Composition, Dynamic Changes, and Carbon Sequestration Effects of Organic Carbon in the Water of a Penaeus vannamei Culture Pond
Abstract
1. Introduction
2. Materials and Methods
2.1. Pond Conditions and Aquaculture Management
2.2. Experimental Design
2.3. Sample Collection and Measurements
2.4. Data Processing and Analysis
3. Results
3.1. Physico-Chemical Indicators in Pond Water during the Aquaculture Period
3.2. Dynamic Changes in Organic Carbon Contents in Pond Water during the Aquaculture Period
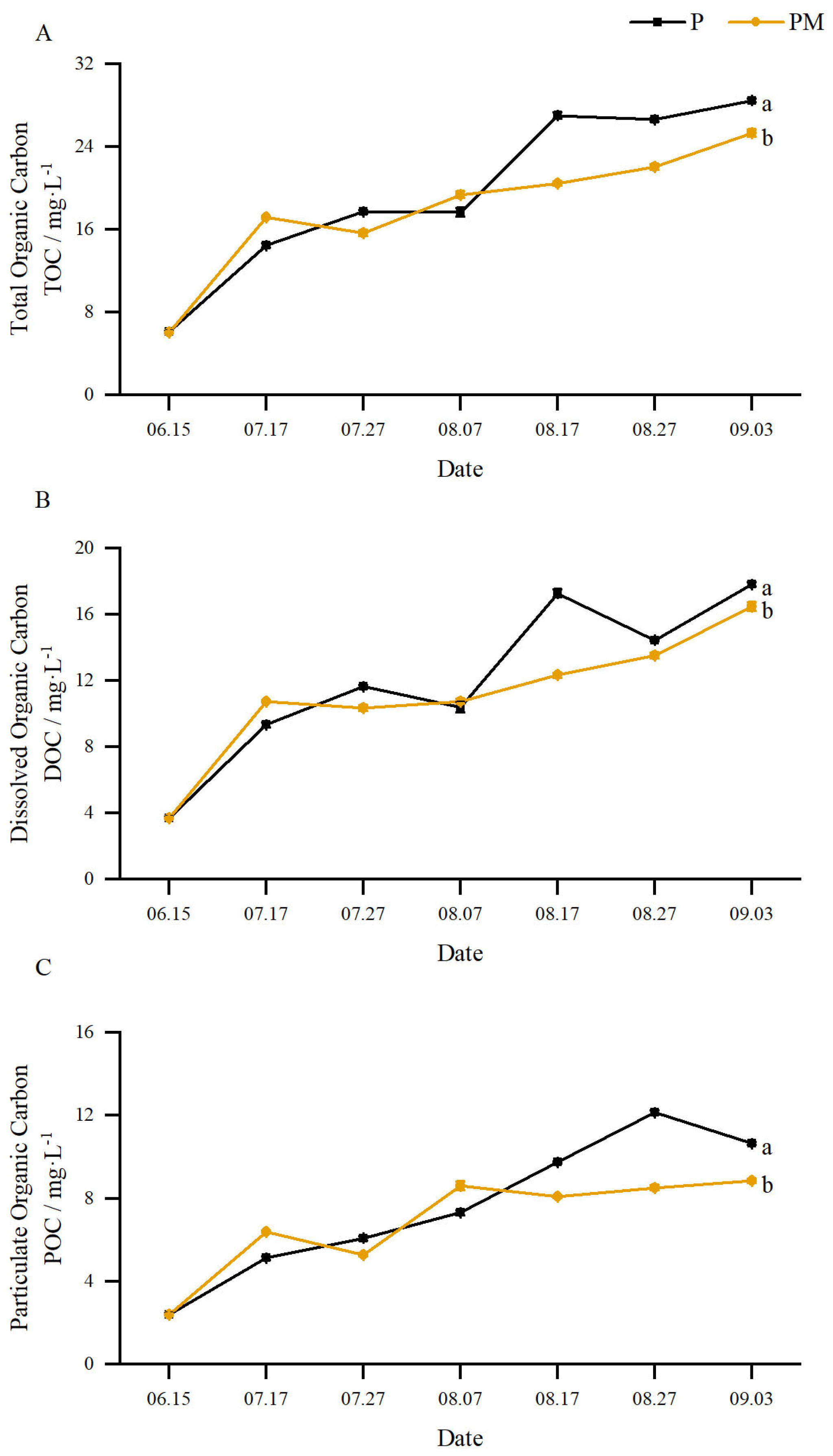
3.2.1. Total Organic Carbon (TOC), Dissolved Organic Carbon (DOC), and Particulate Organic Carbon (POC)
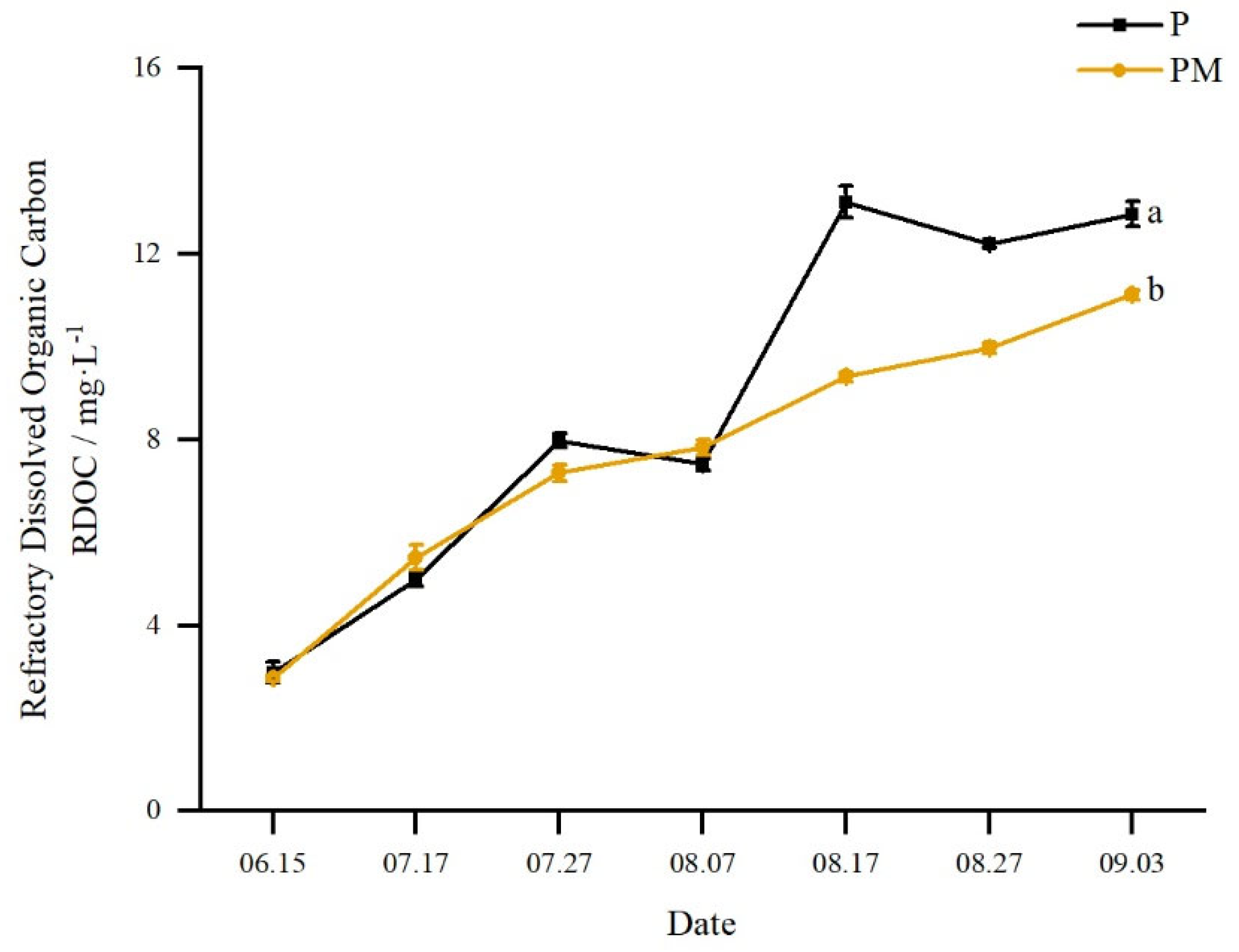
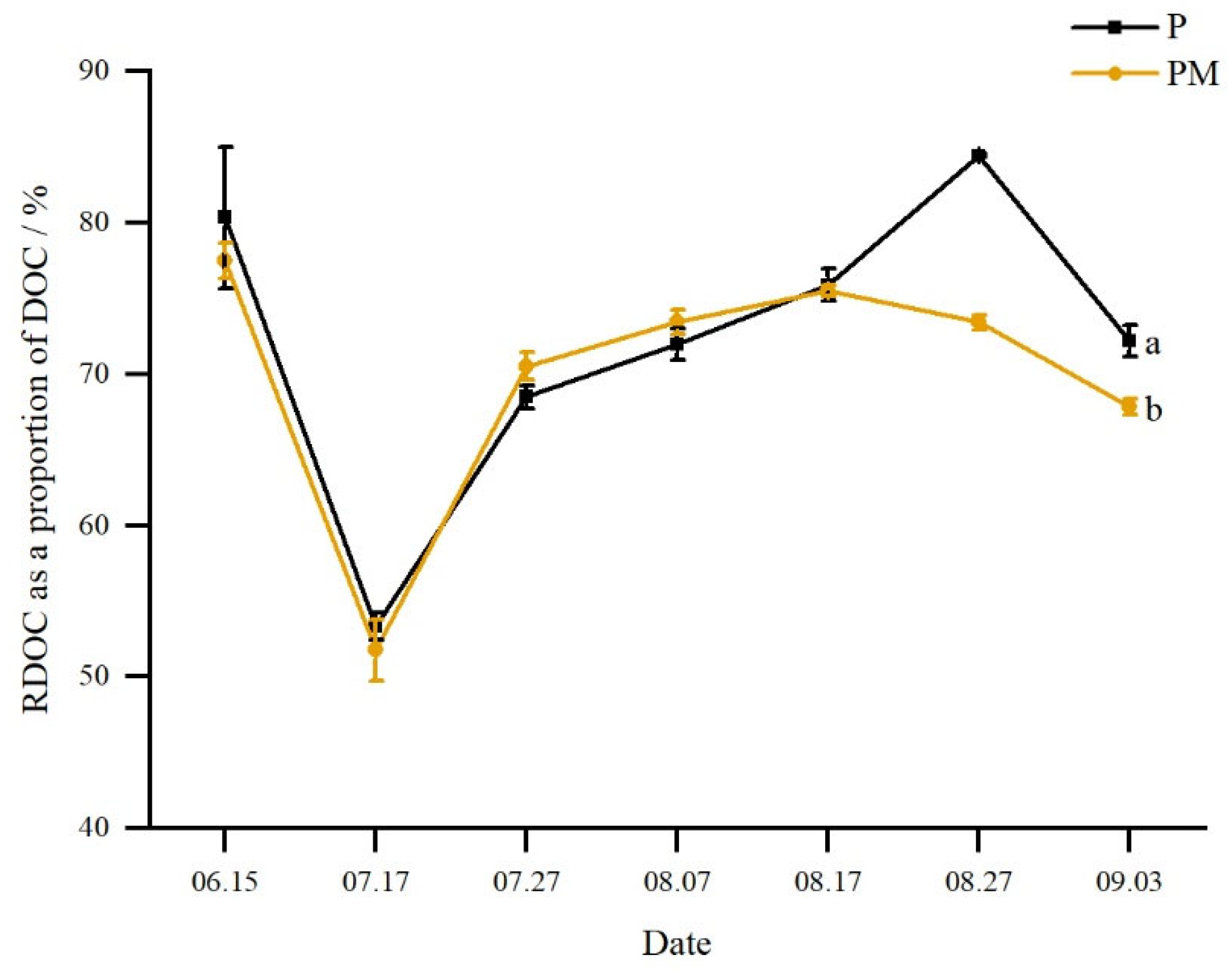
3.2.2. Refractory Dissolved Organic Carbon (RDOC)
3.2.3. Proportions Made up by Different Organic Carbon Components
3.3. Correlation Analysis between Organic Carbon and Environmental Factors in Water
4. Discussion
4.1. Comparison of Organic Carbon Composition and Contents in Different Aquaculture Ecosystems
4.2. Factors Affecting Changes in Refractory Organic Carbon Contents
4.3. Analysis of Carbon Sequestration Effects of Integrated Prawn Aquaculture Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, C.M.; Prairie, Y.T. Prevalence of heterotrophy and atmospheric CO2 emissions from aquatic ecosystems. Ecosystems 2005, 8, 862–870. [Google Scholar] [CrossRef]
- McKinley, G.A.; Fay, A.R.; Lovenduski, N.S.; Pilcher, D.J. Natural variability and anthropogenic trends in the ocean carbon sink. Annu. Rev. Mar. Sci. 2017, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, L.; Yang, P.; Tong, C.; Yang, H.; Tan, L.; Lin, Y.; Lai, D.Y.; Tang, K.W. Seasonal variations in source-sink balance of CO2 in subtropical earthen aquaculture ponds: Implications for carbon emission management. J. Hydrol. 2023, 626, 130330. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, K.W.; Yang, P.; Yang, H.; Tong, C.; Song, C.; Tan, L.; Zhao, G.; Zhou, X.; Sun, D. Assessing carbon greenhouse gas emissions from aquaculture in China based on aquaculture system types, species, environmental conditions and management practices. Agric. Ecosyst. Environ. 2022, 338, 108110. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, L.; Lin, Y.; Yang, H.; Lai, D.Y.; Tong, C.; Zhang, Y.; Tan, L.; Zhao, G.; Tang, K.W. Significant inter-annual fluctuation in CO2 and CH4 diffusive fluxes from subtropical aquaculture ponds: Implications for climate change and carbon emission evaluations. Water Res. 2024, 249, 120943. [Google Scholar] [CrossRef]
- Sidik, F.; Lovelock, C.E. CO2 efflux from shrimp ponds in Indonesia. PLoS ONE 2013, 8, e66329. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.P.; Urbina, M.A.; Wilson, R.W. Lessons from two high CO2 worlds—Future oceans and intensive aquaculture. Glob. Chang. Biol. 2017, 23, 2141–2148. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, Y.; Luo, J.; Hu, C.; Duan, H.; Qiu, Y.; Zhang, M.; Hu, Z.; Xiao, W. Low carbon dioxide emissions from aquaculture farm of lake revealed by long-term measurements. Agric. Ecosyst. Environ. 2024, 363, 108851. [Google Scholar] [CrossRef]
- Liu, X.; Dong, S.; Yu, L.; Shan, H.; Zhang, P.; Wang, F. CO2 fluxes at the water-air interface and their relationship with environmental factors in an integrated Penaeus vannamei—Hardshell clam pond culture system. Period. Ocean. Univ. China 2023, 53, 26–34. [Google Scholar]
- Tang, J.; Jiang, Z.; Mao, Y. Clarification on the definitions and its relevant issues of fisheries carbon sink and carbon sink fisheries. Prog. Fish. Sci. 2022, 43, 1–7. [Google Scholar]
- Jiang, Z.; Fang, J.; Mao, Y.; Jiang, W.; Fang, J.; Lin, F.; Gao, Y.; Du, M.; Li, R. Research progress on the carbon sink function of filter-feeding shellfish mariculture and future scientific issues. Prog. Fish. Sci. 2022, 43, 106–114. [Google Scholar]
- Barber, R.T. Dissolved organic carbon from deep waters resists microbial oxidation. Nature 1968, 220, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Kragh, T.; Søndergaard, M. Production and decomposition of new DOC by marine plankton communities: Carbohydrates, refractory components and nutrient limitation. Biogeochemistry 2009, 96, 177–187. [Google Scholar] [CrossRef]
- Mopper, K.; Stahovec, W.L. Sources and sinks of low molecular weight organic carbonyl compounds in seawater. Mar. Chem. 1986, 19, 305–321. [Google Scholar] [CrossRef]
- Druffel, E.R.M.; Williams, P.M.; Suzuki, Y. Concentrations and radiocarbon signatures of dissolved organic matter in the Pacific Ocean. Geophys. Res. Lett. 1989, 16, 991–994. [Google Scholar] [CrossRef]
- Alperin, M.; Martens, C. Dissolved organic carbon in marine pore waters: A comparison of three oxidation methods. Mar. Chem. 1993, 41, 135–143. [Google Scholar] [CrossRef]
- Arrieta, J.M.; Mayol, E.; Hansman, R.L.; Herndl, G.J.; Dittmar, T.; Duarte, C.M. Dilution limits dissolved organic carbon utilization in the deep ocean. Science 2015, 348, 331–333. [Google Scholar] [CrossRef]
- Hertkorn, N.; Benner, R.; Frommberger, M.; Schmitt-Kopplin, P.; Witt, M.; Kaiser, K.; Kettrup, A.; Hedges, J.I. Characterization of a major refractory component of marine dissolved organic matter. Geochim. Cosmochim. Acta 2006, 70, 2990–3010. [Google Scholar] [CrossRef]
- Jiao, N.; Herndl, G.J.; Hansell, D.A.; Benner, R.; Kattner, G.; Wilhelm, S.W.; Kirchman, D.L.; Weinbauer, M.G.; Luo, T.; Chen, F.; et al. Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nat. Rev. Microbiol. 2010, 8, 593–599. [Google Scholar] [CrossRef]
- Hansell, D.A. Recalcitrant dissolved organic carbon fractions. Annu. Rev. Mar. Sci. 2013, 5, 421–445. [Google Scholar] [CrossRef]
- Servais, P.; Billen, G.; Hascoët, M.-C. Determination of the biodegradable fraction of dissolved organic matter in waters. Water Res. 1987, 21, 445–450. [Google Scholar] [CrossRef]
- Servais, P.; Anzil, A.; Ventresque, C. Simple method for determination of biodegradable dissolved organic carbon in water. Appl. Environ. Microbiol. 1989, 55, 2732–2734. [Google Scholar] [CrossRef] [PubMed]
- Mermillod-Blondin, F.; Simon, L.; Maazouzi, C.; Foulquier, A.; Delolme, C.; Marmonier, P. Dynamics of dissolved organic carbon (DOC) through stormwater basins designed for groundwater recharge in urban area: Assessment of retention efficiency. Water Res. 2015, 81, 27–37. [Google Scholar] [CrossRef]
- A Rhim, J. Characteristics of adsorption and biodegradation of dissolved organic carbon in biological activated carbon pilot plant. Korean J. Chem. Eng. 2006, 23, 38–42. [Google Scholar] [CrossRef]
- Kubo, A.; Yamashita, Y.; Hashihama, F.; Kanda, J. The origin and characteristics of dissolved organic carbon in the highly urbanized coastal waters of Tokyo Bay. J. Oceanogr. 2023, 79, 241–252. [Google Scholar] [CrossRef]
- He, Q.; Xiao, Q.; Fan, J.; Zhao, H.; Cao, M.; Zhang, C.; Jiang, Y. The impact of heterotrophic bacteria on recalcitrant dissolved organic carbon formation in a typical karstic river. Sci. Total. Environ. 2022, 815, 152576. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shang, P.; Chen, S.; Du, Y.; Bonizzoni, M.; Ward, A.K. Discharge and temperature controls of dissolved organic matter (dom) in a forested coastal plain stream. Water 2021, 13, 2919. [Google Scholar] [CrossRef]
- Qualls, R.G.; Haines, B.L. Biodegradability of dissolved organic-matter in forest throughfall, soil solution, and stream water. Soil Sci. Soc. Am. J. 1992, 56, 578–586. [Google Scholar] [CrossRef]
- Chapelle, F.H. The bioavailability of dissolved, particulate, and adsorbed organic carbon in groundwater systems. Groundwater 2020, 59, 226–235. [Google Scholar] [CrossRef]
- Cronan, C.S.; Aiken, G.R. Chemistry and transport of soluble humic substances in forested watersheds of the Adirondack Park, New York. Geochim. Cosmochim. Acta 1985, 49, 1697–1705. [Google Scholar] [CrossRef]
- He, T.; Zhang, F.; Wang, Y.; Chen, X.; Du, J. Characterization of dissolved organic matter in submarine groundwater from a salt marsh in Chongming Island, China. J. Oceanol. Limnol. 2021, 40, 128–141. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Xiong, T.; Tang, K.; He, C.; Shi, Q.; Jiao, N.; Zhang, Y. Carbon sequestration in the form of recalcitrant dissolved organic carbon in a seaweed (kelp) farming environment. Environ. Sci. Technol. 2022, 56, 9112–9122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, J.; Shen, Y.; Xia, Z.; Hu, M.; Wu, T.; Zhuang, M.; Li, Y.; Tong, Y.; Yang, J.; et al. Removable carbon and storage carbon of golden tides. Mar. Pollut. Bull. 2023, 191, 114974. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Zhang, Z.; He, C.; Shi, Q.; Jiao, N.; Zhang, Y. DOC dynamics and bacterial community succession during long-term degradation of Ulva prolifera and their implications for the legacy effect of green tides on refractory DOC pool in seawater. Water Res. 2020, 185, 116268. [Google Scholar] [CrossRef] [PubMed]
- Verdegem, M.C.J.; Bosma, R.H. Water withdrawal for brackish and inland aquaculture, and options to produce more fish in ponds with present water use. Water Policy 2009, 11, 52–68. [Google Scholar] [CrossRef]
- Cao, L.; Diana, J.S.; Keoleian, G.A.; Lai, Q. Life cycle assessment of chinese shrimp farming systems targeted for export and domestic sales. Environ. Sci. Technol. 2011, 45, 6531–6538. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qin, T.; Han, L. A review of research on marine carbon sink fisheries. Resour. Sci. 2018, 40, 161–172. [Google Scholar]
- Jin, Y.; Zhu, B.; Wang, F.; Sun, S.; Wang, P.; Liu, X. Analysis of water chemistry characteristics and main ion controlling factors of lakes in the Nagqu area of the Qinghai–Tibet plateau in summer. Water 2023, 15, 2900. [Google Scholar] [CrossRef]
- Lei, Y. Experiment in the Chemistry of Aquaculture Water Environments; China Agriculture Press: Beijing, China, 2006. [Google Scholar]
- Tranvik, L.J.; Hofle, M.G. Bacterial growth in mixed cultures on dissolved organic carbon from humic and clear waters. Appl. Environ. Microbiol. 1987, 53, 482–488. [Google Scholar] [CrossRef]
- Kubo, A.; Yamamoto-Kawai, M.; Kanda, J. Seasonal variations in concentration and lability of dissolved organic carbon in Tokyo Bay. Biogeosciences 2015, 12, 269–279. [Google Scholar] [CrossRef][Green Version]
- You, K.; Ma, S. Preliminary study on organic carbon in shrimp cultural ecosystems. J. Ocean. Univ. Qingdao 2002, 32, 51–55. [Google Scholar]
- Liu, G. Storage of the organic carbon pools in the shrimp ponds. Acta Ecol. Sin. 2000, 20, 1056–1060. [Google Scholar]
- Hansell, D.A.; Carlson, C.A.; Repeta, D.J.; Schlitzer, R. Dissolved organic matter in the ocean: A controversy stimulates new insights. Oceanography 2009, 22, 202–211. [Google Scholar] [CrossRef]
- Regnier, P.; Friedlingstein, P.; Ciais, P.; Mackenzie, F.T.; Gruber, N.; Janssens, I.A.; Laruelle, G.G.; Lauerwald, R.; Luyssaert, S.; Andersson, A.J.; et al. Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat. Geosci. 2013, 6, 597–607. [Google Scholar] [CrossRef]
- Xiang, H.; Lü, X.-W.; Yang, F.; Yin, L.-H.; Zhu, G.-C. Characteristics of microbial community and operation efficiency in biofilter process for drinking water purification. Huan Jing ke Xue Huanjing Kexue 2011, 32, 1194–1201. [Google Scholar] [PubMed]
- Shen, Y.; Chapelle, F.H.; Strom, E.W.; Benner, R. Origins and bioavailability of dissolved organic matter in groundwater. Biogeochemistry 2014, 122, 61–78. [Google Scholar] [CrossRef]
- Raven, J.A.; Falkowski, P.G. Oceanic sinks for atmospheric CO2. Plant Cell Environ. 1999, 22, 741–755. [Google Scholar] [CrossRef]
- Volk, T.; Hoffert, M.I. Ocean carbon pumps: Analysis of relative strengths and efficiencies in ocean-driven atmospheric CO2 changes. In The Carbon Cycle and Atmospheric CO2: Natural Variations Archean to Present; Wiley: Hoboken, NJ, USA, 1985; pp. 99–110. [Google Scholar]
- Chisholm, S.W. Oceanography—Stirring times in the Southern Ocean. Nature 2000, 407, 685–687. [Google Scholar] [CrossRef]
- Passow, U.; Carlson, C. The biological pump in a high CO2 world. Mar. Ecol. Prog. Ser. 2012, 470, 249–271. [Google Scholar] [CrossRef]
- Jiao, N.; Cai, R.; Zheng, Q.; Tang, K.; Liu, J.; Jiao, F.; Wallace, D.; Chen, F.; Li, C.; Amann, R.; et al. Unveiling the enigma of refractory carbon in the ocean. Natl. Sci. Rev. 2018, 5, 459–463. [Google Scholar] [CrossRef]
- Trolle, D.; Staehr, P.A.; Davidson, T.A.; Bjerring, R.; Lauridsen, T.L.; Søndergaard, M.; Jeppesen, E. Seasonal dynamics of CO2 flux across the surface of shallow temperate lakes. Ecosystems 2011, 15, 336–347. [Google Scholar] [CrossRef]
- McDonough, L.K.; Santos, I.R.; Andersen, M.S.; O’carroll, D.M.; Rutlidge, H.; Meredith, K.; Oudone, P.; Bridgeman, J.; Gooddy, D.C.; Sorensen, J.P.R.; et al. Changes in global groundwater organic carbon driven by climate change and urbanization. Nat. Commun. 2020, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dungait, J.A.; Wei, X.; Ge, T.; Hou, R.; Ouyang, Z.; Zhang, F.; Tian, J. Long-term warming increased microbial carbon use efficiency and turnover rate under conservation tillage system. Soil Biol. Biochem. 2022, 172, 108770. [Google Scholar] [CrossRef]
- Chen, X.; Dong, L.; Zhao, W.; Jian, H.; Wang, J.; Wang, F. The effects of metabolism and temperature on carbon isotope composition of lipids in marine bacterium Shewanella piezotolerans WP3. Chem. Geol. 2022, 606, 120963. [Google Scholar] [CrossRef]
- Guo, Y.; Gu, S.; Wu, K.; Tanentzap, A.J.; Yu, J.; Liu, X.; Li, Q.; He, P.; Qiu, D.; Deng, Y.; et al. Temperature-mediated microbial carbon utilization in China’s lakes. Glob. Chang. Biol. 2023, 29, 5044–5061. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, T.C.; del Giorgio, P.A. Compositional changes in free-living bacterial communities along a salinity gradient in two temperate estuaries. Limnol. Oceanogr. 2002, 47, 453–470. [Google Scholar] [CrossRef]
- Cherrier, J.; Bauer, J.E.; Druffel, E.R.M.; Coffin, R.B.; Chanton, J.P. Radiocarbon in marine bacteria: Evidence for the ages of assimilated carbon. Limnol. Oceanogr. 1999, 44, 730–736. [Google Scholar] [CrossRef]
- Benner, R.; Opsahl, S. Molecular indicators of the sources and transformations of dissolved organic matter in the Mississippi river plume. Org. Geochem. 2001, 32, 597–611. [Google Scholar] [CrossRef]
- Dong, G.C.; Tian, X.L.; Dong, S.L.; Wang, D.P.; Chang, J. An experimental study on energy budget and conversion efficiency in different polyculture modes of shrimp, bivalve and seaweed. J. Ocean. Univ. China 2007, 37, 899–906. [Google Scholar]
- Filgueira, R.; Byron, C.; Comeau, L.; Costa-Pierce, B.; Cranford, P.; Ferreira, J.; Grant, J.; Guyondet, T.; Jansen, H.; Landry, T.; et al. An integrated ecosystem approach for assessing the potential role of cultivated bivalve shells as part of the carbon trading system. Mar. Ecol. Prog. Ser. 2015, 518, 281–287. [Google Scholar] [CrossRef]
| Treatments | Physico-Chemical Indicators in Pond Water during the Aquaculture Period | ||||||
|---|---|---|---|---|---|---|---|
| WT/°C | DO/mg·L−1 | S | Alk/mmol·L−1 | pH | SD/cm | Chl-a/μg·L−1 | |
| P | 26.1 ± 3.1 (22.3~31.2) | 7.53 ± 0.80 (6.52~8.66) | 27.09 ± 1.43 (24.53~28.91) | 1.74 ± 0.48 (1.01~2.53) | 8.55 ± 0.23 (8.26~8.96) | 28.6 ± 7.8 (21.1~42.9) | 110.59 ± 78.21 a (24.51~218.03) |
| PM | 26.1 ± 3.1 (22.2~31.0) | 7.60 ± 1.02 (6.03~8.98) | 25.71 ± 1.19 (24.51~27.79) | 1.92 ± 0.55 (1.03~2.67) | 8.54 ± 0.15 (8.36~8.77) | 31.4 ± 7.8 (20.9~43.6) | 74.20 ± 41.42 b (25.14~124.85) |
| Treatments | Nutrient Contents in Pond Water during the Aquaculture Period | |||||
|---|---|---|---|---|---|---|
| NO3–N/mg·L−1 | NO2–N/mg·L−1 | NH4–N/mg·L−1 | PO4–P/mg·L−1 | TN/mg·L−1 | TP/mg·L−1 | |
| P | 0.032 ± 0.040 (0~0.092) | 0.006 ± 0.003 (0.002~0.008) | 0.090 ± 0.089 (0.024~0.226) | 0.020 ± 0.019 (0.002~0.062) | 5.52 ± 1.89 (2.83~7.97) | 0.28 ± 0.11 (0.12~0.44) |
| PM | 0.040 ± 0.050 (0~0.117) | 0.006 ± 0.002 (0.002~0.009) | 0.068 ± 0.061 (0.015~0.199) | 0.025 ± 0.015 (0.006~0.054) | 5.07 ± 1.22 (2.86~6.62) | 0.42 ± 0.17 (0.23~0.69) |
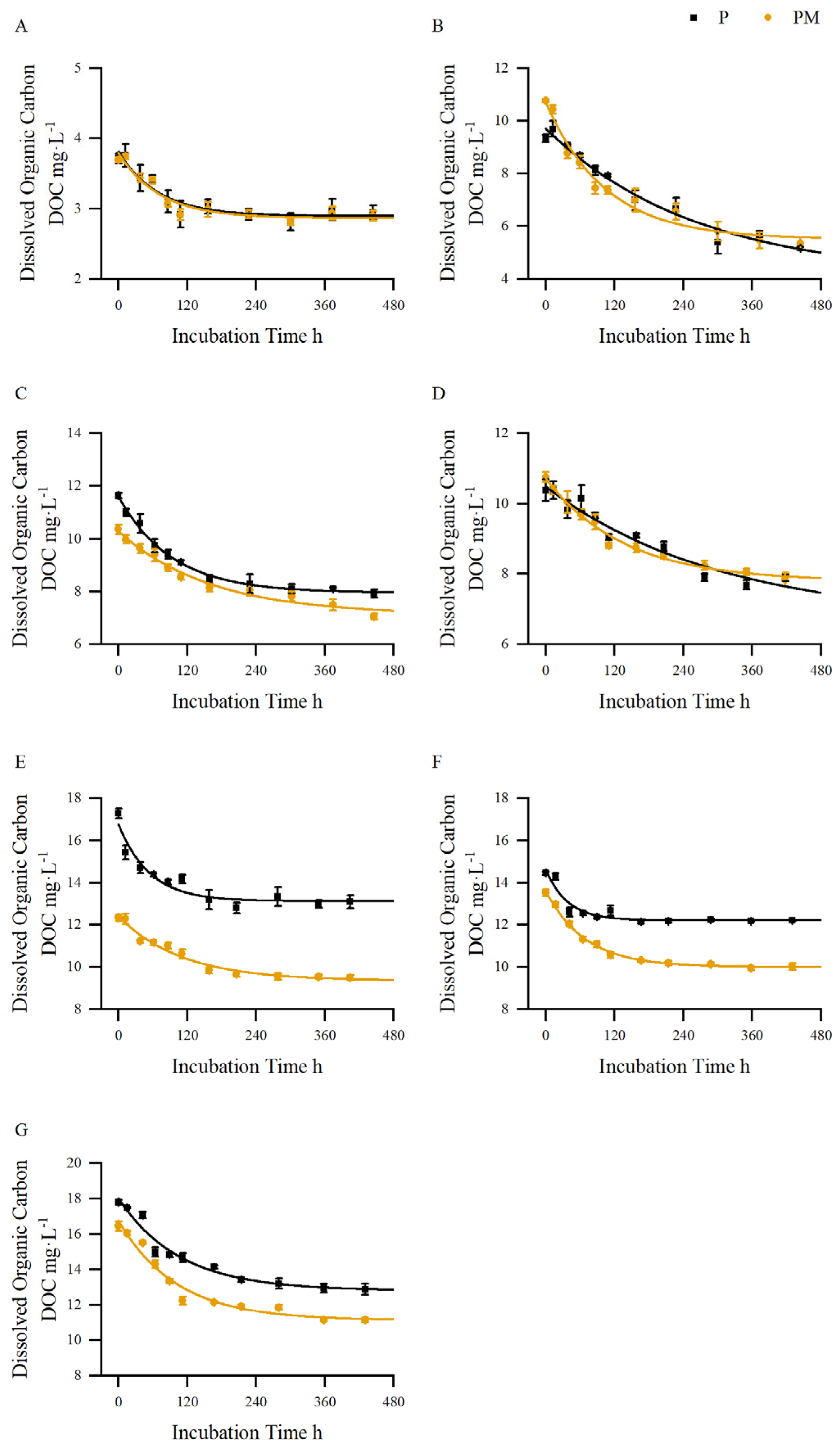
| Dates | Treatments | Functional Equation | R2 |
|---|---|---|---|
| 06.15 | P | y = 0.915 × exp (−x/68.545) + 2.895 | 0.903 |
| PM | y = 0.930 × exp (−x/70.947) + 2.864 | 0.917 | |
| 07.17 | P | y = 5.609 × exp (−x/262.458) + 4.094 | 0.970 |
| PM | y = 5.201 × exp (−x/104.356) + 5.505 | 0.973 | |
| 07.27 | P | y = 3.660 × exp (−x/92.638) + 7.950 | 0.993 |
| PM | y = 3.180 × exp (−x/161.279) + 7.106 | 0.975 | |
| 08.07 | P | y = 3.794 × exp (−x/302.667) + 6.691 | 0.935 |
| PM | y = 2.926 × exp (−x/136.747) + 7.791 | 0.983 | |
| 08.17 | P | y = 3.664 × exp (−x/54.385) + 13.110 | 0.908 |
| PM | y = 3.064 × exp (−x/109.447) + 9.357 | 0.967 | |
| 08.27 | P | y = 2.436 × exp (−x/37.783) + 12.205 | 0.885 |
| PM | y = 3.615 × exp (−x/68.840) + 10.003 | 0.994 | |
| 09.03 | P | y = 5.237 × exp (−x/106.457) + 12.801 | 0.956 |
| PM | y = 5.673 × exp (−x/101.434) + 11.109 | 0.958 |
| Treatments | Items | Date | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 06.15 | 07.17 | 07.27 | 08.07 | 08.17 | 08.27 | 09.03 | Mean ± SD | ||
| P | Time for completion of DOC degradation/h | 67.11 ± 0.91 | 388.07 ± 29.18 | 191.55 ± 6.38 | 278.36 ± 4.32 | 140.94 ± 14.09 | 97.22 ± 7.02 | 243.60 ± 11.17 | 200.98 ± 104.52 a |
| DOC degradation rates/mg·L−1·h−1 | 0.011 ± 0.000 | 0.011 ± 0.001 | 0.019 ± 0.001 | 0.010 ± 0.000 | 0.030 ± 0.003 | 0.023 ± 0.002 | 0.020 ± 0.001 a | 0.018 ± 0.007 | |
| PM | Time for completion of DOC degradation/h | 68.39 ± 2.94 | 240.40 ± 2.94 | 221.34 ± 0.91 | 199.08 ± 2.16 | 188.67 ± 2.89 | 162.08 ± 0.99 | 245.05 ± 1.04 | 189.29 ± 56.36 b |
| DOC degradation rates/mg·L−1·h−1 | 0.012 ± 0.001 | 0.022 ± 0.000 | 0.014 ± 0.000 | 0.015 ± 0.000 | 0.016 ± 0.000 | 0.022 ± 0.000 | 0.022 ± 0.000 b | 0.017 ± 0.004 | |
| Treatments | Components | Contents/mg·L−1 | Proportions to TOC/% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15 June | 17 July | 27 July | 7 August | 17 August | 27 August | 3 September | Mean ± SD | |||
| P | DOC | 3.71 ± 0.07 | 9.34 ± 0.14 | 11.63 ± 0.09 | 10.38 ± 0.31 | 17.27 ± 0.23 | 14.45 ± 0.08 | 17.81 ± 0.13 | 12.08 ± 4.57 | 61.51 ± 3.67 |
| POC | 2.39 ± 0.05 | 5.12 ± 0.08 | 6.07 ± 0.10 | 7.31 ± 0.14 | 9.74 ± 0.06 | 12.15 ± 0.09 | 10.66 ± 0.07 | 7.63 ± 3.17 | 38.49 ± 3.67 | |
| TOC | 6.11 ± 0.12 | 14.46 ± 0.22 | 17.71 ± 0.19 | 17.68 ± 0.43 | 27.01 ± 0.29 | 26.60 ± 0.18 | 28.47 ± 0.20 | 19.72 ± 7.55 | 100.00 | |
| PM | DOC | 3.70 ± 0.03 | 10.75 ± 0.07 | 10.35 ± 0.18 | 10.76 ± 0.15 | 12.34 ± 0.15 | 13.53 ± 0.16 | 16.46 ± 0.26 | 11.13 ± 3.62 | 61.71 ± 3.12 |
| POC | 2.39 ± 0.04 | 6.40 ± 0.05 | 5.29 ± 0.08 | 8.61 ± 0.20 | 8.08 ± 0.07 | 8.52 ± 0.12 | 8.84 ± 0.10 | 6.88 ± 2.20 | 38.29 ± 3.12 | |
| TOC | 6.09 ± 0.07 | 17.15 ± 0.11 | 15.64 ± 0.26 | 19.36 ± 0.34 | 20.42 ± 0.21 | 22.05 ± 0.28 | 25.31 ± 0.35 | 18.00 ± 0.35 | 100.00 | |
| Environment Factors | DOC Contents | POC Contents | RDOC Contents | RDOC Proportion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | PM | Mean | P | PM | Mean | P | PM | Mean | P | PM | Mean | |
| WT | −0.696 ** | −0.840 ** | −0.723 ** | −0.721 ** | NS | −0.523 ** | −0.681 ** | −0.685 ** | −0.644 ** | NS | NS | NS |
| DO | −0.690 ** | −0.886 ** | −0.731 ** | −0.753 ** | −0.506 * | −0.590 ** | −0.664 ** | −0.687 ** | −0.621 ** | NS | NS | NS |
| S | −0.863 ** | −0.575 * | −0.547 ** | −0.848 ** | −0.711 ** | −0.563 ** | −0.855 ** | −0.877 ** | −0.594 ** | −0.605 ** | −0.864 ** | −0.530 ** |
| Alkalinity | 0.751 ** | 0.755 ** | 0.650 ** | 0.717 ** | 0.511 * | 0.523 ** | 0.761 ** | 0.753 ** | 0.631 ** | 0.631 ** | NS | 0.437 ** |
| pH | NS | 0.855 ** | NS | NS | NS | 0.367 * | NS | 0.878 ** | 0.341 * | NS | NS | NS |
| SD | −0.604 ** | −0.859 ** | −0.700 ** | −0.798 ** | −0.823 ** | −0.761 ** | −0.757 ** | −0.977 ** | −0.815 ** | −0.886 ** | −0.596 ** | −0.733 ** |
| Chl-a | 0.685 ** | 0.821 ** | 0.727 ** | 0.903 ** | 0.804 ** | 0.884 ** | 0.821 ** | 0.949 ** | 0.858 ** | 0.848 ** | 0.593 ** | 0.744 ** |
| Dependent Variable | Multiple Linear Regression Equation | R2 | p |
|---|---|---|---|
| DOC contents/mg·L−1 | DOC = 23.828 + 0.023 × Chl-a − 0.496 × WT | 0.733 | <0.05 |
| POC contents/mg·L−1 | POC = 15.524 + 0.025 × Chl-a − 0.384 × S | 0.848 | <0.05 |
| RDOC contents/mg·L−1 | RDOC = 21.780 + 0.028 × Chl-a − 0.220 × WT − 0.369 × S | 0.861 | <0.05 |
| RDOC proportions/% | RDOC proportion = 106.425 + 0.088 × Chl-a − 1.376 × S | 0.616 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Jiang, T.; Shan, H.; Wang, F. Composition, Dynamic Changes, and Carbon Sequestration Effects of Organic Carbon in the Water of a Penaeus vannamei Culture Pond. Water 2024, 16, 721. https://doi.org/10.3390/w16050721
Huang C, Jiang T, Shan H, Wang F. Composition, Dynamic Changes, and Carbon Sequestration Effects of Organic Carbon in the Water of a Penaeus vannamei Culture Pond. Water. 2024; 16(5):721. https://doi.org/10.3390/w16050721
Chicago/Turabian StyleHuang, Chenxiao, Teng Jiang, Hongwei Shan, and Fang Wang. 2024. "Composition, Dynamic Changes, and Carbon Sequestration Effects of Organic Carbon in the Water of a Penaeus vannamei Culture Pond" Water 16, no. 5: 721. https://doi.org/10.3390/w16050721
APA StyleHuang, C., Jiang, T., Shan, H., & Wang, F. (2024). Composition, Dynamic Changes, and Carbon Sequestration Effects of Organic Carbon in the Water of a Penaeus vannamei Culture Pond. Water, 16(5), 721. https://doi.org/10.3390/w16050721










