Regulating Denitrification in Constructed Wetlands: The Synergistic Role of Radial Oxygen Loss and Root Exudates
Abstract
1. Introduction
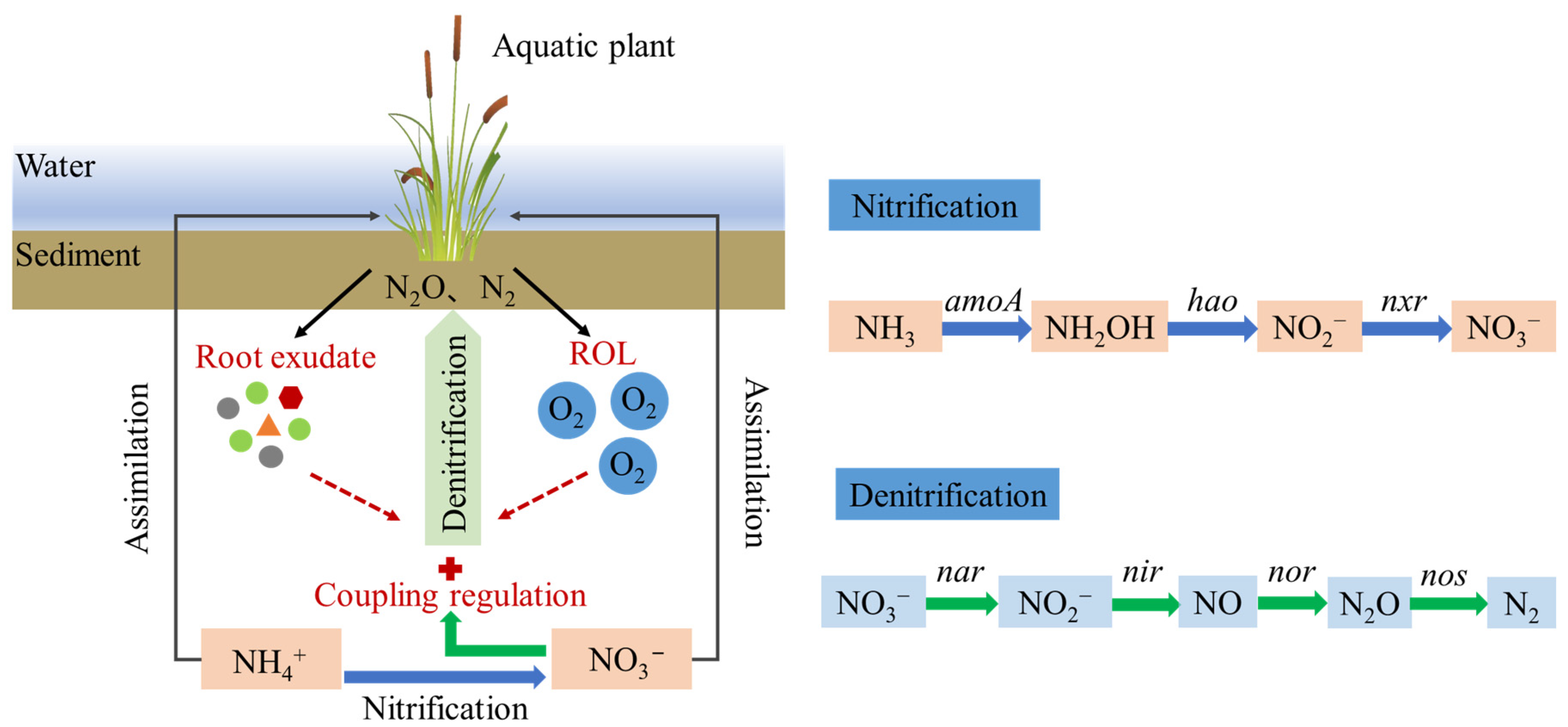
2. Denitrification Process and the Function of Wetland Plants
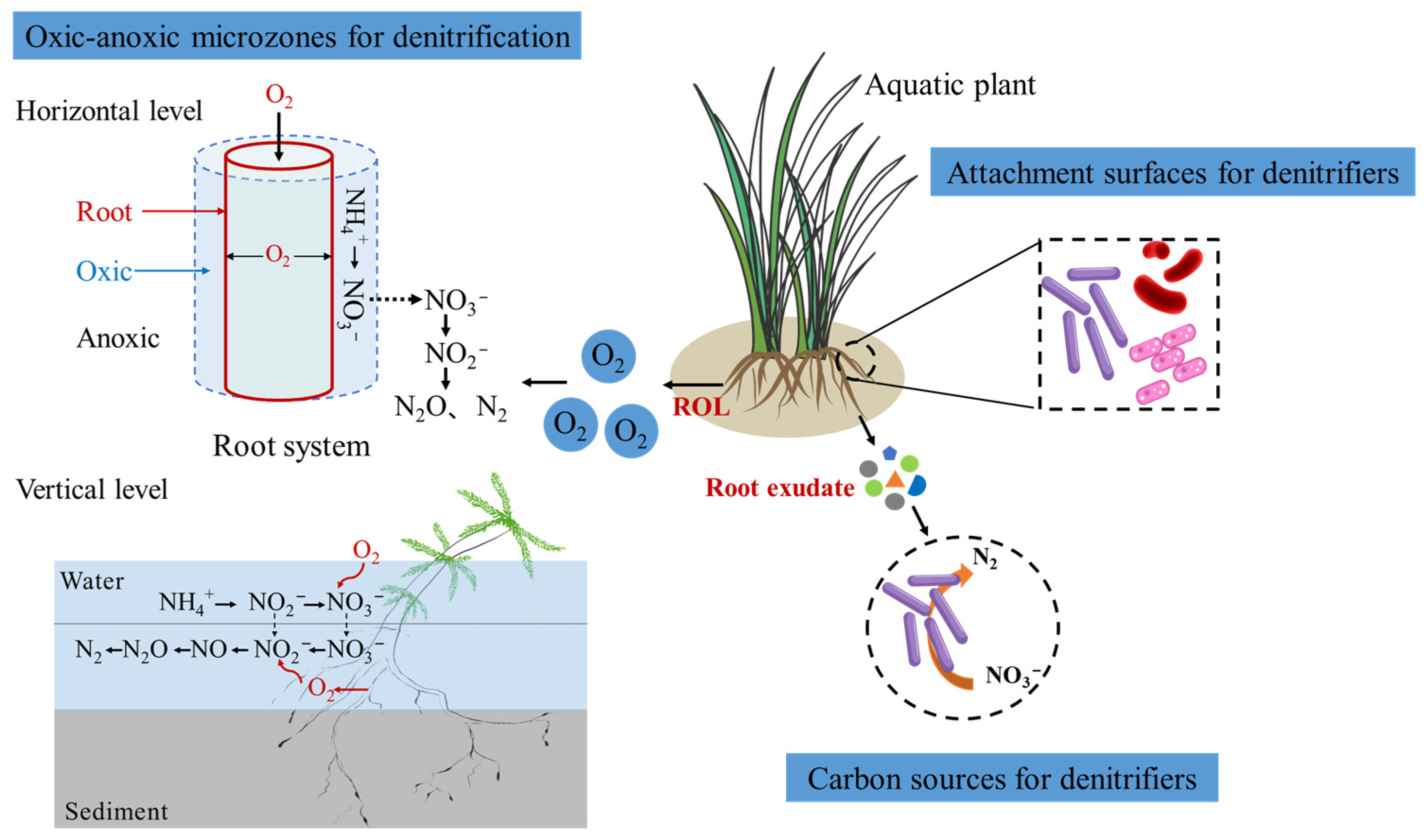
3. Mechanisms of Radial Oxygen Loss in Regulating Denitrification
4. Root Exudates and Their Effects on Denitrification
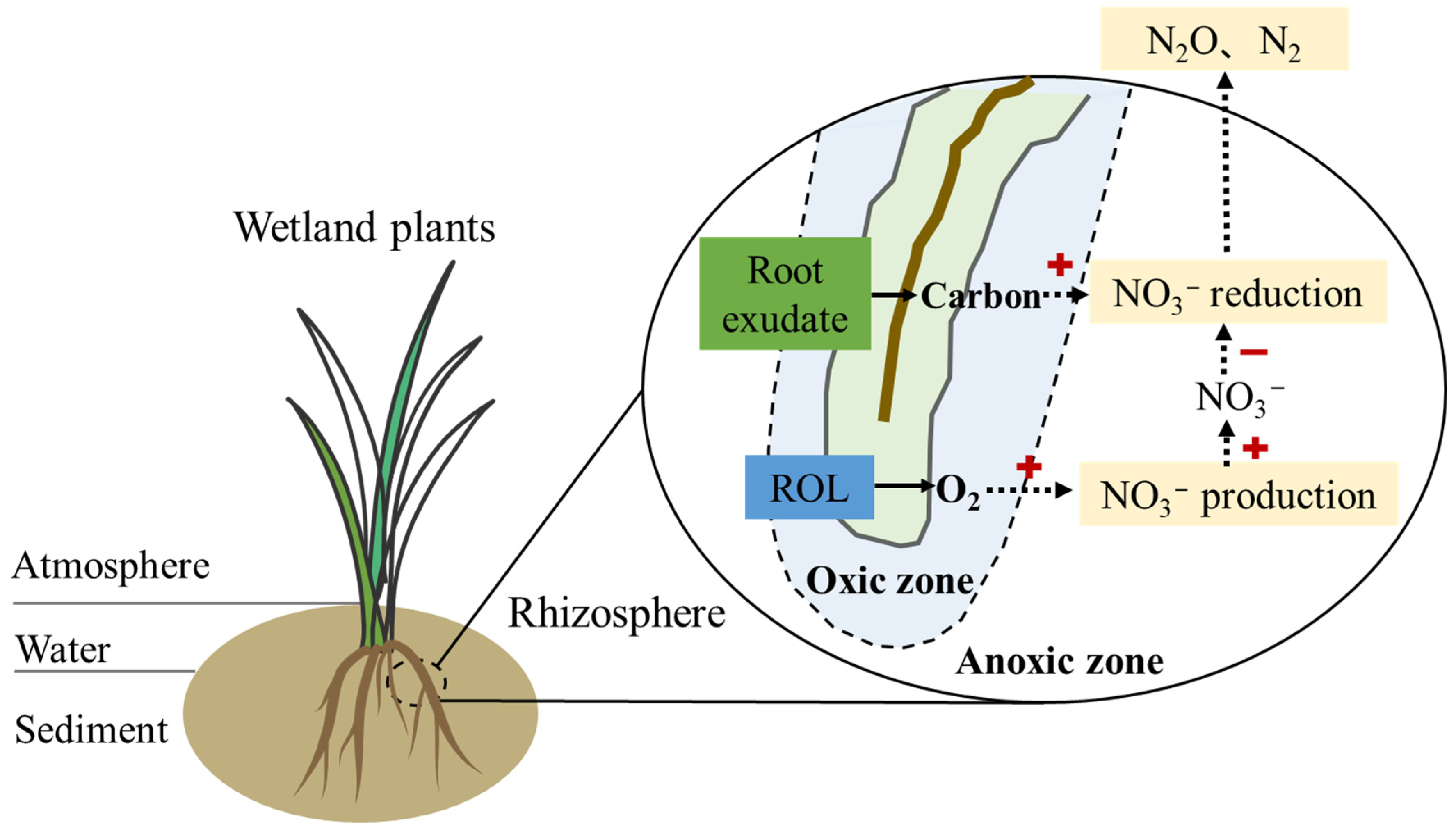
5. Coupling Effects of ROL and Root Exudates on Denitrification
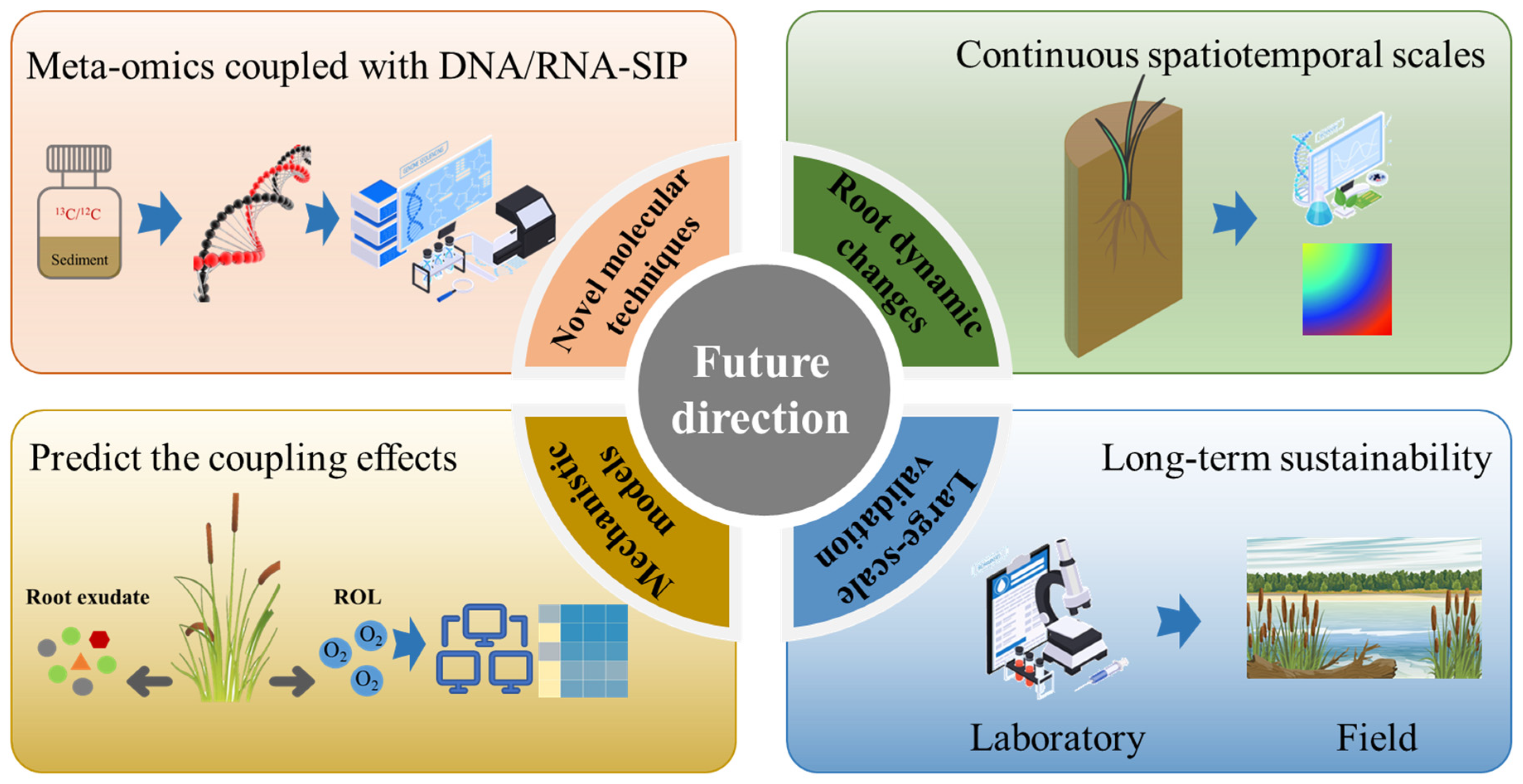
6. Limitations of Conventional Techniques and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holmes, D.E.; Dang, Y.; Smith, J.A. Nitrogen cycling during wastewater treatment. Adv. Appl. Microbiol. 2019, 106, 113–192. [Google Scholar]
- Zhao, X.; Chen, J.; Guo, M.; Li, C.; Hou, N.; Bai, S. Constructed wetlands treating synthetic wastewater in response to day-night alterations: Performance and mechanisms. Chem. Eng. J. 2022, 446, 137460. [Google Scholar] [CrossRef]
- Carabal, N.; Cardoso, L.S.; Padisák, J.; Selmeczy, G.B.; Puche, E.; Rodrigo, M.A. How a constructed wetland within a natural park enhances plankton communities after more than 10 years of operation: Changes over space and time. Environ. Res. 2024, 263, 120114. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, R.; Yan, P.; Wu, S.; Chen, Z.; Zhao, Y.; Cheng, C.; Hu, Z.; Zhuang, L.; Guo, Z. Constructed wetlands for pollution control. Nat. Rev. Earth. Environ. 2023, 4, 218–234. [Google Scholar] [CrossRef]
- Deng, S.; Cun, D.; Lin, R.; Peng, D.; Du, Y.; Wang, A.; Guan, B.; Tan, R.; Chang, J. Enhanced remediation of real agricultural runoff in surface-flow constructed wetlands by coupling composite substrate-packed bio-balls, submerged plants and functional bacteria: Performance and mechanisms. Environ. Res. 2024, 263, 120124. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Wei, D.; Zhang, J.; Hu, J.; Liu, Z.; Li, R. Natural pyrite to enhance simultaneous long-term nitrogen and phosphorus removal in constructed wetland: Three years of pilot study. Water Res. 2019, 148, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chachar, A.; Sun, S.; Peng, Y.; Gu, X.; He, S. Unveiling synergistic enhancement mechanism of nitrogen removal in surface flow constructed wetlands: Utilizing iron scraps and elemental sulfur as integrated electron donors. J. Environ. Manag. 2024, 370, 123006. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Du, Y.; Peng, W.; Zhang, S.; Liu, X.; Wang, S.; Yuan, S.; Kolditz, O. Modeling the impacts of plants and internal organic carbon on remediation performance in the integrated vertical flow constructed wetland. Water Res. 2021, 204, 117635. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, Z.; Kang, Y.; Fan, J.; Zhang, J. Recent advances in the enhanced nitrogen removal by oxygen-increasing technology in constructed wetlands. Ecotox. Environ. Safe 2020, 205, 111330. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, Y.; Ji, X.; Lu, C.; Zhang, J. Influences of plant species and radial oxygen loss on nitrous oxide fluxes in constructed wetlands. Ecol. Eng. 2020, 142, 105644. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, Z.; Hu, Q.; Hu, J.; Ni, C.; Li, F. Using stable isotopes to identify nitrogen transformations and estimate denitrification in a semi-constructed wetland. Sci. Total Environ. 2020, 720, 137628. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Lu, J.; Wang, Y.; Liu, G.; Hua, Y.; Wan, X.; Zhao, J.; Zhu, D. The abundance of nirS-type denitrifiers and anammox bacteria in rhizospheres was affected by the organic acids secreted from roots of submerged macrophytes. Chemosphere 2020, 240, 124903. [Google Scholar] [CrossRef] [PubMed]
- Faulwetter, J.L.; Gagnon, V.; Sundberg, C.; Chazarenc, F.; Burr, M.D.; Brisson, J.; Camper, A.K.; Stein, O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009, 35, 987–1004. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, X.; Fang, Y.; Xu, K.; He, S.; Zhao, M. Autotrophic denitrification in constructed wetlands: Achievements and challenges. Bioresour. Technol. 2020, 318, 123778. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.I.; Hallin, S.; Ecke, F.; Hubalek, V.; Juhanson, J.; Frainer, A.; McKie, B.G. Disentangling the roles of plant functional diversity and plaint traits in regulating plant nitrogen accumulation and denitrification in freshwaters. Funct. Ecol. 2022, 36, 921–932. [Google Scholar] [CrossRef]
- Gu, X.; Chen, D.; Wu, F.; Tang, L.; He, S.; Zhou, W. Function of aquatic plants on nitrogen removal and greenhouse gas emission in enhanced denitrification constructed wetlands: Iris pseudacorus for example. J. Cleaner Prod. 2022, 330, 129842. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Y.; Xie, H.; Yang, Z. Constructed wetlands: A review on the role of radial oxygen loss in the rhizosphere by macrophytes. Water 2018, 10, 678. [Google Scholar] [CrossRef]
- Tang, S.; Liao, Y.; Xu, Y.; Dang, Z.; Zhu, X.; Ji, G. Microbial coupling mechanisms of nitrogen removal in constructed wetlands: A review. Bioresour. Technol. 2020, 314, 123759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, S.; Gu, X.; Yu, Q.; He, S. Role of hydrophytes in constructed wetlands for nitrogen removal and greenhouse gases reduction. Bioresour. Technol. 2023, 388, 129759. [Google Scholar] [CrossRef]
- Pu, Y.; Li, Y.; Zhu, L.; Cheng, Y.; Nuamah, L.; Zhang, H.; Chen, H.; Du, G.; Wang, L.; Song, C. Long-term assessment on performance and seasonal optimal operation of a full-scale integrated multiple constructed wetland-pond system. Sci. Total Environ. 2023, 862, 161219. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wu, S.; Dai, Y.; Liang, W.; Wu, Z. Nitrogen removal from nitrate-laden wastewater by integrated vertical-flow constructed wetland systems. Ecol. Eng. 2013, 58, 192–201. [Google Scholar] [CrossRef]
- Hunt, P.G.; Stone, K.C.; Matheny, T.A.; Poach, M.E.; Vanotti, M.B.; Ducey, T.F. Denitrification of nitrified and non-nitrified swine lagoon wastewater in the suspended sludge layer of treatment wetlands. Ecol. Eng. 2009, 35, 1514–1522. [Google Scholar] [CrossRef]
- Sindilariu, P.-D.; Wolter, C.; Reiter, R. Constructed wetlands as a treatment method for effluents; from intensive trout farms. Aquaculture 2008, 277, 179–184. [Google Scholar] [CrossRef]
- Li, M.; Duan, R.; Hao, W.; Li, Q.; Arslan, M.; Liu, P.; Qi, X.; Huang, X.; El-Din, M.G.; Liang, P. High-rate nitrogen removal from carbon limited wastewater using sulfur-based constructed wetland: Impact of sulfur sources. Sci. Total Environ. 2020, 744, 140969. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, Y.; Tam, N.F.Y.; Wang, Y.; Li, L. Roles of root porosity, radial oxygen loss, Fe plaque formation on nutrient removal and tolerance of wetland plants to domestic wastewater. Water Res. 2014, 50, 147–159. [Google Scholar] [CrossRef]
- Sun, L.; Lu, Y.; Yu, F.; Kronzucker, H.J.; Shi, W. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 2016, 212, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. Res. 2021, 28, 54497–54510. [Google Scholar] [CrossRef]
- Oburger, E.; Schmidt, H. New methods to unravel rhizosphere processes. Trends Plant Sci. 2016, 21, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Poole, P. Shining a light on the dark world of plant root-microbe interactions. Proc. Natl. Acad. Sci. USA 2017, 114, 4281–4283. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Wang, J.Y.; Al-Babili, S. Metabolomics of plant root exudates: From sample preparation to data analysis. Front. Plant Sci. 2022, 13, 1062982. [Google Scholar] [CrossRef] [PubMed]
- Achouak, W.; Haichar, F.E.Z. Stable isotope probing of microbiota structure and function in the plant rhizosphere. Front. Plant Sci. 2019, 2046, 233–243. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Torrens, A.; de la Varga, D.; Ndiaye, A.K.; Folch, M.; Coly, A. Innovative multistage constructed wetland for municipal wastewater treatment and reuse for agriculture in senegal. Water 2020, 12, 3139. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Liu, L.; Zhuang, L.; Zhao, S.; Su, Y.; Li, Y.; Wang, M.; Wang, C.; Xu, L. Microbial nitrogen cycle hotspots in the plant-bed/ditch system of a constructed wetland with N2O mitigation. Environ. Sci. Technol. 2018, 52, 6226–6236. [Google Scholar] [CrossRef]
- Reddy, K.; Patrick Jr, W.; Lindau, C. Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnol. Oceanogr. 1989, 34, 1004–1013. [Google Scholar] [CrossRef]
- Lamers, L.P.; Van Diggelen, J.M.; Op den Camp, H.J.; Visser, E.J.; Lucassen, E.C.; Vile, M.A.; Jetten, M.S.; Smolders, A.J.; Roelofs, J.G. Microbial transformations of nitrogen, sulfur, and iron dictate vegetation composition in wetlands: A review. Front. Microbiol. 2012, 3, 156. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, M.; Liu, F.; Chen, L.; Li, Y.; Li, Y.; Xiao, R.; Wu, J. Seasonality distribution of the abundance and activity of nitrification and denitrification microorganisms in sediments of surface flow constructed wetlands planted with Myriophyllum elatinoides during swine wastewater treatment. Bioresour. Technol. 2018, 248, 89–97. [Google Scholar] [CrossRef]
- Li, C.; Ding, S.; Ma, X.; Chen, M.; Zhong, Z.; Zhang, Y.; Ren, M.; Zhang, M.; Yang, L.; Rong, N.; et al. O2 distribution and dynamics in the rhizosphere of Phragmites australis, and implications for nutrient removal in sediments. Environ. Pollut. 2021, 287, 117193. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, M.; Zhang, T.; Bai, S.; Meng, Y.; Tian, Y.; Yang, J.; Ma, F. Spatiotemporal dynamics of root exudates drive microbial adaptation mechanisms under day-night alterations in constructed wetlands. Chem. Eng. J. 2023, 477, 147311. [Google Scholar] [CrossRef]
- Sun, H.; Xu, S.; Wu, S.; Wang, R.; Zhuang, G.; Bai, Z.; Deng, Y.; Zhuang, X. Enhancement of facultative anaerobic denitrifying communities by oxygen release from roots of the macrophyte in constructed wetlands. J. Environ. Manag. 2019, 246, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Huang, R.; Zeng, J.; Zhao, D.; He, R.; Yu, Z.; Wu, Q.L. Rhizosphere-associated nosZ II microbial community of Phragmites australis and its influence on nitrous oxide emissions in two different regions. J. Soils Sediments 2021, 21, 3326–3341. [Google Scholar] [CrossRef]
- Ruiz-Rueda, O.; Hallin, S.; Baneras, L. Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. FEMS Microbiol. Ecol. 2009, 67, 308–319. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; He, X.; Zhang, S.; Liang, R.; Shen, J. Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci. Total Environ. 2017, 598, 697–703. [Google Scholar] [CrossRef]
- Zhang, N.; Hong, Z.; Qiu, R.; Chao, Y.; Yu, Y.; Dan, A. Removal pathway quantification and co-metabolic mechanism evaluation of alkylphenols from synthetic wastewater by phenolic root exudates in the rhizosphere of Phragmites australis. J. Hazard. Mater. 2022, 424, 127269. [Google Scholar] [CrossRef]
- Vila-Costa, M.; Pulido, C.; Chappuis, E.; Calvino, A.; Casamayor, E.O.; Gacia, E. Macrophyte landscape modulates lake ecosystem-level nitrogen losses through tightly coupled plant-microbe interactions. Limnol. Oceanogr. 2016, 61, 78–88. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Wang, R.; Hu, Y.; Li, W.; Cui, L. Specific root length regulated the rhizosphere effect on denitrification across distinct macrophytes. Geoderma 2024, 449, 117002. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.; Dzakpasu, M.; Gao, Z.; Zhou, W.; Zhu, R.; Xiong, J. Assessment of plants radial oxygen loss for nutrients and organic matter removal in full-scale constructed wetlands treating municipal effluents. Bioresour. Technol. 2022, 360, 127545. [Google Scholar] [CrossRef] [PubMed]
- Salvato, M.; Borin, M.; Doni, S.; Macci, C.; Ceccanti, B.; Marinari, S.; Masciandaro, G. Wetland plants, micro-organisms and enzymatic activities interrelations in treating N polluted water. Ecol. Eng. 2012, 47, 36–43. [Google Scholar] [CrossRef]
- Di, L.; Li, Y.; Nie, L.; Wang, S.; Kong, F. Influence of plant radial oxygen loss in constructed wetland combined with microbial fuel cell on nitrobenzene removal from aqueous solution. J. Hazard. Mater. 2020, 394, 122542. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, J.; Kemp, W. Seasonal and spatial patterns of oxygen production, respiration and root-rhizome release in Potamogeton perfoliatus L. and Zostera marina L. Aquat. Bot. 1991, 40, 109–128. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Laskov, C.; Horn, O.; Hupfer, M. Environmental factors regulating the radial oxygen loss from roots of Myriophyllum spicatum and Potamogeton crispus. Aquat. Bot. 2006, 84, 333–340. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Zhang, J.; Ngo, H.H.; Guo, W.; Liang, S.; Fan, J.; Lu, S.; Wu, H. Optimizations on supply and distribution of dissolved oxygen in constructed wetlands: A review. Bioresour. Technol. 2016, 214, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Brodersen, K.E.; Jakobsen, S.L.; Kühl, M. Optical sensor nanoparticles in artificial sediments–a new tool to visualize O2 dynamics around the rhizome and roots of seagrasses. Environ. Sci. Technol. 2015, 49, 2286–2292. [Google Scholar] [CrossRef]
- Maisch, M.; Lueder, U.; Kappler, A.; Schmidt, C. Iron lung: How rice roots induce iron redox changes in the rhizosphere and create viches for microaerophilic Fe(II)-oxidizing bacteria. Environ. Sci. Technol. Lett. 2019, 6, 600–605. [Google Scholar] [CrossRef]
- Coyotzi, S.; Doxey, A.C.; Clark, I.D.; Lapen, D.R.; Van Cappellen, P.; Neufeld, J.D. Agricultural soil denitrifiers possess extensive nitrite reductase gene diversity. Environ. Microbiol. 2017, 19, 1189–1208. [Google Scholar] [CrossRef]
- Bai, S.; Chen, J.; Guo, M.; Ren, N.; Zhao, X. Vertical-scale spatial influence of radial oxygen loss on rhizosphere microbial community in constructed wetland. Environ. Int. 2023, 171, 107690. [Google Scholar] [CrossRef]
- Wiessner, A.; Kuschk, P.; Kästner, M.; Stottmeister, U. Abilities of helophyte species to release oxygen into rhizospheres with varying redox conditions in laboratory-scale hydroponic systems. Int. J. Phytoremediat. 2002, 4, 1–15. [Google Scholar] [CrossRef]
- Soda, S.; Ike, M.; Ogasawara, Y.; Yoshinaka, M.; Mishima, D.; Fujita, M. Effects of light intensity and water temperature on oxygen release from roots into water lettuce rhizosphere. Water Res. 2007, 41, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Kim, D.-H.; Oh, S.; Moon, H.S. Effects of water level and vegetation on nitrate dynamics at varying sediment depths in laboratory-scale wetland mesocosms. Sci. Total Environ. 2020, 703, 134741. [Google Scholar] [CrossRef]
- Marzocchi, U.; Benelli, S.; Larsen, M.; Bartoli, M.; Glud, R.N. Spatial heterogeneity and short-term oxygen dynamics in the rhizosphere of Vallisneria spiralis: Implications for nutrient cycling. Freshw. Biol. 2019, 64, 532–543. [Google Scholar] [CrossRef]
- Yuan, H.; Cai, Y.; Yang, Z.; Li, Q.; Liu, E.; Yin, H. Phosphorus removal from sediments by Potamogeton crispus: New high-resolution in situ evidence for rhizosphere assimilation and oxidization-induced retention. J. Environ. Sci. 2021, 109, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, Y.; Zhang, Y.; Song, B.; Li, H.; Chen, Z. Effects of root organic exudates on rhizosphere microbes and nutrient removal in the constructed wetlands. Ecol. Eng. 2016, 92, 243–250. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Zhang, Y.; Li, T.; Ge, H.; Xia, S.; Gu, J.; Zhang, H.; Lü, B.; Wu, X. Rice root morphological and physiological traits interaction with rhizosphere soil and its effect on methane emissions in paddy fields. Soil Biol. Biochem. 2019, 129, 191–200. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Fang, J.; Tao, Y.; Liu, J.; Lyu, T.; Yang, X.; Ma, S.; Dong, J.; Dou, H.; Zhang, H. Effects of emergent plants on soil carbon-fixation and denitrification processes in freshwater and brackish wetlands in a watershed in northern China. Geoderma 2023, 430, 116311. [Google Scholar] [CrossRef]
- Langarica-Fuentes, A.; Manrubia, M.; Giles, M.E.; Mitchell, S.; Daniell, T.J. Effect of model root exudate on denitrifier community dynamics and activity at different water-filled pore space levels in a fertilised soil. Soil Biol. Biochem. 2018, 120, 70–79. [Google Scholar] [CrossRef]
- Zhai, X.; Piwpuan, N.; Arias, C.A.; Headley, T.; Brix, H. Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecol. Eng. 2013, 61, 555–563. [Google Scholar] [CrossRef]
- Seitz, V.A.; McGivern, B.B.; Daly, R.A.; Chaparro, J.M.; Borton, M.A.; Sheflin, A.M.; Kresovich, S.; Shields, L.; Schipanski, M.E.; Wrighton, K.C.; et al. Variation in root exudate composition influences soil microbiome membership and function. Appl. Environ. Microbiol. 2022, 88, e00226. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, X.; He, X. Effects of selected root exudate components on nitrogen removal and development of denitrifying bacteria in constructed wetlands. Water 2017, 9, 430. [Google Scholar] [CrossRef]
- Maurer, D.; Malique, F.; Alfarraj, S.; Albasher, G.; Horn, M.A.; Butterbach-Bahl, K.; Dannenmann, M.; Rennenberg, H. Interactive regulation of root exudation and rhizosphere denitrification by plant metabolite content and soil properties. Plant Soil 2021, 467, 107–127. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; Van Der Heijden, M.G. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Hu, Y.; Ding, J.; Ma, Q.; Zong, K.; Yang, Z. Effect of carbon source derived from macrophytes on microbial denitrification in constructed wetlands: Role of plant species. Bioresour. Technol. Rep. 2019, 7, 100217. [Google Scholar] [CrossRef]
- Rummel, P.S.; Well, R.; Pfeiffer, B.; Dittert, K.; Floßmann, S.; Pausch, J. Nitrate uptake and carbon exudation–do plant roots stimulate or inhibit denitrification? Plant Soil 2021, 459, 217–233. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, P.; Wang, Q.; Deng, S.; He, X.; Zhang, X.; Wang, R.; Feng, Q.; Yin, H. Temperature rather than N availability determines root exudation of alpine coniferous forests on the eastern Tibetan Plateau along elevation gradients. Tree Physiol. 2023, 43, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiao, M.; Li, D.; Yin, H.; Liu, Q. Do warming-induced changes in quantity and stoichiometry of root exudation promote soil N transformations via stimulation of soil nitrifiers, denitrifiers and ammonifiers? Eur. J. Soil Biol. 2016, 74, 60–68. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, Z.; Li, B.; Bai, G.; Yang, L.; Chi, Y.; Wang, M.; Ren, Y. Root vertical spatial stress: A method for enhancing rhizosphere effect of plants in subsurface flow constructed wetland. Environ. Res. 2023, 231, 116083. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Texier, S.; Hallet, S.; Bru, D.; Dambreville, C.; Chèneby, D.; Bizouard, F.; Germon, J.C.; Philippot, L. Disentangling the rhizosphere effect on nitrate reducers and denitrifiers: Insight into the role of root exudates. Environ. Microbiol. 2008, 10, 3082–3092. [Google Scholar] [CrossRef]
- Wiesenbauer, J.; Koenig, A.; Gorka, S.; Marchand, L.; Nunan, N.; Kitzler, B.; Inselsbacher, E.; Kaiser, C. A pulse of simulated root exudation alters the composition and temporal dynamics of microbial metabolites in its immediate vicinity. Soil Biol. Biochem. 2024, 189, 109259. [Google Scholar] [CrossRef]
- Benelli, S.; Ribaudo, C.; Bertrin, V.; Bartoli, M.; Fano, E.A. Effects of macrophytes on potential nitrification and denitrification in oligotrophic lake sediments. Aquat. Bot. 2020, 167, 103287. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Ye, C.B.; Zhou, Z.M.; Ni, B.S.; Zhang, X.M.; Liu, H. Unveiling organic loading shock-resistant mechanism in a pilot-scale moving bed biofilm reactor-assisted dual-anaerobic-anoxic/oxic system for effective municipal wastewater treatment. Bioresour. Technol. 2022, 347, 126339. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Ye, Z. Root-induced changes of pH, Eh, Fe(II) and fractions of Pb And Zn in rhizosphere soils of four wetland plants with different radial oxygen losses. Pedosphere 2012, 22, 518–527. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, C.; Li, J.; Liu, Z.; Wang, J. Root plasticity of Populus euphratica seedlings in response to different water table depths and contrasting sediment types. PLoS ONE 2015, 10, e0118691. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yuan, G.; Cao, T.; Ni, L.; Zhang, M.; Wang, S. An alternative mechanism for shade adaptation: Implication of allometric responses of three submersed macrophytes to water depth. Ecol. Res. 2012, 27, 1087–1094. [Google Scholar] [CrossRef]
- Richards, J.H.; Troxler, T.G.; Lee, D.W.; Zimmerman, M.S. Experimental determination of effects of water depth on Nymphaea odorata growth, morphology and biomass allocation. Aquat. Bot. 2011, 95, 9–16. [Google Scholar] [CrossRef]
- Sas, L.; Rengel, Z.; Tang, C. Excess cation uptake, and extrusion of protons and organic acid anions by Lupinus albus under phosphorus deficiency. Plant Sci. 2001, 160, 1191–1198. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, S.; Lin, D.; Guo, C.; Yan, L.; Wang, S.; He, Z. Nitrogen loading affects microbes, nitrifiers and denitrifiers attached to submerged macrophyte in constructed wetlands. Sci. Total Environ. 2018, 622, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Guo, D.; Zhang, J.; Zhang, T.; Yang, L.; Li, B.; Lan, J.; Ren, Y. Construction of an ideotype root system architecture of subsurface flow constructed wetland macrophytes by vertical spatial stress: Strengthening of rhizosphere effects and determination of appropriate substrate depth. Environ. Res. 2024, 259, 119523. [Google Scholar] [CrossRef]
- Yang, L.; Shen, L.; Tao, J.; Xiao, D.; Shi, Q.; Wang, Y.; Zheng, X.; Zhao, M.; Han, W. Effects of plant species diversity and density of Acorus calamus and Reineckea carnea on nitrogen removal and plant growth in constructed wetlands during the cold season. Environ. Eng. Sci. 2024, 29, 161219. [Google Scholar] [CrossRef]
- Hu, X.; Yue, J.; Yao, D.; Zhang, X.; Li, Y.; Hu, Z.; Liang, S.; Wu, H.; Xie, H.; Zhang, J. Plant development alters the nitrogen cycle in subsurface flow constructed wetlands: Implications to the strategies for intensified treatment performance. Water Res. 2023, 246, 120750. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Rahaman, M.H.; Zhai, J.; Makinia, J. Coupling transformation of carbon, nitrogen and sulfur in a long-term operated full-scale constructed wetland. Sci. Total Environ. 2021, 777, 146016. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Dong, J.; Li, C.; Chen, H.; Wang, L.; Lyu, T.; He, H.; Liu, J. Response of microbial community composition and function to emergent plant rhizosphere of a constructed wetland in northern China. Appl. Soil Ecol. 2021, 168, 104141. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Liu, W.; Jiang, H.; Wang, M.; Ge, Y.; Chang, J. Denitrifying bacterial community dominantly drove nitrogen removals in vertical flow constructed wetlands as impacted by macrophyte planting patterns. Chemosphere 2021, 281, 130418. [Google Scholar] [CrossRef]
- Blossfeld, S.; Gansert, D.; Thiele, B.; Kuhn, A.J.; Loesch, R. The dynamics of oxygen concentration, pH value, and organic acids in the rhizosphere of Juncus spp. Soil Biol. Biochem. 2011, 43, 1186–1197. [Google Scholar] [CrossRef]
- Du, L.; Trinh, X.; Chen, Q.; Wang, C.; Wang, H.; Xia, X.; Zhou, Q.; Xu, D.; Wu, Z.B. Enhancement of microbial nitrogen removal pathway by vegetation in Integrated Vertical-Flow Constructed Wetlands (IVCWs) for treating reclaimed water. Bioresour. Technol. 2018, 249, 644–651. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, J.; Hu, F.; Zhang, J.; Chen, P.; Yuan, Z.; Xu, Z. Microbial community succession and responses to internal environmental drivers throughout the operation of constructed wetlands. Environ. Res. 2024, 259, 119522. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, S. A review on nirS-type and nirK-type denitrifiers via a scientometric approach coupled with case studies. Environ. Sci. Proc. Imp. 2022, 24, 221–232. [Google Scholar] [CrossRef]
- Ma, Y.; Zilles, J.; Kent, A. An evaluation of primers for detecting denitrifiers via their functional genes. Environ. Microbiol. 2019, 21, 1196–1210. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, S.; Jiang, Y.; Jiang, C.; Wang, D.; Xu, G.; Yang, D.; Wu, S.; Bai, Z.; Zhuang, G.; et al. Enhancing nitrogen removal from anaerobically-digested swine wastewater through integration of Myriophyllum aquaticum and free nitrous acid-based technology in a constructed wetland. Sci. Total Environ. 2021, 779, 146441. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, H.; Ma, Z.; You, X.; Wei, X.; Li, Y.; Tian, Y. Meta-analyzing the mechanism of pyrogenic biochar strengthens nitrogen removal performance in sulfur-driven autotrophic denitrification system: Evidence from metatranscriptomics. Water Res. 2024, 253, 121296. [Google Scholar] [CrossRef]
- Chen, K.; Feng, J.; Bodelier, P.L.E.; Yang, Z.; Huang, Q.; Delgado-Baquerizo, M.; Cai, P.; Tan, W.; Liu, Y. Metabolic coupling between soil aerobic methanotrophs and denitrifiers in rice paddy fields. Nat. Commun. 2024, 15, 3471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, W.; Wang, X. New Insights into How Increases in Fertility Improve the Growth of Rice at the Seedling Stage in Red Soil Regions of Subtropical China. PLoS ONE 2014, 9, e109161. [Google Scholar] [CrossRef]
- Nyer, S.C.; Volkenborn, N.; Aller, R.C.; Graffam, M.; Zhu, Q.; Price, R.E. Nitrogen transformations in constructed wetlands: A closer look at plant-soil interactions using chemical imaging. Sci. Total Environ. 2022, 816, 151560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Li, X.; Li, J.; Zhao, Z.; Hou, X. Mixed culture of plants improved nutrient removal in constructed wetlands: Response of microbes and root exudates. Environ. Sci. Pollut. Res. 2023, 30, 5861–5872. [Google Scholar] [CrossRef]
- Bognár, Z.; Mosshammer, M.; Brodersen, K.E.; Bollati, E.; Gyurcsányi, R.E.; Kühl, M. Multiparameter sensing of oxygen and pH at biological interfaces via hyperspectral imaging of luminescent sensor nanoparticles. ACS Sens. 2024, 9, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.; Xia, X.; Yang, J.J.; Liu, J.; Remusat, L.; Rumpel, C.; Bloem, E.; Krasny, B.B.; Schnug, E. Speciation and distribution of chromium (III) in rice root tip and mature zone: The significant impact of root exudation and iron plaque on chromium bioavailability. J. Hazard. Mater. 2023, 448, 130992. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.P.; Zhu, Y.F.; Kianfar, E. Nano Biosensors: Properties, applications and electrochemical techniques. J. Mater. Res. Technol.-JMRT 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Cai, B.; Zhang, Y.; Wang, X.; Li, Y. An optimization model for a wetland restoration project under uncertainty. Int. J. Environ. Res. Public Health 2018, 15, 2795. [Google Scholar] [CrossRef]
- Feng, S.; Xu, S.; Zhang, X.; Wang, R.; Ma, X.; Zhao, Z.; Zhuang, G.; Bai, Z.; Zhuang, X. Myriophyllum aquaticum-based surface flow constructed wetlands for enhanced eutrophic nutrient removal-A case study from laboratory-scale up to pilot-scale constructed wetland. Water 2018, 10, 1391. [Google Scholar] [CrossRef]
- Wang, G.; Li, T.; Zhou, Q.; Zhang, X.; Li, R.; Wang, J. Characterization and environmental applications of soil biofilms: A review. Environ. Chem. Lett. 2024, 22, 1989–2011. [Google Scholar] [CrossRef]
- McDaniel, E.A.; Wahl, S.A.; Ishii, S.I.; Pinto, A.; Ziels, R.; Nielsen, P.H.; McMahon, K.D.; Williams, R.B.H. Prospects for multi-omics in the microbial ecology of water engineering. Water Res. 2021, 205, 117608. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Pioli, R.; Stocker, R.; Berry, D. Single-cell stable isotope probing in microbial ecology. ISME Commun. 2022, 2, 55. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kuramae, E.E.; Jia, Z.; Liu, B. Stable isotope probing reveals compositional and functional shifts in active denitrifying communities along the soil profile in an intensive agricultural area. Sci. Total Environ. 2024, 907, 167968. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Htun, N.N.; Schlenz, F.; Kasimati, A.; Verbert, K. A review of visualisations in agricultural decision support systems: An HCI perspective. Comput. Electron. Agric. 2019, 163, 104844. [Google Scholar] [CrossRef]
- Wu, N.; Shi, W.; Zhang, L.; Wang, H.; Liu, W.; Ren, Y.; Li, X.; Gao, Z.; Wang, X. Dynamic alterations and ecological implications of rice rhizosphere bacterial communities induced by an insect-transmitted reovirus across space and time. Microbiome 2024, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, Y.; Zhao, Q.; He, T.; Mao, T.; Zhang, T.; Zhang, L.; Su, W. The quantification of root exudation by an in-situ method based on root morphology over three incubation periods. Front. Plant Sci. 2024, 15, 1423703. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, D.P.; Thompson, D.; Joshi, M.; Mishra, A.K.; Joshi, V. Unraveling the spatio-temporal dynamics of soil and root-associated microbiomes in Texas olive orchards. Sci. Rep. 2024, 14, 18214. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Kim, B.S.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Constructed wetlands for the removal of organic micropollutants from wastewater: Current status, progress, and challenges. Chemosphere 2024, 360, 142364. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Cheng, X.; Jiang, S.; Pan, J.; Zhu, D.; Lu, Z.; Jiang, Y.; Liu, C.; Guo, H.; Xie, J. Unveiling the power of COD/N on constructed wetlands in a short-term experiment: Exploring microbiota co-occurrence patterns and assembly dynamics. Sci. Total Environ. 2024, 912, 169568. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Han, X.; Hu, J.; Chen, H.; Zhai, Y. Jointly considering multi-medium and full-cycle to better reveal distribution and removal of antibiotic resistance genes in long-term constructed wetland. Sci. Total Environ. 2024, 955, 177276. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ge, Z.; Zhou, X.; Li, S.; Li, X.; Tang, J. Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: A global meta-analysis. Glob. Change Biol. 2020, 26, 1638–1653. [Google Scholar] [CrossRef] [PubMed]
- Ealias, A.M.; Meda, G.; Tanzil, K. Recent Progress in Sustainable Treatment Technologies for the Removal of Emerging Contaminants from Wastewater: A Review on Occurrence, Global Status and Impact on Biota. Rev. Environ. Contam. Toxicol. 2024, 262, 16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Zhou, Y.; Jiang, C. Regulating Denitrification in Constructed Wetlands: The Synergistic Role of Radial Oxygen Loss and Root Exudates. Water 2024, 16, 3706. https://doi.org/10.3390/w16243706
Sun H, Zhou Y, Jiang C. Regulating Denitrification in Constructed Wetlands: The Synergistic Role of Radial Oxygen Loss and Root Exudates. Water. 2024; 16(24):3706. https://doi.org/10.3390/w16243706
Chicago/Turabian StyleSun, Haishu, Yuan Zhou, and Cancan Jiang. 2024. "Regulating Denitrification in Constructed Wetlands: The Synergistic Role of Radial Oxygen Loss and Root Exudates" Water 16, no. 24: 3706. https://doi.org/10.3390/w16243706
APA StyleSun, H., Zhou, Y., & Jiang, C. (2024). Regulating Denitrification in Constructed Wetlands: The Synergistic Role of Radial Oxygen Loss and Root Exudates. Water, 16(24), 3706. https://doi.org/10.3390/w16243706






