Newell, G.E.; Newell, R.C. Marine Plankton, a Practical Guide; Hutchinson: London, UK, 1963; 207p. [Google Scholar]
- Kuczynski, D. Rotifers from Reconquista River, Argentina: The genus Brachionus with descriptions of new species. Hydrobiologia 1991, 215, 135–152. [Google Scholar] [CrossRef]
- Pennek, W.R. Freshwater Investigation of the United States, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1978; 803p. [Google Scholar]
- Segers, H. Rotifera of some lakes in the floodplain of the river Niger (Imo State, Nigeria). I. New species and other taxonomic considerations. Hydrobiologia 1993, 250, 39–61. [Google Scholar] [CrossRef]
- Santhanam, R.; Srinivasan, A.A. Manual of Marine Zooplankton; Oxford and IBH publishing Co. pvt. Ltd.: Patpar Ganj, Delhi, 1994. [Google Scholar]
- Segers, H.; Dumont, H.J. Rotifera from Arabia, with descriptions of two new species. Fauna Saudi Arab. 1993, 13, 3–26. [Google Scholar]
- Obuid-Allah, A.H. A Review Article on: Diversity of Cladocera and Copepoda in the Freshwater Bodies of Egypt and Factors Affecting It; Zoology & Entomology Department, Faculty of Science, Assiut University: Assiut, Egypt, 2001. [Google Scholar]
- Marshall, S.M. Protozoa. In Fiches D’identification du Zooplancton; de la Mer, J.H.F., Ed.; Charlottenlund Slot: Charlottenlund, Danemark, 1969. [Google Scholar]
- Yousef, E.A. Taxonomy and description of the female Candonocypris novaezelandiae (Baird, 1843) (Crustacea Ostracoda) from River Nile, Sohag Governorate, Egypt. Egypt. Acad. J. Biolog. Sci. 2010, 2, 61–69. [Google Scholar] [CrossRef][Green Version]
- Yousef, E.A.; Hegab, M.H. Evaluation of Two Novel Feeding Protocols Utilizing Alive and Dried Chlorella vulgaris to Grow Heterocypris salina (Ostracoda: Crustacea). J. Zool. Res. 2017, 1, 10–15. [Google Scholar] [CrossRef]
- Shannon, C.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Ezzat, S.M.; Water, N.; Fouda, A.; Gamal, S. Assessment of some drinking water purification plants efficiency at Great Cairo in Egypt A. Curr. Sci. Int. 2018, 6, 761–776. [Google Scholar]
- Rashad, S.; Abdul Moneem, M.; AEl-Chaghaby, G.; FAbdel-Kader, S. Monitoring water quality and plankton distribution in the River Nile around El-Maadi area in Egypt during 2018. Egypt. J. Aquat. Biol. Fish. 2019, 23, 81–87. [Google Scholar] [CrossRef]
- Uttah, E.; Uttah, C.; Akpa, P.; Ikpeme, E.; Ogbeche, J.; Usip, L.; Asor, J. Bio-survey of Plankton as indicators of water quality for recreational activities in Calabar River, Nigeria. J Appl Sci. Environ. Manag. 2010, 12, 35–42. [Google Scholar] [CrossRef]
- Delince, G. The Ecology of the Fish Pond Ecosystem with Special Reference to Africa; Text Book; Kluwer Academic Publishers: London, UK, 1992; 230p. [Google Scholar]
- El-Serafy, S.S.; Mageed, A.A.; El-Enany, H.R. Impact of flood on the distribution of zooplankton in lake Nasse khors-Egypt. J. Egypt. Acad. Environ. Develop. 2009, 10, 121–141. [Google Scholar]
- Abdel-Satar, A.M.; Ali, M.H.H.; Goher, M.E. Indices of water quality and metal pollution of Nile River Egypt. Egypt. J. Aquat. Res. 2017, 43, 21–29. [Google Scholar] [CrossRef]
- Mola, H.R.A.; Parveen, S. Effect of monsoon on zooplankton variations and environmental characteristics in a tropical fish pond at Aligarh, India. Egypt. J. Aquat. Biol. Fish. 2014, 18, 105–114. [Google Scholar]
- El-Enany, H.R. Ecological Studies on Lake Manzalah with Special References to Their Water Quality and Sediment Productivity. Master’s Thesis, Zoology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt, 2004. [Google Scholar]
- Yadav, S.S.; Kumar, R. Monitoring water quality of Kosi River in Rampur district, Uttar Pradesh, India. Adv. Appl. Sci. Res. 2011, 2, 197–201. [Google Scholar]
- Mola, H.R.; Shehata, M.A. Effect of drains on the distribution of zooplankton at the southeastern part of Lake Manzala, Egypt. Egypt. J. Aquat. Biol. Fish. 2012, 16, 57–68. [Google Scholar] [CrossRef]
- Abdel-Satar, A.M. Water quality assessment of River Nile from Idfo to Cairo. Egypt. J. Aquat. Res. 2005, 31, 200–223. [Google Scholar]
- Van Dijk, G.M.; Van Zanten, B. Seasonal changes in zooplankton abundance in the Lower Rhine during 1987–1991. Hydrobiologia 1995, 52, 29–38. [Google Scholar] [CrossRef]
- Galkovskaja, G.A. Planktonic rotifers and temperature. Hydrobiologia 1987, 147, 307–317. [Google Scholar] [CrossRef]
- Ramadan, S.E.; El-Serehy, H.A.; Farghaly, M.E.; El-Bassat, R.A. Zooplankton community in the Nile River, Egypt. Bull. Nat. Inst. Oceanogr. Fish. 1998, 24, 139–159. [Google Scholar]
- Mageed, A.A. Distribution and long term Historical changes of zooplankton assemblages in lake Manzala (South Mediterranean Sea, Egypt). Egypt. J. Aquat. Res. 2007, 33, 183–192. [Google Scholar]
- Scheinin, M.; Scyphers, S.B.; Kauppi, L.; Heck, K.L., Jr.; Mattila, J. The relationship between vegetation density and its protective value depends on the densities and traits of prey and predators. Oikos 2012, 121, 1093–1102. [Google Scholar] [CrossRef]
- Ahmed, U.; Parveen, S.; Khan, A.A.; Kabir, H.A.; Mola, H.R.A.; Ganai, A.H. Zooplankton population in relation to physicochemical factors of sewage fed Pond of Aligarh (UP), India. Biol. Med. 2011, 3, 336–341. [Google Scholar]
- Emam, W. Preliminary Study on the Impact of Water Pollution in El-Rahawy Drain Dumping in Rosetta Nile Branch on Zooplankton and Benthic Invertebrates. Master’s Thesis, Zoology Department, Faculty of Science, Ain Shams University, Cairo, Egypt, 2006. [Google Scholar]
- Bedair, S.M.E. Environmental Studies on Zooplankton and Phytoplankton in Some Polluted Areas of the River Nile and Their Relation with the Feeding Habit of Fish. Ph.D. Thesis, Faculty of Science, Zagazig University, Zagazig, Egypt, 2003. [Google Scholar]
- El-Bassat, R.A. Ecological Studies on Zooplankton Communities with Particular References to Free Living Protozoa at River Nile—Egypt. Ph.D. Thesis, Zoology Department, Faculty of Science, Ain Shams University, Cairo, Egypt, 2002; 116p. [Google Scholar]
- Abdelmageed, A.A.; Ellah, R.G.A.; Abdel-Satar, A.M.; Gawad, S.S.; Khalifa, N.; Zaher, S.S.; Othman, A.A.; Belal, D.M.; El-Hady, H.H.; Salem, S.G.; et al. Evaluation of the ecological health and food chain on the shores of four River Nile Islands, Egypt. Environ. Monit. Assess. 2022, 194, 309–325. [Google Scholar] [CrossRef]
- Mola, H.R.A. Seasonal and spatial distribution of Brachionus (Pallas, 1966; Eurotatoria: Monogonanta: Brachionidae), a bioindicator of Eutrophication in lake El-Manzalah, Egypt. Biol. Med. 2011, 3, 60–69. [Google Scholar]
- Sharma, K.K.; Kour, S.; Antal, N. Diversity of zooplankton and macrobenthic invertebrates of two perennial ponds in Jammu Region. J. Glob. Biosc. 2015, 4, 1383–1393. [Google Scholar]
- Sladecek, V. Rotifers as indicators of water quality. Hydrobiologia 1983, 100, 169–201. [Google Scholar] [CrossRef]
- El-Shabrawy, G.M.; El-Feqy, F.A.; Mahmoud, N.H.; Gaber, K.M. Comparative analysis of rotifer community in two Rayahs of River Nile, Egypt. J. Egypt. Acad. Soc. Environ. Dev. 2017, 18, 147–159. [Google Scholar]
- Mola, H.R.A.; Ahmed, N.A.M. Zooplankton Community Structure and Diversity Relative to Environmental Variables in the River Nile from Helwan to El-Qanater El-Khayria, Egypt. Int. J. Environ. 2015, 4, 140–150. [Google Scholar]
- Mola, H.R.A.; Parveen, S.; Ganai, A.H.; Kabir, H.A.; Ahmad, U. Longitudinal distribution of zooplankton in the river Nile, Egypt. J. Curr. Sci. 2011, 16, 33–45. [Google Scholar]
- Obuid-Allah, A.H.; Moustafa, S.A.; Hussien, E.; Gaber, M.A.; Mohammad, A.W. Distribution of Zooplankton Community in the River Nile at Esna Barrages, Egypt in relation to some ecological factors. Egypt. J. Aquat. Biol. Fish. 2020, 24, 131–143. [Google Scholar] [CrossRef]
- Pandey, B.N.; Ambasta, O.P.; Thakur, A.K.; Kumar, S.; Kumari, R. Zooplankton diversity in relation to certain physic-chemical parameters of swamp of Kishanganj, Bihar. Environ. Conserv. J. 2009, 10, 9–14. [Google Scholar] [CrossRef]
- El-Enany, H.R. Ecological Studies on Planktonic and Epiphytic Micro-Invertebrates in Lake Nasser, Egypt. Ph.D. Thesis, Zoology Department, Faculty of Science, Benha University, Banha, Egypt, 2009; 311p. [Google Scholar]
- Smol, N.K.; Awillems, J.C.R.; Govaere, J.C.; Sandee, A.J.J. Composition, distribution and biomass of meiobenthos in the Oasterschelde estuary (SW Netherlands). Hydrobiology 1994, 197–217. [Google Scholar] [CrossRef]
- Ramadan, S.E.; Fishar, M.R.; Hassaa, S.H.; Ebeid, A.A.; Abdel Gawad, S.S. Benthic Communities in the River Nile, Egypt III- Meiofauna at Helwan Region. Egypt. J. Aquat. Biol. Fish. 2002, 6, 95–113. [Google Scholar] [CrossRef]
- Stahl, R.; Ramadan, A.B. Environmental Studies on WaterQuality of the Ismailia Canal, Egypt. In Fors-chungszentrum Karlsruhe in der Helmholtz-Gemeinschaft Wis-Senschaftliche Berichte Fzka 7427; Scientific Report; FZKA: Karlsruhe, Germany, 2008; p. 58. [Google Scholar]
- Sharma, A.; Sharma, M. Zooplankton Diversity in Relation to PhysicoChemical Parameters in Subtropical Pond of Jammu, Jammu and Kashmir, India. Biosci. Biotechnol. Res. Asia 2019, 16, 425–439. [Google Scholar] [CrossRef]
- El-Shabrawy, G.M.; Dumont, H.J. Spatial and seasonal variation of the zooplankton in the coastal zone and main khors of Lake Nasser (Egypt). Hydrobiologia 2003, 491, 119–132. [Google Scholar] [CrossRef]
- Mohammad, W.A.; Obuid-Allah, A.H.; Moustafa, A.S.; Gaber, A.M. Seasonal variations in the abundance and diversity of zooplankton community inhabiting River Nile and its branches at Qena governorate, Upper Egypt. Egypt. J. Aquat. Biol. Fish. 2021, 25, 445. [Google Scholar]
- Meshram, D.; Catherine, D.; Badhe, N.; Khedkar, S.; Vijay, R.; Nandy, T. Zooplankton diversity as indicators of pollution in warm monomictic Dal–Nigeen lake. Sustain. Water Resour. Manag. 2018, 4, 897–904. [Google Scholar] [CrossRef]
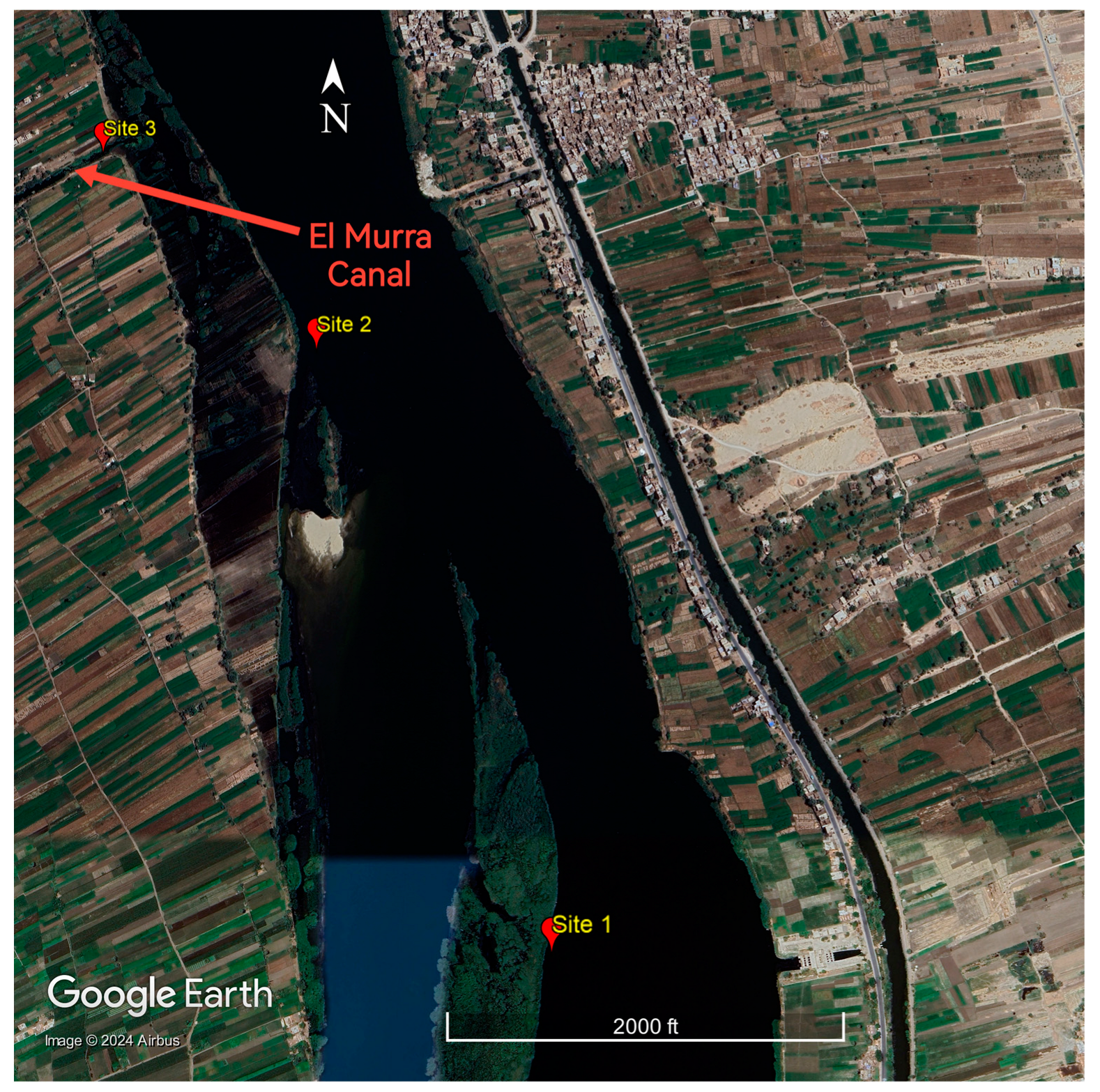
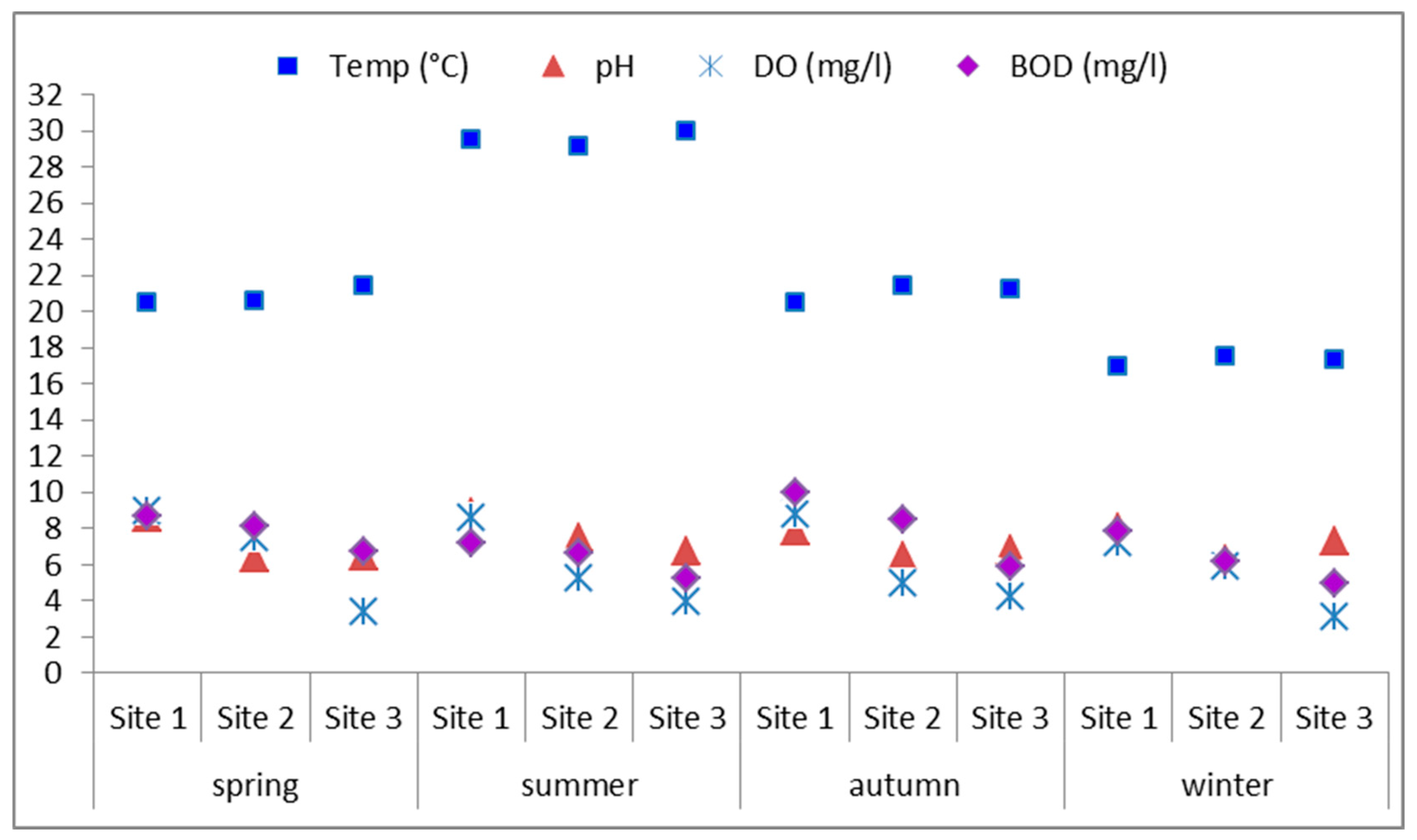
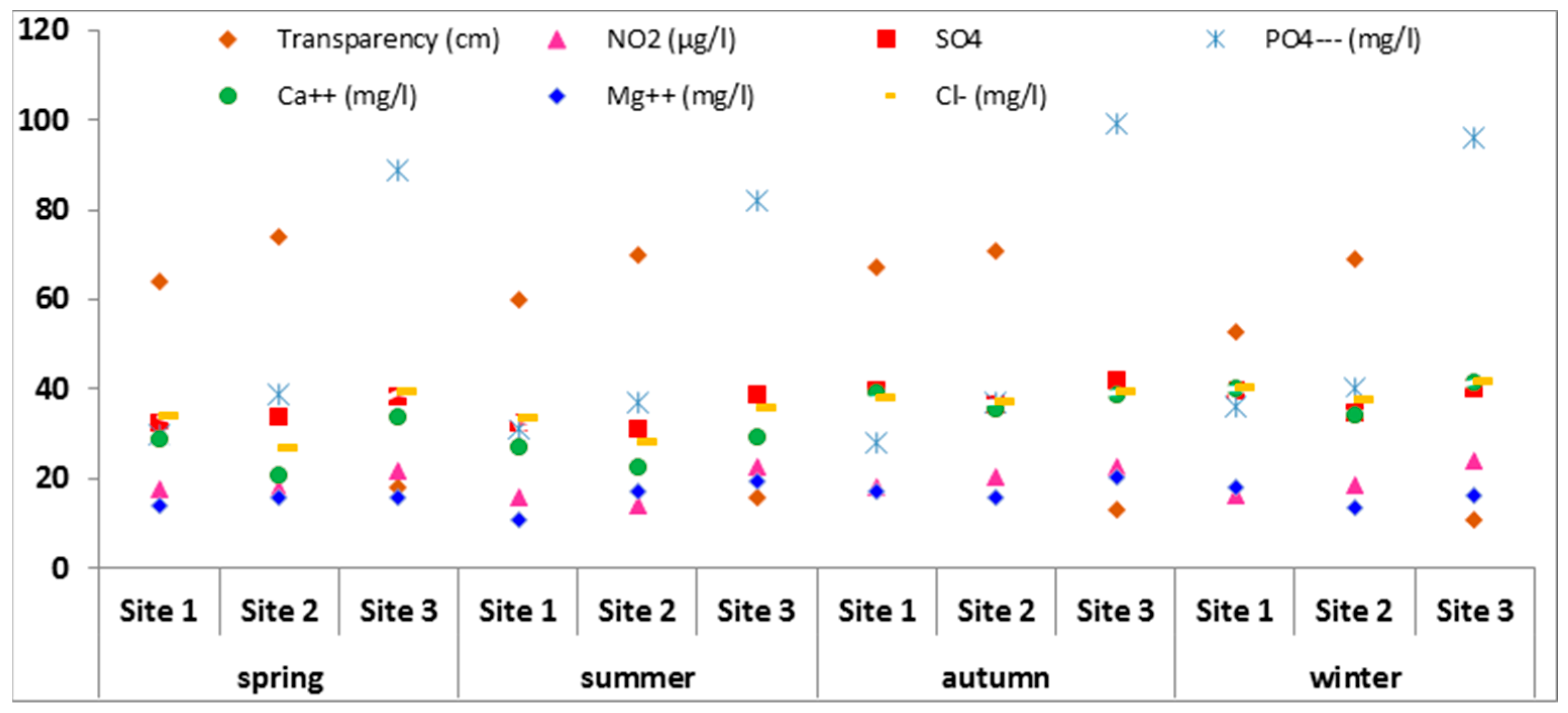
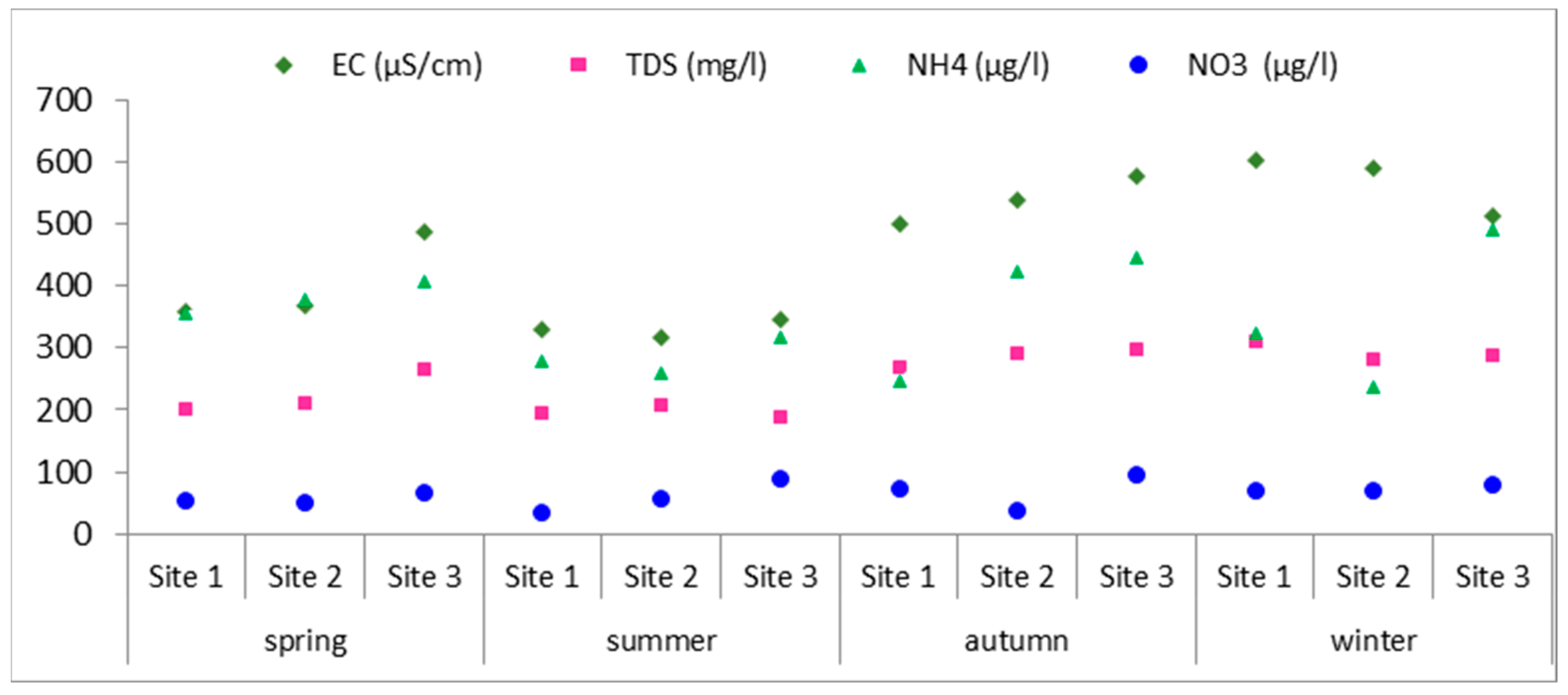
| Season | Spring | Summer | Autumn | Winter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | Site 3 |
| Temp (°C) | 20.45 | 20.6 | 21.4 | 29.5 | 29.1 | 30 | 20.5 | 21.4 | 21.2 | 17 | 17.5 | 17.3 |
| Transparency (cm) | 64 | 74 | 18 | 60 | 70 | 16 | 67 | 71 | 13 | 53 | 69 | 11 |
| pH | 8.62 | 6.38 | 6.45 | 8.89 | 7.45 | 6.71 | 7.87 | 6.47 | 6.83 | 8.05 | 6.30 | 7.29 |
| DO (mg/L) | 8.93 | 7.52 | 3.36 | 8.60 | 5.23 | 3.94 | 8.81 | 4.95 | 4.19 | 7.19 | 5.85 | 3.09 |
| EC (μS/cm) | 359.7 | 367.5 | 488.2 | 331.5 | 317.8 | 347.4 | 499 | 540 | 578 | 603 | 590 | 512 |
| TDS (mg/L) | 200 | 210 | 266 | 195 | 207 | 190 | 270 | 290.5 | 297.5 | 310 | 282.5 | 289 |
| BOD (mg/L) | 8.66 | 8.12 | 6.75 | 7.15 | 6.67 | 5.22 | 9.96 | 8.48 | 5.92 | 7.81 | 6.18 | 4.99 |
| NO3 (μg/L) | 53.6 | 49.9 | 67.6 | 33.8 | 57.8 | 88.2 | 74.1 | 37.9 | 94.7 | 70.4 | 69.2 | 78.3 |
| NO2 (μg/L) | 17.5 | 17.7 | 21.6 | 15.9 | 14.3 | 22.7 | 18.3 | 20.4 | 22.6 | 16.4 | 18.4 | 23.9 |
| NH4 (μg/L) | 354.6 | 378.4 | 405.7 | 278.3 | 259.6 | 318.2 | 247.3 | 422.5 | 447.1 | 322.7 | 238.1 | 491.4 |
| SO42− (mg/L) | 32.75 | 34.05 | 38.45 | 32.35 | 31.21 | 38.67 | 39.96 | 36.51 | 41.94 | 39.53 | 34.73 | 40.33 |
| PO42− (μg/L) | 30 | 39 | 89 | 31 | 37 | 82 | 28 | 37 | 99 | 36 | 40 | 96 |
| Ca2+ (mg/L) | 28.8 | 20.9 | 33.8 | 27.25 | 22.5 | 29.4 | 39.5 | 35.6 | 38.9 | 40.2 | 34.5 | 41.6 |
| Mg2+ (mg/L) | 14.3 | 15.9 | 15.7 | 11.05 | 17.4 | 19.3 | 17.2 | 15.7 | 20.2 | 18.3 | 13.7 | 16.4 |
| Cl− (mg/L) | 34 | 26.7 | 39.5 | 33.6 | 28 | 35.9 | 38 | 37 | 39.3 | 40 | 37.3 | 41.6 |
| Spring | Summer | Autumn | Winter | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa | Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | Site 3 | |
| Rotifera | |||||||||||||
| Anuraeopsis fissa | 48 | 27 | 64 | 39 | 12 | 43 | 34 | 22 | 51 | 28 | 15 | 62 | 445 |
| Brachionus angularis | 100 | 49 | 171 | 78 | 30 | 93 | 158 | 90 | 189 | 266 | 99 | 287 | 1610 |
| Brachionus calyciflorus | 93 | 48 | 100 | 61 | 24 | 71 | 272 | 89 | 162 | 274 | 160 | 298 | 1652 |
| Brachionus caudatus | 25 | 19 | 37 | 18 | 8 | 45 | 16 | 7 | 39 | 9 | 8 | 44 | 275 |
| Brachionus falcatus | 11 | 8 | 6 | 4 | 0 | 2 | 7 | 4 | 13 | 9 | 0 | 11 | 75 |
| Brachionus plicatilis | 113 | 78 | 108 | 52 | 17 | 89 | 103 | 110 | 77 | 216 | 140 | 269 | 1372 |
| Brachionus qudridentatus | 19 | 9 | 22 | 6 | 3 | 6 | 2 | 0 | 6 | 11 | 3 | 15 | 102 |
| Brachionus rubens | 21 | 6 | 8 | 9 | 2 | 13 | 7 | 0 | 14 | 15 | 2 | 9 | 106 |
| Brachionus urceolaris | 14 | 4 | 6 | 2 | 0 | 6 | 5 | 1 | 8 | 0 | 4 | 11 | 61 |
| Brachionus sp. | 22 | 15 | 2 | 4 | 0 | 8 | 6 | 2 | 8 | 6 | 0 | 9 | 82 |
| Cephalodella gibba | 8 | 4 | 13 | 4 | 1 | 20 | 4 | 1 | 33 | 12 | 8 | 40 | 148 |
| Collotheca ornata | 5 | 0 | 13 | 15 | 4 | 3 | 9 | 0 | 11 | 3 | 5 | 12 | 80 |
| Conochilus unicornis | 3 | 0 | 2 | 4 | 2 | 12 | 11 | 5 | 4 | 10 | 2 | 5 | 60 |
| Filinia longiseta | 9 | 11 | 9 | 6 | 2 | 3 | 10 | 7 | 14 | 5 | 4 | 11 | 91 |
| Keratella cochlearis | 98 | 82 | 115 | 71 | 20 | 78 | 105 | 81 | 97 | 211 | 69 | 214 | 1241 |
| Keratella tropica | 164 | 51 | 99 | 52 | 14 | 79 | 136 | 79 | 80 | 216 | 60 | 218 | 1248 |
| Philodina roseola | 1 | 0 | 3 | 3 | 5 | 2 | 7 | 0 | 8 | 5 | 3 | 2 | 39 |
| Polyarthra euryptera | 7 | 0 | 15 | 7 | 0 | 12 | 9 | 0 | 11 | 7 | 0 | 19 | 87 |
| Cladocera | |||||||||||||
| Alona intermedia | 82 | 26 | 45 | 12 | 0 | 18 | 124 | 110 | 93 | 89 | 46 | 52 | 697 |
| Alona rectangula | 62 | 22 | 35 | 7 | 8 | 4 | 54 | 25 | 16 | 24 | 15 | 19 | 291 |
| Bosmina longirostris | 22 | 2 | 12 | 13 | 10 | 8 | 47 | 37 | 26 | 63 | 5 | 27 | 272 |
| Ceriodaphnia reticulata | 94 | 56 | 74 | 18 | 9 | 12 | 112 | 57 | 94 | 104 | 37 | 100 | 767 |
| Chydorus sphaericus | 4 | 0 | 2 | 0 | 3 | 2 | 6 | 0 | 3 | 3 | 0 | 1 | 24 |
| Daphnia longispina | 2 | 0 | 8 | 3 | 0 | 6 | 3 | 2 | 0 | 3 | 0 | 4 | 31 |
| Diaphanosoma excisum | 53 | 20 | 32 | 16 | 4 | 12 | 193 | 53 | 102 | 167 | 44 | 83 | 779 |
| Macrothrix laticornis | 7 | 3 | 0 | 4 | 0 | 0 | 67 | 16 | 39 | 19 | 8 | 14 | 177 |
| Moina micrura | 16 | 1 | 10 | 7 | 5 | 9 | 69 | 6 | 26 | 18 | 5 | 10 | 182 |
| Pleuroxus aduncus | 10 | 0 | 6 | 4 | 1 | 0 | 8 | 13 | 11 | 15 | 16 | 10 | 94 |
| Pleuroxus letourneuxi | 0 | 4 | 3 | 2 | 0 | 0 | 16 | 0 | 12 | 4 | 0 | 0 | 41 |
| Simocephalus expinosus | 5 | 0 | 2 | 11 | 6 | 3 | 28 | 19 | 59 | 26 | 18 | 19 | 196 |
| Copepoda | |||||||||||||
| Afrocyclops gibsoni | 17 | 0 | 0 | 23 | 5 | 15 | 0 | 6 | 8 | 11 | 2 | 0 | 87 |
| Eucyclops serrulatus | 37 | 0 | 20 | 16 | 0 | 0 | 7 | 0 | 0 | 6 | 5 | 0 | 91 |
| Macrocyclops albidus | 40 | 6 | 5 | 8 | 18 | 13 | 12 | 6 | 22 | 21 | 0 | 7 | 158 |
| Mesocyclops ogunnus | 17 | 12 | 12 | 13 | 33 | 11 | 26 | 7 | 18 | 8 | 4 | 4 | 165 |
| Microcylops linjanticus | 61 | 0 | 27 | 58 | 4 | 0 | 19 | 0 | 0 | 13 | 3 | 7 | 192 |
| Paracyclops fimbriatus | 47 | 7 | 0 | 14 | 15 | 5 | 9 | 6 | 8 | 21 | 6 | 5 | 143 |
| Shizopera nilotica | 55 | 0 | 38 | 12 | 0 | 0 | 27 | 12 | 10 | 14 | 0 | 9 | 177 |
| Thermodiaptomus galebi | 54 | 10 | 0 | 20 | 6 | 7 | 18 | 6 | 11 | 17 | 2 | 11 | 162 |
| Thermocyclops consimilis | 69 | 6 | 26 | 15 | 1 | 25 | 11 | 9 | 10 | 29 | 8 | 8 | 217 |
| Tropocyclops confinis | 25 | 8 | 15 | 34 | 0 | 12 | 0 | 0 | 6 | 5 | 0 | 4 | 109 |
| Copepodite stage | 63 | 11 | 31 | 28 | 2 | 6 | 27 | 5 | 16 | 14 | 1 | 4 | 208 |
| Nauplius stage | 88 | 16 | 57 | 73 | 0 | 48 | 42 | 18 | 34 | 24 | 0 | 10 | 410 |
| Ostracoda | |||||||||||||
| Candona neglecta | 4 | 4 | 12 | 6 | 3 | 2 | 11 | 10 | 7 | 14 | 7 | 9 | 89 |
| Candonocypris novaezelandiae | 1 | 2 | 30 | 5 | 7 | 37 | 4 | 10 | 102 | 4 | 5 | 78 | 285 |
| Cyprideis torosa | 11 | 0 | 7 | 0 | 5 | 0 | 64 | 32 | 12 | 4 | 7 | 5 | 147 |
| Cypridopsis vidua | 27 | 6 | 9 | 23 | 7 | 9 | 28 | 36 | 6 | 49 | 21 | 9 | 230 |
| Cyprideis littoralis | 24 | 4 | 17 | 15 | 6 | 3 | 15 | 18 | 2 | 11 | 6 | 9 | 130 |
| Gomphocythere sp. | 3 | 0 | 4 | 0 | 1 | 5 | 12 | 8 | 4 | 9 | 1 | 0 | 47 |
| Heterocypris salina | 14 | 7 | 12 | 13 | 16 | 0 | 46 | 29 | 7 | 24 | 18 | 4 | 190 |
| Heterocypris giesbrechtii | 8 | 5 | 6 | 0 | 0 | 2 | 27 | 4 | 0 | 9 | 5 | 8 | 74 |
| Limnocythere inopinata | 17 | 0 | 2 | 12 | 0 | 0 | 17 | 22 | 8 | 16 | 8 | 11 | 113 |
| Potamocypris variegata | 9 | 1 | 6 | 4 | 0 | 2 | 0 | 23 | 0 | 5 | 0 | 3 | 53 |
| Sclerocypris bicornis | 11 | 3 | 4 | 15 | 0 | 7 | 4 | 6 | 9 | 9 | 4 | 7 | 79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, E.A.; El-Mallah, A.M.; Abdel-Baki, A.-A.S.; Al-Quraishy, S.; Reyad, A.; Abdel-Tawab, H. Effect of Environmental Variables on Zooplankton in Various Habitats of the Nile River. Water 2024, 16, 915. https://doi.org/10.3390/w16070915
Yousef EA, El-Mallah AM, Abdel-Baki A-AS, Al-Quraishy S, Reyad A, Abdel-Tawab H. Effect of Environmental Variables on Zooplankton in Various Habitats of the Nile River. Water. 2024; 16(7):915. https://doi.org/10.3390/w16070915
Chicago/Turabian StyleYousef, Ebtesam A., Almahy M. El-Mallah, Abdel-Azeem S. Abdel-Baki, Saleh Al-Quraishy, Abdulrahman Reyad, and Heba Abdel-Tawab. 2024. "Effect of Environmental Variables on Zooplankton in Various Habitats of the Nile River" Water 16, no. 7: 915. https://doi.org/10.3390/w16070915
APA StyleYousef, E. A., El-Mallah, A. M., Abdel-Baki, A.-A. S., Al-Quraishy, S., Reyad, A., & Abdel-Tawab, H. (2024). Effect of Environmental Variables on Zooplankton in Various Habitats of the Nile River. Water, 16(7), 915. https://doi.org/10.3390/w16070915






