Abstract
Floating aquatic macrophytes have a high level of proficiency in the removal of various contaminants, particularly nutrients, from wastewater. Due to their rapid growth rates, it is imperative to ensure the safe removal of the final biomass from the system. The ultimate macrophyte biomass is composed of lignocellulose and has enhanced nutritional and energy properties. Consequently, it can serve as a viable source material for applications such as the production of bioenergy, fertilizer and animal feed. However, its use remains limited, and in-depth studies are scarce. Here, we provide a comprehensive analysis of floating aquatic macrophytes and their efficacy in the elimination of heavy metals, nutrients and organic pollutants from various types of wastewater. This study offers a wide-ranging scrutiny of the potential use of plant biomasses as feedstock for bioenergy generation, focusing on both biochemical and thermochemical conversion processes. In addition, we provide information regarding the conversion of biomass into animal feed, focusing on ruminants, fish and poultry, the manufacture of fertilizers and the use of treated water. Overall, we offer a clear idea of the technoeconomic benefits of using macrophytes for the treatment of wastewater and the challenges that need to be rectified to make this cradle-to-cradle concept more efficient.
1. Introduction
Water is the most critical natural resource on the planet [1]. The global water crisis has been highlighted as a highly serious ongoing and future issue in the global risk report from the World Economic Forum [2]. Additionally, it has also been anticipated that two-thirds of the world’s population is supposed to face a water shortage within the next two decades [1]. The rapid increase in anthropogenic activities to compensate for the water demand of the growing population has intensified the withdrawal of fresh water [3]. This has resulted in water quality impairment and increased effluent quantities [4,5]. Currently, we are witnessing a universal trend of water mining and usage, taking the line of least resistance regarding water scarcity and substandard effluent quality [6,7].
Wastewater consists of organic matter, nutrients, heavy metals, explosives, radioactive elements and specific organic and inorganic chemicals, including micropollutants and microorganisms, that are above the permissible level unless they pass through a treatment system [8,9,10]. Wastewater, according to its origin, can be broadly categorized as domestic, industrial, agricultural, leachate and stormwater discharge [9]. Domestic wastewater, also known as household sewage, is comprised of waste from the kitchen, shower, toilet, washbasin and laundry. Since the solid content is approximately 0.1%, the availability of toxicants and the huge quantity of water being expelled make domestic sewage treatment more challenging [1,11]. Industrial wastewater is more difficult to treat as it contains various toxicants at high concentrations, and the constituents of industrial wastewater vary depending on the industry [12]. Agricultural runoff is generally the outlet water from farmlands and contains toxicants conveyed from residual fertilizers and other chemicals used in agriculture [11]. Landfill leachate has negative impacts on the environment because of its high levels of organic nitrogen and ammonia [13]. Water flowing across terrestrial areas after rain or snowmelt is considered stormwater runoff; as it does not originate from a single channel, its constituents can largely vary.
When wastewater reaches the environment without prior removal of or reduction in contaminants, it can threaten living organisms [14]. In general, water bodies are the ultimate sites of effluent discharge [15], and therefore, wastewater plays a significant role in controlling the quality of water bodies by influencing their water quality parameters [16]. Toxified water with high organic matter and nutrient levels will lead to oxygen depletion in water bodies due to the stimulated growth of microbes and aquatic macrophytes, technically termed “eutrophication”. Eutrophication is characterized by abrupt algal growth, the development of plankton scum, the death and replacement of fish and other organisms and increased water sedimentation and turbidity. This facilitates the development of pathogenic microorganisms, resulting in the spread of waterborne diseases [17]. Specifically, the availability of nitrogen above the threshold level in water bodies causes blue baby syndrome in infants [18].
The occurrence of heavy metals in water bodies may cause either acute or chronic diseases [19]. After consumption, heavy metals will not be localized to the primary consumer but transmitted to different levels of consumers from their prey in the food chain via biomagnification [20], resulting in the death of the end consumer. Overall, pollutants in water bodies will adversely affect the aquatic ecosystem and result in the collapse of biodiversity, which is not limited to aquatic ecosystems [21].
Globally, it is estimated that approximately 80% of wastewater is disposed of without any adequate treatment [22]. This practice is more prevalent in developing countries because of insufficient treatment and disposal systems [23,24]. Hence, both the scientific community and industrial units must collaborate to develop efficient wastewater treatment and disposal systems.
The treatment of wastewater before its mixing with water bodies is indispensable for a safer environment. Treatments remove or reduce contaminants from wastewater and recover resources whenever possible. Pollutant removal can be accomplished by different treatment methods, such as physical, chemical and biological processes, as shown in Table 1. Each method consists of a variety of treatment techniques [25,26].

Table 1.
Different wastewater treatment methods and respective processes.
The selection of an appropriate treatment method is critical as it needs to meet the selection criteria; predominantly, the treatment should be efficient, economically viable and environmentally friendly. According to a previous study, biological treatment methods are generally more adaptable than other methods [25]. Economic advancement due to no or low energy consumption, eco-friendly attributes and operating flexibility make the biological treatment more feasible despite its minimal shortcomings [27,28]
Phytoremediation is a biological treatment method using plants to eliminate contaminants from wastewater, groundwater and soil [29,30]. Subsequently, wherever possible, the extracted resources, along with the plants, will be used for other purposes, such as animal feed production [8], drug formulation [31], bioenergy production [32] and soil fertility improvement and reclamation [33]. The suitability of macrophytes in post-treatment resource recovery and product development frames the floating phytoremediation process as a cradle-to-cradle (C2C) design, which is a novel approach to eliminate waste and create a circular economy. In this context, floating phytoremediation is regarded as an efficient and environmentally friendly method for the treatment of wastewater [34]. In this paper, we discuss the use of selected floating aquatic macrophytes (FAMs) in the removal of various pollutants and present the post-treatment potential of selected FAMs regarding their contribution to a circular economy. This approach can validate the effects of combining macrophytes with other treatment methods for the protection of water bodies.
1.1. Aquatic Macrophytes
Aquatic macrophytes are a set of diverse photosynthetic organisms that can be seen by the naked eye and belong to different divisions of the kingdom Plantae, such as Chlorophyta, Bryophyta, Pteridophyta and Spermatophyta [35]. The availability of aquatic macrophytes from more primitive divisions is lower than that of vascular macrophytes. As shown in Figure 1, aquatic macrophytes are generally categorized based on their growth forms, such as emergent macrophytes (pickerelweed, tape grass), submerged macrophytes (American pondweed, Chara), free-floating macrophytes (duckweed, pistia) and floating leaf macrophytes (fragrant waterlily, spatterdock) [36].
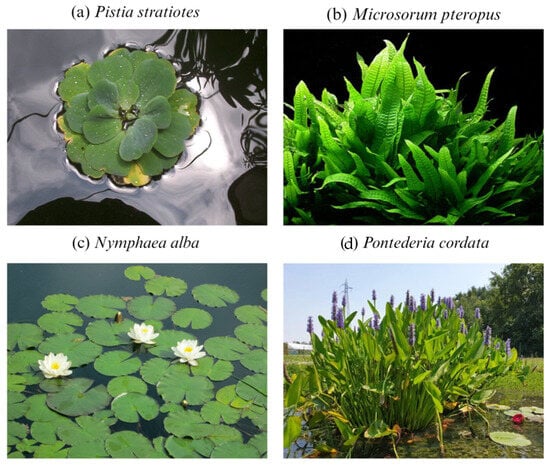
Figure 1.
Different types of aquatic macrophytes.
Aquatic macrophytes use the nutrients available in the water bodies and convert them into biomass [37]. This capacity has been studied in detail to investigate their capability to thrive under various nutrient and pollutant conditions in view of their application in wastewater treatment. As a result, few plant species with remediating potential have been identified so far [38]. The recommended macrophytes are expected to possess some additional qualifications, such as (1) the capability to extract and accumulate, transform, degrade or volatilize contaminants; (2) high growth rates; (3) the simultaneous remediation of multiple pollutants; (4) dense root and shoot systems to support bioaccumulation and biosorption; (5) resistance to pests and disease; and (6) unattractiveness to animals to ensure the cessation of toxicant transformation through the food chain [39,40,41,42].
Macrophytes can remove, transform or stabilize nutrients [43], heavy metals [44], pharmaceutical compounds [45], endocrine-disrupting chemicals (EDCs) [46], radionuclides [47] and microorganisms [26]. This can be achieved via five different phytoremediation techniques, namely phytoextraction, rhizofiltration, phytostabilization, phytodegradation and phytovolatilization [48,49].
Via phytoextraction, the accumulated contaminants are eliminated by harvesting the biomass [50,51]. Phytoextraction is either a continuous process, using hyperaccumulating or fast-growing plants, or an induced process, using chelates to improve the bioavailability of the metals [52,53]. Rhizofiltration is related to the absorption, concentration and precipitation of inorganics (metal ions) and organics [54] through plant roots over a certain period [55,56]. Phytostabilization occurs through sorption, precipitation, complexation and metal valence reduction. Traits, genotypes and root physiology control the mobility and bioavailability of contaminants [50,57,58]. Phytovolatilization deals with eliminating the absorbed contaminants in gaseous form to the atmosphere via evapotranspiration [59].
In phytodegradation, the contaminants are either accumulated in the plant tissue and converted into less toxic compounds through metabolic activities or directly decomposed with the help of enzymes released by the plant roots [60,61]. In addition, the capability of improving dissolved oxygen, competing with microorganisms for food and sunlight and the physical filtration capacity using the dense root system make them more efficient in treating the various types of wastewater efficiently. Macrophytes are proficient in transforming oxygen through roots into the constructed wetland systems, which can accelerate the organic waste degradation and reduce the pollutant loads [62]. On the basis of previous studies, macrophytes can directly absorb organic matter [63] and can also reduce sunlight penetration [64] into the wastewater treatment systems, thereby reducing the establishment and multiplication of pathogenic microorganisms and supressing harmful algal blooming. Physical filtration is another mode of supportive mechanism in the wastewater treatment rendered by macrophytes [65], in which the dense and complex fibrous root systems can filter and trap various sizes of particles.
As phytoremediation processes do not require external energy, macrophyte-based treatment is economically viable and sustainable [38,66,67,68]. This approach can be seen as an alternative to chemical and physical treatments because of the possibility to meet the desired standards established for primary, secondary and tertiary effluents [69]. Macrophytes have been used for the treatment of wastewater for more than four decades [40]. Currently, this method is gaining considerable attention, making it an emerging technology [38].
1.2. Floating Aquatic Macrophytes
Floating macrophytes (FAMs) are a distinctive category of aquatic macrophytes as they are exposed to the atmosphere and can exist as producers either in turbid or high-water-depth conditions. They generally prefer water areas with little or no movement [36] and use phytoextraction, phytodegradation, phytovolatilization and rhizofiltration to remove contaminants [70]. Further, they support microbial decomposition in water bodies by associating with secondary carbon sources and facilitating nitrogen removal through denitrification [71]. Regardless of some drawbacks, such as preventing the photosynthesis of submerged organisms and being a barrier to oxygen dissolution from the atmosphere to the water bodies [36], FAMs play a crucial role in pollutant removal, producing biomass with a high nutritive value [69,70,71,72].
According to previous studies, FAMs can scavenge metal ions [73,74,75,76]. Specifically, the roots are involved in the active uptake of metal ions and their translocation to other tissues [77,78]. The roots also play a key role in facilitating microbial growth by providing a high specific surface area. Passive metal-ion uptake is dominated by the aerial parts that are in contact with the wastewater [77]. In this regard, the most common FAMs, such as water hyacinth, water fern and duckweed, have been extensively studied for their ability to improve wastewater quality. Their rapid growth, hyperaccumulation and availability favour their use in remediation projects [49,79,80].
Water hyacinth (Eichhornia sp.) is a floating aquatic weed, shown in Figure 2a, that belongs to the family Pontederiaceae and is the most commonly used macrophyte due to its high availability and adaptability and high growth rate [81]. Native to South America, it now occurs in almost all tropical countries. Significant amounts of money have been used to control the spread of water hyacinth. The genus Eichhornia contains seven species, namely E. azurea, E. crasspes, E. diversifolia, E. heterosperma, E. natans, E. paniculata and E. paradoxa. Among them, E. crassipes is the most common one [82]. The species of this genus can thrive in hazardous environments and produce biomass amounts of 60–100 t/ha/yr, making them highly effective in treating wastewater and predominant in subsequent resource recovery [83,84].

Figure 2.
Predominant strains of floating aquatic macrophytes.
Duckweed is one of the most abundantly available small angiosperms, shown in Figure 2b, without any distinctive systems or leaves. It belongs to the family Lemnaceae and includes four genera, namely Lemna, Spirodela, Wolffia and Wolfiella, with a total of 37 species [85]. The growth rate of duckweed is higher than that of other large macrophytes. Ziegler, et al. [86] reported that under in vitro conditions, the doubling time of duckweed varies from 1.34 to 4.54 days, with an annual biomass output of 39.2–44 t dw/ha/yr. Duckweed is becoming increasingly popular due to its growth habits and capacity to withstand highly toxic conditions. It can accumulate heavy metals and serves as a metal indicator [87], making it an important plant in the treatment of heavy-metal-contaminated sites [88].
Water fern, also known as mosquito fern, is a tiny aquatic macrophyte, shown in Figure 2c, that occurs in both tropical and sub-tropical regions. It belongs to the genus Azolla and the family Salviniaceae, with two subgenera and six species. Duckweed can fix atmospheric nitrogen through a symbiotic relationship with Anabaena azollae, allowing it to survive in sites with low nitrogen levels [89]. This macrophyte can take up different heavy metals from wastewater streams [90,91,92] and remove large amounts of nitrogen and phosphorous, as high as 2.6 t N/ha/yr and 0.434 t P/ha/yr, and has been effectively used in phytoremediation [93,94]. Azolla is one of the fastest growing aquatic genera, with a doubling time from 5 to 7 days and a biomass production of 93.4–100 t dw/ha/yr [81,95]. Floating aquatic plants can grow in vertical as well as horizontal directions, thereby increasing their photosynthetically active surface area, making them some of the most productive communities [96].
2. Pollutant Removal
Macrophytes have been used in the removal of toxic compounds individually or as components of constructed wetlands to purify various wastewater types [97]. Based on recent studies on the use of macrophytes, there is a trend toward the simultaneous removal of multiple pollutants [22,98]. Different macrophytes have been studied regarding their potential to remove various pollutants, organic matter, nutrients, heavy metals and pathogens. Factors such as plant tolerance, the feasible range of toxicants that plants can accumulate, the concentration of toxicants in the medium and environmental factors largely impact the remediation capability of macrophytes [99]. It is not always appropriate to use living aquatic macrophytes for the continuous removal of harmful pollutants. After becoming saturated with pollutants, pollutant uptake decreases, and the plant will eventually perish due to the detrimental effects of the pollutants on plant growth and metabolism [100]. Therefore, although macrophytes are widely used in wastewater treatment, the application of dead or inactive parts or any substances obtained from biological sources is a better choice in continuous wastewater treatment.
When the biomass is alive during the treatment of wastewater, the process is denoted as “bioaccumulation”, whereas the use of dead biomass is termed “biosorption” [101]. During the former process, the toxic compounds attach in an inter- and intracellular manner, whereas in biosorption, such attachment is extracellular. Absorption, a double-stage active process, is responsible for pollutant removal via bioaccumulation, whereas in biosorption, a single-stage passive process, adsorption controls pollutant removal. Further, while desorption is only partially possible for bioaccumulation, biosorption also includes desorption. Generally, the removal performance is higher in biosorption than in bioaccumulation [102,103].
Water hyacinth can effectively be used in the removal of pollutants by chemical, biological, mechanical or hybrid means [104]. It can eliminate inorganic nitrogen [nitrate (NO3-N), ammonium (NH4-N), and total N)] and phosphorus (PO4−3-P and total P) from nutrient-rich wastewater [96]. Duckweed is another promising macrophyte with high potential in the removal of a wide spectrum of pollutants (organic pollutants, heavy metals, agrochemicals, pharmaceuticals and personal care products, radioactive waste, nanomaterials and hydrocarbons) from wastewater and can thrive in highly contaminated water [96]. Duckweed has been used in the treatment of low-strength domestic wastewater to high/severe-strength industrial wastewater streams to obtain clean, non-potable water [105,106]. Some authors recommend the use of duckweed after the removal or conversion of organic sludge into simple organic and inorganic molecules as they can be easily taken up by this macrophyte [107]. Another aquatic macrophyte with a high potential for pollutant removal is Azolla [108]. In combination with Anabaena azollae, it efficiently removes nutrients even after complete nitrogen depletion. The species A. pinnata can effectively be used in the treatment of domestic and industrial effluents [8,109,110].
Since FAMs grow rapidly and eliminate large amounts of pollutants with high removal rates through their extensive fibrous root system and aerial parts [111], they have been studied extensively regarding the removal of different pollutants such as heavy metals, nutrients and different organic compounds in wastewater. In the following section, we discuss in detail the capacity of different FAMs to remove various pollutants.
2.1. Heavy Metal Removal
Heavy metals are major pollutants in aquatic environments due to their high toxicity, non-degradable nature and bioaccumulation and biomagnification [10,112,113,114]. Aquatic macrophytes play a crucial role in the removal of heavy metals from the aquatic environment [115]. Table 2 shows the heavy metal uptake capacities of different FAMs, either via bioaccumulation or biosorption [101,116,117]. During the uptake of heavy metals at the whole plant and cellular level, plants absorb the metals based on the negative charges of their cell walls. Subsequently, the metals are transported into the cell cytoplasm and partitioned into cell organs or excreted [118]. Plants can accumulate 100,000 times higher concentrations of heavy metals compared to the effluent concentration [119].

Table 2.
Heavy metal uptake by different floating aquatic macrophytes.
Live water hyacinth can remove large amounts of heavy metals through absorption and translocation to shoots and other tissues [141]. Dried water hyacinth and ash obtained from water hyacinth have also been used to adsorb heavy metals from waste streams [121,142,143].
Jones, et al. [120] conducted a study in the British River and reported the most pronounced heavy metal removal (21 heavy metals) using water hyacinth. After an exposure period of 7 h, 63% of Al, 62% of Zn, 47% of Cd, 22% of Mn and 23% of As were removed. Under in situ conditions, the authors reported the removal of Mn, Zn and Cd at 6%, 11% and 15%, respectively.
Bais [144] explored the biosorption ability of the shoots and roots of water hyacinth in the rainy season as well as in winter and summer. Based on the findings, during winter, 31% of Cd was removed via the shoots and 41% via the roots. Bianchi, et al. [136] reported that A. filiculoides can efficiently eliminate Fe and Al, with removal rates of 92% and 96%, respectively, whereas only 10% of Cr could be removed.
When comparing the biosorption capacities of Azolla fliculoides and Hydrilla verticillata regarding the removal of Cu(II), Cr(VI), As(III) and Pb(II), Bind, et al. [145] found that Pb was effectively absorbed by both species, with removal rates of 81.4% and 84.3%, respectively, from a synthetic wastewater stream containing Pb at a concentration of 10 mg L1. The adsorption capacity followed the order Pb(II) > Cu(II) > As(III) > Cr(VI).
Chaudhary and Sharma [129] investigated the efficiency of Lemna gibba in removing Cr and Cd from solutions with varying concentrations under laboratory conditions. The experiments were carried out for 7 and 15 days, and the removal rates were 37.3% to 98.6% for Cr and 81.6% to 94.6% for Cd. The removal capacity of this species decreased with increasing metal concentrations.
Yilmaz and Akbulut [146] evaluated the efficiencies of two different species of duckweed, namely L. minor and L. gibba, regarding metal removal under aeration. The removal rates were Pb 57%, Ni 60%, Mn 60% and Cu 62% for L. minor and for L. gibba. Aeration and the combination of these species increased the removal rates.
2.2. Nutrient Removal
In water bodies, nutrients are essential for the survival of aquatic biomes. However, above certain thresholds, they can become toxic to various organisms. Since aquatic plants can thrive in high nutrient concentrations and produce large amounts of biomass, they remove nutrients from wastewater. Table 3 shows the nutrient uptake capacities of different FAMs. Nitrogen and phosphorous are the key nutrients, accompanied by carbon at a certain level. Excess nitrogen and phosphorous accumulation results in water eutrophication, with negative impacts on ecosystem health. To satisfy the physiological requirement of macro- and micronutrients and to support the epiphytic biofilm growing on the surface, macrophytes will consume nutrients through assimilative uptake, which is the direct method of nutrient removal [147]. Further, the macrophytes can additionally support the treatment system indirectly in nitrogen removal by enhancing the nitrification and denitrification process through generating a spatial oxygen gradient across the treatment system [148].

Table 3.
Nutrient uptake by floating aquatic macrophytes.
Kadir, et al. [156] carried out a preliminary study to determine the appropriate dilution of palm oil mill effluent (POME) to successfully grow L. minor and A. pinnata and to evaluate the corresponding nutrient removal rates. Both species showed high ammonia removal, with rates of 98% and 95.5%, respectively, in 5% POME. Phosphate removal was higher in 10% POME, with 93.3% removal by A. pinnata and 86.7% by L. minor. Overall, A. pinnata showed a significant nutrient reduction in 2.5% POME.
In another study, the authors performed a 2-week experiment to test the nutrient removal capacities of L. minor and A. filiculoides from textile, distillery and domestic wastewater mixtures. There were no significant differences in the nutrient removal rates between the species; A. filiculoides removed 94.6% of phosphorous, and L. minor removed 92% of phosphorous. Total nitrogen was more efficiently removed by A. filiculoides (94.6%) compared to L. minor (92%) [132]. Similarly, Verma and Suthar [164] investigated the capacity of L. gibba to treat sewage; L. gibba removed 42–64% of nitrate and 37–54% of total phosphorous.
Singh, et al. [165] investigated the potential of Eichhornia crassipes in removing nitrogen and phosphorous from glass industry effluent (GIE). This study was supported by a response surface methodology and an artificial neural network for optimization and prediction. Diluting the GIE to 60% and treating it with GIE showed the best results in terms of the removal of total Kjeldahl’s nitrogen (93.9%) and total phosphorus (87.4%).
2.3. Organic Contaminant Removal
Organic pollutants are broadly categorized into two major groups: oxygen-demanding waste and synthetic organic pollutants. Wastewater from municipalities and the food industry, paper mill effluent and animal farm wastewater contain more biodegradable compounds that can be degraded by microorganisms, resulting in a higher oxygen demand and, ultimately, in anoxic conditions. Plants can effectively remove simple organic matter, which requires high oxygen demand during decomposition, and their effectiveness was tested by several researchers. El-Kheir, et al. [166] used L. gibba to treat primary treated sewage and observed BOD (biological oxygen demand) and COD (chemical oxygen demand) decreases by 90.6% and 89.0%, respectively. Another study was carried out by Bhagavanulu, et al. [167] to evaluate the biosorption capacity of the root, stem and leaf powder of water hyacinth. Root and stem powder were effective in turbidity management. The maximum BOD reduction of 49.2% was observed when the root powder was used for 30 min. A COD reduction was observed when a combination of leaf and root powder, in equal amounts, was used. Sahi and Megateli [168] investigated the ability of L. minor to reduce the COD in real dairy wastewater (RDW) and synthetic dairy wastewater (SDW) over a period of 10 days, and this species was more effective in removing COD from RDW (60%) compared to SDW (55.5%). Mamat, et al. [169] determined the efficacy of Azolla pinata in the treatment of palm oil mill wastewater and reported that this species effectively removed 85.89% of the BOD and 80.58% of the COD.
Synthetic organic compounds are produced by synthetic detergents, agrochemicals, specific food additives, pharmaceuticals, synthetic fibers and plastics [170]. Organic pollutants of the category persistent organic pollutants (POPs) are more dangerous because they can remain in the food chain and have a longer half-life [171]. Endocrine disruptive chemicals are a subdivision of synthetic organic compounds with the capacity of creating hormonal imbalances and affecting reproduction development or behaviour in animals and causing irregular endocrine behaviour and cancer in humans [172]. The increase in the amounts of endocrine-disrupting chemicals in most waste streams has resulted in public concerns regarding their elimination [173]. Chlorophenols, bisphenol A, dichlorodiphenyltrichloroethane (DDT), chlorpyrifos, atrazine, 2, 4-D and glyphosate are widely available endocrine-disrupting chemicals [108,174].
Pharmaceuticals are another subclass of synthetic organic pollutants, and their use has increased recently. Anti-inflammatories, antidepressants, antiepileptics, lipid-lowering drugs, β-blockers, anti-ulcer agents, antihistamines and antibiotics [175] are organic pollutants derived from the pharma industry. As an example, diclofenac, a nonsteroidal anti-inflammatory drug, has gained attention because it persists in municipal wastewater [176].
Table 4 shows the capacities of different FAMs to take up organic pollutants. Campos, et al. [177] investigated the efficiency of E. crassipes and Cyprus isocladus in different constructed wetlands to eliminate endocrine disruptors from synthetic municipal wastewater. The removal rates varied from 9.0 to 95.6% for ethinyl estradiol, 29.5 to 91.2% for bisphenol A and 39.1 to 100.0% for the progestin levonorgestrel. Zazouli, et al. [108] reported the removal of bisphenol A by Azolla, with removal rates from 60 to 90%. In a study by Bianchi, et al. [136], by using L. minuta and A. filiculoids, diclofenac was removed at removal rates below 10%, whereas higher rates were observed for the removal of levofloxacin, with rates of 50% and 60%, respectively. Xia and Ma [178] investigated the removal of the phosphorus pesticide ethion with water hyacinth. The plant accounted for 69% of the removal of ethion from the waste stream through uptake and phytodegradation, but the roots and shoots emitted ethion at levels of 74–81% and 55–91%, respectively, in ethion-free medium after a growth period of 7 days. In a study by Balarak [179], 2-chlorophenol (2-CP) and 4-chlorophenol (4-CP) were removed from agropharma waste using Azolla, with removal rates of 71% and 85%, respectively. Garcia-Rodríguez, et al. [180] tested the potential of duckweed to remove carbamazepine, acetaminophen, propranolol, ibuprofen, diclofenac, caffeine, bisphenol A, and 17-a-ethinylestradiol from secondary treated wastewater, and the observed removal rates are promising.

Table 4.
Organic pollutant uptake by floating aquatic macrophytes.
Dyes are another group of organic pollutants in the wastewater stream and are mainly derived from industrial plants and households. According to Kant [196], around 8000 chemicals are associated with dyeing processes and pose risks to environmental and human health. For example, crystal violet, a commonly used dye, is mutagenic and carcinogenic. Color removal from dyes is a serious problem as it consumes more oxygen and increases the BOD value in the waste stream [197]. Kulkarni, et al. [197] studied the biosorption capacity of water hyacinth root powder for the decolorization of crystal violet dye and obtained a Langmuir monolayer biosorption capacity of 322.58 mg/g. These authors further examined the influence of initial pH, initial dye concentration, biosorbent dosage, contact time and temperature on dye removal and found that water hyacinth was an effective biosorbent to remove crystal violet dye from an aqueous solution. Nath, et al. [198] investigated the biosorption capacity of water hyacinth in the removal of industrial dyes such as methylene blue, Congo red, crystal violet and malachite green from aqueous solutions at laboratory scale and observed maximum removal rates of 90%, 88%, 92% and 90%, respectively. According to Padmesh, et al. [199], Azolla can efficiently be used in the removal of acid blue 15 and eliminated 61.3% of this dye from an aqueous solution through biosorption. Durairaj [200] and Imron, et al. [201] tested the effectiveness of L. minor in removing methylene blue and textile acid orange 10, respectively, with contact times of 1 day for methylene blue and 4 days for acid orange. The removal rates were 80.66% for methylene blue and 82.9% for acid orange.
3. Circular Economy in Phytoremediation
The current production perspectives are directly linked to resource extraction and product transformation, which are not sustainable as these resources are limited [202]. Rather than such a linear economy concept, which is based on “take–make–dispose”, the circular economy running in closed loops is more effective in terms of resource sustainability [203]. According to Webster [204], a circular economy is one that is restorative by design and that aims to keep product components and materials at their highest utility and value at all times. Cradle-to-cradle principles and the laws of ecology are the main pillars supporting this concept [205,206]. Regarding the wastewater sector, the circular economy emphasizes the extraction and use of possible resources, including water, as shown in Figure 3.
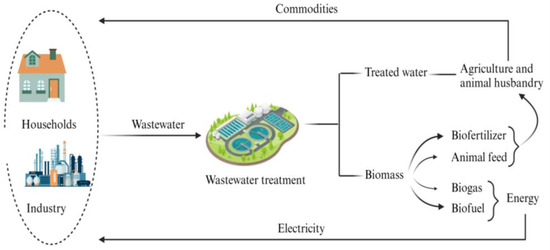
Figure 3.
Implementation of circular economy in FAM wastewater treatment.
Wastewater may contain different components such as energy, nutrients, heavy metals, biopolymers and antibiotics [207,208,209]. Effluents from different sources are rich in different resources, depending on the point of waste generation. Hence, it is obvious that wastewater is a potential source of various resources, and different chemical and physical methods have been employed to extract these resources. For example, Yangui and Abderrabba [210] extracted polyphenols from olive oil wastewater via adsorption, and Li, et al. [211] separated proteins from soybean wastewater using complexation. Li, et al. [212] removed heavy metals using calcination with nitrogen, using magnesium chloride as an additive. However, these processes and energy demanding and not environmentally friendly, calling for “greener” alternatives.
Although macrophytes are commonly used for wastewater treatment, the disposal of the harvested biomass is challenging [213,214]. Since the harvested biomass is rich in nutrients and can be used to produce energy via thermochemical and biochemical processes, it can be directly used to generate fuel, feed or fertilizer [215]. Water hyacinth, Azolla and duckweed are predominant aquatic weeds in Asian countries, with a high potential to remove nutrients from waste streams and to produce high biomass amounts [89]. The biomass production of prominent FAMs is shown in Table 5. Biomass production varies depending on the plant type and the prevailing environmental circumstances. Based on the table, Eichornia crassipes has the highest biomass production capacity, whereas Lemna sp. has the lowest one.

Table 5.
Productivity of prominent floating aquatic macrophytes.
3.1. Bioenergy Production
Excessive consumption, the emission of toxic substances and global warming are major concerns related to the use of fossil fuels [221]. The Paris Climate Agreement of 2015 emphasizes that nations should limit temperature rise to 1.5 °C by any means possible. Due to the high global demand for energy, the production of fossil fuels continues to dominate the energy section, accounting for 81% [222]. However, after continuous growth, the fossil fuel industry, which contributes remarkably to CO2 emissions, is expected to decline. It has been anticipated that 56% of fossil methane gas, 58% of oil and 89% of coal have to be limited to the percentages available in 2018 to ensure a probability of 50% in achieving the Paris Climate agreement of 2015 [223]. This scenario will create a gap between the demand for and supply of global energy. Therefore, to avoid energy shortages, it is crucial to propose alternative energy sources [224]. In this context, the use of renewable energy resources appears to be an effective solution.
Bioenergy can be seen as one of the potential players in the renewable energy context [225]. There are sets of bioenergy production technologies, such as the production of bioethanol, biodiesel and biomethane, that receive more attention in substituting traditional energy sources. Currently, it is difficult to find biomass feedstock of high quality and quantity to produce bioenergy. This trend has shifted the search for biomass away from edible feedstock to lignocellulosic or algal biomass [226]. Several authors encourage the usage of macrophyte biomass as an alternative for first-generation feedstock to produce biofuel. The ability of macrophytes to proliferate rapidly and produce higher biomass amounts via sequestering nutrients from effluents makes them potentially suitable for bioenergy production [89]. The desired biomass constituents, such as proteins, lipids and carbohydrates, along with low lignin and higher cellulose and hemicellulose contents, as shown in Table 6, are advantages [227,228,229]. Macrophytes, which perform well in nutrient uptake from wastewater and are capable of producing efficient biomass feedstock, can potentially be applied in integrated wastewater treatment and bioenergy production [89]. In recent years, bioenergy production via biochemical and thermochemical processes in aquatic macrophytes has received increased attention.

Table 6.
Lignocellulosic biomass composition of specific floating aquatic macrophytes.
3.1.1. Biochemical Conversion
Biochemical conversion is a prominent technology used to produce multiple biofuels, such as bioethanol, biomethanol, biodiesel, Fischer–Tropsch diesel and gaseous fuels such as biomethane and biohydrogen. These end products can be attained through the anaerobic digestion, alcoholic fermentation and acidogenic fermentation of aquatic macrophyte feedstock. Hossain, et al. [242] conducted a study on ethanol production using a biomass of water hyacinth (E. crassipes) and Azolla sp. (A. pinnata) as feed stock for fermentation. Water hyacinth showed a higher ethanol yielding capacity (0.32 g/g) than Azolla sp. (0.20 g/g ethanol). Magdum, et al. [243] and Das, et al. [244] used Pichia stipitis for the production of ethanol from the hydrolysate of water hyacinth and obtained 19.2 and 10.44 g/L, respectively. Su, et al. [245], using duckweed as a substrate for producing higher alcohols, reported that duckweed is a suitable fermentation biomass substrate that requires basic pre-treatment, without the need for supplementary nitrogen or strengthening with redox agents; the production of biofuel from duckweed could be achieved through bioconversion by Clostridium acetobutylicum and Escherichia coli. The biofuels produced are not limited to traditional forms of energy, such as ethanol, and higher-energy alcohols with higher energy yields can also be produced. Xu, et al. [246] investigated the capacity of duckweed to produce ethanol after transferring it from piggery farm effluent to well water and sustaining it for 10 days. The final ethanol production was 6.42 × 103 L/ha, which is 50% higher than that obtained with the use of maize produced in the same area.
Singhal and Rai [247] investigated the biogas production from water hyacinth. The plants were allowed to grow in pulp and paper mill effluent and distillery effluent at various dilutions. Parallel experiments used deionized water as a control. Biogas production was higher in phytoremediation plants than in deionized water plants, and water hyacinth cultivated in 20% pulp and paper mill effluent showed the highest biogas output (23,650,141.4 cc/kg dry weight).
Ramaraj and Unpaprom [248] examined duckweed biogas production at different temperatures. Based on their results, the total biogas yield at ambient temperature was 7863.69 mL/L, whereas the yield under mesophilic conditions (35 °C) was 10,376.59 mL/L, and that under thermophilic conditions (50 °C) was 9981.08 mL/L, with a maximum methane concentration of 64.47%. This study emphasizes that duckweed biomass substrate has the highest biogas production rate in the mesophilic temperature range.
Patil, et al. [249] explored the biogas production efficiency of water hyacinth treated with NaOH, combined with poultry waste and primary sludge. Fresh water hyacinth was used as a control. The highest cumulative biogas yield of 0.38 L/g was obtained from water hyacinth combined with poultry waste. A high methane percentage of 71% was found in the treatment using water hyacinth pretreated with NaOH. Other studies investigating bioenergy production through biochemical conversion are listed in Table 7; most of them focused on biogas production.

Table 7.
Use of floating aquatic macrophytes in bioenergy production.
3.1.2. Thermochemical Conversion
Thermochemical conversion is the process of decomposing biomass into solid, liquid and gaseous fuel products via thermal processing. It encompasses gasification, pyrolysis and hydrothermal liquefaction techniques. Pyrolysis is an anoxic thermochemical process through which the biomass is converted into bio-oil, carbon-rich solids and volatile matter [262]. Gasification is the process of incompletely burning biomass to produce CO, H2 and CH4. The produced mix is called producer gas and used as fuel for engines [263]. Hydrothermal liquefaction is another novel technique for thermochemical conversion in which the biomass is depolymerized to produce biocrude oil and chemicals at a moderate temperature and high pressure [264].
Miranda, et al. [218] explored the influence of temperature on the bio-oil production efficiency of A. filiculoides through hydrothermal liquefaction after participation in treating selenium-rich synthetic wastewater (SeSW). After 5 days of treatment with SeSW, the produced total bio-oil accounted for 15.8%, 21.5% and 16.0%, respectively, at 260, 280 and 300 °C for 15 min. Biswas, et al. [257] investigated the pyrolysis of Azolla sp., Sargassum enerrimum and water hyacinth using a fixed-bed reactor at different temperatures in the vicinity of nitrogen. Azolla sp., S. tenerrimum and water hyacinth produced 38.5, 43.4 and 24.6 weight percentages of liquid yield, respectively, at 400, 450 and 400 °C.
Muradov, et al. [265] analyzed the pyrolysis products of L. punctata and A. filiculoides after their use in swine wastewater treatment. The authors concluded that Azolla and algae produced similar spectra of bio-oils, which were different from the products obtained from duckweed samples. The wide range of petrochemicals and straight-chain C10 and C21 alkanes obtained can be used directly as diesel fuel supplements or as a glycerine-free biodiesel component. Golzary, et al. [266] and Singh, et al. [267] reported the efficient thermochemical conversion of Azolla and water hyacinth to biocrude oil, respectively, with yields of 39% and 29% and 23% and 24.6%, respectively, via hydrothermal liquefaction and pyrolysis.
3.2. Feed Production
With deforestation and the introduction of dwarf plant species, the area of grazing land has declined, along with a decrease in fodder availability.
Commercial feed supplements are being released at a high price to compensate for natural feeding, resulting in increased costs for animal products. In addition, commercial feeds have negative effects on product quality and animal health [268]. When searching for long-term animal feed, macrophytes have been found as a viable alternative to conventional feeds, with promising nutritional values (Table 8) and a high biomass production capacity. In general, it has been evidenced that the control of aquatic weeds consumes more money. Hence, utilizing the macrophytes in animal feed formulations after effective wastewater treatment would be an ideal choice instead of spending more money on both the production of plants, such as maize, sorghum and vegetables, and specialized animal feed and on the control of aquatic weeds [269]. According to de Queiroz, et al. [270], after lifecycle analysis (LCA), they declared that the production of animal feed would be more effective in mitigating freshwater eutrophication and climate change compared to the production of biofuel and biofertilizer production. Therefore, it can be said that the effective utilization of macrophytes or the combination of macrophytes and other nutritional sources in the formulation of the ration would be a more socially, economically and environmentally viable approach.

Table 8.
Nutrition composition of some floating aquatic macrophytes on dry matter basis.
The protein content of Azolla on a dry weight basis can reach 25.4%, with an amino acid profile of 10.2%. However, the carbohydrate and oil contents are comparatively low [284]. Duckweed has a high protein content, which can reach 41% in nutrient-rich media [285,286]. According to its amino acid profile, it is rich in leucine, threonine, valine, phenylalanine and lysine [287]. Specifically, the concentration levels of lysine are close to those of animal protein [288].
The high cellulose and hemicellulose contents of water hyacinth make this plant an energy source for ruminants [289]. In addition, most of these aquatic macrophytes are rich in minerals and vitamins that are essential for the normal functioning of the body. Numerous studies have confirmed the suitability of feeding duckweed, Azolla and water hyacinth to various farm animals such as ruminants, pseudo-ruminants, non-ruminants and fish and shrimp [286,290,291,292,293,294].
3.2.1. Ruminants
Although farmers, particularly in Southeast Asia and probably elsewhere, have developed the use of Azolla as a source of nutrients for livestock, controlled experiments to develop commercial crops are lacking. There are, however, some reports on the use of Azolla as feed supplement for fish and livestock, focusing on fish and domestic animals in which normal feed protein sources have been replaced by Azolla meal on an iso-nitrogenous basis. However, studies on the use of Azolla microphylla as supplementation in the diet of cross-bred cattle are scarce [295].
Pillai, et al. [284] identified an increase in overall milk yield in cattle of up to 15% when they were fed 1.5–2 kg of fresh Azolla per day along with regular feed. Further, the researchers concluded that the increment in milk yield is not only due to the nutrient content of Azolla but also to other components, such as carotenoids, biopolymers and probiotics. An attempt was made to gauge the nutritional impact of Azolla meal in a total mixed ration (TMR) at various dietary levels on the nutrient use and metabolic condition of goats. To this end, goat kids were fed different inclusion levels of Azolla meal (0%, 20% and 40%) mixed with a concentrate mixture and green fodder berseem. The inclusion of 20% Azolla meal resulted in the highest digestibility, and the final weight gain of the goat kids was also significantly higher [296].
The use of duckweed as a ruminant feed source has not received much attention, mainly because of the challenge of gathering enough duckweed for a reliable feed trial. More ruminant studies are, however, expected as the popularity of duckweed grows. Duckweed meal has not been extensively studied as a fodder supplement for ruminants, although duckweed can potentially supply minerals, particularly P and N. A meal for ruminants that includes both fresh duckweed and crop waste may have a balanced nutrient level and can be used in livestock production systems for cattle, sheep and goats [297]. Babayemi, et al. [298] conducted a study in African dwarf goats to determine the potentiality of aquatic fern and duckweed as a protein source for ruminants. According to the initial preference test, goats were more likely to consume dried and fresh duckweed than water fern. Based on the outcomes, in the next step, a balance trial was conducted. Duckweed supplementation considerably increased nitrogen retention compared to the control diet, which consisted of guinea grass only. Another study was conducted to determine the potential use of duckweed in goat nutrition. In this experiment, five different levels of fermented duckweed were incorporated into the diet of goats, namely 0%, 15%, 30%, 45% and 60%. According to the results, a 45% inclusion level resulted in a high efficiency in goats, and it is assumed that such a level can guarantee the supply of sufficient energy and balance the concentration of ammonia and volatile fatty acids in the rumen, thereby optimizing rumen microbial activity [299].
Water hyacinth contains high levels of cellulose and hemicellulose, which could serve as energy sources for ruminants. Fresh water hyacinth has been used as a partial replacement for para grass in diets for cattle [300,301]. The use of wilted water hyacinth in a rice-straw-based diet had a positive effect on feed intake and growth in beef cattle [302]. Water hyacinth can be successfully ensiled with the addition of molasses, rice bran, cassava root and organic acids, and the silages are generally accepted by ruminants. In one study, feeding an ensiled mixture of water hyacinth, rice straw, urea and molasses to dairy cattle resulted in a higher milk yield [303]. Islam, et al. [302] conducted a study on bull cattle to investigate the effect of feeding wilted water hyacinth on growth and nutrient use. Three groups of cattle were fed three different rations (treatments), namely 100% rice straw, 75% rice straw + 25% wilted water hyacinth and 50% rice straw + 50% wilted water hyacinth, along with 2 kg of fresh German grass, 300 g of mustard oil cake and 50 g of common salt per 100 kg of body weight. The daily dry matter intake did not vary significantly among the treatments and fluctuated between 3.15 and 3.41 kg. The authors concluded that the total and daily live weight gain were significantly higher in groups that were given wilted water hyacinth supplementation.
3.2.2. Fish
Water hyacinth, Azolla and duckweed have been recommended as dietary supplements for herbivorous and omnivorous freshwater fish [271,304,305]. However, when the fiber content is above the permissible limit, the corresponding macrophyte will not be recommended [306]. Datta [271] conducted a feeding trial to examine the efficiencies of different inclusion levels of dried Azolla, such as 15%, 25% and 35%, in the diet of Labeo rohita. The inclusion of 25% Azolla resulted in the highest specific growth rate of 0.75%/day and the most pronounced weight gain. The obtained condition factor of all fish involved in the experiment ranged between 1.224 and 1.233, whereas the recommended condition factor is between 0.964 and 1.896. This indicates the good condition of the experimental fish. Additionally, a reduction in fat content was observed with the incorporation of Azolla.
Talukdar, et al. [307] investigated the effect of duckweed as a feed on fish polyculture. One treatment was the control (T2), and in the other treatments, the fish were additionally supplied with duckweed daily at 50% of their body weight (T1). Fish from T1 showed a higher survival rate (90%) than those from T2. The net production from T1 was 6.25 t/ha/yr, and that of T2 was 2.84 t/ha/yr. This study emphasizes the use of duckweed as an economically viable feed in fish polyculture. Along these lines, Kabir, et al. [305] determined the consequences of duckweed supplementation in polyculture diets, using ponds fertilized with cow dung, urea and triple superphosphate to grow silver carp, Thai sharputi, tilapia, common carp and mrigal for 90 days with and without duckweed supplementation. Fish from the pond supplied with duckweed exhibited a higher net production compared to the control.
3.2.3. Poultry
Alalade, et al. [308] explored the effects of supplementing Azolla in the diets of growing pullets, using a complete randomized design for 10 weeks with 120 Nera brown pullets. Azolla meals were incorporated at levels of 0%, 5%, 10% and 15% (treatments) with a regular diet. Weight gain (WG), feed intake, feed conversion ratio, packed cell volume, red blood cell, hemoglobin and white blood cells were not significantly different among the treatments. Age at first lay and egg quality characteristics, except egg yolk weight, were similar for all treatments. Yolk weight was lower in hens fed Azolla meal. Based on these findings, Azolla meal can be added to the diet of growing pullets up to a level of 15%. Basak, et al. [309] reported that Azolla in the ration of broilers improves the live weight gain, production number and protein efficiency of broilers at an inclusion level of 5%.
Khandaker, et al. [310] suggested that incorporating 15% duckweed into the diet of laying ducks, instead of mustard oil cake (MOC), has economic benefits. To determine the optimal amount of duckweed to include, the authors used a group of 84 laying Jinding ducks over a period of 75 days. The diet initially consisted of 15% MOC, which was subsequently modified by duckweed in a progressive manner to 5%, 10% and 15%. The addition of duckweed did not result in any notable decline in live weight gain, egg weight or feed conversion efficiency. However, it did lead to an increase in egg production and overall profitability. Conversely, Men and Yamasaki [311] indicated that modifying a commercial diet by adding 5–25% fresh water hyacinth has a detrimental effect on the growth of ducks. However, from a financial perspective, such a modification would still be considered acceptable.
3.3. Fertigation
With the initiation of the green revolution, the negative impacts of certain agricultural practices have become obvious, with widespread diseases and ecosystem degradation. Intense agriculture has promoted the usage of agrochemicals and artificial fertilizers to obtain higher outputs in limited areas, thus causing soil degradation, water depletion and climate change, among other consequences. Apart from their negative impacts on ecosystems, chemical fertilizers are also costly. The energy requirement for producing 1 kg of nitrogen fertilizer ranges from 51 to 68 MJ [312]. The cost of energy, along with other fixed and variable costs, accounts for a large proportion of the money used for fertilizers and other agrochemicals, which is reflected in the price of agricultural products.
The implementation of a sustainable agriculture can reduce the negative impacts on intensive agricultural systems. Sustainable agriculture is a series of agronomic practices that are eco-friendly, economically viable and socially acceptable. Reducing chemical fertilizer usage and adopting organic farming practices are key strategies of sustainable agriculture. According to Tuomisto, et al. [313] organic farming is the best way to attain sustainability in agriculture because it has the capacity to maintain production, along with a healthier soil and biosphere.
Numerous studies emphasize the potential of macrophytes in the production of organic fertilizer. When considering macrophytes as biofertilizer, they are low cost and eco-friendly. According to de Queiroz, et al. [270], from their LCA analysis, producing biofertilizer will mitigate terrestrial acidification and ozone layer depletion more than producing animal feed. If a community wastewater treatment system persists with FAMs, the end of the treatment of the community itself can utilize the macrophytes for biofertilizer production and can try to be a self-sufficient community.
Azolla spp. is one of the vital species which can be processed as biofertilizer, green manure, compost and biochar. The symbiotic relationship with Anabaena azollae facilitates the plant’s ability to fix atmospheric nitrogen and serve as a nitrogen source [314]. In addition to supplying nitrogen, it can supply other essential elements, vitamins, minerals, essential amino acids, growth promoters and organic compounds [284].
Yao, et al. [315] examined the efficiency of Azolla substitution as a biofertilizer in rice fields instead of synthetic nitrogen fertilizer in a field experiment over 3 years with five treatments, namely control without urea (CK), farmers’ nitrogen practice (FN), farmers’ nitrogen practice combined with Azolla biofertilizer (FNA), reducing the nitrogen level by 25% (RN) and substituting Azolla biofertilizer for 25% nitrogen (RNA). The nitrogen use efficiency was high in RNA and FNA, with 52% and 31%, respectively, and both treatments showed reduced nitrogen loss by 48% and 26%, respectively, along with lower ammonia losses. Treatments with Azolla showed increased nitrogen uptake, with levels 17% (RNA) and 33% (FNA) higher than those observed for FN. In addition, the RNA and FNA treatments showed 8% and 14% higher rice yields compared to the yield observed for FNA.
Duckweed is another floating macrophyte that can thrive in environments with high nitrogen and phosphorous levels. Healthy duckweed can be compared to commercial fertilizer in terms of nitrogen availability and used to increase plant productivity in a sustainable manner. Kreider, et al. [316] incorporated dried duckweed into the soil in microcosm, column and field trials and compared it to compost, diammonium phosphate (DAP) and amendment-free soil (control) in terms of biological nitrogen cycling, nutrient retention and crop yield. According to the results, duckweed N mineralization (25 ± 13%) was higher than that of compost (11 ± 12%) and lower than that of DAP (107 ± 21%) in microcosm tests. In the column study, 2% of the added nitrogen was leached out in the duckweed treatment, whereas 60% of N was leached out from the DAP treatment. Regarding the leaching of phosphate, the duckweed treatment showed a higher level of leaching (56%) than compost (27%) and a lower level than DAP (78%). Crop yield was measured in a field after application to sorghum, and the dry mass yield of forage sorghum was highest in the DAP plots (8.69 ± 0.90 Mg ha−1), followed by the duckweed (8.36 ± 1.26 Mg ha−1) and the amendment-free plots (7.93 ± 0.73 Mg ha−1).
Water hyacinth is another potential macrophyte that is also capable of thriving in a wide range of water quality conditions and can absorb nutrients from water. Since it produces large amounts of biomass, it can become noxious if left in the ecosystem after water treatment. Composting and direct use as green manure would therefore be appropriate methods. Lata and Veenapani [317] prepared manure from water hyacinth by processing it for 3 months and 10 days and investigated the efficiency of incorporating water hyacinth manure (WHM) into the soil by determining the growth parameters of Brassica juncea. Different combinations of water hyacinth were prepared as follows: 100% WHM (1:0), 50% WHM (water hyacinth manure + garden soil, 1:1), FYM’W (water hyacinth manure + farmyard manure,1:3) and CNTR (control; no water hyacinth manure, only garden soil, 0:1). The different treatments differed significantly in terms of plant growth and yield parameters. Maximum yield was obtained in 100% WHM, along with the highest values of some growth parameters, such as the number of inflorescences per plant, number of seeds per plant, root weight and dry weight of pods. The 50% WHM treatment also showed the highest values in shoot, root and whole-plant length. Hence, it was concluded that higher levels of WHM inclusion promote the yield and growth attributes of plants.
3.4. Water Usage
Wastewater treated with macrophytes can be a good water source for agriculture and landscape irrigation, washing, cleaning, industrial purposes and groundwater replenishment [318]. Currently, there is a trend towards the reuse of treated wastewater. According to Galkina and Vasyutina [11], the usage of treated wastewater was nearly eight million cubic meters per day in 2017, with an annual increase of 15%. There are several advantages of the use of treated wastewater for purposes other than drinking, such as the maintenance of available freshwater resources without pollution, the reduction of fertilizer use and economic viability [319].
4. Challenges and Recommendations
Although the use of macrophytes in wastewater treatment and subsequent resource recovery are prominent and productive, there are still opportunities, limitations and threats, as shown in Figure 4. While the strengths and opportunities of this treatment process are obvious, the weaknesses and threats of this treatment method can be seen through a common frame as disadvantages.
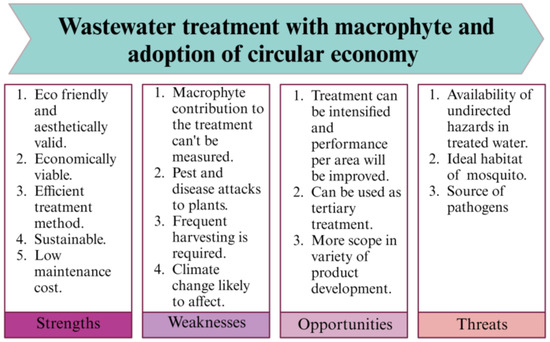
Figure 4.
SWOT analysis of using floating aquatic macrophytes for wastewater treatment and consequent product development.
The common drawback of using macrophytes in wastewater treatment is the complexity of finding the influential depth of the macrophytes in wastewater treatment. In general, the treatment potential of the macrophytes is the sum of the activities of microorganisms, natural decomposition and plant activities. Taking nitrogen as an example, macrophytes are capable of taking up ammonia and nitrate from the wastewater; simultaneously, the algae present in the water use both forms of nitrogen and reduce the nitrogen content. However, the level of ammonia in wastewater is controlled by ammonification and decomposition, and the nitrate concentration in the wastewater is determined by nitrification and denitrification. Therefore, the influence of biotic and abiotic factors makes it more difficult to determine the contribution of macrophytes in pollutant removal [320]. In this case, each component influencing pollutant removal in different types of wastewater should be studied separately.
One of the major issues is the susceptibility of macrophytes to environmental changes; in addition, they are prone to insect and pest attacks. Temperature, relative humidity, sunlight and wind speed are the most influential factors determining the growth of plants. Under sub-optimal conditions, the results are not adequate. Any pest species should therefore be adequately managed to ensure a high efficiency. In most cases, the reason behind the failure of large-scale treatment plants, even if the system worked successfully on a laboratory scale, is the influence of biotic and abiotic factors [321]. To solve these issues, the location of the wastewater treatment plant should be feasible for optimizing the climate to a certain range (e.g., natural shade and accessibility of sprinklers). Furthermore, seasonal variations should be considered when selecting macrophyte species. To overcome the issue of pests and diseases, some plants, such as Chrysanthemum indicum, can be used as border crops, along with organic pesticides, fungicides and bactericides for control.
Various hazards have also been reported when using the products of treated wastewater. As these macrophytes can only treat wastewater up to a certain extent, irrigation using the treated water will result in the accumulation of contaminants in the soil and disturb the soil ecosystem, with potential transfer along the food chain. The use of macrophytes is also risky regarding the production of feed for animals. As an example, duckweed has the capacity to accumulate a variety of heavy metals, which will be transferred along the food chain, threatening human health [297,322]. Macrophytes should therefore be used with caution, and depending on the requirement, treated wastewater can be used.
Although macrophytes are high in nutrients, they may be low in some amino acids such as tryptophan and methionine, necessitating supplementation [323,324]. Another challenge in dealing with macrophyte for wastewater treatment is the fast growth rate [325]. Although they facilitate wastewater treatment, their biomass needs to be removed safely, which is time- and labour-intensive. The need for more space is another issue related to macrophyte growth, and treatment systems can only be used in designated regions and not continuously [286]. To maintain the macrophytes within a defined boundary, continued removal is essential. To this end, an automated system can be fixed to harvest the macrophytes, depending on the time or morphological characteristics.
5. Conclusions
Floating phytoremediation is a green trend to eliminate contaminants from wastewater. This method is more effective than conventional treatment methods as it is economically viable, effective in contaminant removal and environmentally friendly. Aquatic macrophytes can remove various pollutant types, including organic and inorganic compounds. Several macrophytes have been used to decontaminate wastewater in the secondary and tertiary stages of treatment. Floating aquatic macrophytes such as Azolla, duckweed and water hyacinth are the key players in removing contaminants and can be efficient via bioaccumulation and biosorption. The produced macrophyte biomass can, however, threaten environmental health if not handled properly. In some cases, it can be used for the production of bioenergy, animal feed and fertilizer, facilitating a circular economy.
Author Contributions
Conceptualization, S.S. and H.A.H.; methodology, S.S. and H.A.H.; resources, S.R.S.A.; data curation, S.S. and H.A.H.; writing—original draft preparation, S.S.; writing—review and editing, S.S. and H.A.H.; supervision, H.A.H. and S.R.S.A.; funding acquisition, H.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universiti Kebangsaan Malaysia with Geran Universiti Penyelidikan (GUP-2022-028).
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
The authors would like to acknowledge the Universiti Kebangsaan Malaysia for funding this research project through the Geran Universiti Penyelidikan with grant no. GUP-2022-028.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Machineni, L. Review on biological wastewater treatment and resources recovery: Attached and suspended growth systems. Water Sci. Technol. 2019, 80, 2013–2026. [Google Scholar] [CrossRef]
- World Economic Forum. The Global Risks Report; World Economic Forum: Cologny, Switzerland, 2019. [Google Scholar]
- Shah, M.; Hashmi, H.N.; Ali, A.; Ghumman, A.R. Performance assessment of aquatic macrophytes for treatment of municipal wastewater. J. Environ. Health Sci. Eng. 2014, 12, 106. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.G.; Liu, F.C. Pollution in the urban soils of Lianyungang, China, evaluated using a pollution index, mobility of heavy metals, and enzymatic activities. Environ. Monit. Assess. 2017, 189, 34. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Tyagi, S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef]
- Jackson, R.B.; Carpenter, S.R.; Dahm, C.N.; McKnight, D.M.; Naiman, R.J.; Postel, S.L.; Running, S.W. Water in a changing world. Ecol. Appl. 2001, 11, 1027–1045. [Google Scholar] [CrossRef]
- Ahmed, I.; Lateef, A.; Jan, K.; Khan, Y.M. Partial Replacement of Fish Meal with an Aquatic macrophyte, Ceratophyllum demersum in the Diet of Common Carp, Cyprinus carpio var. communis Fingerlings. Aquac. Res. 2024, 2024, 9925913. [Google Scholar] [CrossRef]
- Vymazal, J. The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol. Eng. 2009, 35, 1–17. [Google Scholar] [CrossRef]
- Bind, A.; Goswami, L.; Prakash, V. Comparative analysis of floating and submerged macrophytes for heavy metal (copper, chromium, arsenic and lead) removal: Sorbent preparation, characterization, regeneration and cost estimation. Geol. Ecol. Landsc. 2018, 2, 61–72. [Google Scholar] [CrossRef]
- Galkina, E.; Vasyutina, O. Reuse of treated wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2018, 365, 022047. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Z.; Wu, G.; Wu, Q.; Zhang, F.; Niu, Z.; Hu, H.-Y. Characteristics of water quality of municipal wastewater treatment plants in China: Implications for resources utilization and management. J. Clean. Prod. 2016, 131, 1–9. [Google Scholar] [CrossRef]
- Bodzek, M.; Łobos-Moysa, E.; Zamorowska, M. Removal of organic compounds from municipal landfill leachate in a membrane bioreactor. Desalination 2006, 198, 16–23. [Google Scholar] [CrossRef]
- Dhote, S.; Dixit, S. Water quality improvement through macrophytes—A review. Environ. Monit. Assess 2009, 152, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Reemtsma, T.; Weiss, S.; Mueller, J.; Petrovic, M.; González, S.; Barcelo, D.; Ventura, F.; Knepper, T.P. Polar pollutants entry into the water cycle by municipal wastewater: A European perspective. Environ. Sci. Technol. 2006, 40, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Rangabhashiyam, S.; Verma, P.; Singh, P.; Devi, P.; Kumar, P.; Hussain, C.M.; Gaurav, G.K.; Kumar, K.S. Environmental and health impacts of contaminants of emerging concerns: Recent treatment challenges and approaches. Chemosphere 2021, 272, 129492. [Google Scholar] [CrossRef] [PubMed]
- Klapper, H. Control of Eutrophication in Inland Waters; Ellis Horwood Ltd.: Herts, UK, 1991. [Google Scholar]
- Sampat, P. Groundwater Shock: The Polluting of the World’s Major Freshwater Stores; World Watch: Washington, DC, USA, 2000; pp. 10–22. [Google Scholar]
- Chouhan, B.; Meena, P.; Poonar, N. Effect of heavy metal ions in water on human health. Int. J. Sci. Eng. Res. 2016, 4, 2015–2017. [Google Scholar]
- Liu, J.; Cao, L.; Dou, S. Trophic transfer, biomagnification and risk assessments of four common heavy metals in the food web of Laizhou Bay, the Bohai Sea. Sci. Total Environ. 2019, 670, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Briand, M.J.; Bustamante, P.; Bonnet, X.; Churlaud, C.; Letourneur, Y. Tracking trace elements into complex coral reef trophic networks. Sci. Total Environ. 2018, 612, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Goala, M.; Yadav, K.K.; Alam, J.; Adelodun, B.; Choi, K.S.; Cabral-Pinto, M.M.S.; Hamid, A.A.; Alhoshan, M.; Ali, F.A.A.; Shukla, A.K. Phytoremediation of dairy wastewater using Azolla pinnata: Application of image processing technique for leaflet growth simulation. J. Water Process Eng. 2021, 42, 102152. [Google Scholar] [CrossRef]
- Adelodun, B.; Ajibade, F.O.; Ibrahim, R.G.; Bakare, H.O.; Choi, K.-S. Snowballing transmission of COVID-19 (SARS-CoV-2) through wastewater: Any sustainable preventive measures to curtail the scourge in low-income countries? Sci. Total Environ. 2020, 742, 140680. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, J.; Ge, J. Dynamic game in agriculture and industry cross-sectoral water pollution governance in developing countries. Agric. Water Manag. 2021, 243, 106417. [Google Scholar] [CrossRef]
- Ahammad, S.Z.; Graham, D.W.; Dolfing, J. Wastewater treatment: Biological. In Managing Water Resources and Hydrological Systems; CRC Press: Boca Raton, FL, USA, 2020; pp. 561–576. [Google Scholar]
- Alufasi, R.; Gere, J.; Chakauya, E.; Lebea, P.; Parawira, W.; Chingwaru, W. Mechanisms of pathogen removal by macrophytes in constructed wetlands. Environ. Technol. Rev. 2017, 6, 135–144. [Google Scholar] [CrossRef]
- Akpor, O.; Muchie, M. Bioremediation of polluted wastewater influent: Phosphorus and nitrogen removal. Sci. Res. Essays 2010, 5, 3222–3230. [Google Scholar]
- Thulasisingh, A.; Kumar, S.; Perumal, S.; Kannaiyan, S. Microbial Biofilms in the Treatment of Textile Effluents. In Advanced and Innovative Approaches of Environmental Biotechnology in Industrial Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2023; pp. 83–97. [Google Scholar]
- Sharma, P.; Pandey, S. Status of phytoremediation in world scenario. Int. J. Environ. Bioremediation Biodegrad. 2014, 2, 178–191. [Google Scholar]
- Parmar, S.; Singh, V. Phytoremediation approaches for heavy metal pollution: A review. J. Plant Sci. Res. 2015, 2, 135. [Google Scholar]
- Saxena, M.K.; Singh, N.; Kumar, S.; Mp, D.; Datta, S. Potent pharmaceutical products from aquatic plants—Review. Asian J. Pharm. Clin. Res. 2021, 14, 48–63. [Google Scholar] [CrossRef]
- Alam, S.N.; Singh, B.; Guldhe, A. Aquatic weed as a biorefinery resource for biofuels and value-added products: Challenges and recent advancements. Clean. Eng. Technol. 2021, 4, 100235. [Google Scholar] [CrossRef]
- Antonangelo, J.A.; Sun, X.; Zhang, H. The roles of co-composted biochar (COMBI) in improving soil quality, crop productivity, and toxic metal amelioration. J. Environ. Manag. 2021, 277, 111443. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Yu, C.; Zhou, G. Multifaceted roles of duckweed in aquatic phytoremediation and bioproducts synthesis. GCB Bioenergy 2020, 13, 70–82. [Google Scholar] [CrossRef]
- Toivonen, H.; Huttunen, P. Aquatic macrophytes and ecological gradients in 57 small lakes in southern Finland. Aquat. Bot. 1995, 51, 197–221. [Google Scholar] [CrossRef]
- Srivastava, J.; Gupta, A.; Chandra, H. Managing water quality with aquatic macrophytes. Rev. Environ. Sci. Bio/Technol. 2008, 7, 255–266. [Google Scholar] [CrossRef]
- Escobar, C.; Escobar, A. Duckweed: A tiny aquatic plant with enormous potential for bioregenerative life support systems. In Proceedings of the 47th International Conference on Environmental System, Charleston, SC, USA, 16–20 July 2017. [Google Scholar]
- Mkandawire, M.; Dudel, E.G. Are Lemna spp. effective phytoremediation agents. Bioremediation Biodivers. Bioavailab. 2007, 1, 56–71. [Google Scholar]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Food Secur. Nutr. -Stress. Environ. Exploit. Plants’ Genet. Capab. 2002, 245, 201–213. [Google Scholar]
- Miretzky, P.; Saralegui, A.; Cirelli, A.F. Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina). Chemosphere 2004, 57, 997–1005. [Google Scholar] [CrossRef]
- Couselo, J.L.; Corredoira, E.; Vieitez, A.M.; Ballester, A. Plant tissue culture of fast-growing trees for phytoremediation research. In Plant Cell Culture Protocols; Springer: Berlin/Heidelberg, Germany, 2012; pp. 247–263. [Google Scholar]
- Bruce, S.; Noller, B.; Grigg, A.; Mullen, B.; Mulligan, D.; Ritchie, P.; Currey, N.; Ng, J. A field study conducted at Kidston Gold Mine, to evaluate the impact of arsenic and zinc from mine tailing to grazing cattle. Toxicol. Lett. 2003, 137, 23–34. [Google Scholar] [CrossRef]
- de Vasconcelos, V.M.; de Morais, E.R.C.; Faustino, S.J.B.; Hernandez, M.C.R.; Gaudêncio, H.R.d.S.C.; de Melo, R.R.; Bessa Junior, A.P. Floating aquatic macrophytes for the treatment of aquaculture effluents. Environ. Sci. Pollut. Res. 2021, 28, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.N. Phytohormonal roles in plant responses to heavy metal stress: Implications for using macrophytes in phytoremediation of aquatic ecosystems. Environ. Toxicol. Chem. 2021, 40, 7–22. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Herrera-Melián, J.A.; Sánchez-Suárez, F.; Díaz-Mendoza, V.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Removal of Pharmaceuticals in a Macrophyte Pond-Constructed Wetland System and the Effect of a Low Effluent Recirculation. Water 2022, 14, 2340. [Google Scholar] [CrossRef]
- Bessadok, S.; Kraiem, K.; Arous, F.; Al Souki, K.S.; Tabassi, D.; El Toumi, S.; Jaouani, A. Efficient wastewater treatment and removal of bisphenol A and diclofenac in mesocosm flow constructed wetlands using granulated cork as emerged substrate. Toxics 2023, 11, 81. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, B.; Manchanda, V. Phytoremediation: Role of terrestrial plants and aquatic macrophytes in the remediation of radionuclides and heavy metal contaminated soil and water. Environ. Sci. Pollut. Res. 2015, 22, 946–962. [Google Scholar] [CrossRef]
- Materac, M.; Wyrwicka, A.; Sobiecka, E. Phytoremediation techniques of wastewater treatment. Environ. Biotechnol. 2015, 11, 10–13. [Google Scholar] [CrossRef][Green Version]
- Raklami, A.; Meddich, A.; Oufdou, K.; Baslam, M. Plants—Microorganisms-based bioremediation for heavy metal cleanup: Recent developments, phytoremediation techniques, regulation mechanisms, and molecular responses. Int. J. Mol. Sci. 2022, 23, 5031. [Google Scholar] [CrossRef]
- Chaney, R.L.; Li, Y.-M.; Brown, S.L.; Homer, F.A.; Malik, M.; Angle, J.S.; Baker, A.J.; Reeves, R.D.; Chin, M. Improving metal hyperaccumulator wild plants to develop commercial phytoextraction systems: Approaches and progress. In Phytoremediation of Contaminated Soil and Water; CRC Press: Boca Raton, FL, USA, 2020; pp. 129–158. [Google Scholar]
- Rulkens, W.; Tichy, R.; Grotenhuis, J. Remediation of polluted soil and sediment: Perspectives and failures. Water Sci. Technol. 1998, 37, 27–35. [Google Scholar] [CrossRef]
- Garbisu, C.; Alkorta, I. Phytoextraction: A cost-effective plant-based technology for the removal of metals from the environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Hernández-Allica, J.; Becerril, J.; Amezaga, I.; Albizu, I.; Onaindia, M.; Garbisu, C. Chelate-enhanced phytoremediation of soils polluted with heavy metals. Rev. Environ. Sci. Biotechnol. 2004, 3, 55–70. [Google Scholar] [CrossRef]
- Sikhosana, M.; Botha, A.; Mpenyane-Monyatsi, L.; Coetzee, M.A. Evaluating the effect of seasonal temperature changes on the efficiency of a rhizofiltration system in nitrogen removal from urban runoff. J. Environ. Manag. 2020, 274, 111192. [Google Scholar] [CrossRef] [PubMed]
- Flathman, P.E.; Lanza, G.R. Phytoremediation: Current Views on an Emerging Green Technology. J. Soil Contam. 2010, 7, 415–432. [Google Scholar] [CrossRef]
- Zhao, M.; Duncan, J.R. Removal and recovery of nickel from aqueous solution and electroplating rinse effluent using Azolla filiculoides. Process Biochem. 1998, 33, 249–255. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, J.; Sharp, R. Phytoremediation in engineered wetlands: Mechanisms and applications. Procedia Environ. Sci. 2010, 2, 1315–1325. [Google Scholar] [CrossRef]
- Phusantisampan, T.; Meeinkuirt, W.; Saengwilai, P.; Pichtel, J.; Chaiyarat, R. Phytostabilization potential of two ecotypes of Vetiveria zizanioides in cadmium-contaminated soils: Greenhouse and field experiments. Environ. Sci. Pollut. Res. 2016, 23, 20027–20038. [Google Scholar] [CrossRef]
- Limmer, M.; Burken, J. Phytovolatilization of organic contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, J.; Pu, R. The enhanced removal and phytodegradation of sodium dodecyl sulfate (SDS) in wastewater using controllable water hyacinth. Int. J. Phytoremediation 2019, 21, 1080–1089. [Google Scholar] [CrossRef]
- Wang, J.; Aghajani Delavar, M. Techno-economic analysis of phytoremediation: A strategic rethinking. Sci. Total Environ. 2023, 902, 165949. [Google Scholar] [CrossRef]
- Rehman, F.; Pervez, A.; Mahmood, Q.; Nawab, B. Wastewater remediation by optimum dissolve oxygen enhanced by macrophytes in constructed wetlands. Ecol. Eng. 2017, 102, 112–126. [Google Scholar] [CrossRef]
- Van Engeland, T.; Bouma, T.J.; Morris, E.P.; Brun, F.G.; Peralta, G.; Lara, M.; Hendriks, I.E.; Soetaert, K.; Middelburg, J.J. Potential uptake of dissolved organic matter by seagrasses and macroalgae. Mar. Ecol. Prog. Ser. 2011, 427, 71–81. [Google Scholar] [CrossRef]
- Pettit, N.; Ward, D.; Adame, M.; Valdez, D.; Bunn, S. Influence of aquatic plant architecture on epiphyte biomass on a tropical river floodplain. Aquat. Bot. 2016, 129, 35–43. [Google Scholar] [CrossRef]
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Priya, A.; Avishek, K.; Pathak, G. Assessing the potentials of Lemna minor in the treatment of domestic wastewater at pilot scale. Environ. Monit. Assess. 2012, 184, 4301–4307. [Google Scholar] [CrossRef] [PubMed]
- Ribadiya, B.M.; Mehta, M.J. Treatment of municipal and industrial wastewater by reed bed technology: A low cost treatment approach. Int. J. Eng. Res. Appl. 2014, 12, 15–18. [Google Scholar]
- Lekeufack, M.; Fonkou, T.; Pamo, T.E.; Amougou, A. Removal of faecal bacteria and nutrients from domestic wastewater in a horizontal surface flow wetland vegetated with Echinochloa pyramidalis. Afr. J. Environ. Sci. Technol. 2012, 6, 337–345. [Google Scholar]
- Sooknah, R.D.; Wilkie, A.C. Nutrient removal by floating aquatic macrophytes cultured in anaerobically digested flushed dairy manure wastewater. Ecol. Eng. 2004, 22, 27–42. [Google Scholar] [CrossRef]
- Dhir, B.; Sharmila, P.; Saradhi, P.P. Potential of Aquatic Macrophytes for Removing Contaminants from the Environment. Crit. Rev. Environ. Sci. Technol. 2009, 39, 754–781. [Google Scholar] [CrossRef]
- Hamersley, M.R.; Howes, B.L.; White, D.S.; Johnke, S.; Young, D.; Peterson, S.B.; Teal, J.M. Nitrogen balance and cycling in an ecologically engineered septage treatment system. Ecol. Eng. 2001, 18, 61–75. [Google Scholar] [CrossRef]
- Mitchell, D. Aquatic Vegetation and Its Use and Control; CABI: Hong Kong, China, 1974. [Google Scholar]
- Ater, M.; Ali, N.; Kasmi, H. Tolérance et accumulation du cuivre et du chrome chez deux espèces de lentilles d’eau: Lemna minor L. et Lemna gibba L. Rev. Des Sci. De L’eau/J. Water Sci. 2006, 19, 57–67. [Google Scholar] [CrossRef][Green Version]
- Dirilgen, N.; Inel, Y. Effects of zinc and copper on growth and metal accumulation in duckweed, Lemna minor. Bull. Environ. Contam. Toxicol. 1994, 53, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Bunluesin, S.; Kruatrachue, M.; Pokethitiyook, P.; Lanza, G.; Upatham, E.; Soonthornsarathool, V. Plant screening and comparison of Ceratophyllum demersum and Hydrilla verticillata for cadmium accumulation. Bull. Environ. Contam. Toxicol. 2004, 73, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Sanyahumbi, D.; Duncan, J.R.; Zhao, M.; Van Hille, R. Removal of lead from solution by the non-viable biomass of the water fern Azolla filiculoides. Biotechnol. Lett. 1998, 20, 745–747. [Google Scholar] [CrossRef]
- Maine, M.a.A.; Suñé, N.L.; Lagger, S.C. Chromium bioaccumulation: Comparison of the capacity of two floating aquatic macrophytes. Water Res. 2004, 38, 1494–1501. [Google Scholar] [CrossRef]
- Gupta, M.; Sinha, S.; Chandra, P. Uptake and toxicity op metals in Scirpus lacustris L. and Bacopa monnieri L. J. Environ. Sci. Health Part A 1994, 29, 2185–2202. [Google Scholar]
- Olguín, E.; Hernández, E.; Ramos, I. The effect of both different light conditions and the pH value on the capacity of Salvinia minima Baker for removing cadmium, lead and chromium. Acta Biotechnol. 2002, 22, 121–131. [Google Scholar] [CrossRef]
- Ye, Z.; Whiting, S.; Qian, J.; Lytle, C.; Lin, Z.Q.; Terry, N. Trace Element Removal from Coal Ash Leachate by a 10-Year-Old Constructed Wetland. J. Environ. Qual. 2001, 30, 1710–1719. [Google Scholar] [CrossRef]
- Mohan, S.V.; Mohanakrishna, G.; Chiranjeevi, P.; Peri, D.; Sarma, P.N. Ecologically engineered system (EES) designed to integrate floating, emergent and submerged macrophytes for the treatment of domestic sewage and acid rich fermented-distillery wastewater: Evaluation of long term performance. Bioresour. Technol. 2010, 101, 3363–3370. [Google Scholar] [CrossRef]
- Mishima, D.; Kuniki, M.; Sei, K.; Soda, S.; Ike, M.; Fujita, M. Ethanol production from candidate energy crops: Water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.). Bioresour. Technol. 2008, 99, 2495–2500. [Google Scholar] [CrossRef]
- Ebel, M.; Evangelou, M.W.; Schaeffer, A. Cyanide phytoremediation by water hyacinths (Eichhornia crassipes). Chemosphere 2007, 66, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Y.; Yang, X.E.; Chang, H.Q.; Pu, P.M.; Ding, X.F.; Rengel, Z. Phytoremediation of nitrogen-polluted water using water hyacinth. J. Plant Nutr. 2007, 30, 1753–1765. [Google Scholar] [CrossRef]
- Landolt, E. The family of Lemnaceae-a monographic study. Biosystematic investigations in the family of duckweeds (Lemnaceae). Veroff. Geobot. Inst. Rubel ETH 1987, 2, 566–638. [Google Scholar]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.J. Relative in vitro growth rates of duckweeds (L. emnaceae)–the most rapidly growing higher plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Lenaghan, S.C. Duckweed: A potential phytosensor for heavy metals. Plant Cell Rep. 2022, 41, 2231–2243. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kumar, M.; Sachdeva, S.; Puri, S.K. Aquatic weeds as the next generation feedstock for sustainable bioenergy production. Bioresour. Technol. 2018, 251, 390–402. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy Metal Phytoremediation from Aquatic Ecosystems with Special Reference to Macrophytes. Crit. Rev. Environ. Sci. Technol. 2009, 39, 697–753. [Google Scholar] [CrossRef]
- Rai, P.K.; Tripathi, B.D. Comparative assessment of Azolla pinnata and Vallisneria spiralis in Hg removal from G.B. Pant Sagar of Singrauli Industrial region, India. Environ. Monit. Assess. 2009, 148, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Saxena, S.; Sharma, D.K. Tolerance and phytoaccumulation of chromium by three Azolla species. World J. Microbiol. Biotechnol. 2006, 22, 97–100. [Google Scholar] [CrossRef]
- Arora, A.; Saxena, S. Cultivation of Azolla microphylla biomass on secondary-treated Delhi municipal effluents. Biomass Bioenergy 2005, 29, 60–64. [Google Scholar] [CrossRef]
- Rakhshaee, R.; Khosravi, M.; Ganji, M.T. Kinetic modeling and thermodynamic study to remove Pb (II), Cd (II), Ni (II) and Zn (II) from aqueous solution using dead and living Azolla filiculoides. J. Hazard. Mater. 2006, 134, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kollah, B.; Patra, A.K.; Mohanty, S.R. Aquatic microphylla Azolla: A perspective paradigm for sustainable agriculture, environment and global climate change. Environ. Sci. Pollut. Res. 2016, 23, 4358–4369. [Google Scholar] [CrossRef]
- Lu, Q. Evaluation of Aquatic Plants for Phytoremediation of Eutrophic Stormwaters; University of Florida: Gainesville, FL, USA, 2009. [Google Scholar]
- Akinbile, C.O.; Ogunrinde, T.A.; Che Bt Man, H.; Aziz, H.A. Phytoremediation of domestic wastewaters in free water surface constructed wetlands using Azolla pinnata. Int. J. Phytoremediation 2016, 18, 54–61. [Google Scholar] [CrossRef]
- Mishra, V.K.; Tripathi, B. Accumulation of chromium and zinc from aqueous solutions using water hyacinth (Eichhornia crassipes). J. Hazard. Mater. 2009, 164, 1059–1063. [Google Scholar] [CrossRef]
- Demim, S.; Drouiche, N.; Aouabed, A.; Benayad, T.; Dendene-Badache, O.; Semsari, S. Cadmium and nickel: Assessment of the physiological effects and heavy metal removal using a response surface approach by L. gibba. Ecol. Eng. 2013, 61, 426–435. [Google Scholar] [CrossRef]
- Eccles, H. Removal of heavy metals from effluent streams—Why select a biological process? Int. Biodeterior. Biodegrad. 1995, 35, 5–16. [Google Scholar] [CrossRef]
- Mishra, V.K.; Tripathi, B.; Kim, K.-H. Removal and accumulation of mercury by aquatic macrophytes from an open cast coal mine effluent. J. Hazard. Mater. 2009, 172, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K. Biosorption and bioaccumulation—The prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Uniyal, P.L.; Prasanna, R.; Ahluwalia, A.S. Phytoremediation potential of aquatic macrophyte, Azolla. Ambio 2012, 41, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Roy, S.B.; Mahindrakar, A. Treatment of Water Using Water Hyacinth, Water Lettuce and Vetiver Grass—A Review. Resour. Environ. 2012, 2, 202–215. [Google Scholar] [CrossRef]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Sun, C.; Yu, L.; Zhu, M.; Xu, H.; Zhao, J.; Ma, Y.; Zhou, G. Comparative analysis of duckweed cultivation with sewage water and SH media for production of fuel ethanol. PLoS ONE 2014, 9, e115023. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Moelyowati, I. Duckweed based wastewater treatment (DWWT): Design guidelines for hot climates. Water Sci. Technol. 2001, 43, 291–299. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Mahdavi, Y.; Bazrafshan, E.; Balarak, D. Phytodegradation potential of bisphenolA from aqueous solution by Azolla Filiculoides. J. Environ. Health Sci. Eng. 2014, 12, 66. [Google Scholar] [CrossRef]
- Akinbile, C.O.; Ikuomola, B.T.; Olanrewaju, O.O.; Babalola, T.E. Assessing the efficacy of Azolla pinnata in four different wastewater treatment for agricultural re-use: A case history. Sustain. Water Resour. Manag. 2019, 5, 1009–1015. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Lim, L.B.; Lim, L.-H.; Malik, O.A. Phytoextraction potential of water fern (Azolla pinnata) in the removal of a hazardous dye, methyl violet 2B: Artificial neural network modelling. Int. J. Phytoremediation 2018, 20, 424–431. [Google Scholar] [CrossRef]
- Karman, S.B.; Diah, S.Z.M.; Gebeshuber, I.C. Raw materials synthesis from heavy metal industry effluents with bioremediation and phytomining: A biomimetic resource management approach. Adv. Mater. Sci. Eng. 2015, 2015, 185071. [Google Scholar] [CrossRef]
- Singh, P.K.; Kushwaha, A.; Hans, N.; Gautam, A.; Rani, R. Evaluation of the cytotoxicity and interaction of lead with lead resistant bacterium Acinetobacter junii Pb1. Braz. J. Microbiol. 2019, 50, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Goswami, L.; Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Simultaneous heavy metal removal and anthracene biodegradation by the oleaginous bacteria Rhodococcus opacus. 3 Biotech 2017, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Uysal, Y.; Taner, F. Effect of pH, temperature, and lead concentration on the bioremoval of lead from water using Lemna minor. Int. J. Phytoremediation 2009, 11, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K. Phytoremediation of Hg and Cd from industrial effluents using an aquatic free floating macrophyte Azolla pinnata. Int. J. Phytoremediation 2008, 10, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Mashkani, S.G.; Ghazvini, P.T.M. Biotechnological potential of Azolla filiculoides for biosorption of Cs and Sr: Application of micro-PIXE for measurement of biosorption. Bioresour. Technol. 2009, 100, 1915–1921. [Google Scholar] [CrossRef]
- Hall, J.á. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Mishra, V.K.; Tripathi, B. Concurrent removal and accumulation of heavy metals by the three aquatic macrophytes. Bioresour. Technol. 2008, 99, 7091–7097. [Google Scholar] [CrossRef]
- Jones, J.L.; Jenkins, R.O.; Haris, P.I. Extending the geographic reach of the water hyacinth plant in removal of heavy metals from a temperate Northern Hemisphere river. Sci. Rep. 2018, 8, 11071. [Google Scholar] [CrossRef]
- Mahmood, T.; Malik, S.A.; Hussain, S.T. Biosorption and recovery of heavy metals from aqueous solutions by Eichhornia crassipes (water hyacinth) ash. BioResources 2010, 5, 1244–1256. [Google Scholar] [CrossRef]
- Lu, X.; Kruatrachue, M.; Pokethitiyook, P.; Homyok, K. Removal of cadmium and zinc by water hyacinth, Eichhornia crassipes. Sci. Asia 2004, 30, 103. [Google Scholar] [CrossRef]
- E, P.; Premalatha, R.P.; Davamani, V.; Periasamy, K.; Sebastian, P.; Suganya, K. Biosorption of chromium ions through modified Eichhornia crassipes biomass form the aqueous medium. J. Environ. Biol. 2021, 42, 62–73. [Google Scholar] [CrossRef]
- Saha, P.; Shinde, O.; Sarkar, S. Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytoremediation 2017, 19, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, F.; Adeniran, K.; Egbuna, C. Phytoremediation efficiencies of water hyacinth in removing heavy metals in domestic sewage (A Case Study of University of Ilorin, Nigeria). Int. J. Eng. Sci. 2013, 2, 16–27. [Google Scholar]
- Malar, S.; Sahi, S.V.; Favas, P.J.; Venkatachalam, P. Mercury heavy-metal-induced physiochemical changes and genotoxic alterations in water hyacinths [Eichhornia crassipes (Mart.)]. Environ. Sci. Pollut. Res. 2015, 22, 4597–4608. [Google Scholar] [CrossRef]
- Huynh, A.T.; Chen, Y.-C.; Tran, B.N.T. A small-scale study on removal of heavy metals from contaminated water using water hyacinth. Processes 2021, 9, 1802. [Google Scholar] [CrossRef]
- Ubuza, L.J.A.; Padero, P.C.S.; Nacalaban, C.M.N.; Tolentino, J.T.; Alcoran, D.C.; Tolentino, J.C.; Ido, A.L.; Mabayo, V.I.F.; Arazo, R.O. Assessment of the potential of duckweed (Lemna minor L.) in treating lead-contaminated water through phytoremediation in stationary and recirculated set-ups. Environ. Eng. Res. 2020, 25, 977–982. [Google Scholar] [CrossRef]
- Chaudhary, E.; Sharma, P. Chromium and cadmium removal from wastewater using duckweed—Lemna gibba L. and ultrastructural deformation due to metal toxicity. Int. J. Phytoremediation 2019, 21, 279–286. [Google Scholar] [CrossRef]
- Mechora, Š.; Stibilj, V.; Germ, M. Response of duckweed to various concentrations of selenite. Environ. Sci. Pollut. Res. 2015, 22, 2416–2422. [Google Scholar] [CrossRef]
- Alvarado, S.; Guédez, M.; Lué-Merú, M.P.; Nelson, G.; Alvaro, A.; Jesús, A.C.; Gyula, Z. Arsenic removal from waters by bioremediation with the aquatic plants Water Hyacinth (Eichhornia crassipes) and Lesser Duckweed (Lemna minor). Bioresour. Technol. 2008, 99, 8436–8440. [Google Scholar] [CrossRef]
- Amare, E.; Kebede, F.; Mulat, W. Wastewater treatment by Lemna minor and Azolla filiculoides in tropical semi-arid regions of Ethiopia. Ecol. Eng. 2018, 120, 464–473. [Google Scholar] [CrossRef]
- Al-Khafaji, M.S.; Al-Ani, F.H.; Ibrahim, A.F. Removal of some heavy metals from industrial wastewater by Lemmna minor. KSCE J. Civ. Eng. 2018, 22, 1077–1082. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S. Lead and cadmium removal from water using duckweed—Lemna gibba L.: Impact of pH and initial metal load. Alex. Eng. J. 2015, 54, 1297–1304. [Google Scholar] [CrossRef]
- Teixeira, S.; Vieira, M.; Marques, J.E.; Pereira, R. Bioremediation of an iron-rich mine effluent by Lemna minor. Int. J. Phytoremediation 2014, 16, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Biancalani, A.; Berardi, C.; Antal, A.; Fibbi, D.; Coppi, A.; Lastrucci, L.; Bussotti, N.; Colzi, I.; Renai, L.; et al. Improving the efficiency of wastewater treatment plants: Bio-removal of heavy-metals and pharmaceuticals by Azolla filiculoides and Lemna minuta. Sci. Total Environ. 2020, 746, 141219. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.; Rakhshaee, R.; Ganji, M.T. Pre-treatment processes of Azolla filiculoides to remove Pb (II), Cd (II), Ni (II) and Zn (II) from aqueous solution in the batch and fixed-bed reactors. J. Hazard. Mater. 2005, 127, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, P.; Singh, J.; Kumar, P. Potential of water fern (Azolla pinnata R. Br.) in phytoremediation of integrated industrial effluent of SIIDCUL, Haridwar, India: Removal of physicochemical and heavy metal pollutants. Int. J. Phytoremediation 2020, 22, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Babu, D.J.; Sumalatha, B.; Venkateswarulu, T.; Das, K.M.; Kodali, V.P. Kinetic, equilibrium and thermodynamic studies of biosorption of Chromium (VI) from aqueous solutions using Azolla Filiculoidus. J. Pure Appl. Microbiol. 2014, 8, 3107–3116. [Google Scholar]
- Shafi, N.; Pandit, A.K.; Kamili, A.N.; Mushtaq, B. Heavy metal accumulation by azollapinnata of dal lake ecosystem, India. Development 2015, 1, 8–12. [Google Scholar]
- Jadia, C.D.; Fulekar, M. Phytoremediation of heavy metals: Recent techniques. Afr. J. Biotechnol. 2009, 8. Available online: https://www.ajol.info/index.php/ajb/article/view/59987 (accessed on 21 February 2024).
- Hasan, S.H.; Ranjan, D.; Talat, M. Water hyacinth biomass (WHB) for the biosorption of hexavalent chromium: Optimization of process parameters. BioResources 2010, 5, 563–575. [Google Scholar] [CrossRef]
- Elangovan, R.; Philip, L.; Chandraraj, K. Biosorption of chromium species by aquatic weeds: Kinetics and mechanism studies. J. Hazard. Mater. 2008, 152, 100–112. [Google Scholar] [CrossRef]
- Bais, S. Analysis of heavy metals removal by Eichhornia crassipes (Mart.) Solms. World J. Pharm. Pharm. Sci. 2015, 4, 665–672. [Google Scholar]
- Bind, A.; Kushwaha, A.; Devi, G.; Goswami, S.; Sen, B.; Prakash, V. Biosorption valorization of floating and submerged macrophytes for heavy-metal removal in a multi-component system. Appl. Water Sci. 2019, 9. [Google Scholar] [CrossRef]
- Yilmaz, D.D.; Akbulut, H. Effect of circulation on wastewater treatment by Lemna gibba and Lemna minor (floating aquatic macrophytes). Int. J. Phytoremediation 2011, 13, 970–984. [Google Scholar] [CrossRef]
- Levi, P.S.; Riis, T.; Alnøe, A.B.; Peipoch, M.; Maetzke, K.; Bruus, C.; Baattrup-Pedersen, A. Macrophyte complexity controls nutrient uptake in lowland streams. Ecosystems 2015, 18, 914–931. [Google Scholar] [CrossRef]
- Ruiz-Rueda, O.; Hallin, S.; Baneras, L. Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. FEMS Microbiol. Ecol. 2009, 67, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Chen, J.; Tancioni, L.; Caiola, M.G. Evaluation of the fern Azolla for growth, nitrogen and phosphorus removal from wastewater. Water Res. 2001, 35, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Golzary, A.; Tavakoli, O.; Rezaei, Y.; Karbassi, A. Wastewater treatment by Azolla Filiculoides: A study on color, odor, COD, nitrate, and phosphate removal. Pollution 2018, 4, 69–76. [Google Scholar]
- Muvea, F.; Ogendi, G.; Omondi, S. Nutrient removal efficiency by floating macrophytes; Lemna minor and Azolla pinnata in a constructed wetland. Glob. J. Environ. Sci. Manag. 2019, 5, 415–430. [Google Scholar]
- Anandha Varun, R.; Kalpana, S. Performance analysis of nutrient removal in pond water using Water Hyacinth and Azolla with papaya stem. Int. Res. J. Eng. Technol. 2015, 2, 444–448. [Google Scholar]
- Hazmi, N.I.A.; Hanafiah, M.M. Phytoremediation of livestock wastewater using Azolla Fili culoides and Lemna minor. Environ. Ecosyst. Sci. (EES) 2018, 2, 13–16. [Google Scholar] [CrossRef]
- Iqbal, J.; Saleem, M.; Javed, A. Effect of electrical conductivity (Ec) on growth performance of duckweed at dumpsite leachate. Int. J. Sci. Environ. Technol. 2017, 6, 1989–1999. [Google Scholar]
- Hu, H.; Zhou, Q.; Li, X.; Lou, W.; Du, C.; Teng, Q.; Zhang, D.; Liu, H.; Zhong, Y.; Yang, C. Phytoremediation of anaerobically digested swine wastewater contaminated by oxytetracycline via Lemna aequinoctialis: Nutrient removal, growth characteristics and degradation pathways. Bioresour. Technol. 2019, 291, 121853. [Google Scholar] [CrossRef]
- Kadir, A.A.; Abdullah, S.R.S.; Othman, B.A.; Hasan, H.A.; Othman, A.R.; Imron, M.F.; Ismail, N.; Kurniawan, S.B. Dual function of Lemna minor and Azolla pinnata as phytoremediator for Palm Oil Mill Effluent and as feedstock. Chemosphere 2020, 259, 127468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shi, H.; Liu, Y.; Zhao, H.; Su, H.; Wang, M.; Zhao, Y. The influence of duckweed species diversity on biomass productivity and nutrient removal efficiency in swine wastewater. Bioresour. Technol. 2014, 167, 383–389. [Google Scholar] [CrossRef]
- Valipour, A.; Raman, V.K.; Ahn, Y.-H. Effectiveness of domestic wastewater treatment using a bio-hedge water hyacinth wetland system. Water 2015, 7, 329–347. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Wan, X.; Weng, B.; Huang, Q. Water hyacinth (Eichhornia crassipes) waste as an adsorbent for phosphorus removal from swine wastewater. Bioresour. Technol. 2010, 101, 9025–9030. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Din, M.F.M.; Taib, S.M.; Dahalan, F.A.; Songip, A.R.; Singh, L.; Kamyab, H. The efficient role of aquatic plant (water hyacinth) in treating domestic wastewater in continuous system. Int. J. Phytoremediation 2016, 18, 679–685. [Google Scholar] [CrossRef]
- Kumar, S.; Deswal, S. Phytoremediation capabilities of Salvinia molesta, water hyacinth, water lettuce, and duckweed to reduce phosphorus in rice mill wastewater. Int. J. Phytoremediation 2020, 22, 1097–1109. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, Z.; Liu, M.; Liu, H.; Wang, Y.; Wen, X.; Zhang, Y.; Yan, S. Site test of phytoremediation of an open pond contaminated with domestic sewage using water hyacinth and water lettuce. Ecol. Eng. 2016, 95, 753–762. [Google Scholar] [CrossRef]
- Prasad, R.; Sharma, D.; Yadav, K.D.; Ibrahim, H. Preliminary study on greywater treatment using water hyacinth. Appl. Water Sci. 2021, 11, 88. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S. Synchronized urban wastewater treatment and biomass production using duckweed Lemna gibba L. Ecol. Eng. 2014, 64, 337–343. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, P.; Eid, E.M.; Taher, M.A.; El-Morsy, M.H.; Osman, H.E.; Al-Bakre, D.A.; Kumar, V. Phytoremediation of nitrogen and phosphorus pollutants from glass industry effluent by using water hyacinth (Eichhornia crassipes (Mart.) Solms): Application of RSM and ANN techniques for experimental optimization. Environ. Sci. Pollut. Res. 2023, 30, 20590–20600. [Google Scholar] [CrossRef] [PubMed]
- El-Kheir, W.A.; Ismail, G.; El-Nour, F.; Tawfik, T.; Hammad, D. Assessment of the efficiency of duckweed (Lemna gibba) in wastewater treatment. Int. J. Agric. Biol. 2007, 9, 681–687. [Google Scholar]
- Bhagavanulu, D.D.; Murthy, D.S.; Anjali, C. A Study on the impact of water hyacinth in improving the wastewater properties. Int. J. Civ. Eng. Technol. 2017, 8, 1199–1209. [Google Scholar]
- Sahi, W.; Megateli, S. Evaluation of Lemna minor phytoremediation performance for the treatment of dairy wastewater. Water Pract. Technol. 2023, 18, 1138–1147. [Google Scholar] [CrossRef]
- Mamat, N.Z.; Abdullah, S.R.S.; Hasan, H.A.; Ismail, N.I.; Sharuddin, S.S.N. Polishing of treated palm oil mill effluent using Azolla pinnata. J. Biochem. Microbiol. Biotechnol. 2022, 10, 40–45. [Google Scholar] [CrossRef]
- Yang, D.J.; Zheng, Z.F.; Zhu, H.Y.; Liu, H.W.; Gao, X.P. Titanate nanofibers as intelligent absorbents for the removal of radioactive ions from water. Adv. Mater. 2008, 20, 2777–2781. [Google Scholar] [CrossRef]
- Ene, A.-M. Persistent organic pollutants (pops): Environment persistence and bioaccumulation potential. Sci. Bull. ”Mircea Cel Batran" Nav. Acad. 2014, 17, 115. [Google Scholar]
- Jung, C.; Son, A.; Her, N.; Zoh, K.-D.; Cho, J.; Yoon, Y. Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: A review. J. Ind. Eng. Chem. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Liu, G.; Ma, J.; Li, X.; Qin, Q. Adsorption of bisphenol A from aqueous solution onto activated carbons with different modification treatments. J. Hazard. Mater. 2009, 164, 1275–1280. [Google Scholar] [CrossRef]
- Silva, C.; Gómez, J.; Beristain-Cardoso, R. Simultaneous removal of 2-chlorophenol, phenol, p-cresol and p-hydroxybenzaldehyde under nitrifying conditions: Kinetic study. Bioresour. Technol. 2011, 102, 6464–6468. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Queiroz, S.; Roston, D. Removal of the endocrine disruptors ethinyl estradiol, bisphenol a, and levonorgestrel by a laboratory scale subsurface constructed wetlands. Sci. Total Environ. 2019, 693, 133514. [Google Scholar] [CrossRef]
- Xia, H.; Ma, X. Phytoremediation of ethion by water hyacinth (Eichhornia crassipes) from water. Bioresour. Technol. 2006, 97, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Balarak, D. Application of Azolla Filiculoides biomass for 2-Chlorophenol and 4-Chrorophenol Removal from aqueous solutions. Iran. J. Health Sci. 2013, 1, 43–55. [Google Scholar]
- Garcia-Rodríguez, A.; Matamoros, V.; Fontàs, C.; Salvadó, V. The influence of Lemna sp. and Spirogyra sp. on the removal of pharmaceuticals and endocrine disruptors in treated wastewaters. Int. J. Environ. Sci. Technol. 2014, 12, 2327–2338. [Google Scholar] [CrossRef]
- Yılmaz, Ö.; Taş, B. Feasibility and assessment of the phytoremediation potential of green microalga and duckweed for zeta-cypermethrin removal. Desalination Water Treat. 2021, 209, 131–143. [Google Scholar] [CrossRef]
- Reinhold, D.; Vishwanathan, S.; Park, J.J.; Oh, D.; Saunders, F.M. Assessment of plant-driven removal of emerging organic pollutants by duckweed. Chemosphere 2010, 80, 687–692. [Google Scholar] [CrossRef]
- Allam, A.; Tawfik, A.; Negm, A.; Yoshimura, C.; Fleifle, A. Treatment of drainage water containing pharmaceuticals using duckweed (Lemna gibba). Energy Procedia 2015, 74, 973–980. [Google Scholar] [CrossRef]
- Daud, M.; Ali, S.; Abbas, Z.; Zaheer, I.E.; Riaz, M.A.; Malik, A.; Hussain, A.; Rizwan, M.; Zia-ur-Rehman, M.; Zhu, S.J. Potential of duckweed (Lemna minor) for the phytoremediation of landfill leachate. J. Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Iatrou, E.I.; Gatidou, G.; Damalas, D.; Thomaidis, N.S.; Stasinakis, A.S. Fate of antimicrobials in duckweed Lemna minor wastewater treatment systems. J. Hazard. Mater. 2017, 330, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, D.A.; Scholz, M. Textile dye removal using experimental wetland ponds planted with common duckweed under semi-natural conditions. Environ. Prot. Eng. 2017, 43, 39–60. [Google Scholar] [CrossRef]
- Aswani, M.; Kumar, M.P. A novel water hyacinth based biosorbent for 2, 4-dichlorophenoxyacetic acid (2, 4-D) removal from aqueous solution. Desalin. Water Treat. 2019, 165, 163–176. [Google Scholar] [CrossRef]
- Anudechakul, C.; Vangnai, A.S.; Ariyakanon, N. Removal of chlorpyrifos by water hyacinth (Eichhornia crassipes) and the role of a plant-associated bacterium. Int. J. Phytoremediation 2015, 17, 678–685. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Rajendran, D.; Samivel, D.; Khan, M.N. New approach of dye removal in textile effluent: A cost-effective management for cleanup of toxic dyes in textile effluent by water hyacinth. In Toxicity and Biodegradation Testing. Methods in Pharmacology and Toxicology; Humana Press: New York, NY, USA, 2018; pp. 241–267. [Google Scholar]
- Yan, Y.; Chen, Y.; Xu, X.; Zhang, L.; Wang, G. Effects and removal of the antibiotic sulfadiazine by Eichhornia crassipes: Potential use for phytoremediation. Bull. Environ. Contam. Toxicol. 2019, 103, 342–347. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, X.; Ma, X.; Chen, J. Sustainable removal of formaldehyde using controllable water hyacinth. J. Clean. Prod. 2018, 181, 1–7. [Google Scholar] [CrossRef]
- Ena, A.; Carlozzi, P.; Pushparaj, B.; Paperi, R.; Carnevale, S.; Sacchi, A. Ability of the aquatic fern Azolla to remove chemical oxygen demand and polyphenols from olive mill wastewater. Grasas Y Aceites 2007, 58, 34–39. [Google Scholar] [CrossRef]
- Balarak, D.; Bazrafshan, E.; Mostafapour, F. Equilibrium, kinetic studies on the adsorption of acid green 3 (Ag3) dye onto Azolla filiculoides as adosorbent. Am. Chem. Sci. J. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Taghavi, M.; Mohebi, S.; Balarak, D. Activated carbon derived from Azolla filiculoides fern: A high-adsorption-capacity adsorbent for residual ampicillin in pharmaceutical wastewater. Biomass Convers. Biorefinery 2021, 1–13, ahead of print. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Balarak, D.; Mahdavi, Y. Pyrocatechol removal from aqueous solutions by using Azolla filiculoides. HealthScope 2013, 2, 25–30. [Google Scholar]
- Kant, R. Textile dyeing industry an environmental hazard. J. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Revanth, T.; Acharya, A.; Bhat, P. Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study. Resour.-Effic. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Nath, A.; Chakraborty, S.; Bhattacharjee, C. Bioadsorbtion of industrial dyes from aqueous solution onto water hyacinth (Eichornia crassipes): Equilibrium, kinetic, and sorption mechanism study. Desalination Water Treat. 2014, 52, 1484–1494. [Google Scholar] [CrossRef]
- Padmesh, T.; Vijayaraghavan, K.; Sekaran, G.; Velan, M. Biosorption of Acid Blue 15 using fresh water macroalga Azolla filiculoides: Batch and column studies. Dye. Pigment. 2006, 71, 77–82. [Google Scholar] [CrossRef]
- Durairaj, S. Role of lemna minor lin. In treating the textile industry wastewater, international journal of environmental. Earth Sci. Eng. 2014, 8, 55–59. [Google Scholar]
- Imron, M.F.; Kurniawan, S.B.; Soegianto, A.; Wahyudianto, F.E. Phytoremediation of methylene blue using duckweed (Lemna minor). Heliyon 2019, 5, e02206. [Google Scholar] [CrossRef] [PubMed]
- Lovins, L.H. Rethinking production. In State of the World 2008; Routledge: Oxfordshire, UK, 2012; pp. 60–72. [Google Scholar]
- Guerra-Rodríguez, S.; Oulego, P.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Towards the implementation of circular economy in the wastewater sector: Challenges and opportunities. Water 2020, 12, 1431. [Google Scholar] [CrossRef]
- Webster, K. A Wealth of Flows; Ellen MacArthur Foundation: Isle of Wight, UK, 2015. [Google Scholar]
- McDonough, W.; Braungart, M. Design for the triple top line: New tools for sustainable commerce. Corp. Environ. Strategy 2002, 9, 251–258. [Google Scholar] [CrossRef]
- Commoner, B. The environmental cost of economic growth. Popul. Resour. Environ. 1972, 3, 343–363. [Google Scholar]
- Yu, S.; Miao, C.; Song, H.; Huang, Y.; Chen, W.; He, X. Efficiency of nitrogen and phosphorus removal by six macrophytes from eutrophic water. Int. J. Phytoremediation 2019, 21, 643–651. [Google Scholar] [CrossRef]
- Headley, T.R.; Tanner, C.C. Constructed Wetlands with Floating Emergent Macrophytes: An Innovative Stormwater Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2261–2310. [Google Scholar] [CrossRef]
- Cardwell, A.; Hawker, D.; Greenway, M. Metal accumulation in aquatic macrophytes from southeast Queensland, Australia. Chemosphere 2002, 48, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Yangui, A.; Abderrabba, M. Towards a high yield recovery of polyphenols from olive mill wastewater on activated carbon coated with milk proteins: Experimental design and antioxidant activity. Food Chem. 2018, 262, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Long, J.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Protein recovery and anti-nutritional factor removal from soybean wastewater by complexing with a high concentration of polysaccharides in a novel quick-shearing system. J. Food Eng. 2019, 241, 1–9. [Google Scholar] [CrossRef]
- Li, R.; Zhai, Z.; Li, Y.; Yang, T.; Chen, Y. Kinetic study of heavy metals Cu and Zn removal during sewage sludge ash calcination in air and N2 atmospheres. J. Hazard. Mater. 2018, 347, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Imron, M.F.; Kurniawan, S.B.; Ismail, N.I.; Abdullah, S.R.S. Future challenges in diesel biodegradation by bacteria isolates: A review. J. Clean. Prod. 2020, 251, 119716. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.I.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and opportunities of biocoagulant/bioflocculant application for drinking water and wastewater treatment and its potential for sludge recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef] [PubMed]
- Kwoczynski, Z.; Čmelík, J. Characterization of biomass wastes and its possibility of agriculture utilization due to biochar production by torrefaction process. J. Clean. Prod. 2021, 280, 124302. [Google Scholar] [CrossRef]
- Lahon, D.; Sahariah, D.; Debnath, J.; Nath, N.; Meraj, G.; Farooq, M.; Kanga, S.; Singh, S.; Chand, K. Growth of water hyacinth biomass and its impact on the floristic composition of aquatic plants in a wetland ecosystem of the Brahmaputra floodplain of Assam, India. PeerJ 2023, 11, e14811. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Debusk, W.F. Growth characteristics of aquatic macrophytes cultured in nutrient-enriched water: I. Water hyacinth, water lettuce, and pennywort. Econ. Bot. 1984, 38, 229–239. [Google Scholar] [CrossRef]
- Miranda, A.F.; Biswas, B.; Ramkumar, N.; Singh, R.; Kumar, J.; James, A.; Roddick, F.; Lal, B.; Subudhi, S.; Bhaskar, T. Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnol. Biofuels 2016, 9, 221. [Google Scholar] [CrossRef]
- Brouwer, P.; Schluepmann, H.; Nierop, K.G.; Elderson, J.; Bijl, P.K.; van der Meer, I.; de Visser, W.; Reichart, G.J.; Smeekens, S.; van der Werf, A. Growing Azolla to produce sustainable protein feed: The effect of differing species and CO2 concentrations on biomass productivity and chemical composition. J. Sci. Food Agric. 2018, 98, 4759–4768. [Google Scholar] [CrossRef]
- Costa, M.L.; Santos, M.C.; Carrapiço, F. Biomass characterization of Azolla filiculoides grown in natural ecosystems and wastewater. Hydrobiologia 1999, 415, 323–327. [Google Scholar] [CrossRef]
- Basha, S.A.; Gopal, K.R.; Jebaraj, S. A review on biodiesel production, combustion, emissions and performance. Renew. Sustain. Energy Rev. 2009, 13, 1628–1634. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2019—Analysis-IEA. World Energy Outlook 2019. Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 21 February 2024).
- Welsby, D.; Price, J.; Pye, S.; Ekins, P. Unextractable fossil fuels in a 1.5 °C world. Nature 2021, 597, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Nikhil, G.; Chiranjeevi, P.; Reddy, C.N.; Rohit, M.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Barbora, L.; Bora, D.; Mitra, S.; Rangan, L.; Mahanta, P. An assessment of duckweed as a potential lignocellulosic feedstock for biogas production. Int. Biodeterior. Biodegrad. 2017, 119, 253–259. [Google Scholar] [CrossRef]
- Koutinas, A.; Kanellaki, M.; Bekatorou, A.; Kandylis, P.; Pissaridi, K.; Dima, A.; Boura, K.; Lappa, K.; Tsafrakidou, P.; Stergiou, P.-Y. Economic evaluation of technology for a new generation biofuel production using wastes. Bioresour. Technol. 2016, 200, 178–185. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Wang, Y.; Bai, J.; Bai, H.; Yan, D.; Cao, Y.; Li, Y.; Yu, Z.; Dong, G. Bioelectricity generation, contaminant removal and bacterial community distribution as affected by substrate material size and aquatic macrophyte in constructed wetland-microbial fuel cell. Bioresour. Technol. 2017, 245, 372–378. [Google Scholar] [CrossRef]
- Mohan, S.V.; Mohanakrishna, G.; Chiranjeevi, P. Sustainable power generation from floating macrophytes based ecological microenvironment through embedded fuel cells along with simultaneous wastewater treatment. Bioresour. Technol. 2011, 102, 7036–7042. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, D.; Ren, Z.J. Microbial community structure accompanied with electricity production in a constructed wetland plant microbial fuel cell. Bioresour. Technol. 2015, 195, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ruan, T.; Zeng, R.; Yin, X.-Y.; Zhang, S.-X.; Yang, Z.-H. Water hyacinth (Eichhornia crassipes) biomass as a biofuel feedstock by enzymatic hydrolysis. BioResources 2016, 11, 2372–2380. [Google Scholar] [CrossRef]
- Barua, V.B.; Goud, V.V.; Kalamdhad, A.S. Microbial pretreatment of water hyacinth for enhanced hydrolysis followed by biogas production. Renew. Energy 2018, 126, 21–29. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Liu, H.; Evrendilek, F.; Buyukada, M. Pyrolysis of water hyacinth biomass parts: Bioenergy, gas emissions, and by-products using TG-FTIR and Py-GC/MS analyses. Energy Convers. Manag. 2020, 207, 112552. [Google Scholar] [CrossRef]
- Varanasi, J.L.; Kumari, S.; Das, D. Improvement of energy recovery from water hyacinth by using integrated system. Int. J. Hydrog. Energy 2018, 43, 1303–1318. [Google Scholar] [CrossRef]
- Alalade, O.; Iyayi, E. Chemical composition and the feeding value of Azolla (Azolla pinnata) meal for egg-type chicks. Int. J. Poult. Sci. 2006, 5, 137–141. [Google Scholar]
- Mosha, S. A review on significance of Azolla meal as a protein plant source in finfish culture. J. Aquac. Res. Dev. 2018, 9. [Google Scholar] [CrossRef]
- Gupta, S.K.; Chandra, R.; Dey, D.; Mondal, G.; Shinde, K.P. Study of chemical composition and mineral content of sun dried Azolla pinnata. J. Pharmacogn. Phytochem. 2018, 7, 1214–1216. [Google Scholar]
- Verma, D.; Dey, K.P.S.D. Effect of supplementation of azolla (Azolla pinnata) on productive performance in cattle and economics of farmers: A field study. Pharma Innov. J. 2021, 10, 336–339. [Google Scholar]
- Xu, J.; Deshusses, M.A. Fermentation of swine wastewater-derived duckweed for biohydrogen production. Int. J. Hydrog. Energy 2015, 40, 7028–7036. [Google Scholar] [CrossRef]
- Zhao, X.; Moates, G.; Wellner, N.; Collins, S.; Coleman, M.; Waldron, K. Chemical characterisation and analysis of the cell wall polysaccharides of duckweed (Lemna minor). Carbohydr. Polym. 2014, 111, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Felycia, E.S.; Suryadi, I.; Yi-Hsu, J. Conversion of water hyacinth Eichhornia crassipes into biofuel intermediate: Combination subcritical water and zeolite based catalyst processes. Can Tho Univ. J. Sci. 2016, 14, 64–69. [Google Scholar]
- Shahbazi, A.; Croonenberghs, J.; Wang, L. Thermochemical Liquefaction of Duckweed to Biofuel. In Proceedings of the 2008 Providence, Rhode Island, St. Joseph, MI, USA, 29 June–2 July 2008; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2008. [Google Scholar]
- Hossain, R.; Chowdhury, M.K.; Yeasmin, S.; Hoq, M.M. Production of ethanol using yeast isolates on water hyacinth and azolla. Bangladesh J. Microbiol. 2010, 27, 56–60. [Google Scholar] [CrossRef]
- Magdum, S.; More, S.; Nadaf, A. Biochemical conversion of acid-pretreated water hyacinth (Eichhornia crassipes) to alcohol using Pichia Stipitis NCIM3497. Int. J. Adv. Biotechnol. Res. 2012, 3, 585–590. [Google Scholar]
- Das, S.; Bhattacharya, A.; Haldar, S.; Ganguly, A.; Gu, S.; Ting, Y.; Chatterjee, P. Optimization of enzymatic saccharification of water hyacinth biomass for bio-ethanol: Comparison between artificial neural network and response surface methodology. Sustain. Mater. Technol. 2015, 3, 17–28. [Google Scholar] [CrossRef]
- Su, H.; Zhao, Y.; Jiang, J.; Lu, Q.; Li, Q.; Luo, Y.; Zhao, H.; Wang, M. Use of Duckweed (Landoltia punctata) as a Fermentation Substrate for the Production of Higher Alcohols as Biofuels. Energy Fuels 2014, 28, 3206–3216. [Google Scholar] [CrossRef]
- Xu, J.; Cui, W.; Cheng, J.J.; Stomp, A.-M. Production of high-starch duckweed and its conversion to bioethanol. Biosyst. Eng. 2011, 110, 67–72. [Google Scholar] [CrossRef]
- Singhal, V.; Rai, J. Biogas production from water hyacinth and channel grass used for phytoremediation of industrial effluents. Bioresour. Technol. 2003, 86, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Ramaraj, R.; Unpaprom, Y. Effect of temperature on the performance of biogas production from Duckweed. Chem. Res. J. 2016, 1, 58–66. [Google Scholar]
- Patil, J.H.; AntonyRaj, M.; Gavimath, C. Study on effect of pretreatment methods on biomethanation of water hyacinth. Int. J. Adv. Biotechnol. Res. 2011, 2, 143–147. [Google Scholar]
- Madian, H.R.; Sidkey, N.M.; Abo Elsoud, M.M.; Hamouda, H.I.; Elazzazy, A.M. Bioethanol production from water hyacinth hydrolysate by Candida tropicalis Y-26. Arab. J. Sci. Eng. 2019, 44, 33–41. [Google Scholar] [CrossRef]
- Sayago, U.F.C. Design of a sustainable development process between phytoremediation and production of bioethanol with Eichhornia crassipes. Environ. Monit. Assess. 2019, 191, 221. [Google Scholar] [CrossRef]
- Shanab, S.M.; Hanafy, E.A.; Shalaby, E.A. Water hyacinth as non-edible source for biofuel production. Waste Biomass Valorization 2018, 9, 255–264. [Google Scholar] [CrossRef]
- Brouwer, P.; van der Werf, A.; Schluepmann, H.; Reichart, G.-J.; Nierop, K.G. Lipid yield and composition of Azolla filiculoides and the implications for biodiesel production. Bioenergy Res. 2016, 9, 369–377. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Kumar, P.; Singh, J. Anaerobic digestion of Azolla pinnata biomass grown in integrated industrial effluent for enhanced biogas production and COD reduction: Optimization and kinetics studies. Environ. Technol. Innov. 2020, 17, 100627. [Google Scholar] [CrossRef]
- Chupaza, M.H.; Park, Y.-R.; Kim, S.H.; Yang, J.W.; Jeong, G.-T.; Kim, S.-K. Bioethanol Production from Azolla filiculoides by Saccharomyces cerevisiae, Pichia stipitis, Candida lusitaniae, and Kluyveromyces marxianus. Appl. Biochem. Biotechnol. 2021, 193, 502–514. [Google Scholar] [CrossRef]
- Dohaei, M.; Karimi, K.; Rahimmalek, M.; Satari, B. Integrated biorefinery of aquatic fern Azolla filiculoides for enhanced extraction of phenolics, protein, and lipid and methane production from the residues. J. Clean. Prod. 2020, 276, 123175. [Google Scholar] [CrossRef]
- Biswas, B.; Singh, R.; Krishna, B.B.; Kumar, J.; Bhaskar, T. Pyrolysis of azolla, sargassum tenerrimum and water hyacinth for production of bio-oil. Bioresour. Technol. 2017, 242, 139–145. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Khan, A.A.; Suthar, S. Effect of thermal pre-treatment on co-digestion of duckweed (Lemna gibba) and waste activated sludge on biogas production. Chemosphere 2017, 174, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Chusov, A.; Maslikov, V.; Badenko, V.; Zhazhkov, V.; Molodtsov, D.; Pavlushkina, Y. Biogas potential assessment of the composite mixture from duckweed biomass. Sustainability 2021, 14, 351. [Google Scholar] [CrossRef]
- Lee, C.J.; Yangcheng, H.; Cheng, J.J.; Jane, J.L. Starch characterization and ethanol production of duckweed and corn kernel. Starch-Stärke 2016, 68, 348–354. [Google Scholar] [CrossRef]
- Muradov, N.; Fidalgo, B.; Gujar, A.C.; Ali, T. Pyrolysis of fast-growing aquatic biomass–Lemna minor (duckweed): Characterization of pyrolysis products. Bioresour. Technol. 2010, 101, 8424–8428. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An overview of biomass pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.T. 5—Hydrothermal liquefaction of protein-containing feedstocks. In Direct Thermochemical Liquefaction for Energy Applications; Rosendahl, L., Ed.; Woodhead Publishing: Delhi, India, 2018; pp. 127–168. [Google Scholar]
- Muradov, N.; Taha, M.; Miranda, A.F.; Kadali, K.; Gujar, A.; Rochfort, S.; Stevenson, T.; Ball, A.S.; Mouradov, A. Dual application of duckweed and azolla plants for wastewater treatment and renewable fuels and petrochemicals production. Biotechnol. Biofuels 2014, 7, 30. [Google Scholar] [CrossRef]
- Golzary, A.; Abdoli, M.A.; Yoshikawa, K.; Khodadadi, A.; Karbassi, A. Azolla as a Feedstock for Bio-Refinery: Cultivation, Conversion and Application. In Proceedings of the Qatar Foundation Annual Research Conference Proceedings Volume 2016 Issue 1, Doha, Qatar, 22–23 March 2016; p. EESP2082. [Google Scholar]
- Singh, R.; Balagurumurthy, B.; Prakash, A.; Bhaskar, T. Catalytic hydrothermal liquefaction of water hyacinth. Bioresour. Technol. 2015, 178, 157–165. [Google Scholar] [CrossRef]
- Gouri, M.D.; Sanganal, J.S.; Gopinath, C.; Kalibavi, C. Importance of azolla as a sustainable feed for livestock and poultry-A review. Agric. Rev. 2012, 33, 93–103. [Google Scholar]
- Forte, A.; Fagnano, M.; Fierro, A. Potential role of compost and green manure amendment to mitigate soil GHGs emissions in Mediterranean drip irrigated maize production systems. J. Environ. Manag. 2017, 192, 68–78. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, R.d.C.S.; Maranduba, H.L.; Hafner, M.B.; Rodrigues, L.B.; de Almeida Neto, J.A. Life cycle thinking applied to phytoremediation of dairy wastewater using aquatic macrophytes for treatment and biomass production. J. Clean. Prod. 2020, 267, 122006. [Google Scholar] [CrossRef]
- Datta, S.N. Culture of Azolla and its efficacy in diet of Labeo rohita. Aquaculture 2011, 310, 376–379. [Google Scholar] [CrossRef]
- Hossain, M.; Shimu, S.; Sarker, M.; Ahsan, M.; Banu, M. Biomass growth and composition of Azolla (Azolla pinnata R. BR.) supplemented with inorganic phosphorus in outdoor culture. SAARC J. Agric. 2021, 19, 177–184. [Google Scholar] [CrossRef]
- Adzman, N.; Goh, S.; Johari, A.; Alam, M.Z.; Kamaruddin, M. Preliminary study on Azolla cultivation and characterization for sustainable biomass source. J. Phys. Conf. Ser. 2022, 2259, 012018. [Google Scholar] [CrossRef]
- Shiomi, N.; Kitoh, S. Physiology: Nutrient absorption capacity of Azolla from waste water and use of Azolla plant as biomass. J. Plant Nutr. 1987, 10, 1663–1670. [Google Scholar] [CrossRef]
- Kalita, P.; Mukhopadhyay, P.K.; Mukherjee, A.K. Evaluation of the nutritional quality of four unexplored aquatic weeds from northeast India for the formulation of cost-effective fish feeds. Food Chem. 2007, 103, 204–209. [Google Scholar] [CrossRef]
- Duan, P.; Chang, Z.; Xu, Y.; Bai, X.; Wang, F.; Zhang, L. Hydrothermal processing of duckweed: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2013, 135, 710–719. [Google Scholar] [CrossRef]
- Aguilera-Morales, M.E.; Canales-Martínez, M.M.; Ávila-González, E.; Flores-Ortíz, C.M. Nutrients and bioactive compounds of the Lemna gibba and Ulva lactuca as possible ingredients to functional foods. Lat. Am. J. Aquat. Res. 2018, 46, 709–716. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, Y.; Zhang, G.; Fang, Y.; Xiao, Y.; Zhao, H. Improving production of bioethanol from duckweed (Landoltia punctata) by pectinase pretreatment. Energies 2012, 5, 3019–3032. [Google Scholar] [CrossRef]
- Adelakun, K.; Kehinde, A.; Amali, R.; Ogundiwin, D.; Omotayo, O. Nutritional and phytochemical quality of some tropical aquatic plants. Poultry, Fish. Wildl. Sci. 2016, 4, 1–4. [Google Scholar]
- Hossain, M.E.; Sikder, H.; Kabir, M.H.; Sarma, S.M. Nutritive value of water hyacinth (Eichhornia crassipes). Online J. Anim. Feed Res. 2015, 5, 40–44. [Google Scholar]
- Aboud, A.; Kidunda, R.; Osarya, J. Potential of water hyacinth (Eicchornia crassipes) in ruminant nutrition in Tanzania. Livest. Res. Rural Dev. 2005, 17, 2005. [Google Scholar]
- Mako, A.; Babayemi, O.; Akinsoyinu, A. An evaluation of nutritive value of water hyacinth (Eichhornia crassipes Mart. Solms-Laubach) harvested from different water sources as animal feed. Livest. Res. Rural Dev. 2011, 23, 10. [Google Scholar]
- Malik, A.A.; Aremu, A.; Ayanwale, B.; Ijaiya, A. A nutritional evaluation of water hyacinth {Eichhornia crassipes (Martius) Solms-Laubach} meal diets supplemented with Maxigrain enzyme for growing pullets. Agric. Food Sci. 2016, 10, 18–44. [Google Scholar]
- Pillai, P.K.; Premalatha, S.; Rajamony, S. Azolla-A sustainable feed substitute for livestock. Leisa India 2002, 4, 15–17. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, N.; Phillips, G.C.; Xu, J. Growing Lemna minor in agricultural wastewater and converting the duckweed biomass to ethanol. Bioresour. Technol. 2012, 124, 485–488. [Google Scholar] [CrossRef]
- Mwale, M.; Gwaze, F.R. Characteristics of duckweed and its potential as feed source for chickens reared for meat production: A review. Sci. Res. Essays 2013, 8, 689–697. [Google Scholar]
- Rusoff, L.L.; Blakeney, E.W., Jr.; Culley, D.D., Jr. Duckweeds (Lemnaceae family): A potential source of protein and amino acids. J. Agric. Food Chem. 1980, 28, 848–850. [Google Scholar] [CrossRef]
- Skillicorn, P.; Spira, W.; Journey, W. Duckweed Aquaculture: A New Aquatic Farming System for Developing Countries; CABI: Hong Kong, China, 1993. [Google Scholar]
- Mukherjee, R.; Nandi, B. Improvement of in vitro digestibility through biological treatment of water hyacinth biomass by two Pleurotus species. Int. Biodeterior. Biodegrad. 2004, 53, 7–12. [Google Scholar] [CrossRef]
- Akter, M.; Chowdhury, S.; Akter, Y.; Khatun, M. Effect of duckweed (Lemna minor) meal in the diet of laying hen and their performance. Bangladesh Res. Pub. J 2011, 5, 252–261. [Google Scholar]
- Huque, K.; Chowdhury, S.; Kibria, S. Study on the potentiality of duckweeds as a feed for cattle. Asian-Australas. J. Anim. Sci. 1996, 9, 133–137. [Google Scholar] [CrossRef]
- Effiong, B.; Sanni, A.; Fakunle, J. Effect of partial replacement of fishmeal with duckweed (Lemna pauciscostata) meal on the growth performance of Heterobranchus longifilis fingerlings. Rep. Opin. 2009, 1, 76–81. [Google Scholar]
- Devendra, C.; Leng, R. Feed resources for animals in Asia: Issues, strategies for use, intensification and integration for increased productivity. Asian-Australas. J. Anim. Sci. 2011, 24, 303–321. [Google Scholar] [CrossRef]
- Thu, N.; Dong, N.K. A Study of Water Hyacinth (Eichhornia crassipes) as a Feed Resource for Feeding Growing Rabbits. Available online: https://hostcambodia.com/mekarn/workshops/environ/PDF/kdong.pdf (accessed on 21 February 2024).
- Chatterjee, A.; Sharma, P.; Ghosh, M.; Mandal, M.; Roy, P. Utilization of Azolla microphylla as feed supplement for crossbred cattle. Int. J. Agric. Food Sci. Technol. 2013, 4, 207–214. [Google Scholar]
- Kumari, J.; Kumar, S.; Kumar, K.; Singh, P.K.; MONI, C.; Kumar, P.; Kumari, R. Effect of different level of azolla meal on nutrient utilization and growth performance in goat kids: Influence of azolla meal on nutrient utilization and growth in goat kids. J. AgriSearch 2021, 8, 275–280. [Google Scholar] [CrossRef]
- Van der Spiegel, M.; Noordam, M.; Van der Fels-Klerx, H. Safety of novel protein sources (insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef] [PubMed]
- Babayemi, O.; Bamikole, M.; Omojola, A. Evaluation of the nutritive value and free choice intake of two aquatic weeds (Nephrolepis biserrata and Spirodela polyrhiza) by West African dwarf goats. Trop. Subtrop. Agroecosystems 2006, 6, 15–22. [Google Scholar]
- Tintin Rostini, J.; Achmad, I.Z.; Diyamoko, D. Utilization of duckweed in feed goats on availability of protein and energy balance. Int. J. Agric. Res. (IJAIR) 2016, 4, 694–697. [Google Scholar]
- Biswas, P. Use of water hyacinth (Eichhornia crassipes) in the ration of growing calves. Indian Vet. J. 1988, 65, 496–500. [Google Scholar]
- Thu, N. Effects of water hyacinth (Eichhornia crassipes) in local cattle diets on nutrient utilization, rumen parameters and microbial protein synthesis. In SAADC 2011 Strategies and Challenges for Sustainable Animal Agriculture-Crop Systems, Volume III: Full Papers. Proceedings of the 3rd International Conference on Sustainable Animal Agriculture for Developing Countries, Nakhon Ratchasima, Thailand, 26–29 July 2011; Suranaree University of Technology: Nakhon Ratchasima, Thailand; pp. 422–426.
- Islam, S.; Khan, M.; Islam, M. Effect of feeding wilted water hyacinth (Eichhornia crassipes) on the performance of growing bull cattle. Indian J. Anim. Sci. 2009, 79, 494–497. [Google Scholar]
- Chakraborty, B.; Biswas, P.; Mandal, L.; Banerjee, G. Effect of Feeding Fresh Water Hyacinth (Eichhornia crassipes), or its Silage on the Milk Production in Crossbred Cows. Indian J. Anim. Nutr. 1991, 8, 115–118. [Google Scholar]
- Hertrampf, J.W.; Piedad-Pascual, F. Handbook on Ingredients for Aquaculture Feeds; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Kabir, A.; Hossain, M.; Rahman, M. Use of duckweed as feed for fishes in polyculture. J. Agric. Rural Dev. 2009, 7, 157–160. [Google Scholar] [CrossRef]
- Buddington, R.K. Hydrolysis-resistant organic matter as a reference for measurement of fish digestive efficiency. Trans. Am. Fish. Soc. 1980, 109, 653–656. [Google Scholar] [CrossRef]
- Talukdar, M.; Shahjahan, M.; Rahman, M. Suitability of duckweed (Lemna minor) as feed for fish in polyculture system. Int. J. Agric. Res. Innov. Technol. 2012, 2, 42–46. [Google Scholar] [CrossRef]
- Alalade, O.A.; Iyayi, E.A.; Alalade, T.O. The nutritive value of Azolla (Azolla pinnata) meal in diets for growing pullets and subsequent effect on laying performance. J. Poult. Sci. 2007, 44, 273–277. [Google Scholar] [CrossRef][Green Version]
- Basak, B.; Pramanik, M.A.H.; Rahman, M.S.; Tarafdar, S.U.; Roy, B.C. Azolla (Azolla pinnata) as a feed ingredient in broiler ration. Int. J. Poult. Sci. 2002, 1, 29–34. [Google Scholar]
- Khandaker, T.; Khan, M.J.; Shahjalal, M.; Rahman, M.M. Use of duckweed (Lemna perpusilla) as a protein source feed item in the diet of semi-scavenging jinding layer ducks. J. Poult. Sci. 2007, 44, 314–321. [Google Scholar] [CrossRef]
- Men, B.X.; Yamasaki, S. Use of water hyacinth as partial supplements in diets of growing crossbred common ducks. In Proceedings of the Workshop on the Technology Development for Livestock Production, JIRCAS-CTU; JIRCAS: Ibaraki, Japan, 2005. [Google Scholar]
- McLaughlin, N.; Hiba, A.; Wall, G.; King, D. Comparison of energy inputs for inorganic fertilizer and manure based corn production. Can. Agric. Eng. 2000, 42, 9–18. [Google Scholar]
- Tuomisto, H.L.; Hodge, I.; Riordan, P.; Macdonald, D.W. Does organic farming reduce environmental impacts? A meta-analysis of European research. J. Environ. Manag. 2012, 112, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Carrapiço, F. Azolla as a superorganism. Its implication in symbiotic studies. In Symbioses and Stress; Springer: Berlin/Heidelberg, Germany, 2010; pp. 225–241. [Google Scholar]
- Yao, Y.; Zhang, M.; Tian, Y.; Zhao, M.; Zeng, K.; Zhang, B.; Zhao, M.; Yin, B. Azolla biofertilizer for improving low nitrogen use efficiency in an intensive rice cropping system. Field Crops Res. 2018, 216, 158–164. [Google Scholar] [CrossRef]
- Kreider, A.N.; Fernandez Pulido, C.R.; Bruns, M.A.; Brennan, R.A. Duckweed as an Agricultural Amendment: Nitrogen Mineralization, Leaching, and Sorghum Uptake. J. Environ. Qual 2019, 48, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Lata, N.; Veenapani, D. Response of Water Hyacinth Manure on Growth Attributes and Yield in Brassica Juncea. J. Cent. Eur. Agric. 2011, 12, 336–343. [Google Scholar] [CrossRef][Green Version]
- Mo, W.; Zhang, Q. Energy-nutrients-water nexus: Integrated resource recovery in municipal wastewater treatment plants. J. Environ. Manag. 2013, 127, 255–267. [Google Scholar] [CrossRef]
- Valdes, R.; Aguilera, G.; Tobón, E.; Samaniego, M.; Díaz, J.; Carlos, H. Potential Uses of Treated Municipal Wastewater in a Semiarid Region of Mexico. Sustainability 2019, 11, 2217. [Google Scholar] [CrossRef]
- Ng, Y.S.; Chan, D.J.C. Phytoremediation capabilities of Spirodela polyrhiza, Salvinia molesta and Lemna sp. in synthetic wastewater: A comparative study. Int. J. Phytoremediation 2018, 20, 1179–1186. [Google Scholar] [CrossRef]
- Huang, J.-L.; Chen, Q.; Xu, L.-H. Problems and countermeasures in the application of constructed wetlands. Huan Jing Ke Xue Huanjing Kexue 2013, 34, 401–408. [Google Scholar]
- Chandra, P.; Kulshreshtha, K. Chromium accumulation and toxicity in aquatic vascular plants. Bot. Rev. 2004, 70, 313–327. [Google Scholar] [CrossRef]
- Landesman, L.; Chang, J.; Yamamoto, Y.; Goodwin, J. Nutritional value of wastewater-grown duckweed for fish and shrimp feed. World Aquac. 2002, 33, 39–40. [Google Scholar]
- Men, B.X.; Ogle, B.; Lindberg, J.E. Use of duckweed as a protein supplement for growing ducks. Asian-Australas. J. Anim. Sci. 2001, 14, 1741–1746. [Google Scholar] [CrossRef]
- Zainuddin, N.A.; Md Din, M.F.; Nuida, M.; Abdul Halim, K.; Abdul Salim, N.A.; Elias, S.H.; Mat Lazim, Z. The phytoremediation using water hyacinth and water lettuce: Correlation between sugar content, biomass growth rate, and nutrients. J. Kejuruter. 2022, 34, 915–924. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).