Abstract
Constructed wetland (CW) is a critical ecological engineering for wastewater treatment and improvement of water quality. Nitrogen (N) removal is one of the vital functions of CWs during operation, and N treatment in CWs is mainly affected by aquatic plants and denitrification carried out by microbes. However, due to their low efficiency and instability in N removal, further applications of CWs are limited. The review provides a view of two basic characteristics of aquatic plants, radial oxygen loss (ROL) and root exudates, and their coupled effect on denitrification processes in CWs. First, the role of aquatic plants in denitrification is presented. The individual roles of ROL and root exudates in regulating denitrification, as well as their interaction in this process, have been discussed. Also, the limitation of conventional techniques to reveal interaction between the plant and the microbes has been highlighted. Further research on coupling regulatory mechanisms of ROL and root exudates may be conducted to develop an optimal wetland design and improve biological N removal. This review offers new insights and directions for improving N removal in CWs by utilizing the synergistic effects of plant ROL and root exudates.
1. Introduction
Human activities have severely interfered with the global nitrogen (N) cycle, with excessive application of N fertilizers and N emissions from livestock, domestic, and industrial sources leading to severe water pollution [1]. The discharge of N-containing wastewater not only poses serious threats to human health and triggers a cascade of social and environmental issues, but also exacerbates the growing water scarcity crisis [1]. Constructed wetlands (CWs), as a cost-effective and environmentally friendly wastewater treatment method, have emerged as an efficient approach for wastewater treatment and reclaimed water reuse, particularly in rural and suburban areas [2,3]. They exhibit remarkable application advantages and ecological value in watershed water environment management and advanced wastewater treatment, playing an indispensable role in enhancing the quality of aquatic ecosystems [4,5]. However, the low efficiency and instability of N removal hinder their widespread application [6,7].
As aquatic ecosystems are primarily composed of plants and microorganisms, N removal in CWs depends largely on the uptake by aquatic plants and microbial processes of nitrification and denitrification [8,9]. Plants play a crucial role in CWs by providing attachment surfaces for microbes, releasing oxygen through their roots, and supplying organic carbon through root exudates [10,11]. Even though plants do not take a direct role in microbial nitrification and denitrification, aquatic plant traits can considerably modify the microbial N cycle [10,11]. Therefore, elucidating the effect of aquatic plants on the N cycle has important scientific value for understanding plant–microbe interactions and holds vital practical significance for wetland design optimization and N removal enhancement.
Denitrification is one of the main pathways for N removal, during which nitrate is converted to gaseous nitrogen, thus being completely removed from systems [12,13]. The denitrification process in CWs is affected by various environmental variables including oxygen content, nitrate supply, and the availability of carbon sources [14,15]. Aquatic plants could influence these variables because of their special characteristics, among these, radial oxygen loss (ROL) and root exudates are considered the most important factors [16,17]. The most notable feature of CWs is that they are located in regions that experience intermittent or persistent flooding [11,18]. To survive in hypoxic environments, plants transport oxygen produced during photosynthesis to the rhizosphere through the aerenchyma, thereby satisfying their respiratory requirements and discharging a portion of the oxygen into the root zone. This process, known as radial oxygen loss (ROL), is a unique adaptation of aquatic plants to waterlogging [18]. ROL reduces root damage caused by the accumulation of harmful reductive compounds such as ferrous iron and sulfide [8,18]. Most of the literature has focused on the multi-functions of aquatic plants in CWs, as it provides colonizing habitats for microorganisms, influences the conditions of the rhizosphere, and alters the availability of nutrients [19,20]. Nevertheless, the synergistic effect of ROL and root exudates on denitrification is not yet fully understood.
Despite the potential of CWs for wastewater treatment, their N removal efficiency is often hampered by various challenges, such as seasonal variations and site-specific factors. Seasonal variations in temperature, precipitation, and plant growth can significantly affect the performance of CWs [21,22]. For example, lower temperatures in winter reduce microbial activity and plant growth, leading to lower N removal rates [8]. Site-specific factors, such as influent wastewater characteristics, hydraulic loading rates, and substrate properties, also affect the effectiveness of ROL and root exudates in promoting denitrification [23,24,25]. Moreover, the effectiveness of ROL and root exudates in enhancing N removal may vary depending on the plant species, growth stage, and environmental conditions [26,27]. These challenges highlight the need for a better understanding of the complex interactions between plants, microbes, and environmental factors in CWs to optimize their design and operation for efficient N removal.
Furthermore, conventional techniques used to study the role of ROL and root exudates in regulating denitrification in CWs have limitations. First, the collection, purification, and identification of root exudates need improvement. Although hydroponics is the most widely used method for collecting root exudates, as it prevents the uptake of soil particles, it fails to provide an accurate representation of field conditions [28]. Second, continuously tracking and visualizing the spatiotemporal dynamics of plant roots remains a challenge. Despite the great potential of advanced techniques such as isotope imaging, chemical imaging, and microbial imaging, data acquisition is labor-intensive, expensive, and restricted to limited time points and sample points [29]. Final, the primary bottleneck in studying plant–microbial interactions lies in the difficulty of observing and investigating the root system without disturbance [29,30]. To bridge these technological gaps, novel approaches such as stable isotope probing, real-time monitoring techniques, and metabolomics are needed to link plant traits with microbial functions and elucidate the coupling effects of ROL and root exudates on denitrification [31,32]. Developing mechanistic models that simulate the dynamics of ROL and root exudates under different environmental conditions can further advance our understanding of their interactions and guide the optimization of CWs for enhanced N removal.
This review aims to illustrate the linkage of ROL, root exudates, and the denitrification process in CWs. The role of aquatic plants in denitrification was discussed. The mechanisms of ROL and root exudates affecting denitrification were then thoroughly investigated. Furthermore, it sheds light on the coupling effect of ROL and root exudates on denitrification. Final, the limitations of traditional methods and the future direction of research are analyzed. This review presents an innovative idea to enhance N removal from CWs by harnessing the combined effects of plant ROL and root exudates.
2. Denitrification Process and the Function of Wetland Plants
Denitrification is a sequential process including four enzymatic reactions catalyzed by nitrate, nitrite, nitric oxide, and nitrous oxide reductases [33]. It is mediated by various denitrifying bacteria, fungi, and archaea which use nitrate as an electron acceptor during respiration in the absence of oxygen. Denitrifying microbes gain their energy through electron transport via the cytochrome system [33].
CWs are aquatic systems composed of plants and microorganisms and exhibit unique characteristics impacting the processes of the N cycle [34]. First, as a result of the prolonged or intermittent flooding conditions, CWs are characterized by hotspots concerning water chemistry and microbial N cycle. [35]. Second, CWs are unique in that they include complex interaction interfaces, particularly in the rhizosphere [36]. Third, CWs display temporal and spatial heterogeneities, which are further moderated by the rhizosphere effect [37]. Seasonal variations in temperature and root growth stage can alter the extent of ROL and the composition of root exudates, leading to fluctuations in redox conditions and carbon supply for denitrifying communities [38,39]. In addition to temporal heterogeneity, spatial heterogeneities in oxygen and nutrient distribution within the rhizosphere also play a crucial role in regulating denitrification. Oxygen availability tends to decrease with increasing distance from the root surface, creating a gradient of oxic to anoxic conditions. This gradient allows for the coexistence of nitrifying and denitrifying communities, facilitating coupled nitrification-denitrification processes [26]. The spatial variation in root exudates can also influence N cycling processes. Zhao et al. (2023) found that higher levels of root exudates in the upper layers of wetlands led to enhanced carbon and N metabolism in CWs. These findings highlight the differences in root-induced microbial dynamics and pollutant removal in CWs, providing valuable mechanistic insights into the adaptation of microbiota in response to spatiotemporal changes in rhizosphere [40].
Aquatic plants impact denitrification through alteration of environmental conditions and interactions with denitrifying microbes [41]. A meta-analysis across 419 wetlands showed that plants increased the rate of denitrification by an average of 55%, and their effect differed significantly among plant communities [41]. The beneficial effects of plants on denitrification have been documented in several studies. For instance, the Phragmites australis rhizospheres had substantially higher potential denitrification rates and a greater abundance of denitrifiers [42]. Similarly, Typha latifolia increased the abundance and activity of denitrifiers in the CWs [43]. Aquatic plants may contribute to the increased denitrification process through several mechanisms (Figure 1). First, the plant can enhance the abundance and activity of the microbes in its surroundings by establishing a niche for the microbes to access the root surfaces [31,32]. Second, plants may secrete root exudates, which act as a carbon source for denitrifiers [33]. Root exudates, which contain a variety of organic compounds such as sugars, amino acids, and organic acids, serve as carbon sources for denitrifying microorganisms in the rhizosphere [28]. The composition and quantity of root exudates can selectively enrich specific denitrifying taxa, thus shaping the microbial community structure [44]. For instance, Zhang et al. (2022) found that phenolic root exudates from Phragmites australis significantly enriched denitrifying bacteria [45]. Third, plant ROL creates an oxic-anoxic interface at the horizontal or vertical scale, which is crucial for the coupling of nitrification-denitrification processes. The oxygen released by roots supports the growth of nitrifying bacteria, which convert ammonium to nitrate, subsequently serving as a substrate for denitrification in adjacent anoxic zones [26,28]. Moreover, macrophyte species with higher ROL capacity enhanced denitrification, attributed to the increased prevalence of ammonia oxidizers and the strengthened coupling between nitrifying and denitrifying communities [46].
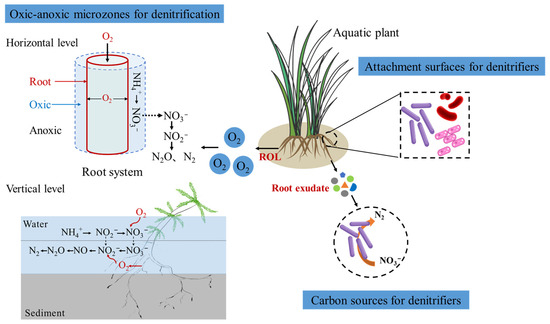
Figure 1.
The positive effect of plant ROL and root exudate on the denitrification process in CWs.
The influence of wetland plants on the denitrification process may vary depending on the plant species and its specific traits. Root morphology, such as root porosity, root length, and size, can affect the extent of ROL and the surface area available for microbial colonization [47,48]. For instance, plants with deeper and more extensive root systems, like Phragmites australis, may create larger oxic zones and support higher rates of coupled nitrification-denitrification [39]. Furthermore, the composition and quantity of root exudates can differ among plant species, leading to variations in carbon substrate availability for denitrifying communities [44]. Some species, such as Typha latifolia, have been shown to release higher amounts of easily degradable organic compounds, which can stimulate denitrification activity more effectively than species with less labile exudates [49]. Therefore, the selection of plant species with traits that promote denitrification can be a key consideration in optimizing N removal in CWs.
3. Mechanisms of Radial Oxygen Loss in Regulating Denitrification
ROL can create aerobic, anaerobic, and facultative aerobic microzones around the roots for the cultivation of various microorganisms supporting diverse biogeochemical processes in the rhizosphere [50]. ROL varies significantly among different wetland plants. Several studies have reported ROL rates from submerged plants in natural wetlands, ranging from 0.5 to 5.2 g/m2/d [51,52]. Laskov et al. (2006) [53] calculated a ROL between 0.15 and 0.60 g/m2/d by analyzing data from 200 plants. However, the multiple methods used to measure ROL can substantially affect the magnitude of the reported rates, leading to potential controversies in the estimation of ROL. Despite these methodological challenges, most published studies indicated that the ROL of aquatic plants is typically less than 5.0 g/m2/d [54]. Plant roots do not continuously release oxygen throughout their entire growth process, resulting in the construction of a dynamic and heterogeneous niche in the rhizosphere. Consequently, one of the primary research priorities is to improve and optimize ROL measurement methods to enable dynamic and synchronous visual monitoring of plant ROL. Koren et al. (2015) [55] employed optical sensor nanoparticles to dynamically monitor the ROL of seagrass in real time, enabling full visualization of the complex ROL state. This technique exceeded the limitations of previous microelectrode-based methods, which could provide only localized real-time monitoring. This advancement is crucial for studying key physicochemical parameters of submerged plant roots, offering a more comprehensive understanding of the dynamic ROL in the rhizosphere.
ROL induces an oxidation-reduction microenvironment in the root zone, providing a suitable niche for aerobic and anaerobic microorganisms. This process makes the plant rhizosphere a hotspot for pollutant removal and the N cycle. Positive relationships between ROL and N removal rates have therefore been reported [26,48]. ROL may influence the microbial communities in such a way that it couples nitrification and denitrification. As oxygen diffuses from the root tips and laterals into the surrounding environment, it creates a gradient of oxygen availability, with the highest concentrations found closest to the root surface [26]. The oxygen gradient in the rhizosphere creates distinct microzones, including aerobic zones near the roots, facultative aerobic zones at intermediate distances, and anaerobic zones farther from the root system [56]. The spatial distribution of these microzones plays a crucial role in regulating the sequential processes of nitrification and denitrification [41].
Due to their different sensitivity to oxygen, denitrifying bacteria and nitrifying bacteria occupy different ecological niches within the rhizosphere [57]. Moreover, plant ROL can create oxygen gradients in CWs that enable denitrifying communities to find suitable habitats in the facultative anaerobic regions. For example, Sun et al. (2019) showed that Myriophyllum aquaticum had a ROL of 0.3 g/m2/d, and such a level of ROL developed a distinct gradient for denitrification. It was described by a significant increase in the abundance of denitrifying bacteria from sediment to the water column and finally to the rhizoplane [41]. Similarly, Bai et al. (2023) reported a decline in ROL with the increasing water depth, resulting a vertical oxygen gradient in the rhizosphere. This could thus facilitate denitrification through the formation of an optimal niche for denitrifiers such as Ralstonia and Hydrogenophaga [58].
The variability of ROL among wetland plants is influenced by several factors, including plant species, environmental conditions, and root growth stages. Some species, such as Phragmites australis and Typha latifolia, are known to release more oxygen from their roots compared to others, like Juncus effusus [59]. This variability in ROL can lead to differences in the extent of aerobic and anaerobic microzones in the rhizosphere, ultimately affecting denitrification rates [41]. Environmental conditions, such as temperature, water level, and nutrient availability, can also impact ROL [53]. Higher temperatures generally increase root respiration and oxygen demand, potentially reducing the amount of oxygen available for release into the rhizosphere [60]. Water level fluctuations can alter the redox conditions in the sediment, with higher water levels limiting oxygen diffusion and promoting anaerobic conditions favorable for denitrification [61]. Nutrient availability, particularly N, can influence root growth and development, which in turn affects ROL [62]. Root growth stages also play a role in ROL variability. During active growth periods, plants may allocate more resources to root development and release more oxygen into the rhizosphere compared to dormant or senescent stages [41]. In the root structure of Phragmites australis, emerging main roots and lateral roots exhibit root O2 leakage, and O2 concentrations can reach values of 30–50 μmol/L. Interestingly, the main root stopped releasing O2 over time, while the lateral root showed only a weak O2 leak over time [39]. Methodological challenges in measuring ROL can have implications for denitrification research. Traditional methods, such as microelectrodes, provide localized measurements of oxygen concentrations but may not capture the full spatial and temporal variability of ROL [29]. The development of non-invasive, high-resolution methods for measuring ROL is crucial for better understanding its role in regulating denitrification and optimizing N removal in CWs [63].
4. Root Exudates and Their Effects on Denitrification
Root exudates are the complex mixture of organic compounds excreted through roots into the surrounding environment. These compounds comprise primary metabolites like sugars, amino acids, and organic acids, as well as various secondary compounds [64]. Microorganisms may use root exudates as carbon and energy sources, trigger their growth and activities, and thereby participate in different biogeochemical processes within the rhizosphere [40,65,66].
Root exudates containing low molecular weight (LMW) organic compounds are widely studied and are recognized as easily accessible sources of carbon and energy for microorganisms. In most plants, LMW organic compounds consist primarily of sugars, amino acids, and organic acids, with sugars being the most abundant, followed by amino acids and organic acids [51]. These LMW organic compounds provide electron donors for the denitrification process in wetlands [67]. These easily degradable organic substances can stimulate the synthesis of denitrifying enzymes, thereby increasing the denitrification rate. For example, Adrian et al. (2018) found that the addition of artificial root exudates increased denitrification rates and community structure, particularly affecting nirS and nosZ-I genes [68]. By measuring the quantity of dissolved organic carbon secreted from the roots of wetland plants such as Phragmites australis, Juncus effusus, and Iris pseudacorus, it was found that their average organic carbon secretion rate ranged from 4.3 to 12.2 μg/g root DM/h, potentially enhancing the denitrification process in subsurface flow CWs by 94–267 kg N/(hm2·a) [69]. The composition of root exudates, which includes various organic compounds, can selectively enrich specific microbial groups, thereby shaping the structure and diversity of the rhizosphere microbiome [70]. A diverse microbial community is essential for establishing a more stable and efficient denitrification function, as different microbial groups may possess complementary metabolic capabilities and adapt to varying environmental conditions [66]. These studies highlight the importance of root exudate composition complexity in maintaining a diverse community of denitrifying microorganisms, which is crucial for optimizing N removal in CWs [20].
The composition of root exudates significantly influences the growth and development of denitrifying microbial communities. For example, the secretion of sucrose and glucose by the roots of aquatic plants such as Phragmites australis, Typha angustifolia, and Cyperus alternifolius provides essential nutrients for denitrifying microorganisms. These exudates have been reported to largely determine the population density and distribution patterns of denitrifying bacteria, especially in wetlands with low pollution levels [44]. Because of the complex and diverse nature of the components in root exudates, their impact on denitrification processes differs. Wu et al. (2017) discovered that organic acids and sugars present in the root exudates favored denitrification. Substances with electron-donating groups (e.g., tartaric acid) and easily degradable organic matter (e.g., glucose) are more favorable for utilization by denitrifying communities. In contrast, amino acids were found to make little contribution toward N removal [71]. In the work by Maurer et al. (2021), it was noted that higher organic acid concentration may enhance the activities of denitrifying enzymes while lyxose inhibits the denitrification process [72].
The compositions of root exudates depend on the plant types, root growth, and environmental conditions [73,74]. Different plant species, due to their unique physiological and metabolic characteristics, can secrete different types and amounts of organic compounds. For example, root exudate release rates in Acorus calamus were highest, followed by Scirpus tabernaemontani and Phragmites australis, with contributions to bacterial denitrification varying by species [75]. Root-derived carbon may stimulate denitrification in plants, but N uptake become the controlling factors with increasing plant and root growth [76]. Environmental conditions, such as temperature and nutrient status, can also influence the quantity and composition of root exudates [58]. For instance, root exudate rates positively correlated with mean air temperature [77]. Climate warming-induced changes in root exudation quantity stimulate the growth and reproduction of N transformation bacteria, accelerating organic matter decomposition and N transformations [78]. Plants and microbes actively regulate root exudation of primary metabolites by altering concentration gradients and diffusion rates based on their nutrient status [79].
5. Coupling Effects of ROL and Root Exudates on Denitrification
ROL and root exudates are two important characteristics of wetland plants that can significantly influence the denitrification process in CWs, and previous studies have focused on the separate role of ROL and root exudates in denitrification. However, since wetland plants secrete oxygen and root exudates simultaneously, the mechanisms underlying their coupling effects have not yet been elucidated (Figure 2) [64].
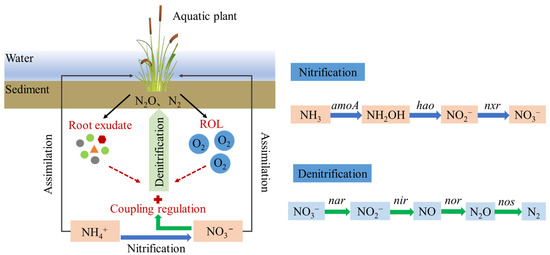
Figure 2.
Coupled regulation of denitrification in wetland plant rhizosphere by ROL and root exudates.
ROL and root exudates might synergistically impact the denitrification process in CWs. First, ROL can enhance the aerobic degradation of root exudates. The oxygen released in the rhizosphere promotes the mineralization of easily degradable organic matter in root exudates by aerobic microorganisms, producing more low-molecular-weight organic substances, such as organic acids and sugars [18]. These substances can be directly absorbed and utilized by denitrifying bacteria, providing electron donors for the denitrification process [80,81,82]. Second, the microaerobic environment formed by ROL is conducive to the growth of facultative anaerobic denitrifying bacteria [41]. The microaerobic environment generated by ROL allows these bacteria to colonize the rhizosphere and switch to denitrification metabolism once oxygen is depleted [83], thereby improving their utilization efficiency of root exudates [84]. Furthermore, ROL may also affect the composition and availability of root exudates by altering the rhizosphere pH. Studies have shown that ROL can lead to a decrease in rhizosphere pH due to the oxidation of reduced compounds, such as ferrous iron and sulfides [85]. The acidification of the rhizosphere can enhance the solubility and availability of nutrients, including phosphorus and micronutrients, which may stimulate wetland plant growth and root exudation [85]. These changes in root exudate composition and availability can increase the activity and abundance of denitrifying bacteria in the rhizosphere, potentially impacting the denitrification process [71].
Environmental factors have significant impacts on the synergy between ROL and root exudates and the denitrification process. Temperature is a key factor because it affects plant growth, root exudate release, and microbial activity. Studies have shown that higher temperatures generally promote root growth and exudate release while accelerating microbial metabolism of these exudates [78]. Therefore, an increase in temperature may enhance the synergy between ROL and root exudates, promoting the denitrification process. Water depth is also an important environmental factor because it influences the growth of wetland plants and ROL. In deeper water, wetland plants may allocate more biomass to stems and leaves compared to roots and rhizomes, which may reduce the ROL and root exudate [86,87]. This may weaken the synergy between ROL and root exudates. However, the impact of water depth on the synergy between ROL and root exudates and denitrification is not always straightforward. An appropriate water depth may increase plant biomass allocation to root structures. For example, Richards et al. (2011) found that plants in 30 cm water depth had significantly greater biomass allocated to rhizomes [88]. Furthermore, in some cases, moderate water depth can create anaerobic conditions favorable for denitrification [61]. Therefore, the impact of water depth may depend on specific conditions and the balance between these opposing effects. Nutrient availability, particularly the availability of N and phosphorus, also has important effects on the synergy between ROL and root exudates. N and phosphorus deficiency may stimulate plants to secrete more organic acids and other compounds to increase nutrient solubility and uptake [89]. These exudates can provide more carbon sources for denitrifying bacteria [28]. At the same time, nutrient deficiency may limit plant growth and ROL, so the interaction between nutrient availability and ROL and root exudates may be more complex.
The coupling effect of ROL and root exudate on denitrification is dynamic. Such effects are amended with seasons and plant growth stages [90,91,92,93]. The interaction between ROL and root exudates together controls the availability of nitrate; therefore, a complex interlinkage affects the denitrification process (Figure 3). Plant development profoundly affected water parameters through increased root activities, including ROL and root exudates. NH4+-N removal efficiency was highest at the mature stage, while NO3−-N removal performed best at the seedling stage. The mature stage demonstrated the highest relative abundances of nitrification and anammox-related microbes attributed to enhanced ROL intensity creating high DO microhabitats. Conversely, the seedling and wilting stages exhibited the highest relative abundances of denitrification and dissimilatory nitrate reduction to ammonium-related microbes, explained by the absence of ROL and biological denitrification inhibitors from root exudates [93]. Seasonal changes also significantly influence the coupling effects of ROL and root exudates. During the growing season, higher temperatures and light availability generally promote plant growth and metabolic activities, resulting in increased release of ROL and root exudates [78]. These seasonal fluctuations affect the interactions between ROL and root exudates and may result in seasonal variations in N removal efficiency in CWs. In the long term, the cumulative effect of coupling between ROL and root exudate may become more significant. For example, the roots of perennial wetland plants are continuously releasing ROL and root exudates, thus providing a stable habitat and carbon source for denitrifying bacteria [43]. Moreover, the denitrification activity promoted by ROL and root exudates may enhance over time as the denitrifying microbial community adapts and optimizes its metabolic pathways [94]. However, further quantitative studies are needed to investigate the dynamic coupling effects of ROL and root exudates on denitrification under different scenarios.
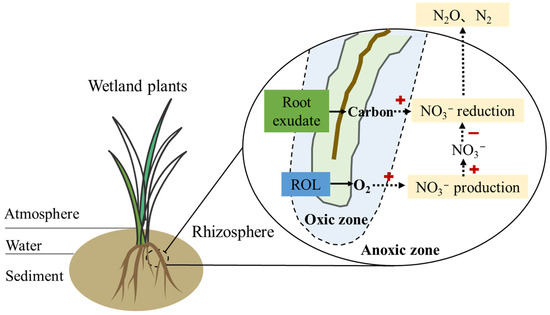
Figure 3.
A conceptual diagram of the effects of ROL and root exudates on nitrate availability within the wetland plants.
Recent studies have reported the synergistic effects of ROL and root exudates on microbial communities. For instance, Fang et al. (2021) have documented that ROL can promote the growth of aerobic microbes by increasing the oxidation-reduction potential in sediments. Meanwhile, root exudates offer carbon and energy for microbial development. Hence, ROL and organic carbons interactively shape microbial composition and metabolic processes, resulting in distinct characteristics compared with the non-rhizosphere [95]. Ma et al. (2021) [8] integrated ROL and internal organic carbon (IOC) into CW Model No.1 that simulated synthetic wastewater treatment. This innovative approach allowed them to explore the synergistic spatiotemporal coupling of ROL and IOC secretion by plant roots and its impact on N removal. The results indicated that in summer, the removal rates of ammonia and TN were greater than 90%, while they decreased to 55% and 45% in unplanted wetlands during winter, respectively, with approximately 85% and 78% removal rates being registered in the planted wetland. The present study underlines the co-action of ROL and IOC on N removal. The concurrent release of oxygen and organic carbons from the aquatic plants directly fuels the sequential processes of nitrification and denitrification.
ROL, root exudates, microbial processes, and environmental factors together constitute a complex interaction network that comprehensively influences the denitrification process in CWs. A better understanding of the interaction mechanism between these factors is essential to optimize the denitrification performance of CWs. ROL can modulate the activity and abundance of denitrifiers by altering oxygen level, pH, and nutrient bioavailability [41,96]. The easily degradable carbon sources and some secondary metabolites in root exudates can directly stimulate the growth and metabolism of denitrifying microorganisms and increase the denitrification rate [68,70]. Root exudates can also regulate the expression of nitrifying and denitrifying genes, affecting microbial functions [71]. Environmental factors such as temperature, pH, and hydraulic loads also significantly affect ROL, root exudates, and microbial processes, which in turn regulate the denitrification process of CWs [78,97].
6. Limitations of Conventional Techniques and Future Perspectives
Most research on the role of ROL and root exudates in the regulation of denitrification has traditionally relied on methods such as high-throughput sequencing to identify denitrifying communities [98,99]. However, this technique often fails to provide a comprehensive picture of the denitrifying communities in CWs. Denitrification is widely distributed among phylogenetically diverse microorganisms, making it challenging to comprehensively characterize all denitrifying microbial communities utilizing the 16S rRNA gene as a molecular marker [57]. Alternatively, the employment of denitrification functional genes as molecular markers is constrained by primer bias and the incompleteness of annotation databases, hindering the accurate resolution of functionally active denitrifying microbial populations [100,101]. Consequently, current conventional techniques are limited to describing the denitrifying communities in CWs, failing to identify the core active denitrifiers and the key genes controlling the denitrification process. This limitation hinders in-depth investigations into the regulatory mechanisms of ROL and root exudates on denitrifying communities. Therefore, complementary methods, such as meta-omics and stable isotope probing, are needed to link bacterial diversity with their actual denitrification performance in CWs. Meta-omics and stable isotope probing techniques have unique advantages in addressing the limitations of conventional methods. Metagenomics enables a comprehensive and unbiased revelation of microbial community composition by directly sequencing all DNA without relying on primers [102]. Metatranscriptomics, by directly sequencing RNA, can more comprehensively reveal active functional genes in microbial communities [103]. Stable isotope probing (SIP) techniques, such as DNA-SIP and RNA-SIP, can directly track microbial taxa involved in specific metabolic processes by incorporating stable isotope-labeled substrates into environmental samples. This approach can reveal key microbial taxa in specific ecological processes that are difficult to detect using conventional methods [104]. For instance, Coyotzi et al. (2017) combined DNA-SIP and metagenomics to reveal the potential role of Janthinobacterium as an active denitrifier that cannot reduce N2O, which may significantly impact agricultural soil N2O emissions. This study was the first to use DNA-SIP to identify active denitrifying bacteria in agricultural soils [57].
Another limitation of current studies on ROL and root exudates in CWs is the lack of data on ROL and root exudates on continuous spatiotemporal scales. The measurement and characterization of ROL and root exudates are often carried out at special time points and zones [97,105]. However, the oxygen release and root exudates of plant roots are complicated and dynamic; a single data point could not represent the real mechanisms of plant–microbe interactions [28]. Consequently, there is an urgent requirement for novel technologies for the continuous characterization of ROL and root exudates in real time [106]. Furthermore, previous studies focus on the effect of aquatic plants or root exudates on denitrification [96,107]. However, wetland plants release both oxygen and root exudates during actual growth. Due to the complexity and dynamics of the plant rhizosphere, the two factors of ROL and root exudates are difficult to separate, which limits the study of coupled regulatory mechanisms on the denitrification process in situ [8]. Therefore, it is necessary to develop mathematical models to simulate the oxygen and root exudate secretion of plant roots to study the coupling regulatory mechanisms.
In recent years, various emerging technologies have provided new possibilities for continuous spatiotemporal data collection of ROL and root exudates. For example, optical sensors can enable in situ continuous monitoring of ROL [108]. Microfluidic technologies can miniaturize and automate the collection of root exudates, and could directly image the plant–microbe interactions in real time [28]. Combined with advanced imaging techniques, such as nanoscale secondary ion mass spectrometry (NanoSIMS), the spatiotemporal distribution of root exudates in the rhizosphere can be visualized non-destructively [109]. Furthermore, emerging biosensor technologies, such as nanobiosensors, hold promise for in situ real-time monitoring of root exudates [110]. These innovative technologies are expected to provide powerful tools for continuous spatiotemporal data collection of ROL and root exudates, thereby greatly enhancing our understanding of plant–microbe interactions. Continuous spatiotemporal data can also guide the establishment and optimization of mathematical models. By incorporating the dynamic changes of ROL and root exudates into biogeochemical models, the N removal process in CWs can be simulated and predicted with greater accuracy, enabling a more comprehensive assessment of the impacts of environmental factors and plant characteristics [8]. Model predictions can guide the design and optimization of CWs, such as selecting plant species with high ROL and root exudate release and optimizing operating conditions [111]. Moreover, continuous spatiotemporal data can guide the design and interpretation of field experiments. By validating mathematical models based on continuous spatiotemporal data under field conditions, the effectiveness and limitations of the models in practical applications can be evaluated [112]. Field experimental results, in turn, can provide a basis for further improvement and optimization of the models. Through the optimization of models and field experiments, more robust and reliable design and optimization strategies for CWs can be developed.
Future research on the coupling effects of ROL and root exudates on denitrification in CWs should focus on the following areas (Figure 4). First, establishing and applying innovative molecular technologies, such as DNA/RNA stable isotope probing, metagenomics, and metatranscriptomics [113,114,115], to link the denitrifying communities with their actual function in the rhizosphere of diverse plant species. These tools empower researchers to clarify the core active denitrifiers and clarify the vital genes and pathways associated with denitrification [116]. Second, analyzing the root dynamics of various plant species under different environmental conditions at continuous spatial and temporal scales [117,118], and understanding how ROL and root exudates impact denitrification in CWs. This investigation aims to elucidate the interactions between plant traits and the denitrification process [119,120], thereby offering insights into choosing the optimal plant species for maximizing N removal in CWs. Furthermore, developing mechanistic models that simulate the root exudates and ROL from plants based on plant traits and environmental conditions [121,122]. These models can realize the separation and combination of ROL and root exudate and study their effect on denitrification under different environmental conditions. Finally, through laboratory mechanism research, the dominant aquatic plants for field research and large-scale waste treatment experiment verification can be screened out to testify for the laboratory research result [123]. Investigation into these research gaps can provide deeper details on how ROL and root exudates together affect the denitrification process under different scenarios. Such mechanisms can provide theoretical guidance in developing efficient N removal technology in CWs. Management of natural wetlands, which is so imperative in governing the global N cycle on aquatic systems, can also be partly guided by this knowledge [124,125].
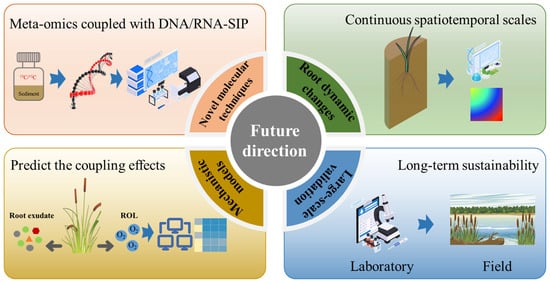
Figure 4.
Future research direction of coupling effect of ROL and root exudate on denitrification of CWs.
7. Conclusions
CWs are an important technology for wastewater treatment. However, the performance of CWs is often limited by the low efficiency and variability in N removal. It has been well-recognized that aquatic plants can affect denitrification regulation through their ROL and root exudates. However, the relationship between ROL and root exudates in denitrification remains unclear. An in-depth understanding of the coupling mechanisms between ROL and root exudates faces several challenges. First, the release of ROL and root exudates is regulated by various biotic and abiotic factors, such as plant species, growth stage, root morphology, and environmental conditions, and the complex interactions among these factors are still unclear. Second, the spatiotemporal dynamics of ROL and root exudates in the rhizosphere are difficult to accurately characterize, limiting our understanding of their ecological effects. Furthermore, the feedback regulation mechanisms between ROL-root exudates and plant–microbial metabolism remain to be elucidated. Therefore, research on the relationship between ROL and root exudates is a key focus and challenge for future plant–microbe interaction studies.
Future research should focus on the following aspects: (1) develop new in situ monitoring technologies, such as microelectrode arrays, microfluidic chips, nanobiosensors, to improve the resolution of the spatiotemporal dynamics of ROL and root exudates of wetland plants; (2) integrate multi-omics technologies and SIP to deeply reveal the coupled mechanism of ROL and root exudates on the microbial communities; (3) construct multi-scale mathematical models to elucidate the quantitative relationship between the coupling effect of ROL and root exudates and its regulatory mechanism on key biogeochemical processes; (4) strengthen field studies to evaluate the ecological significance of the interaction between ROL and root exudates and its contribution to the ecosystem service function of CWs.
A better understanding of the coupling mechanism between ROL and root exudates can guide the design and optimization of CWs, such as screening plant species with high ROL and root exudates and regulating hydraulic conditions to promote the development of beneficial microbial communities. This knowledge can also provide inspiration for other environmental management practices, including soil remediation, sustainable agriculture, and ecosystem restoration. By regulating plant–microbe interactions, it is expected to achieve in situ remediation of polluted environments and the improvement of ecosystem services. Elucidating the interaction mechanism between ROL and root exudates will help guide the development of rhizosphere engineering strategies and optimize the rhizosphere microbiome. This knowledge can be used to improve crop nutrient use efficiency and stress resistance, ultimately contributing to sustainable agricultural development by targeting the regulation of ROL and root exudates.
Author Contributions
Conceptualization, H.S. and C.J.; resources, Y.Z. and C.J.; writing—original draft preparation, H.S.; writing—review and editing, C.J.; supervision, C.J.; funding acquisition, H.S. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (nos. 52300027), Guangdong Basic and Applied Basic Research Foundation [grant number 2022A1515110943] and the Science Foundation of China Urban Construction Design & Research Institute Co., Ltd. (Y09E24009).
Data Availability Statement
No new data were created or analyzed in this review. Data sharing is not applicable to this article.
Conflicts of Interest
Author Y.Z. was employed by the company China Urban Construction Design & Research Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Holmes, D.E.; Dang, Y.; Smith, J.A. Nitrogen cycling during wastewater treatment. Adv. Appl. Microbiol. 2019, 106, 113–192. [Google Scholar]
- Zhao, X.; Chen, J.; Guo, M.; Li, C.; Hou, N.; Bai, S. Constructed wetlands treating synthetic wastewater in response to day-night alterations: Performance and mechanisms. Chem. Eng. J. 2022, 446, 137460. [Google Scholar] [CrossRef]
- Carabal, N.; Cardoso, L.S.; Padisák, J.; Selmeczy, G.B.; Puche, E.; Rodrigo, M.A. How a constructed wetland within a natural park enhances plankton communities after more than 10 years of operation: Changes over space and time. Environ. Res. 2024, 263, 120114. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, R.; Yan, P.; Wu, S.; Chen, Z.; Zhao, Y.; Cheng, C.; Hu, Z.; Zhuang, L.; Guo, Z. Constructed wetlands for pollution control. Nat. Rev. Earth. Environ. 2023, 4, 218–234. [Google Scholar] [CrossRef]
- Deng, S.; Cun, D.; Lin, R.; Peng, D.; Du, Y.; Wang, A.; Guan, B.; Tan, R.; Chang, J. Enhanced remediation of real agricultural runoff in surface-flow constructed wetlands by coupling composite substrate-packed bio-balls, submerged plants and functional bacteria: Performance and mechanisms. Environ. Res. 2024, 263, 120124. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Wei, D.; Zhang, J.; Hu, J.; Liu, Z.; Li, R. Natural pyrite to enhance simultaneous long-term nitrogen and phosphorus removal in constructed wetland: Three years of pilot study. Water Res. 2019, 148, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chachar, A.; Sun, S.; Peng, Y.; Gu, X.; He, S. Unveiling synergistic enhancement mechanism of nitrogen removal in surface flow constructed wetlands: Utilizing iron scraps and elemental sulfur as integrated electron donors. J. Environ. Manag. 2024, 370, 123006. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Du, Y.; Peng, W.; Zhang, S.; Liu, X.; Wang, S.; Yuan, S.; Kolditz, O. Modeling the impacts of plants and internal organic carbon on remediation performance in the integrated vertical flow constructed wetland. Water Res. 2021, 204, 117635. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, Z.; Kang, Y.; Fan, J.; Zhang, J. Recent advances in the enhanced nitrogen removal by oxygen-increasing technology in constructed wetlands. Ecotox. Environ. Safe 2020, 205, 111330. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, Y.; Ji, X.; Lu, C.; Zhang, J. Influences of plant species and radial oxygen loss on nitrous oxide fluxes in constructed wetlands. Ecol. Eng. 2020, 142, 105644. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, Z.; Hu, Q.; Hu, J.; Ni, C.; Li, F. Using stable isotopes to identify nitrogen transformations and estimate denitrification in a semi-constructed wetland. Sci. Total Environ. 2020, 720, 137628. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Lu, J.; Wang, Y.; Liu, G.; Hua, Y.; Wan, X.; Zhao, J.; Zhu, D. The abundance of nirS-type denitrifiers and anammox bacteria in rhizospheres was affected by the organic acids secreted from roots of submerged macrophytes. Chemosphere 2020, 240, 124903. [Google Scholar] [CrossRef] [PubMed]
- Faulwetter, J.L.; Gagnon, V.; Sundberg, C.; Chazarenc, F.; Burr, M.D.; Brisson, J.; Camper, A.K.; Stein, O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009, 35, 987–1004. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, X.; Fang, Y.; Xu, K.; He, S.; Zhao, M. Autotrophic denitrification in constructed wetlands: Achievements and challenges. Bioresour. Technol. 2020, 318, 123778. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.I.; Hallin, S.; Ecke, F.; Hubalek, V.; Juhanson, J.; Frainer, A.; McKie, B.G. Disentangling the roles of plant functional diversity and plaint traits in regulating plant nitrogen accumulation and denitrification in freshwaters. Funct. Ecol. 2022, 36, 921–932. [Google Scholar] [CrossRef]
- Gu, X.; Chen, D.; Wu, F.; Tang, L.; He, S.; Zhou, W. Function of aquatic plants on nitrogen removal and greenhouse gas emission in enhanced denitrification constructed wetlands: Iris pseudacorus for example. J. Cleaner Prod. 2022, 330, 129842. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Y.; Xie, H.; Yang, Z. Constructed wetlands: A review on the role of radial oxygen loss in the rhizosphere by macrophytes. Water 2018, 10, 678. [Google Scholar] [CrossRef]
- Tang, S.; Liao, Y.; Xu, Y.; Dang, Z.; Zhu, X.; Ji, G. Microbial coupling mechanisms of nitrogen removal in constructed wetlands: A review. Bioresour. Technol. 2020, 314, 123759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, S.; Gu, X.; Yu, Q.; He, S. Role of hydrophytes in constructed wetlands for nitrogen removal and greenhouse gases reduction. Bioresour. Technol. 2023, 388, 129759. [Google Scholar] [CrossRef]
- Pu, Y.; Li, Y.; Zhu, L.; Cheng, Y.; Nuamah, L.; Zhang, H.; Chen, H.; Du, G.; Wang, L.; Song, C. Long-term assessment on performance and seasonal optimal operation of a full-scale integrated multiple constructed wetland-pond system. Sci. Total Environ. 2023, 862, 161219. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wu, S.; Dai, Y.; Liang, W.; Wu, Z. Nitrogen removal from nitrate-laden wastewater by integrated vertical-flow constructed wetland systems. Ecol. Eng. 2013, 58, 192–201. [Google Scholar] [CrossRef]
- Hunt, P.G.; Stone, K.C.; Matheny, T.A.; Poach, M.E.; Vanotti, M.B.; Ducey, T.F. Denitrification of nitrified and non-nitrified swine lagoon wastewater in the suspended sludge layer of treatment wetlands. Ecol. Eng. 2009, 35, 1514–1522. [Google Scholar] [CrossRef]
- Sindilariu, P.-D.; Wolter, C.; Reiter, R. Constructed wetlands as a treatment method for effluents; from intensive trout farms. Aquaculture 2008, 277, 179–184. [Google Scholar] [CrossRef]
- Li, M.; Duan, R.; Hao, W.; Li, Q.; Arslan, M.; Liu, P.; Qi, X.; Huang, X.; El-Din, M.G.; Liang, P. High-rate nitrogen removal from carbon limited wastewater using sulfur-based constructed wetland: Impact of sulfur sources. Sci. Total Environ. 2020, 744, 140969. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, Y.; Tam, N.F.Y.; Wang, Y.; Li, L. Roles of root porosity, radial oxygen loss, Fe plaque formation on nutrient removal and tolerance of wetland plants to domestic wastewater. Water Res. 2014, 50, 147–159. [Google Scholar] [CrossRef]
- Sun, L.; Lu, Y.; Yu, F.; Kronzucker, H.J.; Shi, W. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 2016, 212, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. Res. 2021, 28, 54497–54510. [Google Scholar] [CrossRef]
- Oburger, E.; Schmidt, H. New methods to unravel rhizosphere processes. Trends Plant Sci. 2016, 21, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Poole, P. Shining a light on the dark world of plant root-microbe interactions. Proc. Natl. Acad. Sci. USA 2017, 114, 4281–4283. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Wang, J.Y.; Al-Babili, S. Metabolomics of plant root exudates: From sample preparation to data analysis. Front. Plant Sci. 2022, 13, 1062982. [Google Scholar] [CrossRef] [PubMed]
- Achouak, W.; Haichar, F.E.Z. Stable isotope probing of microbiota structure and function in the plant rhizosphere. Front. Plant Sci. 2019, 2046, 233–243. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Torrens, A.; de la Varga, D.; Ndiaye, A.K.; Folch, M.; Coly, A. Innovative multistage constructed wetland for municipal wastewater treatment and reuse for agriculture in senegal. Water 2020, 12, 3139. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Liu, L.; Zhuang, L.; Zhao, S.; Su, Y.; Li, Y.; Wang, M.; Wang, C.; Xu, L. Microbial nitrogen cycle hotspots in the plant-bed/ditch system of a constructed wetland with N2O mitigation. Environ. Sci. Technol. 2018, 52, 6226–6236. [Google Scholar] [CrossRef]
- Reddy, K.; Patrick Jr, W.; Lindau, C. Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnol. Oceanogr. 1989, 34, 1004–1013. [Google Scholar] [CrossRef]
- Lamers, L.P.; Van Diggelen, J.M.; Op den Camp, H.J.; Visser, E.J.; Lucassen, E.C.; Vile, M.A.; Jetten, M.S.; Smolders, A.J.; Roelofs, J.G. Microbial transformations of nitrogen, sulfur, and iron dictate vegetation composition in wetlands: A review. Front. Microbiol. 2012, 3, 156. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, M.; Liu, F.; Chen, L.; Li, Y.; Li, Y.; Xiao, R.; Wu, J. Seasonality distribution of the abundance and activity of nitrification and denitrification microorganisms in sediments of surface flow constructed wetlands planted with Myriophyllum elatinoides during swine wastewater treatment. Bioresour. Technol. 2018, 248, 89–97. [Google Scholar] [CrossRef]
- Li, C.; Ding, S.; Ma, X.; Chen, M.; Zhong, Z.; Zhang, Y.; Ren, M.; Zhang, M.; Yang, L.; Rong, N.; et al. O2 distribution and dynamics in the rhizosphere of Phragmites australis, and implications for nutrient removal in sediments. Environ. Pollut. 2021, 287, 117193. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, M.; Zhang, T.; Bai, S.; Meng, Y.; Tian, Y.; Yang, J.; Ma, F. Spatiotemporal dynamics of root exudates drive microbial adaptation mechanisms under day-night alterations in constructed wetlands. Chem. Eng. J. 2023, 477, 147311. [Google Scholar] [CrossRef]
- Sun, H.; Xu, S.; Wu, S.; Wang, R.; Zhuang, G.; Bai, Z.; Deng, Y.; Zhuang, X. Enhancement of facultative anaerobic denitrifying communities by oxygen release from roots of the macrophyte in constructed wetlands. J. Environ. Manag. 2019, 246, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Huang, R.; Zeng, J.; Zhao, D.; He, R.; Yu, Z.; Wu, Q.L. Rhizosphere-associated nosZ II microbial community of Phragmites australis and its influence on nitrous oxide emissions in two different regions. J. Soils Sediments 2021, 21, 3326–3341. [Google Scholar] [CrossRef]
- Ruiz-Rueda, O.; Hallin, S.; Baneras, L. Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. FEMS Microbiol. Ecol. 2009, 67, 308–319. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; He, X.; Zhang, S.; Liang, R.; Shen, J. Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci. Total Environ. 2017, 598, 697–703. [Google Scholar] [CrossRef]
- Zhang, N.; Hong, Z.; Qiu, R.; Chao, Y.; Yu, Y.; Dan, A. Removal pathway quantification and co-metabolic mechanism evaluation of alkylphenols from synthetic wastewater by phenolic root exudates in the rhizosphere of Phragmites australis. J. Hazard. Mater. 2022, 424, 127269. [Google Scholar] [CrossRef]
- Vila-Costa, M.; Pulido, C.; Chappuis, E.; Calvino, A.; Casamayor, E.O.; Gacia, E. Macrophyte landscape modulates lake ecosystem-level nitrogen losses through tightly coupled plant-microbe interactions. Limnol. Oceanogr. 2016, 61, 78–88. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Wang, R.; Hu, Y.; Li, W.; Cui, L. Specific root length regulated the rhizosphere effect on denitrification across distinct macrophytes. Geoderma 2024, 449, 117002. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.; Dzakpasu, M.; Gao, Z.; Zhou, W.; Zhu, R.; Xiong, J. Assessment of plants radial oxygen loss for nutrients and organic matter removal in full-scale constructed wetlands treating municipal effluents. Bioresour. Technol. 2022, 360, 127545. [Google Scholar] [CrossRef] [PubMed]
- Salvato, M.; Borin, M.; Doni, S.; Macci, C.; Ceccanti, B.; Marinari, S.; Masciandaro, G. Wetland plants, micro-organisms and enzymatic activities interrelations in treating N polluted water. Ecol. Eng. 2012, 47, 36–43. [Google Scholar] [CrossRef]
- Di, L.; Li, Y.; Nie, L.; Wang, S.; Kong, F. Influence of plant radial oxygen loss in constructed wetland combined with microbial fuel cell on nitrobenzene removal from aqueous solution. J. Hazard. Mater. 2020, 394, 122542. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, J.; Kemp, W. Seasonal and spatial patterns of oxygen production, respiration and root-rhizome release in Potamogeton perfoliatus L. and Zostera marina L. Aquat. Bot. 1991, 40, 109–128. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Laskov, C.; Horn, O.; Hupfer, M. Environmental factors regulating the radial oxygen loss from roots of Myriophyllum spicatum and Potamogeton crispus. Aquat. Bot. 2006, 84, 333–340. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Zhang, J.; Ngo, H.H.; Guo, W.; Liang, S.; Fan, J.; Lu, S.; Wu, H. Optimizations on supply and distribution of dissolved oxygen in constructed wetlands: A review. Bioresour. Technol. 2016, 214, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Brodersen, K.E.; Jakobsen, S.L.; Kühl, M. Optical sensor nanoparticles in artificial sediments–a new tool to visualize O2 dynamics around the rhizome and roots of seagrasses. Environ. Sci. Technol. 2015, 49, 2286–2292. [Google Scholar] [CrossRef]
- Maisch, M.; Lueder, U.; Kappler, A.; Schmidt, C. Iron lung: How rice roots induce iron redox changes in the rhizosphere and create viches for microaerophilic Fe(II)-oxidizing bacteria. Environ. Sci. Technol. Lett. 2019, 6, 600–605. [Google Scholar] [CrossRef]
- Coyotzi, S.; Doxey, A.C.; Clark, I.D.; Lapen, D.R.; Van Cappellen, P.; Neufeld, J.D. Agricultural soil denitrifiers possess extensive nitrite reductase gene diversity. Environ. Microbiol. 2017, 19, 1189–1208. [Google Scholar] [CrossRef]
- Bai, S.; Chen, J.; Guo, M.; Ren, N.; Zhao, X. Vertical-scale spatial influence of radial oxygen loss on rhizosphere microbial community in constructed wetland. Environ. Int. 2023, 171, 107690. [Google Scholar] [CrossRef]
- Wiessner, A.; Kuschk, P.; Kästner, M.; Stottmeister, U. Abilities of helophyte species to release oxygen into rhizospheres with varying redox conditions in laboratory-scale hydroponic systems. Int. J. Phytoremediat. 2002, 4, 1–15. [Google Scholar] [CrossRef]
- Soda, S.; Ike, M.; Ogasawara, Y.; Yoshinaka, M.; Mishima, D.; Fujita, M. Effects of light intensity and water temperature on oxygen release from roots into water lettuce rhizosphere. Water Res. 2007, 41, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Kim, D.-H.; Oh, S.; Moon, H.S. Effects of water level and vegetation on nitrate dynamics at varying sediment depths in laboratory-scale wetland mesocosms. Sci. Total Environ. 2020, 703, 134741. [Google Scholar] [CrossRef]
- Marzocchi, U.; Benelli, S.; Larsen, M.; Bartoli, M.; Glud, R.N. Spatial heterogeneity and short-term oxygen dynamics in the rhizosphere of Vallisneria spiralis: Implications for nutrient cycling. Freshw. Biol. 2019, 64, 532–543. [Google Scholar] [CrossRef]
- Yuan, H.; Cai, Y.; Yang, Z.; Li, Q.; Liu, E.; Yin, H. Phosphorus removal from sediments by Potamogeton crispus: New high-resolution in situ evidence for rhizosphere assimilation and oxidization-induced retention. J. Environ. Sci. 2021, 109, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, Y.; Zhang, Y.; Song, B.; Li, H.; Chen, Z. Effects of root organic exudates on rhizosphere microbes and nutrient removal in the constructed wetlands. Ecol. Eng. 2016, 92, 243–250. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Zhang, Y.; Li, T.; Ge, H.; Xia, S.; Gu, J.; Zhang, H.; Lü, B.; Wu, X. Rice root morphological and physiological traits interaction with rhizosphere soil and its effect on methane emissions in paddy fields. Soil Biol. Biochem. 2019, 129, 191–200. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Fang, J.; Tao, Y.; Liu, J.; Lyu, T.; Yang, X.; Ma, S.; Dong, J.; Dou, H.; Zhang, H. Effects of emergent plants on soil carbon-fixation and denitrification processes in freshwater and brackish wetlands in a watershed in northern China. Geoderma 2023, 430, 116311. [Google Scholar] [CrossRef]
- Langarica-Fuentes, A.; Manrubia, M.; Giles, M.E.; Mitchell, S.; Daniell, T.J. Effect of model root exudate on denitrifier community dynamics and activity at different water-filled pore space levels in a fertilised soil. Soil Biol. Biochem. 2018, 120, 70–79. [Google Scholar] [CrossRef]
- Zhai, X.; Piwpuan, N.; Arias, C.A.; Headley, T.; Brix, H. Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecol. Eng. 2013, 61, 555–563. [Google Scholar] [CrossRef]
- Seitz, V.A.; McGivern, B.B.; Daly, R.A.; Chaparro, J.M.; Borton, M.A.; Sheflin, A.M.; Kresovich, S.; Shields, L.; Schipanski, M.E.; Wrighton, K.C.; et al. Variation in root exudate composition influences soil microbiome membership and function. Appl. Environ. Microbiol. 2022, 88, e00226. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, X.; He, X. Effects of selected root exudate components on nitrogen removal and development of denitrifying bacteria in constructed wetlands. Water 2017, 9, 430. [Google Scholar] [CrossRef]
- Maurer, D.; Malique, F.; Alfarraj, S.; Albasher, G.; Horn, M.A.; Butterbach-Bahl, K.; Dannenmann, M.; Rennenberg, H. Interactive regulation of root exudation and rhizosphere denitrification by plant metabolite content and soil properties. Plant Soil 2021, 467, 107–127. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; Van Der Heijden, M.G. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Hu, Y.; Ding, J.; Ma, Q.; Zong, K.; Yang, Z. Effect of carbon source derived from macrophytes on microbial denitrification in constructed wetlands: Role of plant species. Bioresour. Technol. Rep. 2019, 7, 100217. [Google Scholar] [CrossRef]
- Rummel, P.S.; Well, R.; Pfeiffer, B.; Dittert, K.; Floßmann, S.; Pausch, J. Nitrate uptake and carbon exudation–do plant roots stimulate or inhibit denitrification? Plant Soil 2021, 459, 217–233. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, P.; Wang, Q.; Deng, S.; He, X.; Zhang, X.; Wang, R.; Feng, Q.; Yin, H. Temperature rather than N availability determines root exudation of alpine coniferous forests on the eastern Tibetan Plateau along elevation gradients. Tree Physiol. 2023, 43, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiao, M.; Li, D.; Yin, H.; Liu, Q. Do warming-induced changes in quantity and stoichiometry of root exudation promote soil N transformations via stimulation of soil nitrifiers, denitrifiers and ammonifiers? Eur. J. Soil Biol. 2016, 74, 60–68. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, Z.; Li, B.; Bai, G.; Yang, L.; Chi, Y.; Wang, M.; Ren, Y. Root vertical spatial stress: A method for enhancing rhizosphere effect of plants in subsurface flow constructed wetland. Environ. Res. 2023, 231, 116083. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Texier, S.; Hallet, S.; Bru, D.; Dambreville, C.; Chèneby, D.; Bizouard, F.; Germon, J.C.; Philippot, L. Disentangling the rhizosphere effect on nitrate reducers and denitrifiers: Insight into the role of root exudates. Environ. Microbiol. 2008, 10, 3082–3092. [Google Scholar] [CrossRef]
- Wiesenbauer, J.; Koenig, A.; Gorka, S.; Marchand, L.; Nunan, N.; Kitzler, B.; Inselsbacher, E.; Kaiser, C. A pulse of simulated root exudation alters the composition and temporal dynamics of microbial metabolites in its immediate vicinity. Soil Biol. Biochem. 2024, 189, 109259. [Google Scholar] [CrossRef]
- Benelli, S.; Ribaudo, C.; Bertrin, V.; Bartoli, M.; Fano, E.A. Effects of macrophytes on potential nitrification and denitrification in oligotrophic lake sediments. Aquat. Bot. 2020, 167, 103287. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Ye, C.B.; Zhou, Z.M.; Ni, B.S.; Zhang, X.M.; Liu, H. Unveiling organic loading shock-resistant mechanism in a pilot-scale moving bed biofilm reactor-assisted dual-anaerobic-anoxic/oxic system for effective municipal wastewater treatment. Bioresour. Technol. 2022, 347, 126339. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Ye, Z. Root-induced changes of pH, Eh, Fe(II) and fractions of Pb And Zn in rhizosphere soils of four wetland plants with different radial oxygen losses. Pedosphere 2012, 22, 518–527. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, C.; Li, J.; Liu, Z.; Wang, J. Root plasticity of Populus euphratica seedlings in response to different water table depths and contrasting sediment types. PLoS ONE 2015, 10, e0118691. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yuan, G.; Cao, T.; Ni, L.; Zhang, M.; Wang, S. An alternative mechanism for shade adaptation: Implication of allometric responses of three submersed macrophytes to water depth. Ecol. Res. 2012, 27, 1087–1094. [Google Scholar] [CrossRef]
- Richards, J.H.; Troxler, T.G.; Lee, D.W.; Zimmerman, M.S. Experimental determination of effects of water depth on Nymphaea odorata growth, morphology and biomass allocation. Aquat. Bot. 2011, 95, 9–16. [Google Scholar] [CrossRef]
- Sas, L.; Rengel, Z.; Tang, C. Excess cation uptake, and extrusion of protons and organic acid anions by Lupinus albus under phosphorus deficiency. Plant Sci. 2001, 160, 1191–1198. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, S.; Lin, D.; Guo, C.; Yan, L.; Wang, S.; He, Z. Nitrogen loading affects microbes, nitrifiers and denitrifiers attached to submerged macrophyte in constructed wetlands. Sci. Total Environ. 2018, 622, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Guo, D.; Zhang, J.; Zhang, T.; Yang, L.; Li, B.; Lan, J.; Ren, Y. Construction of an ideotype root system architecture of subsurface flow constructed wetland macrophytes by vertical spatial stress: Strengthening of rhizosphere effects and determination of appropriate substrate depth. Environ. Res. 2024, 259, 119523. [Google Scholar] [CrossRef]
- Yang, L.; Shen, L.; Tao, J.; Xiao, D.; Shi, Q.; Wang, Y.; Zheng, X.; Zhao, M.; Han, W. Effects of plant species diversity and density of Acorus calamus and Reineckea carnea on nitrogen removal and plant growth in constructed wetlands during the cold season. Environ. Eng. Sci. 2024, 29, 161219. [Google Scholar] [CrossRef]
- Hu, X.; Yue, J.; Yao, D.; Zhang, X.; Li, Y.; Hu, Z.; Liang, S.; Wu, H.; Xie, H.; Zhang, J. Plant development alters the nitrogen cycle in subsurface flow constructed wetlands: Implications to the strategies for intensified treatment performance. Water Res. 2023, 246, 120750. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Rahaman, M.H.; Zhai, J.; Makinia, J. Coupling transformation of carbon, nitrogen and sulfur in a long-term operated full-scale constructed wetland. Sci. Total Environ. 2021, 777, 146016. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Dong, J.; Li, C.; Chen, H.; Wang, L.; Lyu, T.; He, H.; Liu, J. Response of microbial community composition and function to emergent plant rhizosphere of a constructed wetland in northern China. Appl. Soil Ecol. 2021, 168, 104141. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Liu, W.; Jiang, H.; Wang, M.; Ge, Y.; Chang, J. Denitrifying bacterial community dominantly drove nitrogen removals in vertical flow constructed wetlands as impacted by macrophyte planting patterns. Chemosphere 2021, 281, 130418. [Google Scholar] [CrossRef]
- Blossfeld, S.; Gansert, D.; Thiele, B.; Kuhn, A.J.; Loesch, R. The dynamics of oxygen concentration, pH value, and organic acids in the rhizosphere of Juncus spp. Soil Biol. Biochem. 2011, 43, 1186–1197. [Google Scholar] [CrossRef]
- Du, L.; Trinh, X.; Chen, Q.; Wang, C.; Wang, H.; Xia, X.; Zhou, Q.; Xu, D.; Wu, Z.B. Enhancement of microbial nitrogen removal pathway by vegetation in Integrated Vertical-Flow Constructed Wetlands (IVCWs) for treating reclaimed water. Bioresour. Technol. 2018, 249, 644–651. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, J.; Hu, F.; Zhang, J.; Chen, P.; Yuan, Z.; Xu, Z. Microbial community succession and responses to internal environmental drivers throughout the operation of constructed wetlands. Environ. Res. 2024, 259, 119522. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, S. A review on nirS-type and nirK-type denitrifiers via a scientometric approach coupled with case studies. Environ. Sci. Proc. Imp. 2022, 24, 221–232. [Google Scholar] [CrossRef]
- Ma, Y.; Zilles, J.; Kent, A. An evaluation of primers for detecting denitrifiers via their functional genes. Environ. Microbiol. 2019, 21, 1196–1210. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, S.; Jiang, Y.; Jiang, C.; Wang, D.; Xu, G.; Yang, D.; Wu, S.; Bai, Z.; Zhuang, G.; et al. Enhancing nitrogen removal from anaerobically-digested swine wastewater through integration of Myriophyllum aquaticum and free nitrous acid-based technology in a constructed wetland. Sci. Total Environ. 2021, 779, 146441. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, H.; Ma, Z.; You, X.; Wei, X.; Li, Y.; Tian, Y. Meta-analyzing the mechanism of pyrogenic biochar strengthens nitrogen removal performance in sulfur-driven autotrophic denitrification system: Evidence from metatranscriptomics. Water Res. 2024, 253, 121296. [Google Scholar] [CrossRef]
- Chen, K.; Feng, J.; Bodelier, P.L.E.; Yang, Z.; Huang, Q.; Delgado-Baquerizo, M.; Cai, P.; Tan, W.; Liu, Y. Metabolic coupling between soil aerobic methanotrophs and denitrifiers in rice paddy fields. Nat. Commun. 2024, 15, 3471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, W.; Wang, X. New Insights into How Increases in Fertility Improve the Growth of Rice at the Seedling Stage in Red Soil Regions of Subtropical China. PLoS ONE 2014, 9, e109161. [Google Scholar] [CrossRef]
- Nyer, S.C.; Volkenborn, N.; Aller, R.C.; Graffam, M.; Zhu, Q.; Price, R.E. Nitrogen transformations in constructed wetlands: A closer look at plant-soil interactions using chemical imaging. Sci. Total Environ. 2022, 816, 151560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Li, X.; Li, J.; Zhao, Z.; Hou, X. Mixed culture of plants improved nutrient removal in constructed wetlands: Response of microbes and root exudates. Environ. Sci. Pollut. Res. 2023, 30, 5861–5872. [Google Scholar] [CrossRef]
- Bognár, Z.; Mosshammer, M.; Brodersen, K.E.; Bollati, E.; Gyurcsányi, R.E.; Kühl, M. Multiparameter sensing of oxygen and pH at biological interfaces via hyperspectral imaging of luminescent sensor nanoparticles. ACS Sens. 2024, 9, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.; Xia, X.; Yang, J.J.; Liu, J.; Remusat, L.; Rumpel, C.; Bloem, E.; Krasny, B.B.; Schnug, E. Speciation and distribution of chromium (III) in rice root tip and mature zone: The significant impact of root exudation and iron plaque on chromium bioavailability. J. Hazard. Mater. 2023, 448, 130992. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.P.; Zhu, Y.F.; Kianfar, E. Nano Biosensors: Properties, applications and electrochemical techniques. J. Mater. Res. Technol.-JMRT 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Cai, B.; Zhang, Y.; Wang, X.; Li, Y. An optimization model for a wetland restoration project under uncertainty. Int. J. Environ. Res. Public Health 2018, 15, 2795. [Google Scholar] [CrossRef]
- Feng, S.; Xu, S.; Zhang, X.; Wang, R.; Ma, X.; Zhao, Z.; Zhuang, G.; Bai, Z.; Zhuang, X. Myriophyllum aquaticum-based surface flow constructed wetlands for enhanced eutrophic nutrient removal-A case study from laboratory-scale up to pilot-scale constructed wetland. Water 2018, 10, 1391. [Google Scholar] [CrossRef]
- Wang, G.; Li, T.; Zhou, Q.; Zhang, X.; Li, R.; Wang, J. Characterization and environmental applications of soil biofilms: A review. Environ. Chem. Lett. 2024, 22, 1989–2011. [Google Scholar] [CrossRef]
- McDaniel, E.A.; Wahl, S.A.; Ishii, S.I.; Pinto, A.; Ziels, R.; Nielsen, P.H.; McMahon, K.D.; Williams, R.B.H. Prospects for multi-omics in the microbial ecology of water engineering. Water Res. 2021, 205, 117608. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Pioli, R.; Stocker, R.; Berry, D. Single-cell stable isotope probing in microbial ecology. ISME Commun. 2022, 2, 55. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kuramae, E.E.; Jia, Z.; Liu, B. Stable isotope probing reveals compositional and functional shifts in active denitrifying communities along the soil profile in an intensive agricultural area. Sci. Total Environ. 2024, 907, 167968. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Htun, N.N.; Schlenz, F.; Kasimati, A.; Verbert, K. A review of visualisations in agricultural decision support systems: An HCI perspective. Comput. Electron. Agric. 2019, 163, 104844. [Google Scholar] [CrossRef]
- Wu, N.; Shi, W.; Zhang, L.; Wang, H.; Liu, W.; Ren, Y.; Li, X.; Gao, Z.; Wang, X. Dynamic alterations and ecological implications of rice rhizosphere bacterial communities induced by an insect-transmitted reovirus across space and time. Microbiome 2024, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, Y.; Zhao, Q.; He, T.; Mao, T.; Zhang, T.; Zhang, L.; Su, W. The quantification of root exudation by an in-situ method based on root morphology over three incubation periods. Front. Plant Sci. 2024, 15, 1423703. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, D.P.; Thompson, D.; Joshi, M.; Mishra, A.K.; Joshi, V. Unraveling the spatio-temporal dynamics of soil and root-associated microbiomes in Texas olive orchards. Sci. Rep. 2024, 14, 18214. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Kim, B.S.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Constructed wetlands for the removal of organic micropollutants from wastewater: Current status, progress, and challenges. Chemosphere 2024, 360, 142364. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Cheng, X.; Jiang, S.; Pan, J.; Zhu, D.; Lu, Z.; Jiang, Y.; Liu, C.; Guo, H.; Xie, J. Unveiling the power of COD/N on constructed wetlands in a short-term experiment: Exploring microbiota co-occurrence patterns and assembly dynamics. Sci. Total Environ. 2024, 912, 169568. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Han, X.; Hu, J.; Chen, H.; Zhai, Y. Jointly considering multi-medium and full-cycle to better reveal distribution and removal of antibiotic resistance genes in long-term constructed wetland. Sci. Total Environ. 2024, 955, 177276. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ge, Z.; Zhou, X.; Li, S.; Li, X.; Tang, J. Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: A global meta-analysis. Glob. Change Biol. 2020, 26, 1638–1653. [Google Scholar] [CrossRef] [PubMed]
- Ealias, A.M.; Meda, G.; Tanzil, K. Recent Progress in Sustainable Treatment Technologies for the Removal of Emerging Contaminants from Wastewater: A Review on Occurrence, Global Status and Impact on Biota. Rev. Environ. Contam. Toxicol. 2024, 262, 16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).