Degradation of Water Pollutants by Biochar Combined with Advanced Oxidation: A Systematic Review
Abstract
1. Introduction
| Method | Free Radical | Mechanism | Reference | |
|---|---|---|---|---|
| Photocatalysis | •OH, •O2−, 1O2 | Photocatalyst + UV/vis → h+ + e− | (1) | [10] |
| h+ + H2O → •OH + H+ | (2) | |||
| e− + O2 → •O2− | (3) | |||
| •O2− + H+ + e− → H2O2 | (4) | |||
| H2O2 + e− → •OH + OH− | (5) | |||
| Fenton process | •OH | Fe2+ + H2O2 → Fe3+ + •OH + OH− | (6) | [2] |
| Fe3+ + H2O2 → Fe2+ + OOH• + H+ | (7) | |||
| Fe2+ + •OH → Fe3+ + OH− | (8) | |||
| •OH + •OH → H2O2 | (9) | |||
| H2O2 + •OH → H2O + OOH• | (10) | |||
| •OH + OOH• → H2O + O2 | (11) | |||
| Mn+ + H2O2 → M(n+1)+ + OH− + •OH | (12) | |||
| Fe(OH)2+ + hv → Fe2+ + •OH | (13) | |||
| e− + Fe3+ → Fe2+ | (14) | |||
| h+ + H2O → H+ + •OH | (2) | |||
| O2 + 2H+ + 2e− → H2O2 | (15) | |||
| H2O2 + e− → •OH + OH− | (5) | |||
| Electrocatalysis | •OH, 1O2, SO4−• | C–O + PMS → C=O + SO4−• | (16) | [11,12,13] |
| C=O + e− → C–O | (17) | |||
| SO4−• + H2O → •OH + H+ + SO42− | (18) | |||
| SO4−• + H2O → 1O2 + H+ + SO42− | (19) | |||
| Ozonation | •OH, | 3O3 + OH− + H+ → •OH + 4O2 | (20) | [14] |
| Ultrasonic | •OH | ))) + H2O → •OH + •H | (21) | [15,16,17] |
| •OH + •H → H2O | (22) | |||
| •OH + •OH → H2O2 | (9) | |||
| •H + •H → H2 | (23) | |||
| ))) + O2 → O + O | (24) | |||
| O + H2O → •OH + •OH | (25) | |||
| •H + O2 → •OOH | (26) | |||
| •OOH + •OOH → H2O2 + O2 | (27) | |||
| PS | •OH, •O2−, 1O2, SO4−• | S2O82− + e− + UV/vis → SO4−• + SO42− | (28) | [20,21,22,23,24,25,26,27] |
| S2O82− + •O2− + UV/vis → SO4−• + SO42− + O2 | (29) | |||
| HSO5− + heat → SO4−• + •OH | (30) | |||
| S2O82− + heat → 2SO4−• | (31) | |||
| SO4−• + H2O → SO42−+ •OH + H+ | (32) | |||
| S2O82− + ))) → 2SO4−• | (33) | |||
| S2O82− + •OH → HSO4− + SO4−• + 1/2O2 | (34) | |||
| S2O82− + e− → SO42− + SO4−• | (35) | |||
| S2O82− + H2O → SO42− + HO2− + 2H+ | (36) | |||
| S2O82− + HO2− → SO42− + SO4−• + H+ + •O2− | (37) | |||
| S2O82− + Mn+ → M(n+1)+ + SO4−• + SO42− | (38) | |||
| HSO5− + SO52− → HSO4−+ SO42− + 1O2 | (39) | |||
| S2O82− → 2SO4−• | (40) | |||
| SO4−• + H2O → •HSO42−+ •OH | (41) | |||
| 2•OH → H2O + 1/21O2 | (42) | |||
2. Preparation of Biochar
2.1. Feedstock
2.2. Biochar Preparation Method
2.2.1. Pyrolysis
2.2.2. Co-Precipitation
2.2.3. Hydrothermal
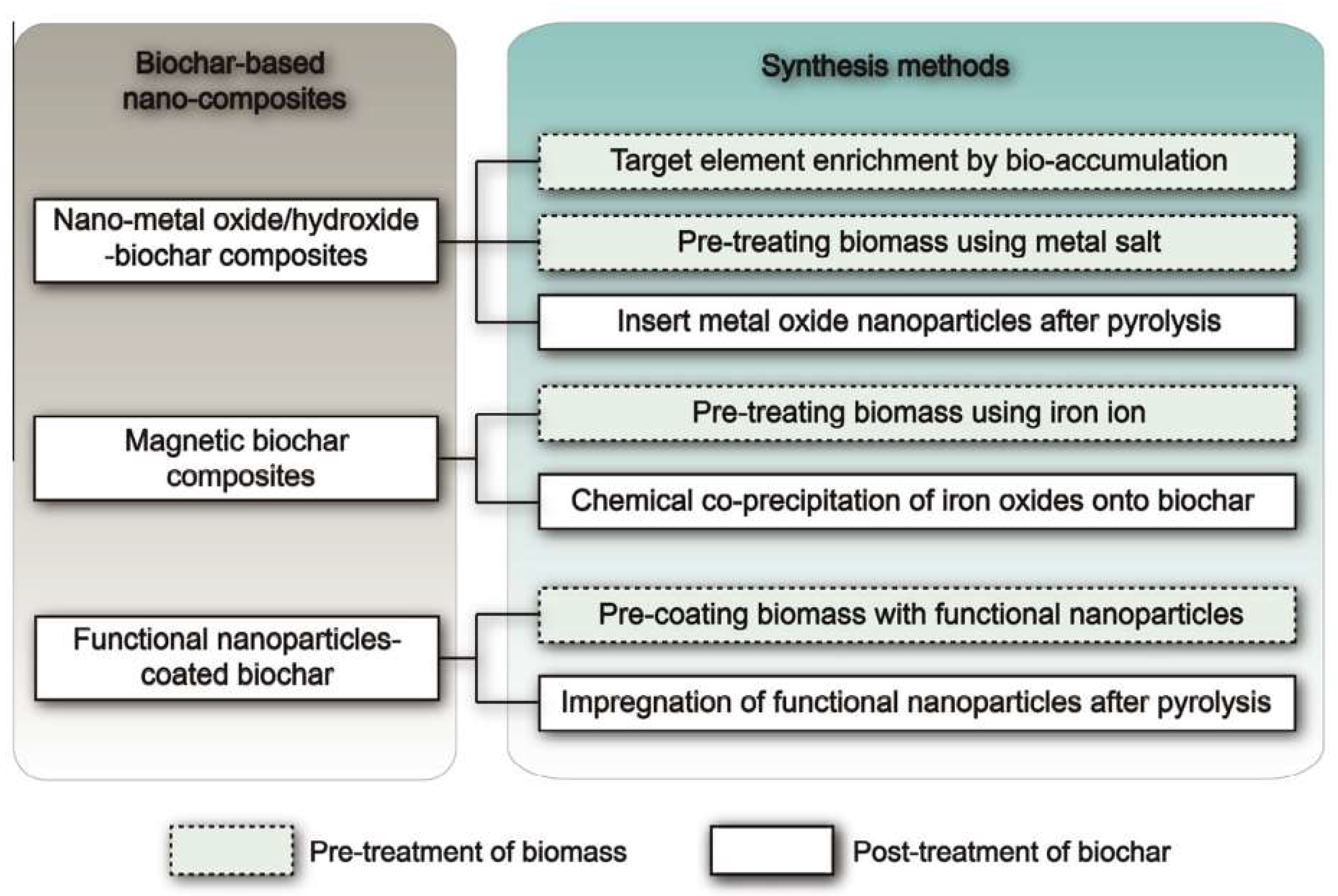
2.3. Modification of Biochar
3. Metals Loading
3.1. Co-Based Catalysts
3.2. Cu-Based Catalysts
3.3. Fe-Based Catalysts
3.4. Mixed-Metal Catalysts
4. Heteroatomic Doping
4.1. Nitrogen Doping
4.2. Sulfur Doping
4.3. Boron Doping
4.4. Other Atoms Doping
4.5. Metal and Non-Metal Based Biochar
5. Catalytic Mechanisms
5.1. Free Radical Pathway
5.1.1. Surface Functional Groups
| Biomass | Pyrolysis Temperature (°C) | N-Functional Groups | Refs. |
|---|---|---|---|
| Chlorella vulgaris | 600–900 | Pyrrolic-N, quaternary-N, pyridinic-N | [209] |
| Phragmites australis | 450 | Pyridinic-N, pyrrolic-N | [142] |
| Nannochloropsis sp. Spirulina platensis Enteromorpha prolifera | 400–800 | Pyridinic-N, pyrrolic-N, quaternary-N | [210] |
| Bamboo | 600 | Pyridinic-N, pyrrolic-N, quaternary-N, | [211] |
| Wheat straw | 300–800 | Pyridinic-N, amine-N, pyrrolic-N, quaternary-N, NH4-N | [212] |
| Corn straw | 600 | Pyridinic-N, pyrrolic/pyridonic-N, graphitic-N, | [213] |
| Corn straw | 600–800 | Pyridinic-N pyrrolic-N pyridonic-N, graphitic-N | [214] |
| Straw | 300–400 | Nitrile-N, pyridinic-N pyrrolic-N, amine-N NH4-N, NO3-N NO2 -N | [215] |
| A Mixture of sewage, cattle manure and eucalyptus wood chips | 250–550 | NH4-N, amine-N | [216] |
5.1.2. Role of PFRs
5.1.3. π-π Bonds and Hydrogen Bonds
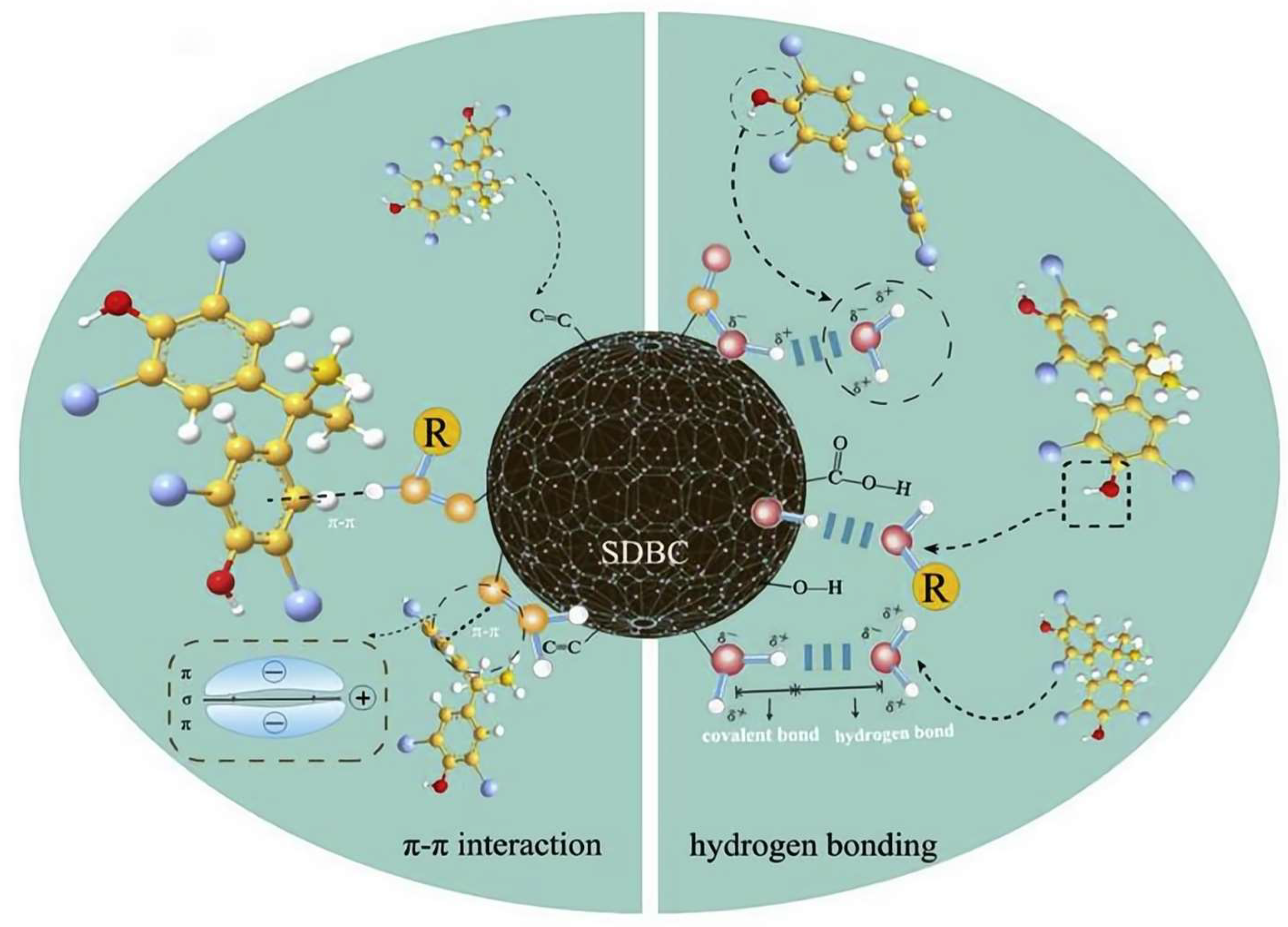
5.1.4. Defect Sites
5.2. Non-Radical Pathways
5.2.1. Electronic Transfer
5.2.2. Single Line State Oxygen
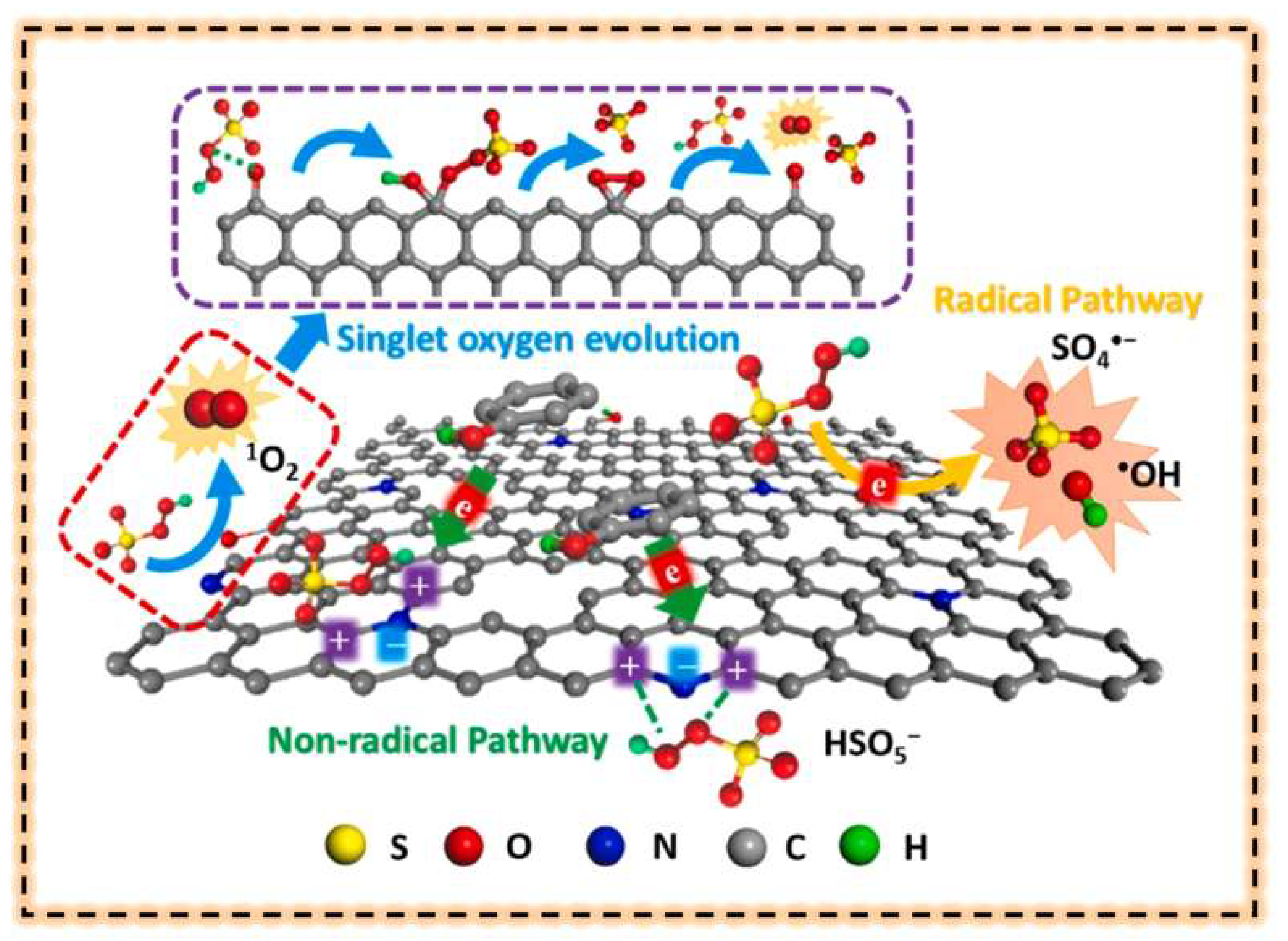
5.2.3. Surface Activation
6. Conclusions
- (1)
- The choice of raw materials for biochar, the process of preparation, and the modification of biochar all have a substantial impact on the structure and characteristics of the material. Pyrolysis stands out as the most frequently employed method in biochar production, and the elevated temperature during this process significantly affects the overall performance of the biochar. Metal-doped catalysts, especially mixed-metal-doped catalysts, have a variety of applications such as a high REDOX activity of persulfate activation, versatility, ferromagnetism, and photocatalytic and antibacterial properties. OCGs present on biochar (e.g., -OH and -COOH) and the metals loaded on them react with PS to form free radicals. Therefore, they are suitable for use in heterogeneous catalysts or mixed systems consisting of different AOPs. In catalyst doped with heteroatoms, the heteroatoms not only promote the transfer of electrons, but also generate new active sites. During the degradation of pollutants by AOPs, it is important to consider that the process primarily involves the use of free radicals (such as SO4−•, •OH, and •O2−) as well as non-free radical pathways (like 1O2). The selection of a specific pathway is heavily influenced by the active sites found on the surface of biochar;
- (2)
- The mechanism of biochar combined with advanced oxidation to degrade AOPs involves both radical and non-radical pathways. Active sites that produce 1O2 consist of OCGs and defects, with OCGs helping to enhance the electron transfer process. Electron transfer from PFRs can lead to the generation of •OH, SO4−•, and •O2−. Furthermore, •OH is associated with -OH and oxygen-centered PFRs, while •O2− is linked to quinone structures and carbon-centered PFRs. The adsorption mechanism of biochar for AOPs is driven by hydrogen bonds and π-π interactions. The graphitization degree, pore structure, and specific surface area of biochar impact both the electron transfer and adsorption capabilities of the catalyst, thus playing a crucial role in AOPs’ activation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Q.; Zhao, X.; Yang, X.J. China’s decadal pollution census. Nature 2017, 543, 491. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of dyes from aqueous solution by Fenton processes: A review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef] [PubMed]
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Faheem; Du, J.; Kim, S.H.; Hassan, M.A.; Irshad, S.; Bao, J. Application of biochar in advanced oxidation processes: Supportive, adsorptive, and catalytic role. Environ. Sci. Pollut. Res. 2020, 27, 37286–37312. [Google Scholar] [CrossRef]
- Romero, V.; Acevedo, S.; Marco, P.; Giménez, J.; Esplugas, S. Enhancement of Fenton and photo-Fenton processes at initial circumneutral pH for the degradation of the β-blocker metoprolol. Water Res. 2016, 88, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; He, X.; Khan, H.M.; Shah, N.S.; Dionysiou, D.D. Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82−/Fe2+ and UV/HSO5−/Fe2+ processes: A comparative study. Chem. Eng. J. 2013, 218, 376–383. [Google Scholar] [CrossRef]
- Li, Y.; Pan, L.; Zhu, Y.; Yu, Y.; Wang, D.; Yang, G.; Yuan, X.; Liu, X.; Li, H.; Zhang, J. How does zero valent iron activating peroxydisulfate improve the dewatering of anaerobically digested sludge? Water Res. 2019, 163, 114912. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Wang, J.; Deng, Y.; Liu, Y.; Feng, H.; Chen, S.; Ren, X. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism. Chem. Eng. J. 2019, 364, 146–159. [Google Scholar] [CrossRef]
- Yu, X.; Lin, X.; Li, W.; Feng, W. Effective Removal of Tetracycline by Using Biochar Supported Fe3O4 as a UV-Fenton Catalyst. Chem. Res. Chin. Univ. 2019, 35, 79–84. [Google Scholar] [CrossRef]
- Kelly, J.; McDonnell, C.; Skillen, N.; Manesiotis, P.; Robertson, P.K. Enhanced photocatalytic degradation of 2-methyl-4-chlorophenoxyacetic acid (MCPA) by the addition of H2O2. Chemosphere 2021, 275, 130082. [Google Scholar] [CrossRef]
- Wang, K.; Liang, G.; Waqas, M.; Yang, B.; Xiao, K.; Zhu, C.; Zhang, J. Peroxymonosulfate enhanced photoelectrocatalytic degradation of ofloxacin using an easily coated cathode. Sep. Purif. Technol. 2020, 236, 116301. [Google Scholar] [CrossRef]
- Wu, S.; Hu, Y.H. A comprehensive review on catalysts for electrocatalytic and photoelectrocatalytic degradation of antibiotics. Chem. Eng. J. 2021, 409, 127739. [Google Scholar] [CrossRef]
- Farissi, S.; Abubakar, G.A.; Akhilghosh, K.A.; Muthukumar, A.; Muthuchamy, M. Sustainable application of electrocatalytic and photo-electrocatalytic oxidation systems for water and wastewater treatment: A review. Environ. Monit. Assess. 2023, 195, 1447. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, H.; Masubuchi, Y.; Minagawa, K.; Ogata, T.; Takimoto, J.-i.; Koyama, K. A reaction kinetics model of water sonolysis in the presence of a spin-trap. Ultrason. Sonochemistry 1999, 5, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Boffito, D.; Crocellà, V.; Pirola, C.; Neppolian, B.; Cerrato, G.; Ashokkumar, M.; Bianchi, C. Ultrasonic enhancement of the acidity, surface area and free fatty acids esterification catalytic activity of sulphated ZrO2–TiO2 systems. J. Catal. 2013, 297, 17–26. [Google Scholar] [CrossRef]
- Joseph, J.M.; Destaillats, H.; Hung, H.-M.; Hoffmann, M.R. The sonochemical degradation of azobenzene and related azo dyes: Rate enhancements via Fenton’s reactions. J. Phys. Chem. A 2000, 104, 301–307. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Wan, D.; Huang, J.; Liu, Y. Peroxymonosulfate-enhanced photocatalysis by carbonyl-modified g-C3N4 for effective degradation of the tetracycline hydrochloride. Sci. Total Environ. 2020, 749, 142313. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, Y.; Wei, J.; Li, M.; Xu, D.; Lai, B. Bisphenol S degradation by visible light assisted peroxymonosulfate process based on BiOI/B4C photocatalysts with Z-scheme heterojunction. Chem. Eng. J. 2021, 417, 129188. [Google Scholar] [CrossRef]
- Zu, M.; Zhang, S.; Liu, C.; Liu, P.; Li, D.-S.; Xing, C.; Zhang, S. Portable wastewater treatment system based on synergistic photocatalytic and persulphate degradation under visible light. Sci. China Mater. 2021, 64, 1952–1963. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, Y.; Zeng, H.; Liang, Y.; Ma, J.; Lu, X. Ultrasound-enhanced zero-valent copper activation of persulfate for the degradation of bisphenol AF. Chem. Eng. J. 2019, 378, 122143. [Google Scholar] [CrossRef]
- Fagan, W.P.; Zhao, J.; Villamena, F.A.; Zweier, J.L.; Weavers, L.K. Synergistic, aqueous PAH degradation by ultrasonically-activated persulfate depends on bulk temperature and physicochemical parameters. Ultrason. Sonochemistry 2020, 67, 105172. [Google Scholar] [CrossRef] [PubMed]
- Sizykh, M.; Batoeva, A. Effect of anions on the oxidation of organic compounds with ultrasonically activated persulfate. Russ. J. Phys. Chem. A 2016, 90, 1298–1300. [Google Scholar] [CrossRef]
- Aseman-Bashiz, E.; Sayyaf, H. Synthesis of nano-FeS2 and its application as an effective activator of ozone and peroxydisulfate in the electrochemical process for ofloxacin degradation: A comparative study. Chemosphere 2021, 274, 129772. [Google Scholar] [CrossRef]
- Santos, A.; Fernandez, J.; Rodriguez, S.; Dominguez, C.; Lominchar, M.; Lorenzo, D.; Romero, A. Abatement of chlorinated compounds in groundwater contaminated by HCH wastes using ISCO with alkali activated persulfate. Sci. Total Environ. 2018, 615, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Guo, X.; Duan, W.; Cheng, X.; Zhang, X.; Li, Z. Degradation of high molecular weight polyacrylamide by alkali-activated persulfate: Reactivity and potential application in filter cake removal before cementing. J. Pet. Sci. Eng. 2019, 174, 70–79. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Z.; Huang, C.; Wang, P.; Huang, S.; Yang, X.; Cheng, H.; Zheng, X.; Tian, H.; Liu, Z. A novel 3D Co/Mo co-catalyzed graphene sponge-mediated peroxymonosulfate activation for the highly efficient pollutants degradation. Sep. Purif. Technol. 2022, 301, 122035. [Google Scholar] [CrossRef]
- Dai, L.; Wu, B.; Tan, F.; He, M.; Wang, W.; Qin, H.; Tang, X.; Zhu, Q.; Pan, K.; Hu, Q. Engineered hydrochar composites for phosphorus removal/recovery: Lanthanum doped hydrochar prepared by hydrothermal carbonization of lanthanum pretreated rice straw. Bioresour. Technol. 2014, 161, 327–332. [Google Scholar] [CrossRef]
- Dai, L.; Tan, F.; Wu, B.; He, M.; Wang, W.; Tang, X.; Hu, Q.; Zhang, M. Immobilization of phosphorus in cow manure during hydrothermal carbonization. J. Environ. Manag. 2015, 157, 49–53. [Google Scholar] [CrossRef]
- Dai, L.; Yang, B.; Li, H.; Tan, F.; Zhu, N.; Zhu, Q.; He, M.; Ran, Y.; Hu, G. A synergistic combination of nutrient reclamation from manure and resultant hydrochar upgradation by acid-supported hydrothermal carbonization. Bioresour. Technol. 2017, 243, 860–866. [Google Scholar] [CrossRef]
- de Souza, D.I.; Dottein, E.M.; Giacobbo, A.; Rodrigues, M.A.S.; de Pinho, M.N.; Bernardes, A.M. Nanofiltration for the removal of norfloxacin from pharmaceutical effluent. J. Environ. Chem. Eng. 2018, 6, 6147–6153. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Chen, W.-Y.; Zhang, Y.; Sun, B.; Pan, W.-P. Derivation of oxygen-containing functional groups on biochar under non-oxygen plasma for mercury removal. Fuel 2020, 275, 117879. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef] [PubMed]
- Wijitkosum, S. Biochar derived from agricultural wastes and wood residues for sustainable agricultural and environmental applications. Int. Soil Water Conserv. Res. 2022, 10, 335–341. [Google Scholar] [CrossRef]
- Sun, J.; Norouzi, O.; Mašek, O. A state-of-the-art review on algae pyrolysis for bioenergy and biochar production. Bioresour. Technol. 2022, 346, 126258. [Google Scholar] [CrossRef]
- Aravind, S.; Kumar, P.S.; Kumar, N.S.; Siddarth, N. Conversion of green algal biomass into bioenergy by pyrolysis. A review. Environ. Chem. Lett. 2020, 18, 829–849. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Yan, S.; Li, Y.; Zhang, P.; Ren, P.; Wang, M.; Kuang, S. Biochar-amended composting of lincomycin fermentation dregs promoted microbial metabolism and reduced antibiotic resistance genes. Bioresour. Technol. 2023, 367, 128253. [Google Scholar] [CrossRef]
- Akdeniz, N. A systematic review of biochar use in animal waste composting. Waste Manag. 2019, 88, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Akca, M.O.; OK, S.S.; Deniz, K.; Mohammedelnour, A.; Kibar, M. Spectroscopic characterisation and elemental composition of biochars obtained from different agricultural wastes. J. Agric. Sci. 2021, 27, 426–435. [Google Scholar] [CrossRef]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.; Nidheesh, P.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Zhang, Y.; Mao, X.; Tan, W.; Zhang, Y.; Wang, X.; Chang, M.; Guo, R.; Xi, B. Perdisulfate-assisted advanced oxidation of 2, 4-dichlorophenol by bio-inspired iron encapsulated biochar catalyst. J. Colloid Interface Sci. 2021, 592, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.J.; Miller, D.J. Transport model with radiative heat transfer for rapid cellulose pyrolysis. Ind. Eng. Chem. Res. 1988, 27, 1775–1783. [Google Scholar] [CrossRef]
- Anthony, D.B.; Howard, J.B. Coal devolatilization and hydrogastification. AIChE J. 1976, 22, 625–656. [Google Scholar] [CrossRef]
- Phuong, D.T.M.; Loc, N.X. Rice straw biochar and magnetic rice straw biochar for safranin O adsorption from aqueous solution. Water 2022, 14, 186. [Google Scholar] [CrossRef]
- Saravanan, P.; Vinod, V.; Sreedhar, B.; Sashidhar, R. Gum kondagogu modified magnetic nano-adsorbent: An efficient protocol for removal of various toxic metal ions. Mater. Sci. Eng. C 2012, 32, 581–586. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Kalantary, R.R.; Gholami, M.; Jafari, A.J.; Kermani, M.; Asgharnia, H. TiO2-decorated magnetic biochar mediated heterogeneous photocatalytic degradation of tetracycline and evaluation of antibacterial activity. Biomass Convers. Biorefinery 2021, 13, 8949–8959. [Google Scholar] [CrossRef]
- Steele, W.V.; Appelman, E.H. The standard enthalpy of formation of peroxymonosulfate (HSO5−) and the standard electrode potential of the peroxymonosulfate-bisulfate couple. J. Chem. Thermodyn. 1982, 14, 337–344. [Google Scholar] [CrossRef]
- Rani, S.K.; Easwaramoorthy, D.; Bilal, I.M.; Palanichamy, M. Studies on Mn (II)-catalyzed oxidation of α-amino acids by peroxomonosulphate in alkaline medium-deamination and decarboxylation: A kinetic approach. Appl. Catal. A Gen. 2009, 369, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, G.; Chen, H.; Li, X. Magnetic biochar catalyst derived from biological sludge and ferric sludge using hydrothermal carbonization: Preparation, characterization and its circulation in Fenton process for dyeing wastewater treatment. Chemosphere 2018, 191, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Lee, S.Y.; Lee, Y.J.; Choi, J.-W. Ultrasound-assisted heterogeneous Fenton-like process for bisphenol A removal at neutral pH using hierarchically structured manganese dioxide/biochar nanocomposites as catalysts. Ultrason. Sonochemistry 2019, 57, 22–28. [Google Scholar] [CrossRef]
- Ma, H.; Li, J.-B.; Liu, W.-W.; Miao, M.; Cheng, B.-J.; Zhu, S.-W. Novel synthesis of a versatile magnetic adsorbent derived from corncob for dye removal. Bioresour. Technol. 2015, 190, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Lv, S.; Dou, J.; Kong, M.; Dai, D.; Si, C.; Liu, G. The efficient adsorption removal of Cr (vi) by using Fe3O4 nanoparticles hybridized with carbonaceous materials. RSC Adv. 2015, 5, 60033–60040. [Google Scholar] [CrossRef]
- Khataee, A.; Gholami, P.; Kalderis, D.; Pachatouridou, E.; Konsolakis, M. Preparation of novel CeO2-biochar nanocomposite for sonocatalytic degradation of a textile dye. Ultrason. Sonochemistry 2018, 41, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Abdul, G.; Zhu, X.; Chen, B. Structural characteristics of biochar-graphene nanosheet composites and their adsorption performance for phthalic acid esters. Chem. Eng. J. 2017, 319, 9–20. [Google Scholar] [CrossRef]
- Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H. A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Gao, Y.; Li, A. Facile synthesis of nano ZnO/ZnS modified biochar by directly pyrolyzing of zinc contaminated corn stover for Pb (II), Cu (II) and Cr (VI) removals. Waste Manag. 2018, 79, 625–637. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Dong, C.; Sun, Y. Synthesis of magnetic biochar composites for enhanced uranium (VI) adsorption. Sci. Total Environ. 2019, 651, 1020–1028. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.; Wu, S.; Nie, H.; Wang, Y. Phosphorus recovery from biogas fermentation liquid by Ca–Mg loaded biochar. J. Environ. Sci. 2015, 29, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.A.; Zhu, J.; Muhammad, N.; Sheng, T.; Xu, X. Effect of synthesis methods on magnetic Kans grass biochar for enhanced As (III, V) adsorption from aqueous solutions. Biomass Bioenergy 2014, 71, 299–310. [Google Scholar] [CrossRef]
- Yu, J.-X.; Wang, L.-Y.; Chi, R.-A.; Zhang, Y.-F.; Xu, Z.-G.; Guo, J. Competitive adsorption of Pb2+ and Cd2+ on magnetic modified sugarcane bagasse prepared by two simple steps. Appl. Surf. Sci. 2013, 268, 163–170. [Google Scholar] [CrossRef]
- Wang, M.; Sheng, G.; Qiu, Y. A novel manganese-oxide/biochar composite for efficient removal of lead (II) from aqueous solutions. Int. J. Environ. Sci. Technol. 2015, 12, 1719–1726. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman Jr, C.U. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, S.; Qian, Y.; Jia, H.; Gao, P.; Kang, Y.; Lichtfouse, E. Formation of persistent free radicals in sludge biochar by hydrothermal carbonization. Environ. Chem. Lett. 2021, 19, 2705–2712. [Google Scholar] [CrossRef]
- Madduri, S.; Elsayed, I. Novel oxone treated hydrochar for the removal of Pb (II) and methylene blue (MB) dye from aqueous solutions. Chemosphere 2020, 260, 127683. [Google Scholar] [CrossRef] [PubMed]
- Kohzadi, S.; Marzban, N.; Libra, J.A.; Bundschuh, M.; Maleki, A. Removal of RhB from water by Fe-modified hydrochar and biochar–An experimental evaluation supported by genetic programming. J. Mol. Liq. 2023, 369, 120971. [Google Scholar] [CrossRef]
- Petrović, J.; Ercegović, M.; Simić, M.; Kalderis, D.; Koprivica, M.; Milojković, J.; Radulović, D. Novel Mg-doped pyro-hydrochars as methylene blue adsorbents: Adsorption behavior and mechanism. J. Mol. Liq. 2023, 376, 121424. [Google Scholar] [CrossRef]
- Jais, F.M.; Ibrahim, S.; Chee, C.Y.; Ismail, Z. Solvothermal growth of the bimetal organic framework (NiFe-MOF) on sugarcane bagasse hydrochar for the removal of dye and antibiotic. J. Environ. Chem. Eng. 2021, 9, 106367. [Google Scholar] [CrossRef]
- Lee, H.-S.; Shin, H.-S. Competitive adsorption of heavy metals onto modified biochars: Comparison of biochar properties and modification methods. J. Environ. Manag. 2021, 299, 113651. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Lv, G.-H.; Bai, W.-B.; Liu, Q.; Zhang, Y.-C.; Song, J.-Q. Modification and use of biochar from wheat straw (Triticum aestivum L.) for nitrate and phosphate removal from water . Desalination Water Treat. 2016, 57, 4681–4693. [Google Scholar]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar pyrolytically produced from municipal solid wastes for aqueous As (V) removal: Adsorption property and its improvement with KOH activation. Bioresour. Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, H.; Lyu, H.; Wang, L.; Huang, H.; Nan, Q.; Tang, J. UV modification of biochar for enhanced hexavalent chromium removal from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 10808–10819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Chen, C.; Li, F.; Shen, K. Effects of UV-modified biochar derived from phytoremediation residue on Cd bioavailability and uptake in Coriandrum sativum L. in a Cd-contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 17395–17404. [Google Scholar] [CrossRef]
- Yuvaraj, A.; Thangaraj, R.; Karmegam, N.; Ravindran, B.; Chang, S.W.; Awasthi, M.K.; Kannan, S. Activation of biochar through exoenzymes prompted by earthworms for vermibiochar production: A viable resource recovery option for heavy metal contaminated soils and water. Chemosphere 2021, 278, 130458. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; Cai, N.; Wang, J.; Shen, L.; Shi, H.; Yang, D.; Feng, X.; Yu, F. Porous carbon nanofibers loaded with copper-cobalt bimetallic particles for heterogeneously catalyzing peroxymonosulfate to degrade organic dyes. J. Environ. Chem. Eng. 2021, 9, 106003. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Wang, H. Superior fenton-like degradation of tetracycline by iron loaded graphitic carbon derived from microplastics: Synthesis, catalytic performance, and mechanism. Sep. Purif. Technol. 2021, 270, 118773. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Si, Q.; Luo, H.; Zhao, Q.; Ren, N. Sludge-derived biochar as efficient persulfate activators: Sulfurization-induced electronic structure modulation and disparate nonradical mechanisms. Appl. Catal. B Environ. 2020, 279, 119361. [Google Scholar] [CrossRef]
- Tan, X.-F.; Liu, Y.-G.; Gu, Y.-L.; Xu, Y.; Zeng, G.-M.; Hu, X.-J.; Liu, S.-B.; Wang, X.; Liu, S.-M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Stathatos, E.; Dionysiou, D.D. Heterogeneous activation of oxone using Co3O4. J. Phys. Chem. B 2005, 109, 13052–13055. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Qiao, X.; Wang, D.; Cai, X. Performance of nano-Co3O4/peroxymonosulfate system: Kinetics and mechanism study using Acid Orange 7 as a model compound. Appl. Catal. B: Environ. 2008, 80, 116–121. [Google Scholar] [CrossRef]
- Guo, W.; Su, S.; Yi, C.; Ma, Z. Degradation of antibiotics amoxicillin by Co3O4-catalyzed peroxymonosulfate system. Environ. Prog. Sustain. Energy 2013, 32, 193–197. [Google Scholar] [CrossRef]
- Shi, P.; Su, R.; Zhu, S.; Zhu, M.; Li, D.; Xu, S. Supported cobalt oxide on graphene oxide: Highly efficient catalysts for the removal of Orange II from water. J. Hazard. Mater. 2012, 229, 331–339. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Hsu, F.-K.; Lee, W.-D. Magnetic cobalt–graphene nanocomposite derived from self-assembly of MOFs with graphene oxide as an activator for peroxymonosulfate. J. Mater. Chem. A 2015, 3, 9480–9490. [Google Scholar]
- Yang, Q.; Choi, H.; Al-Abed, S.R.; Dionysiou, D.D. Iron–cobalt mixed oxide nanocatalysts: Heterogeneous peroxymonosulfate activation, cobalt leaching, and ferromagnetic properties for environmental applications. Appl. Catal. B Environ. 2009, 88, 462–469. [Google Scholar] [CrossRef]
- Muhammad, S.; Saputra, E.; Sun, H.J.d.; Izidoro, C.; Fungaro, D.A.; Ang, H.M.; Tadé, M.O.; Wang, S. Coal fly ash supported Co3O4 catalysts for phenol degradation using peroxymonosulfate. Rsc. Adv. 2012, 2, 5645–5650. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Magnetic Fe3O4/carbon sphere/cobalt composites for catalytic oxidation of phenol solutions with sulfate radicals. Chem. Eng. J. 2014, 245, 1–9. [Google Scholar] [CrossRef]
- Shukla, P.; Sun, H.; Wang, S.; Ang, H.M.; Tadé, M.O. Co-SBA-15 for heterogeneous oxidation of phenol with sulfate radical for wastewater treatment. Catal. Today 2011, 175, 380–385. [Google Scholar] [CrossRef]
- Shukla, P.; Wang, S.; Singh, K.; Ang, H.; Tadé, M.O. Cobalt exchanged zeolites for heterogeneous catalytic oxidation of phenol in the presence of peroxymonosulphate. Appl. Catal. B Environ. 2010, 99, 163–169. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: The pivotal roles of persistent free radicals and ecotoxicity assessment. J. Hazard. Mater. 2020, 398, 122768. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Zuo, X.; Huang, Y.; Cai, T.; Ding, D. Biochar modification significantly promotes the activity of Co3O4 towards heterogeneous activation of peroxymonosulfate. Chem. Eng. J. 2018, 354, 856–865. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Li, J.; Hao, Q.; Li, X.; Liu, F. Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols. Environ. Pollut. 2020, 257, 113610. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lua, S.-K.; Dong, Z.; Lim, T.-T. Rational design of hierarchically-structured CuBi2O4 composites by deliberate manipulation of the nucleation and growth kinetics of CuBi2O4 for environmental applications. Nanoscale 2016, 8, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, J.; Wu, D.; Zhou, Z.; Deng, Y.; Zhang, T.; Shih, K. Efficient degradation of sulfamethazine with CuCo2O4 spinel nanocatalysts for peroxymonosulfate activation. Chem. Eng. J. 2015, 280, 514–524. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Deng, L. Performance of CuO/Oxone system: Heterogeneous catalytic oxidation of phenol at ambient conditions. Chem. Eng. J. 2011, 178, 239–243. [Google Scholar] [CrossRef]
- Kušić, H.; Koprivanac, N.; Selanec, I. Fe-exchanged zeolite as the effective heterogeneous Fenton-type catalyst for the organic pollutant minimization: UV irradiation assistance. Chemosphere 2006, 65, 65–73. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, G.; Liu, Y.; Di, J.; Wang, Y.; Zhao, Z.; Sun, Q.; Xu, C.; Gao, J.; Duan, A. Hierarchical macro-meso-microporous ZSM-5 zeolite hollow fibers with highly efficient catalytic cracking capability. Sci. Rep. 2014, 4, 7276. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Wang, Y.; Le Roux, J.; Yang, Y.; Croué, J.-P. Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation. Environ. Sci. Technol. 2014, 48, 5868–5875. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Huang, W.; Wei, X.; Huang, W. Biochar supported CuO composites used as an efficient peroxymonosulfate activator for highly saline organic wastewater treatment. Sci. Total Environ. 2020, 721, 137764. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lin, Q.; Zhang, X.; Huang, Z.; Fu, H.; Xiao, R.; Liu, S.-S. Determining the key factors of nonradical pathway in activation of persulfate by metal-biochar nanocomposites for bisphenol A degradation. Chem. Eng. J. 2020, 391, 123555. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R. Green one-spot synthesis of hydrochar supported zero-valent iron for heterogeneous Fenton-like discoloration of dyes at neutral pH. J. Mol. Liq. 2020, 320, 114421. [Google Scholar] [CrossRef]
- Hussain, I.; Li, M.; Zhang, Y.; Li, Y.; Huang, S.; Du, X.; Liu, G.; Hayat, W.; Anwar, N. Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol. Chem. Eng. J. 2017, 311, 163–172. [Google Scholar] [CrossRef]
- Liu, C.-M.; Diao, Z.-H.; Huo, W.-Y.; Kong, L.-J.; Du, J.-J. Simultaneous removal of Cu2+ and bisphenol A by a novel biochar-supported zero valent iron from aqueous solution: Synthesis, reactivity and mechanism. Environ. Pollut. 2018, 239, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, F.; He, S. The degradation of decabromodiphenyl ether in the e-waste site by biochar supported nanoscale zero-valent iron/persulfate. Ecotoxicol. Environ. Saf. 2019, 183, 109540. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-F.; Ling, L.-L.; Chen, W.-J.; Liu, W.-J.; Li, D.-C.; Jiang, H. High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors. Chem. Eng. J. 2019, 359, 572–583. [Google Scholar] [CrossRef]
- Luo, H.; Lin, Q.; Zhang, X.; Huang, Z.; Liu, S.; Jiang, J.; Xiao, R.; Liao, X. New insights into the formation and transformation of active species in nZVI/BC activated persulfate in alkaline solutions. Chem. Eng. J. 2019, 359, 1215–1223. [Google Scholar] [CrossRef]
- Volpe, A.; Pagano, M.; Mascolo, G.; Lopez, A.; Ciannarella, R.; Locaputo, V. Simultaneous Cr (VI) reduction and non-ionic surfactant oxidation by peroxymonosulphate and iron powder. Chemosphere 2013, 91, 1250–1256. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M.; Manshouri, M. Textile wastewater decolorization by zero valent iron activated peroxymonosulfate: Compared with zero valent copper. J. Environ. Chem. Eng. 2014, 2, 1846–1851. [Google Scholar] [CrossRef]
- Pi, Z.; Li, X.; Wang, D.; Xu, Q.; Tao, Z.; Huang, X.; Yao, F.; Wu, Y.; He, L.; Yang, Q. Persulfate activation by oxidation biochar supported magnetite particles for tetracycline removal: Performance and degradation pathway. J. Clean. Prod. 2019, 235, 1103–1115. [Google Scholar] [CrossRef]
- Rong, X.; Xie, M.; Kong, L.; Natarajan, V.; Ma, L.; Zhan, J. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation. Chem. Eng. J. 2019, 372, 294–303. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Xu, S.; Fu, H.; Li, Z.; Li, K.; Sheng, K.; Du, J.; Lu, X.; Li, X. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4. Chem. Eng. J. 2020, 387, 124094. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Hung, C.-M. Synthesis of magnetic biochar from bamboo biomass to activate persulfate for the removal of polycyclic aromatic hydrocarbons in marine sediments. Bioresour. Technol. 2017, 245, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Yan, J.; Qian, L.; Chen, Y.; Han, L.; Su, A.; Zhang, W.; Ni, H.; Chen, M. Degradation of 1, 4-dioxane by biochar supported nano magnetite particles activating persulfate. Chemosphere 2017, 184, 609–617. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Yu, G.; Xie, S.; Li, C.; Lai, D.; Li, Z.; You, F.; Wang, Y. The synthesis of heterogeneous Fenton-like catalyst using sewage sludge biochar and its application for ciprofloxacin degradation. Sci. Total Environ. 2019, 654, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Hou, H.; Liang, S.; Qiu, J.; Tao, S.; Yang, L.; Yu, W.; Xiao, K.; Liu, B.; Hu, J. Sludge-derived biochar with multivalent iron as an efficient Fenton catalyst for degradation of 4-Chlorophenol. Sci. Total Environ. 2020, 725, 138299. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, X.; Xie, R.; Zhang, Y.; Jin, Y.; Jiang, W. A novel porous biochar-supported Fe-Mn composite as a persulfate activator for the removal of acid red 88. Sep. Purif. Technol. 2020, 250, 117232. [Google Scholar] [CrossRef]
- Fu, H.; Ma, S.; Zhao, P.; Xu, S.; Zhan, S. Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: Structure dependence and mechanism. Chem. Eng. J. 2019, 360, 157–170. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Ding, D.; Cai, T. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor. Appl. Catal. B Environ. 2019, 254, 312–320. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, M.; Cao, Q.; Sun, P.; Chen, Y.; Meng, F. The superoxide radicals’ production via persulfate activated with CuFe2O4@ Biochar composites to promote the redox pairs cycling for efficient degradation of o-nitrochlorobenzene in soil. J. Hazard. Mater. 2020, 400, 122887. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Zhang, Q.; Qiu, Y.; Meng, L.; Wei, X.; Sang, W.; Tao, J. Insight into the degradation of Orange G by persulfate activated with biochar modified by iron and manganese oxides: Synergism between Fe and Mn. J. Water Process Eng. 2020, 37, 101470. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Q.; Zhang, C.; Wang, R.; Deng, R.; Luo, H.; Li, T.; Li, J.; Chen, S.; Liu, C. Mn doped magnetic biochar as persulfate activator for the degradation of tetracycline. Chem. Eng. J. 2020, 391, 123532. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Li, W.; Zhong, R.; Lan, Y.; Guo, J. The efficient degradation of sulfisoxazole by singlet oxygen (1O2) derived from activated peroxymonosulfate (PMS) with Co3O4–SnO2/RSBC. Environ. Res. 2020, 187, 109665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, Z.; Ye, C.; Deng, J.; Ma, X.; Xu, Y.; Wang, L.; Tang, Z.; Luo, H.; Li, X. Magnetic Co/Fe nanocomposites derived from ferric sludge as an efficient peroxymonosulfate catalyst for ciprofloxacin degradation. Chem. Eng. J. 2022, 432, 134180. [Google Scholar] [CrossRef]
- Xin, S.; Liu, G.; Ma, X.; Gong, J.; Ma, B.; Yan, Q.; Chen, Q.; Ma, D.; Zhang, G.; Gao, M. High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: Economical synthesis, catalytic performance and mechanism. Appl. Catal. B: Environ. 2021, 280, 119386. [Google Scholar] [CrossRef]
- Zeng, L.; Li, W.; Cheng, J.; Wang, J.; Liu, X.; Yu, Y. N-doped porous hollow carbon nanofibers fabricated using electrospun polymer templates and their sodium storage properties. Rsc Adv. 2014, 4, 16920–16927. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H. The lithium ions storage behavior of heteroatom-mediated echinus-like porous carbon spheres: From co-doping to multi-atom doping. J. Colloid Interface Sci. 2020, 567, 54–64. [Google Scholar] [CrossRef]
- Niu, W.; Li, L.; Liu, X.; Wang, N.; Liu, J.; Zhou, W.; Tang, Z.; Chen, S. Mesoporous N-doped carbons prepared with thermally removable nanoparticle templates: An efficient electrocatalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 5555–5562. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Dai, Y.; Han, X.; Xing, B.; Zhu, L.; Qiu, K.; Wang, S. A novel approach for preparing in-situ nitrogen doped carbon via pyrolysis of bean pulp for supercapacitors. Energy 2021, 216, 119227. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Cui, Z.; Chen, D.; Pang, X.; Zhang, Q.; Shao, F.; Dong, H.; Yu, L.; Dong, L. Tailoring in-situ N, O, P, S-doped soybean-derived porous carbon with ultrahigh capacitance in both acidic and alkaline media. Renew. Energy 2021, 163, 375–385. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Z.; Li, M.; Liu, L.; Wang, Y.; Lv, W.; Qin, Z.; Zhao, X.; Zhu, P.; Wang, G. FeS-decorated hierarchical porous N, S-dual-doped carbon derived from silica-ionogel as an efficient catalyst for oxygen reduction reaction in alkaline media. Electrochim. Acta 2018, 265, 221–231. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.; Liu, Y.; Fan, L.-Z. Biowaste-derived 3D honeycomb-like porous carbon with binary-heteroatom doping for high-performance flexible solid-state supercapacitors. J. Mater. Chem. A 2018, 6, 160–166. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Wu, Q.; Luo, H.; Zhao, Q.; Si, Q.; Sseguya, F.; Ren, N. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-D.; Lisak, G.; Webster, R.D.; Liang, Y.-N.; Veksha, A.; Giannis, A.; Moo, J.G.S.; Lim, J.-W.; Lim, T.-T. Insights into the thermolytic transformation of lignocellulosic biomass waste to redox-active carbocatalyst: Durability of surface active sites. Appl. Catal. B Environ. 2018, 233, 120–129. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Liu, P.; Bai, X.; Du, X.; Jin, P.; Yang, L.; Jin, X.; Shi, X.; Wang, Y. Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants. Chem. Eng. J. 2020, 387, 124065. [Google Scholar] [CrossRef]
- Terrell, E.; Garcia-Perez, M. Application of nitrogen-based blowing agents as an additive in pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2019, 137, 203–211. [Google Scholar] [CrossRef]
- Ding, D.; Yang, S.; Qian, X.; Chen, L.; Cai, T. Nitrogen-doping positively whilst sulfur-doping negatively affect the catalytic activity of biochar for the degradation of organic contaminant. Appl. Catal. B: Environ. 2020, 263, 118348. [Google Scholar] [CrossRef]
- Li, B.; Li, K. Effect of nitric acid pre-oxidation concentration on pore structure and nitrogen/oxygen active decoration sites of ethylenediamine-modified biochar for mercury (II) adsorption and the possible mechanism. Chemosphere 2019, 220, 28–39. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, C.W.; Phiri, I.; Ko, J.M. New design for Polyaniline@ Multiwalled carbon nanotubes composites with bacteria doping for supercapacitor electrodes. Polymer 2020, 210, 123014. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Ma, F.; Wang, L.; Duan, X.; Wang, S. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, W.; He, C.; Wen, H.; Cai, Z.; Wang, Z.; Chen, Z.; Liu, W. Microwave-assisted preparation of nitrogen-doped biochars by ammonium acetate activation for adsorption of acid red 18. Appl. Surf. Sci. 2018, 433, 222–231. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, X.; Li, X.; Jia, C.Q.; Jiang, W. Preparation of high-yield N-doped biochar from nitrogen-containing phosphate and its effective adsorption for toluene. RSC Adv. 2018, 8, 30171–30179. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer. Appl. Catal. B Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, W.; Wang, X.; Tong, W.; Li, P.; Zhou, H.; Wang, Y.; Zhang, Y. Molten salt induced nitrogen-doped biochar nanosheets as highly efficient peroxymonosulfate catalyst for organic pollutant degradation. Environ. Pollut. 2020, 260, 114053. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Li, R.; Zhang, C.; Ge, Y.; Cao, G.; Ma, M.; Duan, X.; Wang, S.; Ren, N.-q. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019, 159, 77–86. [Google Scholar] [CrossRef]
- Yan, P.; Liu, J.; Yuan, S.; Liu, Y.; Cen, W.; Chen, Y. The promotion effects of graphitic and pyridinic N combinational doping on graphene for ORR. Appl. Surf. Sci. 2018, 445, 398–403. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Ravidhas, C.; Anitha, B.; Moses Ezhil Raj, A.; Ravichandran, K.; Sabari Girisun, T.; Mahalakshmi, K.; Saravanakumar, K.; Sanjeeviraja, C. Effect of nitrogen doped titanium dioxide (N-TiO2) thin films by jet nebulizer spray technique suitable for photoconductive study. J. Mater. Sci. Mater. Electron. 2015, 26, 3573–3582. [Google Scholar] [CrossRef]
- Patel, N.; Jaiswal, R.; Warang, T.; Scarduelli, G.; Dashora, A.; Ahuja, B.; Kothari, D.; Miotello, A. Efficient photocatalytic degradation of organic water pollutants using V–N-codoped TiO2 thin films. Appl. Catal. B Environ. 2014, 150, 74–81. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T. Nitrogen complex species and its chemical nature in TiO2 for visible-light sensitized photocatalysis. Chem. Phys. 2007, 339, 57–63. [Google Scholar] [CrossRef]
- Tavares, C.; Marques, S.; Viseu, T.; Teixeira, V.; Carneiro, J.; Alves, E.; Barradas, N.; Munnik, F.; Girardeau, T.; Rivière, J.-P. Enhancement in the photocatalytic nature of nitrogen-doped PVD-grown titanium dioxide thin films. J. Appl. Phys. 2009, 106, 113535. [Google Scholar] [CrossRef]
- Li, C.; Gu, M.; Gao, M.; Liu, K.; Zhao, X.; Cao, N.; Feng, J.; Ren, Y.; Wei, T.; Zhang, M. N-doping TiO2 hollow microspheres with abundant oxygen vacancies for highly photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2022, 609, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Ju, X.; He, T.; Chen, T.; Zhou, Y.; Zhang, C.; Wang, J.; Xu, Y.; Yao, B.; Zhuang, W. Sulfur-doped porous carbon as high-capacity anodes for lithium and sodium ions batteries. J. Alloys Compd. 2021, 863, 158078. [Google Scholar] [CrossRef]
- Yaglikci, S.; Gokce, Y.; Yagmur, E.; Aktas, Z. The performance of sulphur doped activated carbon supercapacitors prepared from waste tea. Environ. Technol. 2019, 41, 36–48. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. B-doped graphitic porous biochar with enhanced surface affinity and electron transfer for efficient peroxydisulfate activation. Chem. Eng. J. 2020, 396, 125119. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Peroxymonosulfate activation by Co9S8@ S and N co-doped biochar for sulfamethoxazole degradation. Chem. Eng. J. 2020, 385, 123933. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Zhou, M.; Su, X.; Sun, J. High-efficiency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation. J. Hazard. Mater. 2020, 397, 122764. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C.; Ni, Y.; Su, M.; Xu, Z. A new sight on hydrogenation of F and NF doped {0 0 1} facets dominated anatase TiO2 for efficient visible light photocatalyst. Appl. Catal. B Environ. 2012, 127, 28–35. [Google Scholar] [CrossRef]
- Zhong, Q.; Lin, Q.; Huang, R.; Fu, H.; Zhang, X.; Luo, H.; Xiao, R. Oxidative degradation of tetracycline using persulfate activated by N and Cu codoped biochar. Chem. Eng. J. 2020, 380, 122608. [Google Scholar] [CrossRef]
- Ren, T.-L.; Ma, X.-W.; Wu, X.-Q.; Yuan, L.; Lai, Y.-L.; Tong, Z.-H. Degradation of imidazolium ionic liquids in a thermally activated persulfate system. Chem. Eng. J. 2021, 412, 128624. [Google Scholar] [CrossRef]
- Mani, P.; Kim, Y.; Lakhera, S.K.; Neppolian, B.; Choi, H. Complete arsenite removal from groundwater by UV activated potassium persulfate and iron oxide impregnated granular activated carbon. Chemosphere 2021, 277, 130225. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.M.; Rodriguez, V.; Montero, E.; Romero, A.; Santos, A. Abatement of dichloromethane using persulfate activated by alkali: A kinetic study. Sep. Purif. Technol. 2020, 241, 116679. [Google Scholar] [CrossRef]
- Kim, D.-G.; Ko, S.-O. Effects of thermal modification of a biochar on persulfate activation and mechanisms of catalytic degradation of a pharmaceutical. Chem. Eng. J. 2020, 399, 125377. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, T.; Huang, Q.; Zhan, M.; Li, X.; Yan, J. Activation of persulfate by CO2-activated biochar for improved phenolic pollutant degradation: Performance and mechanism. Chem. Eng. J. 2020, 380, 122519. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, Y.; Tsang, D.C.; Iris, K.; Fan, J.; Clark, J.H.; Zhou, Y.; Cao, X.; Gao, B.; Ok, Y.S. A sustainable biochar catalyst synergized with copper heteroatoms and CO2 for singlet oxygenation and electron transfer routes. Green Chem. 2019, 21, 4800–4814. [Google Scholar] [CrossRef]
- Gupta, V.; Moradi, O.; Tyagi, I.; Agarwal, S.; Sadegh, H.; Shahryari-Ghoshekandi, R.; Makhlouf, A.; Goodarzi, M.; Garshasbi, A. Study on the removal of heavy metal ions from industry waste by carbon nanotubes: Effect of the surface modification: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 93–118. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Xu, R.-K.; Xiao, S.-C.; Yuan, J.-H.; Zhao, A.-Z. Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues. Bioresour. Technol. 2011, 102, 10293–10298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiao, X.; Chen, B.; Zhu, L. Quantification of chemical states, dissociation constants and contents of oxygen-containing groups on the surface of biochars produced at different temperatures. Environ. Sci. Technol. 2015, 49, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.-H.; Zhang, W.-X.; Liang, J.-Y.; Huang, S.-T.; Dong, F.-X.; Yan, L.; Qian, W.; Chu, W. Removal of herbicide atrazine by a novel biochar based iron composite coupling with peroxymonosulfate process from soil: Synergistic effect and mechanism. Chem. Eng. J. 2021, 409, 127684. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Sun, C.; Wan, J.; He, H.; Wang, F.; Dai, Y.; Yang, S.; Lin, Y.; Zhan, X. Insights into removal of tetracycline by persulfate activation with peanut shell biochar coupled with amorphous Cu-doped FeOOH composite in aqueous solution. Environ. Sci. Pollut. Res. 2019, 26, 2820–2834. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Han, L.; Gao, W.; Xue, S.; Chen, M. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Huong, P.T.; Jitae, K.; Al Tahtamouni, T.; Tri, N.L.M.; Kim, H.-H.; Cho, K.H.; Lee, C. Novel activation of peroxymonosulfate by biochar derived from rice husk toward oxidation of organic contaminants in wastewater. J. Water Process Eng. 2020, 33, 101037. [Google Scholar] [CrossRef]
- Meng, H.; Nie, C.; Li, W.; Duan, X.; Lai, B.; Ao, Z.; Wang, S.; An, T. Insight into the effect of lignocellulosic biomass source on the performance of biochar as persulfate activator for aqueous organic pollutants remediation: Epicarp and mesocarp of citrus peels as examples. J. Hazard. Mater. 2020, 399, 123043. [Google Scholar] [CrossRef] [PubMed]
- Klupfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox properties of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef]
- Chen, N.; Huang, Y.; Hou, X.; Ai, Z.; Zhang, L. Photochemistry of hydrochar: Reactive oxygen species generation and sulfadimidine degradation. Environ. Sci. Technol. 2017, 51, 11278–11287. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.; Alvim-Ferraz, M.; Dias, J. Activated carbon modifications to enhance its water treatment applications. An overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, H.; Chen, Y.; Xia, M.; Chen, X.; Chen, H. Transformation of nitrogen and evolution of N-containing species during algae pyrolysis. Environ. Sci. Technol. 2017, 51, 6570–6579. [Google Scholar] [CrossRef]
- Ding, W.; Wei, Z.; Chen, S.; Qi, X.; Yang, T.; Hu, J.; Wang, D.; Wan, L.J.; Alvi, S.F.; Li, L. Space-confinement-induced synthesis of pyridinic-and pyrrolic-nitrogen-doped graphene for the catalysis of oxygen reduction. Angew. Chem. 2013, 125, 11971–11975. [Google Scholar] [CrossRef]
- Saha, D.; Kienbaum, M.J. Role of oxygen, nitrogen and sulfur functionalities on the surface of nanoporous carbons in CO2 adsorption: A critical review. Microporous Mesoporous Mater. 2019, 287, 29–55. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Y.; Gao, B.; Cao, X. N-doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red. Chem. Eng. J. 2019, 368, 564–572. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Sun, C.; He, H.; Dai, Y.; Yang, S.; Lin, Y.; Zhan, X.; Li, Q.; Zhou, Y. Catalytic degradation of diatrizoate by persulfate activation with peanut shell biochar-supported nano zero-valent iron in aqueous solution. Int. J. Environ. Res. Public Health 2018, 15, 1937. [Google Scholar] [CrossRef]
- Pan, L.; Cao, Y.; Zang, J.; Huang, Q.; Wang, L.; Zhang, Y.; Fan, S.; Tang, J.; Xie, Z. Preparation of iron-loaded granular activated carbon catalyst and its application in tetracycline antibiotic removal from aqueous solution. Int. J. Environ. Res. Public Health 2019, 16, 2270. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Sui, Z.; Zhang, Y.; Sun, B.; Pan, W.-P. Enhanced mercury removal by transplanting sulfur-containing functional groups to biochar through plasma. Fuel 2019, 253, 703–712. [Google Scholar] [CrossRef]
- Huang, S.; Liang, Q.; Geng, J.; Luo, H.; Wei, Q. Sulfurized biochar prepared by simplified technic with superior adsorption property towards aqueous Hg (II) and adsorption mechanisms. Mater. Chem. Phys. 2019, 238, 121919. [Google Scholar] [CrossRef]
- Hsi, H.-C.; Tsai, C.-Y.; Kuo, T.-H.; Chiang, C.-S. Development of low-concentration mercury adsorbents from biohydrogen-generation agricultural residues using sulfur impregnation. Bioresour. Technol. 2011, 102, 7470–7477. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Wang, J.J.; Zhou, B.; Mikhael, J.E.; DeLaune, R.D. Removing mercury from aqueous solution using sulfurized biochar and associated mechanisms. Environ. Pollut. 2019, 244, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.; Solis, K.L.; An, H.-R.; Hong, Y.; Igalavithana, A.D.; Ok, Y.S. Sustainable removal of Hg (II) by sulfur-modified pine-needle biochar. J. Hazard. Mater. 2020, 388, 122048. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ptacek, C.J.; Elena, K.M.; Blowes, D.W.; Gould, W.D.; Finfrock, Y.Z.; Wang, A.O.; Landis, R.C. Evaluation of mercury stabilization mechanisms by sulfurized biochars determined using X-ray absorption spectroscopy. J. Hazard. Mater. 2018, 347, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.A.; Anirudhan, T. Removal of mercury (II) from aqueous solutions and chlor-alkali industry effluent by steam activated and sulphurised activated carbons prepared from bagasse pith: Kinetics and equilibrium studies. J. Hazard. Mater. 2002, 92, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Peng, T.; Li, G.; Wang, S.; Duan, L.; Mulder, J.; Cornelissen, G.; Cheng, Z.; Yang, S.; Hou, D. Sulfur-modified rice husk biochar: A green method for the remediation of mercury contaminated soil. Sci. Total Environ. 2018, 621, 819–826. [Google Scholar] [CrossRef]
- Lefebvre, D.D.; Kelly, D.; Budd, K. Biotransformation of Hg (II) by cyanobacteria. Appl. Environ. Microbiol. 2007, 73, 243–249. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, B. Sulfonated graphene nanosheets as a superb adsorbent for various environmental pollutants in water. Environ. Sci. Technol. 2015, 49, 7364–7372. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M.N.V. Cadmium minimization in rice. A review. Agron. Sustain. Dev. 2014, 34, 155–173. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, X.; Wang, X.; Feng, K.; Su, J.; Dong, J. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M.; Shi, L.; Wu, C.; Li, W.; An, W.; Liu, Z.; Xue, S. Effect of sulfur and sulfur-iron modified biochar on cadmium availability and transfer in the soil–rice system. Chemosphere 2019, 222, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Higashikawa, F.S.; Conz, R.F.; Colzato, M.; Cerri, C.E.P.; Alleoni, L.R.F. Effects of feedstock type and slow pyrolysis temperature in the production of biochars on the removal of cadmium and nickel from water. J. Clean. Prod. 2016, 137, 965–972. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Huang, M.; Wang, X.; Liu, C.; Fang, G.; Gao, J.; Wang, Y.; Zhou, D. Facile ball milling preparation of sulfur-doped carbon as peroxymonosulfate activator for efficient removal of organic pollutants. J. Environ. Chem. Eng. 2021, 9, 106536. [Google Scholar] [CrossRef]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Y.; Dong, H.; Xiao, S.; Xiao, J.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q.; Li, L. A comparative study on the activation of persulfate by mackinawite@ biochar and pyrite@ biochar for sulfamethazine degradation: The role of different natural iron-sulfur minerals doping. Chem. Eng. J. 2022, 448, 137620. [Google Scholar] [CrossRef]
- Maliutina, K.; Tahmasebi, A.; Yu, J. Pressurized entrained-flow pyrolysis of microalgae: Enhanced production of hydrogen and nitrogen-containing compounds. Bioresour. Technol. 2018, 256, 160–169. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Yang, H.; Xia, M.; Li, K.; Chen, X.; Chen, H. Co-pyrolysis of lignocellulosic biomass and microalgae: Products characteristics and interaction effect. Bioresour. Technol. 2017, 245, 860–868. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Yang, H.; Li, K.; Chen, X.; Chen, H. Investigation on biomass nitrogen-enriched pyrolysis: Influence of temperature. Bioresour. Technol. 2018, 249, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tan, Z.; Ye, Z. Transformation and transport mechanism of nitrogenous compounds in a biochar “preparation–returning to the field” process studied by employing an isotope tracer method. ACS Sustain. Chem. Eng. 2018, 6, 1780–1791. [Google Scholar] [CrossRef]
- Yu, J.; Maliutina, K.; Tahmasebi, A. A review on the production of nitrogen-containing compounds from microalgal biomass via pyrolysis. Bioresour. Technol. 2018, 270, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Cui, G.; Liu, Z.; Duo, L.; Zhang, G.; Xing, B. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J. Environ. Manag. 2016, 176, 61–68. [Google Scholar] [CrossRef]
- Yuan, S.; Tan, Z.; Huang, Q. Migration and transformation mechanism of nitrogen in the biomass–biochar–plant transport process. Renew. Sustain. Energy Rev. 2018, 85, 1–13. [Google Scholar] [CrossRef]
- Wang, T.; Arbestain, M.C.; Hedley, M.; Bishop, P. Chemical and bioassay characterisation of nitrogen availability in biochar produced from dairy manure and biosolids. Org. Geochem. 2012, 51, 45–54. [Google Scholar] [CrossRef]
- Lomnicki, S.; Truong, H.; Vejerano, E.; Dellinger, B. Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter. Environ. Sci. Technol. 2008, 42, 4982–4988. [Google Scholar] [CrossRef] [PubMed]
- Vejerano, E.; Lomnicki, S.M.; Dellinger, B. Formation and stabilization of combustion-generated, environmentally persistent radicals on Ni (II) O supported on a silica surface. Environ. Sci. Technol. 2012, 46, 9406–9411. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
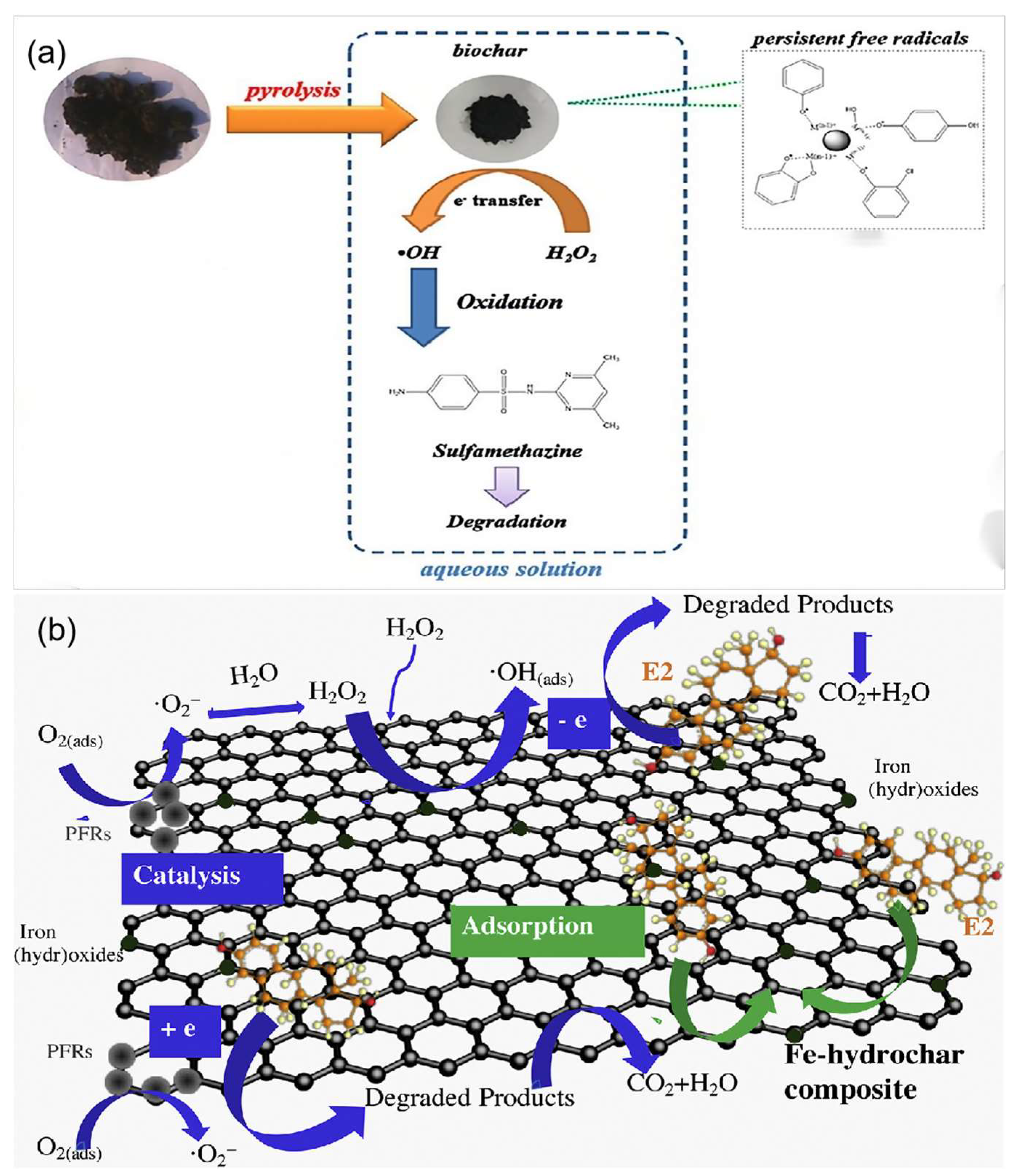
- Deng, R.; Luo, H.; Huang, D.; Zhang, C. Biochar-mediated Fenton-like reaction for the degradation of sulfamethazine: Role of environmentally persistent free radicals. Chemosphere 2020, 255, 126975. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, S.; Nulaji, G.; Tao, K.; Wang, F.; Sharma, V.K.; Wang, C. Environmentally persistent free radicals in soils of past coking sites: Distribution and stabilization. Environ. Sci. Technol. 2017, 51, 6000–6008. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, G.; Gao, Y.; Zhang, L.; Ok, Y.S.; An, T. Persistent free radicals in carbon-based materials on transformation of refractory organic contaminants (ROCs) in water: A critical review. Water Res. 2018, 137, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Sun, Y.; Du, W.; Tang, Y.; Liu, Q.; Zhang, Z.; Doherty, W.; Frost, R.L.; Qian, G.; Tsang, D.C. Formation, characteristics, and applications of environmentally persistent free radicals in biochars: A review. Bioresour. Technol. 2019, 281, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Liu, C.; Gao, J.; Dionysiou, D.D.; Zhou, D. Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ. Sci. Technol. 2015, 49, 5645–5653. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Liu, C.; Dionysiou, D.D.; Wang, Y.; Zhou, D. Key role of persistent free radicals in hydrogen peroxide activation by biochar: Implications to organic contaminant degradation. Environ. Sci. Technol. 2014, 48, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, Z.; Zhang, H.; Chen, T.; Qiu, Y.; Xu, Z.; Yin, D. Efficient removal of several estrogens in water by Fe-hydrochar composite and related interactive effect mechanism of H2O2 and iron with persistent free radicals from hydrochar of pinewood. Sci. Total Environ. 2019, 658, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, W.; Yin, R.; Du, J.; Wu, Q.; Luo, H.; Liu, B.; Sseguya, F.; Ren, N. Biochar-induced Fe (III) reduction for persulfate activation in sulfamethoxazole degradation: Insight into the electron transfer, radical oxidation and degradation pathways. Chem. Eng. J. 2019, 362, 561–569. [Google Scholar] [CrossRef]
- He, W.; Zhu, Y.; Zeng, G.; Zhang, Y.; Wang, Y.; Zhang, M.; Long, H.; Tang, W. Efficient removal of perfluorooctanoic acid by persulfate advanced oxidative degradation: Inherent roles of iron-porphyrin and persistent free radicals. Chem. Eng. J. 2020, 392, 123640. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Huang, Y.; Ding, Z.; Chen, Y.; Hu, X. Insight into biomass feedstock on formation of biochar-bound environmentally persistent free radicals under different pyrolysis temperatures. RSC Adv. 2022, 12, 19318–19326. [Google Scholar] [CrossRef]
- Zhong, D.; Zhang, Y.; Wang, L.; Chen, J.; Jiang, Y.; Tsang, D.C.; Zhao, Z.; Ren, S.; Liu, Z.; Crittenden, J.C. Mechanistic insights into adsorption and reduction of hexavalent chromium from water using magnetic biochar composite: Key roles of Fe3O4 and persistent free radicals. Environ. Pollut. 2018, 243, 1302–1309. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Huang, D.-L.; Liu, Y.-G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.-M.; Gong, X.-M.; Duan, A.; Zhang, Q. Recent advances in biochar-based catalysts: Properties, applications and mechanisms for pollution remediation. Chem. Eng. J. 2019, 371, 380–403. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Geng, M.; Chen, D.; Lin, H.; Zhang, H. Catalytic oxidation of clofibric acid by peroxydisulfate activated with wood-based biochar: Effect of biochar pyrolysis temperature, performance and mechanism. Chem. Eng. J. 2019, 374, 1253–1263. [Google Scholar] [CrossRef]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Chen, W.; Duan, L.; Wang, L.; Zhu, D. Adsorption of hydroxyl-and amino-substituted aromatics to carbon nanotubes. Environ. Sci. Technol. 2008, 42, 6862–6868. [Google Scholar] [CrossRef]
- Tang, J.; Lv, H.; Gong, Y.; Huang, Y. Preparation and characterization of a novel graphene/biochar composite for aqueous phenanthrene and mercury removal. Bioresour. Technol. 2015, 196, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, Y.; He, Y.; Liu, X.; Xu, B.; Yu, J.; Dai, C.; Huang, A.; Pang, Y.; Luo, L. Analyses of tetracycline adsorption on alkali-acid modified magnetic biochar: Site energy distribution consideration. Sci. Total Environ. 2019, 650, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bai, X.; Yuan, Y.; Zhang, Y.; Sun, J. Printing and dyeing sludge derived biochar for activation of peroxymonosulfate to remove aqueous organic pollutants: Activation mechanisms and environmental safety assessment. Chem. Eng. J. 2022, 446, 136942. [Google Scholar] [CrossRef]
- Xu, L.; Fu, B.; Sun, Y.; Jin, P.; Bai, X.; Jin, X.; Shi, X.; Wang, Y.; Nie, S. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application. Chem. Eng. J. 2020, 400, 125870. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Li, J.; Zhu, Y.; Yu, H.; Peng, Y. Adsorption and co-adsorption of tetracycline and doxycycline by one-step synthesized iron loaded sludge biochar. Chemosphere 2019, 236, 124254. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem. Eng. J. 2019, 356, 350–358. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; He, Y.; Peng, X. Efficient removal of tetrabromobisphenol A (TBBPA) using sewage sludge-derived biochar: Adsorptive effect and mechanism. Chemosphere 2020, 251, 126370. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, H.; Ao, Z.; Zhou, L.; Wang, G.; Wang, S. Unveiling the active sites of graphene-catalyzed peroxymonosulfate activation. Carbon 2016, 107, 371–378. [Google Scholar] [CrossRef]
- Geng, A.; Xu, L.; Gan, L.; Mei, C.; Wang, L.; Fang, X.; Li, M.; Pan, M.; Han, S.; Cui, J. Using wood flour waste to produce biochar as the support to enhance the visible-light photocatalytic performance of BiOBr for organic and inorganic contaminants removal. Chemosphere 2020, 250, 126291. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Feng, H.; Zou, J.; Wang, J.; Feng, C.; Zhu, X.; Ouyang, X. Hierarchical porous biochar from shrimp shell for persulfate activation: A two-electron transfer path and key impact factors. Appl. Catal. B Environ. 2020, 260, 118160. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, Y.; Yan, J.; Qian, L.; Han, L.; Chen, M. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1, 4-dioxane: Important role of biochar defect structures. Chem. Eng. J. 2019, 370, 614–624. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal-and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Tade, M.; Wang, S. Metal-free activation of persulfate by cubic mesoporous carbons for catalytic oxidation via radical and nonradical processes. Catal. Today 2018, 307, 140–146. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, L.; Wang, J.; Yu, J.; Feng, H.; Lu, Y.; Chen, Y.; Liu, Y.; Wang, J.; Xie, Q. Enhanced surface activation process of persulfate by modified bagasse biochar for degradation of phenol in water and soil: Active sites and electron transfer mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124904. [Google Scholar] [CrossRef]
- He, J.; Xiao, Y.; Tang, J.; Chen, H.; Sun, H. Persulfate activation with sawdust biochar in aqueous solution by enhanced electron donor-transfer effect. Sci. Total Environ. 2019, 690, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, M.; Gong, Q.; Wang, X.; Cai, J.; Wang, S.; Chen, Z. One-step preparation of ZVI-sludge derived biochar without external source of iron and its application on persulfate activation. Sci. Total Environ. 2020, 714, 136728. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, C.; Kim, M.; Kim, S.; Choi, W. Ambient-temperature catalytic degradation of aromatic compounds on iron oxide nanorods supported on carbon nanofiber sheet. Appl. Catal. B Environ. 2019, 259, 118066. [Google Scholar] [CrossRef]
- Yi, Q.; Ji, J.; Shen, B.; Dong, C.; Liu, J.; Zhang, J.; Xing, M. Singlet oxygen triggered by superoxide radicals in a molybdenum cocatalytic Fenton reaction with enhanced REDOX activity in the environment. Environ. Sci. Technol. 2019, 53, 9725–9733. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, X.; Liu, S.; Liu, Y.; Zeng, G.; Ye, S.; Yin, Z.; Hu, X.; Liu, N. Catalytic degradation of estrogen by persulfate activated with iron-doped graphitic biochar: Process variables effects and matrix effects. Chem. Eng. J. 2019, 378, 122141. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Zhu, Y.-A.; Peng, W.; Wang, S.; Duan, X. Synergy of nitrogen doping and structural defects on hierarchically porous carbons toward catalytic oxidation via a non-radical pathway. Carbon 2019, 155, 268–278. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, L.; Li, X.; Yan, T.; Song, W.; Hou, T.; Tong, C.; Mu, J.; Xu, M. Goethite/biochar-activated peroxymonosulfate enhances tetracycline degradation: Inherent roles of radical and non-radical processes. Sci. Total Environ. 2021, 783, 147102. [Google Scholar] [CrossRef]
- Pang, K.; Sun, W.; Ye, F.; Yang, L.; Pu, M.; Yang, C.; Zhang, Q.; Niu, J. Sulfur-modified chitosan derived N, S-co-doped carbon as a bifunctional material for adsorption and catalytic degradation sulfamethoxazole by persulfate. J. Hazard. Mater. 2022, 424, 127270. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Z.; Wang, H.; Jia, Y.; Xu, N.; Wang, X.; Wang, J.; Zhang, C.; Tian, T.; Shen, W. Peroxydisulfate activation using B-doped biochar for the degradation of oxytetracycline in water. Appl. Surf. Sci. 2022, 599, 153917. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Zhang, J.; Qin, Y.; Wu, Y.; Zhou, M.; Sun, J. Efficient removal of tetracycline by H2O2 activated with iron-doped biochar: Performance, mechanism, and degradation pathways. Chin. Chem. Lett. 2022, 33, 2105–2110. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, H.; Wang, S. Metal-free carbocatalysis in advanced oxidation reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Xing, B.; Dong, J.; Yang, G.; Jiang, N.; Liu, X.; Yuan, J. An insight into N, S-codoped activated carbon for the catalytic persulfate oxidation of organic pollutions in water: Effect of surface functionalization. Appl. Catal. A Gen. 2020, 602, 117714. [Google Scholar] [CrossRef]
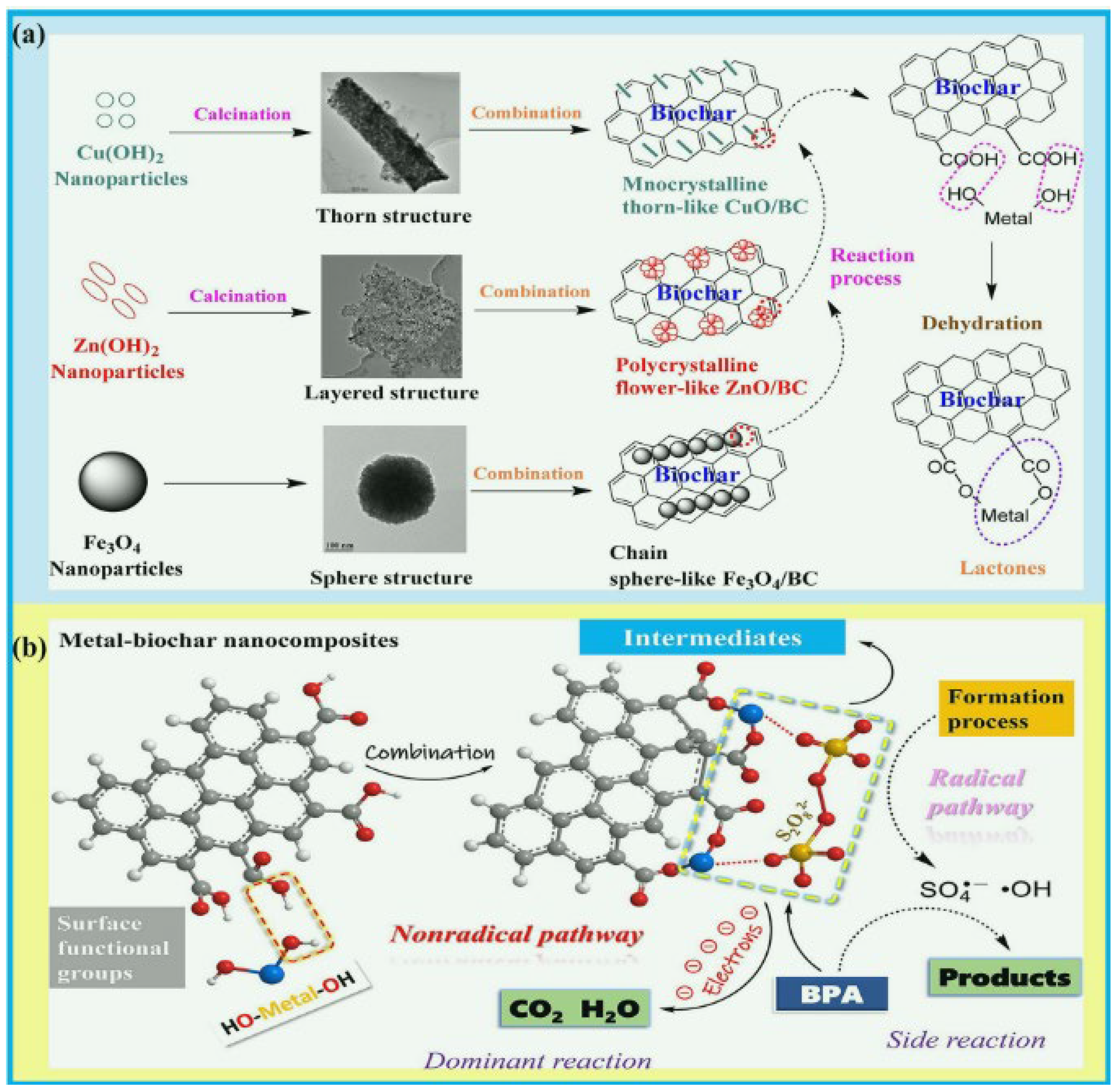
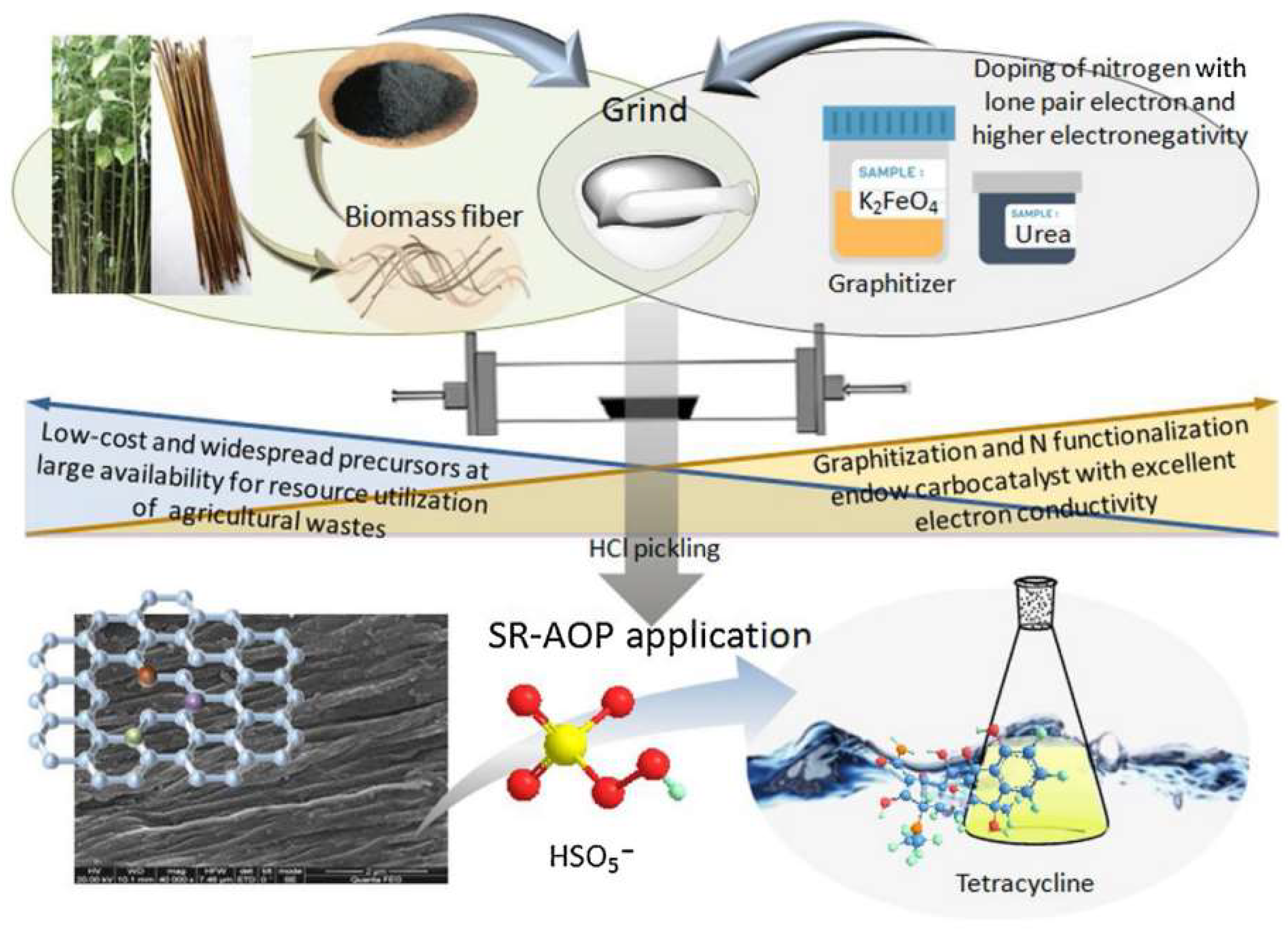
| Method | Biomass | Catalyst | Characteristics and Advantages | Removal Property | Reference |
|---|---|---|---|---|---|
| Pyrolysis | Wheat straw | CeO2 | Higher BET, average pore size and pore volume. | The removal rate was high (95.8%), and the removal rate was still 87% after 5 cycles of experiment. | [56] |
| Wood | Graphene oxide | Higher BET, porous structure and thermal stability. | More effcient removal of organic pollutants through π-π EDA interaction and the maximum adsorption capacity was 30.78 mg·g−1. | [57] | |
| Waste walnut shell | TiO2 | Strong interaction between BC and TiO2 effectively promotes the transfer of e− in TiO2. | The decolorization rate reached 96.88%, and the activity was still high after 5 catalytic experiments. | [58] | |
| Corn stover | ZnO/ZnS | Higher BET, porous structure and total pore volume. | The maximum adsorption capacities were 135.8, 91.2 and 24.5 mg·g−1. | [59] | |
| Rice husk | Fe3O4 | presents a superior magnetic response. | Displayed a preeminent adsorption performance for U(VI). | [60] | |
| Co-precipitation | Corncob | MgCl2 and CaCl2 | High surface area, mesoporous structure. | The adsorption of P reached 326.63 mg·g−1. | [61] |
| Kans grass straw (Saccharum spontaneum) | FeSO4•7H2O and FeCl3•6H2O | MGKB has ferromagnetism, high thermal stability, higher surface porosity, high surface area, and large total pore volume. | When pH = 13.5, the adsorption capacities of As(III) and As(V) are 2.004 mg·g−1 and 3.132 mg·g−1. The adsorption effect is best at this time. | [62] | |
| Sugarcane baggase (SCB) | FeCl3 and FeSO4 | It has a porous structure with a large number of carboxyl groups and negative charges on the surface. | The adsorption capacities of Pb2+ and Cd2+ were 1.2 and 1.1 mmol·g−1, respectively. When C(Pb): C(Cd) is greater than or equal to 4:1, the magnetic adsorbent can selectively adsorb Pb2+. | [63] | |
| Peeled pine wood (Pinus massoniana) | Hydrous-manganese oxide (HMO) | High BET and porosity. The surface hydroxyl group increases and the pHPZC(zero charge point pH) of the carbon decreases. | When pH = 5.00, the removal efficiency of lead (II) increased from 6.4 to 98.9%. | [64] | |
| Oak wood and oak bark | Fe2(SO4)3•nH2O (n: 6–9) and FeSO4 | High surface area, large porosity, high MS value. | The adsorption capacities of MOWBC and MOBBC were Pb2+ 10.13 and 2.87 mg·g−1 and Pb2+: 30.2 and Cd2+: 7.4 mg·g−1. | [65] | |
| Hydrothermal | Fresh olive waste | / | Carboxyl and carbonyl groups increased by about 300%. | Removes approximately 100% of methylene blue and Congo red. The three adsorption cycles have a repeat utilization rate of about 80%. | [66] |
| Pine wood | / | The optimum carboxylic acid content of hydrocarbon surface was obtained by oxyketone treatment. | The maximum adsorption capacity of MB was 86.7 mg·g−1 and Pb(II) was 46.7 mg·g−1. | [67] | |
| wheat straw | Fe | Iron-modified hydrocarbons have higher voids, roughness and specific surface area. | Under the conditions of initial pH 6, concentration of RhB 5 mg·L−1, concentration of RhB 1 g·L−1 and adsorption time 90 min, the optimal adsorption efficiency of fe modified hydrocarbons for RHB is 91%. | [68] | |
| Mg-doped grape pomace Mg-doped corn cob Mg-doped Miscanthus × giganteus | Mg | Hydrogen bonding, π-π interactions, electrostatic interactions, and surface complexation all play a role | Their adsorbability is 289.65 mg·g−1, 262.30 mg·g−1, and 232.48 mg·g−1. | [69] | |
| sugarcane bagasse | Ni, Fe | High BET and contains a large number of carboxyl and metal carboxyl groups, hydrogen bonds, π-π or π-eda interactions, surface complexation, and acid-base interactions. | Qmax = 395.9 mg·g−1 for CV dye and 568.1 mg·g−1 for TCQmax. After 4 regeneration cycles, it has good recyclability. | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, F.; Liu, J.; Xiang, Z.; Fan, W.; Liu, J.; Wang, J.; Wang, Y.; Wang, L.; Xi, B. Degradation of Water Pollutants by Biochar Combined with Advanced Oxidation: A Systematic Review. Water 2024, 16, 875. https://doi.org/10.3390/w16060875
Kong F, Liu J, Xiang Z, Fan W, Liu J, Wang J, Wang Y, Wang L, Xi B. Degradation of Water Pollutants by Biochar Combined with Advanced Oxidation: A Systematic Review. Water. 2024; 16(6):875. https://doi.org/10.3390/w16060875
Chicago/Turabian StyleKong, Fanrong, Jin Liu, Zaixin Xiang, Wei Fan, Jiancong Liu, Jinsheng Wang, Yangyang Wang, Lei Wang, and Beidou Xi. 2024. "Degradation of Water Pollutants by Biochar Combined with Advanced Oxidation: A Systematic Review" Water 16, no. 6: 875. https://doi.org/10.3390/w16060875
APA StyleKong, F., Liu, J., Xiang, Z., Fan, W., Liu, J., Wang, J., Wang, Y., Wang, L., & Xi, B. (2024). Degradation of Water Pollutants by Biochar Combined with Advanced Oxidation: A Systematic Review. Water, 16(6), 875. https://doi.org/10.3390/w16060875






