A Novel Generalized Clapeyron Equation-Based Model for Capturing the Soil Freezing Characteristics Curve of Saline Soil: Validation by Small Sample Lab and Field Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Modelling
2.1.1. The Combined Model
- (1)
- The GCE
- (2)
- The Salt Exclusion Model
- (3)
- The Combined Model
2.1.2. The GCE-Salt Model
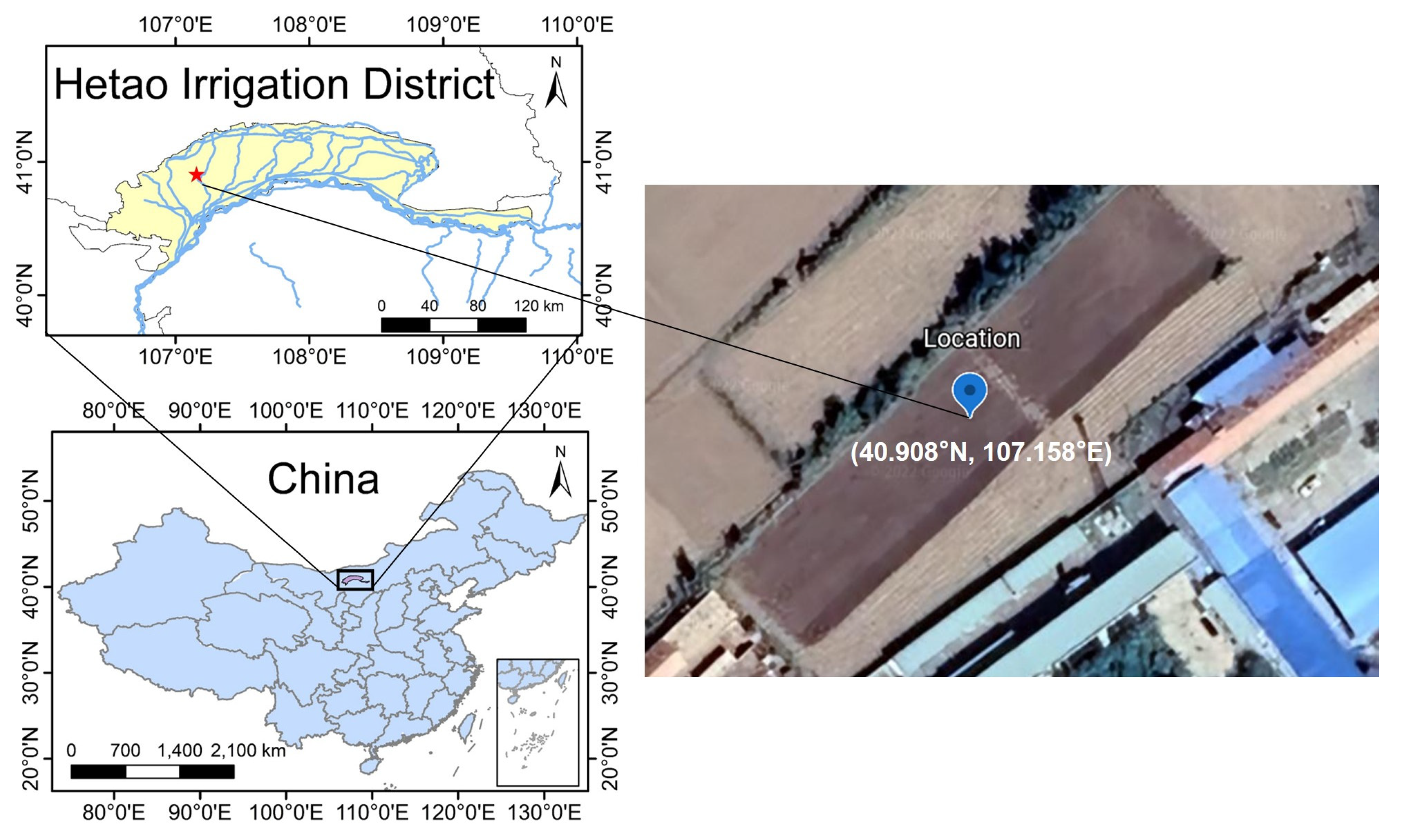
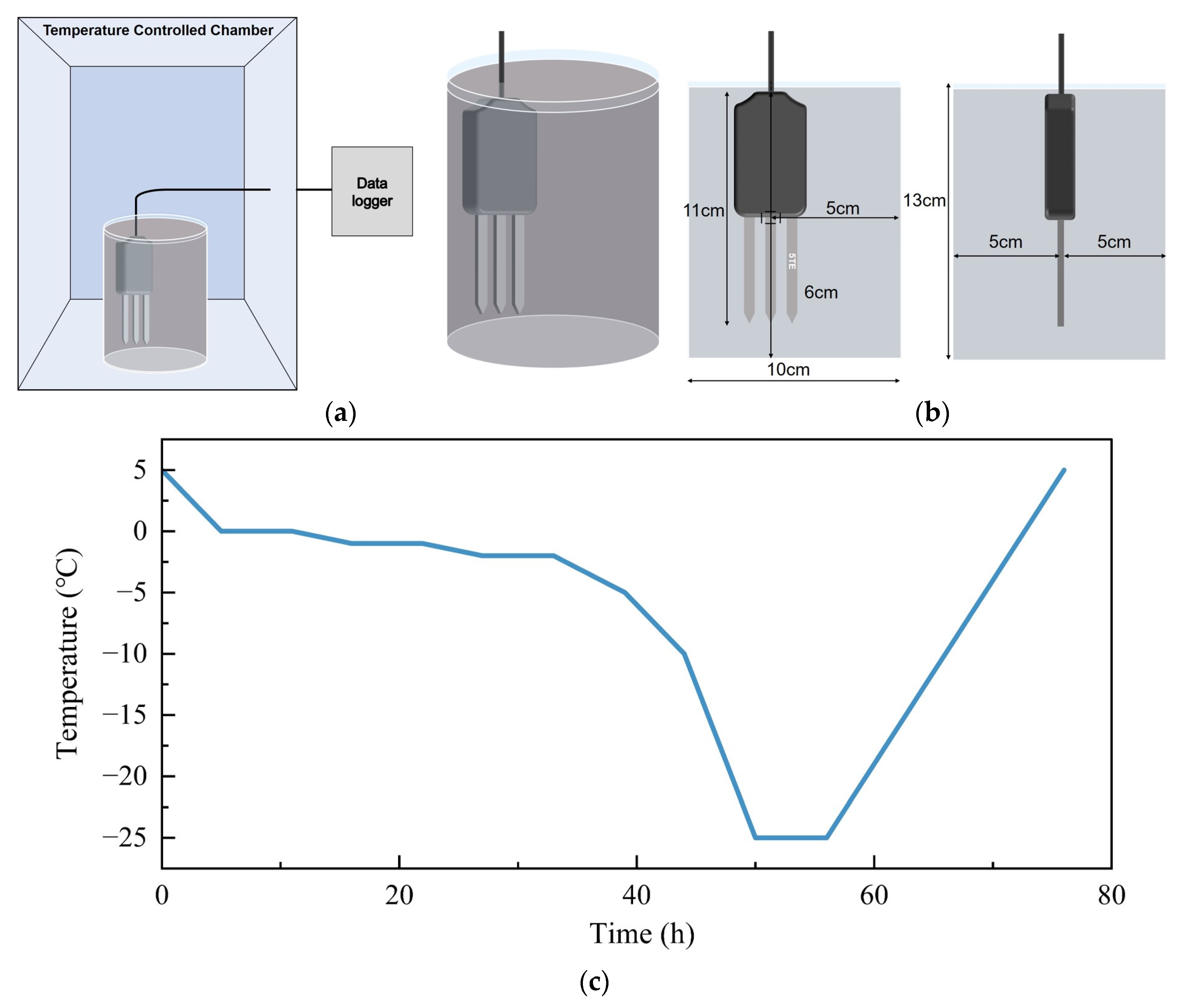
2.2. Lab and Field Experiments
2.2.1. Laboratory Experiments
2.2.2. Field Experiment
3. Results and Discussion
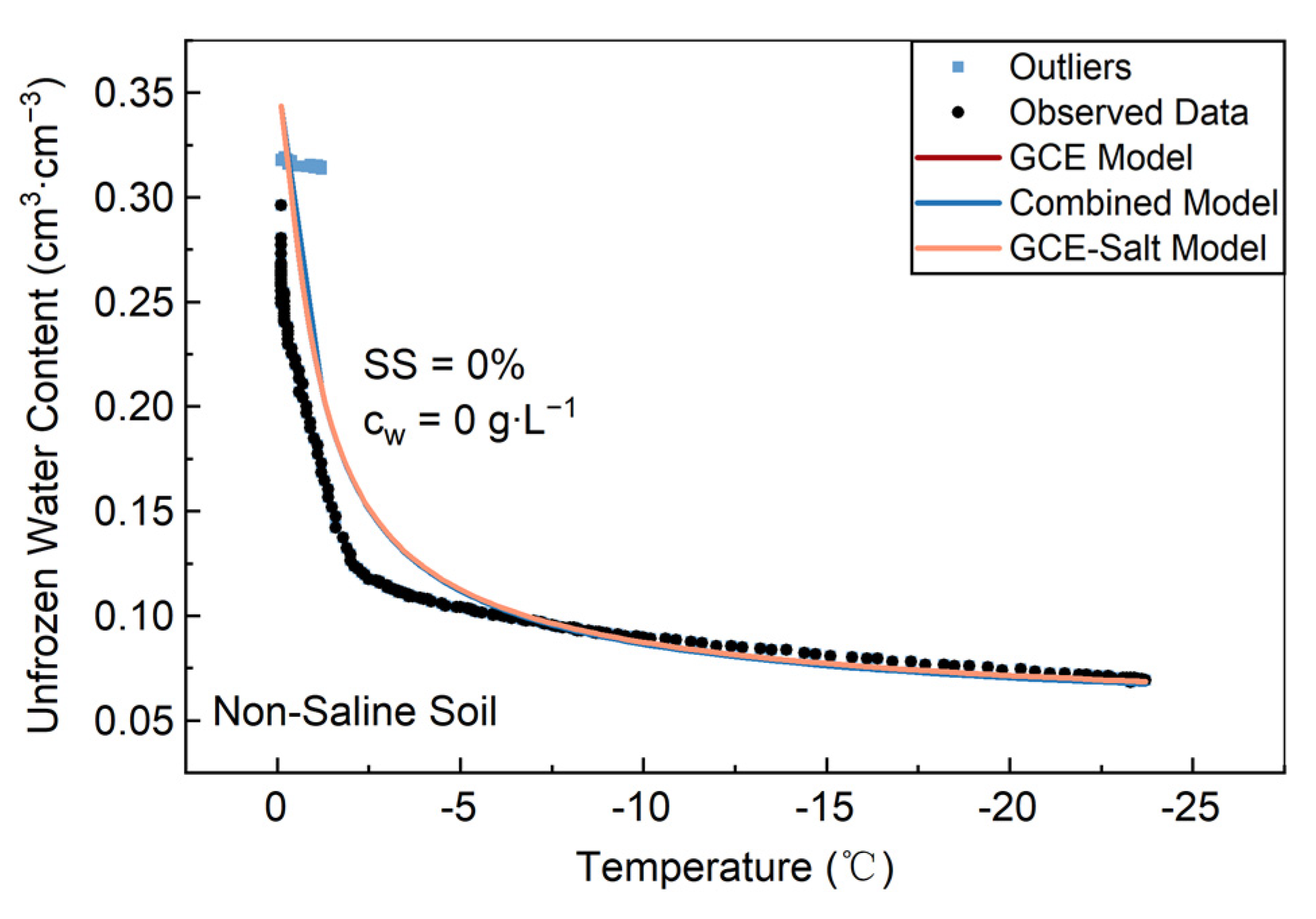
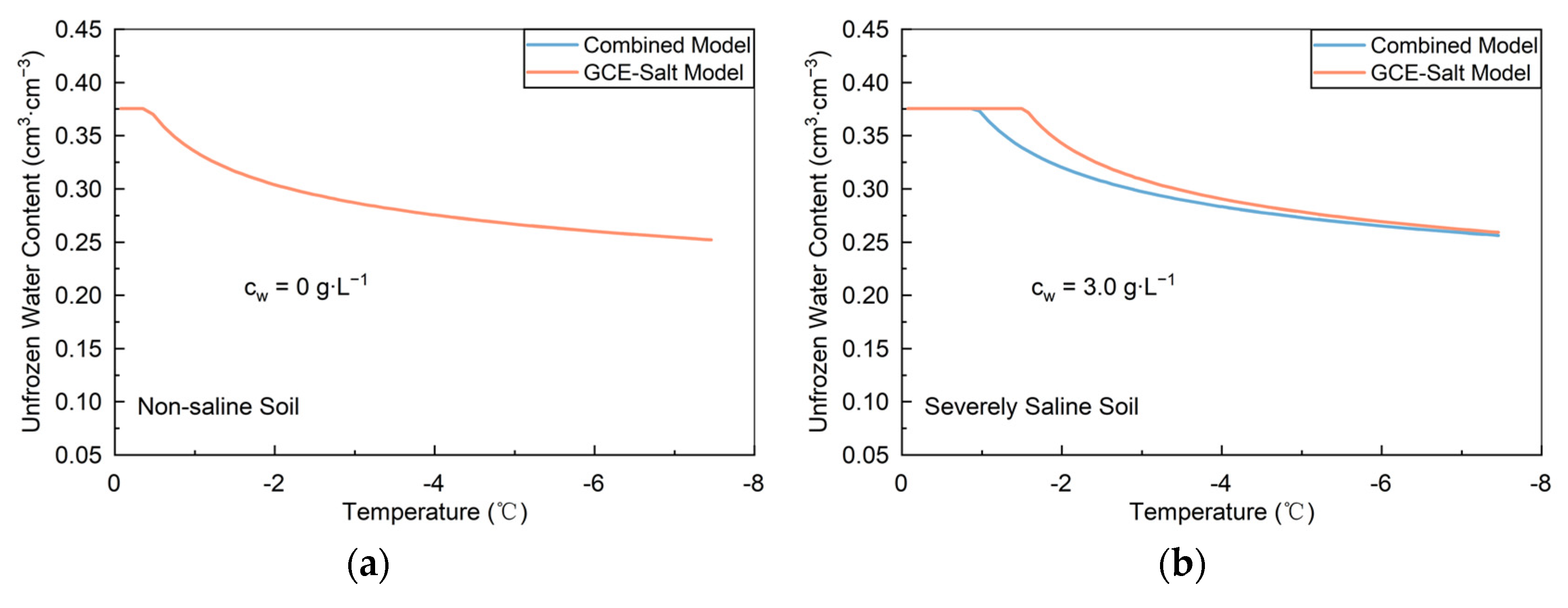
3.1. Lab Non-Saline Soil
3.2. Saline Soil
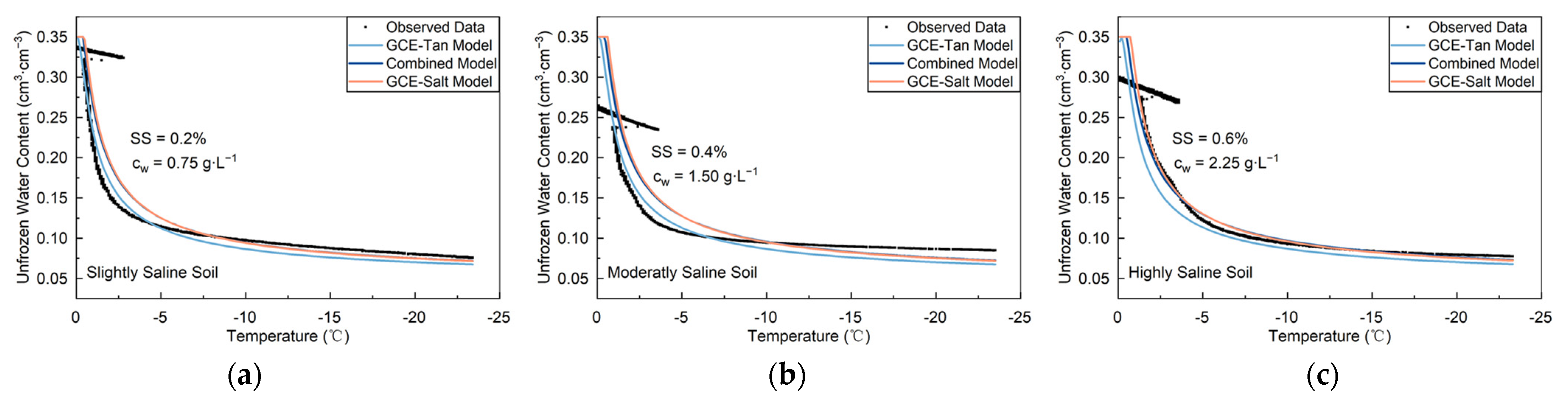
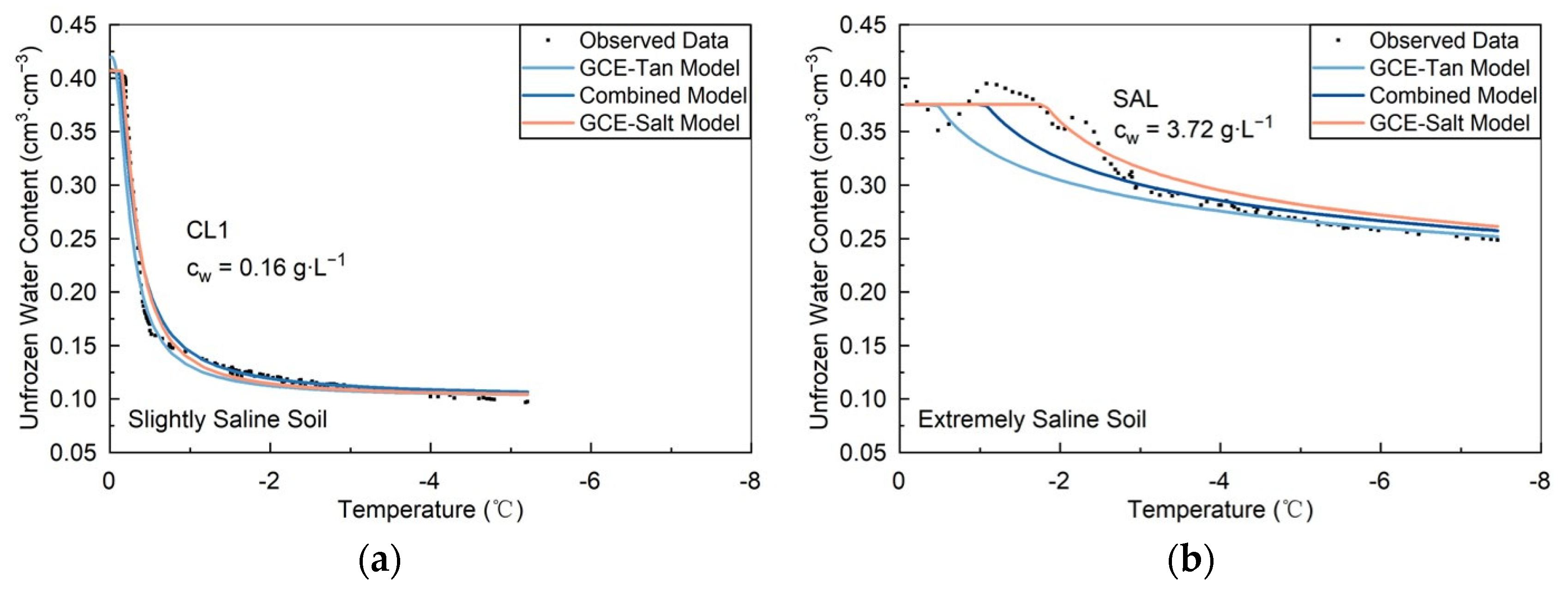
3.2.1. Lab Slightly to Highly Saline Soils
3.2.2. Field Extremely and Slightly Saline Soils
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amankwah, S.K.; Ireson, A.M.; Maulé, C.; Brannen, R.; Mathias, S.A. A Model for the Soil Freezing Characteristic Curve That Represents the Dominant Role of Salt Exclusion. Water Resour. Res. 2021, 57, e2021WR030070. [Google Scholar] [CrossRef]
- He, H.; Dyck, M.; Zhao, Y.; Si, B.; Jin, H.; Zhang, T.; Lv, J.; Wang, J. Evaluation of five composite dielectric mixing models for understanding relationships between effective permittivity and unfrozen water content. Cold Reg. Sci. Technol. 2016, 130, 33–42. [Google Scholar] [CrossRef]
- Gao, B.; Yang, D.; Qin, Y.; Wang, Y.; Li, H.; Zhang, Y.; Zhang, T. Change in frozen soils and its effect on regional hydrology, upper Heihe basin, northeastern Qinghai–Tibetan Plateau. Cryosphere 2018, 12, 657–673. [Google Scholar] [CrossRef]
- Kong, F.; Nie, L.; Xu, Y.; Rui, X.; He, Y.; Zhang, T.; Wang, Y.; Du, C.; Bao, C. Effects of freeze-thaw cycles on the erodibility and microstructure of soda-saline loessal soil in Northeastern China. Catena 2022, 209, 105812. [Google Scholar] [CrossRef]
- Gharedaghloo, B.; Berg, S.J.; Sudicky, E.A. Water freezing characteristics in granular soils: Insights from pore-scale simulations. Adv. Water Resour. 2020, 143, 103681. [Google Scholar] [CrossRef]
- Krzeminska, D.; Bloem, E.; Starkloff, T.; Stolte, J. Combining FDR and ERT for monitoring soil moisture and temperature patterns in undulating terrain in south-eastern Norway. Catena 2022, 212, 106100. [Google Scholar] [CrossRef]
- Ding, B.; Zhang, Y.; Yu, X.; Jia, G.; Wang, Y.; Zheng, P.; Li, Z. Comparative study of seasonal freeze–thaw on soil water transport in farmland and its shelterbelt. Catena 2023, 225, 106982. [Google Scholar] [CrossRef]
- Xu, J.; Ren, J.; Wang, Z.; Wang, S.; Yuan, J. Strength behaviors and meso-structural characters of loess after freeze-thaw. Cold Reg. Sci. Technol. 2018, 148, 104–120. [Google Scholar] [CrossRef]
- Hou, R.; Li, T.; Fu, Q.; Liu, D.; Li, M.; Zhou, Z.; Li, L.; Yan, J. Characteristics of water–heat variation and the transfer relationship in sandy loam under different conditions. Geoderma 2019, 340, 259–268. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Wang, X.; Huo, Z. Response of soil compaction to the seasonal freezing-thawing process and the key controlling factors. Catena 2020, 184, 104247. [Google Scholar] [CrossRef]
- Ye, W.; Li, C. The consequences of changes in the structure of loess as a result of cyclic freezing and thawing. Bull. Eng. Geol. Environ. 2018, 78, 2125–2138. [Google Scholar] [CrossRef]
- Ren, J.; Vanapalli, S.K.; Han, Z. Soil freezing process and different expressions for the soil-freezing characteristic curve. Sci. Cold Arid Reg. 2017, 9, 221–228. [Google Scholar] [CrossRef]
- Xiao, Z.; Lai, Y.; Zhang, M. Study on the freezing temperature of saline soil. Acta Geotech. 2017, 13, 195–205. [Google Scholar] [CrossRef]
- Wu, M.; Tan, X.; Huang, J.; Wu, J.; Jansson, P.-E. Solute and water effects on soil freezing characteristics based on laboratory experiments. Cold Reg. Sci. Technol. 2015, 115, 22–29. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Hu, L. A theoretical model of the soil freezing characteristic curve for saline soil. J. Hydrol. 2023, 622, 129639. [Google Scholar] [CrossRef]
- Chen, J.; Mei, S.; Irizarry, J.T.; Rempel, A.W. A Monte Carlo Approach to Approximating the Effects of Pore Geometry on the Phase Behavior of Soil Freezing. J. Adv. Model. Earth Syst. 2020, 12, e2020MS002117. [Google Scholar] [CrossRef]
- Bi, J.; Wang, G.; Wu, Z.; Wen, H.; Zhang, Y.; Lin, G.; Sun, T. Investigation on unfrozen water content models of freezing soils. Front. Earth. Sci. 2023, 10, 1039330. [Google Scholar] [CrossRef]
- Chai, M.; Zhang, J.; Zhang, H.; Mu, Y.; Sun, G.; Yin, Z. A method for calculating unfrozen water content of silty clay with consideration of freezing point. Appl. Clay Sci. 2018, 161, 474–481. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Sugimoto, M. Pulsed NMR Measurements of Unfrozen Water Content in Partially Frozen Soil. J. Cold Reg. Eng. 2020, 34, 04020013. [Google Scholar] [CrossRef]
- Teng, J.; Zhong, Y.; Zhang, S.; Sheng, D. A mathematic model for the soil freezing characteristic curve: The roles of adsorption and capillarity. Cold Reg. Sci. Technol. 2021, 181, 103178. [Google Scholar] [CrossRef]
- Bittelli, M.; Flury, M.; Campbell, G.S. A thermodielectric analyzer to measure the freezing and moisture characteristic of porous media. Water Resour. Res. 2003, 39, 1041. [Google Scholar] [CrossRef]
- Nishimura, S.; Gens, A.; Olivella, S.; Jardine, R.J. THM-coupled finite element analysis of frozen soil: Formulation and application. Geotechnique 2009, 59, 159–171. [Google Scholar] [CrossRef]
- Zhou, J. Experimental Study on Characteristics of Unfrozen Water in Clay Mineral and Its Effect on Sand Thawing Characteristics; China University of Mining and Technology: Xuzhou, China, 2020. [Google Scholar]
- Wen, Z.; Ma, W.; Feng, W.; Deng, Y.; Wang, D.; Fan, Z.; Zhou, C. Experimental study on unfrozen water content and soil matric potential of Qinghai-Tibetan silty clay. Environ. Earth Sci. 2011, 66, 1467–1476. [Google Scholar] [CrossRef]
- Dall’Amico, M.; Endrizzi, S.; Gruber, S.; Rigon, R. A robust and energy-conserving model of freezing variably-saturated soil. Cryosphere 2011, 5, 469–484. [Google Scholar] [CrossRef]
- Anderson, D.M.; Tice, A.R. Predicting Unfrozen Water Contents in Frozen Soils from Surface Area Measurements. Highw. Res. Rec. 1972, 393, 12–18. [Google Scholar]
- Michalowski, R.L.; Zhu, M. Frost heave modelling using porosity rate function. Int. J. Numer. Anal. Methods Geomech. 2006, 30, 703–722. [Google Scholar] [CrossRef]
- Michalowski, R.L. A constitutive model of saturated soils for frost heave simulations. Cold Reg. Sci. Technol. 1993, 22, 47–63. [Google Scholar] [CrossRef]
- Osterkamp, T.E.; Romanovsky, V.E. Freezing of the Active Layer on the Coastal Plain of the Alaskan Arctic. Permafr. Periglac. Process. 1997, 8, 23–44. [Google Scholar] [CrossRef]
- McKenzie, J.M.; Voss, C.I.; Siegel, D.I. Groundwater flow with energy transport and water–ice phase change: Numerical simulations, benchmarks, and application to freezing in peat bogs. Adv. Water Resour. 2007, 30, 966–983. [Google Scholar] [CrossRef]
- Kozlowski, T. A semi-empirical model for phase composition of water in clay–water systems. Cold Reg. Sci. Technol. 2007, 49, 226–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Carey, S.K.; Quinton, W.L. Evaluation of the algorithms and parameterizations for ground thawing and freezing simulation in permafrost regions. J. Geophys. Res. 2008, 113, 116. [Google Scholar] [CrossRef]
- Nicolsky, D.J.; Romanovsky, V.E.; Panda, S.K.; Marchenko, S.S.; Muskett, R.R. Applicability of the ecosystem type approach to model permafrost dynamics across the Alaska North Slope. J. Geophys. Res. Earth Surf. 2017, 122, 50–75. [Google Scholar] [CrossRef]
- Weng, L.; Wu, Z.; Liu, Q.; Chu, Z.; Zhang, S. Evolutions of the unfrozen water content of saturated sandstones during freezing process and the freeze-induced damage characteristics. Int. J. Rock Mech. Min. Sci 2021, 142, 104757. [Google Scholar] [CrossRef]
- Westermann, S.; Boike, J.; Langer, M.; Schuler, T.V.; Etzelmüller, B. Modeling the impact of wintertime rain events on the thermal regime of permafrost. Cryosphere 2011, 5, 945–959. [Google Scholar] [CrossRef]
- Li, B.; Huang, L.; Lv, X.; Ren, Y. Variation features of unfrozen water content of water-saturated coal under low freezing temperature. Sci. Rep. 2021, 11, 15398. [Google Scholar] [CrossRef]
- Qin, Y.; Li, G.; Qu, G. A formula for the unfrozen water content and temperature of frozen soils. In Cold Regions Engineering 2009: Cold Regions Impacts on Research, Design, and Construction; 2009; pp. 155–161. Available online: https://ascelibrary.org/doi/10.1061/41072%28359%2918 (accessed on 15 January 2023).
- Kong, L.; Wang, Y.; Sun, W.; Qi, J. Influence of plasticity on unfrozen water content of frozen soils as determined by nuclear magnetic resonance. Cold Reg. Sci. Technol. 2020, 172, 102993. [Google Scholar] [CrossRef]
- Kurylyk, B.L.; Watanabe, K. The mathematical representation of freezing and thawing processes in variably-saturated, non-deformable soils. Adv. Water Resour. 2013, 60, 160–177. [Google Scholar] [CrossRef]
- Ma, T.; Wei, C.; Xia, X.; Zhou, J.; Chen, P. Soil Freezing and Soil Water Retention Characteristics: Connection and Solute Effects. J. Perform. Constr. Fac. 2017, 31, D4015001. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, C.; Lai, Y.; Wei, H.; Tian, H. Application of the Generalized Clapeyron Equation to Freezing Point Depression and Unfrozen Water Content. Water Resour. Res. 2018, 54, 9412–9431. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, X.; Wei, C.; Pei, W. Unified Soil Freezing Characteristic for Variably-Saturated Saline Soils. Water Resour. Res. 2020, 56, e2019WR026648. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Yu, X.; Wang, T.; Feng, R. A Simplified Model for the Phase Composition Curve of Saline Soils Considering the Second Phase Transition. Water Resour. Res. 2021, 57, e2020WR028556. [Google Scholar] [CrossRef]
- Wan, X.; Yang, Z. Pore water freezing characteristic in saline soils based on pore size distribution. Cold Reg. Sci. Technol. 2020, 173, 103030. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Liu, J.; Liu, J.; Chen, W.; Zhang, Q.; Wang, T. Crystallization deformation and phase transitions of coarse-grained sulfate saline soils upon cooling. Cold Reg. Sci. Technol. 2023, 209, D4015001. [Google Scholar] [CrossRef]
- Tan, X.; Wu, M.; Huang, J.; Wu, J.; Chen, J. Similarity of soil freezing characteristic and soil water characteristic: Application in saline frozen soil hydraulic properties prediction. Cold Reg. Sci. Technol. 2020, 173, 102876. [Google Scholar] [CrossRef]
- Xu, X.; Oliphant, J.L.; Tice, A.R. Prediction of unfrozen water content in frozen soil by a two-point or one-point method. In Proceedings of the 4th International Symposium on Ground Freezing, Sapporo, Japan, 5–7 August 1985; Volume 2, pp. 83–87. [Google Scholar]
- Banin, A.; Anderson, D.M. Effects of Salt Concentration Changes During Freezing on the Unfrozen Water Content of Porous Materials. Water Resour. Res. 1974, 10, 124–128. [Google Scholar] [CrossRef]
- Bi, J.; Wu, Z.; Lu, Y.; Wen, H.; Zhang, Y.; Shen, Y.; Wei, T.; Wang, G. Study on soil freezing characteristic curve during a freezing-thawing process. Front. Earth. Sci. 2023, 10, 1007342. [Google Scholar] [CrossRef]
- Jin, X.; Yang, W.; Gao, X.; Zhao, J.Q.; Li, Z.; Jiang, J. Modeling the Unfrozen Water Content of Frozen Soil Based on the Absorption Effects of Clay Surfaces. Water Resour. Res. 2020, 56, e2020WR027482. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Lai, Y.; Chen, Q.; He, X.; Zhang, H.; Liu, X. Predicting the Soil Freezing Characteristic From the Particle Size Distribution Based on Micro-Pore Space Geometry. Water Resour. Res. 2022, 58, e2021WR030782. [Google Scholar] [CrossRef]
- Dall’Amico, M. Coupled Water and Heat Transfer in Permafrost Modeling. Doctoral Thesis, University of Trento, Trento, Italy, 2010. [Google Scholar]
- Sheshukov, A.Y.; Nieber, J.L. One-dimensional freezing of nonheaving unsaturated soils: Model formulation and similarity solution. Water Resour. Res. 2011, 47, W11519. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X. Physically Based Equation for Phase Composition Curve of Frozen Soils. Transp. Res. Rec. J. Transp. Res. Board 2013, 2349, 93–99. [Google Scholar] [CrossRef]
- Mu, Q.Y.; Ng, C.W.W.; Zhou, C.; Zhou, G.G.D.; Liao, H.J. A new model for capturing void ratio-dependent unfrozen water characteristics curves. Comput. Geotech. 2018, 101, 95–99. [Google Scholar] [CrossRef]
- Wen, H.; Bi, J.; Guo, D. Evaluation of the calculated unfrozen water contents determined by different measured subzero temperature ranges. Cold Reg. Sci. Technol. 2020, 170, 102927. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Y.; Huo, Z.; Liu, Z.; Liu, G.; Wang, S.; Lin, Y.; Wu, P. Salt Accumulation during Cropping Season in an Arid Irrigation Area with Shallow Water Table Depth: A 10-Year Regional Monitoring. Water 2022, 14, 1664. [Google Scholar] [CrossRef]
- Fuchs, M.; Campbell, G.S.; Papendick, R.I. An Analysis of Sensible and Latent Heat Flow in a Partially Frozen Unsaturated Soil. Soil Sci. Soc. Am. J. 1978, 42, 379–385. [Google Scholar] [CrossRef]
- Wan, X.; Liu, E.; Qiu, E. Study on ice nucleation temperature and water freezing in saline soils. Permafr. Periglac. Process. 2020, 32, 119–138. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Pronk, P. Fluidized Bed Heat Exchangers to Prevent Fouling in Ice Slurry Systems and Industrial Crystallizers. Doctoral Thesis, Universiteit Delft, Delft, The Netherlands, 2006. [Google Scholar]
- Chen, H.; Chen, K.; Yang, M.; Xu, P. A fractal capillary model for multiphase flow in porous media with hysteresis effect. Int. J. Multiphas. Flow 2020, 125, 103208. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Wang, P.; Huo, Z.; Wang, C. The Effect of Salinity on Soil Seasonal Freezing-Thawing Process in Areas with Shallow Water Table Depth. Unpublished results. 2023. [Google Scholar]
- DRIPD. Measuring Soil Salinity. Available online: https://www.agric.wa.gov.au/soil-salinity/measuring-soil-salinity (accessed on 9 January 2023).
- Meter. Measurement Volume of Meter Volumetric Water Content Sensors. Available online: https://www.metergroup.com/en/meter-environment (accessed on 15 January 2023).
- Wang, X. Monitoring and Simulation of Water, Heat and Salt Process under Freezing-Thawing Conditions in Saline Soil. Doctoral Thesis, China Agricultural University: Beijing, China, 2021. [Google Scholar]
- Liu, G.; Wang, C.; Wang, X.; Huo, Z.; Liu, J. Growing season water and salt migration between abandoned lands and adjacent croplands in arid and semi-arid irrigation areas in shallow water table environments. Agric. Water Manag. 2022, 274, 107968. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, L.; Ren, T. Measuring soil freezing characteristic curve with thermo-time domain reflectometry. Eur. J. Soil Sci. 2023, 74, e13335. [Google Scholar] [CrossRef]


| Soil Types | Particle Distribution/% | Specific Surface Area m2·g−1 | Bulk Density g·cm−3 | ||
|---|---|---|---|---|---|
| 0.01–2.00 µm | 2.00–50.00 µm | 50.00–2000.00 µm | |||
| Sandy loam a | 6.078 | 61.258 | 32.644 | 0.743 | 1.5 |
| SITE | Sand (%) a | Silt (%) a | Clay (%) a | Texture d | ρb (g∙cm−3) a | θr (cm3∙cm−3) b | θs (cm3∙cm−3) b | α (m−1) c | n c |
|---|---|---|---|---|---|---|---|---|---|
| SAL | 35.39 | 58.03 | 6.57 | silt loam | 1.51 | 0.046 | 0.479 | 0.080 | 1.171 |
| CL1 | 25.66 | 61.76 | 12.58 | silt loam | 1.53 | 0.101 | 0.450 | 0.047 | 2.354 |
| Parameter | α (m−1) | n | m | θs (cm3∙cm−3) | θr (cm3∙cm−3) |
|---|---|---|---|---|---|
| Value | 0.014 | 1.69 | 0.41 | 0.40 | 0.046 |
| Site | SS (%) | cw (g·L−1) | Model | RMSE (cm3∙cm−3) | NSE |
|---|---|---|---|---|---|
| CL1 | 0.05 | 0.16 | GCE-Tan Model | 0.098 | 0.94 |
| Combined Model | 0.017 | 0.98 | |||
| GCE-Salt Model | 0.013 | 0.99 | |||
| SAL | 1.18 | 3.72 | GCE-Tan Model | 0.029 | 0.62 |
| Combined Model | 0.017 | 0.86 | |||
| GCE-Salt Model | 0.013 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, X.; Han, J.; Wang, C.; Zhang, C.; Huo, Z. A Novel Generalized Clapeyron Equation-Based Model for Capturing the Soil Freezing Characteristics Curve of Saline Soil: Validation by Small Sample Lab and Field Experiments. Water 2024, 16, 670. https://doi.org/10.3390/w16050670
Wang L, Wang X, Han J, Wang C, Zhang C, Huo Z. A Novel Generalized Clapeyron Equation-Based Model for Capturing the Soil Freezing Characteristics Curve of Saline Soil: Validation by Small Sample Lab and Field Experiments. Water. 2024; 16(5):670. https://doi.org/10.3390/w16050670
Chicago/Turabian StyleWang, Liwen, Xianghao Wang, Juan Han, Chaozi Wang, Chenglong Zhang, and Zailin Huo. 2024. "A Novel Generalized Clapeyron Equation-Based Model for Capturing the Soil Freezing Characteristics Curve of Saline Soil: Validation by Small Sample Lab and Field Experiments" Water 16, no. 5: 670. https://doi.org/10.3390/w16050670
APA StyleWang, L., Wang, X., Han, J., Wang, C., Zhang, C., & Huo, Z. (2024). A Novel Generalized Clapeyron Equation-Based Model for Capturing the Soil Freezing Characteristics Curve of Saline Soil: Validation by Small Sample Lab and Field Experiments. Water, 16(5), 670. https://doi.org/10.3390/w16050670








