Hydrogeochemistry, Water Quality, and Health Risk Analysis of Phreatic Groundwater in the Urban Area of Yibin City, Southwestern China
Abstract
1. Introduction
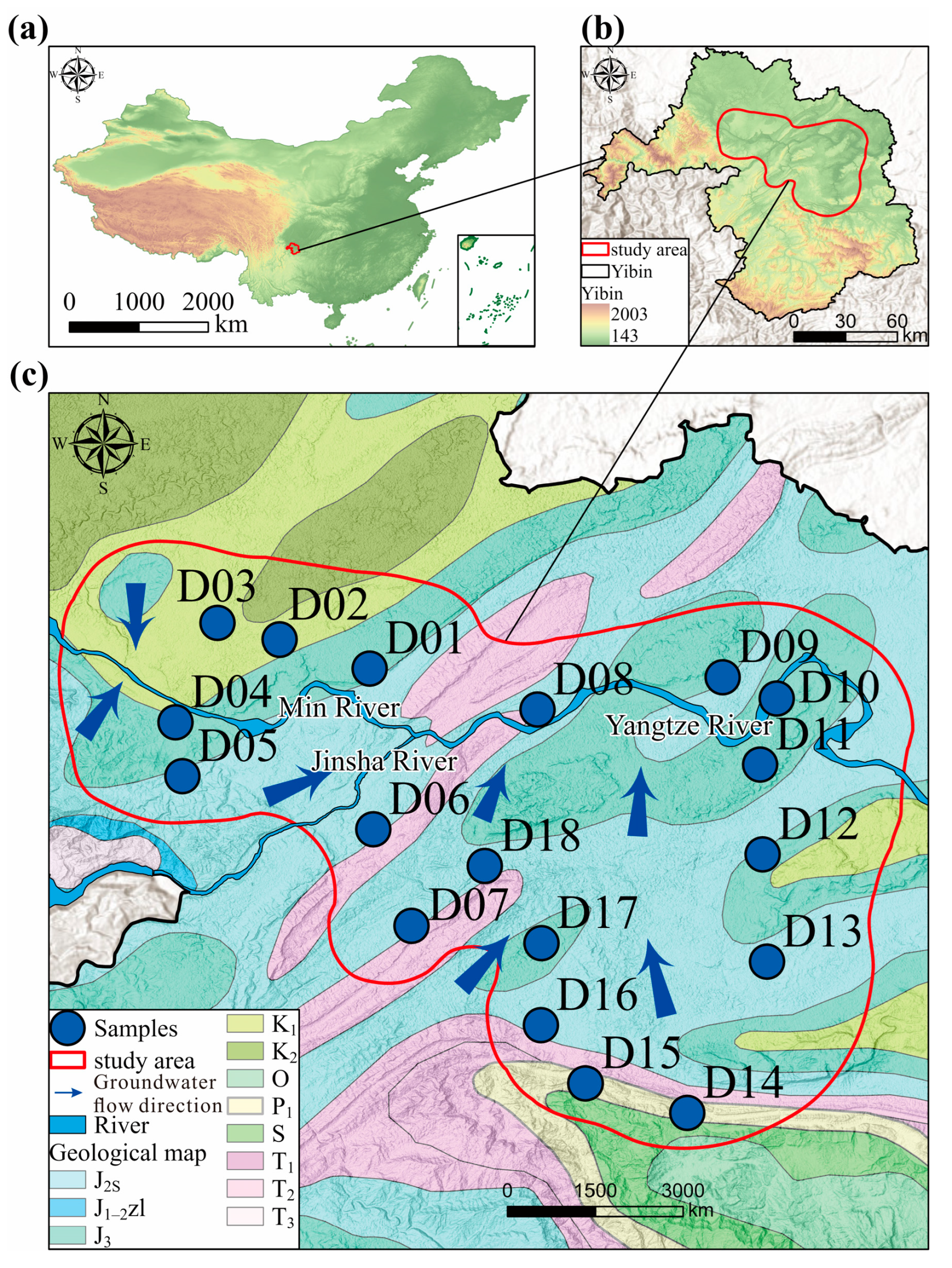
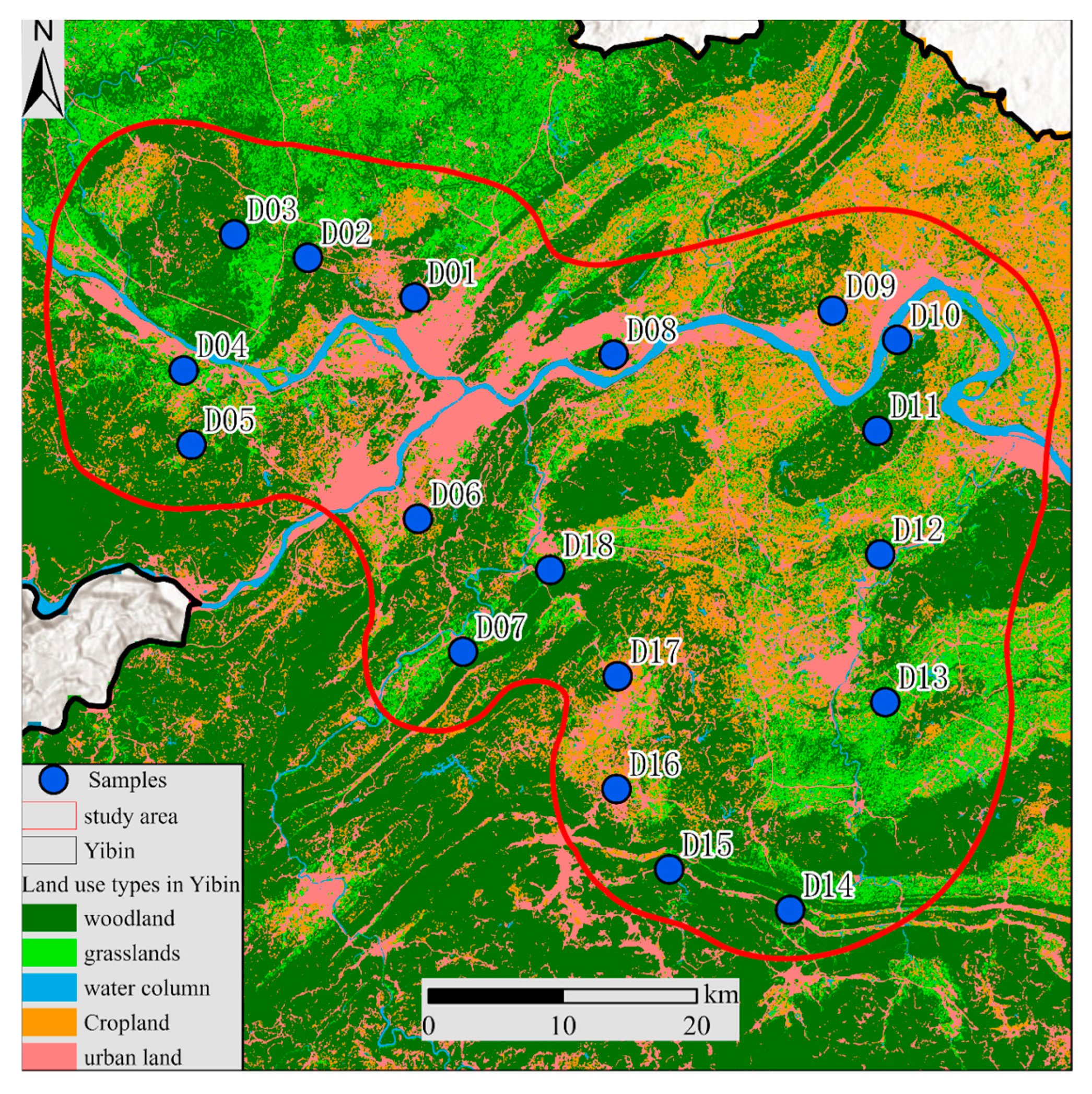
2. Study Area
3. Materials and Methods
3.1. Data Sources
3.2. Hydrogeochemical Characteristics Analysis
3.3. Entropy-Weighted Water Quality Index (EWQI)
- Establish the initial evaluation index matrix. This matrix consolidates and presents the collected groundwater-related parameters. Let the number of sampling points be m and a certain evaluation variable be n; thus, the initial evaluation index matrix is denoted as X. In the evaluation index matrix, Xij represents the value of the j-th evaluation indicator for the i-th sampling point.
- Data normalization processing. Since the nature of each evaluation indicator varies and their magnitudes differ, it is necessary to normalize the data of each indicator to eliminate their units. Positive indicators are processed using Equation (2), while negative indicators are processed using Equation (3).where yij is the standardized value of the j-th indicator for the i-th sample, xij is the original value of the j-th indicator for the i-th sample, and max(xj) and min(xj) are the original maximum and minimum values of the corresponding indicator, respectively.
- Determine the weights of each evaluation factor. If there are m samples to be evaluated in the study, and n (where n = 1~i) evaluation variables, then the entropy value of the j-th evaluation variable is denoted as wj.In this step, Pij represents the ratio of the indicator value in a specific column of the standardized evaluation index matrix to the sum of the values in that column. The additional term 10−4 is a correction parameter, aimed at preventing the formula from becoming meaningless when Pij equals 0, which could affect subsequent calculations.
- Evaluation index calculation. The water quality ratio qij is calculated based on the concentration Cj of each parameter j and the corresponding permissible limit Sj. A separate calculation method is used for the pH value. Finally, the EWQI is derived by summing the weighted ratios of all parameters. Based on the calculated EWQI, groundwater can be classified into five categories: (1) Excellent (EWQI ≤ 50); (2) Good (50 < EWQI ≤ 100); (3) Moderate (100 < EWQI ≤ 150); (4) Poor (150 < EWQI ≤ 200); (5) Very Poor (EWQI > 200) [46].where Sj is the permissible limit for parameter j based on China’s groundwater quality standards in this study ([7]).
3.4. Health Risk Assessment Model
3.5. Uncertainty and Sensitivity Analysis
4. Results and Discussion
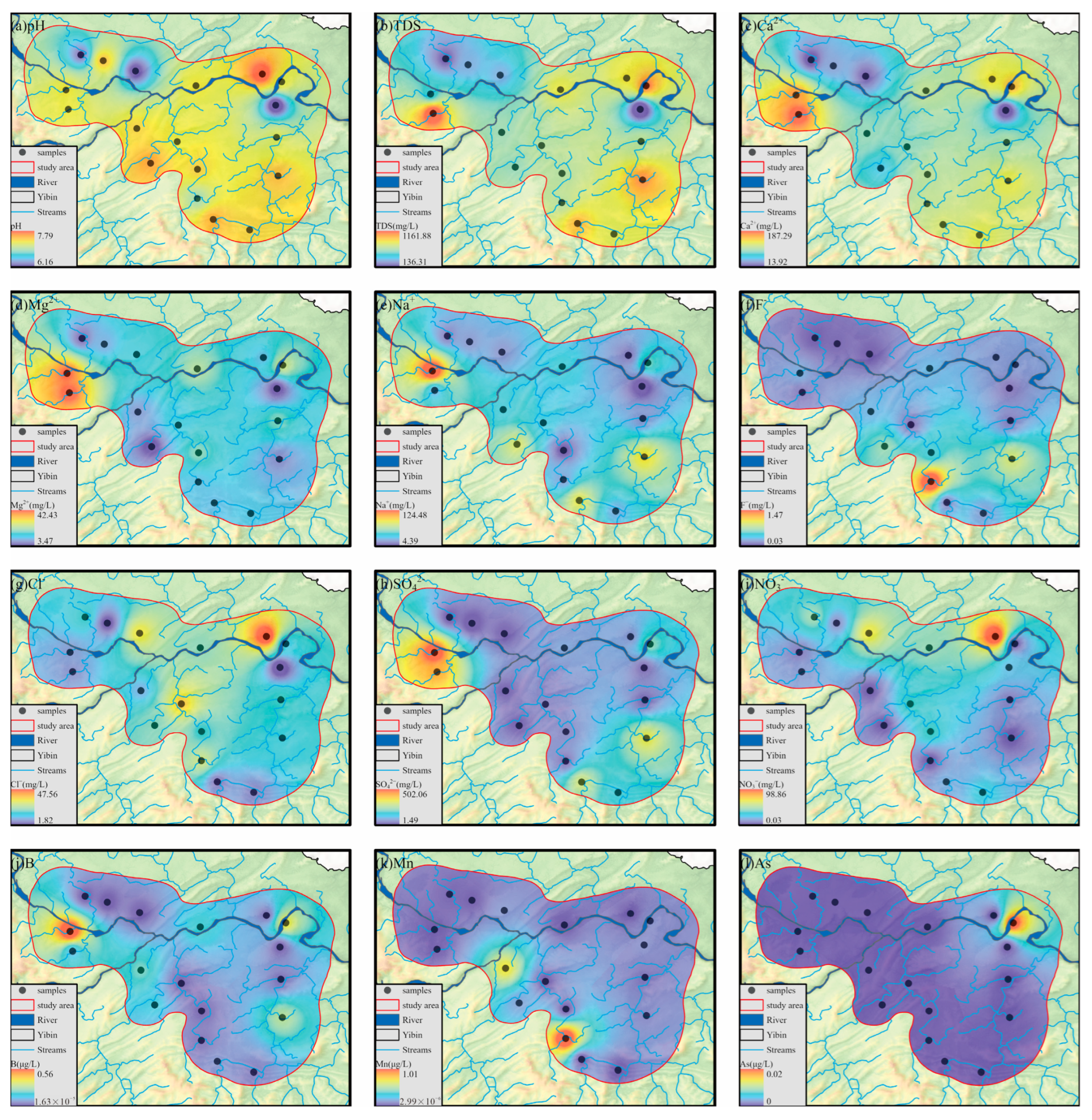
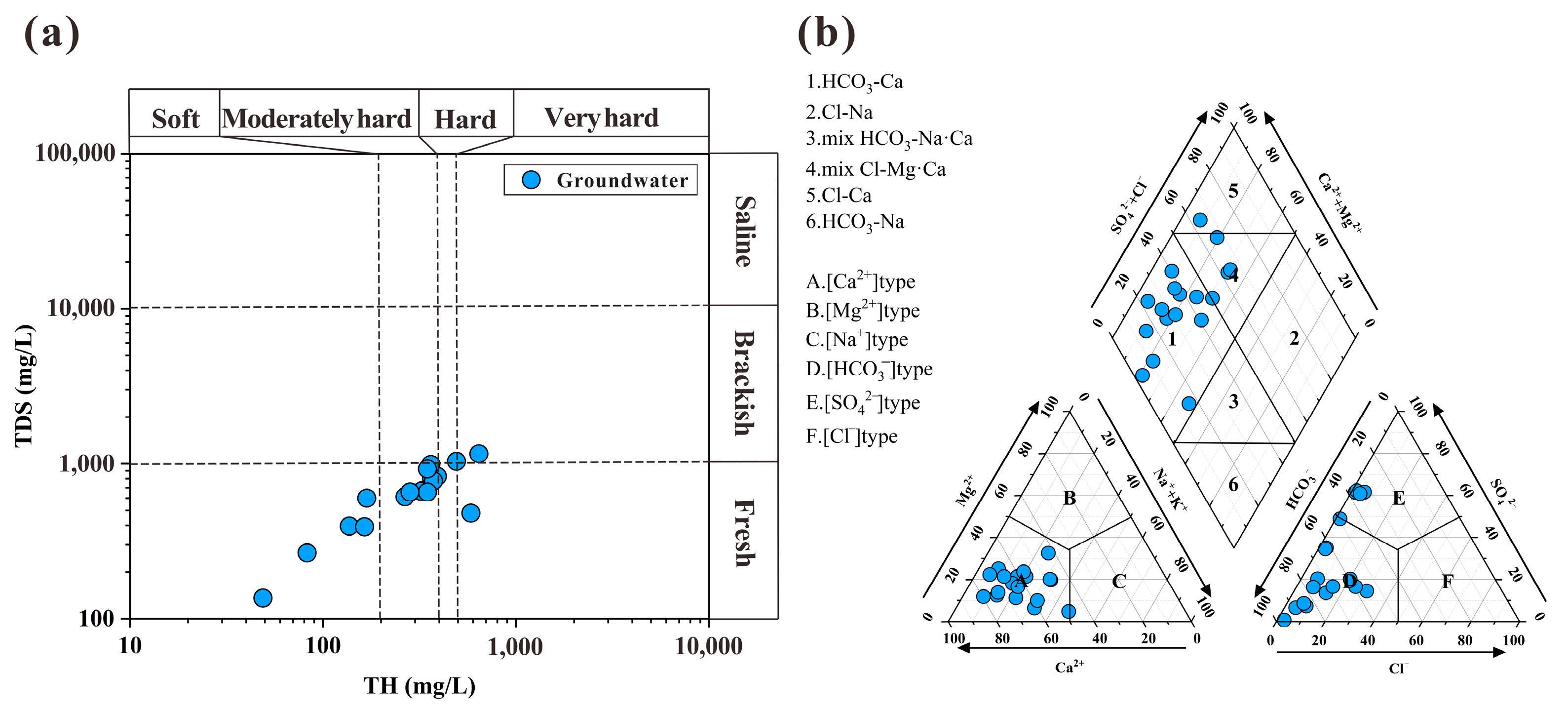
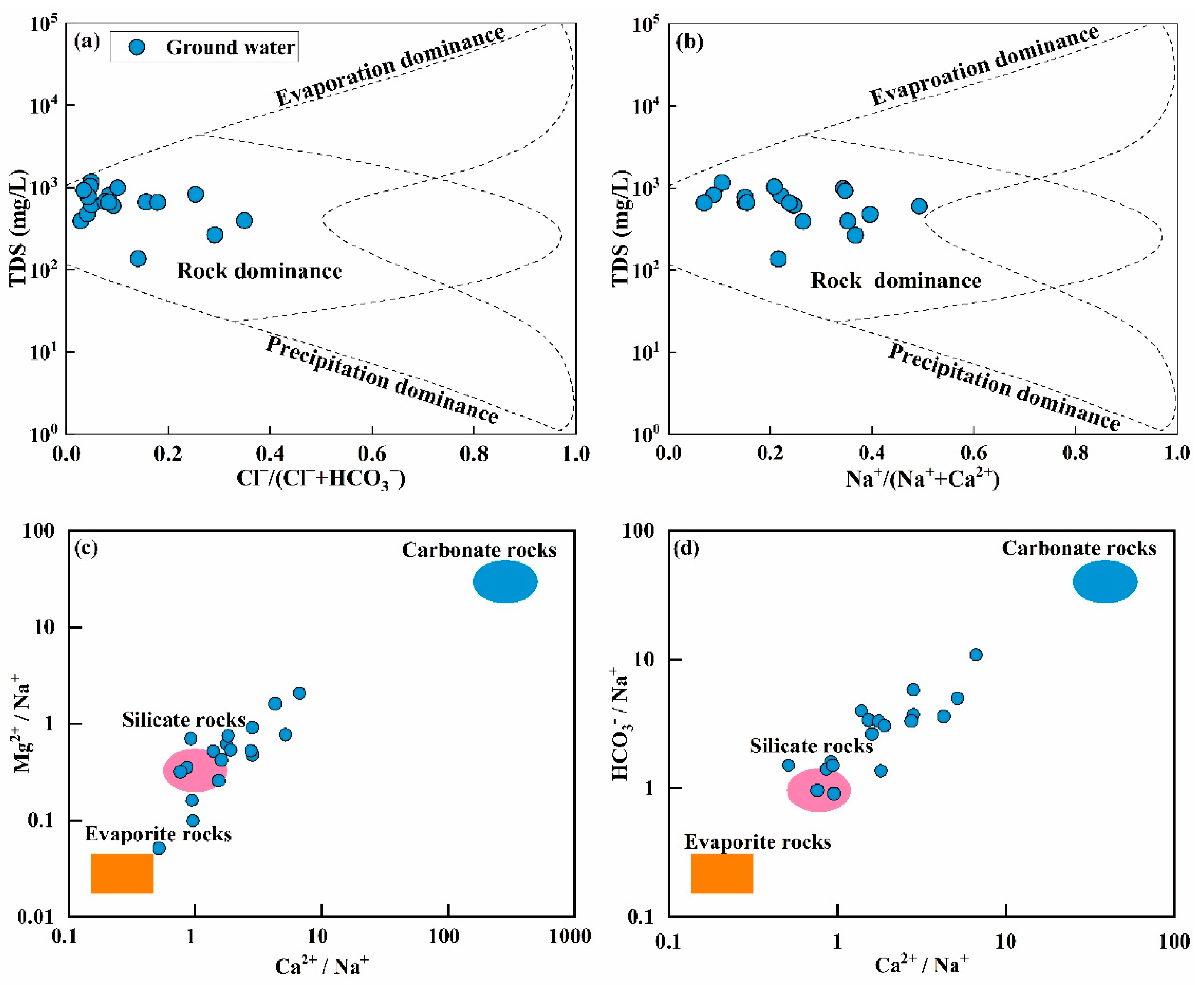
4.1. Hydrogeochemical Characteristics and Driving Factors of Groundwater
4.2. Reverse Hydrogeochemical Simulation
- Selection of simulation path
- 2.
- Determination of probable mineral phases
- 3.
- Saturation index (SI)
- 4.
- The result of hydrogeochemical simulation
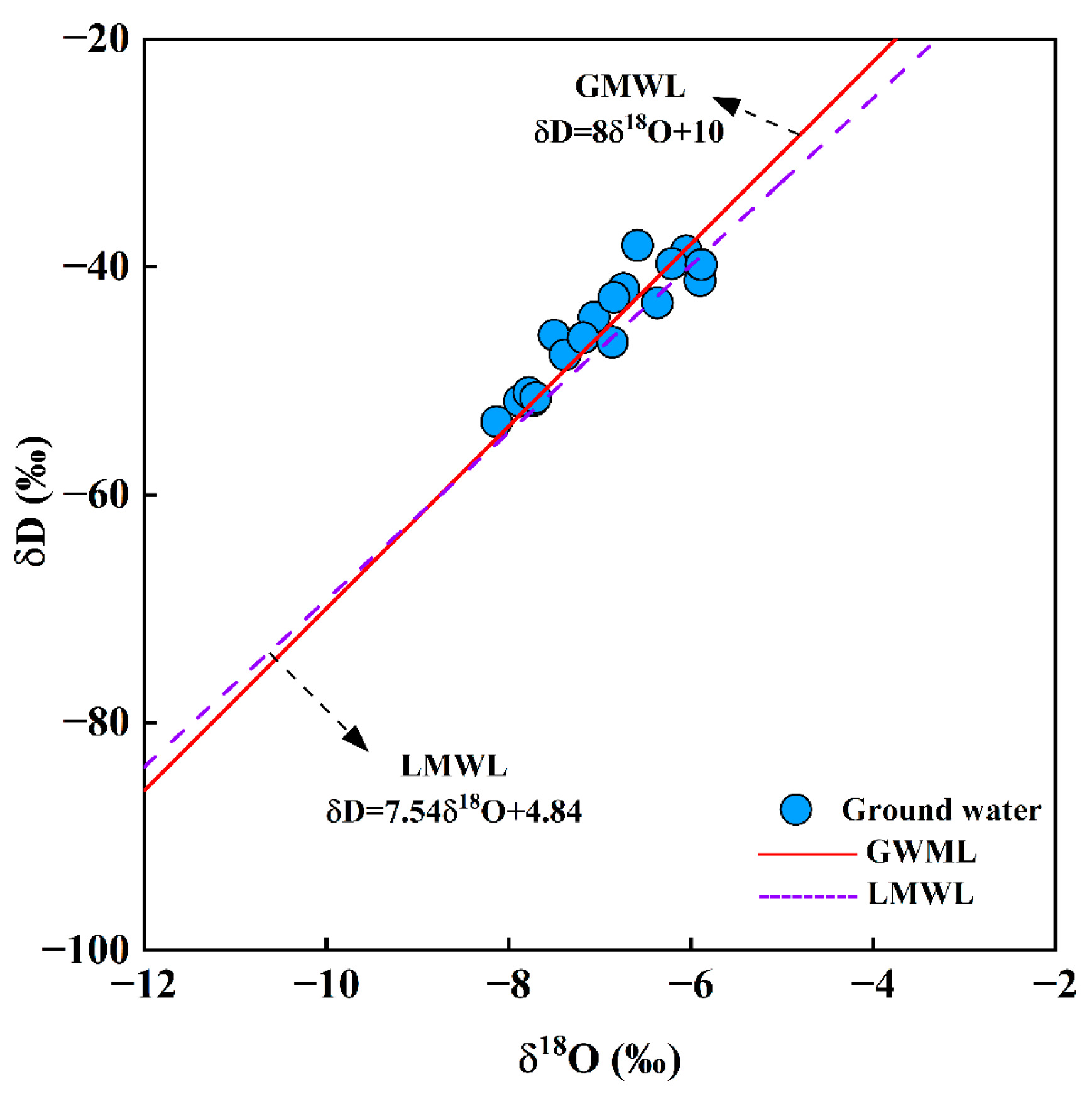
4.3. Isotopic Characterization Analysis
4.3.1. Recharge Source
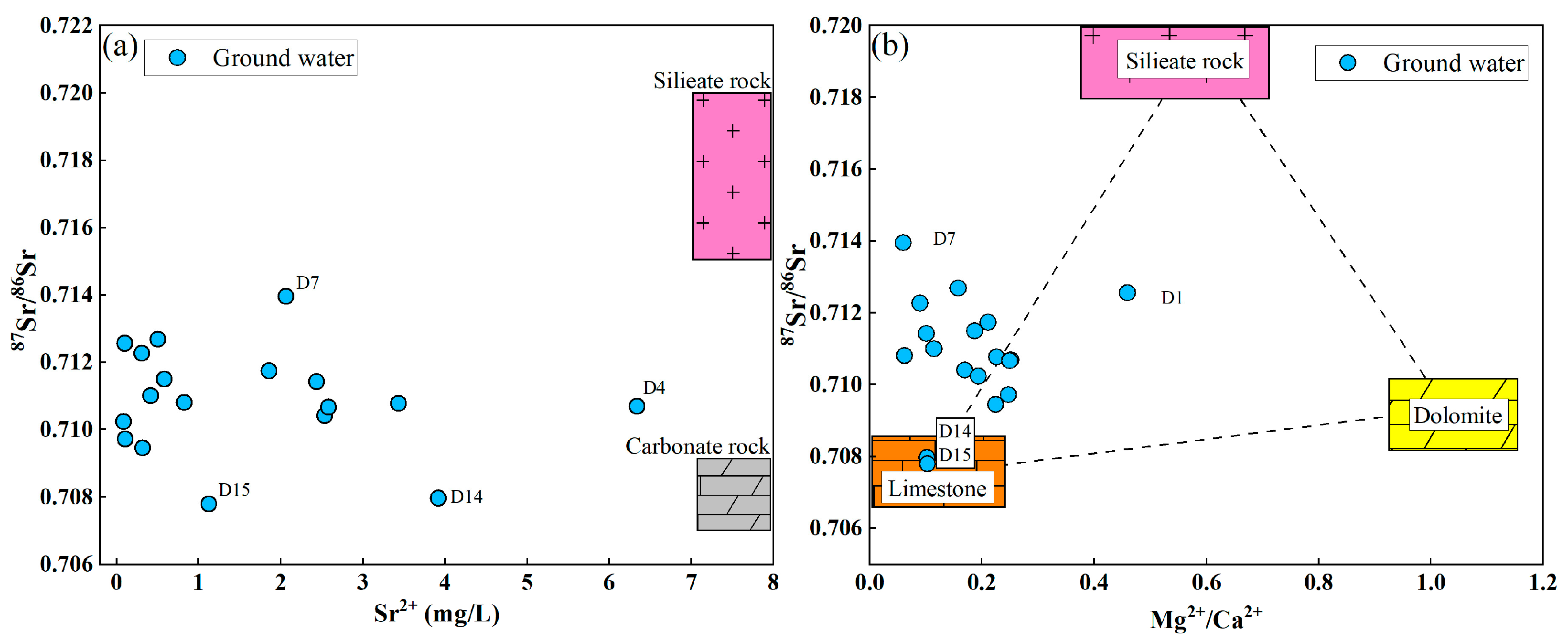
4.3.2. Ion Source Analysis
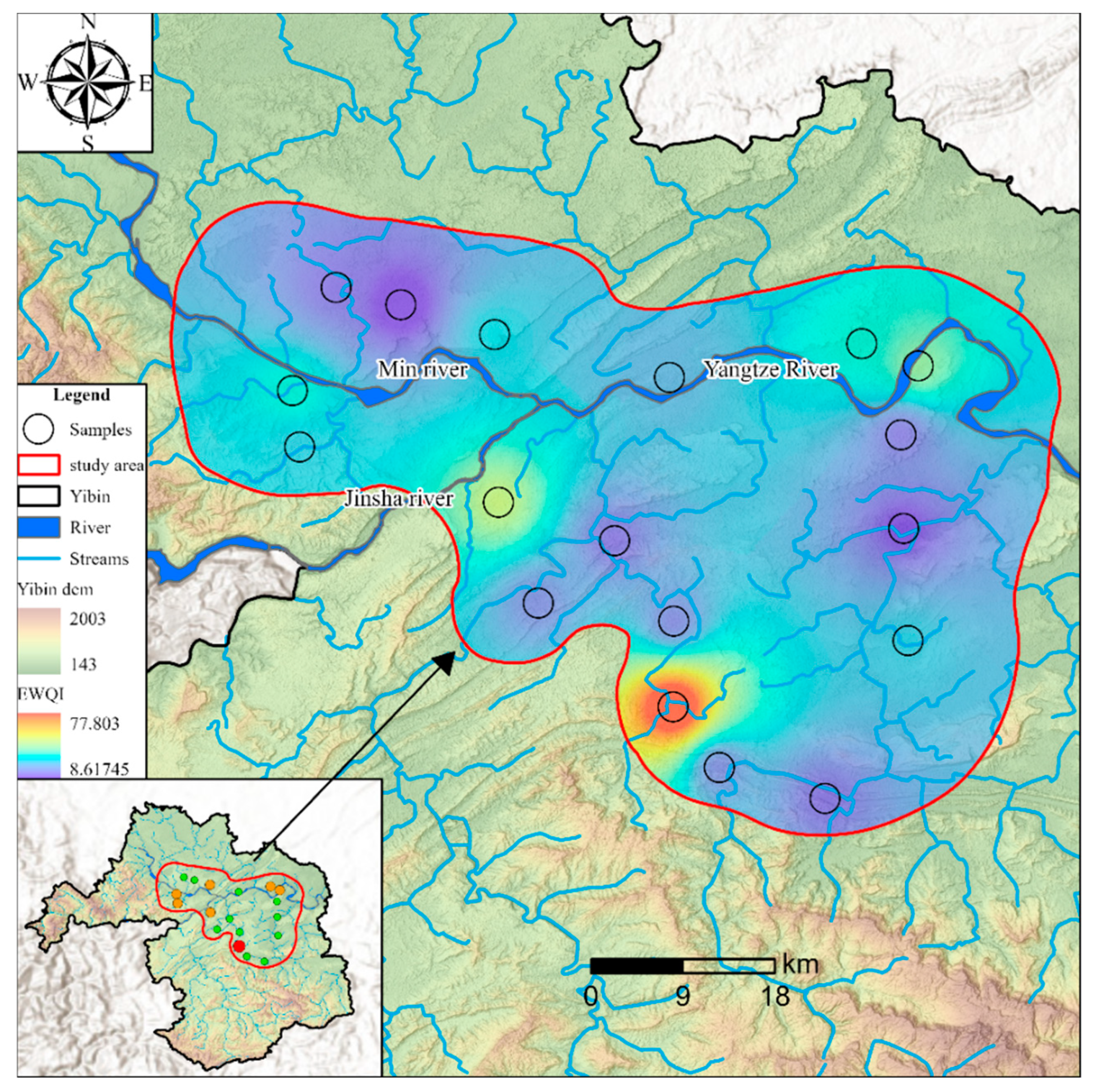
4.4. Entropy-Weighted Water Quality Index (EWQI)
4.5. Health Risk Assessment
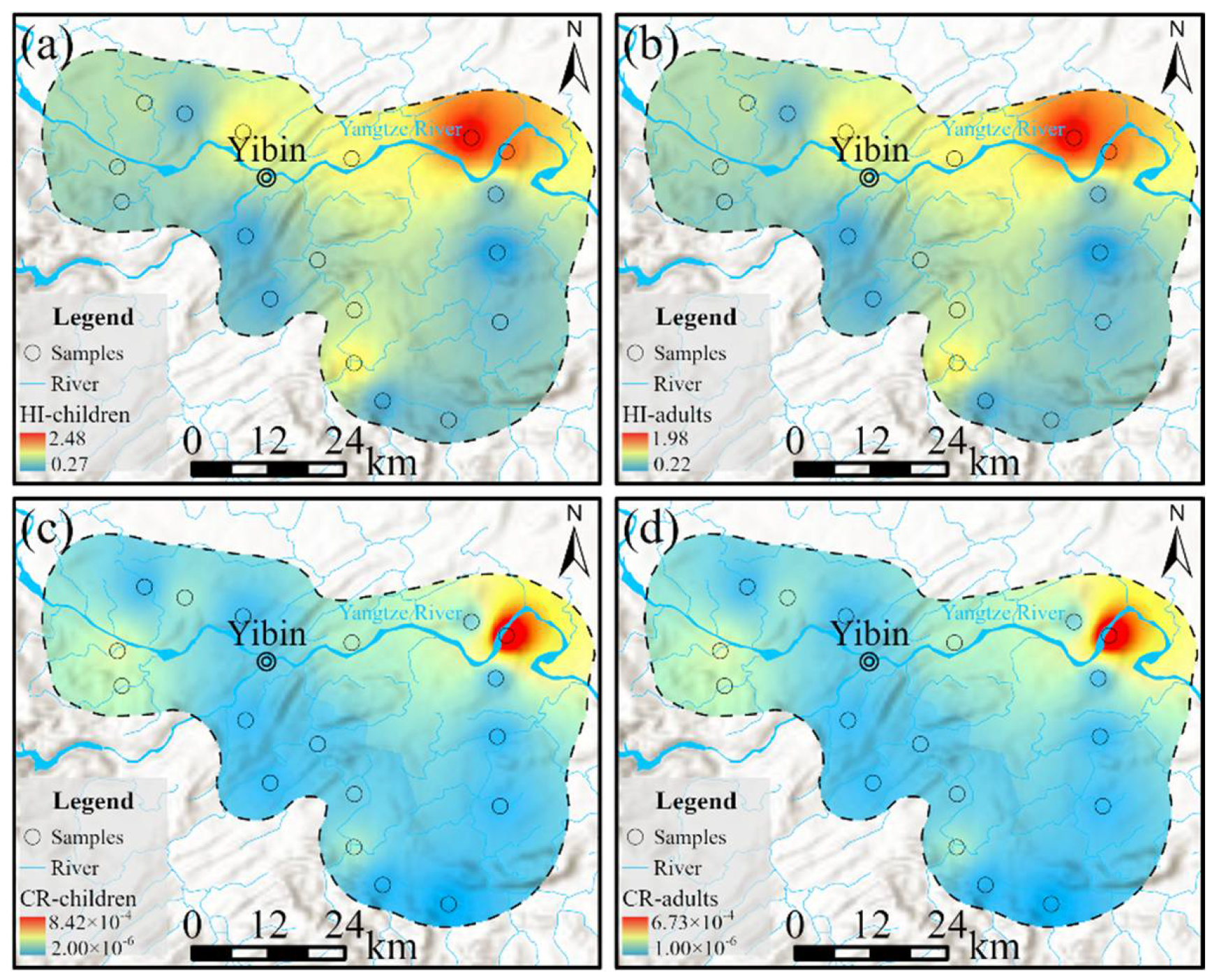
4.5.1. Deterministic Characteristics of Health Risk
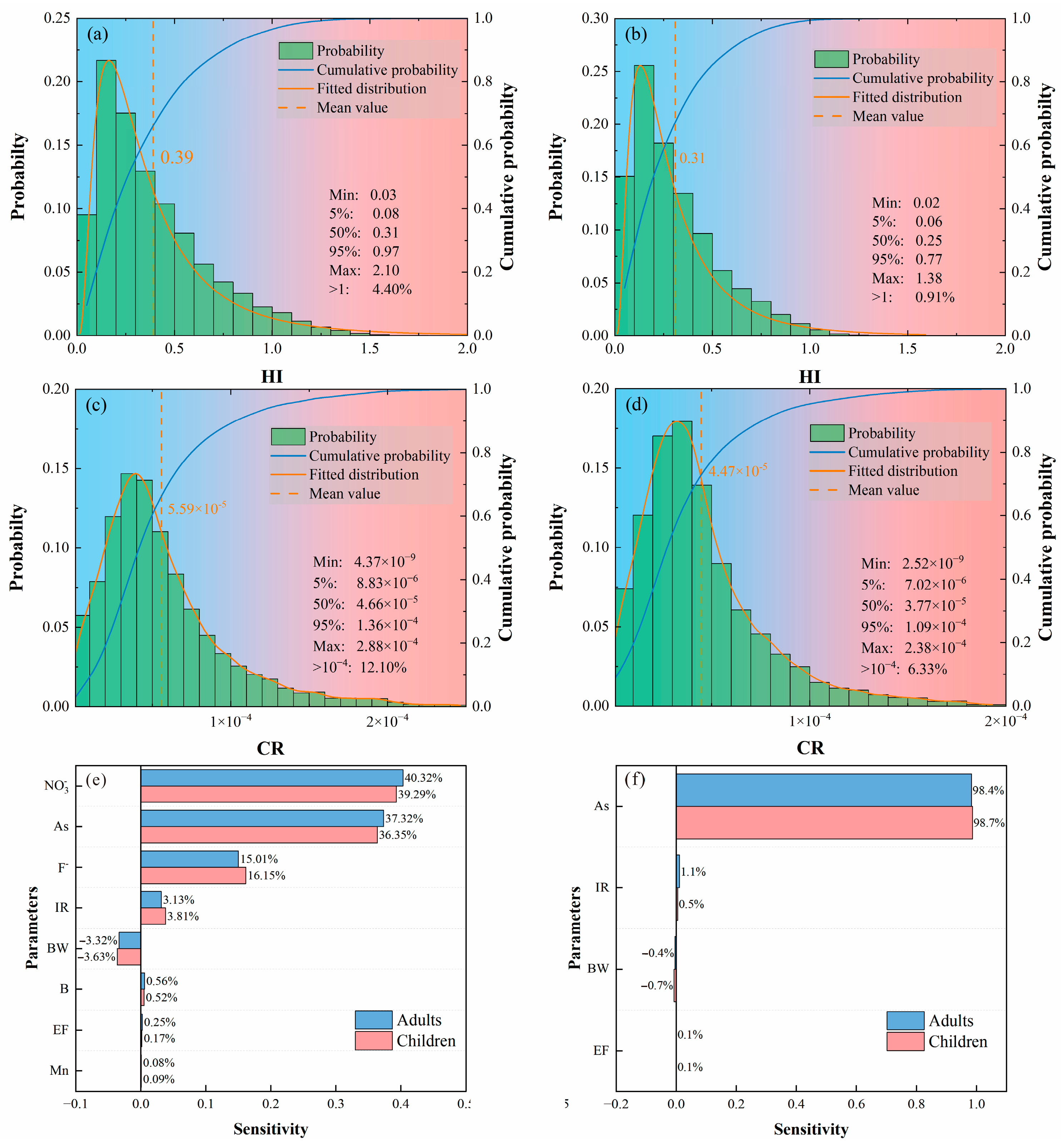
4.5.2. Probabilistic Analysis of Health Risk
4.5.3. Sensitivity Analysis
4.6. Drinking Water Protection Measures and Recommendations
- Given the high hardness of groundwater in the study area, it is recommended that all drinking water undergo uniform softening and purification before consumption.
- Conduct lectures and educational programs on groundwater health risks to raise residents’ awareness of water-related health issues. Encourage the installation of household water purification systems and recommend that residents consume purified groundwater.
- The single location with relatively poor water quality is situated near the industrial park in the southern area of Tongluo Town. As such, local factories should enhance their wastewater purification and treatment processes to reduce the impact of discharged pollutants on groundwater quality.
- Non-carcinogenic health risks associated with groundwater are primarily influenced by NO3− and As, with As being the predominant factor in carcinogenic risks. Therefore, focused control and purification efforts for NO3− and As are particularly important. Given that these contaminants primarily stem from agricultural and industrial activities, increased regulation and preventive measures in these sectors are essential.
5. Conclusions
- There are significant exceedances of pH, TDS, NO3−, Mn, and As in the groundwater. The hardness of the water exhibits a trend ranging from soft to very hard, with the dominant hydrochemical type being HCO3-Ca. Strong cation exchange processes have occurred in the groundwater, with Na⁺ and K⁺ ions likely originating from the dissolution of silicates or cation exchange. The Ca2⁺ and Mg2⁺ ions are jointly controlled by the dissolution of carbonate and silicate minerals, primarily through the dissolution of calcite. The mineral saturation index of the groundwater indicates that dolomite and calcite are in a supersaturated state, with the saturation index of calcite being higher than that of dolomite.
- The primary source of groundwater in the study area is atmospheric precipitation, and the evaporation process in the region is not significant. The Gibbs diagram indicates that water–rock interactions play a dominant role in the formation of the hydrochemical mechanisms of groundwater in the study area, with evaporation and precipitation not being prominent influences. The Sr in the groundwater is likely controlled by the dissolution of carbonate rocks, with its ion sources mainly influenced by the weathering and dissolution of limestone.
- The overall water quality in the study area is good, with only one sample rated as relatively good, accounting for 5.56% of the total samples. Some areas along the riverbanks show a declining trend in water quality. The only point with relatively poor water quality is located near the industrial area in Tongluo Town in the southern part of the study area, suggesting that industrial activities in the south have severely impacted the local groundwater quality, necessitating attention to prevention and remediation measures.
- In the study area, 72.22% of the groundwater samples have non-carcinogenic health risks below the limit of one, while 66.67% of the samples have carcinogenic health risks below the limit of 1.00 × 10−4. The maximum, average, and exceedance ratios of probabilistic results are all lower than those of deterministic results, although the overall trend is similar. Non-carcinogenic risks are primarily influenced by NO3− and As, followed by F−. Children exhibit slightly higher sensitivity to body weight (BW) and intake rate (IR) than adults, while adults are more sensitive to NO3− and As than children. In carcinogenic risks, the concentration of As has a dominant effect. Despite the overall good water quality in the study area, there are still significant human health risks, indicating that the management and control of groundwater will be crucial for reducing these health risks.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uddin, M.J.; Jeong, Y.K. Urban river pollution in Bangladesh during last 40 years: Potential public health and ecological risk, present policy, and future prospects toward smart water management. Heliyon 2021, 7, e06107. [Google Scholar] [CrossRef]
- Trebuch, L.M.; Timmer, J.; van de Graaf, J.; Janssen, M.; Fernandes, T.V. Making waves: How to clean surface water with photogranules. Water Res. 2024, 260, 121875. [Google Scholar] [CrossRef]
- Abbasnia, A.; Yousefi, N.; Mahvi, A.H.; Nabizadeh, R.; Radfard, M.; Yousefi, M.; Alimohammadi, M. Evaluation of groundwater quality using water quality index and its suitability for assessing water for drinking and irrigation purposes: Case study of Sistan and Baluchistan province (Iran). Hum. Ecol. Risk Assess. Int. J. 2019, 25, 988–1005. [Google Scholar] [CrossRef]
- Mukherjee, I.; Singh, U.K. Groundwater fluoride contamination, probable release, and containment mechanisms: A review on Indian context. Environ. Geochem. Health 2018, 40, 2259–2301. [Google Scholar] [CrossRef]
- Al-Mussawi, W.H. Assessment of Groundwater Quality in UMM ER Radhuma Aquifer (Iraqi Western Desert) by Integration Between Irrigation Water Quality Index and GIS. J. Univ. Babylon 2014, 22, 201–217. [Google Scholar]
- Wang, S.; Chen, J.; Zhang, S.; Bai, Y.; Zhang, X.; Jiang, W.; Yang, S. Shallow groundwater quality and health risk assessment of fluoride and arsenic in Northwestern Jiangsu Province, China. Appl. Water Sci. 2024, 14, 119. [Google Scholar] [CrossRef]
- GB/T 14848-2017; GAQS (General Administration of Quality Supervision) and SAC (Standardization Administration of China): Standard for Groundwater Quality. Standardization Administration of the PRC: Beijing, China, 2017.
- Harris, R.C.; Skinner, A.C. Controlling diffuse pollution of groundwater from agriculture and industry. J. Inst. Water Environ. Manag. 1992, 6, 569–574. [Google Scholar] [CrossRef]
- Tokatli, C. Health risk assessment of toxic metals in surface and groundwater resources of a significant agriculture and industry zone in Turkey. Environ. Earth Sci. 2021, 80, 156. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Wu, X.; Yan, Y.; Wei, C.; Luo, M.; Xiao, Y.; Zhang, Y. Evaluation of Groundwater Quality for Drinking and Irrigation Purposes Using GIS-Based IWQI, EWQI and HHR Model. Water 2023, 15, 2233. [Google Scholar] [CrossRef]
- Cervantes-Servin, A.I.; Arora, M.; Peterson, T.J.; Pettigrove, V. Seasonal estimation of groundwater vulnerability. Sci. Rep. 2023, 13, 9720. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, A.J.; McKenzie, J.M.; Hamel, E.; Rudolph, D.L.; Mulligan, B.; de Grandpré, I. Groundwater vulnerability in the Yukon and Northwest Territories, Canada. Hydrogeol. J. 2024, 32, 341–346. [Google Scholar] [CrossRef]
- Maggi, F.T.; Fiona HMTubiello, F.N. Agricultural pesticide land budget and river discharge to oceans. Nature 2023, 620, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, W.; Qiu, Y.; Chen, S.; Wang, D.; Luo, Y.; Qu, S.; Gao, R.; Xue, B.; Wang, G.; et al. Identifying the spatio-seasonal pattern of hydrochemical evolution and surface water-groundwater interaction in a large urban river basin, Northwest China. Sci. Total Environ. 2024, 944. [Google Scholar] [CrossRef]
- Zhang, C.; Dionysiou, D.D.; Wen, R.; Zhang, H.; Wan, X.; Wang, X.; Li, F.; Li, Y.; Zhou, Q.; Ying, G.-G.; et al. Inference of emission history of neonicotinoid pesticides from marine sediment cores impacted by riverine runoff of a developed agricultural region: The Pearl River Basin, China. Water Res. 2022, 218, 118475. [Google Scholar] [CrossRef] [PubMed]
- Othax, N.; Peluso, F.; Castelain, J.G. Health risk due to fluorides, nitrates and arsenic in groundwater: The case of the city of Tres Arroyos, Argentina. Rev. Int. Contam. Ambient. 2014, 30, 27–41. [Google Scholar]
- Tan, H.; Zhang, C.; Li, J.; Zeng, M.; Cheng, Y. Human Health Risk Assessment of Elevated Fe and Mn Intake in Groundwater in Yangtze Catchment. Groundwater 2024, 62, 226–235. [Google Scholar] [CrossRef]
- Jing, X.; Wang, X.; Zhou, J.; Lu, Y. Spatiotemporal variations and health risks of arsenic in soils of the Pearl River Basin, China. Sci. Total Environ. 2024, 951, 175393. [Google Scholar] [CrossRef] [PubMed]
- Adimalla, N.; Qian, H. Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol. Environ. Saf. 2019, 176, 153–161. [Google Scholar] [CrossRef]
- Vlachou, C.; Hofstädter, D.; Rauscher-Gabernig, E.; Griesbacher, A.; Fuchs, K.; König, J. Probabilistic risk assessment of nitrates for Austrian adults and estimation of the magnitude of their conversion into nitrites. Food Chem. Toxicol. 2020, 145, 111719. [Google Scholar] [CrossRef]
- Mosaferi, M.; Jahani Moghaddam, H.; Shaker Khatibi, M.; Esmat Saatloo, S.M.; Nemati Mansour, S.; Nazmara, S. Spatial variation and quantitative screening level assessment of human risk from boron exposure in groundwater resources of western edge of the Lake Urmia, Iran. Int. J. Environ. Health Res. 2020, 30, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, X.; Zheng, X.; Qiang, C.; Zhang, X. Human health risk assessment and spatial distribution of fluoride from shallow groundwater in a region of southwest China. J. Water Supply Res. Technol.-AQUA 2020, 69, 733–747. [Google Scholar] [CrossRef]
- Aboud, A.A.; Tidball, A.M.; Kumar, K.K.; Neely, M.D.; Ess, K.C.; Erikson, K.M.; Bowman, A.B. Genetic risk for Parkinson’s disease correlates with alterations in neuronal manganese sensitivity between two human subjects. NeuroToxicology 2012, 33, 1443–1449. [Google Scholar] [CrossRef][Green Version]
- Teschke, R. Copper, Iron, Cadmium, and Arsenic, All Generated in the Universe: Elucidating Their Environmental Impact Risk on Human Health Including Clinical Liver Injury. Int. J. Mol. Sci. 2024, 25, 6662. [Google Scholar] [CrossRef]
- Tang, L.; Yao, R.; Zhang, Y.; Ding, W.; Wang, J.; Kang, J.; Liu, G.; Zhang, W.; Li, X. Hydrochemical analysis and groundwater suitability for drinking and irrigation in an arid agricultural area of the Northwest China. J. Contam. Hydrol. 2023, 259, 104256. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Shen, S.-L.; Zhou, A. Indices and models of surface water quality assessment: Review and perspectives. Environ. Pollut. 2022, 308, 119611. [Google Scholar] [CrossRef]
- Adimalla, N. Application of the Entropy Weighted Water Quality Index (EWQI) and the Pollution Index of Groundwater (PIG) to Assess Groundwater Quality for Drinking Purposes: A Case Study in a Rural Area of Telangana State, India. Arch. Environ. Contam. Toxicol. 2021, 80, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Umar, R.; Ahmad, I. Assessment of groundwater quality using Entropy-Weighted Quality Index (EWQI) and multivariate statistical techniques in Central Ganga plain, India. Environ. Dev. Sustain. 2024, 26, 1615–1643. [Google Scholar] [CrossRef]
- USEPA. Exposure Factors Handbook, 2011 ed; United States Environmental Protection Agency: Washington, DC, USA, 2011. [Google Scholar]
- Yao, R.; Zhang, Y.; Yan, Y.; Wu, X.; Uddin, M.G.; Wei, D.; Huang, X.; Tang, L. Natural background level, source apportionment and health risk assessment of potentially toxic elements in multi-layer aquifers of arid area in Northwest China. J. Hazard. Mater. 2024, 479, 135663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Du, Q.; Guan, Q.; Luo, H.; Shan, Y.; Shao, W. A Monte Carlo simulation-based health risk assessment of heavy metals in soils of an oasis agricultural region in northwest China. Sci. Total Environ. 2023, 857, 159543. [Google Scholar] [CrossRef]
- Zhang, T.S.; Chen, X.H.; Jiang, Z.Y.; Lan, G.Z. Occurrence of Groundwater and Its Influence Factors in Red Bed Region, Sichuan Basin—A Case Study of Nanchong City. Acta Geol. Sichuan 2005, 25, 97–100. [Google Scholar]
- Li, X.F. Environmental Problems of Water Resources and Sustainable Development Management in Sichuan. Reform Econ. Syst. 2002, 6, 16–18. (In Chinese) [Google Scholar]
- Han, B.; Zhou, W.; Chen, R.; Tian, S.; Gong, H.; Wang, Y.; Xu, Q.; Bian, M. Multi-factor analysis of the quality of cellar mud of Luzhou-flavor liquor in Yibin production area. Food Sci. Nutr. 2024, 12, 5231–5249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, B.; Hu, X. A value assessment system for the conservation and utilization of decentralized distilleries in the architectural heritage of the brewing industry: A case study of Yibin city, China. J. Asian Archit. Build. Eng. 2024. [Google Scholar] [CrossRef]
- Wei, L.; Kong, D.; Luo, Y.; Jia, Y.; Shu, Q. Groundwater Occurrence Environment in the Red Beds of Southern Sichuan and the Hydro-chemical Characteristic:A Case Study in Pingshan County,Yibin City. Explor. Eng. Rock Soil Drill. Tunneling 2018, 45, 145–150. [Google Scholar]
- Apollaro, C.; Buccianti, A.; Vespasiano, G.; Vardè, M.; Fuoco, I.; Barca, D.; Bloise, A.; Miriello, D.; Cofone, F.; Servidio, A.; et al. Comparative geochemical study between the tap waters and the bottled mineral waters in Calabria (Southern Italy) by compositional data analysis (CoDA) developments. Appl. Geochem. 2019, 107, 19–33. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.; Liu, J.; Yang, S.; Wu, X.; Wang, Y. Hydrochemical appraisal and suitability for irrigation and portable purposes in eastern Himalayan syntaxis of Tibet Plateau. Phys. Chem. Earth Parts A/B/C 2024, 136, 103713. [Google Scholar] [CrossRef]
- Petelet-Giraud, E.; Négrel, P.; Casanova, J. Tracing surface water mixing and groundwater inputs using chemical and isotope fingerprints (delta O-18-delta H-2, Sr-87/Sr-86) at basin scale: The Loire River (France). Appl. Geochem. 2018, 97, 279–290. [Google Scholar] [CrossRef]
- Blum, J.D.; Erel, Y.; Brown, K. 87Sr/86Sr ratios of sierra nevada stream waters: Implications for relative mineral weathering rates. Geochim. Cosmochim. Acta 1993, 57, 5019–5025. [Google Scholar] [CrossRef]
- Gautam, U.; Tiwari, V.; Tripathi, V.K. Evaluation of groundwater quality of Prayagraj city using entropy water quality index (EWQI) and new integrated water quality index (IWQI). Sustain. Water Resour. Manag. 2022, 8, 57. [Google Scholar] [CrossRef]
- Paliwal, K.V.; Delhi, N. Irrigation with saline water. Water Technol. Cent. Indian Agric. Res. Inst 1975, 39, 1973. [Google Scholar] [CrossRef]
- Li, P.; Qian, H.; Wu, J. Groundwater Quality Assessment Based on Improved Water Quality Index in Pengyang County, Ningxia, Northwest China. J. Chem. 2010, 7, S209–S216. [Google Scholar]
- Spandana, M.P.; Suresh, K.R.; Prathima, B. Developing an Irrigation Water Quality Index for Vrishabavathi Command Area. Int. J. Eng. Res. Technol 2013, 2, 821–830. [Google Scholar]
- Luo, Y.; Xiao, Y.; Hao, Q.; Zhang, Y.; Zhao, Z.; Wang, S.; Dong, G. Groundwater geochemical signatures and implication for sustainable development in a typical endorheic watershed on Tibetan plateau. Environ. Sci. Pollut. Res. 2021, 28, 48312–48329. [Google Scholar] [CrossRef]
- You, D.; Zhou, Y.; Wang, B.; Li, K.; Chang, J.X.; Lu, C. Groundwater health risk assessment of North China Plain based on Monte Carlo model sensitivity analysis and morphological analysis: A case study of Shijiazhuang City. Water Environ. Res. 2024, 96. [Google Scholar] [CrossRef]
- Alharbi, T.; El-Sorogy, A.S. Groundwater quality and health risk assessment of nitrate and fluoride in Al Qaseem area, Saudi Arabia. Open Chem. 22, 20240042. [CrossRef]
- Aralu, C.C.; Okoye, P.A.C.; Abugu, H.O.; Ochiagha, K.E.; Egbueri, J.C. Evaluating the seasonal variations of risks associated with potentially toxic elements in underground water sources near a dumpsite in Awka, Nigeria. J. Hazard. Mater. Adv. 2024, 15, 100440. [Google Scholar] [CrossRef]
- Freeze, R.A.; Cherry, J.A. Groundwater; Prentice-Hall: Englewood Cliffs, NJ, USA, 1979; p. 370. [Google Scholar]
- Apollaro, C.; Caracausi, A.; Paternoster, M.; Randazzo, P.; Aiuppa, A.; De Rosa, R.; Fuoco, I.; Mongelli, G.; Muto, F.; Vanni, E.; et al. Fluid geochemistry in a low-enthalpy geothermal field along a sector of southern Apennines chain (Italy). J. Geochem. Explor. 2020, 219, 106618. [Google Scholar] [CrossRef]
- Feth, J.H. Mechanisms controlling world water chemistry—evaporation-crystallization process. Science 1971, 172, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Wen, X.H.; Wu, Y.Q.; Wu, J. Hydrochemical characteristics of groundwater in the Zhangye Basin, Northwestern China. Environ. Geol. 2008, 55, 1713–1724. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, D.; Zhou, P.; Qu, S.; Liao, F.; Wang, G. Hydrochemical characteristics of groundwater and dominant water–rock interactions in the Delingha Area, Qaidam Basin, Northwest China. Water 2020, 12, 836. [Google Scholar] [CrossRef]
- Zongxing, L.; Qi, F.; Song, Y.; Wang, Q.J.; Yang, J.; Yongge, L.; Jianguo, L.; Xiaoyan, G. Stable isotope composition of precipitation in the south and north slopes of Wushaoling Mountain, northwestern China. Atmos. Res. 2016, 182, 87–101. [Google Scholar] [CrossRef]
- Liu, J.D.; Zhao, Y.C.; Liu, E.K.; Wang, D. Discussion on the stable isotope time-space distribution law of china atmospheric precipitation. Site Investig. Sci. Technol. 1997, 3, 34–39. (In Chinese) [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhihua, H.U.; Jianji, T.I.A.N.; Fei, L.Y.U. Isotope characteristics of lithium-rich hot springs and its genesis in Batang area of western Sichuan. World Nucl. Geosci. 2024, 41, 88–104. (In Chinese) [Google Scholar]
- Tang, Y.; Wang, X.; Pang, X.; Chen, S.; Zhang, X.; Sun, P. Hydrochemical characteristics and genesis of Xujiahe Formation formation water in Jianyang,central Sichuan: Evidence from hydrochemistry and strontium isotope. Fault-Block Oil Gas Field 2024, 31, 444–452. (In Chinese) [Google Scholar]
- Hernández-Terrones, L.M.; Street, J.; Null, K.; Paytan, A. Groundwater chemistry and Sr isotope ratios shed light on connectivity and water-rock interactions in the coastal aquifer of the Caribbean coast, Mexico. Cont. Shelf Res. 2021, 212, 104293. [Google Scholar] [CrossRef]
- Pu, J.; Daoxian, Y.; Cheng, Z.; Heping, Z. Identifying the Sources of Solutes in Karst Groundwater in Chongqing, China: A Combined Sulfate and Strontium Isotope Approach. J. Geol. Engl. Ed. 2012, 86, 980–992. [Google Scholar]
- Wang, Y.; Yuan, X.; Zhang, Y.; Zhang, X.; Xiao, Y.; Duo, J.; Huang, X.; Sun, M.; Lv, G. Hydrochemical, D–O–Sr isotopic and electromagnetic characteristics of geothermal waters from the Erdaoqiao area, SW China: Insights into genetic mechanism and scaling potential. Ore Geol. Rev. 2023, 158, 105486. [Google Scholar] [CrossRef]
- Yuan, R.; Li, Z.; Guo, S. Health risks of shallow groundwater in the five basins of Shanxi, China: Geographical, geological and human activity roles. Environ. Pollut. 2023, 316, 120524. [Google Scholar] [CrossRef]
- Zheng, Y.; Wei, D.; Gan, J.; Zou, L.; Zhu, R.; Zhang, Y. Hydrochemical insights, water quality, and human health risk assessment of groundwater in a coastal area of southeastern China. Environ. Monit. Assess. 2024, 196, 959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P. Appraisal of shallow groundwater quality with human health risk assessment in different seasons in rural areas of the Guanzhong Plain (China). Environ. Res. 2022, 207, 112210. [Google Scholar] [CrossRef]
- Kaur, L.; Rishi, M.S.; Siddiqui, A.U. Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat, Haryana, India. Environ. Pollut. 2020, 259, 113711. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Yao, R.; Wei, D.; Huang, X.; Luo, M.; Wei, C.; Chen, S.; Yang, C. Natural background levels, source apportionment and health risks of potentially toxic elements in groundwater of highly urbanized area. Sci. Total Environ. 2024, 935, 173276. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qian, H.; Zhou, Y.; Chen, J.; Wang, H.; Ren, W.; Qu, W. Cumulative health risk assessment of multiple chemicals in groundwater based on deterministic and Monte Carlo models in a large semiarid basin. J. Clean. Prod. 2022, 352, 131567. [Google Scholar] [CrossRef]



| Parameters | Unit | Mn | B | As | NO3− | F− |
|---|---|---|---|---|---|---|
| RfD | mg/(kg·d) | 0.14 | 0.2 | 0.0003 | 1.6 | 0.06 |
| SF | kg·d/mg | - | - | 1.5 | - | - |
| Path | Na+ | Mg2+ | K+ | Ca2+ | F− | Cl− | SO42− | HCO3− | pH |
|---|---|---|---|---|---|---|---|---|---|
| D3 | 15.70 | 5.83 | 4.31 | 23.54 | 0.02 | 16.81 | 15.55 | 70.34 | 6.38 |
| D10 | 46.39 | 26.06 | 1.82 | 154.03 | 0.17 | 12.86 | 201.55 | 451.67 | 7.25 |
| Mineral Phase | Chemical Equation |
|---|---|
| Gypsum | CaSO4 = Ca2+ + SO42− |
| Calcite | CaCO3 = Ca2+ + CO32− |
| Dolomite | CaMg(CO3)2 = Ca2+ + Mg2+ + 2CO32− |
| Halite | NaCl = Na+ + Cl− |
| Fluorite | CaF2 = Ca2+ + 2F− |
| CO2 (g) | CO2 + H2O = H2CO3 |
| Path | Samples | Calcite | Dolomite | Fluorite | Gypsum | Halite | CO2 (g) |
|---|---|---|---|---|---|---|---|
| D3 → D10 | D03 | −1.72 | −3.70 | −4.80 | −2.74 | −8.12 | −1.45 |
| D10 | 0.58 | 0.73 | −2.35 | −1.13 | −7.82 | −1.55 |
| Path | Calcite | Dolomite | Fluorite | Gypsum | Halite | CO2 (g) |
|---|---|---|---|---|---|---|
| D3 → D10 | 5.971 × 10−2 | 2.693 × 10−2 | 7.499 × 10−5 | 1.054 × 10−1 | 5.841 × 10−3 | 2.447 × 10−1 |
| Parameters | HI | CR | ||
|---|---|---|---|---|
| Children | Adults | Children | Adults | |
| Min | 0.27 | 0.22 | 2.00 × 10−6 | 1.00 × 10−6 |
| 5% | 0.39 | 0.31 | 1.70 × 10−5 | 1.40 × 10−5 |
| Median | 0.74 | 0.59 | 5.10 × 10−5 | 4.10 × 10−5 |
| Mean | 0.93 | 0.74 | 1.20 × 10−4 | 9.60 × 10−5 |
| 95% | 2.11 | 1.69 | 3.07 × 10−4 | 2.45 × 10−4 |
| Max | 2.48 | 1.98 | 8.42 × 10−4 | 6.73 × 10−4 |
| Unacceptable | 27.78% | 27.78% | 33.33% | 33.33% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yu, J.; Yang, S.; Zhang, Y.; Hu, Q.; Xu, X.; Wang, Y.; Wang, Y.; Luo, H.; Xie, Z. Hydrogeochemistry, Water Quality, and Health Risk Analysis of Phreatic Groundwater in the Urban Area of Yibin City, Southwestern China. Water 2024, 16, 3599. https://doi.org/10.3390/w16243599
Wu X, Yu J, Yang S, Zhang Y, Hu Q, Xu X, Wang Y, Wang Y, Luo H, Xie Z. Hydrogeochemistry, Water Quality, and Health Risk Analysis of Phreatic Groundwater in the Urban Area of Yibin City, Southwestern China. Water. 2024; 16(24):3599. https://doi.org/10.3390/w16243599
Chicago/Turabian StyleWu, Xiangchuan, Jinhai Yu, Shiming Yang, Yunhui Zhang, Qili Hu, Xiaojun Xu, Ying Wang, Yangshuang Wang, Huan Luo, and Zhan Xie. 2024. "Hydrogeochemistry, Water Quality, and Health Risk Analysis of Phreatic Groundwater in the Urban Area of Yibin City, Southwestern China" Water 16, no. 24: 3599. https://doi.org/10.3390/w16243599
APA StyleWu, X., Yu, J., Yang, S., Zhang, Y., Hu, Q., Xu, X., Wang, Y., Wang, Y., Luo, H., & Xie, Z. (2024). Hydrogeochemistry, Water Quality, and Health Risk Analysis of Phreatic Groundwater in the Urban Area of Yibin City, Southwestern China. Water, 16(24), 3599. https://doi.org/10.3390/w16243599







