Abstract
Trace elements in hot-spring waters are indicators for tracing hydrochemical processes, such as the deep circulation of geothermal water, the degree of water–rock interaction, redox reactions, the contamination of shallow water by thermal water, etc. In this study, 74 hot springs in the Xianshuihe–Xiaojiang Fault Zone (XSHF-XJF) were chosen for an investigation of the geochemical characteristics of trace elements using classic geochemical tools and multivariate statistical analysis. The results indicated (1) the hot-spring waters were mainly derived from atmospheric precipitation as indicated by δD and δ18O values that generally increased with decreasing elevations; (2) the high concentrations of B, As, Fe, and Mn in the waters, as well as the values of the Water Quality Index (WQI), indicated that the water quality was spatially heterogeneous and the hot-spring waters are not entirely suitable for drinking; and (3) B/Cl ratios showed that hot springs have different reservoir sources in the XSHF and XJF, respectively. The leaching of surrounding rock during water circulation contributed predominantly to the trace elements of the study’s waters. Diversity of lithology was the main factor affecting their concentrations. In addition, deep circulation controlled by the fault could influence trace-element enrichment. Our results offer a guide for the exploration and use of geothermal resources.
1. Introduction
Hot springs are the surface manifestation of deep hydrothermal fluids, which frequently present in regions of active volcanism and intense tectonic activity [1,2]. In the past decade, geothermal energy has been increasingly emphasized because of its advantages like renewable and large reserves, and it is widely exploited for power generation, tourism, physiotherapy, heating, etc. [3,4,5].
Due to the continuous northeastward thrusting of the Indian Plate and eastward subduction of the Myanmar Plate, the tectonic activity of the fault system at the eastern boundary of the Sichuan–Yunnan rhombic block is very active, and destructive earthquakes occur frequently [6,7]. Moreover, the fault system belongs to the Himalayan–Tibetan geothermal belt, with abundant geothermal springs [8,9].
In comparison with groundwater or surface water, trace elements, including typical elements (As, B, Li, etc.) and heavy elements (Fe, Mn, Cd, etc.), usually show enrichment in hot-spring waters, which play a crucial role in determining the genesis of hot springs and explaining the circulation process of thermal waters [10,11]. For example, the high concentrations of F, As, B, Li, and heavy metals, as are found in geothermal water with strong magmatic activity in Turkey [11], Chile [12], Tibet [13], Tengchong [14], and Taiwan [10], are usually constrained by multiple factors such as the type of host rock in the water–rock reaction, the input of magmatic fluids(e.g., B, Li, etc.), and secondary processes (e.g., precipitation of solid phases). Moreover, the mixing of geothermal waters, which contain high concentrations of trace elements, with surface waters can result in problems linking to drinking-water quality and environmental contamination [15,16,17].
The XSHF-XJF, as an important active fault zone at the eastern edge of the Sichuan–Yunnan rhombic block, is surrounded by a large amount of geothermal resources [18]. In this region, a series of investigations of the hydrochemical features of hot springs [19,20,21], gas isotope characteristics [22], the genesis of geothermal water [23], and the relationship between fluids and seismic activity [24,25] were conducted using the approaches of hydrogeochemistry and isotopes and the methods of geophysical exploration. Furthermore, high concentrations of B, Li, and F in the Kangding area were reported [26]. However, the geochemical characteristics of trace elements in hot springs throughout the fault zone and their environmental effects are lacking so far. Therefore, this paper aims at analyzing the source and enrichment mechanism of trace elements in hot springs and evaluating the water quality of thermal waters in terms of trace elements. Our results could contribute to understanding the geochemical characteristics of trace elements in hot springs throughout the fault zone, providing insights for studies of trace elements in geothermal systems occurring in strike-slip fault zones across the world, and providing a new reference for the exploitation of hot-spring waters in the XSHF-XJF.
2. Geological Setting
The study area is on the eastern margin of the Tibetan Plateau (Figure 1a). It is an important left-lateral strike-slip fault and seismic zone on the Chinese mainland, including the Xianshuihe Fault (XSHF), Anninghe Fault (ANHF), Zemuhe Fault (ZMHF) and Xiaojiang Fault (XJF) (Figure 1b). The widely developed faults create channels for the intrusion of rock bodies and a favorable geological environment for the storage and transportation of geothermal resources. The XSHF is a NW–SE-trending left-lateral strike-slip fault, which is located between Ganzi and Shimian in the Sichuan province. The regional geological setting includes Triassic sedimentary strata composed of sandstone, siltstone, and mudstone, Precambrian rocks composed of metamorphic rocks, and Mesozoic intrusive rocks dominated by granite and diorite [27]. The ANHF is NS trending and extends for about 200 km along the Anninghe valley, intersecting with the XSHF. The ZMHF is NNW trending, dipping northeast. The bedrocks in the AZF (ANHF-ZMHF) are mainly composed of metamorphic rocks formed by Cambrian granite and rhyolite; the Cenozoic strata (Late Tertiary to Early Quaternary) are mainly composed of interbedded mudstones and sandstones, with thin layers of coal [28]. The XJF is an SN-trending, left-slip active fault. The rocks of fault walls are mostly carbonate rocks and sedimentary rocks [29].
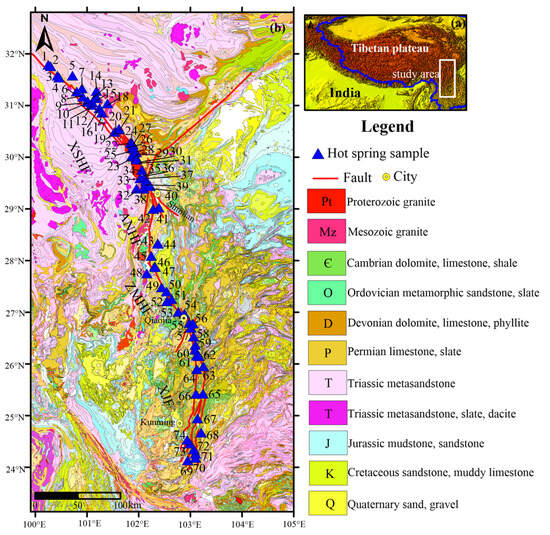
Figure 1.
(a) Localization of the area of this study; (b) geological map with the sampling sites.
In the study area, most hot springs occur along the XSHF-XJF, and the geothermal resources are most developed in the XSHF. The hot springs geographically look like the scatted beads in the Tertiary strata and Yanshanian–Himalayan granites, and they are mostly medium–high-temperature (30–90 °C) hot springs. The hot springs in the ANHF-XJF are unevenly distributed, mainly developed in carbonate and sedimentary rock strata, with temperatures generally below 60 °C, belonging to the medium–low-temperature hot springs. Furthermore, due to the predominantly plateau-type monsoon climate, precipitation is annually concentrated from May to September. The surface water system is well developed due to atmospheric precipitation and ice meltwater. The groundwater in solidified host rocks can be classified into bedrock fissure water in granite, clastic fissure water with sandstone as the dominant water-bearing lithology, and carbonate fissure water [30].
3. Sampling and Methods
3.1. Sampling and Analysis Methods
In total, 74 water samples were obtained from hot springs in the XSHF-XJF during 2018–2019. After filtrating with a 0.45 µm membrane, the samples were collected in polyethylene terephthalate (PET) bottles (50 mL) for the laboratory analysis. Portable equipment was used to measure the pH and temperature of water in the field. The major elements were tested using a Dionex ICS-900 (Dionex, Sunnyvale, CA, USA) ion chromatograph fitted with an AS40 automatic sampler in the Key Laboratory of Earthquake Prediction, China Earthquake Administration, with a detection limit of 0.01 mg/L [31]. The concentrations of HCO3− and CO32− were titrated with a ZDJ-100 potentiometric titrator (Kehuan, Nanjing, China) using the 0.05 mol/L HCl titration 0.1% methyl orange and 1% phenolphthalein procedures (reproducibility within ±2%). The following equation was used to compute the ion balance error in water samples (Equation (1)):
The trace-element analysis was conducted with an element type inductively coupled plasma mass spectrometer (ICP-MS) at the Test Center of the Institute of Nuclear Industry Geology [32]. The concentration of As was determined with an LC-6500 atomic fluorescence photometer (Haiguang, Beijing, China), with a detection limit of 0.60 μg/L. The SiO2 concentration of the samples was analyzed using an inductively coupled plasma emission spectrometer Optima-5300 DV (PerkinElmer, Hopkinton, MA, USA). The δ18O and δD were analyzed with a Finnigan MAT253 (Thermo Electron-Finnigan, San Jose, CA, USA) mass spectrometer using the TC/EA (Temperature Conversion/Elemental Analyzer) method. They are expressed as permille, standardized using V-SMOW, with analytical uncertainty of ±0.2‰ and ±1‰, respectively [33].
3.2. Water Quality Index
The Water Quality Index (WQI), which is a mathematical tool used to assess the combined impact of specific environments, was applied to assess the cumulative impacts of trace elements in hot springs in this study. It can be calculated as follows (Equations (2) and (3)) [34]:
where Ci is the concentration of trace elements analyzed in the water sample, Qi is the limit value of the water quality standard of the elements, and PI stands for the exceedance index of a trace element i, relative to the standard limit value. Considering the geological background of the study area, the trace-element concentrations were assessed using the China Standards for Drinking Water Quality [35]. The values of the reference standard were listed in Table S3. The WQI is divided into four categories: WQI ≤ 1 (within the standard limit); 1 < WQI ≤ 2 (slightly exceeding the standard limit); 2 < WQI ≤ 3 (moderately exceeding the standard limit); and WQI > 3 (heavily exceeding the standard limit) [36].
3.3. Statistical Analysis
Using the different water parameters, a descriptive statistical analysis, correlation analysis, and factor analysis (FA) were performed using SPSS 22.0 software. For FA, the data were verified for suitability using Bartlett tests and KMO (Kaiser-Meyer-Olkin), which can take values between 0 and 1. A value close to 1 indicates a good fit for FA. The value of the KMO test in this study is 0.540, and the significance level of the Bartlett test is less than 0.01, suggesting that the data have a certain correlation and are suitable for FA.
4. Results
4.1. Hydrochemical Compositions
The results of the physico-chemical parameters of the water samples are given in Table S1. The outlet temperatures of the water samples range from 11.3 °C to 77.0 °C, with the majority being hot springs, except for Nos. 1, 4, 5, 8, 9, 10, and 11 of the water samples (T < 25 °C). The pH values range from 6.49 to 9.50. TDS values of the water samples range from 113.45 to 2247.98 mg/L, 134.69 to 6331.29 mg/L, and 115.27 to 1486.79 mg/L in the XSHF, AZF, and XJF, respectively. HCO3− is the most prominent anion in the XSHF and AZF, and the main cation is Na+. The thermal waters in the XSHF are HCO3-Na, while those in AZF are HCO3-Ca (Mg) and HCO3(SO4)-Na (Ca) [20,21]. In the XJF, the main anions are SO42− and HCO3−, and Ca2+ and Mg2+ are the dominant cations in the thermal waters, resulting in water types of SO4-Ca (Mg) and HCO3-Ca (Mg) [19].
The statistical results of trace elements of the water samples are shown in Table S2 and Figure 2 with data of rivers on the surface near the study area [34,37] for comparison. The concentrations of Co, Cu, Ni, Pb, and Tl in the water samples are generally low. In contrast, the concentrations of B, Fe, Li, and Mn in the study area range from 5.6 to 6366 μg/L; 0.66 to 10,520 μg/L; 1.29 to 4547 μg/L; and 0.07 to 1754 μg/L, respectively, which are generally higher than those in surface water (Figure 2). Arsenic concentrations range from <0.60 to 530 μg/L. Compared with the typical magma-impacted geothermal system (e.g., Yangbajing, Rehai, etc.) [38,39], the concentrations of B, Li, and As in the water samples are relatively low, indicating that the input of magmatic fluids into the hot-spring waters is limited.
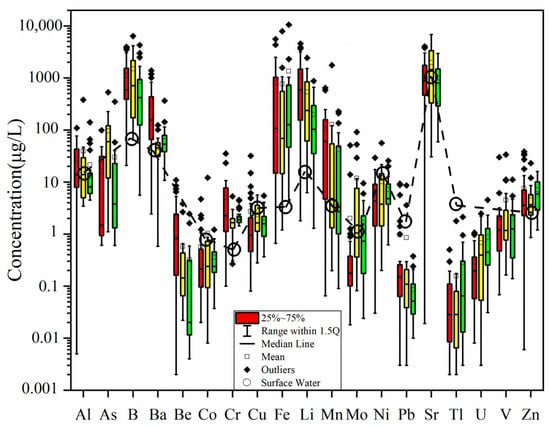
Figure 2.
Trace-element concentrations in the water samples in the XSHF-XJF. The red color stands for the samples from the XSHF, yellow color for the samples from the AZF, and green color for the samples from the XJF. Surface water data are taken from the mean values of the content of the element.
The δD and δ18O values of the water samples are listed in Table S1. δD and δ18O values in the XSHF range from −115‰ to −82‰ and −19.20‰ to −11.40‰; δD and δ18O values in the AZF from −118‰ to −84‰ and −15.60‰ to −11.50‰; and δ D and δ18O values in the XJF from −117% to −81‰ and −15.40 to −11.10‰, respectively.
4.2. Water Quality Assessment
The results of the water quality evaluation were shown in Table S3 and Figure 3. Apparently, high concentrations of As, Mn, B, and Fe are found in the hot-spring waters in the XSHF-XJF, greatly exceeding the standard values and hinting they are relatively enriched in the waters. Additionally, high concentrations of Be, Ba, and Ni are also found in some water samples (e.g., Nos. 21, 22, 42, etc.). The WQI values of water samples in the XSHF-XJF range from 0.0 to 5.4 (Table S3). Based on the WQI, the trace elements in the water samples of Nos. 1, 4, 8, 19, 20, 45, and 54 slightly exceed the standard limit (1 < WQI ≤ 2). While the trace elements in the water sample 67 and 46 moderately exceed the standard limit (2 < WQI ≤ 3), and in the water samples of Nos. 42, 51, and 65 they heavily exceed the standard limit (WQI > 3). In general, the water quality is spatially heterogeneous in the study area, and hot-spring waters are not entirely suitable for drinking.
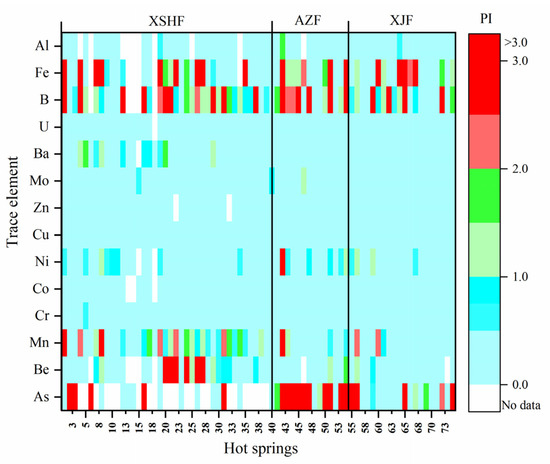
Figure 3.
Heat map of the PI of trace elements in the hot-spring waters in the XSHF-XJF.
5. Discussion
5.1. Origin and Isotopic Characteristics of Hot-Spring Waters
The isotopic compositions of hydrogen and oxygen in the hot-spring waters are recognized as efficient proxies for tracing the origin and geochemical evolution of water [40]. The data of δD and δ18O for hot springs and surface waters in the study area are plotted in Figure 4, along with the Global Meteoric Water Line (GMWL, δD = 8δ18O + 10) [41] and Local Meteoric Water Line in the Tibetan Plateau (LMWL, δD = 8.41δ18O + 16.72) [42]. Both hot-spring water samples and surface water samples are scattered around the GMWL and LMWL, indicating that the source of their recharge is atmospheric precipitation. However, the values of δ18O and δD are generally lower in thermal waters compared to surface waters at the same or nearby locations, indicating that the surface water and surrounding atmospheric precipitation are not the direct source of recharge to hot springs, which are recharged by the infiltration of distant atmospheric precipitation transported through faults and secondary faults.
Additionally, an interesting phenomenon is that the δD and δ18O values of water samples in the XSHF are more negative compared to those in the AZF and XJF. The East Asian monsoon is a key source of water vapor, and as it passes through the high terrain, the heavy isotopes of water (18O and D) are prioritized for removal based on a Rayleigh fractionation mode [43]. There is a gradual decrease in the topographic elevation from northwest to southeast in the study area, resulting in a more depleted isotope composition in the thermal waters than in the XSHF. This explanation is supported by the fact that the recharge elevation in the XSHF (mean: 3.3 km) is higher than that in the AZF (mean: 2.5 km) and XJF (mean: 2.4 km) [20,21]. Furthermore, most of the hot-spring samples shift slightly to the right of LMWL, namely positive δ18O drift, which can be attributed to oxygen isotope exchange between thermal waters and surrounding carbonate or silicate rocks, which are enriched with δ18O [44]. This process can be influenced by higher thermal storage temperatures, a longer circulation time, or higher rock-to-water ratios [45]. However, it is not clear what factors control δ18O drift. A few of the water samples show a δ18O left shift that may be caused by 18O exchange during the dissolution of CO2 from a deep source [22].
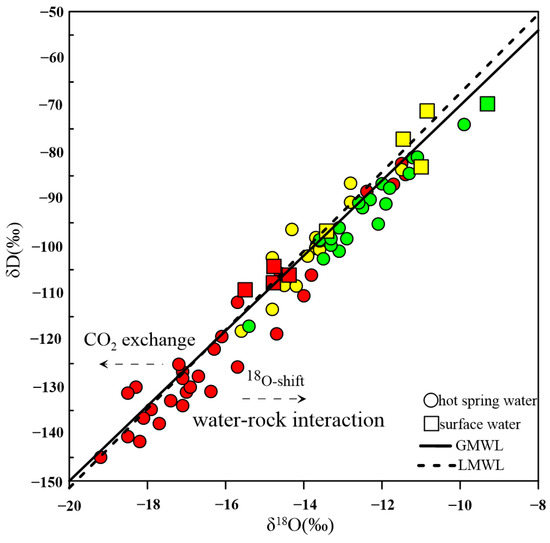
Figure 4.
Plot of δD and δ18O of the hot springs and surface waters in the XSHF-XJF. The red color stands for the samples from the XSHF, yellow color for the samples from the AZF, and green color for the samples from the XJF. Dotted line stands for the LMWL and solid line for the GMWL. The data of surface waters from [46].
5.2. Hydrogeochemical Characteristics of Hot Springs
5.2.1. Water–Rock Reaction and Major Ion Variations
The ion compositions of groundwater depend mainly on the dissolution of mineral components during water–rock interactions [47]. The molar ratio of Na++K+/ Cl− generated using the halite dissolution is generally equal to 1. However, most of the water samples fall below y = x (Figure 5a), indicating that excess Na+ and K+ mainly originate from the weathering and dissolution of silicate minerals (Equations (4) and (5)). Some of the samples (Nos. 42, 49, 52, and 54) from AZF fall on the line of halite dissolution, indicating that they may have been contributed to by the dissolution of halite.
4NaAlSi3O8 (Albite) + 4CO2 + 6H2O ⟶ 4Na+ + 4HCO3− + 8SiO2 + Al2Si2O5(OH)4
4KAlSi3O8 (K-feldspar) + 4CO2 + 6H2O ⟶ 4K+ + 4HCO3− + 8SiO2 + Al2Si2O5(OH)4
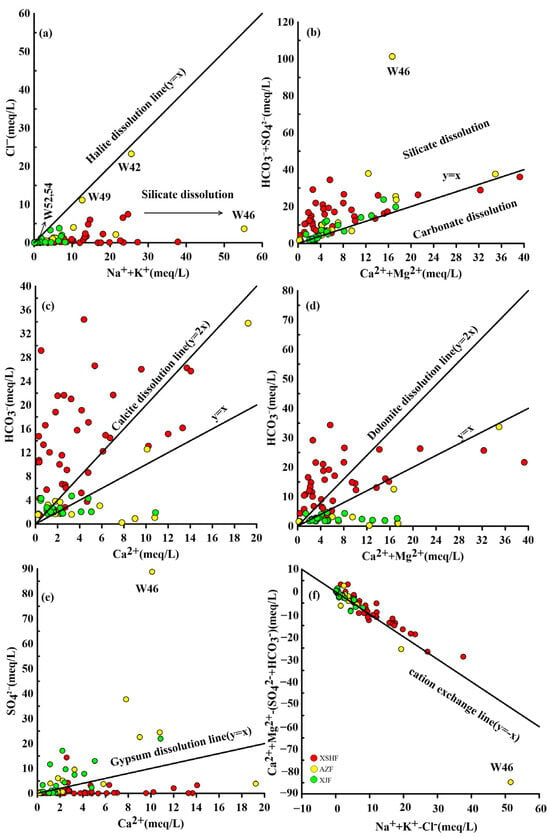
Figure 5.
The plots of major ions for the water samples, (a) (Na+ + K+) vs. Cl−; (b) (Ca2+ + Mg2+) vs. (SO42− + HCO3−); (c) Ca2+ vs. HCO3−; (d) (Ca2+ + Mg2+) vs. HCO3−; (e) Ca2+ vs. SO42−; (f) (Na+ + K+ − Cl−) vs. ((Ca2+ + Mg2+) − (SO42− + HCO3−)).
In general, in addition to halite dissolution, seawater and deep fluids can also be sources of Cl− [48,49]. Most of the Cl− concentrations of the water samples are higher (>3 mg/L), especially in the high temperature hot springs (Table S1), so the Cl− contribution of seawater is neglected because the mean value of Cl− contribution is 0.92 mg/L in continental meteoric water and TDS is generally less than 20 mg/L [50]. And, the concentrations of Cl− in groundwater from meteoric water dissolving igneous rocks range from 2 to 4 mg/L [48]. Thus, the high concentrations of Cl− in water samples can be attributed to the deep fluids mixing and the dissolution of rocks (e.g., granite) in the water–rock reaction.
Ca2+, Mg2+, and HCO3− are commonly obtained through the dissolution of carbonate minerals (Equations (6) and (7)), resulting in Ca2+/HCO3− and (Ca2++Mg2+)/HCO3− molar ratios of 1:2 [51].
CaCO3 (calcite) + CO2+H2O ⟶ Ca2+ + 2HCO3−
CaMg (CO3)2 (dolomite) + CO2+H2O ⟶ Ca2+ + Mg2+ + 4HCO3−
In this study, most of the water samples in the XSHF are almost close to the y-axis side (Figure 5c,d). Previous studies [22] have found that many gases spilled from the hot springs in the XSHF-AZF, which are mainly mantle-derived CO2. Thus, excess HCO3− may be due to the dissolution of sodium silicate minerals promoted by CO2 (Equations (4) and (5)). Some of the water samples in the AZF and XJF are located below the y = x, suggesting that excess Ca2+ and Mg2+ are likely to derive from the dissolution of Ca-Mg materials (Equation (8)), rather than carbonate dissolution alone. The Figure 5b displays that the majority of water samples are scattered above y = x, indicating that dissolution of silicate minerals is the main hydrogeochemical process controlling the ion concentration of the hot-spring waters.
CaAlSi2O8 (Anorthite) + 8H+ ⟶ 2Al3+ + Ca2+ + 2H4SiO4
Furthermore, the dissolution of gypsum in groundwater could also contribute to Ca2+ (Equation (9)). Figure 5e illustrates that gypsum may be the source of Ca2+ in the hot-spring waters. However, some of samples in the AZF and XJF are scattered above the gypsum dissolution line, indicating that the excess SO42− may originate from the hydrolysis of sulfides in the strata.
CaSO4 + 2H2O ⟶ Ca2+ + SO42− + 2H2O
In the Na+ + K+ − Cl− vs. (Ca2+ + Mg2+) − (SO42− + HCO3−) diagram (Figure 5f), most of the samples are scattered along the 1:1 line, hinting that the cation-exchange process between deep fluids and secondary silicate minerals (e.g., kaolinite, illite, montmorillonite, etc.) may affect the chemical composition of the water. But, water sample 46 is not significantly affected by cation exchange (Figure 5f). It is the Na-SO4 type that is exposed in granite. The high concentrations of Na+ (Figure 5a) come from the hydrolysis of silicates at high temperatures, and high concentrations of SO42− (Figure 5b,e) come from the oxidation of sulfides (e.g., pyrite) in granite.
5.2.2. Mineral Saturation Index
The mineral saturation index (SI) could be used to qualitatively recognize the minerals that tend to precipitate or dissolve during water–rock interactions [52]. A positive or negative values for the SI can indicate whether the water is saturated or unsaturated with a particular mineral. The PHREEQC software 2.12.5 was utilized to determine the mineral saturation indices of potential minerals in the circulation path of thermal waters, considering the temperature and pH. The results were presented in Table S4.
Almost all the water samples are undersaturated with respect to albite, anorthite, Ca-montmorillo, K-feldspar, halite, carbonate minerals (rhodochrosite, siderite, witherite), and sulfate minerals (anhydrite, barite, celestite, gypsum), indicating that the dissolution of those minerals could not be the main processes for the hydrochemical formation for the hot-spring waters. In contrast, the thermal waters exhibit an oversaturation of aragonite, calcite, and dolomite, indicating the contribution of deep fluids enriched in CO2 that degassed with the decrease of hydrostatic pressure during upwelling. Oxides and hydroxides, such as manganite and pyrolusite, show either oversaturation or undersaturation in the water samples, which may reflect variations of the surrounding rock and Fe and Mn origins.
5.3. Geochemistry of Trace Element
As aforementioned, B, As, Fe, and Mn are relatively enriched in hot-spring waters in the XSHF-XJF, and Li and Sr are also typical trace components in geothermal systems. In the following section, the source characterizations of trace elements (B, Li, As, Sr, Fe, Mn) in the hot-spring waters are discussed using multivariate statistical methods. According to the results of the factor analysis (Figure 6a), the first four eigenvalues together accounted for 73.4% of the total variability of the hydrochemical processes. F1 explains 37.1% of the total variation with strong positive loading on Li, K+, Na+, B, T, Cl−, and HCO3−. It shows a general trend in hydrochemical characterization, probably reflecting the mixture of the surface water (Figure 2) and deep fluids (e.g., magmatic and tectonic fluids), as well as the dissolution of silicate. F2, explaining 15.4% of the variance, is related to Mn, Ca2+, HCO3−, Cl−, and Fe. It characterizes the effect of Fe, Mn, and carbonite dissolution. F3, explaining 13.6% of the variance, is related to SO42−, As, and Sr and characterizes the influence of sulfate. F4, explaining only 7.3% of the variance, is positively correlated with Mg2+, NO3− and negatively correlated with pH and mainly characterizes the anthropogenic effect. Interestingly, most of the samples from the XSHF have high scores for F1 and F2, while most of the samples in the AZF and XJF (except for sample No.42) have high scores for F3 and F4, as indicated by the factor scores result (Figure 6b). Therefore, it is inferred that trace elements in the hot springs in the XSHF-XJF have different sources and controlling factors.
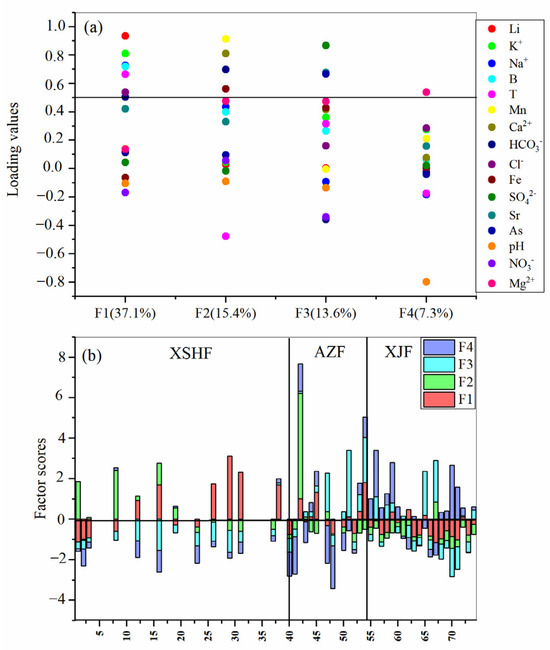
Figure 6.
(a) Results of factor analysis of hydrochemical parameters; (b) distribution of factor scores of water samples in the XSHF-XJF.
5.3.1. Sources and Controlling Factors of Trace Elements in the XSHF
In the XSHF, the concentrations of trace elements (B, Li, Fe, and Mn) in the hot-spring waters are generally higher (Figure 2). Granites of different periods are the main thermal reservoirs in this area, and F1 also shows that silicate mineral dissolution influences the chemical compositions of hot springs. The concentrations of B in water samples range from 20.7 to 3943 μg/L, with a mean value of 1045.33 μg/L. Boron in thermal waters is usually controlled by leaching from the host rocks and the input of deep fluids owing to its soluble and incompatible nature [53]. It correlates positively with Na+ (r = 0.63), K+ (r = 0.76), and Cl− (r = 0.64) (Figure 7), indicating that their sources are similar. Previous studies indicate that felsic igneous rocks (e.g., granites and rhyolites) in the Tibetan Plateau usually have greater B contents than sedimentary and metamorphic rocks [54]. High concentrations of B (>1 mg/L) are found in the Kangding segment developed by Cenozoic granite and in water samples of Nos. 1, 4, 12, 16, 19, and 20 exposed to Cretaceous and Tertiary granites in the northern part of the fault. Therefore, the dissolution of granites should be an important source of B for the hot springs. Also, B and Cl are conservative components of thermal waters, and their ratios are often used to determine a common reservoir [55]. In Figure 8, the values of the B/Cl ratio in the water samples in the southern parts of the XSHF (Kangding-moxi segment) are lower than those in the north. Based on the geological and magnetotelluric evidence, there are conductors (resistivity < 10 Ωm) at a depth of 10 km below the Kangding geothermal field, which are interpreted as an underlying zone of partial melt that provides continuous heat and fluids for the geothermal field [23], and there are higher shear stresses in this area, which are more favorable to the extension of faults into deep water rather than the tensile stress, increasing the permeability of the fault [56]. The study on hydrogeochemistry also shows that the presence of magma beneath Kangding, with parent geothermal fluids (Cl concentrations of 1056 mg/L) mixing with shallow groundwater, results in higher Cl concentrations in the hot-spring waters [57]. So, B/Cl ratios could reflect that hot springs have two different reservoir sources. Additionally, B in the hot-spring waters correlates positively with HCO3− (r = 0.36) (Figure 7), which may reveal the influence of deep water–CO2–rock reactions on concentrations of B. Since most water samples have pH values below 8 (Table S1), it is likely that B is present as H3BO3 [58]. The experiments on the leaching of borate minerals demonstrate that when CO2 concentration rises, the solubility of B in waters increases, producing H3BO3 [59]. So, the strong release of deep CO2 gas may promote the enrichment of B in the hot springs.
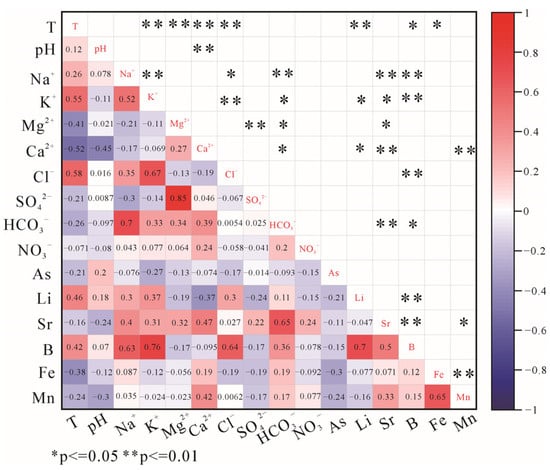
Figure 7.
Pearson correlation diagram for trace elements and major ions in the XSHF.
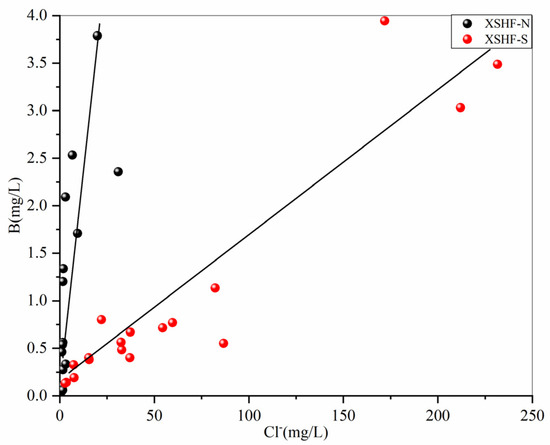
Figure 8.
Plot of B vs. Cl in the hot-spring waters in the XSHF.
The concentrations of Li range from 1.79 to 4547 μg/L, with the mean value of 1009.65 μg/L. Lithium belongs to alkali metals that tend to be more enriched in acidic rocks [60]. It correlates positively with K+ (r = 0.37) and B (r = 0.70) (Figure 7), indicating that they have similar sources. The Li concentrations of granite in the XSHF range from 10.70 to 48.00 ppm [54], which can provide abundant Li for the hot-spring waters. So, the hydrolysis of silicate minerals (e.g., lepidolite) may be the main source of Li.
The concentrations of Fe and Mn in the hot springs show large variability, ranging from 0.662 to 5569 μg/L, and 0.065 to 6424 μg/L, respectively. The No.4 water sample contains the highest Fe concentration (5569 μg/L), and No.8 has the highest Mn concentration (642 μg/L). A high concentration of Fe in water samples may related to groundwater flowing through iron deposits in the Triassic strata in the area [61,62]. The water samples, being supersaturated for goethite and hematite, also support the high content of Fe in hot springs. Manganese correlates positively with Fe (r = 0.65) (Figure 7), indicating that they may be concomitant. The concentrations of Sr range from 0.019 to 4016 μg/L. Strontium correlates positively with Ca2+ (r = 0.47), Mg2+ (r = 0.32), HCO3− (r = 0.65), and Na+ (r = 0.40) (Figure 7), suggesting that the dissolution of Sr-bearing minerals in granite and carbonate rocks may provide a large amount of Sr for the hot-spring waters.
5.3.2. Sources and Controlling Factors of Trace Elements in the AZF and XJF
The concentrations of As and Sr in hot springs in the ANHF-XJF are higher compared to the XSHF (Figure 2). Strontium is positively correlated with Ca2+, Mg2+, Na+, and SO42− in the AZF and XJF (Figure 9 and Figure 10). Strontium can form sulfate and carbonate minerals, and can also be integrated into carbonates, sulphates, and aluminum silicates to replace calcium, so it is more abundant in rocks with high calcium content [63]. The ZMHF is dominated by Cambrian and Ordovician dolomite and limestone, whereas the XJF is surrounded by carbonate rocks, limestone, and sandstone. Therefore, the concentrations of Sr in hot springs in the ANHF-XJF are much higher than those in the XSHF, which is caused by the former flowing through a large distribution of carbonate rocks.
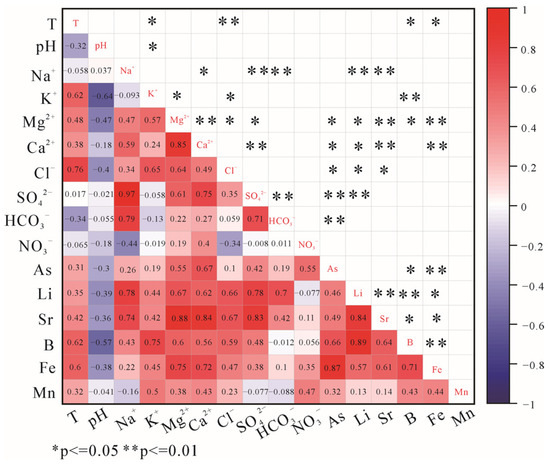
Figure 9.
Pearson correlation diagram for trace elements and major ions in the AZF.
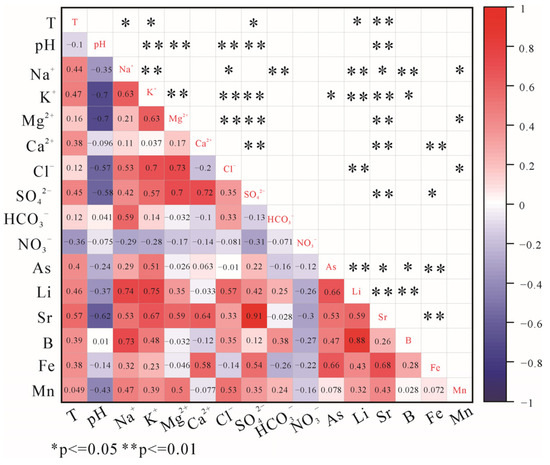
Figure 10.
Pearson correlation diagram for trace elements and major ions in the XJF.
Arsenic concentrations in water samples in the AZF and XJF range from 1.11 to 530 μg/L, and 0.6 to 349 μg/L, respectively. Arsenic is significantly and positively correlated with Mg2+ (r = 0.55), Ca2+ (r = 0.67) in the AZF (Figure 9), and with K+ (r = 0.50), Sr (r = 0.53) in the XJF (Figure 10), suggesting that it also may originate from water–rock interactions. High concentrations of As are related to the dissolution of pyrite and arsenopyrite in shale, siltstone, and interbedded Tertiary lignite. However, it is not significant with SO42− in the AZF and not correlated with SO42− in the XJF (Figure 9 and Figure 10), which indicates that sulfide minerals are not the major mediators of As migration in the hot-spring waters. The hydrated iron oxide in the aquifer has a high As adsorption capability owing to its large specific surface area [11]. Goethite and hematite have a tendency to precipitate (SI > 0), implying that large amounts of As can be adsorbed. Arsenic is significantly and positively correlated with Fe (Figure 9 and Figure 10), further illustrating that it may be sorbed to the surfaces of iron-bearing minerals. Therefore, As is most likely released by the reductive breakdown of iron oxides under high-temperature and alkaline environments.
The concentrations of Fe in hot springs range from 8.25 to 7867 μg/L, and 4.23 to 10,520 μg/L, respectively. It is positively correlated with Mg2+ (r = 0.75) and Ca2+ (r = 0.72) in the AZF (Figure 9) and correlated with Ca2+ (r = 0.58) and SO42− (r = 0.54) in the XJF (Figure 10), which is inferred to be derived from the dissolution of pyrite from the lignite (Equation (10))
2FeS2 + 7O2 + 2H2O ⟶ 2Fe2+ + 4SO42− + 4H+
Furthermore, because carbonates are present in the strata, the free sulfuric acid produced by the oxidation of pyrite will react with carbonates (Equation (11)), which may also be a reason for the high concentration of Fe, SO42−, Ca2+, and Mg2+ in hot springs in the XJF.
H2SO4 + (Ca, Mg) CO3 ⟶ (Ca, Mg)2+ + SO42− + CO2 + H2O
The concentration ranges of B and Li in the AZF are 11.1–6366 μg/L, and 6.49–2425 μg/L, respectively. The ionic components of hot springs were influenced by a combination of silicates, carbonates, and evaporites in the AZF. Boron correlates positively with K+ (r = 0.75), Mg2+ (r = 0.6), Cl− (r = 0.59), and Li correlates positively with Na+ (r = 0.74), Ca2+ (r = 0.62), HCO3− (r = 0.7), SO42− (r = 0.78), Cl− (r = 0.66) (Figure 9). So, B and Li may be derived from water–rock interactions between thermal water and granite, dolomite, and other sedimentary rocks. In the XJF, B and Li concentrations range from 5.6 to 4262 μg/L, and 1.29 to 872 μg/L, respectively, and the dissolution of carbonate minerals has the greatest impact on the hot springs. However, B and Li are weakly correlated with Ca2+, Mg2+, and HCO3−, but are significantly positively correlated with Na+, K+, and Cl− in the water samples (Figure 10), suggesting that they are likely derived from the leaching of reservoir rocks in the deep. Additionally, water samples from No. 55 to No. 60 in the northern segment of the XJF have low B/Cl ratios (mean: 0.016) with high concentrations of Cl, while B/Cl ratios (mean: 0.1) are higher in the southern one. According to geophysical research, anomalies of low velocity and high Poisson’s ratio are present at the northern segment (26.5° N) of the XJF and are interpreted as fluid intrusions (partial melt of deep material) [64]. The XJF served as a channel of groundwater in the crust, which could be influenced by hot materials in this region. Geological and geodetic studies show that the slip rate of the southern fault in the XJF is low and may not be sufficient to create and maintain permeable fluid pathways [65] and that the high-resistivity cap on top of the fault zone conductors may prevent further flow of deep fluids into the shallow crust [66]. Moreover, the circulation depth in the northern region of the XJF is not higher than that in the southern [19]. Thus, water samples in the northern region with high concentrations of Cl (>100 mg/L) may be strongly influenced by deep fluids. B/Cl ratios could also reflect that hot springs are affected by different reservoirs. Additionally, F4 implies that human activities may also have an influence on the components of hot springs in the AZF-XJF.
5.3.3. Influence of Fault-Controlled Circulation Depth on Trace Elements
The concentrations of trace elements in geothermal waters are usually influenced by the circulation depth controlled by faults, resulting in a vertical distribution [67]. Figure 11 illustrates the relationship between circulation depth and trace-element concentration. The concentrations of B and Li increase with the increasing circulation depth of hot-spring waters. Strontium concentrations show a positive trend with circulation depth in the XJF, while it has a negative trend with circulation depth in the XSHF (Figure 11b). The geothermal reservoirs in the XSHF present dual structure characteristics [26]. Deep geothermal water, enriched with elements such as Na, K, and Li, is primarily associated with granite reservoirs. Whereas, shallow geothermal water is associated with limestone reservoirs, with high concentrations of Ca and Mg [68]. Therefore, with a shallower circulation depth, the chemical composition is significantly influenced by carbonate rocks, and Sr concentrations are higher owing to the stronger affinity between Sr and Ca. As for the concentration of Fe, Mn, and As, they are not significantly controlled by the circulation depth, except in the XJF, which may be affected by other factors (e.g., dissolution and precipitation of minerals, adsorption and desorption, etc.) [69]. The circulation depth of hot springs in the AZF is generally low, and the concentrations of trace elements are highly variable because of the complex geological conditions in this area. As mentioned above, the circulation depth of hot springs can affect the trace-element concentration, especially B and Li. Deep circulation of thermal waters would promote the upwelling of deep geothermal fluids and enhance water–rock interactions, which in turn affects their concentrations.
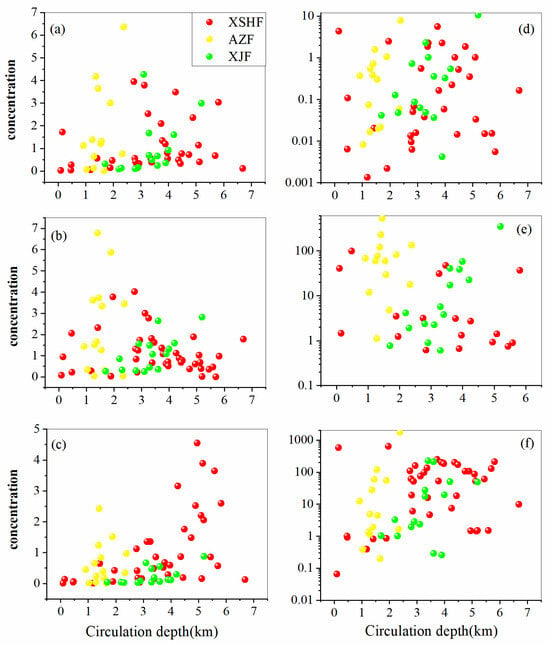
Figure 11.
The scatter plot of circulation depth against (a) B, (b) Sr, (c) Li, (d) Fe, (e) As, and (f) Mn. The data of circulation depth from [19,20,21].
5.4. Conceptual Model of Hot Springs in the XSF-XJF
Deep and large fault zones and secondary fractures play an essential role in the convective transfer of geothermal water, which can control the circulation path of geothermal water [70]. The emergence and circulation of hot springs are controlled by the XSHF-XJF and the secondary fractures (Figure 12), and its recharge is of meteoric water based on the values of δD and δ18O. Previous geological and geophysical studies show that the deep heat source for geothermal water is related to the frictional heat generation from active faults, decay of radioactive elements, and high conductivity and low-velocity zones associated with partial melting of the upper crust [71]. Due to the constrain imposed by the lithology of thermal reservoirs and the degree of water–rock interaction, the composition of hot-spring waters appears to be controlled by a specific geologic environment. In the XSHF, the hot springs are mainly influenced by deep granite and shallow carbonate rocks, with high circulation depths (0.1~6.7 km) and a mean value of 3.4 km. The Kangding–Moxi segment has a higher circulation depth (1.9~5.8 km). High concentrations of B and Li mainly originate from leaching of granite and are controlled by circulation depth. The contribution of the input of deep fluids to them is also not negligible, especially in the Kangding area. Interaction with surrounding rocks, containing Fe and Mn minerals at high temperatures, during the upwelling of hot-spring water results in high concentrations of Fe and Mn. In the XJF, the surrounding rocks of the hot springs are mostly interbedded with carbonates, limestone, and mudstone, and the circulation depth (1.7~5.2 km) is lower compared to XSHF. Sedimentary rocks and Tertiary lignite provide an important source of Fe and As in hot springs, and B may originate from deep thermal reservoirs. Trace-element concentrations are significantly controlled by the circulation depth. The hot-spring waters in the AZF have a much lower circulation depth (0.8~2.4 km), and the chemical compositions of hot-spring waters vary significantly owing to complex stratigraphic lithology.
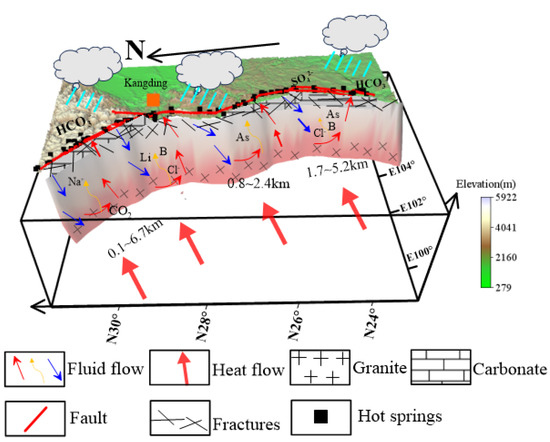
Figure 12.
Conceptual model of hot-spring-water formation pattern in the XSHF-XJF.
6. Conclusions
In this study, the geochemical characteristics of trace elements in hot-spring waters were investigated in the XSHF-XJF, and the conclusions were as follows:
- (1)
- The hot-spring water was recharged by atmospheric precipitation, and the isotope compositions were more depleted in the XSHF owing to the elevation effect.
- (2)
- High concentrations of B, Fe, Mn, and As were found in the XSHF-XJF, and the values of WQI indicated that the water quality was spatially heterogeneous and the hot-spring waters were not entirely suitable for drinking.
- (3)
- The leaching of the surrounding rock during water–rock interaction was the main source of trace elements, and the diversity of stratigraphic lithology was the main factor affecting their concentrations. High concentrations of B and Li were mainly derived from the dissolution of granite. Arsenic concentrations were affected by the reductive breakdown of iron oxides in the ANHF-XJF. In addition, B/Cl ratios showed that hot springs have different reservoir sources in the XSHF and XJF, respectively, and circulation depth could influence the concentrations of trace elements.
The results of this study indicated the importance of trace elements in the circulation of thermal waters, especially in geothermal systems controlled by faults. Although this area has abundant geothermal resources, it is important to be aware of the high concentration of trace elements (e.g., As) in the exploitation process. Consequently, future work could carry out studies on species variation and the fate of toxic elements in geothermal systems, as well as on the possible pollution of nearby rivers.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/w16050680/s1, Table S1: Field and analytical data of major elements and stable isotopes (δD, δ18O) on water samples; Table S2: Analytical data of trace elements on hot-spring water sample; Table S3: The results of the water quality assessment using the measurements of trace elements.; Table S4: Saturation indices values of thermal water samples with respect to minerals.
Author Contributions
Conceptualization, B.Y. and X.Z.; methodology, B.Y.; software, B.Y.; validation, Z.Z., Y.W. and Y.Y.; formal analysis, G.X. and S.C.; investigation, X.Z. and Y.L.; resources, D.Q. and F.Z.; data curation, M.H., J.T., J.D. (Jinyuan Dong) and J.L.; writing—original draft preparation, B.Y.; writing—review and editing, X.Z.; visualization, B.Y.; supervision, X.Z. and J.D. (Jianguo Du); funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by National Key Research and Development Project (2023YFC3012005-1, 2022YFC2204301), Central Public-interest Scientific Institution Basal Research Fund (CEAIEF2022030200, CEAIEF20230602, CEAIEF20230503), the National Natural Science Foundation of China (41673106, 4193000170, U2039207) and Open Foundation of the United Laboratory of High-Pressure Physics and Earthquake Science (2022HPPES05), IGCP Project 724.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martínez-Florentino, T.A.K.; Esteller-Alberich, M.V.; Expósito, J.L.; Domínguez-Mariani, E.; Morales-Arredondo, J.I. Hydrogeochemistry and geothermometry of thermal springs in the eastern Trans-Mexican Volcanic Belt. Geothermics 2021, 96, 102176. [Google Scholar] [CrossRef]
- Welch, A.H.; Westjohn, D.B.; Helsel, D.R.; Wanty, R.B. Arsenic in groundwater of the United States: Occurrence and geochemistry. Groundwater 2000, 38, 589–604. [Google Scholar] [CrossRef]
- Cacciapuoti, S.; Luciano, M.A.; Megna, M.; Annunziata, M.C.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P.; et al. The role of thermal water in chronic skin diseases management: A review of the literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef]
- Chandrajith, R.; Barth, J.A.C.; Subasinghe, N.D.; Merten, D.; Dissanayake, C.B. Geochemical and isotope characterization of geothermal spring waters in Sri Lanka: Evidence for steeper than expected geothermal gradients. J. Hydrol. 2013, 476, 360–369. [Google Scholar] [CrossRef]
- Wang, J.; Pang, Z.; Cheng, Y.; Huang, Y.; Jiang, G.; Lu, Z.; Kong, Y. Current state, utilization and prospective of global geothermal energy. Sci. Technol. Rev. 2023, 41, 5–11. [Google Scholar]
- Liu, W.; Guan, L.F.; Liu, Y.; Xie, X.A.; Zhang, M.L.; Chen, B.Y.; Xu, S.; Sano, Y. Fluid geochemistry and geothermal anomaly along the Yushu-Ganzi-Xianshuihe fault system, eastern Tibetan Plateau: Implications for regional seismic activity. J. Hydrol. 2022, 607, 127554. [Google Scholar] [CrossRef]
- Tapponnier, P.; Xu, Z.Q.; Roger, F.; Meyer, B.; Arnaud, N.; Wittlinger, G.; Yang, J.S. Geology-Oblique stepwise rise and growth of the Tibet platea. Science 2001, 294, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, J.; Pang, Z.; Hu, S.; Tian, J.; Bao, S. The eastern Tibetan Plateau geothermal belt, western China: Geology, geophysics, genesis, and hydrothermal system. Tectonophysics 2017, 717, 433–448. [Google Scholar] [CrossRef]
- Liao, Z. Thermal Springs and Geothermal Energy in the Qinghai-Tibetan Plateau and the Surroundings; Springer: Singapore, 2018. [Google Scholar]
- Maity, J.P.; Chen, C.Y.; Bundschuh, J.; Bhattacharya, P.; Mukherjee, A.; Chang, Y.F. Hydrogeochemical reconnaissance of arsenic cycling and possible environmental risk in hydrothermal systems of Taiwan. Groundw. Sustain. Dev. 2017, 5, 1–13. [Google Scholar] [CrossRef]
- Okan, Ö.; Kalender, L.; Çetindag, B. Trace-element hydrogeochemistry of thermal waters of Karakocan (Elazig) and Mazgirt (Tunceli), eastern Anatolia, Turkey. J. Geochem. Explor. 2018, 194, 29–43. [Google Scholar] [CrossRef]
- Daniele, L.; Taucare, M.; Viguier, B.; Arancibia, G.; Aravena, D.; Roquer, T.; Sepúlveda, J.; Molina, E.; Delgado, A.; Muñoz, M.; et al. Exploring the shallow geothermal resources in the Chilean southern Volcanic Zone: Insight from the Liquine thermal springs. J. Geochem. Explor. 2020, 218, 106611. [Google Scholar] [CrossRef]
- Liu, M.L.; Guo, Q.H.; Luo, L.; He, T. Environmental impacts of geothermal waters with extremely high boron concentrations: Insight from a case study in Tibet, China. J. Volcanol. Geotherm. Res. 2020, 397, 106887. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhou, X.; Liu, Y.; Xu, H.F.; Wu, Y.Q.; Zhuo, L.Y. Major, trace and rare earth elements geochemistry of geothermal waters from the Rehai high-temperature geothermal field in Tengchong of China. Appl. Geochem. 2020, 119, 104639. [Google Scholar] [CrossRef]
- Bundschuh, J.; Maity, J.P. Geothermal arsenic: Occurrence, mobility and environmental implications. Renew. Sustain. Energ. Rev. 2015, 42, 1214–1222. [Google Scholar] [CrossRef]
- Mukherjee, I.; Singh, U.K. Fluoride abundance and their release mechanisms in groundwater along with associated human health risks in a geologically heterogeneous semi-arid region of east India. Microchem. J. 2020, 152, 104304. [Google Scholar] [CrossRef]
- Baba, A.; Uzelli, T.; Sozbilir, H. Distribution of geothermal arsenic in relation to geothermal play types: A global review and case study from the Anatolian plate (Turkey). J. Hazard. Mater. 2021, 414, 125510. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.; Pang, Z.; Hu, S.; Wu, Y.; Bao, S. Distribution and genesis of the eastern Tibetan Plateau geothermal belt, western China. Environ. Earth Sci. 2016, 76, 31. [Google Scholar] [CrossRef]
- Li, C.H.; Zhou, X.C.; Yan, Y.C.; Ouyang, S.P.; Liu, F.L. Hydrogeochemical characteristics of hot springs and their short-term seismic precursor anomalies along the Xiaojiang Fault Zone, southeast Tibet Plateau. Water 2021, 13, 2638. [Google Scholar] [CrossRef]
- Tian, J.; Zhou, X.C.; Yan, Y.C.; He, M.; Li, J.C.; Dong, J.Y.; Liu, F.L.; Ouyang, S.P.; Li, Y.; Tian, L.; et al. Earthquake-induced impulsive release of water in the fractured aquifer system: Insights from the long-term hydrochemical monitoring of hot springs in the southeast Tibetan Plateau. Appl. Geochem. 2023, 148, 105553. [Google Scholar] [CrossRef]
- Yan, Y.C.; Zhou, X.C.; Liao, L.X.; Tian, J.; Li, Y.; Shi, Z.M.; Liu, F.L.; Ouyang, S.P. Hydrogeochemical characteristic of geothermal water and precursory anomalies along the Xianshuihe Fault Zone, southwestern China. Water 2022, 14, 550. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, M.L.; Chen, B.Y.; Liu, Y.; Cao, C.H.; Xu, W.; Zheng, G.D.; Zhou, X.C.; Lang, Y.C.; Sano, Y.; et al. Hydrothermal He and CO2 degassing from a Y-shaped active fault system in eastern Tibetan Plateau with implications for seismogenic processes. J. Hydrol. 2023, 620, 129482. [Google Scholar] [CrossRef]
- Cheng, Y.Z.; Pang, Z.H.; Kong, Y.L.; Chen, X.B.; Wang, G.J. Imaging the heat source of the Kangding high-temperature geothermal system on the Xianshuihe fault by magnetotelluric survey. Geothermics 2022, 102, 102386. [Google Scholar] [CrossRef]
- Li, B.; Shi, Z.; Wang, G.; Liu, C. Earthquake-related hydrochemical changes in thermal springs in the Xianshuihe Fault zone, Western China. J. Hydrol. 2019, 579, 124175. [Google Scholar] [CrossRef]
- Du, J.G.; Cheng, W.Z.; Zhang, Y.L.; Jie, C.L.; Guan, Z.J.; Liu, W.; Bai, L.P. Helium and carbon isotopic compositions of thermal springs in the earthquake zone of Sichuan, southwestern China. J. Asian Earth Sci. 2006, 26, 533–539. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Xu, M.; Li, X.; Qi, J.H.; Zhang, Q.; Guo, J.; Yu, L.L.; Zhao, R. Hydrochemical characteristics and multivariate statistical analysis of natural water system: A case study in Kangding county, southwestern China. Water 2018, 10, 80. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yuan, D.X.; He, S.Y.; Zhang, M.L.; Zhang, J.G. Geochemical features of the geothermal CO2-water-carbonate rock system and analysis on its CO2 sources-Examples from Huanglong Ravine and Kangding, Sichuan, and Xiage, Zhongdian, Yunnan. Sci. China Earth Sci. 2000, 43, 569–576. [Google Scholar] [CrossRef]
- Ren, Z.; Lin, A.; Rao, G. Late Pleistocene–Holocene activity of the Zemuhe Fault on the southeastern margin of the Tibetan Plateau. Tectonophysics 2010, 495, 324–336. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, G. Evaluation of the permeability properties of the Xiaojiang Fault Zone using hot springs and water wells. Geophys. J. Int. 2017, 209, 1526–1533. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Liao, X.; Zhang, Y.H. Hydrogeochemical characteristics and conceptual model of the geothermal waters in the Xianshuihe Fault Zone, southwestern China. Int. J. Environ. Res. Public Health 2020, 17, 500. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, X.; Du, J.; Xie, C.; Liu, L.; Li, Y.; Yi, L.; Liu, H.; Cui, Y. Hydrochemical characteristics of hot spring waters in the Kangding district related to the Lushan MS=7.0 earthquake in Sichuan, China. Nat. Hazards Earth Syst. Sci. 2015, 15, 1149–1156. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, L.S.; Chang, Y.; Fan, Z.W.; Guo, D.F. Determination trace elements in rock samples containing refractory minerals by pressurization-microwave inductively coupled plasma mass spectrometry. Uranium Geol. 2018, 34, 105–111, (In Chinese with English Abstract). [Google Scholar]
- Liu, H.B.; Jing, G.S.; Li, J.J.; Han, J.; Zhang, J.F.; Zhang, J.; Zhong, F.W.; Guo, D.Q. Determination of stable isotope composition in uranium geological samples. Word Nuclear Geosci. 2013, 30, 174–179, (In Chinese with English Abstract). [Google Scholar]
- Qu, B.; Zhang, Y.; Kang, S.; Sillanpää, M. Water quality in the Tibetan Plateau: Major ions and trace elements in rivers of the “Water Tower of Asia”. Sci. Total Environ. 2019, 649, 571–581. [Google Scholar] [CrossRef]
- GB 5749–2022; Standards for Drinking Water Quality. Ministry of Health of the People’s Republic of China: Beijing, China, 2022.
- Ma, Y.Q.; Shi, Y.; Qin, Y.W.; Zheng, B.H.; Zhao, Y.M.; Zhang, L. Temporal-spatial distribution and pollution assessment of heavy metals in the upper reaches of Hunhe River (Qingyuan section), northeast China. Environ. Sci. 2014, 35, 108–116. [Google Scholar]
- Huang, X.; Sillanpää, M.; Duo, B.; Gjessing, E.T. Water quality in the Tibetan Plateau: Metal contents of four selected rivers. Environ. Pollut. 2008, 156, 270–277. [Google Scholar] [CrossRef]
- Elenga, H.I.; Tan, H.; Su, J.; Yang, J. Origin of the enrichment of B and alkali metal elements in the geothermal water in the Tibetan Plateau: Evidence from B and Sr isotopes. Geochemistry 2021, 81, 125797. [Google Scholar] [CrossRef]
- Guo, Q.; Planer-Friedrich, B.; Liu, M.; Li, J.; Zhou, C.; Wang, Y. Arsenic and thioarsenic species in the hot springs of the Rehai magmatic geothermal system, Tengchong volcanic region, China. Chem. Geol. 2017, 453, 12–20. [Google Scholar] [CrossRef]
- Bénard, B.; Famin, V.; Agrinier, P.; Aunay, B.; Lebeau, G.; Sanjuan, B.; Vimeux, F.; Bardoux, G.; Dezayes, C. Origin and fate of hydrothermal fluids at Piton des Neiges volcano (Réunion Island): A geochemical and isotopic (O, H, C, Sr, Li, Cl) study of thermal springs. J. Volcanol. Geotherm. Res. 2020, 392, 106682. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Kong, Y.L.; Wang, K.; Li, J.; Pang, Z.H. Stable isotopes of precipitation in China: A consideration of moisture sources. Water 2019, 11, 1239. [Google Scholar] [CrossRef]
- Xu, Q.; Hoke, G.D.; Jing, L.Z.; Ding, L.; Wang, W.; Yang, Y. Stable isotopes of surface water across the Longmenshan margin of the eastern Tibetan Plateau. Geochem. Geophys. Geosyst. 2014, 15, 3416–3429. [Google Scholar] [CrossRef]
- Yi, L.; Qi, J.; Li, X.; Xu, M.; Zhang, X.; Zhang, Q.; Tang, Y. Geochemical characteristics and genesis of the high-temperature geothermal systems in the north section of the Sanjiang Orogenic belt in southeast Tibetan Plateau. J. Volcanol. Geotherm. Res. 2021, 414, 107244. [Google Scholar] [CrossRef]
- Pang, Z.; Kong, Y.; Li, J.; Tian, J. An isotopic geoindicator in the hydrological cycle. Procedia Earth Planet. Sci. 2017, 17, 534–537. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, L.S.; Liu, S.W.; Yang, Y.; Shi, D.Y. Characteristics of hydrogen and oxygen stable isotopes of hot springs in Xianshuihe-Anninghe fault zone, Sichuan, Province, China. Acta Petrol. 2021, 37, 589–598, (In Chinese with English Abstract). [Google Scholar]
- Das, P.; Maya, K.; Padmalal, D. Hydrochemistry, geothermometry and origin of the low temperature thermal springs of South Konkan region, India. Geothermics 2021, 90, 101997. [Google Scholar] [CrossRef]
- Cui, Y.J.; Sun, F.X.; Liu, L.; Xie, C.; Li, J.; Chen, Z.; Li, Y.; Du, J.G. Contribution of deep-earth fluids to the geothermal system: A case study in the Arxan volcanic region, northeastern China. Front. Earth Sci. 2023, 10, 996583. [Google Scholar] [CrossRef]
- Guo, Q. Hydrogeochemistry of high-temperature geothermal systems in China: A review. Appl. Geochem. 2012, 27, 1887–1898. [Google Scholar] [CrossRef]
- Cui, Y.J.; Sun, F.X.; Du, J.G. Methods for identification of seismic geochemical precursors and source partitioning of hot spring fluids in eastern Chinese Mainland. J. Seismol. Res. 2022, 45, 199–216, (In Chinese with English Abstract). [Google Scholar]
- Zhu, X.Q.; Liu, L.; Lan, F.N.; Li, J.; Hou, S.T. Hydrogeochemistry characteristics of groundwater in the Nandong Karst water system, China. Atmosphere 2022, 13, 604. [Google Scholar] [CrossRef]
- Davraz, A.; Nalbantçilar, M.T.; Önden, I. Hydrogeochemical characteristics and trace element of geothermal systems in central Anatolia, Turkey. J. Afr. Earth Sci. 2022, 195, 104666. [Google Scholar] [CrossRef]
- Liu, M.; Guo, Q.; Wu, G.; Guo, W.; She, W.; Yan, W. Boron geochemistry of the geothermal waters from two typical hydrothermal systems in southern Tibet (China): Daggyai and Quzhuomu. Geothermics 2019, 82, 190–202. [Google Scholar] [CrossRef]
- Lai, S.C.; Qin, J.F.; Long, X.P.; Li, Y.F.; Ju, Y.J.; Zhu, R.Z.; Zhao, S.W.; Zhang, Z.Z.; Zhu, Y.; Wang, J.B. Neoproterozoic gabbro-granite association from the Micangshan area, northern Yangtze Block: Implication for crustal growth in an active continental margin. Geol. J. 2018, 53, 2471–2486. [Google Scholar] [CrossRef]
- Tassi, F.; Aguilera, F.; Darrah, T.; Vaselli, O.; Capaccioni, B.; Poreda, R.J.; Huertas, A.D. Fluid geochemistry of hydrothermal systems in the Arica-Parinacota, Tarapaca and Antofagasta regions (northern Chile). J. Volcanol. Geotherm. Res. 2010, 192, 1–15. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, Z.; Xu, S.; Barry, P.H.; Sano, Y.; Zhang, L.; Halldórsson, S.A.; Chen, A.-T.; Cheng, Z.; Liu, C.-Q.; et al. Linking deeply-sourced volatile emissions to plateau growth dynamics in southeastern Tibetan Plateau. Nat. Commun. 2021, 12, 4157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, G.; Sagoe, G.; Li, Y. Major hydrogeochemical processes controlling the composition of geothermal waters in the Kangding geothermal field, western Sichuan Province. Geothermics 2018, 75, 154–163. [Google Scholar] [CrossRef]
- Bernard, R.; Taran, Y.; Pennisi, M.; Tello, E.; Ramirez, A. Chloride and boron behavior in fluids of Los Humeros geothermal field (Mexico): A model based on the existence of deep acid brine. Appl. Geochem. 2011, 26, 2064–2073. [Google Scholar] [CrossRef]
- Erdogan, Y.; Aksu, M.; Demirbas, A.; Abali, Y. Analyses of boronic ores and sludges and solubilities of boron minerals in CO2-saturated water. Resour. Conserv. Recycl. 1998, 24, 275–283. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Ruan, C.; Sagoe, G.; Li, J. Enrichment mechanisms of lithium for the geothermal springs in the southern Tibet, China. J. Hydrol. 2022, 612, 128022. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, T.; Santosh, M.; Li, H.; Li, J.; Zhang, Z.; Song, X.; Wang, M. Spatio-temporal distribution and tectonic settings of the major iron deposits in China: An overview. Ore Geol. Rev. 2014, 57, 247–263. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Y.; Hou, Z.; Cooke, D.R.; Danyushevsky, L.; Dominy, S.C.; Shuping, Y. A model for carbonatite hosted REE mineralisation—The Mianning–Dechang REE belt, Western Sichuan Province, China. Ore Geol. Rev. 2015, 70, 595–612. [Google Scholar] [CrossRef]
- Kaasalainen, H.; Stefánsson, A. The chemistry of trace elements in surface geothermal waters and steam, Iceland. Chem. Geol. 2012, 330, 60–85. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z. Correlations of the deep structural characteristics, tidal stress variation and earthquake initiation along the Xianshuihe-Anninghe fault zone. Chin. J. Geophy. 2020, 63, 928–943, (In Chinese with English Abstract). [Google Scholar]
- Shen, Z.; Lü, J.; Wang, M.; Bürgmann, R. Contemporary crustal deformation around the southeast borderland of the Tibetan Plateau. J. Geophys. Res. Solid Earth 2005, 110, 409. [Google Scholar] [CrossRef]
- Li, X.; Bai, D.; Ma, X.; Chen, Y.; Varentsov, I.M.; Xue, G.; Xue, S.; Lozovsky, I. Electrical resistivity structure of the Xiaojiang strike-slip fault system (SW China) and its tectonic implications. J. Asian Earth Sci. 2019, 176, 57–67. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhang, W.; Wang, G.L.; Zhao, J.Y.; Yue, G.F. Distribution and genetic mechanism of high arsenic geothermal water in the Batang area, Western Sichuan. Geothermics 2021, 97, 102232. [Google Scholar] [CrossRef]
- Guo, Q.; Pang, Z.; Wang, Y.; Tian, J. Fluid geochemistry and geothermometry applications of the Kangding high-temperature geothermal system in eastern Himalayas. Appl. Geochem. 2017, 81, 63–75. [Google Scholar] [CrossRef]
- Negri, A.; Daniele, L.; Aravena, D.; Muñoz, M.; Delgado, A.; Morata, D. Decoding fjord water contribution and geochemical processes in the Aysen thermal springs (Southern Patagonia, Chile). J. Geochem. Explor. 2018, 185, 1–13. [Google Scholar] [CrossRef]
- Blomgren, V.J.; Crossey, L.J.; Karlstrom, K.E.; Fischer, T.P.; Darrah, T.H. Hot spring hydrochemistry of the Rio Grande rift in northern New Mexico reveals a distal geochemical connection between Valles Caldera and Ojo Caliente. J. Volcanol. Geotherm. Res. 2019, 387, 106663. [Google Scholar] [CrossRef]
- Bai, D.H.; Unsworth, M.J.; Meju, M.A.; Ma, X.B.; Teng, J.W.; Kong, X.R.; Sun, Y.; Sun, J.; Wang, L.F.; Jiang, C.S.; et al. Crustal deformation of the eastern Tibetan plateau revealed by magnetotelluric imaging. Nat. Geosci. 2010, 3, 358–362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).