Abstract
Bicarbonate ions (HCO3−) are abundant in karst water with poor lead (Pb) utilization and biodegradation. This study aimed to investigate the mechanism of HCO3− on the Pb removal efficiency and uptake ability of Pontederia crassipes (a widespread hydrophyte in the karst area) from karst water. The Pb concentration, Pontederia crassipes morphology, and functional group were detected. As the HCO3− molarity in karst water increased (3, 4, and 5 mmol/L), the removal of Pb increased (85.31%, 93.28%, and 95.16%), whereas the bioconcentration amount of Pb decreased (573, 501, and 301 mg/kg), mainly due to the insoluble PbCO3 and Pb (OH)2. The Pb bioconcentration factor was the highest (15,564) at 4 mmol/L HCO3− due to the maximum strength of cation exchange and cell wall protein C=O. High HCO3− molarities changed the variety of positive ions of cation exchange (HCO3− ≤ 4 mmol/L: Na, K, and Mg; HCO3− > 4 mmol/L: Mg and K), and relieved the breaking of roots, stomatal closure, and vascular system shrinking. Moreover, high HCO3− molarities diminished the C≡C oxidation, enlarged the displacement of SO42− and C-O, and stimulated the methyl transfer reaction and the bonding between -CH3 and Pb.
1. Introduction
Recently, excessive activities of industry, farming, and humankind have resulted in the deterioration of karst water quality in the karst area [1]. The karst area has a complicated topography, widespread carbonate rock formation, and rich karst water. Karst water is slightly alkaline. It contains numerous bicarbonate ions (HCO3−) and calcium ions (Ca2+). HCO3-Ca type is the major hydrochemical type due to the chemical erosion of karst water to carbonate rocks. The aquifer in the karst area belongs to karst aquifer with double spatial structure [2]; thus, karst water is hard to restore after being polluted by toxic and stable heavy metals. Karst water reveals low bioavailability and a biodegradation of heavy metals. Heavy metal pollution in karst water threatens the karst ecological environment and brings harm to people, and this problem is becoming more and more serious. Nowadays, restoring karst water contaminated with heavy metal is pressing and necessary around the world [3].
Lead (Pb), a popular heavy metal pollutant, is a cause for concern in karst water pollution restoration due to its high toxicity, good stability, and difficult degradation [4]. The main pollution source of Pb in karst water is industrial wastewater containing Pb. Pb affects the physiological activities of aquatic plants and damages the organs of human beings after Pb uptake [5,6,7].
Pontederia crassipes is a widespread hydrophyte and has developed a root system for the uptake of nutrients and pollutants [8,9]. Pontederia crassipes can tolerate strong acid and alkaline environments, and rapidly grows in wastewater, where it can accumulate heavy metals [10,11]. Studies have shown that Pontederia crassipes can enrich lots of heavy metals in a short time, and it is one of the most useful aquatic plants for purifying water [12,13,14]. Pontederia crassipes has been used for water Pb restoration, and the effect is remarkable [15].
Bicarbonate ion (HCO3−) is the main form of inorganic carbon in natural water for the photosynthesis of aquatic plants [16,17,18,19]. HCO3− provides a sufficient growth matrix for aquatic plants to promote their growth [20], and the high molarity of HCO3− has an obvious “fertilization effect” on aquatic plants [21]. The molarity of HCO3− in karst water is higher than that in non-karst water, which provides a suitable environment for aquatic plants to grow [22]. The high molarity of HCO3− makes karst water slightly alkaline, which affects the metabolism of aquatic plants [23] and the biogeochemical behavior of heavy metals [10]. The removal of heavy metals in karst water by aquatic plants is higher than that in non-karst water, even if the concentrations of heavy metals in karst water are lower than those in non-karst water [24,25,26]. Zhang et al. [26] reported that HCO3− in karst water may lead to the higher removal of heavy metals by aquatic plants in karst water, whereas the effect and mechanism of HCO3− on the Pb removal efficiency and uptake ability of aquatic plants in karst water is still not clear.
Thus, this study aimed to investigate the effect and mechanism of HCO3− on the Pb removal and uptake ability of Pontederia crassipes in karst water, and performed experiments to study the Pb removal and absorption mechanism of Pontederia crassipes in karst water at different HCO3− molarities. This study reveals how HCO3− regulates Pb removal from karst water using Pontederia crassipes, and lays the foundation for the use of HCO3− in Pb restoration from karst water using Pontederia crassipes. We believe that this study makes an important contribution to the literature because previous research has yet to determine the mechanism of HCO3− on Pb removal from karst water using living Pontederia crassipes plants.
2. Materials and Methods
2.1. Materials
Pontederia crassipes plants that grow in the natural karst water environment were collected from Huixian karst wetland in Guilin, Guangxi, China, where Pontederia crassipes plants are plentiful and widespread. The dirt on the plant surface and the decaying plant parts were cleared at the beginning. The plants were then swilled with running water and distilled water repeatedly in sequence. Finally, the plants were steeped in running water for a week so that the uptake of inorganic carbon from the natural karst water environment could be depleted. Inorganic carbon promotes the growth of Pontederia crassipes and heavy metal absorption by Pontederia crassipes [27,28], which may cause experimental error. The weight of Pontederia crassipes used in the experiments was 150 g, and the mass of the leaf and stem was three times that of the root. The height of Pontederia crassipes was 30 cm. The concentrations of Ca, Mg, K, Na, and Pb in Pontederia crassipes were 13,275, 2325, 28,950, 1198, and 2.46 mg/kg, respectively. The experimental water body was collected from the Huixian Mamian-Shizishan subterranean river outlet. The pH of the experimental karst water was 7.25. The molarity of HCO3− was 3 mmol/L. The concentration of Pb was not detected because it was below the limit of detection (<0.07 μg/L).
2.2. Methods
This study conducted hydroponic experiments at 25 ± 3 °C under natural lighting in the Huixian greenhouse. The experimental boxes were made of transparent polyethylene plastic with the same shape and size. In this study, one hundred and fifty grams of Pontederia crassipes that met the above experimental requirements and eight liters of experimental karst water were put into each experimental box. The concentration of Pb in the experimental karst water was set to 0.5 mg/L. The molarities of HCO3− in the karst water were set to 3, 4, and 5 mmol/L. The concentration of Pb (0.5 mg/L) and the three molarities of HCO3− (3, 4, and 5 mmol/L) were achieved by adding an appropriate amount of Pb(NO3)2 (0.0064 g) and NaHCO3 (0.6721 g and 1.3442 g) to each experimental box after sufficiently dissolving the compounds using distilled water, respectively. The experimental period was twenty-four days. Three replicates and one control were set for each experiment. Deionized water was periodically put into every box to replenish the water the Pontederia crassipes consumed so that the volume of water in each experimental box could remain stable. Figure 1 shows the experimental setup. The Pb concentration in the experimental karst water was detected every eight days using an inductively coupled plasma mass spectrometer (ICP-MS, iCAP Q, Thermo Fisher, Waltham, MA, USA) based on GB/T 5750.6-2023 (The National Standard of the People’s Republic of China: Standard examination methods for drinking water, Part 6, Metal and metalloid indices. State Administration for Market Regulation, Standardization Administration of China: Beijing, China, 2023) [29]. The element content in the root, leaf, and stem of Pontederia crassipes was tested using ICP-MS (iCAP Q, Thermo Fisher, Waltham, MA, USA) after they were cleaned, divided, measured, dried, ground, and sieved, referring to GB/T 5009.268-2016 (The National Standard of the People’s Republic of China: National food safety standard, Determination of multiple elements in foods. National Health and Family Planning Commission, People’s Republic of China, State Food and Drug Administration, Beijing, China, 2016) [30]. The morphological characteristics of the plant root, leaf, and stem were determined using a scanning electron microscope (SEM, JEM-6490 LV, JEOL, Tokyo, Japan) after they were rinsed, separated, freeze-dried, and metal-sprayed. The functional group in Pontederia crassipes that reacted with Pb from karst water with different molarities of HCO3− was detected using a Fourier-transform infrared spectrometer (FTIR, Spectrum TWO, Perkin Elmer, Waltham, MA, USA) with the potassium bromide compression technique [31].

Figure 1.
Experimental setup.
2.3. Data Analysis
The removal rate of Pb(q), the bioconcentration amount of Pb (BCA), the bioconcentration factor (BCF), and the translocation factor (TF) of Pontederia crassipes to Pb were, respectively, calculated using the following Formulas (1)–(4) [31].
Removal rate (q):
q = (C0 − Cw)/C0 × 100%
Bioconcentration amount (BCA):
BCA = CP − CP0
Bioconcentration factor (BCF) of Pontederia crassipes to Pb:
BCF = CP/Cw
Translocation factor (TF) of Pontederia crassipes to Pb:
where C0 is the concentration of Pb in water before the experiment (mg/L); Cw is the Pb concentration in the experimental karst water (mg/kg); CP is the content of Pb in a part (root, leaf, or stem) of Pontederia crassipes (mg/kg); CP0 is the initial Pb content in a part (root, leaf or stem) of Pontederia crassipes (mg/kg); Csl is the Pb content in the stem and leaf of Pontederia crassipes (mg/kg); Cr is the content of Pb in the root of Pontederia crassipes (mg/kg). Cw was detected using ICP-MS (iCAP Q, Thermo Fisher, Waltham, MA, USA) based on GB/T 5750.6-2023 (The National Standard of the People’s Republic of China: Standard examination methods for drinking water, Part 6, Metal and metalloid indices. State Administration for Market Regulation, Standardization Administration of China: Beijing, China, 2023) [29]. CP, Csl, and Cr were tested using ICP-MS (iCAP Q, Thermo Fisher, Waltham, MA, USA) based on GB/T 5009.268-2016 (The National Standard of the People’s Republic of China: National food safety standard, Determination of multiple elements in foods. National Health and Family Planning Commission, People’s Republic of China, State Food and Drug Administration, Beijing, China, 2016) [30].
TF = Csl/Cr
The images of removal, element content distribution, SEM, and FTIR spectra were obtained via Origin 2017 (OriginLab, Northampton, MA, USA). The significant statistical differences between the different experiments were tested via SPSS 19 (IBM, Armonk, NY, USA) with ANOVA (alpha = 0.05). The Tukey test was performed as a post hoc test using SPSS 19 (IBM, Armonk, NY, USA) to identify significant differences between treatments. The statistical correlation was performed using SPSS 19 (IBM, Armonk, NY, USA) with the Spearman rank correlation analysis according to Zhou et al. [31].
3. Results and Discussion
3.1. Effect of HCO3− Molarity on Pb Removal in Karst Water
As shown in Figure 2, the removal of Pb in karst water at different molarities of HCO3− by Pontederia crassipes increased (85.31%, 93.28%, and 95.16%) with the increase in HCO3− molarities (3, 4, and 5 mmol/L). The statistical difference among the removal of Pb in karst water at different HCO3− molarities was significant (p = 0.000 < 0.05). This result showed that the removal of Pb from karst water by Pontederia crassipes was affected by the HCO3− molarity. A high molarity of HCO3− was more conducive to Pb removal by Pontederia crassipes from karst water. The removal of Pb in karst water at 3, 4, and 5 mmol/L HCO3− all increased with the extension of culture time. The first 8 days contributed the most to the removal of Pb, which was 65.19%, 83.81%, and 83.92%, respectively, in karst water at 3, 4, and 5 mmol/L HCO3−. The results obtained in this study suggest that a high molarity of HCO3− improves the removal of Pb in karst water at the beginning of the experiments. Li et al. [32] showed that the optimal pH was 5 for Pb in a single-metal system of water using the dehydrated root powder of long-root Pontederia crassipes, which differed from our results, possibly due to the different properties between dead Pontederia crassipes and the living Pontederia crassipes used in this study. Li [27] reported that the removal process of Pb using living Pontederia crassipes is more complicated than that using powder. Chen et al. [33] reported that pH affects the growth of aquatic plants and the combination of heavy metals with H+ or OH−. HCO3− contributes most to the pH of water and a high molarity of HCO3− makes water slightly alkaline, which provides suitable conditions for Pontederia crassipes to grow and remove Pb from water [23]. This should be examined in future research.
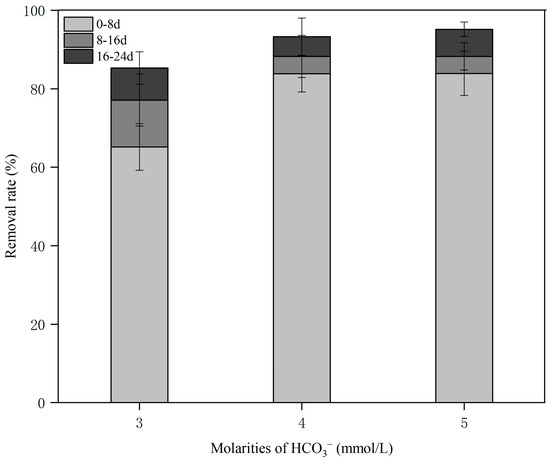
Figure 2.
The lead (Pb) removal in karst water at different molarities of bicarbonate ion (HCO3−) by Pontederia crassipes.
3.2. Effect of HCO3− Molarity on Pb Accumulation in Pontederia crassipes
The contents of elements in different parts of Pontederia crassipes in karst water at different HCO3− molarities are listed in Table 1. Moreover, Table 2 shows the bioconcentration amount of Pb, the bioconcentration factor, and the translocation factor of Pontederia crassipes to Pb. The bioconcentration amount of Pb in Pontederia crassipes from karst water at 3, 4, and 5 mmol/L HCO3− was 573, 501, and 301 mg/kg, respectively. The statistical difference among the bioconcentration amounts of Pb was significant (p = 0.011 < 0.05). This result indicated that the bioconcentration amount of Pb in Pontederia crassipes decreased with the increase of HCO3− molarities in karst water. Moreover, the trend between the bioconcentration amount of Pb in Pontederia crassipes and the removal of Pb in karst water with the change of HCO3− molarity was the opposite. The statistical correlations between bioconcentration amount and the removal of Pb were significant at 0.01 confidence level (bilateral). The initial pH of the experimental karst water was 7.25, and the molarity of HCO3− in the experimental karst water was 3 mmol/L. The pH values of the experimental water with 4 mmol/L and 5 mmol/L HCO3− were slightly higher than 7.25 after 0.6721 g NaHCO3 and 1.3442 g NaHCO3 were sufficiently dissolved using distilled water and were, respectively, added to the experimental karst water, to make the molarity of HCO3− in experimental water achieve 4 mmol/L and 5 mmol/L. The pH values of the experimental water with 3, 4, and 5 mmol/L were 8.54, 8.67, and 8.79 on the eighth day, respectively, and pH decreased as the culture time increased from 8 days to 24 days. On the twenty-fourth day of the experiments, the pH value of the experimental water with 3 mmol/L HCO3− was less than 8.5; however, the pH values of the experimental water with 4 mmol/L and 5 mmol/L HCO3− were both more than 8.5 (Figure 3). Some soluble lead (Pb) in karst water with 4 mmol/L and 5 mmol/L HCO3− transformed to the insoluble compounds (PbCO3 and Pb(OH)2), which cannot be absorbed by Pontederia crassipes. This is mainly due to the increase in HCO3−. Cheng et al. [34] found that HCO3− improved the pH of water. The pH of natural water is primarily related to carbonate balance and the concentration of dissolved CO2. When the pH value of water is 8.5, the lead complexes are mainly composed of 2% (PbCl+ + PbSO4), 10% PbCO3, and 88% PbOH+ [35]. In this study, a high molarity of HCO3− made Pb difficult to dissolve and move in karst water, and thus reduced the concentration of Pb in karst water and the absorption of Pb by Pontederia crassipes. Therefore, the removal of Pb in karst water increased but the bioconcentration amount of Pb in Pontederia crassipes decreased with the increase in HCO3− molarities in karst water.

Table 1.
Contents of elements in different parts of Pontederia crassipes after Pb uptake in karst water at different HCO3− molarities (mg/kg).

Table 2.
Pb uptake in different parts of Pontederia crassipes in karst water at different HCO3− molarities.
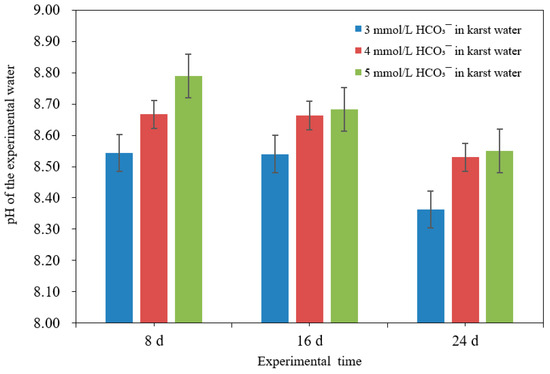
Figure 3.
pH of the experimental water during the experiments.
The bioconcentration factor (BCF) is one of the important parameters to evaluate the accumulation ability of the aquatic plant to the heavy metal. BCF > 1 represents the plant is a kind of heavy metal accumulator plant [26]. The statistical difference among the bioconcentration factor values was significant (p = 0.000 < 0.05). The bioconcentration factor of Pontederia crassipes to Pb (15,564) was the highest in karst water at 4 mmol/L HCO3−, indicating that a high molarity of HCO3− in karst water improved the Pb accumulation ability of Pontederia crassipes to Pb, but the accumulation ability reduced when the molarity of HCO3− was 5 mmol/L. The translocation factor (TF) indicates the aquatic plant transport ability to the accumulated heavy metal. TF > 1 represents that the aquatic plant reveals high transport ability [36]. The statistical difference among the translocation factor values was significant (p = 0.000 < 0.05). In this study, the translocation factor values of Pontederia crassipes to Pb in karst water at 3, 4, and 5 mmol/L HCO3− were all less than 1 (0.100, 0.104, and 0.146), showing that the Pontederia crassipes transport ability to Pb in karst water at different molarities of HCO3− was low. A high molarity of HCO3− in karst water affected the Pontederia crassipes transport ability to Pb. This is due to the common strategy and ability of Pontederia crassipes to prevent Pb damage to the leaf and young stem, which are used for photosynthesis, in order to survive better in the environment [37,38,39,40]. Cheng et al. [34] found that HCO3− affected the photosynthetic parameters of Pontederia crassipes, and the photosynthesis of Pontederia crassipes was mainly restricted by the absence of stomas, which can support the results of this study.
3.3. Ion Exchange Analysis
The contents of essential metal elements in Pontederia crassipes after Pb uptake in karst water at different HCO3− molarities are shown in Figure 4. The results showed that the content of Pb and Ca in Pontederia crassipes increased after Pb uptake in karst water at 3, 4, and 5 mmol/L HCO3−. However, the content of Mg and K in Pontederia crassipes decreased after Pb uptake in karst water at 3, 4, and 5 mmol/L HCO3−. The content of Na in Pontederia crassipes decreased in karst water at 3 mmol/L and 4 mmol/L HCO3−, but increased in karst water at 5 mmol/L HCO3−. Therefore, the cation exchange was rationalized to postulate that the Pb cation exchange with Mg, Na, and K occurred in karst water at 3 mmol/L and 4 mmol/L HCO3−, but the Pb cation exchange with Mg and K occurred in karst water at 5 mmol/L HCO3−. The results of this study found that a high molarity of HCO3− in karst water affected the variety of positive ions of cation exchange. Li et al. [41] showed that the chromium (Cr) and copper (Cu) cation exchange with Ca, Mg, and K occurred when accumulating Cr and Cu using the root powder of Pontederia crassipes. Zheng [42] also reported that the accumulation of Cu resulted in the depletion of Ca, indicating that the cation exchange between Ca and Cu occurred during the accumulation of Cu by roots of Pontederia crassipes. The absorption of Ca and the depletion of Mg and K in Pontederia crassipes reached the maximum when the molarity of HCO3− was 4 mmol/L in karst water, suggesting that the maximum strength of cation exchange occurred in karst water at 4 mmol/L HCO3− when Pontederia crassipes accumulated Pb, which explained why the bioconcentration factor of Pontederia crassipes to Pb was the highest in karst water at 4 mmol/L HCO3−. In this study, a high molarity of HCO3− in karst water affected the strength and the variety of positive ions of the cation exchange that occurred in the Pb uptake by Pontederia crassipes.
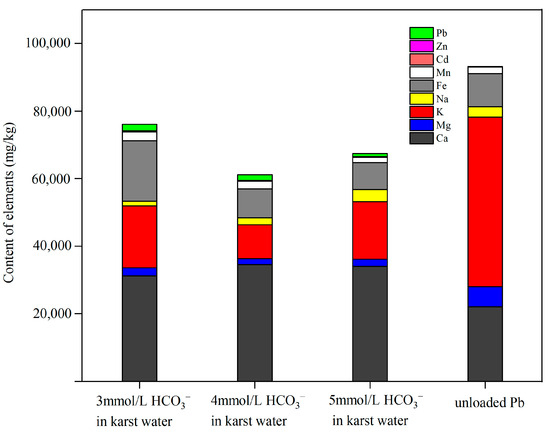
Figure 4.
Content distribution of elements in Pontederia crassipes after Pb uptake in karst water at different HCO3− molarities.
3.4. Morphology Analysis
The morphological characteristics of the Pontederia crassipes root, leaf, and stem were determined after the experiments. The SEM pictures of the root, leaf, and stem in karst water at 3 mmol/L and 4 mmol/L HCO3− are shown in Figure 5.
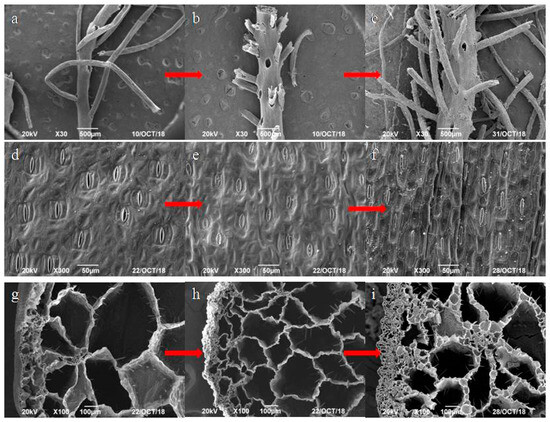
Figure 5.
(a,d,g) SEM pictures of the root, leaf, and stem before experiments; (b,e,h) the root, leaf, and stem in the experimental karst water at 3 mmol/L HCO3−; and (c,f,i) the root, leaf, and stem in the experimental karst water at 4 mmol/L HCO3−.
The root is a significant organ for the uptake of nutrients in the growth of Pontederia crassipes. Moreover, it is the vital place for purifying karst water quality. The developed root system is made up of the fibrous root and main root. Numerous voids exist between the fibrous root and the main root of Pontederia crassipes (Figure 5a), which help the root to exchange gas and enrich Pb. This is because the root cell wall prevents large molecules from entering the cell [8], and the large surface formed by the root and water promotes the Pb uptake by roots [14]. As shown in Figure 5b,c, the voids in the root had no significant changes in karst water at 3 mmol/L and 4 mmol/L HCO3−. The fibrous roots in karst water at 3 mmol/L HCO3− were broken off more seriously than that in karst water at 4 mmol/L HCO3−, suggesting that Pb slightly damaged the morphology of the root; moreover, a high molarity of HCO3− in karst water alleviated Pb damage to the root.
The leaf is the main organ that Pontederia crassipes uses to conduct photosynthesis, transpiration, and food production. There are numerous stomas on the leaf epidermis (Figure 5d). The developed stomas improve the leaf transpiration and photosynthesis, and promote the Pb uptake by the root in karst water more effectively [8]. As shown in Figure 5e,f, almost all stomas closed in karst water at 3 mmol/L and 4 mmol/L HCO3−. The significant Pb toxicity to the leaf decreased the number of opening stomas. Similar results also showed that heavy metals seriously damaged the stomas of the leaf [43,44]. Moreover, the closure degree of stomas in karst water at 3 mmol/L HCO3− was more serious than that in karst water at 4 mmol/L HCO3−. The results suggest that Pb seriously damaged the morphological characteristics of the leaf, and a high molarity of HCO3− in karst water could relieve the Pb toxicity to the leaf.
The stem includes the epidermis with intercellular space, cortex with aerenchyma, medulla formed by parenchymatous cells, and cancellous vascular bundles. The bundle sheaths surround the stem medulla, constituting an intricate vascular system. The structures together make up interconnected airways and air spaces of varying sizes (Figure 5g). As shown in Figure 5h, the stem cortex was atrophied and the ring structure volume became smaller in karst water at 3 mmol/L HCO3−. As shown in Figure 5i, the structural damage of the stem cortex in karst water at 4 mmol/L HCO3− was smaller than that in karst water at 3 mmol/L HCO3−, indicating that Pb caused serious toxicity to the morphology of the stem, and a high molarity of HCO3− in karst water could reduce the Pb damage to the stem.
A high molarity of HCO3− in karst water reduced the breaking of roots, stomatal closure, and vascular system shrinking of Pontederia crassipes. In this study, the Pb damage to the root, leaf, and stem was weakened with the increase in HCO3− molarities in karst water, suggesting that a high molarity of HCO3− in karst water alleviated the Pb damage to the root, leaf, and stem. The Pb damage to the morphology of the root in karst water at different molarities of HCO3− was smaller than that of the leaf and stem, showing that the root was more tolerant to Pb than the leaf and stem in karst water at different molarities of HCO3−. Therefore, increasing the molarity of HCO3− can relieve Pb toxicity to Pontederia crassipes morphology by reducing the Pb uptake by Pontederia crassipes.
3.5. Functional Group Analysis
The transmittance spectra of the Pontederia crassipes root after Pb uptake in karst water at 3, 4, and 5 mmol/L HCO3− molarities are shown in Figure 6. Helpful information about the functional group can be presented in the FTIR spectrum [45]. Figure 6 showed several intense feature bands of the functional groups combined with Pb by the Pontederia crassipes root. The spectral bands at 534, 1033, 1651, 2347, 2927, and 3453 cm−1 were, respectively, assigned to stretching vibration peaks of SO42−, C–O, protein C=O, C≡C, –CH3, and O–H [31,46,47].
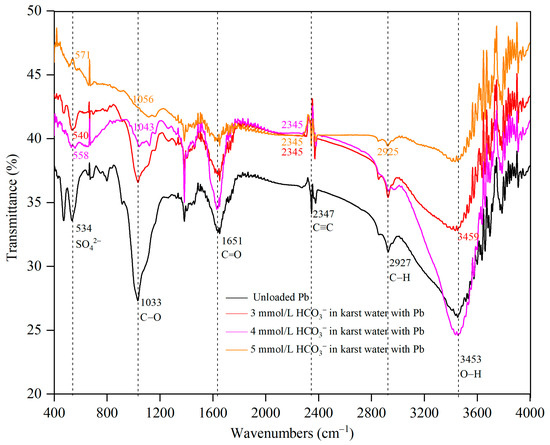
Figure 6.
Transmittance spectra of the Pontederia crassipes root after Pb uptake in karst water at different HCO3− molarities.
As shown in Figure 6, the shapes of the FTIR spectra, and the intensity and shift of several spectral peaks were different after Pb uptake in karst water at 3, 4, and 5 mmol/L HCO3−. The SO42− stretching vibration peak, respectively, shifted from 534 cm−1 to 540, 558, and 571 cm−1 in karst water at 3, 4, and 5 mmol/L HCO3−. The peak shift of SO42− increased, indicating that SO42− worked more strongly with the increase in HCO3− molarities in karst water. No peak of protein C=O on the cell wall at 1651 cm−1 had any displacement; however, the peak intensity of C=O was the strongest when the molarity of HCO3− in karst water was 4 mmol/L, showing that structural changes to the cell wall occurred when protein C=O combined with Pb [32]; moreover, protein C=O had the strongest protective effect on the cell wall and reduced the Pb toxicity to Pontederia crassipes root to the greatest extent, thus resulting in the highest bioconcentration factor of Pontederia crassipes to Pb in karst water at 4 mmol/L HCO3−. All peaks of C≡C at 2347 cm−1 shifted to 2345 cm−1 in karst water at 3, 4, and 5 mmol/L HCO3−; however, the peak intensity reduced with the increase in HCO3− molarities in karst water, showing that the increase in HCO3− molarities in karst water decreased the C≡C oxidation. In this study, the results showed that the functions of SO42−, protein C=O, and C≡C all changed in the Pb uptake by Pontederia crassipes root with the increase in HCO3− molarities in karst water. The O–H stretching vibration peak at 3453 cm−1 shifted to 3459 cm−1 only in karst water at 3 mmol /L HCO3−, indicating that O–H bonded with Pb only in karst water at 3 mmol /L HCO3−. The C–O stretching vibration peaks at 1033 cm−1, respectively, shifted to 1043 cm−1 and 1056 cm−1 in karst water at 4 mmol/L and 5 mmol /L HCO3−. The peak shift of C–O increased, suggesting that C–O interacted differently with Pb in karst water at 4 mmol/L and 5 mmol/L HCO3−. The O-H stretching vibration and C–O stretching vibration suggest that there is alcoholic hydroxyl in Pontederia crassipes [47]. However, alcoholic hydroxyl in the Pontederia crassipes root had no significant effect on Pb uptake because there was only an O–H or C–O stretching vibration in karst water at each molarity of HCO3−. The symmetric stretching vibration peak of –CH3 at 2927 cm−1 shifted to 2925 cm−1 only in karst water at 5 mmol/L HCO3−, showing that –CH3 bonded with Pb and formed methyl compounds through the methyl transfer reaction only in karst water at 5 mmol/L HCO3−. The methyl transfer reaction is closely associated with detoxification [48]. The results in this study suggest that a high molarity of HCO3− in karst water promoted the bonding between –CH3 and Pb and the methyl transfer reaction. Furthermore, a high molarity of HCO3− relieved the Pb toxicity to the Pontederia crassipes morphology through the methyl transfer reaction in Pb uptake.
The molarity of HCO3− in karst water played a vital role in regulating the functional group when Pontederia crassipes combined with Pb. Different kinds of functional groups worked in the Pb uptake by Pontederia crassipes; moreover, the same functional group in the Pontederia crassipes root from karst water at different HCO3− molarities worked distinctly (i.e., SO42−, C≡C, and protein C=O). Alcoholic hydroxyl in the Pontederia crassipes root had no significant effect on the increase in HCO3− molarities in karst water. Protein C=O on the cell wall was the most effective when the molarity of HCO3− was 4 mmol/L. A high molarity of HCO3− in karst water promoted the bonding between –CH3 and Pb and the methyl transfer reaction, which relieved the Pb toxicity to the Pontederia crassipes morphology.
4. Conclusions
This study performs original research on the mechanism of HCO3− on Pb removal and absorption using Pontederia crassipes from karst water and lays the foundation for the use of HCO3− in karst water Pb restoration using Pontederia crassipes. High HCO3− molarities increased the removal of Pb (85.31%, 93.28%, and 95.16%) in karst water using Pontederia crassipes at the beginning of the experiments, but they decreased the bioaccumulation amount of Pb (573, 501, and 301 mg/kg) due to the insoluble PbCO3 and Pb(OH)2. The Pb bioconcentration factor (15,564) and the strength of cation exchange reached the maximum, and protein C=O on the cell wall worked the most strongly in 4 mmol/L HCO3− karst water. High HCO3− molarities in karst water affected both the strength and variety of positive ions of the cation exchange, reduced the Pb toxicity to the morphology of Pontederia crassipes root, leaf, and stem, and controlled the functional group when Pontederia crassipes combined with Pb. High HCO3− molarities reduced the oxidation of C≡C, and promoted the bonding between –CH3 and Pb and the methyl transfer reaction. SO42−, protein C=O, and C≡C played different roles, and alcoholic hydroxyl had no significant effect. Therefore, this study suggests that increasing HCO3− supply can effectively remediate Pb pollution in karst water. Based on the conclusions of this study, we recommend carrying out field experiments on the remediation of Pb pollution in water using Pontederia crassipes, and actively promoting the remediation technology. Furthermore, it is necessary to research the resource utilization of Pontederia crassipes after experiments to control the secondary pollution.
Author Contributions
Conceptualization, J.Z., Z.J. and X.Q.; formal analysis, J.Z.; investigation, J.Z. and L.Z.; methodology, J.Z.; resources, Z.J.; software, J.Z.; supervision, Z.J. and X.Q.; validation, J.Z.; writing—original draft, J.Z.; writing—review and editing, J.Z., Z.J. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research and Development Fund Project of China Institute of Geo-Environmental Monitoring (No.20220105), the National Natural Science Foundation of China (41571203), and the China Geological Survey’s Project (DD20190343).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Li, B.; Zhang, H.; Long, J.; Fan, J.; Wu, P.; Chen, M.; Liu, P.; Li, T. Migration mechanism of pollutants in karst groundwater system of tailings impoundment and management control effect analysis: Gold mine tailing impoundment case. J. Clean. Prod. 2022, 350, 131434. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Jiang, Z.-C.; Xu, G.-L.; Qin, X.-Q.; Huang, Q.-B.; Zhang, L.-K. Major Ionic Characteristics and Controlling Factors of Karst Groundwater at Xiangshui, Chongzuo. Environ. Sci. 2019, 40, 2143–2151. [Google Scholar]
- Zhan, Z.-J.; Chen, F.; Yang, P.-H.; Ren, J.; Zhang, H.-Y.; Liu, D.-W.; Lan, J.-C.; Zhang, Y. Comparison on the Hydrogeochemical Characteristics of Typical Karst Groundwater System in Southwest China, a Case of Qingmuguan and Laolongdong in Chongqing. Environ. Sci. 2016, 37, 3365–3374. [Google Scholar]
- Ji, M.C.; Zhang, J.Q.; Peng, Y.; Ma, Q.Y. Research on the resistances of several kinds of hydrophyte to Lead in hydroponic condition. Biotechnol. Bull. 2017, 33, 120–125. [Google Scholar]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Sun, Z.G.; Mou, X.J.; Tong, C.; Wang, C.Y.; Xie, Z.L.; Song, H.L.; Sun, W.G.; Lv, Y.C. Spatial variations and bioaccumulation of heavy metals in intertidal zone of the Yellow River estuary, China. CATENA 2015, 126, 43–52. [Google Scholar] [CrossRef]
- Saxena, G.; Bharagava, R.N. Organic and inorganic pollutants in industrial wastes, their ecotoxicological effects, health hazards and bioremediation approaches. In Environmental Pollutants and Their Bioremediation Approaches, 1st ed.; Bharagava, R.N., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 23–56. [Google Scholar]
- Han, L.H.; Zhang, Y.P.; Di, X.M.; Huang, A.L.; Liu, C. Anatomical characteristics and ecological adaptability of vegetative organs of three invasive plants. Jiangsu Agric. Sci. 2018, 46, 92–95. [Google Scholar]
- Gui, Z.; Shan, Y.; Liu, C. Flow velocity evolution through a floating rigid cylinder array under unidirectional flow. J. Hydrol. 2023, 617, 128915–128929. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Li, B.-K. Removal of pharmaceuticals and personal care products by Eichhornia crassipe and Pistia stratiotes. J. Taiwan Inst. Chem. Eng. 2016, 58, 318–323. [Google Scholar] [CrossRef]
- Yi, Z.J.; Yao, J.; Chen, H.L.; Wang, F.; Yuan, Z.M.; Liu, X. Uranium biosorption from aqueous solution onto Pontederia crassipes. J. Environ. Radioact. 2016, 154, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mahamadi, C.; Nharingo, T. Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Pontederia crassipes in binary and ternary systems. Bioresour. Technol. 2010, 101, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Tel-Or, E.; Forni, C. Phytoremediation of hazardous toxic metals and organics by photosynthetic aquatic systems. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2011, 145, 224–235. [Google Scholar] [CrossRef]
- Feng, W.; Xiao, K.; Zhou, W.B.; Zhu, D.W.; Zhou, Y.Y.; Yuan, Y.N.; Xiao, N.D.; Wan, A.Q.; Hua, Y.M.; Zhao, J.W. Analysis of utilization technologies for Pontederia crassipes biomass harvested after restoration of wastewater. Bioresour. Technol. 2017, 223, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Suryandari, M.K.; Hariati, A.M.; Mahmudi, M. Removal of Lead from water by Pontederia crassipes (Mart.) Solms. Imp. J. Interdiscip. Res. 2017, 3, 2387–2392. [Google Scholar]
- Jiang, H.S.; Liao, Z.Y.; Li, W. Photosynthetic inorganic carbon utilization strategies and their ecological adaptability in aquatic plants. Plant Sci. J. 2023, 41, 847–856. [Google Scholar]
- Maberly, S.C.; Gontero, B. Ecological imperatives for aquatic CO2-concentrating mechanisms. J. Exp. Bot. 2017, 68, 3797–3814. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Exogenous Inorganic Carbon Sources in Plant Photosynthesis. Biol. Rev. 1970, 45, 167–220. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J. CO2 concentrating mechanisms and environmental change. Aquat. Bot. 2014, 118, 24–37. [Google Scholar] [CrossRef]
- Hu, G.; Wang, P.; Cao, J.H.; Zhang, C.L.; Mo, B.Q. Utilization of dissolved inorganic carbon by Hydrilla verticillate in karst water and its growth of the response. Environ. Sci. Technol. 2016, 35, 349–356. [Google Scholar]
- Liu, Z.; Dreybrodt, W.; Wang, H. A new direction in effective accounting for the atmospheric CO2 budget: Considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms. Earth-Sci. Rev. 2010, 99, 162–172. [Google Scholar] [CrossRef]
- Sun, H.L.; Han, C.H.; Liu, Z.H.; Wei, Y.; Ma, S.; Bao, Q.; Zhang, Y.; Yan, H. Nutrient limitations on primary productivity and phosphorus removal by biological carbon pumps in dammed karst rivers: Implications for eutrophication control. J. Hydrol. 2022, 607, 127480. [Google Scholar] [CrossRef]
- Zeng, Z.Y.; Yan, H.; Sun, H.L.; Liu, Z.H. Theoretical calculation of aquatic photosynthesis contribution ratio and the controlling factors of diurnal vatiations of hydrochemistry and δ~(13) C_(DIC) in the outlets and inlets of travertine pools at Baishuitai, Yunnan, China. Carsologica Sin. 2016, 35, 605–613. [Google Scholar]
- Xue, P.Y.; Yan, C.Z.; Cao, Y.L.; Wei, Q.S. Toxic effects of copper and arsenic and their compound pollution on Hydrilla verticillate (L.f.) Royle. Res. Environ. Sci. 2011, 24, 1052–1058. [Google Scholar]
- Saha, P.; Shinde, O.; Sarkar, S. Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytoremediat. 2017, 19, 87–96. [Google Scholar] [CrossRef]
- Zhang, L.K.; Qin, X.Q.; Huang, Q.B.; Liu, P.Y.; Shan, X.J. Aquatic plants bioremediation to groundwater contaminated by mines in karst areas. Carsologica Sin. 2017, 36, 743–750. [Google Scholar]
- Li, Q. Research on Removal Mechanisms of Four Kinds of Heavy Metals in Water by Long-Root Pontederia crassipes and Its Recycling; Kunming University Science and Technology: Kunming, China, 2015. [Google Scholar]
- Wang, P. Effects of Typical Aquatic Plants on the Stability of Inorganic Carbon in Karst Aquatic Ecosystem; China University of Geosciences: Beijing, China, 2016. [Google Scholar]
- GB/T 5750.6-2023; The National Standard of the People’s Republic of China: Standard Examination Methods for Drinking Water, Part 6, Metal and Metalloid Indices. State Administration for Market Regulation, Standardization Administration of China: Beijing, China, 2023.
- GB/T 5009.268-2016; The National Standard of the People’s Republic of China: National Food Safety Standard, Determination of Multiple Elements in Foods. National Health and Family Planning Commission, People’s Republic of China, State Food and Drug Administration: Beijing, China, 2016.
- Zhou, J.M.; Jiang, Z.C.; Xu, G.L.; Qin, X.Q.; Huang, Q.B.; Zhang, L.K. Effects and Mechanisms of Calcium Ion Addition on Lead Removal from Water by Pontederia crassipes. Int. J. Environ. Res. Public Health 2020, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, B.; Lin, P.; Zhou, J.L.; Zhan, J.H.; Shen, Q.Y.; Pan, X.J. Adsorption of heavy metal from aqueous solution by dehydrated root powder of Long-root Pontederia crassipes. Int. J. Phytorem. 2014, 18, 103–109. [Google Scholar] [CrossRef]
- Chen, L.; Li, C.G.; Li, F.M.; Zhong, Y.X.; Hu, H.Y.; Gao, S.Q.; Zhou, W.D.; Sun, Z.S. Review on water purification ability of aquatic ecological restoration plants. Environ. Pollut. Control 2022, 44, 1079–1084. [Google Scholar]
- Cheng, B.B.; Chen, S.Y.; Yue, L.R. Effects of NaHCO3 stress on morphological indices and photosynthetic parameters of purple root Eichhornia crassipes. Guihaia 2020, 40, 1781–1789. [Google Scholar]
- Chen, J.S.; Deng, B.S.; Tao, S.; Cheng, Y.Q. Environmental Geochemistry; The Ocean Publishing Company: Beijing, China, 1990. [Google Scholar]
- Guan, B.T.H.; Mohamat-Yusuff, F.; Halimoon, N.; Yong, C.S.Y. Uptake of Mn and Cd by wild Water Spinach and their bioaccumulation and translocation factors. Environ. Asia 2017, 10, 44–51. [Google Scholar]
- Baker, A.J.M. Accumulators and excluders strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Landberg, T.; Greger, T. Difference in uptake and tolerance to heavy metal in Salix from unpolluted and polluted areas. Appl. Geochem. 1996, 11, 175–180. [Google Scholar] [CrossRef]
- Sarital, S.; Rohit, S.; Shraddha, S. Comparative studies on accumulation of Cr from metal solution and tannery effluent under repeated metal exposure by aquatic plants: Its toxic effects. Environ. Monit. Assess. 2002, 80, 17–31. [Google Scholar]
- Woldemichael, D.; Zewge, F.; Leta, S. Potential of water Hyacinth (Eichhornia crassipes (Mart.) Solms) for the removal of chromium from tannery effluent in constructed pond system. SINET: Ethiop. J. Sci. 2011, 34, 49–62. [Google Scholar]
- Li, X.S.; Liu, S.L.; Na, Z.Y.; Lu, D.N.; Liu, Z. Adsorption, concentration, and recovery of aqueous heavy metal ions with the root powder of Pontederia crassipes. Ecol. Eng. 2013, 60, 160–166. [Google Scholar] [CrossRef]
- Zheng, J.C. The Performance and Mechanism of Removal of Heavy Metals from Water by Water Hyacinth Roots as a Biosorbent Material; University of Science and Technology of China: Hefei, China, 2010. [Google Scholar]
- Brunet, J.; Repellin, A.; Varrault, G.; Terryn, N.; Zuily-Fodil, Y. Lead accumulation in the roots of grass pea (Lathyrus sativus L.): A novel plant for phytoremediation systems? Comptes Rendus Biol. 2008, 331, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Malar, S.; Vikram, S.S.; Favas, P.J.C.; Perumal, V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Pontederia crassipes (Mart.)]. Bot. Stud. 2014, 55, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Wang, B.; Guo, X.-J.; Zou, C.-W.; Tan, X.-D. Investigating adsorption performance of heavy metals onto humic acid from sludge using Fourier-transform infrared combined with two-dimensional correlation spectroscopy. Environ. Sci. Pollut. Res. 2019, 26, 9842–9850. [Google Scholar] [CrossRef]
- Lim, S.F.; Zheng, Y.M.; Zou, S.W.; Chen, J.P. Characterization of copper adsorption onto an alginate encapsulated magnetic sorbent by a combined FT IR, XPS and mathematical modeling study. Environ. Sci. Technol. 2008, 42, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, C.; Zhu, Q.; Huang, T.; Cai, Y.; Li, N.; Liu, J.; Tan, X. Characterization of dissolved organic matter from biogas residue composting using spectroscopic techniques. Waste Manag. 2018, 78, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.B.; Xin, C.H.; Zhao, H.; Zhang, Q.; Chi, J.L.; Guo, J.B. The role of DNA methylation in plant response to heavy metal stress. Seed 2016, 25, 43–46. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).