Effect and Mechanism of Bicarbonate Ion on Lead Absorption in Pontederia crassipes from Karst Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Data Analysis
3. Results and Discussion
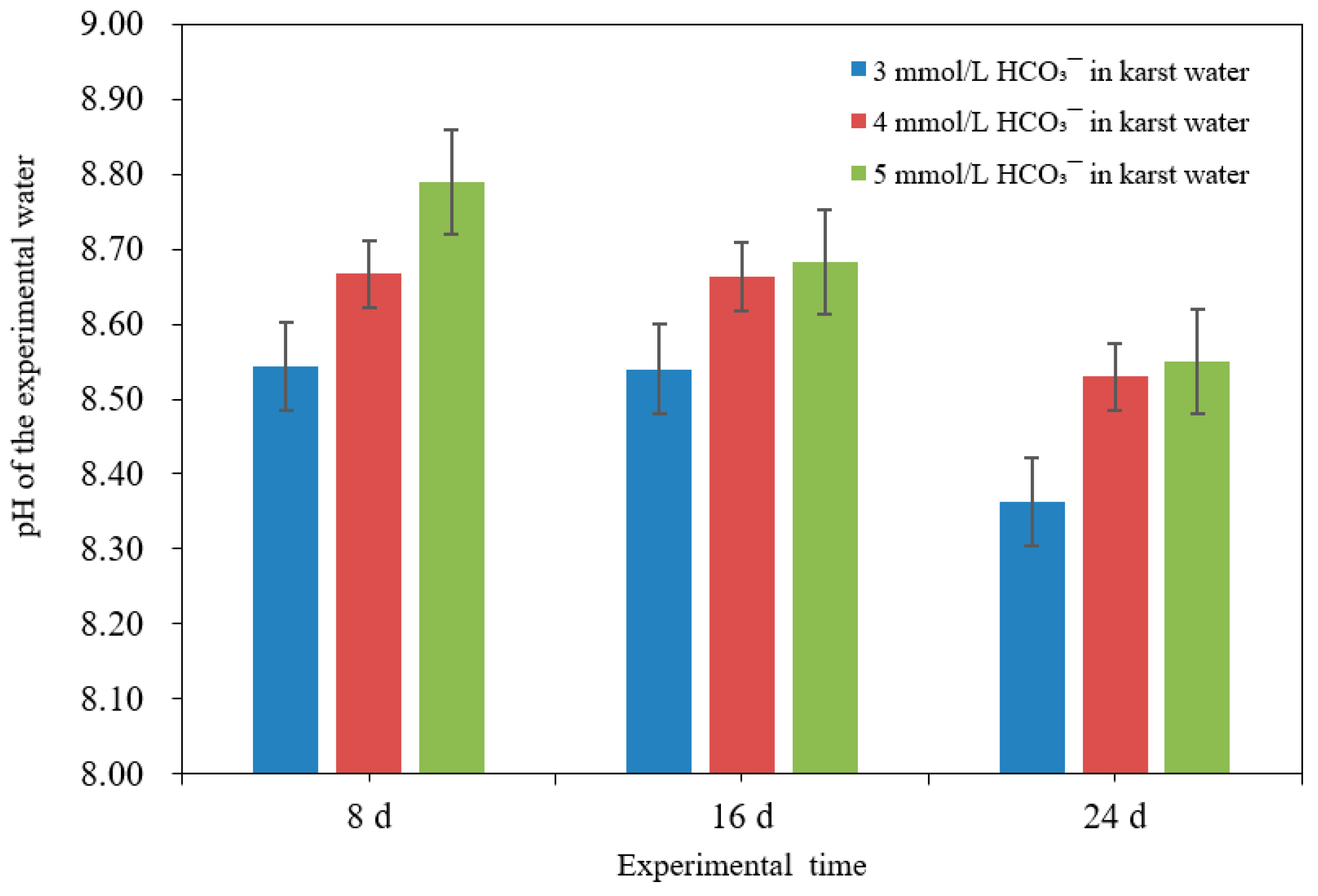
3.1. Effect of HCO3− Molarity on Pb Removal in Karst Water
3.2. Effect of HCO3− Molarity on Pb Accumulation in Pontederia crassipes
3.3. Ion Exchange Analysis
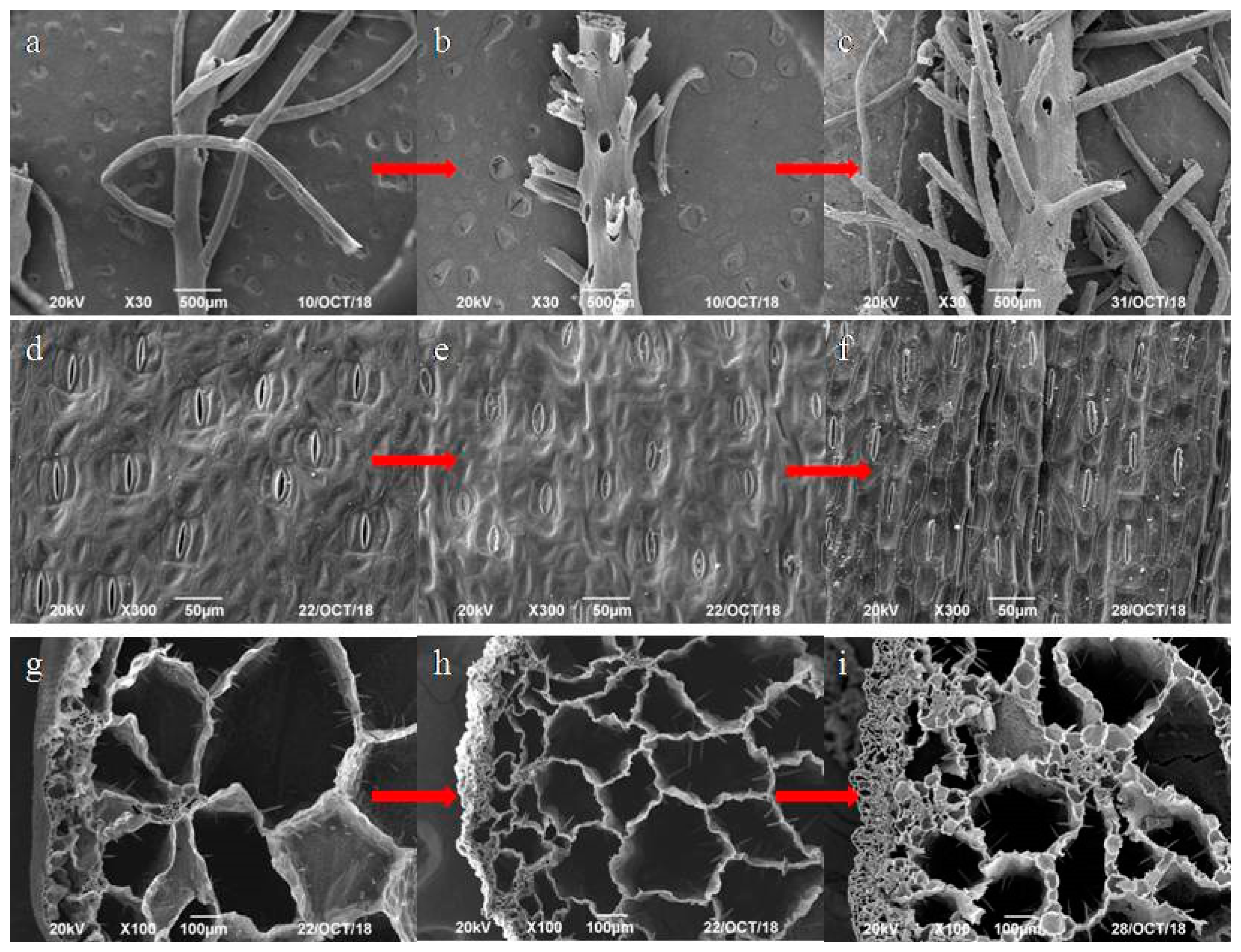
3.4. Morphology Analysis
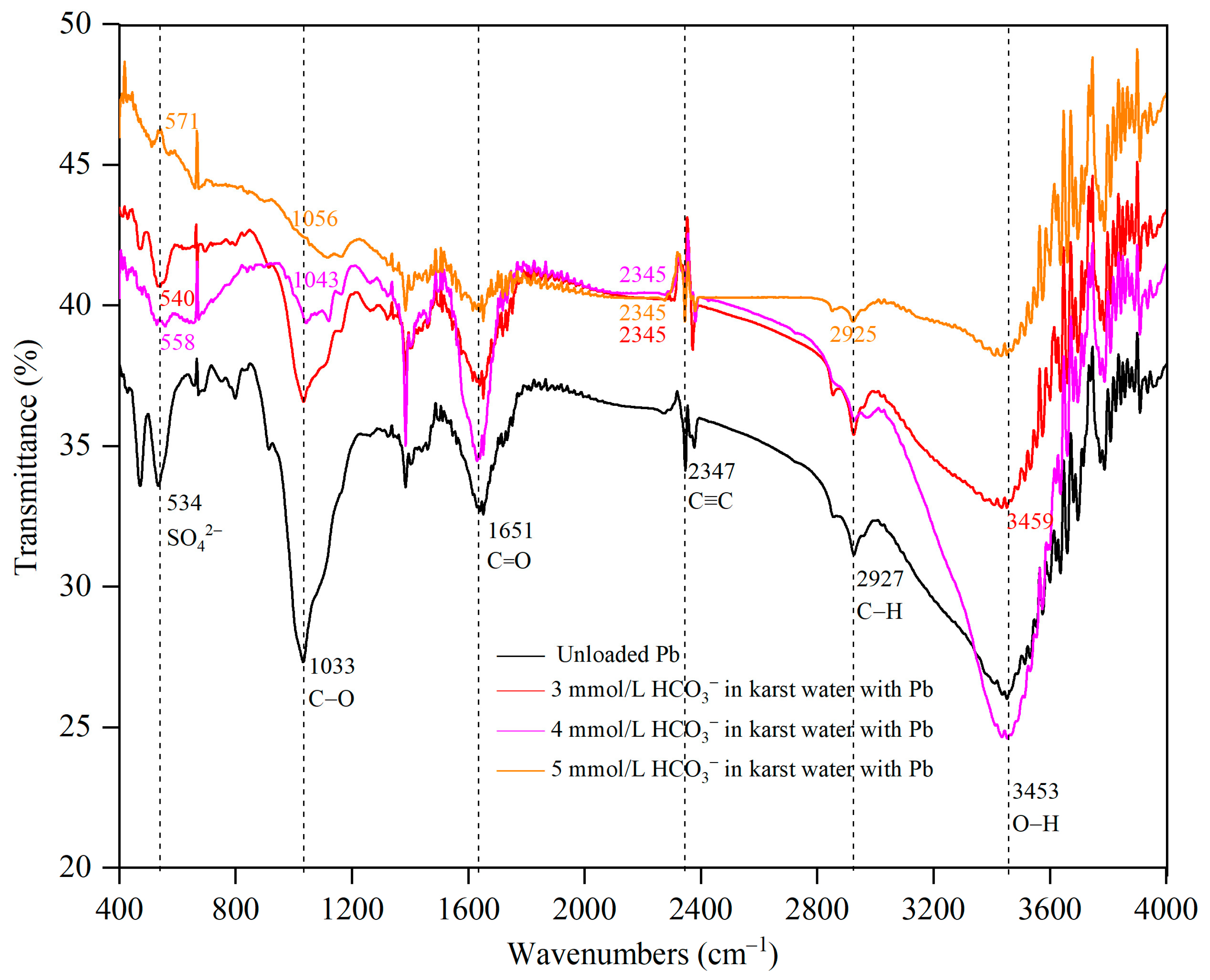
3.5. Functional Group Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Zhang, H.; Long, J.; Fan, J.; Wu, P.; Chen, M.; Liu, P.; Li, T. Migration mechanism of pollutants in karst groundwater system of tailings impoundment and management control effect analysis: Gold mine tailing impoundment case. J. Clean. Prod. 2022, 350, 131434. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Jiang, Z.-C.; Xu, G.-L.; Qin, X.-Q.; Huang, Q.-B.; Zhang, L.-K. Major Ionic Characteristics and Controlling Factors of Karst Groundwater at Xiangshui, Chongzuo. Environ. Sci. 2019, 40, 2143–2151. [Google Scholar]
- Zhan, Z.-J.; Chen, F.; Yang, P.-H.; Ren, J.; Zhang, H.-Y.; Liu, D.-W.; Lan, J.-C.; Zhang, Y. Comparison on the Hydrogeochemical Characteristics of Typical Karst Groundwater System in Southwest China, a Case of Qingmuguan and Laolongdong in Chongqing. Environ. Sci. 2016, 37, 3365–3374. [Google Scholar]
- Ji, M.C.; Zhang, J.Q.; Peng, Y.; Ma, Q.Y. Research on the resistances of several kinds of hydrophyte to Lead in hydroponic condition. Biotechnol. Bull. 2017, 33, 120–125. [Google Scholar]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Sun, Z.G.; Mou, X.J.; Tong, C.; Wang, C.Y.; Xie, Z.L.; Song, H.L.; Sun, W.G.; Lv, Y.C. Spatial variations and bioaccumulation of heavy metals in intertidal zone of the Yellow River estuary, China. CATENA 2015, 126, 43–52. [Google Scholar] [CrossRef]
- Saxena, G.; Bharagava, R.N. Organic and inorganic pollutants in industrial wastes, their ecotoxicological effects, health hazards and bioremediation approaches. In Environmental Pollutants and Their Bioremediation Approaches, 1st ed.; Bharagava, R.N., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 23–56. [Google Scholar]
- Han, L.H.; Zhang, Y.P.; Di, X.M.; Huang, A.L.; Liu, C. Anatomical characteristics and ecological adaptability of vegetative organs of three invasive plants. Jiangsu Agric. Sci. 2018, 46, 92–95. [Google Scholar]
- Gui, Z.; Shan, Y.; Liu, C. Flow velocity evolution through a floating rigid cylinder array under unidirectional flow. J. Hydrol. 2023, 617, 128915–128929. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Li, B.-K. Removal of pharmaceuticals and personal care products by Eichhornia crassipe and Pistia stratiotes. J. Taiwan Inst. Chem. Eng. 2016, 58, 318–323. [Google Scholar] [CrossRef]
- Yi, Z.J.; Yao, J.; Chen, H.L.; Wang, F.; Yuan, Z.M.; Liu, X. Uranium biosorption from aqueous solution onto Pontederia crassipes. J. Environ. Radioact. 2016, 154, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mahamadi, C.; Nharingo, T. Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Pontederia crassipes in binary and ternary systems. Bioresour. Technol. 2010, 101, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Tel-Or, E.; Forni, C. Phytoremediation of hazardous toxic metals and organics by photosynthetic aquatic systems. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2011, 145, 224–235. [Google Scholar] [CrossRef]
- Feng, W.; Xiao, K.; Zhou, W.B.; Zhu, D.W.; Zhou, Y.Y.; Yuan, Y.N.; Xiao, N.D.; Wan, A.Q.; Hua, Y.M.; Zhao, J.W. Analysis of utilization technologies for Pontederia crassipes biomass harvested after restoration of wastewater. Bioresour. Technol. 2017, 223, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Suryandari, M.K.; Hariati, A.M.; Mahmudi, M. Removal of Lead from water by Pontederia crassipes (Mart.) Solms. Imp. J. Interdiscip. Res. 2017, 3, 2387–2392. [Google Scholar]
- Jiang, H.S.; Liao, Z.Y.; Li, W. Photosynthetic inorganic carbon utilization strategies and their ecological adaptability in aquatic plants. Plant Sci. J. 2023, 41, 847–856. [Google Scholar]
- Maberly, S.C.; Gontero, B. Ecological imperatives for aquatic CO2-concentrating mechanisms. J. Exp. Bot. 2017, 68, 3797–3814. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Exogenous Inorganic Carbon Sources in Plant Photosynthesis. Biol. Rev. 1970, 45, 167–220. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J. CO2 concentrating mechanisms and environmental change. Aquat. Bot. 2014, 118, 24–37. [Google Scholar] [CrossRef]
- Hu, G.; Wang, P.; Cao, J.H.; Zhang, C.L.; Mo, B.Q. Utilization of dissolved inorganic carbon by Hydrilla verticillate in karst water and its growth of the response. Environ. Sci. Technol. 2016, 35, 349–356. [Google Scholar]
- Liu, Z.; Dreybrodt, W.; Wang, H. A new direction in effective accounting for the atmospheric CO2 budget: Considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms. Earth-Sci. Rev. 2010, 99, 162–172. [Google Scholar] [CrossRef]
- Sun, H.L.; Han, C.H.; Liu, Z.H.; Wei, Y.; Ma, S.; Bao, Q.; Zhang, Y.; Yan, H. Nutrient limitations on primary productivity and phosphorus removal by biological carbon pumps in dammed karst rivers: Implications for eutrophication control. J. Hydrol. 2022, 607, 127480. [Google Scholar] [CrossRef]
- Zeng, Z.Y.; Yan, H.; Sun, H.L.; Liu, Z.H. Theoretical calculation of aquatic photosynthesis contribution ratio and the controlling factors of diurnal vatiations of hydrochemistry and δ~(13) C_(DIC) in the outlets and inlets of travertine pools at Baishuitai, Yunnan, China. Carsologica Sin. 2016, 35, 605–613. [Google Scholar]
- Xue, P.Y.; Yan, C.Z.; Cao, Y.L.; Wei, Q.S. Toxic effects of copper and arsenic and their compound pollution on Hydrilla verticillate (L.f.) Royle. Res. Environ. Sci. 2011, 24, 1052–1058. [Google Scholar]
- Saha, P.; Shinde, O.; Sarkar, S. Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytoremediat. 2017, 19, 87–96. [Google Scholar] [CrossRef]
- Zhang, L.K.; Qin, X.Q.; Huang, Q.B.; Liu, P.Y.; Shan, X.J. Aquatic plants bioremediation to groundwater contaminated by mines in karst areas. Carsologica Sin. 2017, 36, 743–750. [Google Scholar]
- Li, Q. Research on Removal Mechanisms of Four Kinds of Heavy Metals in Water by Long-Root Pontederia crassipes and Its Recycling; Kunming University Science and Technology: Kunming, China, 2015. [Google Scholar]
- Wang, P. Effects of Typical Aquatic Plants on the Stability of Inorganic Carbon in Karst Aquatic Ecosystem; China University of Geosciences: Beijing, China, 2016. [Google Scholar]
- GB/T 5750.6-2023; The National Standard of the People’s Republic of China: Standard Examination Methods for Drinking Water, Part 6, Metal and Metalloid Indices. State Administration for Market Regulation, Standardization Administration of China: Beijing, China, 2023.
- GB/T 5009.268-2016; The National Standard of the People’s Republic of China: National Food Safety Standard, Determination of Multiple Elements in Foods. National Health and Family Planning Commission, People’s Republic of China, State Food and Drug Administration: Beijing, China, 2016.
- Zhou, J.M.; Jiang, Z.C.; Xu, G.L.; Qin, X.Q.; Huang, Q.B.; Zhang, L.K. Effects and Mechanisms of Calcium Ion Addition on Lead Removal from Water by Pontederia crassipes. Int. J. Environ. Res. Public Health 2020, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, B.; Lin, P.; Zhou, J.L.; Zhan, J.H.; Shen, Q.Y.; Pan, X.J. Adsorption of heavy metal from aqueous solution by dehydrated root powder of Long-root Pontederia crassipes. Int. J. Phytorem. 2014, 18, 103–109. [Google Scholar] [CrossRef]
- Chen, L.; Li, C.G.; Li, F.M.; Zhong, Y.X.; Hu, H.Y.; Gao, S.Q.; Zhou, W.D.; Sun, Z.S. Review on water purification ability of aquatic ecological restoration plants. Environ. Pollut. Control 2022, 44, 1079–1084. [Google Scholar]
- Cheng, B.B.; Chen, S.Y.; Yue, L.R. Effects of NaHCO3 stress on morphological indices and photosynthetic parameters of purple root Eichhornia crassipes. Guihaia 2020, 40, 1781–1789. [Google Scholar]
- Chen, J.S.; Deng, B.S.; Tao, S.; Cheng, Y.Q. Environmental Geochemistry; The Ocean Publishing Company: Beijing, China, 1990. [Google Scholar]
- Guan, B.T.H.; Mohamat-Yusuff, F.; Halimoon, N.; Yong, C.S.Y. Uptake of Mn and Cd by wild Water Spinach and their bioaccumulation and translocation factors. Environ. Asia 2017, 10, 44–51. [Google Scholar]
- Baker, A.J.M. Accumulators and excluders strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Landberg, T.; Greger, T. Difference in uptake and tolerance to heavy metal in Salix from unpolluted and polluted areas. Appl. Geochem. 1996, 11, 175–180. [Google Scholar] [CrossRef]
- Sarital, S.; Rohit, S.; Shraddha, S. Comparative studies on accumulation of Cr from metal solution and tannery effluent under repeated metal exposure by aquatic plants: Its toxic effects. Environ. Monit. Assess. 2002, 80, 17–31. [Google Scholar]
- Woldemichael, D.; Zewge, F.; Leta, S. Potential of water Hyacinth (Eichhornia crassipes (Mart.) Solms) for the removal of chromium from tannery effluent in constructed pond system. SINET: Ethiop. J. Sci. 2011, 34, 49–62. [Google Scholar]
- Li, X.S.; Liu, S.L.; Na, Z.Y.; Lu, D.N.; Liu, Z. Adsorption, concentration, and recovery of aqueous heavy metal ions with the root powder of Pontederia crassipes. Ecol. Eng. 2013, 60, 160–166. [Google Scholar] [CrossRef]
- Zheng, J.C. The Performance and Mechanism of Removal of Heavy Metals from Water by Water Hyacinth Roots as a Biosorbent Material; University of Science and Technology of China: Hefei, China, 2010. [Google Scholar]
- Brunet, J.; Repellin, A.; Varrault, G.; Terryn, N.; Zuily-Fodil, Y. Lead accumulation in the roots of grass pea (Lathyrus sativus L.): A novel plant for phytoremediation systems? Comptes Rendus Biol. 2008, 331, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Malar, S.; Vikram, S.S.; Favas, P.J.C.; Perumal, V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Pontederia crassipes (Mart.)]. Bot. Stud. 2014, 55, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Wang, B.; Guo, X.-J.; Zou, C.-W.; Tan, X.-D. Investigating adsorption performance of heavy metals onto humic acid from sludge using Fourier-transform infrared combined with two-dimensional correlation spectroscopy. Environ. Sci. Pollut. Res. 2019, 26, 9842–9850. [Google Scholar] [CrossRef]
- Lim, S.F.; Zheng, Y.M.; Zou, S.W.; Chen, J.P. Characterization of copper adsorption onto an alginate encapsulated magnetic sorbent by a combined FT IR, XPS and mathematical modeling study. Environ. Sci. Technol. 2008, 42, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, C.; Zhu, Q.; Huang, T.; Cai, Y.; Li, N.; Liu, J.; Tan, X. Characterization of dissolved organic matter from biogas residue composting using spectroscopic techniques. Waste Manag. 2018, 78, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.B.; Xin, C.H.; Zhao, H.; Zhang, Q.; Chi, J.L.; Guo, J.B. The role of DNA methylation in plant response to heavy metal stress. Seed 2016, 25, 43–46. [Google Scholar]



| Molarities of HCO3− in Karst Water (mmol/L) | Parts of Plant | Ca | Mg | K | Na | Fe | Pb | Zn | Cd | Mn |
|---|---|---|---|---|---|---|---|---|---|---|
| Root | 13,401 ± 53 ij | 1157 ± 10 g | 3021 ± 25 i | 690 ± 5.2 h | 15,036 ± 102 a | 1771 ± 11 a | 212 ± 1.1 a | 0.45 ± 0.0025 a | 2320 ± 15 a | |
| 3 | Leaf and stem | 17,800 ± 70 e | 1251 ± 9.0 e | 15,301 ± 60 d | 663 ± 7.5 i | 2895 ± 21 f | 177 ± 1.0 g | 78 ± 0.52 g | 0.093 ± 0.00053 h | 302 ± 2.1 i |
| Whole plant | 16,700 ± 65 f | 1228 ± 9.2 ef | 12,231 ± 51 e | 670 ± 6.9 i | 5930 ± 41 e | 576 ± 3.5 d | 111 ± 0.66 d | 0.18 ± 0.0010 e | 806 ± 5.3 e | |
| Root | 13,514 ± 62 i | 793 ± 8.0 j | 1849 ± 23 j | 996 ± 6.3 f | 8158 ± 76 c | 1536 ± 8.6 b | 180 ± 1.5 b | 0.29 ± 0.0012 c | 1965 ± 12 b | |
| 4 | Leaf and stem | 21,000 ± 75 a | 1024 ± 7.0 h | 8137 ± 25 g | 1051 ± 6.8 e | 476 ± 3 j | 159 ± 1.2 h | 50 ± 0.43 j | 0.07 ± 0.0062 i | 250 ± 1.3 j |
| Whole plant | 19,128 ± 72 c | 966 ± 7.2 i | 6565 ± 24 h | 1037 ± 6.7 e | 2396 ± 21 h | 503 ± 3.0 e | 82.4 ± 0.70 f | 0.13 ± 0.00076 f | 679 ± 4.0 f | |
| Root | 14,633 ± 58 h | 710 ± 9.0 k | 1577 ± 20 k | 1319 ± 5.7 c | 7474 ± 56 d | 843 ± 6.4 c | 152 ± 1.3 c | 0.25 ± 0.0011 d | 1231 ± 12 d | |
| 5 | Leaf and stem | 19,400 ± 73 b | 1390 ± 8.0 d | 15,488 ± 86 d | 2285 ± 6.2 a | 493 ± 3.2 j | 123 ± 1.1 i | 43 ± 0.36 k | 0.07 ± 0.0065 i | 287 ± 1.8 i |
| Whole plant | 18,208 ± 69 d | 1220 ± 8.2 f | 12,010 ± 69 f | 2044 ± 6.1 b | 2238 ± 16.4 i | 303 ± 2.4 f | 70 ± 0.60 h | 0.12 ± 0.00076 g | 523 ± 4.3 h | |
| Root | 6600 ± 69 k | 4200 ± 10 a | 17,400 ± 28 c | 2300 ± 5.4 a | 9442 ± 78 b | 7.7 ± 0.051 j | 98 ± 0.83 e | 0.36 ± 0.0025 b | 1817 ± 16 c | |
| Unloaded | Leaf and stem | 15,500 ± 65 g | 1700 ± 8.0 c | 32,800 ± 131 a | 830 ± 6.1 g | 279 ± 1.6 k | 0.73 ± 0.0062 j | 39 ± 0.22 l | 0.037 ± 0.00012 j | 192 ± 1.2 k |
| Whole plant | 13,275 ± 66 ij | 2325 ± 8.5 b | 28,950 ± 105 b | 1198 ± 5.9 d | 2570 ± 21 g | 2.5 ± 0.017 j | 54 ± 0.37 i | 0.12 ± 0.00063 g | 598 ± 4.9 g |
| Molarities of HCO3− in Karst Water (mmol/L) | Parts of Plant | Bioconcentration Amount (BCA) (mg/kg) | Bioconcentration Factor (BCF) | Translocation Factor (TF) |
|---|---|---|---|---|
| 3 | Root | 1763 ± 15 a | 30,360 ± 226 c | \ |
| Leaf and stem | 177 ± 1.2 g | 3039 ± 24 i | \ | |
| Whole plant | 573 ± 4.6 d | 9866 ± 74 f | 0.100 ± 0.00076 c | |
| 4 | Root | 1528 ± 15 b | 47,497 ± 276 a | \ |
| Leaf and stem | 158 ± 1.4 g | 4922 ± 32 h | \ | |
| Whole plant | 501 ± 4.8 e | 15,564 ± 93 d | 0.104 ± 0.00053 b | |
| 5 | Root | 835 ± 7.5 c | 41,438 ± 246 b | \ |
| Leaf and stem | 122 ± 1.1 h | 6031 ± 38 g | \ | |
| Whole plant | 301 ± 2.7 f | 14,901 ± 90 e | 0.146 ± 0.00056 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Jiang, Z.; Qin, X.; Zhang, L. Effect and Mechanism of Bicarbonate Ion on Lead Absorption in Pontederia crassipes from Karst Water. Water 2024, 16, 529. https://doi.org/10.3390/w16040529
Zhou J, Jiang Z, Qin X, Zhang L. Effect and Mechanism of Bicarbonate Ion on Lead Absorption in Pontederia crassipes from Karst Water. Water. 2024; 16(4):529. https://doi.org/10.3390/w16040529
Chicago/Turabian StyleZhou, Jinmei, Zhongcheng Jiang, Xiaoqun Qin, and Liankai Zhang. 2024. "Effect and Mechanism of Bicarbonate Ion on Lead Absorption in Pontederia crassipes from Karst Water" Water 16, no. 4: 529. https://doi.org/10.3390/w16040529
APA StyleZhou, J., Jiang, Z., Qin, X., & Zhang, L. (2024). Effect and Mechanism of Bicarbonate Ion on Lead Absorption in Pontederia crassipes from Karst Water. Water, 16(4), 529. https://doi.org/10.3390/w16040529






