The Influence of the Biofiltration Method on the Efficiency of Ammonium Nitrogen Removal from Water in Combined Sorption and Nitrification Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject of Research
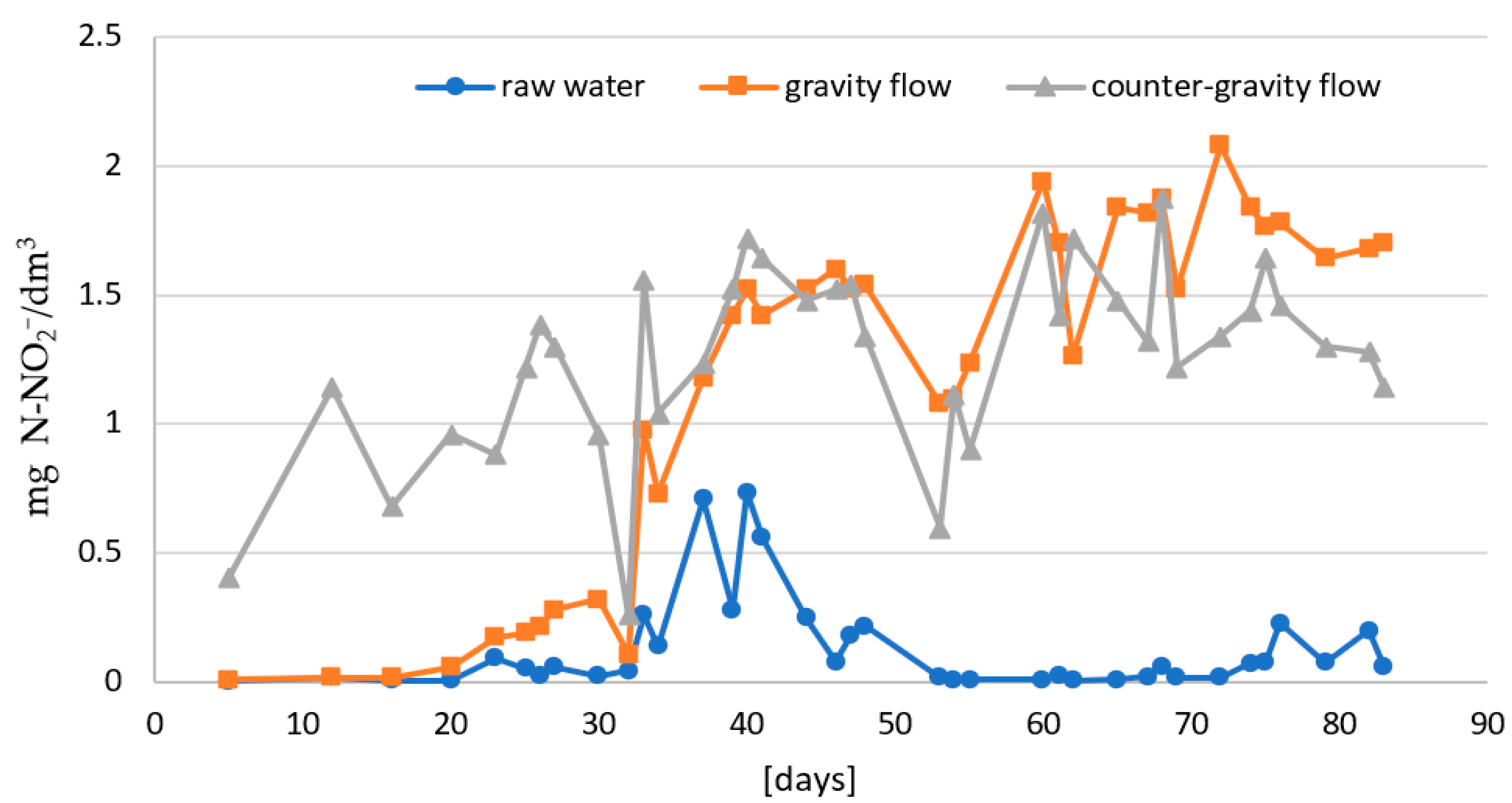
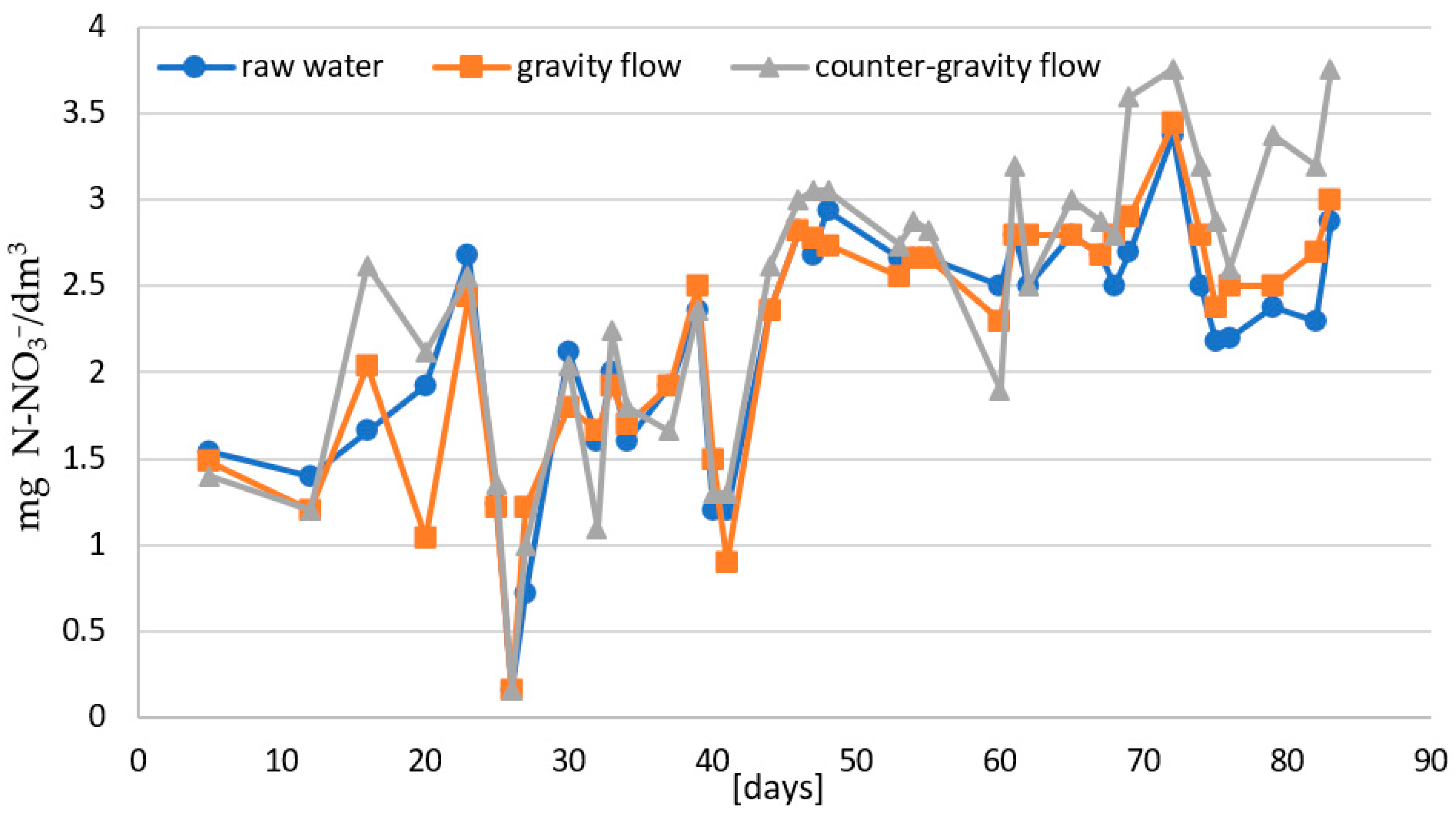
- The first stage consisted of analyzing the course of the ammonium nitrogen removal efficiency process depending on the flow direction of purified water in single-stage biofiltration. The process was carried out with gravity and counter-gravity flow at 14–22 °C.
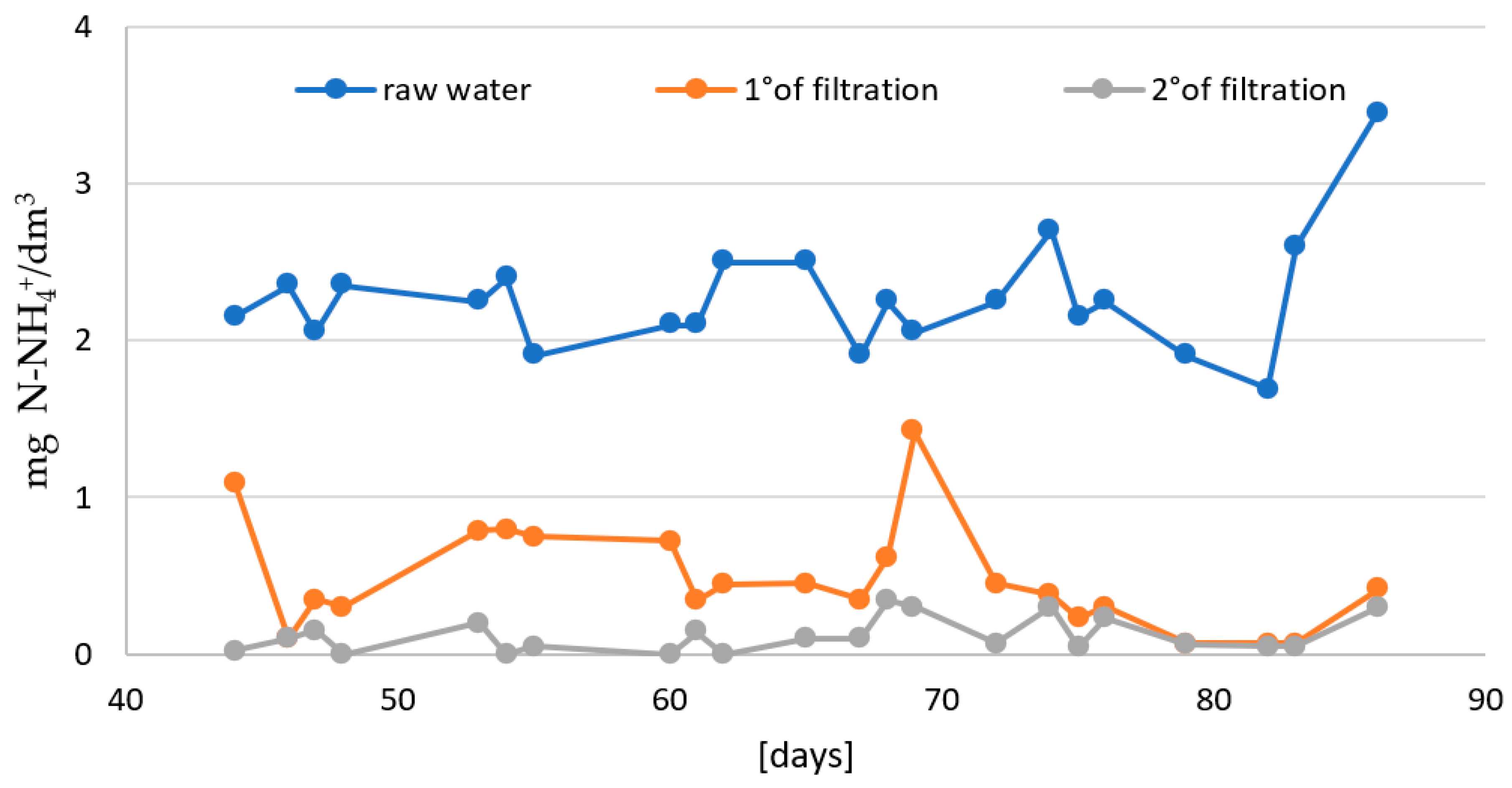
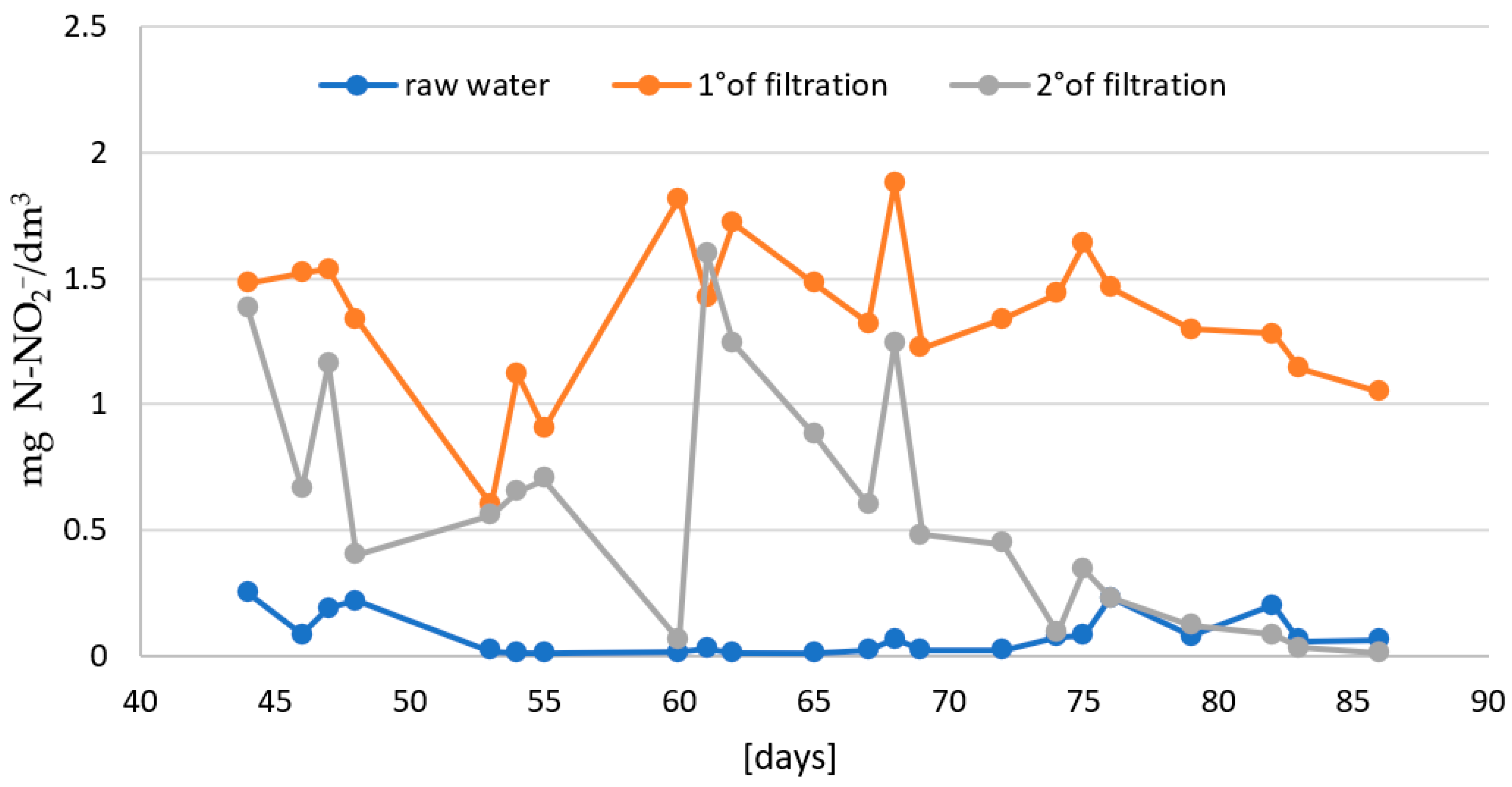
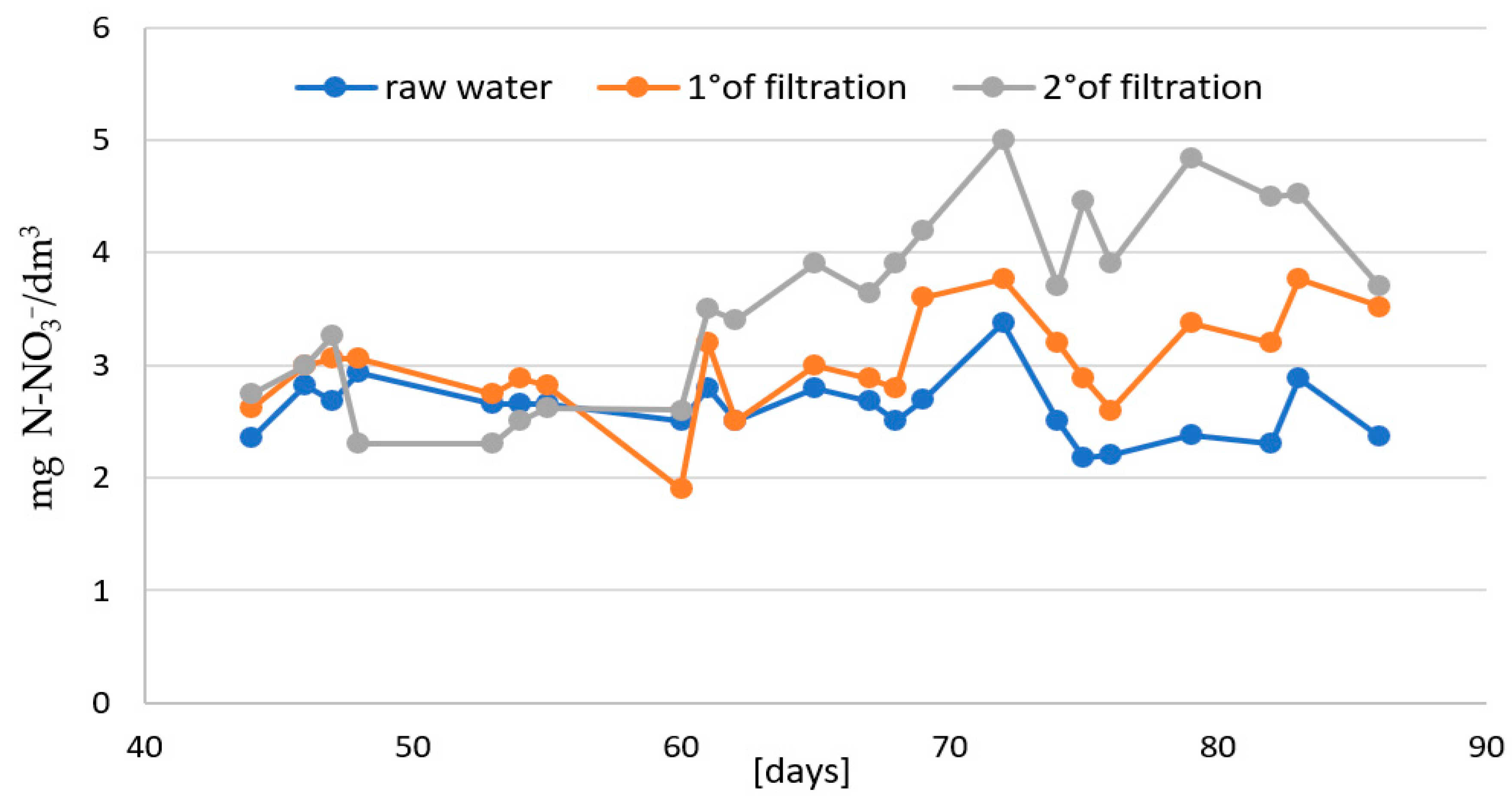
- In the second stage: the effectiveness of two-stage biofiltration with counter-gravity flow was verified (Figure 1).
2.2. Biofilter Parameters and Characteristics of the Filter Material
- height of the filter bed: 1.2 m,
- filter bed diameter: 35 mm,
- granulation of the filter bed: 1–3 mm,
- filtration speed: 2.5 m/h.
2.3. Characteristics of Water Subjected to Biofiltration
2.4. Determination of the Physicochemical Quality of Filtered Water
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mądrecka-Witkowska, B.; Komorowska-Kaufman, M.; Pruss, A.; Holc, D.; Trzebny, A.; Dabert, M. Microbial community of the initial stage of biologically active carbon filters’ work and its role in organic matter removal from water. Arch. Environ. Protect. 2023, 49, 67–77. [Google Scholar]
- Kirisits, M.J.; Emelko, M.B.; Pinto, A.J. Applying Biotechnology for Drinking Water Biofiltration: Advancing Science and Practice. Curr. Opin. Biotechnol. 2019, 57, 197–204. [Google Scholar] [CrossRef]
- Holc, D.; Mądrecka-Witkowska, B.; Komorowska-Kaufman, M.; Szeląg-Wasielewska, E.; Pruss, A.; Cybulski, Z. The application of different methods for indirect microbial development assessment in pilot scale drinking water biofilters. Arch. Environ. Protect. 2023, 47, 37–49. [Google Scholar] [CrossRef]
- Domoń, A.; Papciak, D.; Tchórzewska-Cieślak, B. Influence of Water Treatment Technology on the Stability of Tap Water. Water 2023, 15, 911. [Google Scholar] [CrossRef]
- Sadiq, R.; Rodriguez, M. Disinfection By-Products (DBPs) in Drinking Water and Predictive Models for Their Occurrence: A Review. Sci. Total Environ. 2004, 321, 21–46. [Google Scholar] [CrossRef] [PubMed]
- Subari, F.; Abdullah, S.R.S.; Hasan, H.A.; Rahman, N.A. Biological removal of ammonia by naturally grown bacteria in sand biofilter. MJAS 2018, 22, 346–352. [Google Scholar]
- Yushchenko, V.; Velyugo, E.; Romanovski, V. Influence of Ammonium Nitrogen on the Treatment Efficiency of Underground Water at Iron Removal Stations. Groundw. Sustain. Dev. 2023, 22, 100943. [Google Scholar] [CrossRef]
- Radu, G.; Racoviteanu, G. Removing Ammonium from Water Intended for Human Consumption. A Review of Existing Technologies. IOP Conf. Ser. Earth Environ. Sci. 2021, 664, 012029. [Google Scholar] [CrossRef]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill: Boston, MA, USA, 2007. [Google Scholar]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection Methods of Ammonia Nitrogen in Water: A Review. TrAC Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Regulation of the Minister of Health from December 7, 2017 on the Quality of Water Intended for Human Consumption (in Polish). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20170002294/O/D20172294.pdf (accessed on 15 January 2024).
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31998L0083 (accessed on 15 January 2024).
- McCarty, P.L. What Is the Best Biological Process for Nitrogen Removal: When and Why? Environ. Sci. Technol. 2018, 52, 3835–3841. [Google Scholar] [CrossRef]
- Paul, D.; Banerjee, A. Technologies for Biological and Bioelectrochemical Removal of Inorganic Nitrogen from Wastewater: A Review. Nitrogen 2022, 3, 298–313. [Google Scholar] [CrossRef]
- Kosgey, K.; Zungu, P.V.; Bux, F.; Kumari, S. Biological Nitrogen Removal from Low Carbon Wastewater. Front. Microbiol. 2022, 13, 968812. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.C.; Haas, C.N.; Sales, C.M. Nitrification in Premise Plumbing: A Review. Water 2020, 12, 830. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, L.; Tian, X.; Yin, Y. Seasonal Variation of Bacterial Community in Biological Aerated Filter for Ammonia Removal in Drinking Water Treatment. Water Res. 2017, 123, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, C.N.; McCarty, P.L.; Parkin, G.F. Chemistry for Environmental Engineering and Science, 5th ed.; McGraw-Hill: Boston, MA, USA, 2007. [Google Scholar]
- Zhang, Y.; Love, N.; Edwards, M. Nitrification in Drinking Water Systems. Crit. Rev. Environ. Sci. Technol. 2009, 39, 153–208. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Li, D.-T.; Yang, H. Investigation of Total Bacterial and Ammonia-Oxidizing Bacterial Community Composition in a Full-Scale Aerated Submerged Biofilm Reactor for Drinking Water Pretreatment in China. FEMS Microbiol. Lett. 2007, 268, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-W.; Qin, Y.-Y.; Ren, H.-Q.; Li, D.-T.; Yang, H. Seasonal Variation in Communities of Ammonia-Oxidizing Bacteria Based on Polymerase Chain Reaction—Denaturing Gradient Gel Electrophoresis in a Biofilm Reactor for Drinking Water Pretreatment. Can. J. Microbiol. 2008, 54, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Baribeau, H.; Regan, J. Microbiology, Isolation, and Detection of Nitrifying Microorganisms. In Nitrification Prevention and Control in Drinking Water, 2nd ed.; American Water Works Association: Denver, CO, USA, 2013; pp. 97–125. [Google Scholar]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of Nitrifiers and Evaluation of Partial Nitrification for Wastewater Treatment: A Review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Brock Biology of Microorganisms; Pearson: Boston, MA, USA, 2015. [Google Scholar]
- Daims, H.; Lücker, S.; Wagner, M. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef]
- Lu, S.; Liu, J.; Li, S.; Biney, E. Analysis of Up-Flow Aerated Biological Activated Carbon Filter Technology in Drinking Water Treatment. Environ. Technol. 2013, 34, 2345–2351. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Cornejo-Ponce, L.; Rajendran, R.; Manavalan, K.; Femilaa Rajan, V.; Awad, F. A Review on Biofiltration Techniques: Recent Advancements in the Removal of Volatile Organic Compounds and Heavy Metals in the Treatment of Polluted Water. Bioengineered 2022, 13, 8432–8477. [Google Scholar] [CrossRef]
- Papciak, D.; Zamorska, J.; Piech, A. Effect of iron(III) on effectiveness of ammonium nitrogen removal in process of nitrification in chalcedonite beds. In Proceedings of the XXth Jubilee-National, VIIIth International Scientific and Technical Conference Water Supply and Water Qual, Poznan, Poland, 15–18 June 2008; pp. 579–589. [Google Scholar]
- Bar-Zeev, E.; Belkin, N.; Liberman, B.; Berman, T.; Berman-Frank, I. Rapid Sand Filtration Pretreatment for SWRO: Microbial Maturation Dynamics and Filtration Efficiency of Organic Matter. Desalination 2012, 286, 120–130. [Google Scholar] [CrossRef]
- Gomes, I.B.; Simões, M.; Simões, L.C. An Overview on the Reactors to Study Drinking Water Biofilms. Wat. Res. 2014, 62, 63–87. [Google Scholar] [CrossRef]
- Liu, Y.; Capdeville, B. Specific Activity of Nitrifying Biofilm in Water Nitrification Process. Water Res. 1996, 30, 1645–1650. [Google Scholar] [CrossRef]
- Lytle, D.A.; White, C.; Williams, D.; Koch, L.; Nauman, E. Innovative Biological Water Treatment for the Removal of Elevated Ammonia. J. AWWA 2013, 105, E524–E539. [Google Scholar] [CrossRef]
- De Vet, W.W.J.M.; Van Loosdrecht, M.C.M.; Rietveld, L.C. Phosphorus Limitation in Nitrifying Groundwater Filters. Wat. Res. 2012, 46, 1061–1069. [Google Scholar] [CrossRef]
- Lopato, L.; Röttgers, N.; Binning, P.J.; Arvin, E. Heterogeneous Nitrification in a Full-Scale Rapid Sand Filter Treating Groundwater. J. Environ. Eng. 2013, 139, 375–384. [Google Scholar] [CrossRef]
- Papciak, D.; Domoń, A.; Puszkarewicz, A.; Kaleta, J. The Use of Chalcedonite as a Biosorption Bed in the Treatment of Groundwater. Appl. Sci. 2019, 9, 751. [Google Scholar] [CrossRef]
- Pruss, A. Research on the influence of changes in the thickness of the biological membrane on the grains of the filter bed on oxygen consumption during the removal of ammonium nitrogen from water. Environ. Protect. 2007, 1, 35–39. (In Polish) [Google Scholar]
- Yu, X.; Qi, Z.; Zhang, X.; Yu, P.; Liu, B.; Zhang, L.; Fu, L. Nitrogen Loss and Oxygen Paradox in Full-Scale Biofiltration for Drinking Water Treatment. Wat. Res. 2007, 41, 1455–1464. [Google Scholar] [CrossRef]
- AbuKhadra, M.R.; Eid, M.H.; Allam, A.A.; Ajarem, J.S.; Almalki, A.M.; Salama, Y. Evaluation of Different Forms of Egyptian Diatomite for the Removal of Ammonium Ions from Lake Qarun: A Realistic Study to Avoid Eutrophication. Environ. Pollut. 2020, 266, 115277. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Value | Parameter | Value |
|---|---|---|---|
| SiO3 | 68–73% | Specific gravity | 2.10–2.25 kg/m3 |
| Al2O3 | 9–12% | Bulk density of products depending on grain size | 0.4–0.9 t/m3 |
| Fe2O3 | 4–6% | Compressive strength | 25–60 MPa |
| Porosity | 25–35% | Thermal conductivity | 0.25–0.3 kcal/m∙°C |
| Absorbability | 20–30% | Loss on ignition (900 °C) | 7–10% |
| Parameter | Unit | Min | Max |
|---|---|---|---|
| Ammonium nitrogen | mg N-NH4+/dm3 | 1.60 | 3.50 |
| Nitrite nitrogen | mg N-NO2−/dm3 | 0.004 | 0.23 |
| Nitrate nitrogen | mg N-NO3−/dm3 | 0.16 | 3.38 |
| Dissolved oxygen | mg O2/dm3 | 6.18 | 7.80 |
| Temperature | °C | 14 | 22 |
| Turbidity | NTU | 0 | 5 |
| Color | mg Pt/dm3 | 0 | 11 |
| Parameter | Gravity Flow | Counter-Gravity Flow | |
|---|---|---|---|
| Efficiency of ammonia nitrogen removal [%] | Min | 0 | 31 |
| Max | 99 | 97 | |
| Amount of ammonium nitrogen removed [mg N-NH4+/dm3] | Min | 0 | 0.63 |
| Max | 2.52 | 2.53 | |
| Mean | 1.37 | 1.65 | |
| Achieving normative requirements for N-NH4+ [day] | from 73 days | from 73 days | |
| Start of the phase I of the nitrification process [day] | 23 | 5 | |
| Start of the phase II of the nitrification process [day] | 62 | 40 |
| Parameter | Gravity Flow | Counter-Gravity Flow | |
|---|---|---|---|
| Total theoretical oxygen consumption [mg O2/dm3] | Min | 1.21 | 1.03 |
| Max | 3.52 | 5.97 | |
| Mean | 2.28 | 3.3 | |
| Actual oxygen consumption [mg O2/dm3] | Min | 1.48 | 0.39 |
| Max | 5.37 | 2.5 | |
| Mean | 4.15 | 1.66 | |
| The actual amount of oxygen for each 1 mg N removed in the biofiltration process [mg O2/dm3] | Phase I | 1.02 | 0.34 |
| Phase II | 3.47 | 1.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papciak, D.; Domoń, A.; Zdeb, M. The Influence of the Biofiltration Method on the Efficiency of Ammonium Nitrogen Removal from Water in Combined Sorption and Nitrification Processes. Water 2024, 16, 722. https://doi.org/10.3390/w16050722
Papciak D, Domoń A, Zdeb M. The Influence of the Biofiltration Method on the Efficiency of Ammonium Nitrogen Removal from Water in Combined Sorption and Nitrification Processes. Water. 2024; 16(5):722. https://doi.org/10.3390/w16050722
Chicago/Turabian StylePapciak, Dorota, Andżelika Domoń, and Monika Zdeb. 2024. "The Influence of the Biofiltration Method on the Efficiency of Ammonium Nitrogen Removal from Water in Combined Sorption and Nitrification Processes" Water 16, no. 5: 722. https://doi.org/10.3390/w16050722
APA StylePapciak, D., Domoń, A., & Zdeb, M. (2024). The Influence of the Biofiltration Method on the Efficiency of Ammonium Nitrogen Removal from Water in Combined Sorption and Nitrification Processes. Water, 16(5), 722. https://doi.org/10.3390/w16050722








