Assessment of Effluent Wastewater Quality and the Application of an Integrated Wastewater Resource Recovery Model: The Burgersfort Wastewater Resource Recovery Case Study
Abstract
1. Introduction
2. Materials and Methods
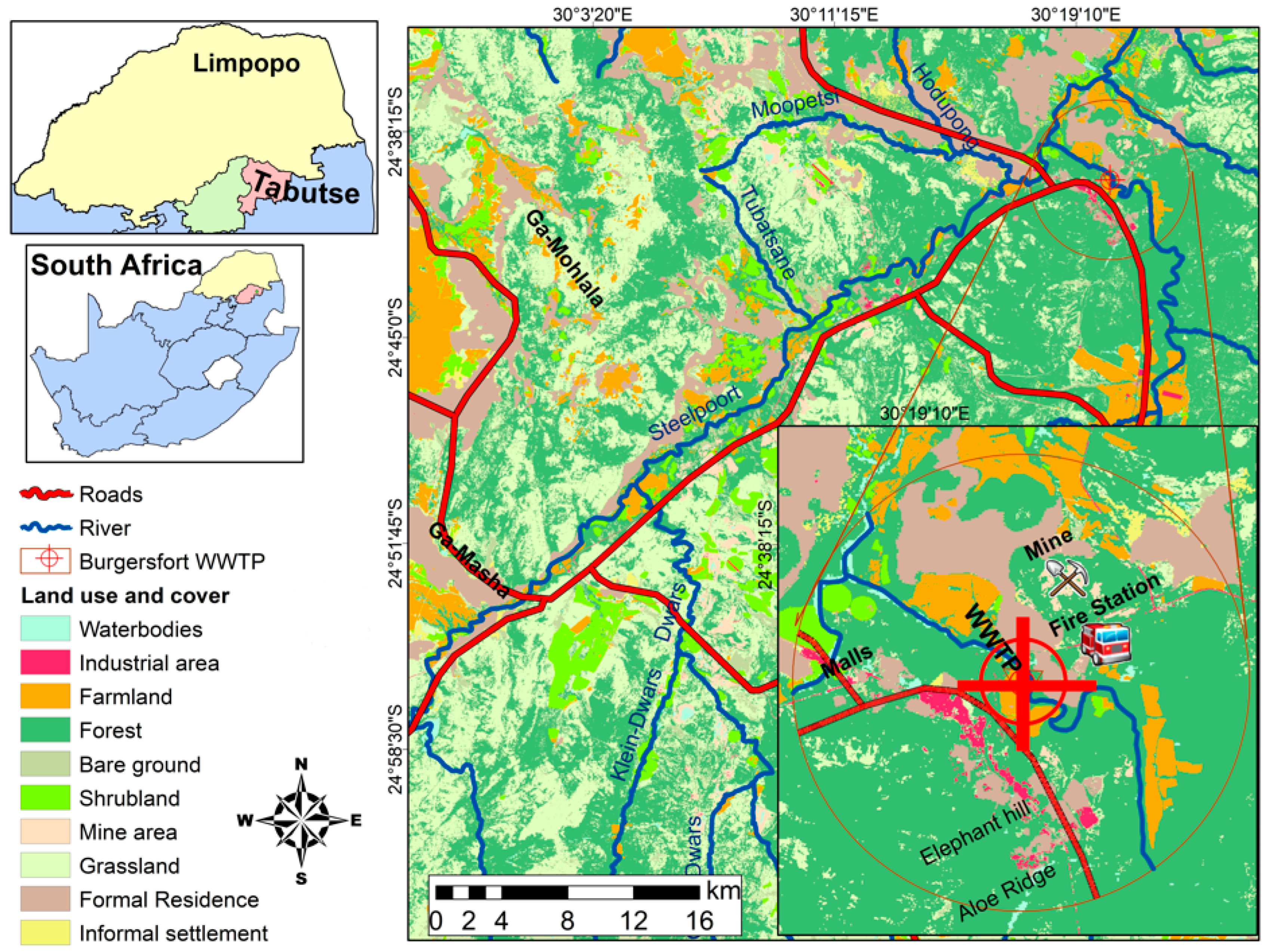
2.1. Study Area
2.2. Sample Site and Collection
2.3. Measurements
2.3.1. Measurement of Physical, Chemical, and Microbiological Variables
2.3.2. Quality Assurance and Quality Control
2.4. Data Analysis
2.4.1. Statistical Analysis
2.4.2. Assessment of Overall Wastewater Quality Using the Weighted Arithmetic Water Quality Index
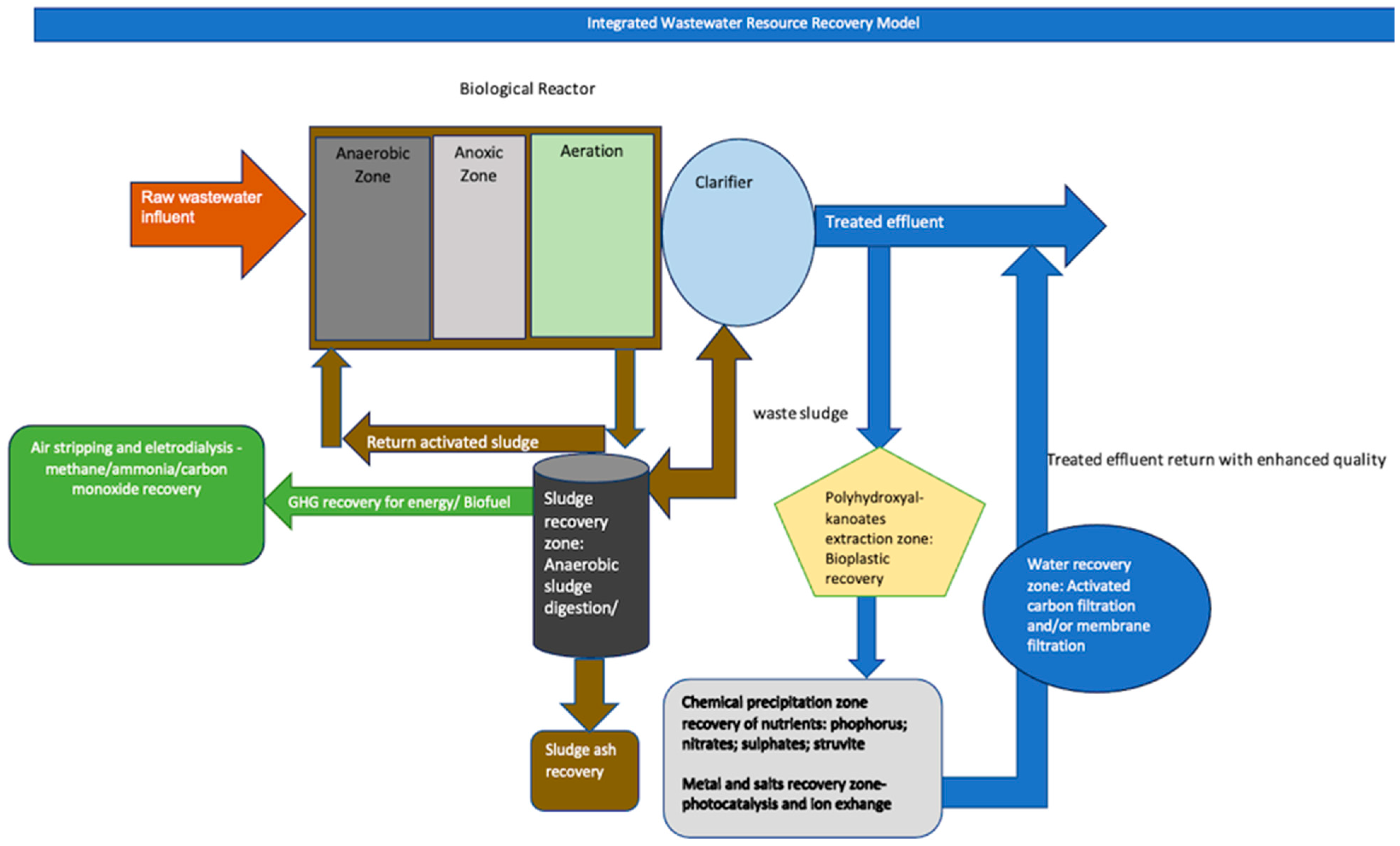
2.4.3. Application of the Integrated Wastewater Resource Recovery Model for the Burgersfort Wastewater Treatment Facility Effluent
3. Results and Discussion
3.1. Results of the Physicochemical and Microbiological Variables
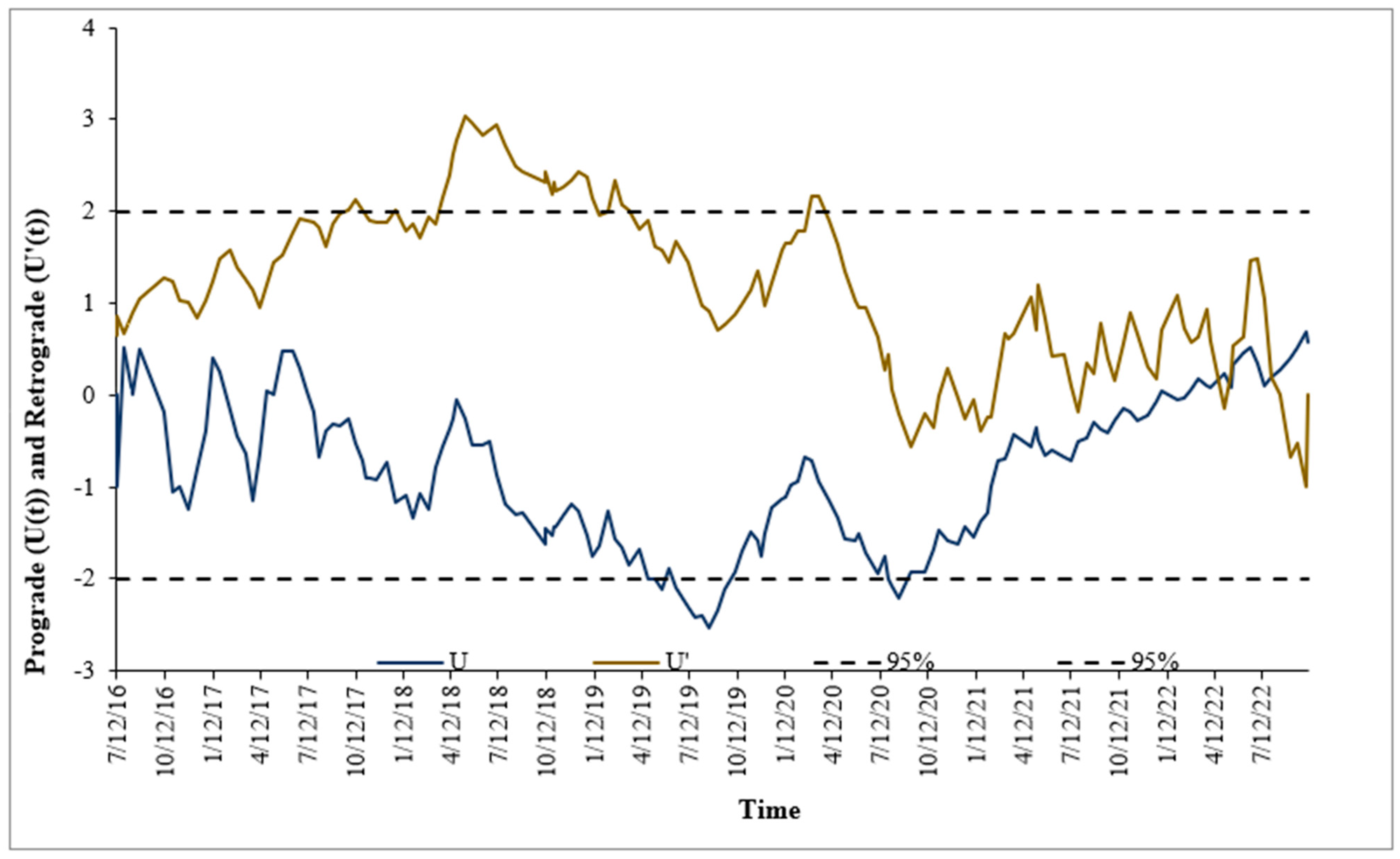
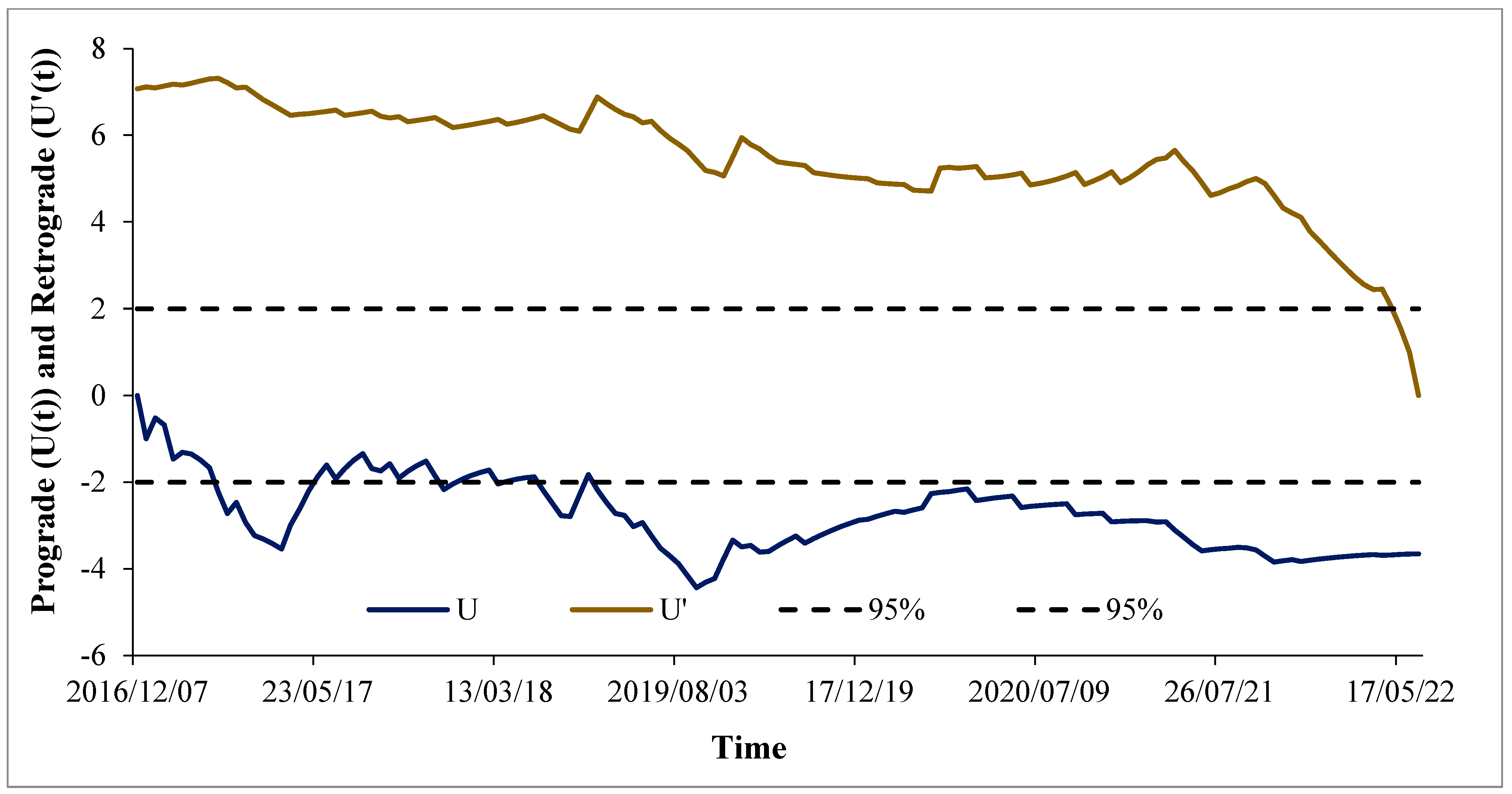
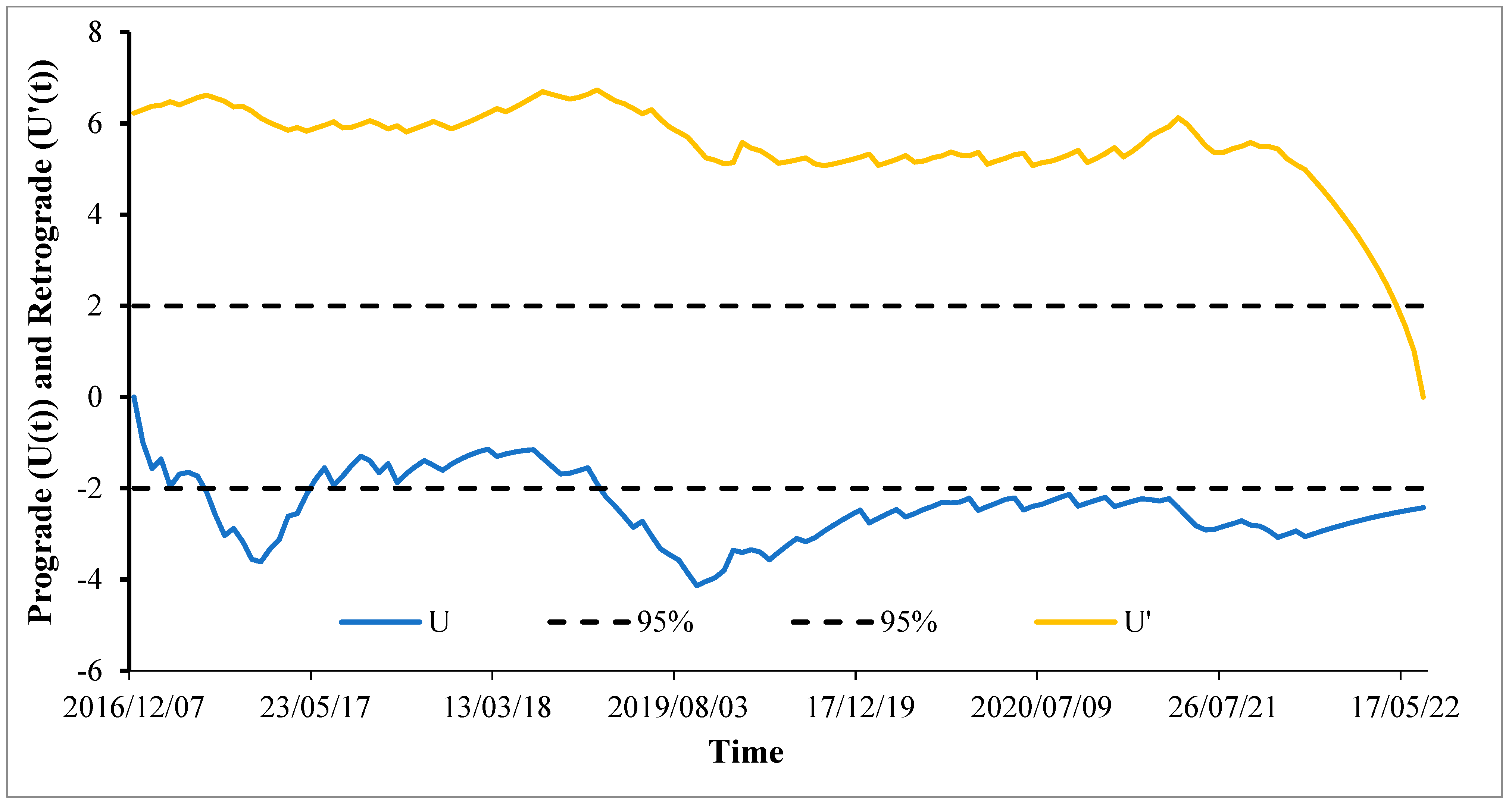
3.2. Mann–Kendall Sequential Trend Analysis Results
3.3. Principal Component Analysis Results for Physicochemical Variables
3.4. Principal Component Analysis Results for Microbiological Variables and Free Chlorine
3.5. Spearman’s Rank-Order Correlation Coefficient Analysis for Physicochemical and Microbiological Variables
3.6. Wastewater Quality Index
3.7. Integrated Wastewater Resource Recovery Model Results
4. Conclusions
- The IWWRR model developed in this study was used to propose possible resource recovery technologies that could be implemented in a WWRF that utilizes the BNR treatment system to recover reusable resources from wastewater effluent, thereby improving on the current wastewater treatment processes and thus reducing the pollution of water resources. The application of this model to the Burgersfort WWRF case study provided evidence of the prevalence of recoverable resources in the effluent, such as nutrients, water, sludge, metals, bioplastics, and biofuel.
- Based on the IWWRR model results, the different technologies that could be incorporated in a WWRF to recover valuable resources from wastewater effluent include the anaerobic digestion of manure or incineration to produce biofuel, the use of struvite precipitation to recover nitrogen and phosphorus, and photocatalysis and ion exchange recovery methods to recover salts and precious metals. However, even though plastic traces were not tested in the case study of Burgersfort effluent, based on the sludge production and poor water quality, and land-use activities in the study area, the IWWRR model suggests the recovery of bioplastics through PHA extraction from bacterial cells by selective digestion of bacterial biomass from wastewater to produce bioplastics that are biodegradable and environmentally friendly.
- On the other hand, the assessment of the effluent quality from the WRRF case study identified NH3, COD, TC, FC, and E. coli water quality variables as critically non-compliant and suggests severe pollution threats to the receiving water body. Such findings from the effluent quality assessment were confirmed by the WWQI results, which revealed a very bad water quality rating for NH3 and COD, as well as all the microbiological variables, TC, FC, and E. coli. If effluent with such poor water quality is disposed into water resources, there is bound to be organic pollution threats, high probability of eutrophication, as well as microbial pollution threats to the environment.
- Based on the findings of this study, we recommend that wastewater resource recovery technologies for nutrients, water, sludge, metals, bioplastics, and biofuel need to be incorporated into the BNR process treatment system as per the results of the water quality and the IWWRR model. By doing so, the pollutants within the effluent of the WRRF will be significantly reduced, and the wastewater treatment facility will be transformed into a wastewater resource recovery facility, thus protecting the receiving water body, the environment, and ensuring the management of wastewater in a holistic and integrated way. The model developed in this study can contribute to the enhancement of effluent quality from WRRFs worldwide and can further be used as a tool in water resource pollution control, water, and food security, as well as an increase in nutrient and water recycling for agricultural purposes.
5. Recommendations for Further Research
- The precise determination of the existence of bioplastics and metal traces in the sludge and wastewater effluent would have added value to this study and directly link such contaminants to factories and mining industries. Therefore, further research is required where the sludge samples and effluent samples are both analyzed for such contaminants to inform decision-making processes in wastewater resource recovery practices; this would have increased this study’s dimensions.
- The availability of raw influent wastewater data samples could have added more value to this study. The raw wastewater data were unavailable on record because raw water samples were not regularly monitored. The raw influent data samples would have informed the level of pollution received at the plant, and if long-term records were kept, this would have informed the need to improve the treatment process much quicker than with the treated effluent quality data. Therefore, more research on wastewater resource recovery is needed that includes long-term assessments of raw influent wastewater quality data and final effluent wastewater quality data.
- A more complex study with diversified sampling positions such as sampling and analysis from different stages of the biological reactor would inform the concentration changes of nutrients or other substances during the reaction process. This would lead to a precise determination of the treatment component that is less efficient in treating the wastewater.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaimaa-Satae, M.A.; Abdul-Kareem, L.; Abdul-Hussein, N.A. New approach for treatment of pollutants in municipal waste water. Turk. J. Physiother. Rehabil. 2019, 32, 11537–11551. Available online: https://sustainability.uobabylon.edu.iq/uploaded/Sustain_2021_1088914.pdf (accessed on 1 June 2023).
- United Nations World Water Assessment Programme. The United Nations World Water Development Report 2017. Wastewater: The Untapped Resource; UNESCO: Paris, France, 2017; Available online: https://reliefweb.int/attachments/ffa40d52-7333-3d19-9f45-3fa8c95d6f17/247153e.pdf (accessed on 1 June 2023).
- Onu, M.A.; Ayeleru, O.O.; Oboirien, B.; Olubambi, P.A. Challenges of wastewater generation and management in sub-Saharan Africa: A Review. Environ. Chall. 2023, 11, 100686. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Oberholster, P.F.; Truter, C.; Botha, A.M. Assessing Rivers Self-Purification Capacity Down WWTPs in a Lowland Catchment Using a Phosphorus Sensitive Index. In Advances in Environmental Research; Daniels, J.A., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 160–182. [Google Scholar]
- Montwedi, M.; Munyaradzi, M.; Pinoy, L.; Dutta, A.; Ikumi, D.S.; Motoasca, E.; Van der Bruggen, B. Resource recovery from and management of wastewater in rural South Africa: Possibilities and practices. J. Water Process. Eng. 2021, 40, 101978. [Google Scholar] [CrossRef]
- Phungela, T.T.; Maphanga, T.; Chidi, B.S.; Madonsela, B.S.; Shale, K. The impact of wastewater treatment effluent on Crocodile River quality in Ehlanzeni District, Mpumalanga province, South Africa. S. Afr. J. Sci. 2022, 118, 61–71. [Google Scholar] [CrossRef]
- Department of Water and Sanitation, South Africa. National Water and Sanitation Master Plan. Volume 2: Plan to Action. Ready for the Future and Ahead of the Curve; USA, 2018. Available online: https://www.dws.gov.za/National%20Water%20and%20Sanitation%20Master%20Plan/Documents/Volume2%20(Printed%20version%20).pdf (accessed on 1 June 2023).
- Department of Water and Sanitation, South Africa. Green Drop Watch Report: Technical Assessment of the Condition of Municipal Wastewater Conveyance and Treatment Systems in South Africa; Pretoria, South Africa, 2023. Available online: https://ws.dws.gov.za/IRIS/releases/GDWR.pdf (accessed on 1 April 2023).
- Radebe, B. Competent People to be Recruited to Resolve Challenge in Hammanskraal. SABC News. 9 June 2023. Available online: https://www.sabcnews.com/sabcnews/competent-people-to-be-recruited-to-resolve-challenge-in-hammanskraal (accessed on 14 August 2023).
- Oberholster, P.J.; Botha, A.-M.; Chamier, J.; De Klerk, A.R. Longitudinal trends in water chemistry and phytoplankton assemblage downstream of the Riverview WWTP in the Upper Olifants River. Ecohydrol. Hydrobiol. 2013, 13, 41–51. [Google Scholar] [CrossRef]
- Ratola, N.; Cincinelli, A.; Alves, A.; Katsoyiannis, A. Occurrence of organic microcontaminants in the wastewater treatment process. A mini review. J. Hazard. Mater. 2012, 239–240, 1–18. [Google Scholar] [CrossRef]
- Mohan, T.R.; Chanakya, H.M.; Mohan Kumar, M.S.; Rao, L. Achieving biological nutrient removal in an old sewage treatment plant through process modifications—A simulation and experimental study. J. Water Process Eng. 2022, 45, 102461. [Google Scholar] [CrossRef]
- Solon, K.; Flores-Alsina, X.; Mbamba, C.K.; Ikumi, D.; Volcke, E.I.P.; Vaneechaute, C.; Ekama, G.; Vanrolleghem, P.A.; Batstone, D.J.; Gernaey, K.V.; et al. Plant-wide modelling of phosphorus transformations in wastewater treatment systems: Impacts of control and operational strategies. Water Res. 2017, 113, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Kehrein, P.; Van Loosdrecht, M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A critical review of resource recovery from municipal wastewater treatment plants—Market supply potentials, technologies and bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef]
- Afzal, M.; Arslan, M.; Müller, J.A.; Shabir, G.; Islam, E.; Tahseen, R.; Anwar-ul-Haq, M.; Hashmat, A.J.; Iqbal, S.; Khan, Q.M. Floating treatment wetlands as a suitable option for large-scale wastewater treatment. Nat. Sustain. 2019, 2, 863–871. [Google Scholar] [CrossRef]
- Borne, K.E.; Fassman, E.A.; Tanner, C.C. Floating treatment wetland retrofit to improve stormwater pond performance for suspended solids, copper and zinc. Ecol. Eng. 2013, 54, 173–182. [Google Scholar] [CrossRef]
- Bu, F.; Xu, X. Planted floating bed performance in treatment of eutrophic river water. Environ. Monit. Assess. 2013, 185, 9651–9662. [Google Scholar] [CrossRef] [PubMed]
- Oberholster, P.; Cheng, P.-H.; Genthe, B.; Steyn, M. The environmental feasibility of low-cost algae-based sewage treatment as a climate change adaptation measure in rural areas of SADC countries. J. Appl. Phycol. 2018, 31, 355–363. [Google Scholar] [CrossRef]
- Muga, E.M.; Mihelcic, R.J. Sustainability of wastewater treatment technologies. J. Environ. Manag. 2008, 88, 437–447. [Google Scholar] [CrossRef]
- Sucu, S.; Van Schaik, M.O.; Esmeli, R.; Ouelhadj, D.; Holloway, T.; Williams, J.B.; Cruddas, P.; Martinson, D.B.; Chen, W.; Cappon, H.J. A conceptual framework for a multi-criteria decision support tool to select technologies for resource recovery from urban wastewater. J. Environ. Manag. 2021, 300, 113608. [Google Scholar] [CrossRef] [PubMed]
- La Para, T.M.; Allenman, J.E.; Pope, P.G. Miniaturized closed reflux, colorimetric method for the determination of chemical oxygen demand. Waste Manag. 2000, 20, 295–298. [Google Scholar] [CrossRef]
- Belcher, R.; Macdonald, A.M.G.; Parry, E. On mohr’s method for the determination of chlorides. Anal. Chim. Acta 2002, 16, 524–529. [Google Scholar] [CrossRef]
- Kinzelman, J.L.; Singh, A.; Ng, C.; Pond, K.R.; Bagley, R.C.; Gradus, S. Use of IDEXX Colilert-18® and Quanti-Tray/2000 as a rapid and simple enumeration method for the implementation of recreational water monitoring and notification programs. Lake Reserv. Manag. 2005, 21, 73–77. [Google Scholar] [CrossRef]
- Owusu-Ansah, E.d.G.J.; Sampson, A.; Amponsah, S.K.; Abaidoo, R.C. Performance, compliance and reliability of waste stabilization pond: Effluent discharge quality and environmental protection agency standards in Ghana. Res. J. Appl. Sci. Eng. Technol. 2015, 10, 1293–1302. [Google Scholar] [CrossRef]
- Department of Water Affairs, South Africa. Discharge Limits and Conditions Set Out in the National Water Act; Government Gazette 20526: Pretoria, South Africa, 1999. [Google Scholar]
- Ayoub, M.; El-Morsy, A. Applying the wastewater quality index for assessing the effluent quality of recently upgraded Meet Abo El-koum Wastewater Treatment Plant. J. Ecol. Eng. 2021, 22, 128–133. [Google Scholar] [CrossRef]
- Azmi, A.A.; Zamanhuri, N.A.; Yahya, A. Precious metals recovery from electroplating wastewater: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012024. [Google Scholar] [CrossRef]
- Wu, H.; Vaneeckhaute, C. Nutrient recovery from wastewater: A review on the integrated physicochemical technologies of ammonia stripping, adsorption and struvite precipitation. Chem. Eng. J. 2022, 433, 133664. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Wastewater Treatment Manuals—Primary, Secondary and Tertiary Treatment; EPA: Wexford, Ireland, 1997; Available online: https://sanihub.info/wp-content/uploads/2023/08/EPA-Ireland_1997_Waste-water-treatment-manuals-primary-secondary-and-tertiary-treatment.pdf (accessed on 1 May 2023).
- Department of Water Affairs, South Africa. Quality of Domestic Water Supply. Volume 4: Treatment Guide. Pretoria. 2002. Available online: http://www.dwa.gov.za/iwqs/AssessmentGuides/TreatmentGuide/TreatmentGuide.pdf (accessed on 1 June 2023).
- Alisawi, H.A.O. Performance of wastewater treatment during variable temperature. Appl. Water Sci. 2020, 10, 12–21. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; Metcalf & Eddy, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Ritter, J.A. Water Quality: Principles and Practices of Water Supply Operations, 4th ed.; American Water Works Association: Denver, CO, USA, 2010. [Google Scholar]
- Edokpayi, J.N.; Odiyo, J.O.; Durowoju, O.S. Impact of Wastewater on Surface Water Quality in Developing Countries: A Case Study of South Africa. In Water Quality; Tutu, H., Ed.; InTechOpen: London, UK, 2017; pp. 401–416. [Google Scholar] [CrossRef]
- Gupta, V.; Shekhawat, S.S.; Kulshreshtha, N.M.; Gupta, A.B. A systematic review on chlorine tolerance among bacteria and standardization of their assessment protocol in wastewater. Water Sci. Technol. 2022, 86, 261–291. [Google Scholar] [CrossRef]
- Madkour, A.G.; Hamed, M.M.; Dar, M.A. Removal of ammonia and orthophosphate from domestic wastewater using marine actinomycetes. Egypt. J. Aquat. Biol. Fish. 2019, 23, 455–465. Available online: https://ejabf.journals.ekb.eg/article_48322_6d4e225e5e5458ac35e6021826e334ba.pdf (accessed on 30 April 2023). [CrossRef]
- Saleh, B.A.; Kayi, H. Prediction of chemical oxygen demand from the chemical composition of wastewater by artificial neural networks. J. Phys. Conf. Ser. 2021, 1818, 012035. [Google Scholar] [CrossRef]
- Halicki, W.; Halicki, M. From domestic sewage to potable water quality: New approach in organic matter removal using natural treatment systems for wastewater. Water 2022, 14, 1909. [Google Scholar] [CrossRef]
- Faragò, M.; Damgaard, A.; Madsen, J.A.; Andersen, J.K.; Thornberg, D.; Andersen, M.H.; Rygaard, M. From wastewater treatment to water resource recovery: Environmental and economic impacts of full-scale implementation. Water Res. 2021, 204, 117554. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Haripavan, N.; Basha, S.R.; Babu, G.V. Removal of ammonia and nitrates from contaminated water by using solid waste bio-adsorbents. Curr. Res. Chem. Biol. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Hariri, M.B.; Botte, G.G. Simultaneous removal of ammonia and nitrate from wastewater using pulse electrolysis technique. J. Electrochem. Soc. 2023, 170, 67–74. [Google Scholar] [CrossRef]
- Makuwa, S.; Tlou, M.; Fosso-Kankeu, E.; Green, E. The effects of dry versus wet season on the performance of a wastewater treatment plant in Northwest Province, South Africa. Water SA 2022, 48, 54–67. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, J.; Zeng, G.; Yang, R.; Xu, Z.; Zhou, Z.; Lyu, S. Insights into the removal of organic contaminants by calcium sulfite activation with Fe (III): Performance, kinetics, and mechanism. Water Res. 2022, 221, 118792. [Google Scholar] [CrossRef] [PubMed]
- Kokina, K.; Mezule, L.; Gruskevica, K.; Neilands, R.; Golovko, K.; Juhna, T. Impact of rapid pH changes on activated sludge process. Appl. Sci. 2022, 12, 5754. [Google Scholar] [CrossRef]
- Zerva, I.; Remmas, N.; Kagalou, I.; Melidis, P.; Ariantsi, M.; Sylaois, G.; Ntougias, S. Effect of chlorination on microbiological quality of effluent of a full-scale wastewater treatment plant. Life 2021, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Yoo, J.-S.; Kim, S.; Lee, H.J.; Ahn, K.-H.; Kim, I.S. Relationship between the electric conductivity and phosphorus concentration variations in an enhanced biological nutrient removal process. Water Sci. Technol. 2007, 2, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Mathur, L.; Kim, I.-H.; Bhardwaj, A.; Singh, B.; Park, J.-Y.; Song, S.-J. Structural and electrical properties of novel phosphate based composite based composite electrolyte for low-temperature fuel cell. Compos. Part B Eng. 2020, 202, 18–24. [Google Scholar] [CrossRef]
- Yadu, A.; Sahariah, B.P.; Anandkumar, J. Influence of COD/ammonia ratio on simultaneous removal of NH4+-N and COD in surface water using moving bed batch reactor. J. Water Process Eng. 2018, 22, 66–72. [Google Scholar] [CrossRef]
- Venâncio, L.V.L.; Farinha, A.S.F.; Gomes, M.T.S.R. Analysing sulphate and chloride in mineral drinking water by flow injection analysis with a single acoustic wave sensor. Talanta 2018, 189, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.C.; Chan, S.-K.; Shim, H. Effect of chloride on biological nutrient removal from wastewater. J. Appl. Sci. Environ. Sanit. 2008, 2, 85–92. [Google Scholar]
- Bekink, M.J.; Nozaic, D.J. Assessment of a chlorine dioxide proprietary product for water and wastewater disinfection. Water SA 2013, 39, 157–165. [Google Scholar] [CrossRef][Green Version]
- Bega, S. Invasive Water Hyacinth Explodes on Hartbeespoort Dam. Mail & Guardian. 27 January 2023. Available online: https://mg.co.za/environment/2023-01-27-invasive-water-hyacinth-explodes-on-hartbeespoort-dam/?amp (accessed on 16 August 2023).
- Raut, S.B.; Anaokar, G.S.; Dharnaik, A.S. Determination of wastewater quality index of municipal wastewater treatment plant using fuzzy rule base. Eur. J. Adv. Eng. Technol. 2017, 4, 733–738. Available online: https://ejaet.com/PDF/4-10/EJAET-4-10-733-738.pdf (accessed on 30 May 2023).
- Jamshidzadeh, Z.; Barzi, M.T. Wastewater quality index (WWQI) as an assessment tool of treated wastewater quality for agriculture: A case of North Wastewater Treatment Plant effluent of Isfahan. Environ. Sci. Pollut. Res. 2020, 27, 7366–7378. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S. Handbook of Water Purity and Quality; Academic Press: New York, NY, USA, 2009. [Google Scholar]
- Water Environment Federation. Operation of Municipal Wastewater Treatment Plants. Volume 2: Liquid Processes; McGraw-Hill Education: New York, NY, USA, 2008. [Google Scholar]
- Gowd, S.C.; Ramakrishna, S.; Rajendran, K. Wastewater in India: An untapped and under-tapped resource for nutrient recovery towards attaining a sustainable circular economy. Chemosphere 2022, 291, 132753. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, S.; Olaniran, A.O. Treated wastewater effluent as a sources of microbial pollution of surface water resources. Int. J. Environ. Res. Public Health 2014, 11, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Al-Qodah, Z.; Al-Zoubi, H.; Hudaib, B.; Omar, W.; Soleimani, M.; Abu-Romman, S.; Frontistis, Z. Sustainable vs. Conventional Approach for Oil Wastewater Mangement: A Review of the State of the Art. Water 2022, 14, 1695. [Google Scholar] [CrossRef]


| Wastewater Quality Index Level | Wastewater Quality Rating |
|---|---|
| 0–25 | Excellent |
| 26–50 | Good |
| 51–75 | Regular |
| 76–100 | Bad |
| ≥101 | Very bad |
| Descriptive Analysis and Water Quality Guidelines | Physical Water Quality Variables | Chemical Water Quality Variables | Microbiological Water Quality Variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC (μS/cm) | pH | Temperature (°C) | Free Chlorine (mg/L) | NO3− (mg/L) | −PO4−3 (mg/L) | NH3 (mg/L) | COD (mg/L) | SO42− (mg/L) | Cl− (mg/L) | TC (MPN/100 mL) | FC (MPN/100 mL) | E. coli (MPN/100 mL) | |
| EPA limits | 75 | 6–9 | <30 | N/A | 50 | N/A | 1 | 250 | N/A | N/A | 400 | N/A | 10 |
| DWA limits | 70–150 | 5.5–9.5 | N/A | 0.25 | 15 | 10 | 3 | 75 | N/A | N/A | N/A | 1000 | N/A |
| Burgersfort WRRF limits | <150 | 5.5–7.5 | N/A | 0.30 | 15 | 10 | 1 | 30 | N/A | N/A | 1000 | 1000 | 1000 |
| Minimum | 70.4 | 6.4 | 14.0 | 0.1 | 0.1 | 69.9 | 0.1 | 149 | 158 | 1.5 | 1 | 1 | 1 |
| Maximum | 219.5 | 8.0 | 27.1 | 2.13 | 33.4 | 159 | 48.5 | 968 | 767 | 398.1 | 2.5 × 106 | 2.4 × 106 | 2.4 × 106 |
| Mean | 121.6 | 7.2 | 21.5 | 1.00 | 1.5 | 3.9 | 13.5 | 250.2 | 51.6 | 85.6 | 1.8 × 105 | 1.5 × 105 | 1.4 × 105 |
| Standard deviation | 24.2 | 0.3 | 2.7 | 0.4 | 4.7 | 6.3 | 12.9 | 167.7 | 65.2 | 55.6 | 3.0 × 105 | 2.3 × 105 | 2.2 × 105 |
| Kurtosis | 1.8 | 0.7 | 0.3 | 0.4 | 19.4 | 73.8 | −0.6 | 2.1 | 92.9 | 8.8 | 3.0 × 105 | 72.3 | 75.6 |
| Skewness | 0.8 | −0.3 | −0.7 | −0.2 | 4.2 | 7.5 | 0.6 | 1.4 | 8.6 | 2.1 | 6.4 | 7.2 | 7.5 |
| Rotated Component Matrix (a) | ||||
|---|---|---|---|---|
| Component Factor | ||||
| Variables | 1 | 2 | 3 | 4 |
| NH3 | 0.5809 | −0.3100 | 0.0658 | −0.2613 |
| COD | −0.2444 | 0.8044 | −0.0863 | −0.26969 |
| EC | 0.1169 | 0.8319 | 0.1706 | 0.203424 |
| NO3− | −0.1884 | −0.0671 | −0.0032 | 0.832893 |
| −PO4−3 | −0.1258 | −0.0296 | 0.0073 | −0.38417 |
| pH | 0.7426 | 0.0405 | 0.0130 | 0.370187 |
| SO42− | 0.0167 | −0.1387 | 0.8218 | −0.01921 |
| Temperature | −0.7109 | −0.0217 | −0.0260 | 0.0155 |
| Cl− | 0.0511 | 0.2272 | 0.7408 | 0.002734 |
| Rotated Component Matrix (a) | ||
|---|---|---|
| Component Factor | ||
| Variables | 1 | 2 |
| Free chlorine | 0 | 1 |
| TC | 0.851 | 0.031 |
| FC | 0.964 | −0.006 |
| E. coli | 0.967 | −0.029 |
| Spearman’s Rho Nonparametric Analysis | Physical Water Quality Variables | Chemical Water Quality Variables | Microbiological Water Quality Variables | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | pH | Temp | NH3 | COD | Cl− | NO3− | SO42− | −PO4−3 | Free Chlorine | TC | FC | E. coli | ||
| EC | CC | 1 | 0.09 | −0.05 | −0.09 | 0.33 | 0.35 | 0.14 | 0.09 | 0.21 | - | - | - | - |
| p value | - | 0.27 | 0.50 | 0.23 | 4.7 × 10−5 | 5.1 × 10−6 | 0.10 | 0.22 | 0.006 | - | - | - | - | |
| pH | CC | 0.09 | 1 | −0.21 | 0.21 | −0.18 | 0.04 | 0.16 | 0.08 | −0.06 | - | - | - | - |
| p value | 0.27 | - | 0.006 | 0.007 | 0.03 | 0.65 | 0.06 | 0.34 | 0.45 | - | - | - | - | |
| Tempera-ture | CC | −0.05 | −0.21 | 1 | −0.17 | 0.16 | −0.07 | −0.07 | −0.07 | 0.02 | - | - | - | - |
| p value | 0.50 | 0.006 | - | 0.04 | 0.05 | 0.39 | 0.36 | 0.40 | 0.82 | - | - | - | - | |
| NH3 | CC | −0.09 | 0.21 | −0.17 | 1 | −0.27 | 0.13 | 0.09 | 0.15 | 0.16 | - | - | - | - |
| p value | 0.23 | 0.007 | 0.04 | - | 0.001 | 0.111 | 0.27 | 0.05 | 0.04 | - | - | - | - | |
| COD | CC | 0.33 | −0.18 | 0.16 | −0.27 | 1 | 0.07 | −0.01 | −0.09 | 0.08 | - | - | - | - |
| p value | 4.7 × 10−5 | 0.03 | 0.05 | 0.001 | - | 0.34 | 0.87 | 0.28 | 0.34 | - | - | - | - | |
| CC | 0.35 | 0.04 | −0.07 | 0.13 | 0.07 | 1 | 0.34 | 0.38 | 0.15 | - | - | - | - | |
| p value | 5.1 × 10−6 | 0.65 | 0.39 | 0.111 | 0.34 | - | 3.1 × 105 | 4.3 × 107 | 0.06 | - | - | - | - | |
| − | CC | 0.14 | 0.16 | −0.07 | 0.09 | −0.01 | 0.34 | 1 | 0.18 | 0.19 | - | - | - | - |
| p value | 0.10 | 0.06 | 0.36 | 0.27 | 0.87 | 3.1 × 105 | - | 0.03 | 0.02 | - | - | - | - | |
| 2− | CC | 0.18 | 0.08 | −0.07 | 0.15 | −0.09 | 0.38 | 0.18 | 1 | 0.02 | - | - | - | - |
| p value | 0.03 | 0.34 | 0.40 | 0.05 | 0.28 | 4.3 × 107 | 0.03 | - | 0.77 | - | - | - | - | |
| −3 | CC | 0.21 | −0.06 | 0.02 | 0.16 | 0.08 | 0.15 | 0.19 | 0.02 | 1 | - | - | - | - |
| p value | 0.006 | 0.45 | 0.82 | 0.04 | 0.34 | 0.06 | 0.02 | 0.77 | - | - | - | - | - | |
| Free chlorine | CC | - | - | - | - | - | - | - | - | - | 1 | 0.07 | 0.07 | 0.03 |
| p value | - | - | - | - | - | - | - | - | - | - | 0.42 | 0.39 | 0.67 | |
| TC | CC | - | - | - | - | - | - | - | - | - | 0.07 | 1 | 0.87 | 0.87 |
| p value | - | - | - | - | - | - | - | - | - | 0.42 | - | 4 × 10−46 | 2 × 10−46 | |
| FC | CC | - | - | - | - | - | - | - | - | - | 0.07 | 0.87 | 1 | 0.95 |
| p value | - | - | - | - | - | - | - | - | - | 0.39 | 4 × 10−46 | - | 4 × 10−75 | |
| E. coli | CC | - | - | - | - | - | - | - | - | - | 0.04 | 0.88 | 0.95 | 1 |
| p value | - | - | - | - | - | - | - | - | - | 0.67 | 2 × 10−45 | 4 × 10−75 | - | |
| Variables | Wastewater Quality Level | Wastewater Quality Index Rating |
|---|---|---|
| EC | 43.2 | Good |
| pH | 70 | Regular |
| Temperature | 42.9 | Good |
| NH3 | 260 | Very bad |
| COD | 4880 | Very bad |
| NO3− | 4.3 | Excellent |
| −PO4−3 | 10 | Excellent |
| Free chlorine | 5.7 | Excellent |
| TC | 35.505 | Very bad |
| FC | 29.900 | Very bad |
| E. coli | 28.100 | Very bad |
| Variable | Principal Component Analysis Significant Loading | WWQI Rating | Resource Recovery Technology | Recoverable Resource |
|---|---|---|---|---|
| EC | >0.3 | Good | Electroplating—Ion exchange | Metals |
| pH | >0.3 | Excellent | Electroplating—Photocatalysis | Metals |
| NH3 | >0.3 | Very bad | Air stripping and electrodialysis | Ammonia gas |
| COD | >0.3 | Very bad | Sludge digestion Sludge incineration Polyhydroxyalkanoates extraction Air stripping and electrodialysis | Sludge Methane gas Carbon monoxide gas Bioplastics Biofuel |
| NO3− | >0.3 | Excellent | Chemical precipitation—Struvite precipitation | Nitrogen gas and phosphorus |
| SO42− | >0.3 | N/A | Electroplating– Ion exchange then air stripping | Metals and sulphur |
| Cl− | >0.3 | N/A | Electroplating—Photocatalysis/ Ion exchange | Chlorides/Salts Metals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maremane, S.; Belle, G.; Oberholster, P. Assessment of Effluent Wastewater Quality and the Application of an Integrated Wastewater Resource Recovery Model: The Burgersfort Wastewater Resource Recovery Case Study. Water 2024, 16, 608. https://doi.org/10.3390/w16040608
Maremane S, Belle G, Oberholster P. Assessment of Effluent Wastewater Quality and the Application of an Integrated Wastewater Resource Recovery Model: The Burgersfort Wastewater Resource Recovery Case Study. Water. 2024; 16(4):608. https://doi.org/10.3390/w16040608
Chicago/Turabian StyleMaremane, Sekato, Gladys Belle, and Paul Oberholster. 2024. "Assessment of Effluent Wastewater Quality and the Application of an Integrated Wastewater Resource Recovery Model: The Burgersfort Wastewater Resource Recovery Case Study" Water 16, no. 4: 608. https://doi.org/10.3390/w16040608
APA StyleMaremane, S., Belle, G., & Oberholster, P. (2024). Assessment of Effluent Wastewater Quality and the Application of an Integrated Wastewater Resource Recovery Model: The Burgersfort Wastewater Resource Recovery Case Study. Water, 16(4), 608. https://doi.org/10.3390/w16040608









