Adsorptive Elimination of Cu(II) Ions from Aqueous Solution onto Chitosan Modified with Uracil
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
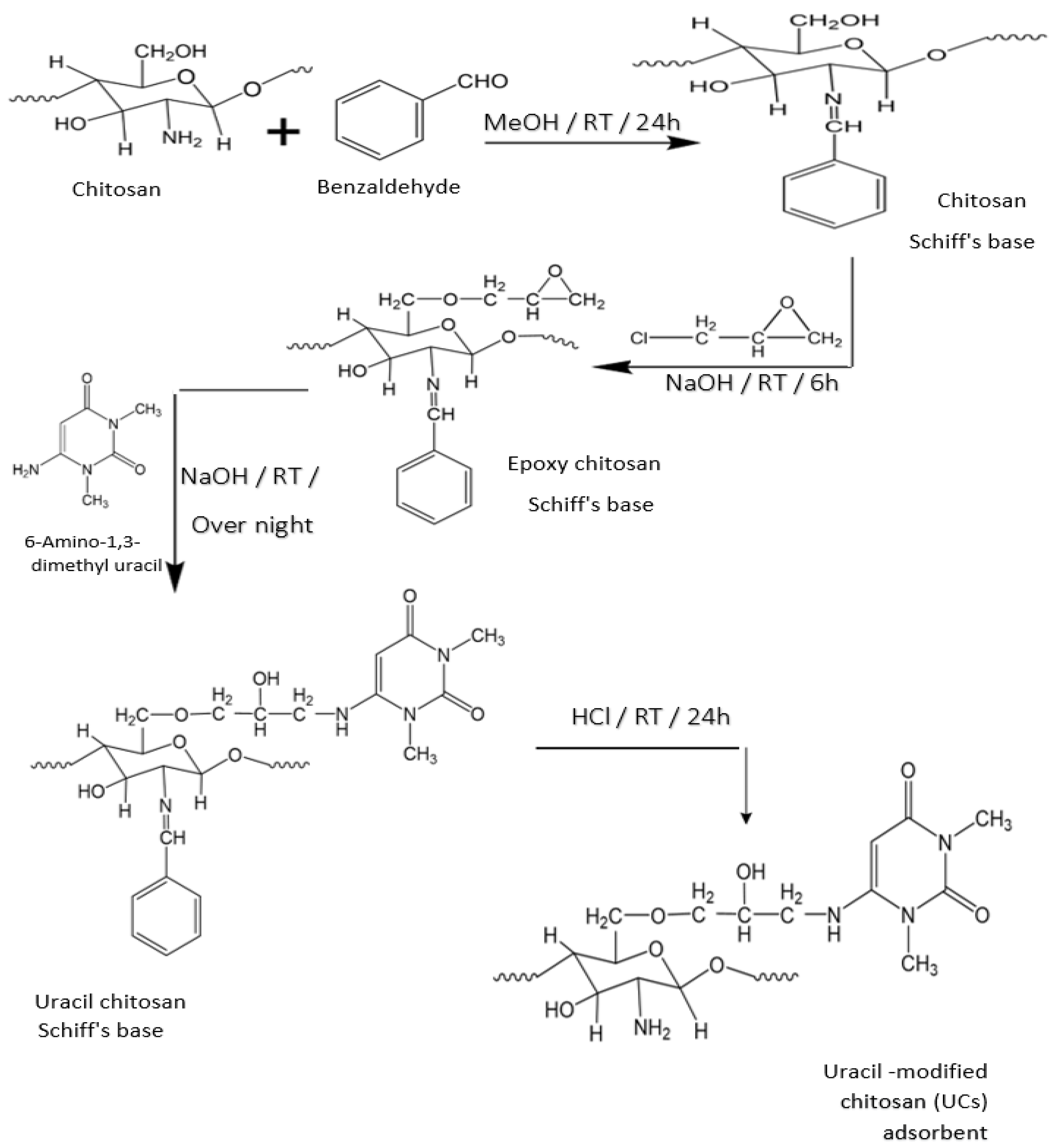
2.2. Preparation of Chitosan Modified with Uracil (UCs)
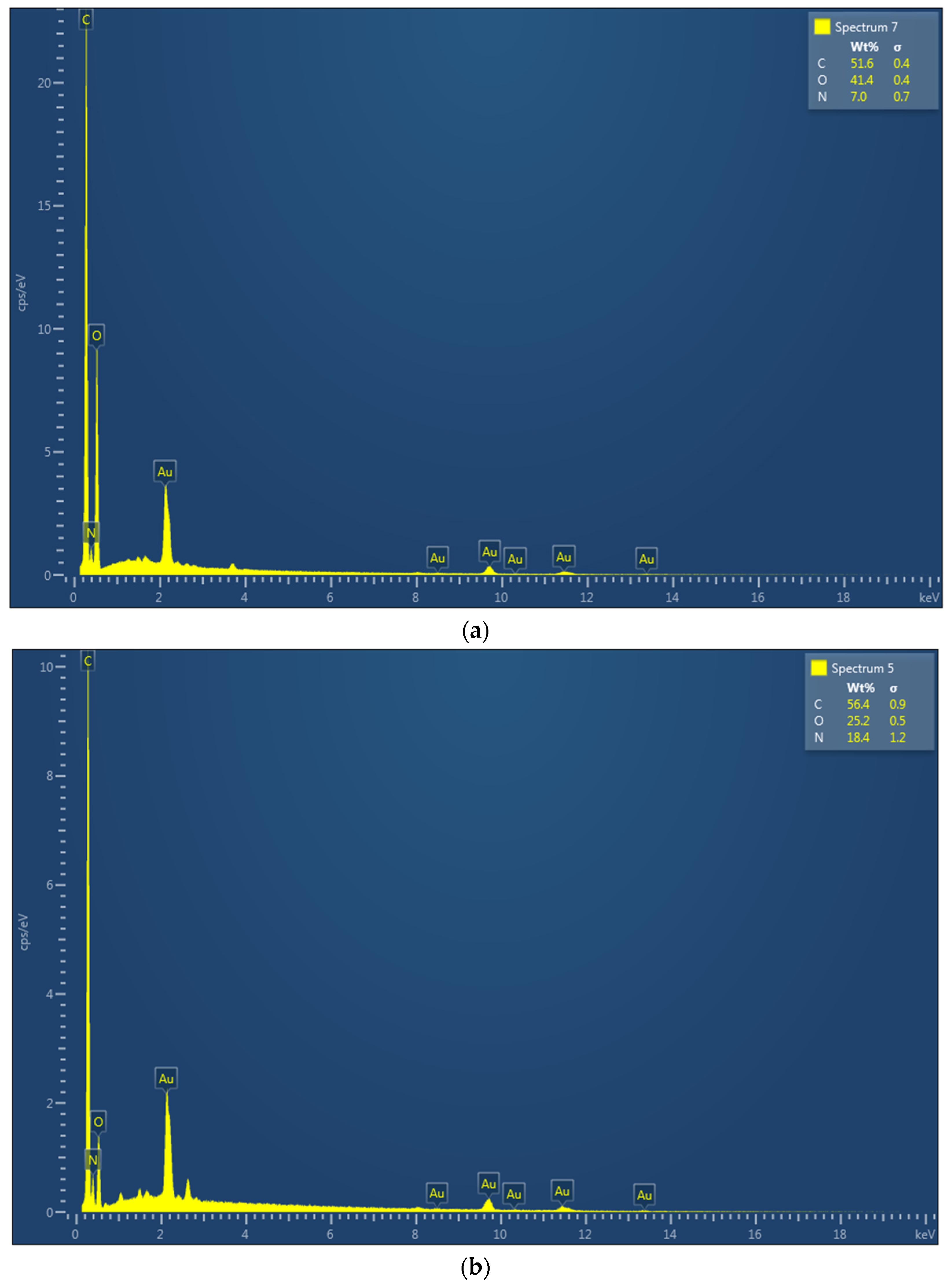
2.3. Characterization of UCs
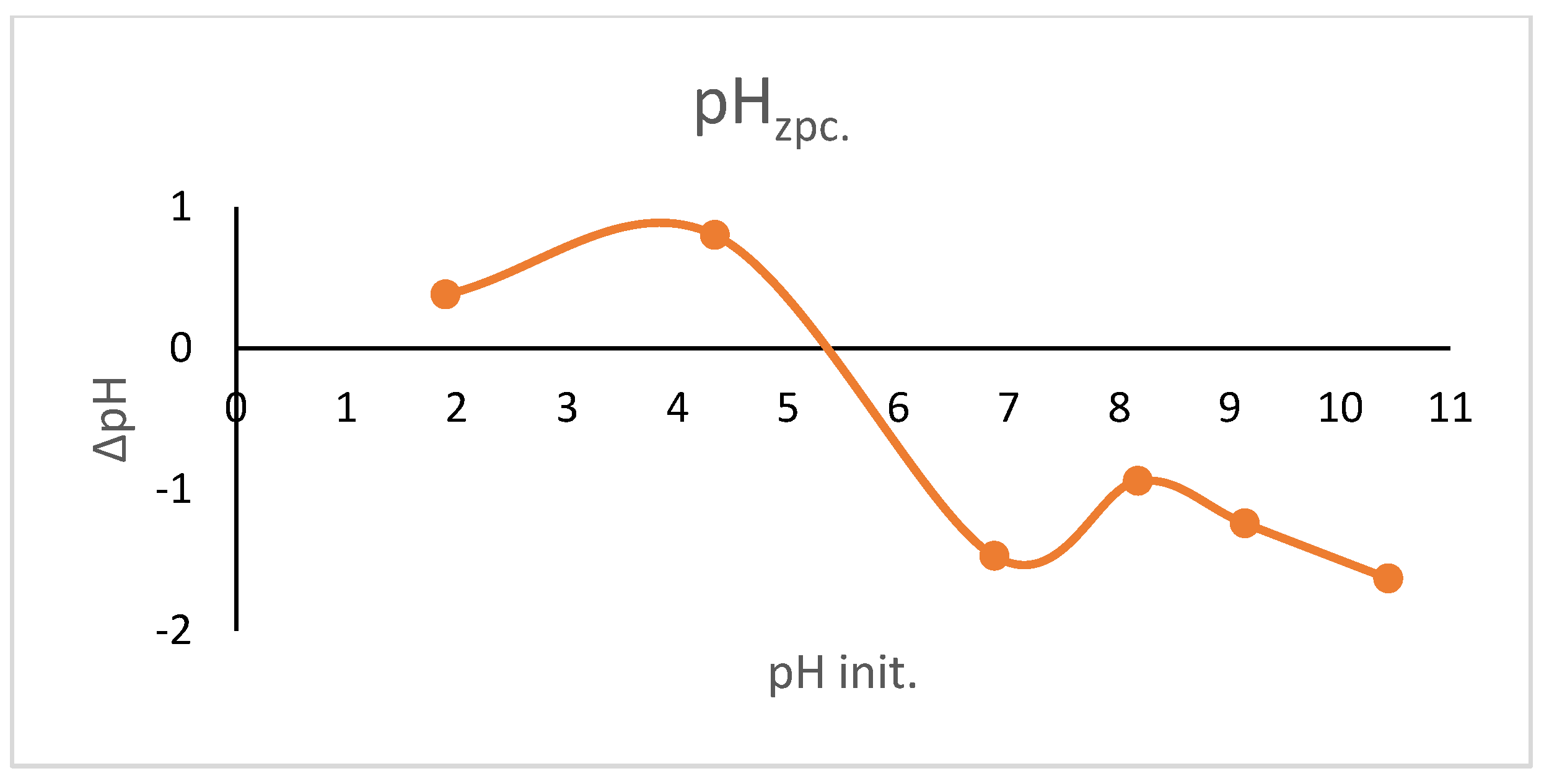
2.4. pH Value of Zero-Point Charge (pHzpc)
2.5. Adsorption Measurements
2.6. Adsorption Kinetics
- Pseudo-First-Order Model
- Pseudo-Second-Order Model
- Elovich Model
- Intraparticle Diffusion Model (the Weber–Morrison model)
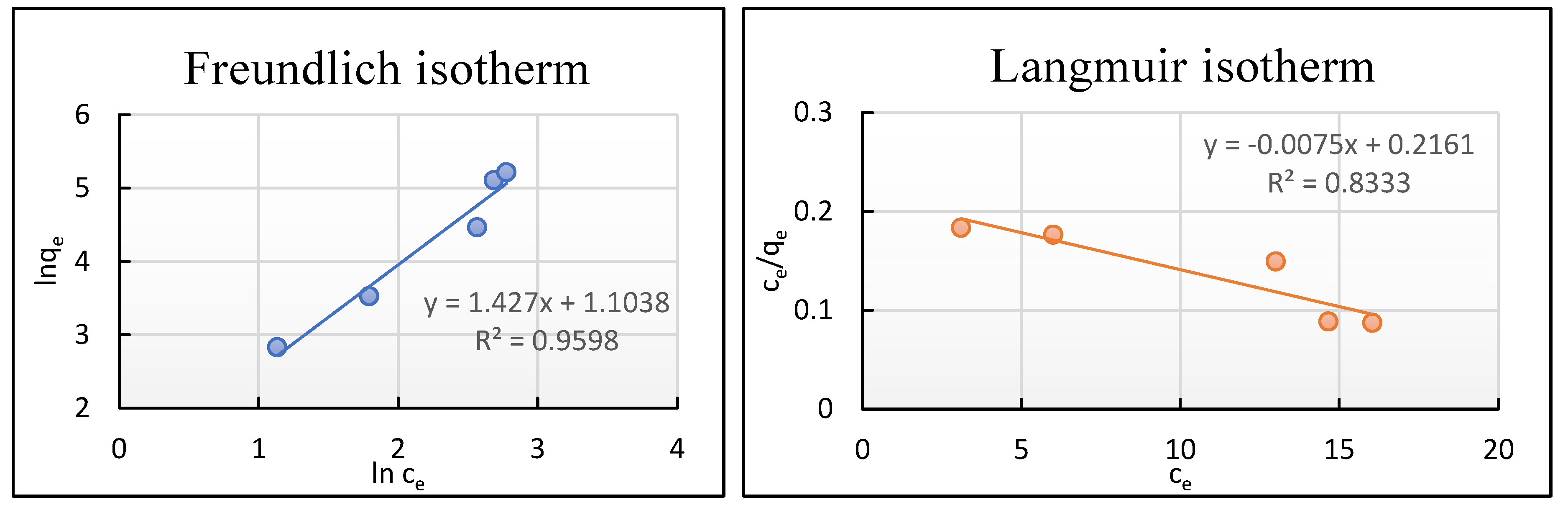
2.7. Adsorption Isotherms
- The Langmuir isotherm model can be expressed by Equations (13)–(15).
- The Freundlich isotherm model can be expressed by Equations (16) and (17).
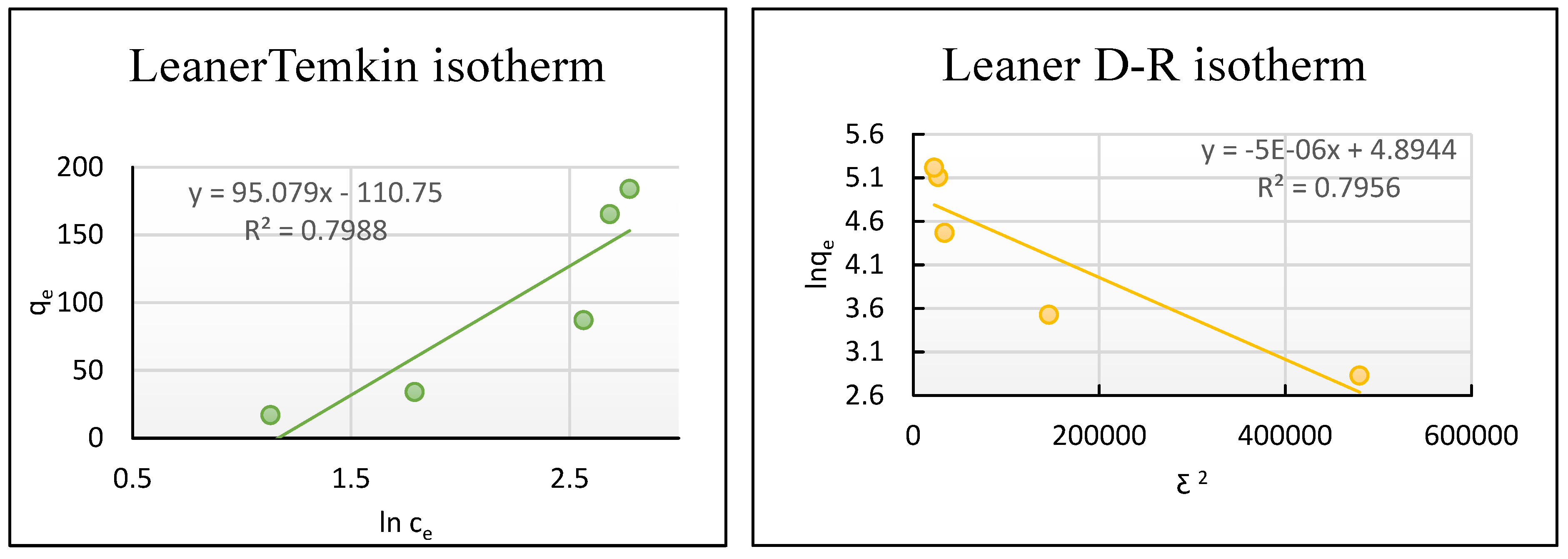
- Temkin isotherm Model
- Dubinin–Radushkevich (D-R) isotherm Model
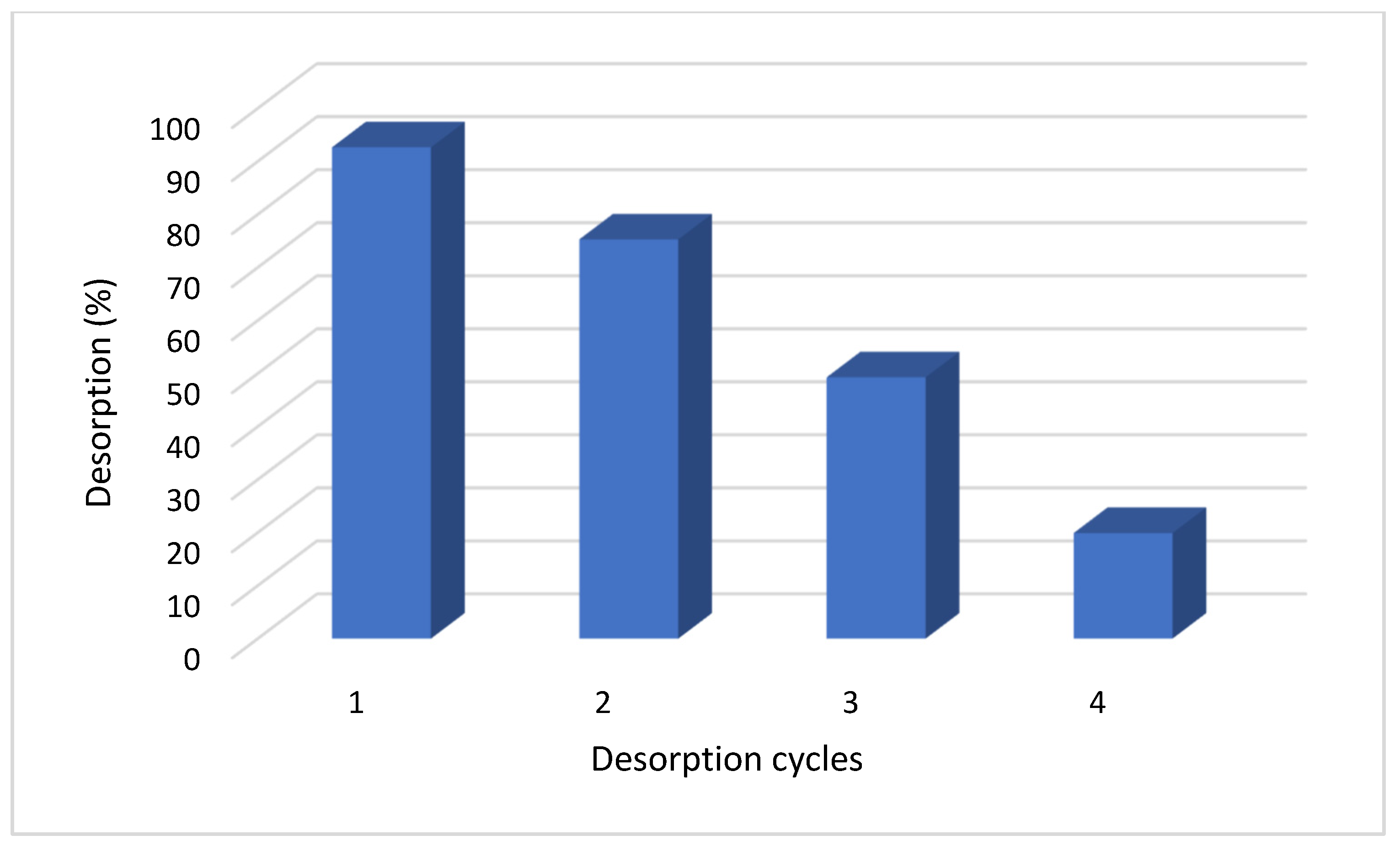
2.8. Desorption Behavior
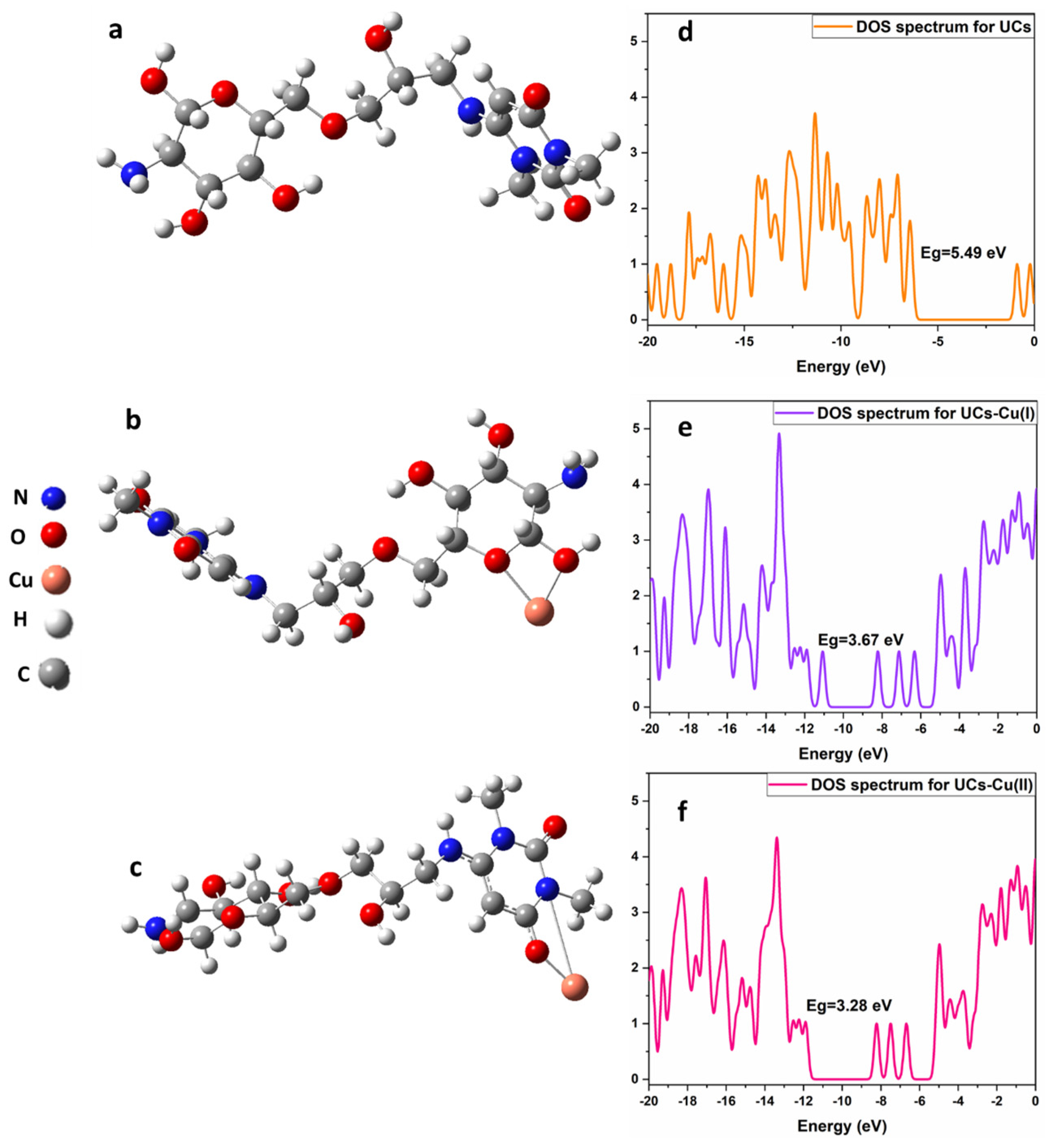
2.9. Theoretical Calculation Details
3. Results and Discussion
3.1. pH of Zero-Point Charge (pHzpc)
3.2. Adsorption Optimization
3.2.1. Influence of the Initial Cu(II) Ions Solution Concentrations
3.2.2. Impact of the Temperature
3.2.3. Influence of pH of the Medium
3.2.4. Effect of UCs Dose
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.5. Desorption Assessment
3.6. Adsorption Mechanism
3.7. Comparison of Different Adsorbents’ Capacities for Adsorbing Cu(II) Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, R.J.E.; Rosana, P.; Rui, A.R.B. Cadmium (II) and zinc (II) adsorption by the aquatic moss Fontinalis antipyretica: Effect of temperature, pH and water hardness. Water Res. 2004, 38, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Renu, M.A.; Kailash, S. Methodologies for removal of heavy metal ions from wastewater: An overview. Interdiscip. Environ. Rev. 2017, 18, 124–142. [Google Scholar] [CrossRef]
- Ningchuan, F.; Xueyi, G.; Sha, L.; Yanshu, Z.; Jianping, L. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar]
- Patrycja, M.K.; Paulina, S.; Ewa, K. Removal of heavy metals from aqueous solutions with the use of lignins and biomass. Fibres Text. East. Eur. 2022, 30, 99–111. [Google Scholar]
- Hu, Z.; Lei, L.; Li, Y.; Ni, Y. Chromium adsorption on high performance activated. Sep. Purif. Technol. 2003, 31, 13–18. [Google Scholar] [CrossRef]
- Homa, G.; Mehrnoosh, A.; Tazkieh, G.; Behzad, A.; Saeed, M. Rapid and Effective removal of heavy metal ions from aqueous solution using nanostructured clay particles. Results Surf. Interfaces 2023, 10, 100097. [Google Scholar]
- Vimala, R.; Nilanjana, D. Biosorbtion of cadmium (II) and lead (II) from aqueous solutions using mushrooms: A comparative study. J. Hazard. Mater. 2009, 168, 376–382. [Google Scholar] [CrossRef]
- Demirbaş, Ö.; Karadağ, A.; Alkan, M.; Doğan, M. Removal of copper ions from aqueous Solutions by hazelnuts. J. Hazard. Mater. 2008, 153, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Cho, H.J.; Yim, J.H.; Kim, J.M.; Jeon, J.K.; Sohn, J.M.; Yoo, K.S.; Kim, S.S.; Park, Y.K. Removal of Cu(II)-ion over amine-functionalized mesoporous silica materials. J. Ind. Eng. Chem. 2011, 17, 504–509. [Google Scholar] [CrossRef]
- Chang, S.H.; Teng, T.T.; Ismail, N. Extraction of Cu (II) from aqueous solutions by vegetable oil-based organic solvents. J. Hazard. Mater. 2010, 181, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Manahan, S.E. Environmental Chemistry, 5th ed.; Lewis Publishers: Chelsea, MI, USA, 1991. [Google Scholar]
- Atalay, E.; Gode, F.; Sharma, Y.C. Removal of selected toxic metals by a modified adsorbent. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2010, 14, 132–138. [Google Scholar] [CrossRef]
- Guijuan, J.I.; Weiwei, B.A.O.; Guimei, G.A.O.; Baichao, A.N.; Shucai, G.A.N. Removal of Cu (II) from aqueous solution using a novel crosslinked alumina-chitosan hybrid adsorbent. Chin. J. Chem. Eng. 2012, 20, 641–648. [Google Scholar]
- Reza, B.L.; Hamid, R.Z.; Mohammad, E.B. Removal of Cu(II) Ions from aqueous solutions by low-cost natural hydroxyapatite/chitosan composite: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2014, 45, 1642–1648. [Google Scholar]
- Uchenna, O.; Mamookho, M.; Lukhanyo, M.; Nkemdinma, U.O.; Tendani, S.; Vuyo, M. Toxic metal implications on agricultural soils, plants, animals, aquatic life and human health. Int. J. Environ. Res. Public Health 2020, 17, 2204. [Google Scholar] [CrossRef]
- Wolfgang, S.; Ralf, W. Wilson disease: More complex than just simply a copper overload condition? A narrative review. AME Med. J. 2022, 7, 22–24. [Google Scholar]
- Abdel-Motaal, M.; Aldakhili, D.A.; Elmaaty, A.A.; Sharaky, M.; Mourad, M.A.E.; Alzahrani, A.Y.A.; Mohamed, N.A.; Al-Karmalawy, A.A. Design and synthesis of novel tetrabromophthalimide derivatives as potential tubulin inhibitors endowed with apoptotic induction for cancer treatment. Drug Dev. Res. 2024, 85, e22197. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Mohan, M.R.M.K.; Raja, S.R.I.; Agilandeswari, D.; Nageswararao, M. Water quality and risk assessment of copper content in drinking water stored in copper container. Appl. Water Sci. 2022, 12, 27. [Google Scholar]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives. a review of the structure-activity relationship. Biomacromolecules 2017, 13, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.S.; Wan, F.S. Adsorption characterization of Pb (II) and Cu (II) ions onto chitosan-tripolyphosphate beads: Kinetic, equilibrium and thermodynamic studies. J. Environ. Manag. 2010, 91, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Alfuraydi, R.T.; Al-Harby, N.F.; Alminderej, F.M.; Elmehbad, N.Y.; Mohamed, N.A. Poly (Vinyl Alcohol) Hydrogels Boosted with Cross-Linked Chitosan and Silver Nanoparticles for Efficient Adsorption of Congo Red and Crystal Violet Dyes. Gels 2023, 9, 882. [Google Scholar] [CrossRef]
- Annu, S.; Ahmed, S.; Ikram, S. Chitin and Chitosan: History, Composition and Properties, Chitosan; Ahmed, S., Ikram, S., Eds.; Scrivener & Wiley: Hoboken, NJ, USA, 2017; pp. 3–24. [Google Scholar]
- Wen, L.; Chen, X.; Chen, C.; Yang, R.; Gong, M.; Zhang, Y.; Fu, Q. Ice-templated porous polymer/UiO-66 monolith for Congo red adsorptive removal. Arab. J. Chem. 2020, 13, 5669–5678. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Benhalima, T.; Lerari, D. Sustainable hydrogel nanocomposites based on grafted chitosan and clay for effective adsorption of cationic dye. Int. J. Mater. Metall. Eng. 2020, 14, 5–15. [Google Scholar]
- Elmehbad, N.Y.; Mohamed, N.A.; Abd El-Ghany, N.A.; Abdel-Aziz, M.M. Evaluation of the in vitro anti-inflammatory and anti-Helicobacter pylori activities of chitosan-based biomaterials modified with copper oxide nanoparticles. Int. J. Biol. Macromol. 2023, 253, 127277. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A. Synthesis, characterization and evaluation of in vitro potential antimicrobial efficiency of new chitosan hydrogels and their CuO nanocomposites. Int. J. Biol. Macromol. 2024, 276, 133810. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, A.; Ramya, R.; Ramasubramaniam, S.; Sudha, P.N. Studies on removal of Cr(VI) and Cu(II) ions using chitosan grafted-polyacrylonitrile. Arch. Appl. Sci. Res. 2011, 3, 424–435. [Google Scholar]
- Xiao, X.; Liang, H.; Chen, W.; Wang, Z. Synthesis and adsorption behavior of chitosan-coated MnFe2O4 nanoparticles for trace heavy metal ions removal. Appl. Surf. Sci. 2013, 285, 498–504. [Google Scholar] [CrossRef]
- Tonetti, C.; Ferrero, F.; Periolatto, M.; Vineis, C.; Varesano, A.; Mazzuchetti, G. Chitosan coated cotton textiles for copper and chromium ions adsorption. In Proceedings of the 9th International Izmir Textile and Apparel Symposium, Izmir, Turkey, 2–5 April 2014; pp. 121–126. [Google Scholar]
- Lv, L.; Chen, N.; Feng, C.; Zhanga, J.; Li, M. Heavy metal ions removal from aqueous solution by xanthate-modified cross-linked magnetic chitosan/poly(vinyl alcohol) particle. RSC Adv. 2017, 7, 27992–28000. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Abd Karim, K.J.; Wan, I.W.A.; Hadi, J.B. Equilibrium, kinetic and mechanism studies of Cu(II) and Cd(II) ions adsorption by modified chitosan beads. Int. J. Biol. Macromol. 2018, 116, 255–263. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption of copper ions onto chitosan/poly(vinyl alcohol) beads functionalized with poly(ethylene glycol). Carbohydr. Polym. 2020, 234, 115890. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Taleghanib, H.G.; Khanjani, J. Efficient removal of copper ion from aqueous solution using crosslinked chitosan grafted with polyaniline. Int. J. Eng. 2021, 34, 305–312. [Google Scholar]
- Pałasz, A.; Cież, D. In search of uracil derivatives as bioactive agents. Uracils and fused uracils: Synthesis, biological activity and applications. Eur. J. Med. Chem. 2015, 97, 582–611. [Google Scholar] [CrossRef] [PubMed]
- Zare, A.; Ghobadpoor, A.; Safdari, T. Preparation, characterization and utilization of a novel dicationic molten salt as catalyst for the synthesis of bis(6-amino-1,3-dimethyluracil-5-yl)methanes. Res. Chem. Intermed. 2020, 46, 1319–1327. [Google Scholar] [CrossRef]
- Belyaev, D.V.; Chizhov, D.L.; Rusinov, G.L.; Charushin, V.N. Synthesis of 2-Substituted 6-(Polyfluoromethyl)pyrimidine-4-carbaldehyde Acetals. Russ. J. Org. Chem. 2019, 55, 879–882. [Google Scholar] [CrossRef]
- Whittleton, S.R.; Hunter, K.C.; Wetmore, S.D. Effects of hydrogen bonding on the acidity of uracil derivatives. J. Phys. Chem. A 2004, 108, 7709–7718. [Google Scholar] [CrossRef]
- Noikham, M.; Yotphan, S. Copper-Catalyzed Regioselective Direct C–H Thiolation and Thiocyanation of Uracils. Eur. J. Org. Chem. 2019, 2019, 2759–2766. [Google Scholar] [CrossRef]
- Das, S.; Kalita, S.J.; Bharali, P.; Konwar, B.K.; Das, B.; Thakur, A.J. Organic reactions in ‘green surfactant’: An avenue to bisuracil derivative. ACS Sustain. Chem. Eng. 2013, 1, 1530–1536. [Google Scholar] [CrossRef]
- Aguiar, H.; Sant’ana, A.; Temperini, M.; Corio, P.; Cunha, F. Surface enhanced Raman spectroscopy analysis of the adsorption of 2-thiouracil to Au, Ag and Cu electrodes: Surface potential dependence. Vib. Spectrosc. 2006, 40, 127–132. [Google Scholar] [CrossRef]
- Ghabbour, E.A.; Davies, G.; Dunfee, R.L.; Smith, N.A.; Vozzella, M.E. Adsorption of nucleic acid constituent uracil on copper(II)-loaded, solid peat and soil-derived humic acids. Can. J. Soil Sci. 2001, 81, 309–316. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Synthesis and characterization of novel uracil-modified chitosan as a promising adsorbent for efficient removal of Congo red dye. Polymers 2022, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Kavianinia, I.; Plieger, P.G.; Kandile, N.G.; Harding, D.R. Preparation and characterization of an amphoteric chitosan derivative employing trimellitic anhydride chloride and its potential for colon targeted drug delivery system. Mater. Today Commun. 2015, 3, 78–86. [Google Scholar] [CrossRef]
- Shukla, K.; Verma, A.; Verma, L.; Rawat, S.; Singh, J. A novel approach to utilize used disposable paper cups for the development of adsorbent and its application for the malachite green and rhodamine-B dyes removal from aqueous solutions. Nat. Environ. Pollut. Technol. 2020, 19, 57–70. [Google Scholar]
- Antony, V.S.; Shobana, N.; Durga, S.; Chamarthy, S. Utilization of chitosan coated superparamagnetic iron oxide nanoparticles for chromium removal. Appl. Water Sci. 2018, 8, 192. [Google Scholar]
- Soon, C.Y.; Rahman, N.A.; Tee, Y.B.; Talib, R.A.; Tan, C.H.; Abdan, K.; Chan, E.W.C. Electrospun biocomposite: Nanocellulose and chitosan entrapped within a poly (hydroxyalkanoate) matrix for Congo red removal. J. Mater. Res. Technol. 2019, 8, 5091–5102. [Google Scholar] [CrossRef]
- Taha, A.A.; Ahmed, A.M.; Abdel-Rahman, H.H.; Abouzeid, F.M.; Abdel Maksoud, M.O. Removal of nickel ions by adsorption on nano bentonite: Equilibrium, kinetic and thermodynamics. J. Dispers. Sci. Technol. 2017, 38, 757–767. [Google Scholar] [CrossRef]
- Najim, T.S.; Elais, N.J.; Dawood, A.A. Adsorption of copper and iron using low cost material as adsorbent. J. Chem. 2009, 6, 161–168. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; Makhfouk, M.E.; Qourzal, S. Biosorption characteristics of cadmium and lead onto eco-Friendly dried cactus (Opuntia ficus indica) cladodes. J. Environ. Chem. Eng. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef]
- Shahat, A.; Awual, M.R.; Naushad, M. Functional ligand anchored nanomaterial based facial adsorbent for cobalt(II) detection and removal from water samples. Chem. Eng. J. 2015, 271, 155–163. [Google Scholar] [CrossRef]
- Awual, M.R. Assessing of lead(III) capturing from contaminated wastewater using ligand doped conjugate adsorbent. Chem. Eng. J. 2016, 289, 65–73. [Google Scholar] [CrossRef]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Adsorption of Cu (II) and Co (II) from aqueous solution using lignosulfonate/chitosan adsorbent. Int. J. Biol. Macromol. 2020, 163, 120–127. [Google Scholar] [CrossRef]
- Esposito, A.; Pagnanelli, F.; Lodi, A.; Solisio, C.; Vegliò, F. Biosorption of heavy metals by sphaerotilus natans: An equilibrium study at different pH and biomass concentrations. Hydrometallurgy 2001, 60, 129–141. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Cr (VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Ola, A. Kinetic and isotherm studies of copper (II) removal from wastewater using various adsorbents. Egypt. J. Aquat. Res. 2007, 33, 125–143. [Google Scholar]
- Vijayaraghavan, K.; Teo, T.T.; Balasubramanian, R.; Joshi, U.M. Application of sargassum biomass to remove heavy metal ions from synthetic multimetal solutions and urban storm water runoff. J. Hazard. Mater. 2009, 164, 1019–1023. [Google Scholar] [CrossRef]
- Demirbas, E.; Dizge, N.; Sulak, M.; Kobya, M. Adsorption kinetics and equilibrium of copper from aqueous solutions using hazelnut shell activated carbon. Chem. Eng. J. 2009, 148, 480–487. [Google Scholar] [CrossRef]
- Šljivić, M.; Smičiklas, I.; Pejanović, S.; Plećaš, I. Comparative study of Cu2+ adsorption on a zeolite, a clay and a diatomite from Serbia. Appl. Clay Sci. 2009, 43, 33–40. [Google Scholar] [CrossRef]
- Shahwan, T.; Erten, H.N. Thermodynamic parameters of Cs+ sorption on natural clays. J. Radioanal. Nucl. Chem. 2002, 253, 115–120. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, R.; Yao, J.; Mao, D.; Xing, C.; Wang, D. Removal of Cd2+ from aqueous solutions by hydroxyapatite. Catal. Today 2008, 139, 94–99. [Google Scholar] [CrossRef]
- Hasany, S.M.; Chaudhary, M.H. Sorption potential of Hare River sand for the removal of antimony from acidic aqueous solution. Appl. Radiat. Isot. 1996, 47, 467–471. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Yu, X.Y.; Luo, T.; Jia, Y.; Liu, J.H.; Huang, X.J. Γ-Fe2O3 nanoparticles encapsulated millimeter-sized magnetic chitosan beads for removal of Cr (VI) from water: Thermodynamics, kinetics, regeneration, and uptake mechanisms. J. Chem. Eng. Data. 2013, 58, 3142–3149. [Google Scholar] [CrossRef]
- Cai, L.; Ying, D.; Liang, X.; Zhu, M.; Lin, X.; Xu, Q.; Cai, Z.; Xu, X.; Zhang, L. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 2021, 410, 128404. [Google Scholar] [CrossRef]
- Ngueagni, P.T.; Woumfo, E.D.; Kumar, P.S.; Siéwé, M.; Vieillard, J.; Brun, N.; Nkuigue, P.F. Adsorption of Cu(II) ions by modified horn core: Effect of temperature on adsorbent preparation and extended application in river water. J. Mol. Liq. 2020, 298, 112023. [Google Scholar] [CrossRef]
- Yang, L.W.; Qi, P.Y.; Feng, Q.C.; Hua, X.G.; Jie, H.J.; Liang, Z.C.; Lai, B. Enhanced adsorption/photocatalytic removal of Cu (II) from wastewater by a novel magnetic chitosan and bismuth tungstate coated by silver (MCTS-Ag/Bi2WO6) composite. Chemosphere 2021, 263, 128120. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Hu, J.; Wang, J.L. Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J. Hazard. Mater. 2012, 221–222, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kannamba, B.; Reddy, K.L.; AppaRao, B.V. Removal of Cu(II) from aqueous solutions using chemically modified chitosan. J. Hazard. Mater. 2010, 175, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Mohammadzai, I.U.; Khan, A.; Shah, Z.; Hassan, W.; Ali, N. Removal of toxic metals with activated carbon prepared from Salvadora persica. Arab. J. Chem. 2017, 10, 2205–2212. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.; Muñiz-Valencia, B.P.A.A.R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Alrasheedi, M.; Mohammed, A.E.M.; Soliman, S.M.; Mohamed, N.A. Effective Removal of Cu (II) Ions from Aqueous Solution by Cross-Linked Chitosan-Based Hydrogels. Water 2024, 16, 2324. [Google Scholar] [CrossRef]


| Kinetic Models | Parameters | UCs |
|---|---|---|
| qe·exp (mg g−1) | 99.65 | |
| Pseudo-first-order model | R2 | 0.752 |
| qe·cal (mg g−1) | 1.948 | |
| k1 (min−1) | 0.009 | |
| Pseudo-second-order model | R2 | 1 |
| qe·cal (mg g−1) | 100 | |
| k2 (10−5) (g mg−1 min−1) | 0.011 | |
| h (mg g−1 min−1) | 96.15 | |
| Elovich ln t | R2 | 0.924 |
| β (g mg−1) | 0.593 | |
| α (1023) (mg g−1 min−1) | 4.108 | |
| Intraparticle diffusion (the Weber–Morrison model) qt = (kint t1/2) + C | R2 | 0.840 |
| k (mg g−1 min−1/2) | 0.483 | |
| Models | Parameter | UCs |
|---|---|---|
| Langmuir | qmax (mg g−1) | 133.33 |
| RL | 0.126–0.590 | |
| KL (L mg−1) | 0.035 | |
| R2 | 0.833 | |
| Freundlich | 1/n | 1.427 |
| Kf (mg g−1) | 3.016 | |
| R2 | 0.960 | |
| Temkin qe = BTInKT + BTIn Ce | BT (kJ mol−1) | 95.79 |
| KT (L g−1) | 3.205 | |
| R2 | 0.799 | |
| D-R Ln qe = ln (qm) − βε2 | qm (mg g−1) | 133.49 |
| ε | 22.452 | |
| β × 10−6 | 5 | |
| kJ mol−1 | 0.316 | |
| R2 | 0.796 |
| Adsorbent | qmax (mg g−1) | Temperature (°C) | Cu(II) Ions Conc. (mg L−1) | Adsorbent Dose (g) | pH | Ref. |
|---|---|---|---|---|---|---|
| Cross-linked chitosan grafted with polyaniline | 131.58 | 20–40 | 100 | 0.05 | 6 | [42] |
| Quaternized chitosan@chitosan cationic polyelectrolyte microsphere | 687.6 | 25 | 0–2000 | 0.075 | 5 | [64] |
| Horn core calcined at 400 °C | 99.98 | 25 | 100–500 | 0.02 | 5 | [65] |
| Magnetic chitosan@bismuth tungstate coated by silver | 181.8 | 20–40 | 20–120 | 0.02 | 6 | [66] |
| Xanthate-modified magnetic chitosan | 34.5 | 25 | 100 | - | 5 | [67] |
| Epichlorohydrin cross-linked xanthate chitosan | 43.47 | 50 | 100 | - | 5 | [68] |
| Medicinal plant (Salvadora persica) | 74.30 | 25 | 100 | - | 4 | [69] |
| Pecan nutshell (Carya illinoinensis) | 23.37 | 30 | - | - | 5 | [70] |
| H1 | 96.20 | 25 | 100 | 0.01 | 6 | [71] |
| H2 | 97.59 | 25 | 100 | 0.01 | 6 | [71] |
| UCs | 99.65 | 25 | 100 | 0.01 | 6 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrasheedi, M.; Mohammed, A.e.M.E.; Al-harby, N.F.; Khedr, G.E.; Mohamed, N.A. Adsorptive Elimination of Cu(II) Ions from Aqueous Solution onto Chitosan Modified with Uracil. Water 2024, 16, 3695. https://doi.org/10.3390/w16243695
Alrasheedi M, Mohammed AeME, Al-harby NF, Khedr GE, Mohamed NA. Adsorptive Elimination of Cu(II) Ions from Aqueous Solution onto Chitosan Modified with Uracil. Water. 2024; 16(24):3695. https://doi.org/10.3390/w16243695
Chicago/Turabian StyleAlrasheedi, Muneera, Ard elshifa M. E. Mohammed, Nouf F. Al-harby, Ghada E. Khedr, and Nadia A. Mohamed. 2024. "Adsorptive Elimination of Cu(II) Ions from Aqueous Solution onto Chitosan Modified with Uracil" Water 16, no. 24: 3695. https://doi.org/10.3390/w16243695
APA StyleAlrasheedi, M., Mohammed, A. e. M. E., Al-harby, N. F., Khedr, G. E., & Mohamed, N. A. (2024). Adsorptive Elimination of Cu(II) Ions from Aqueous Solution onto Chitosan Modified with Uracil. Water, 16(24), 3695. https://doi.org/10.3390/w16243695






