Innovative Solutions for Water Treatment: Unveiling the Potential of Polyoxazoline Polymer Activated Carbon Composite for Efficient Elimination of Lead Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Procedure of Poly 2-methoxycarbonylpropyl-2-oxazoline-activated Carbon Composite
2.3. Instruments
2.4. Pb (II) Ions’ Removal Procedure
3. Results and Discussion
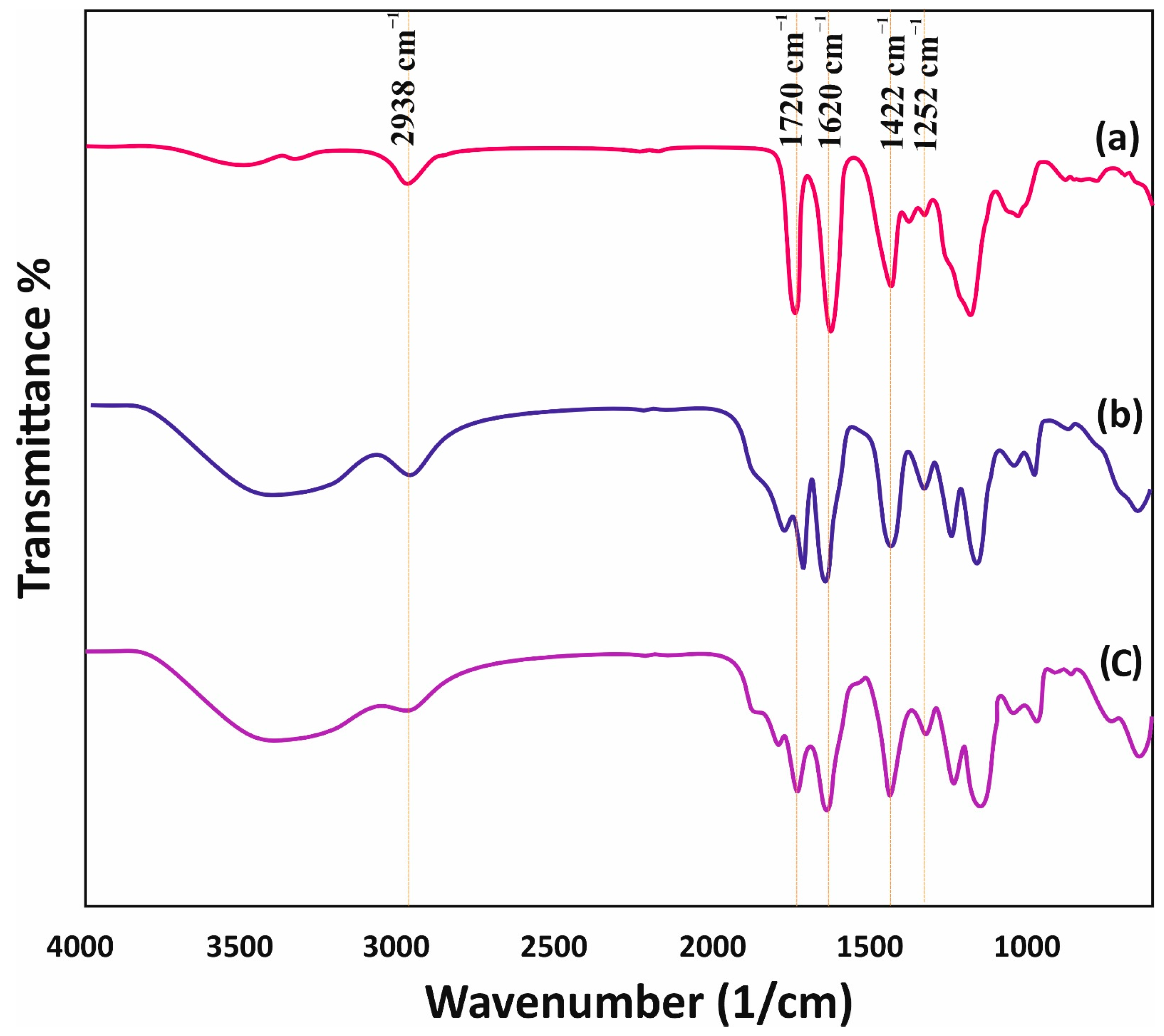
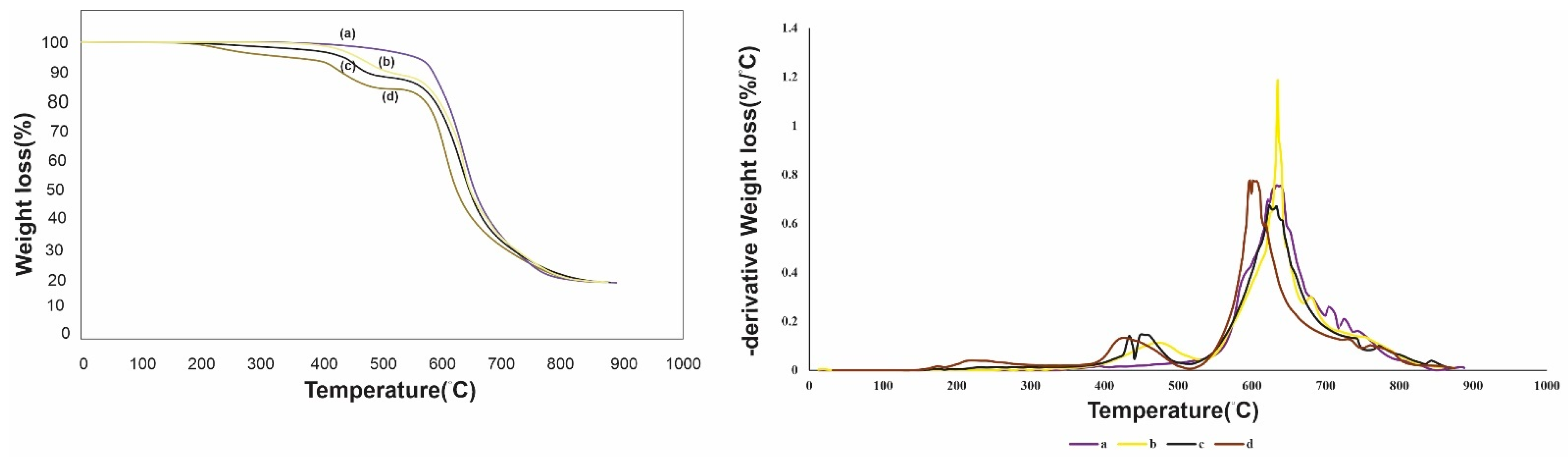
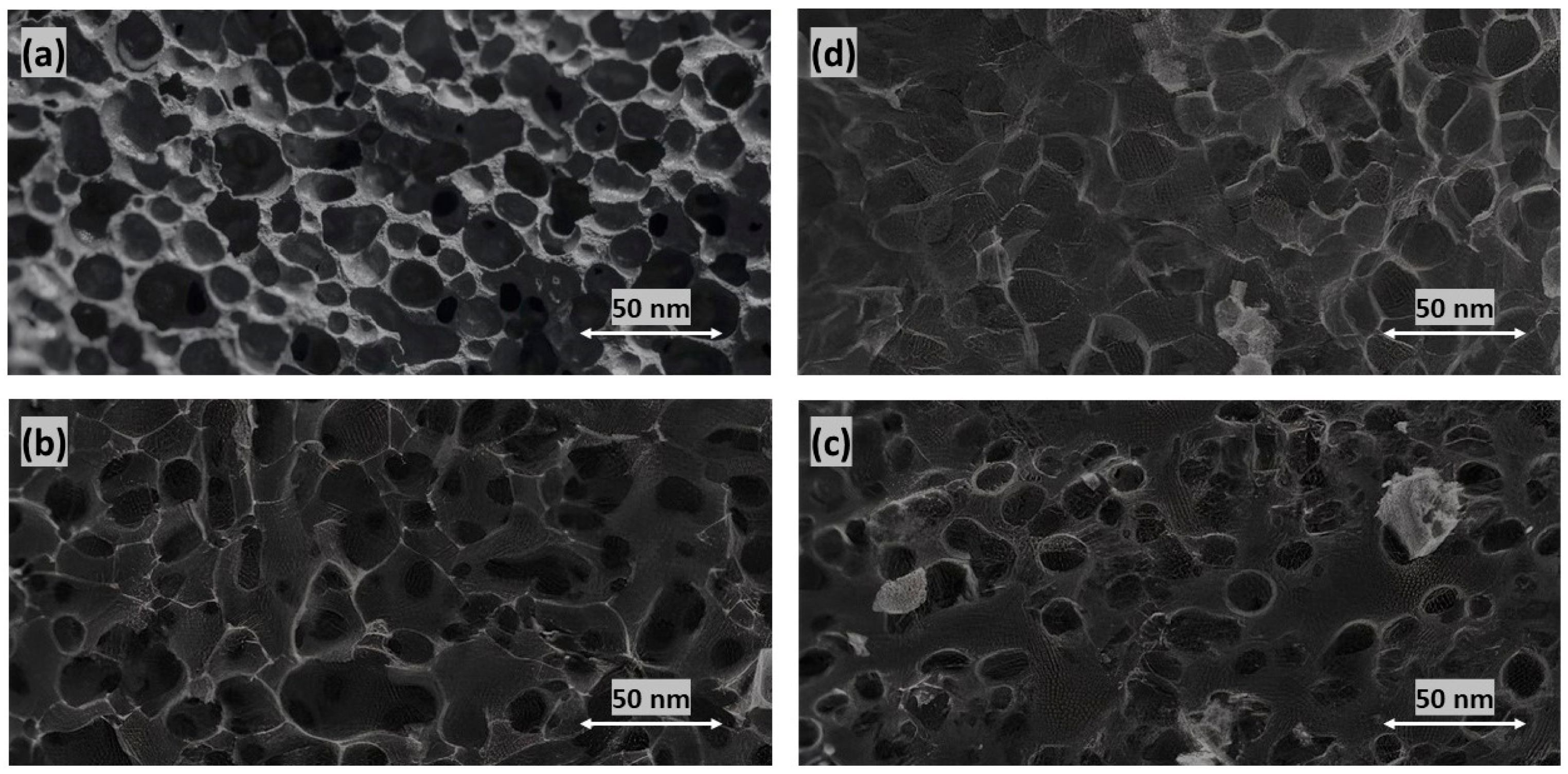
3.1. Synthesis and Characterization of Adsorbents
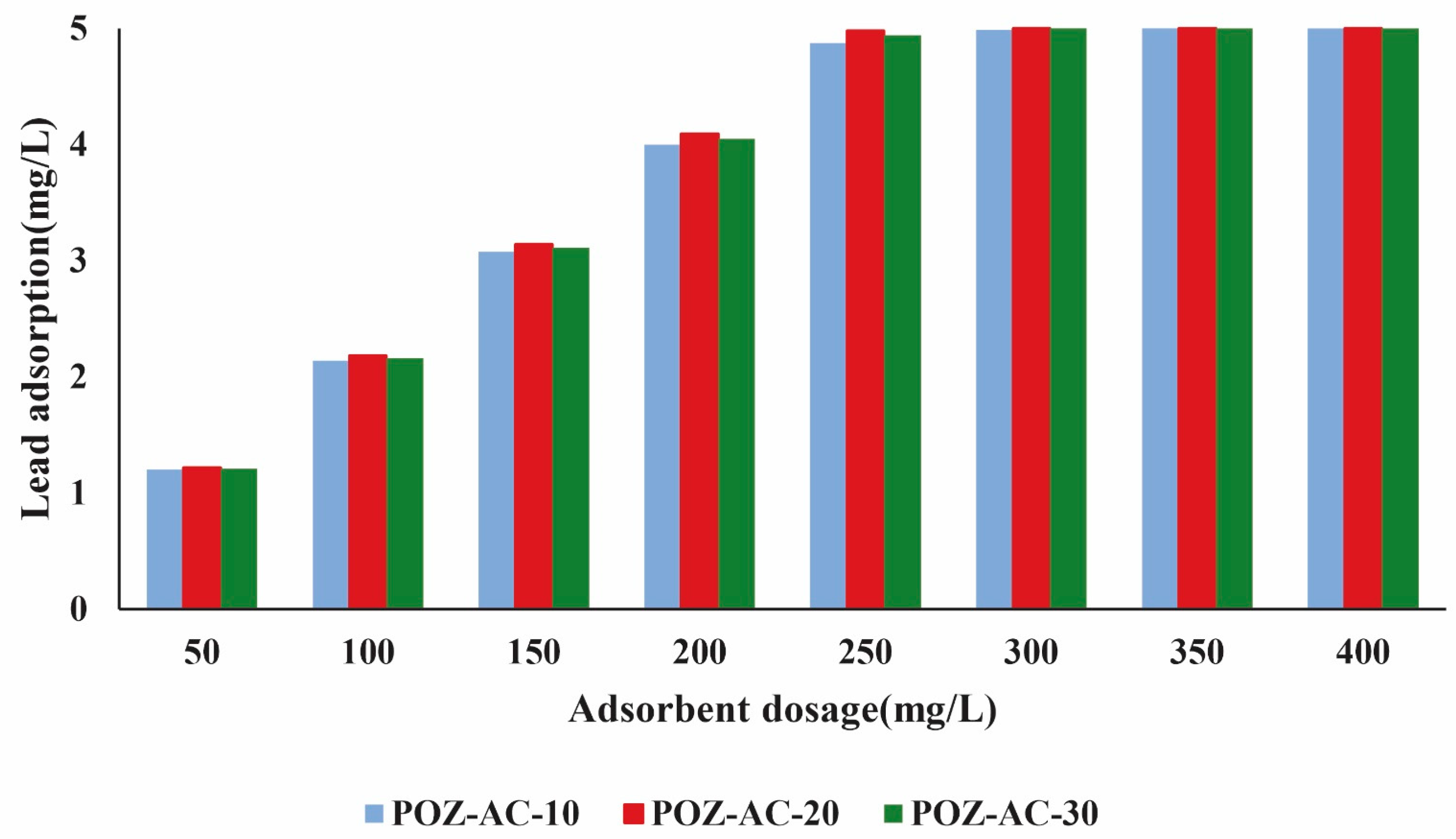
3.2. Investigation of the Adsorbent Dosage on Lead Ions’ Removal Performance
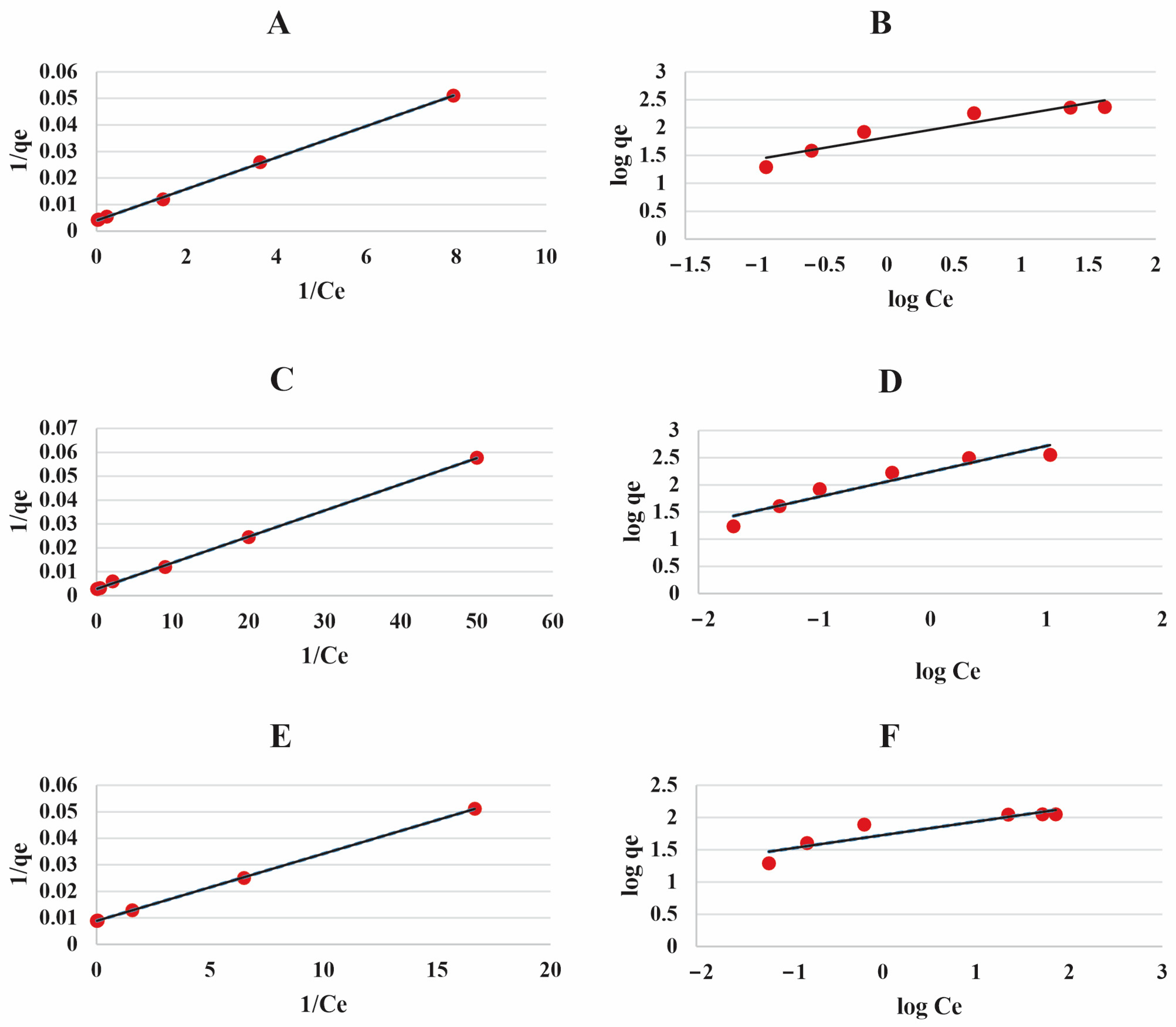
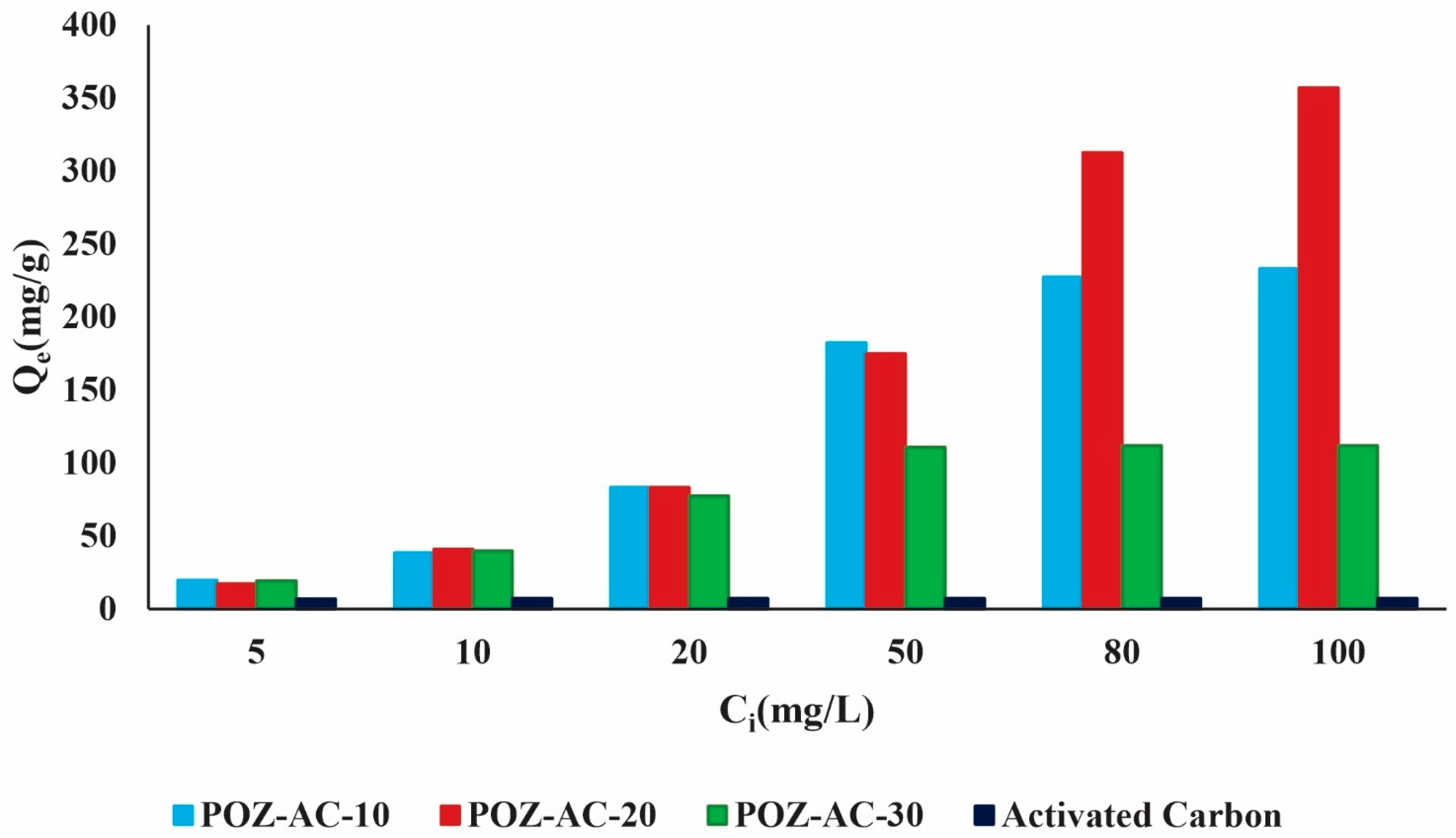
3.3. Investigation of Lead Ions’ Removal Using Isotherm Analysis
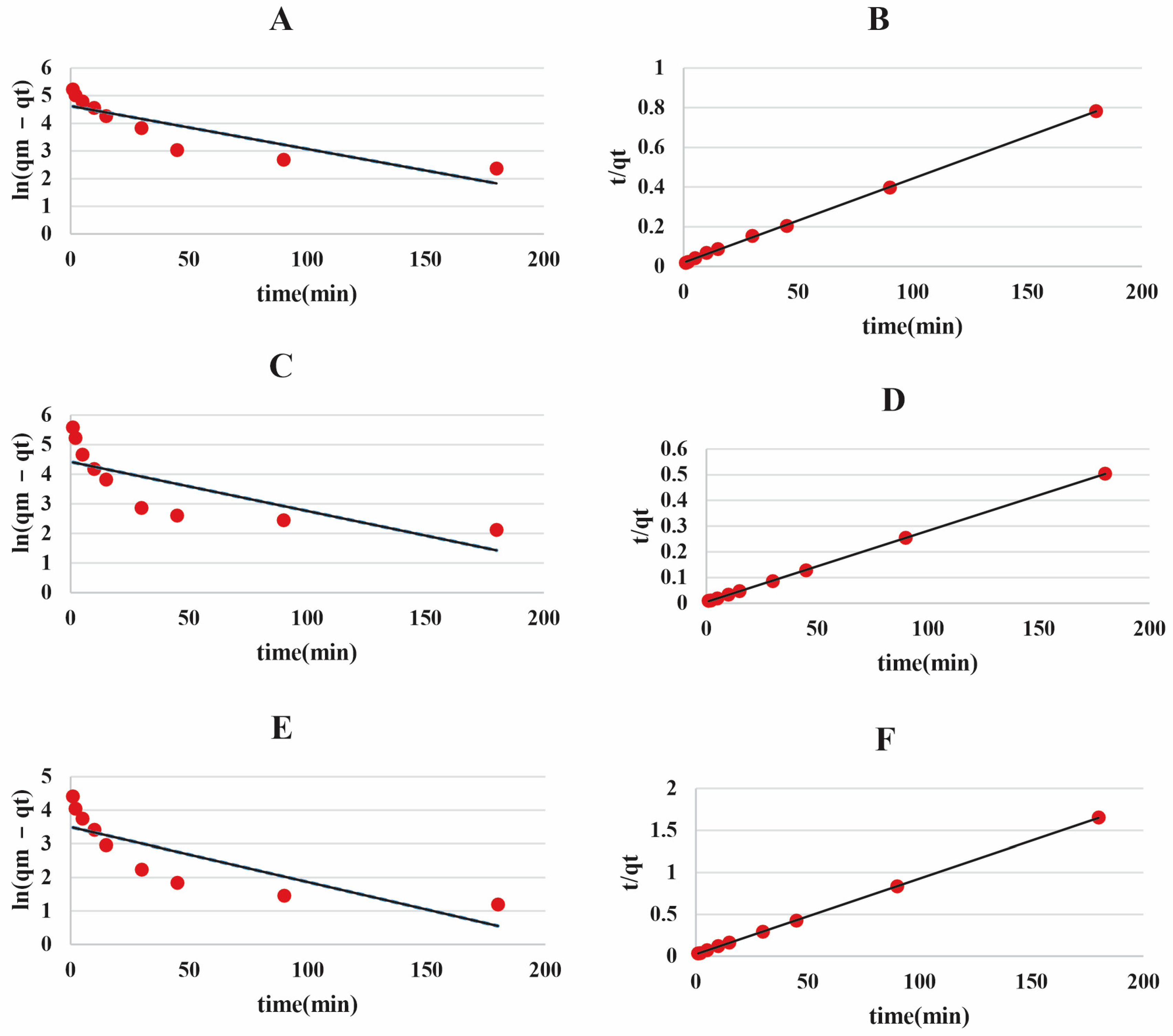
3.4. Investigation of the Kinetic Behavior
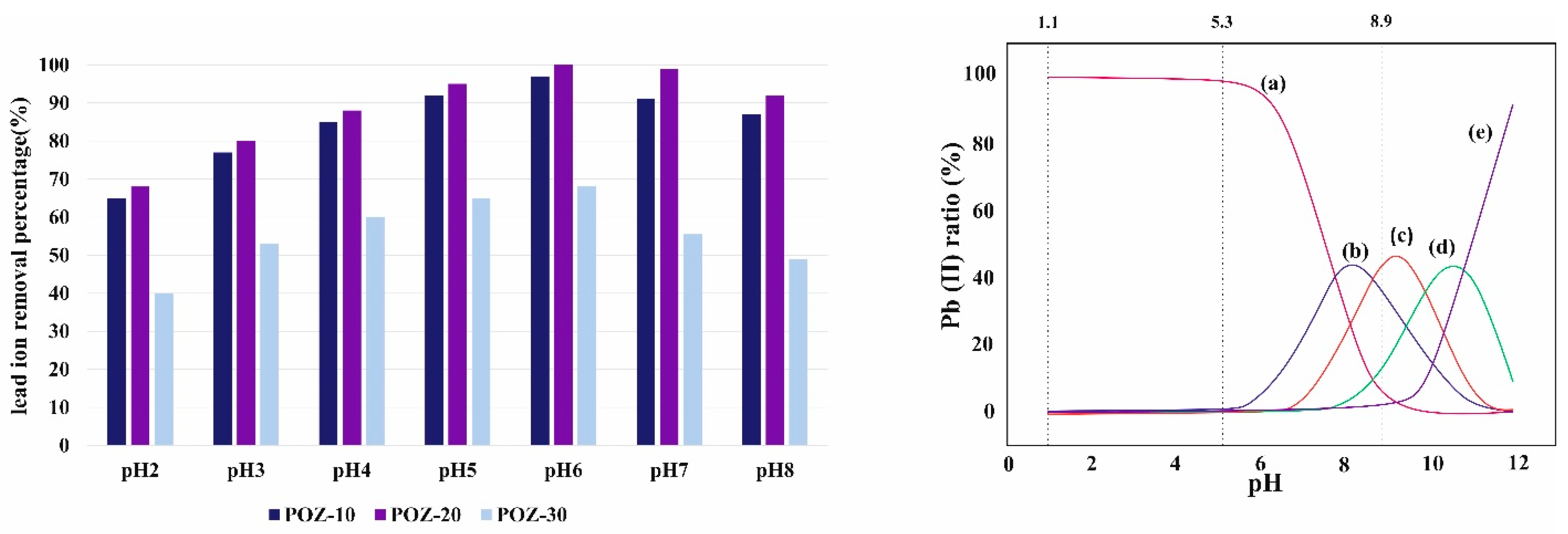
3.5. Investigation of the pH Impact on Lead Ions’ Removal Performance
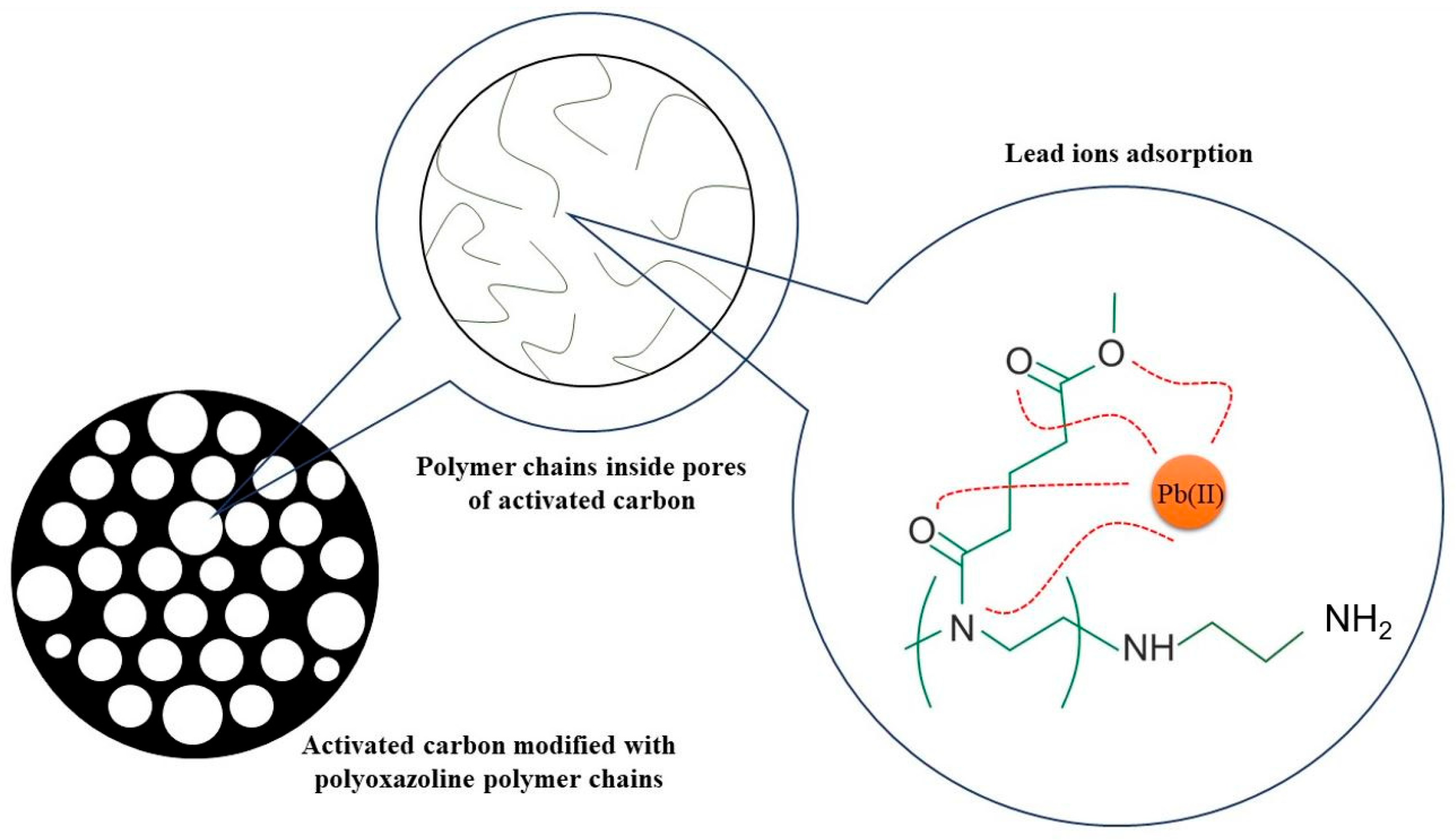
3.6. Adsorption Mechanism
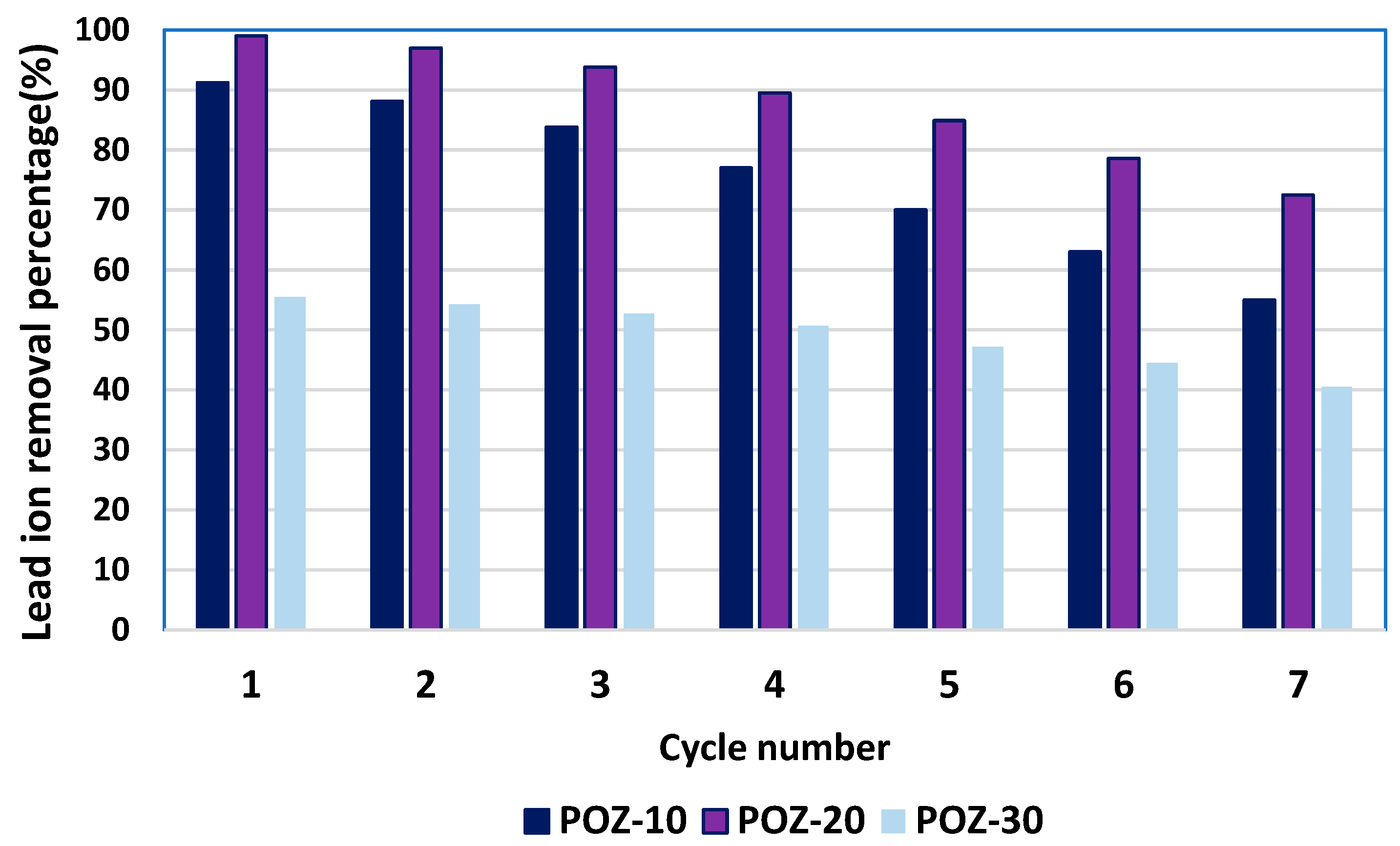
3.7. Investigation of Adsorbent Reusability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousazadeh, B.; Mohammadi, N.; Khosravi-Nikou, M. Synthesis and characterization of porous activated carbons derived from lotus nut and their performance for CO2 adsorption. Int. J. Environ. Sci. Technol. 2024, 1–16. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI-containing Mg(OH)2 nanowaste. Angew. Chem. 2008, 120, 5701–5704. [Google Scholar] [CrossRef]
- Basak, S.; Dey, B.; Bhattacharyya, B. Demand side management for solving environment constrained economic dispatch of a microgrid system using hybrid MGWOSCACSA algorithm. CAAI Trans. Intell. Technol. 2022, 7, 256–267. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Zhu, X.; Yin, L.; Yin, Z.; Li, X.; Zheng, W. Calculation of carbon emissions in wastewater treatment and its neutralization measures: A review. Sci. Total Environ. 2023, 912, 169356. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, B.; Mohammadi, N.; Hamoule, T. Phosphate removal from aqueous environment using iron oxide/activated carbon composite: Ziziphus nuts derived activated carbon as a new precursor. Iran. J. Chem. Eng. (IJChE) 2021, 18. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Mansfield, T.J.; Gibson, J.M. Protecting wastewater workers from disease risks: Personal protective equipment guidelines. Water Environ. Res. 2020, 92, 524–533. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Li, Y.; Li, Y.; Zhou, H.; Fan, Y. A general linear free energy relationship for predicting partition coefficients of neutral organic compounds. Chemosphere 2020, 247, 125869. [Google Scholar] [CrossRef]
- Shafqat, S.; Majeed, H.; Javaid, Q.; Ahmad, H.F. Standard ner tagging scheme for big data healthcare analytics built on unified medical corpora. J. Artif. Intell. Technol. 2022, 2, 152–157. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef]
- Liu, X. Real-world data for the drug development in the digital era. J. Artif. Intell. Technol. 2022, 2, 42–46. [Google Scholar] [CrossRef]
- Abdullayeva, F.J. Internet of Things-based healthcare system on patient demographic data in Health 4.0. CAAI Trans. Intell. Technol. 2022, 7, 644–657. [Google Scholar] [CrossRef]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of heavy metals and metalloids from water using drinking water treatment residuals as adsorbents: A review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef]
- Hamoule, T.; Mohammadi, N.; Mousazadeh, B.; Fakhari, H. Simultaneous improvement of water stability and performance of HKUST-1 for fast cadmium removal from the water environment: Synthesis, characterization, RSM optimization, thermodynamics and kinetics. Int. J. Environ. Sci. Technol. 2023, 20, 11913–11930. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Anwar, J.; Shafique, U.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb (II) and Cd (II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Siddiqui, M. Low level lead exposure and oxidative stress: Current opinions. Clin. Chim. Acta 2007, 383, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Şölener, M.; Tunali, S.; Özcan, A.S.; Özcan, A.; Gedikbey, T. Adsorption characteristics of lead (II) ions onto the clay/poly (methoxyethyl) acrylamide (PMEA) composite from aqueous solutions. Desalination 2008, 223, 308–322. [Google Scholar] [CrossRef]
- Yi, T.; Shi, M.; Zhu, H. Medical data publishing based on average distribution and clustering. CAAI Trans. Intell. Technol. 2022, 7, 381–394. [Google Scholar] [CrossRef]
- Awual, M.R. An efficient composite material for selective lead (II) monitoring and removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 103087. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Namasivayam, C. Agricutural by-product as metal adsorbent: Sorption of lead (II) from aqueous solution onto coirpith carbon. Environ. Technol. 2000, 21, 1091–1097. [Google Scholar] [CrossRef]
- Bandehali, S.; Parvizian, F.; Moghadassi, A.; Hosseini, S.; Shen, J. Fabrication of thin film-PEI nanofiltration membrane with promoted separation performances: Cr, Pb and Cu ions removal from water. J. Polym. Res. 2020, 27, 94. [Google Scholar] [CrossRef]
- Deng, X.; Lü, L.; Li, H.; Luo, F. The adsorption properties of Pb (II) and Cd (II) on functionalized graphene prepared by electrolysis method. J. Hazard. Mater. 2010, 183, 923–930. [Google Scholar] [CrossRef]
- Pang, F.M.; Kumar, P.; Teng, T.T.; Omar, A.M.; Wasewar, K.L. Removal of lead, zinc and iron by coagulation–flocculation. J. Taiwan Inst. Chem. Eng. 2011, 42, 809–815. [Google Scholar] [CrossRef]
- Feng, J.; Yang, Z.; Zeng, G.; Huang, J.; Xu, H.; Zhang, Y.; Wei, S.; Wang, L. The adsorption behavior and mechanism investigation of Pb (II) removal by flocculation using microbial flocculant GA1. Bioresour. Technol. 2013, 148, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yao, X.; Yu, X.; Mao, L.; Zeng, Y.; Wu, F.; Guo, S.; He, G. Flotation Performance and Adsorption Mechanism of Cerussite with Phenylpropenyl Hydroxamic Acid Collector. Minerals 2023, 13, 1315. [Google Scholar] [CrossRef]
- Yu, X.; Mao, L.; Xie, H.; Yao, X.; He, G.; Huang, Z. Flotation Behavior and Adsorption Mechanism of Phenylpropyl Hydroxamic Acid As Collector Agent in Separation of Fluorite from Calcite. Langmuir 2023, 39, 5936–5943. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef]
- Yuna, Z. Review of the natural, modified, and synthetic zeolites for heavy metals removal from wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Wang, Z.; Zhao, Y.; Xia, Q.; Qiu, J.; Wang, H.; Wang, J. Ionic liquid hybrid metal–organic frameworks for efficient adsorption and selective separation of ammonia at high temperature. Chem. Eng. J. 2023, 464, 142728. [Google Scholar] [CrossRef]
- Mohammadi, N.; Mousazadeh, B.; Hamoule, T. Synthesis and characterization of NH2-SiO2@Cu-MOF as a high-performance adsorbent for Pb ion removal from water environment. Environ. Dev. Sustain. 2021, 23, 1688–1705. [Google Scholar] [CrossRef]
- Zheng, X.; Ni, C.; Xiao, W.; Liang, Y.; Li, Y. Ionic liquid grafted polyethersulfone nanofibrous membrane as recyclable adsorbent with simultaneous dye, heavy metal removal and antibacterial property. Chem. Eng. J. 2022, 428, 132111. [Google Scholar] [CrossRef]
- Ragheb, E.; Shamsipur, M.; Jalali, F.; Mousavi, F. Modified magnetic-metal organic framework as a green and efficient adsorbent for removal of heavy metals. J. Environ. Chem. Eng. 2022, 10, 107297. [Google Scholar] [CrossRef]
- Viegas, T.X.; Bentley, M.D.; Harris, J.M.; Fang, Z.; Yoon, K.; Dizman, B.; Weimer, R.; Mero, A.; Pasut, G.; Veronese, F.M. Polyoxazoline: Chemistry, properties, and applications in drug delivery. Bioconjugate Chem. 2011, 22, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Aoi, K.; Okada, M. Polymerization of oxazolines. Prog. Polym. Sci. 1996, 21, 151–208. [Google Scholar] [CrossRef]
- Simon, L.; Marcotte, N.; Devoisselle, J.; Begu, S.; Lapinte, V. Recent advances and prospects in nano drug delivery systems using lipopolyoxazolines. Int. J. Pharm. 2020, 585, 119536. [Google Scholar] [CrossRef] [PubMed]
- Mahand, S.N.; Aliakbarzadeh, S.; Moghaddam, A.; Moghaddam, A.S.; Kruppke, B.; Nasrollahzadeh, M.; Khonakdar, H.A. Polyoxazoline: A review article from polymerization to smart behaviors and biomedical applications. Eur. Polym. J. 2022, 178, 111484. [Google Scholar] [CrossRef]
- Sahin, Z.M.; Kohlan, T.B.; Atespare, A.E.; Yildiz, M.; Unal, S.; Dizman, B. Polyoxazoline-modified graphene oxides with improved water and epoxy resin dispersibility and stability towards composite applications. J. Appl. Polym. Sci. 2022, 139, e52406. [Google Scholar] [CrossRef]
- Portier, É.; Azemar, F.; Benkhaled, B.T.; Bardeau, J.-F.; Faÿ, F.; Réhel, K.; Lapinte, V.; Linossier, I. Poly (oxazoline) for the design of amphiphilic silicone coatings. Prog. Org. Coat. 2021, 153, 106116. [Google Scholar] [CrossRef]
- Cegłowski, M.; Marien, Y.W.; Smeets, S.; De Smet, L.; D’hooge, D.R.; Schroeder, G.; Hoogenboom, R. Molecularly Imprinted Polymers with Enhanced Selectivity Based on 4-(Aminomethyl) pyridine-Functionalized Poly (2-oxazoline) s for Detecting Hazardous Herbicide Contaminants. Chem. Mater. 2021, 34, 84–96. [Google Scholar] [CrossRef]
- Soleimani, M.; Abdalisousan, A.; Manshad, A.K.; Sajadiyan, V.A. Advanced polymer-based surfactant for improved heat and salinity stability in enhanced oil recovery processes. J. Surfactants Deterg. 2024. [Google Scholar] [CrossRef]
- Soleimani, M.; Abdalisousan, A.; KhaksarManshad, A.; Sajadiyan, V.A. Synthesis, characterization, and mechanistic study of a new highly-stable comb-like polymeric surfactant in enhanced oil recovery. Geoenergy Sci. Eng. 2024, 234, 212542. [Google Scholar] [CrossRef]
- Yao, X.; Yu, X.; Wang, L.; Zeng, Y.; Mao, L.; Liu, S.; Xie, H.; He, G.; Huang, Z.; Liu, Z. Preparation of cinnamic hydroxamic acid collector and study on flotation characteristics and mechanism of scheelite. Int. J. Min. Sci. Technol. 2023, 33, 773–781. [Google Scholar] [CrossRef]
- Soleimani, M.; Abdalisousan, A.; Khaksar Manshad, A.; Sajadiyan, V.A. Polymeric Surfactant Flooding for Improved Oil Recovery: Optimized Wettability and Fluid Mobility. Iran. J. Chem. Chem. Eng. 2023, in press. [Google Scholar]
- Bouten, P.J.; Lava, K.; van Hest, J.C.; Hoogenboom, R. Thermal properties of methyl ester-containing poly(2-oxazoline)s. Polymers 2015, 7, 1998–2008. [Google Scholar] [CrossRef]
- Prakash, M.O.; Raghavendra, G.; Ojha, S.; Panchal, M. Characterization of porous activated carbon prepared from arhar stalks by single step chemical activation method. Mater. Today Proc. 2021, 39, 1476–1481. [Google Scholar] [CrossRef]
- Nayak, S. Water purification: Removal of Heavy metals Using Metal-Organic Frameworks (MOFs). In Metal-Organic Frameworks in Biomedical and Environmental Field; Springer: Cham, Switzerland, 2021; pp. 239–268. [Google Scholar]
- Wu, Y.; Xu, G.; Liu, W.; Yang, J.; Wei, F.; Li, L.; Zhang, W.; Hu, Q. Postsynthetic modification of copper terephthalate metal-organic frameworks and their new application in preparation of samples containing heavy metal ions. Microporous Mesoporous Mater. 2015, 210, 110–115. [Google Scholar] [CrossRef]
- Saleem, H.; Rafique, U.; Davies, R.P. Investigations on post-synthetically modified UiO-66-NH2 for the adsorptive removal of heavy metal ions from aqueous solution. Microporous Mesoporous Mater. 2016, 221, 238–244. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, C.; He, Y.; Yang, C.; Li, X.; Zheng, J.; Wang, S. Facile preparation of magnetic Zr-MOF for adsorption of Pb (II) and Cr (VI) from water: Adsorption characteristics and mechanisms. Chem. Eng. J. 2021, 415, 128923. [Google Scholar] [CrossRef]
- Luo, X.; Ding, L.; Luo, J. Adsorptive removal of Pb (II) ions from aqueous samples with amino-functionalization of metal–organic frameworks MIL-101 (Cr). J. Chem. Eng. Data 2015, 60, 1732–1743. [Google Scholar] [CrossRef]
- Singh, S.; Basavaraju, U.; Naik, T.S.K.; Behera, S.K.; Khan, N.A.; Singh, J.; Singh, L.; Ramamurthy, P.C. Graphene oxide-based novel MOF nanohybrid for synergic removal of Pb (II) ions from aqueous solutions: Simulation and adsorption studies. Environ. Res. 2023, 216, 114750. [Google Scholar] [CrossRef]
- Huang, Z.; Xiong, C.; Ying, L.; Wang, W.; Wang, S.; Ding, J.; Lu, J. Facile synthesis of a MOF-derived magnetic CoAl-LDH@ chitosan composite for Pb (II) and Cr (VI) adsorption. Chem. Eng. J. 2022, 449, 137722. [Google Scholar] [CrossRef]
- Soltani, R.; Pelalak, R.; Pishnamazi, M.; Marjani, A.; Albadarin, A.B.; Sarkar, S.M.; Shirazian, S. A novel and facile green synthesis method to prepare LDH/MOF nanocomposite for removal of Cd (II) and Pb (II). Sci. Rep. 2021, 11, 1609. [Google Scholar] [CrossRef]
- Ozdes, D.; Duran, C.; Senturk, H.B. Adsorptive removal of Cd (II) and Pb (II) ions from aqueous solutions by using Turkish illitic clay. J. Environ. Manag. 2011, 92, 3082–3090. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, A.; Ozdes, D.; Duran, C.; Bulut, V.N.; Soylak, M.; Senturk, H.B. Biosorption of Pb (II) ions from aqueous solution by pine bark (Pinus brutia Ten.). Chem. Eng. J. 2009, 153, 62–69. [Google Scholar] [CrossRef]

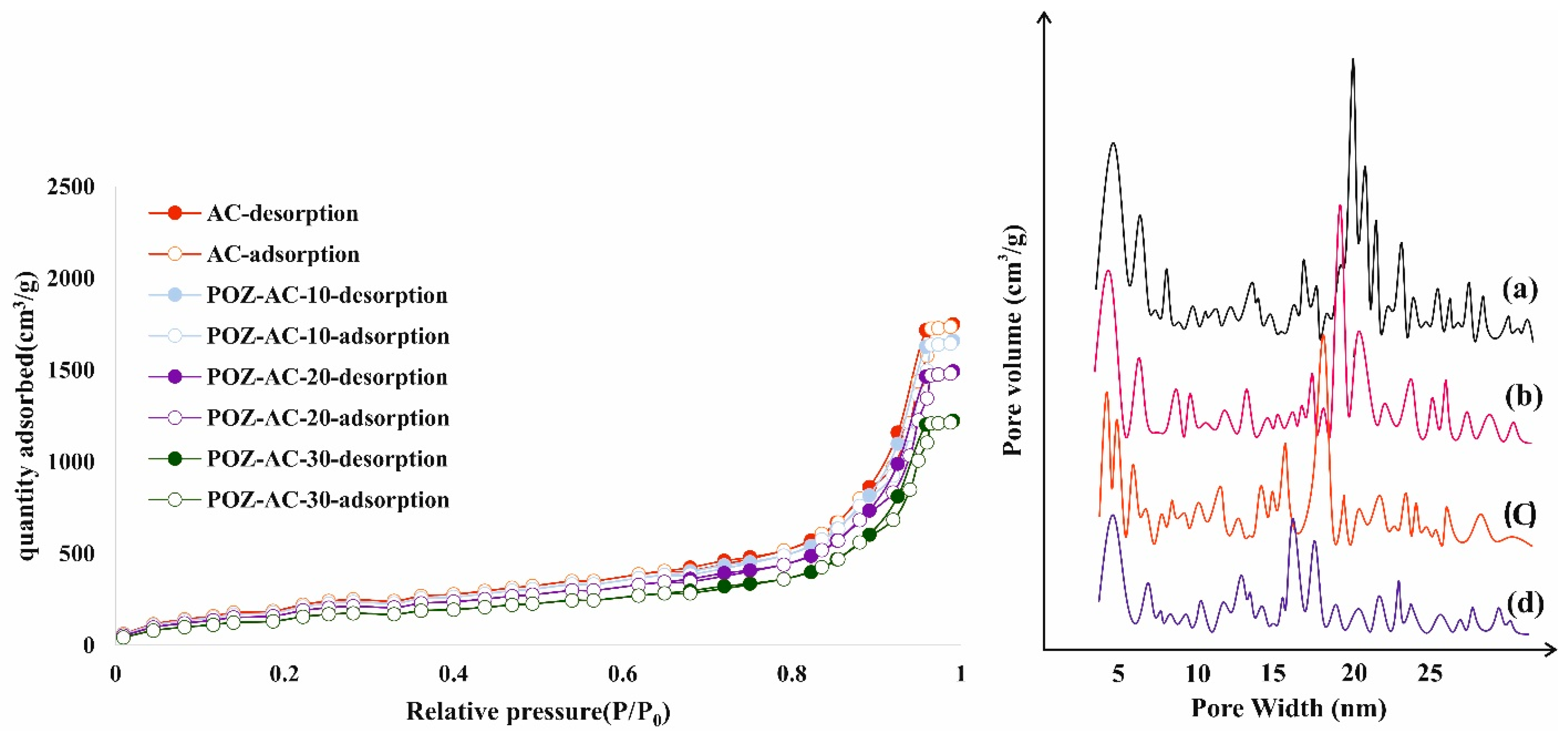


| Material | SBET (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|
| Activated carbon | 443.80 | 0.970 |
| POZ-AC-10 | 420.90 | 0.811 |
| POZ-AC-20 | 378.40 | 0.652 |
| POZ-AC-30 | 311.20 | 0.341 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| (mg g−1) | (L mg−1) | (mg g−1) | ||||
| POZ-AC-10 | 240.7 | 0.7 | 0.989 | 2.44 | 67.17 | 0.901 |
| POZ-AC-20 | 365.5 | 2.5 | 0.997 | 2.1 | 173.38 | 0.913 |
| POZ-AC-30 | 112.3 | 0.35 | 0.994 | 4.77 | 53.32 | 0.829 |
| Adsorbents | Lead Ion Adsorption Capacity (mg/g) | References |
|---|---|---|
| NH2-SiO2@Cu-MOF | 166.7 | [30] |
| thiol-functionalized [Cu4O(BDC)]n | 38.7 | [47] |
| UiO-66-NHC(S)NHMe | 49 | [48] |
| Ni0.6Fe2.4O4-UiO-66-PEI | 273.2 | [49] |
| MIL-101 | 15.8 | [50] |
| UiO-66-NDC/GO | 254.45 | [51] |
| Co-Al-LDH@CS/Fe3O4 | 558.84 | [52] |
| LDH/MOF NC | 301.4 | [53] |
| POZ-AC-20 | 365 | Present study |
| Materials | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| Experimental (mg g−1) | Theoretical (mg g−1) | K1 (1/min) | R2 | Theoretical | K2 (g/mg·min) | R2 | |
| POZ-AC-10 | 233.1 | 102.15 | 0.0155 | 0.7589 | 238.09 | 0.0009 | 0.9995 |
| POZ-AC-20 | 357.19 | 83 | 0.0166 | 0.592 | 357.14 | 0.0015 | 0.9999 |
| POZ-AC-30 | 111.9 | 33.11 | 0.0164 | 0.6759 | 111.11 | 0.0034 | 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amari, A.; Boujelbene, M.; Sami, F.M.; Elboughdiri, N.; Sonawane, C.; Naganna, S.R.; Sammen, S.S. Innovative Solutions for Water Treatment: Unveiling the Potential of Polyoxazoline Polymer Activated Carbon Composite for Efficient Elimination of Lead Ions. Water 2024, 16, 466. https://doi.org/10.3390/w16030466
Amari A, Boujelbene M, Sami FM, Elboughdiri N, Sonawane C, Naganna SR, Sammen SS. Innovative Solutions for Water Treatment: Unveiling the Potential of Polyoxazoline Polymer Activated Carbon Composite for Efficient Elimination of Lead Ions. Water. 2024; 16(3):466. https://doi.org/10.3390/w16030466
Chicago/Turabian StyleAmari, Abdelfattah, Mohamed Boujelbene, Fatima Moayad Sami, Noureddine Elboughdiri, Chandrakant Sonawane, Sujay Raghavendra Naganna, and Saad Sh. Sammen. 2024. "Innovative Solutions for Water Treatment: Unveiling the Potential of Polyoxazoline Polymer Activated Carbon Composite for Efficient Elimination of Lead Ions" Water 16, no. 3: 466. https://doi.org/10.3390/w16030466
APA StyleAmari, A., Boujelbene, M., Sami, F. M., Elboughdiri, N., Sonawane, C., Naganna, S. R., & Sammen, S. S. (2024). Innovative Solutions for Water Treatment: Unveiling the Potential of Polyoxazoline Polymer Activated Carbon Composite for Efficient Elimination of Lead Ions. Water, 16(3), 466. https://doi.org/10.3390/w16030466











